Abstract
Purpose
Somatic B-RAF gene mutation has been identified in many malignancies and detected at a high frequency in cutaneous malignant melanoma. However, the significance of the B-RAF mutation (B-RAFmt) in terms of its prognostic and predictive capabilities for treatment response or disease outcome is not known. We hypothesized that circulating serum B-RAFmt (B-RAFsmt) at V600E, detected in serum, predicts response in melanoma patients receiving concurrent biochemotherapy.
Experimental Design
A real-time clamp quantitative reverse transcription-PCR assay was designed to assess B-RAFsmt by peptide nucleic acid clamping and a locked nucleic acid hybrid probe. Normal (n = 18) and American Joint Committee on Cancer stage I to IV melanoma patients (n = 103) were evaluated. These included stage IV patients (n = 48) with blood drawn before and after biochemotherapy. Patients were classified as biochemotherapy responders or nonresponders. Responders (n = 24) had a complete or partial response following biochemotherapy; nonresponders (n = 24) developed progressive disease.
Results
Of the 103 melanoma patients, 38 (37%) had B-RAFsmt DNA, of which 11 of 34 (32%) were stage I or II, and 27 of 69 (39%)were stage III or IV. Of the 48 biochemotherapy patients,10 of 24 (42%) patients were positive for the B-RAFsmt in the respective responder and nonresponder groups before treatment. After biochemotherapy, B-RAFsmt was detected in only 1of 10 patients (10%) in the responder group and 7 of 10 patients (70%) in the nonresponder group. B-RAFsmt is associated with significantly worse (P = 0.039) overall survival in patients receiving biochemotherapy.
Conclusion
These studies show the presence and utility of circulating B-RAFsmt DNA in melanoma patients.
The management of cutaneous melanoma continues to pose a significant challenge. Clinical prognostic factors have not been shown to predict disease recurrence and overall survival in patients with metastatic disease. Adjuvant therapy for melanoma can have major side effects and can be associated with significant morbidity. In addition, it has been difficult to identify which patients will respond to the few treatment options available and to predict disease recurrence and progression.
Over the last decade, advances in melanoma translational research have attempted to identify key components in molecular and genetic alterations that affect the progression of this disease (1). High-throughput genomic approaches have been focused on identifying gene aberrations in the RAS-RAF-mitogen-activated protein/extracellular signal-regulated kinase (MAP/ERK) and (MEK)-ERK-MAP kinase (MAPK) signaling pathways because they have been shown to regulate cellular differentiation, proliferation, and apoptosis (2–4).
B-RAF mutations (B-RAFmt) have been reported at a high frequency in melanoma, thyroid, and lung cancer (5–8). B-RAF encodes a serine/threonine kinase downstream for RAS in the MAPK pathway that transduces regulatory signals from RAS through MAPK (8–11). B-RAFmt have been found at multiple sites, whereby clustering around exons 11 and 15 of the gene in the kinase domain is quite frequent (5, 6, 12). In our recent study evaluating the frequency of B-RAFmt in melanoma progression, we found that the V600E (formerly V599E) amino acid missense mutation resulting from a 1796T → A transversion in exon 15 of B-RAF was the predominant mutation in the tumors assessed, and the mutation was found in 31% of primary melanoma and 57% of metastatic melanoma tumors (13). Because this mutation has been shown to significantly increase kinase activity and occurs at a significantly higher frequency than other gene mutations found in melanoma, such as N-RAS, p16INK4a, and p53 (5, 14, 15), we hypothesized that the presence of circulating DNA with B-RAFmt at V600E in the serum of melanoma patients may be clinically relevant. We have previously shown that circulating DNA in the serum of melanoma patients has clinical utility as a marker for disease progression, identification of occult recurrences, and predicting response to surgical and adjuvant therapy (16–18). Mori et al. showed a correlation between circulating methylated DNA in serum and disease progression and showed an association between circulating methylated DNA and response to biochemotherapy (16, 19).
In this study, we developed a peptide nucleic acid (PNA) clamp– and locked nucleic acid (LNA) probe technique–based quantitative real-time PCR assay to detect serum-circulating B-RAFsmt DNA of melanoma patients. The detection of single base pair mutations in circulating DNA requires a very sensitive assay because the frequency of circulating B-RAFsmt DNA will be low. PNAs and LNAs are high-affinity DNA synthetic analogues that hybridize with complementary DNA (20). PNAs have N-(2-aminoethyl)-glycine units as backbones. PNA-DNA hybrids are more stable than those for cDNA-DNA and are highly sensitive and specific in distinguishing single base pair mismatches. In addition, as PNA oligomers cannot function as primers in PCR reactions, they are used as blockers to prevent amplification of wild-type DNA templates (21). LNAs also have higher affinity to DNA than cDNA and were incorporated into our assay for their specificity in recognizing single base pair mismatches. LNA-DNA chimeras can be constructed for use as primers or probes. Highly specific detection of B-RAFsmt was achieved using a specific PNA clamping and LNA hybridizing probe.
The purpose of this study was to determine whether we could identify B-RAF V600E mutation on exon 15 as circulating DNA in the serum of melanoma patients and to determine whether quantitative detection of the B-RAFmt could have potential clinical applicability in evaluating noninvasive disease progression or quantitative evaluation of therapeutic maneuvers. To date, detection of B-RAFmt (V600E) in melanoma tissue has not shown any significant correlation to disease outcome, although B-RAFmt in metastatic melanoma can be frequently detected in >55% of patients. In this study, we detected amounts of circulating mutant DNA with high sensitivity and specificity. To further investigate the possible clinical implications of the presence of the B-RAFsmt DNA, we collected sera from patients before and after treatment with biochemotherapy. We hypothesized that the presence of B-RAFsmt in posttreatment serum may indicate absence of response to treatment.
Materials and Methods
Patients and cell line
Fifty-five patients with different American Joint Committee on Cancer stages of melanoma were assessed for B-RAFsmt. In addition, 50 stage IV melanoma patients who received biochemotherapy were also included for the treatment response study. Blood was drawn within 1 week before the start of biochemotherapy and within 4 weeks after the last cycle. The median completed cycles of biochemotherapy were six for the responder group and three for the nonresponder group. The maximum number of cycles received were six cycles, as previously described (22, 23). These patients were further divided into two groups based on their response to biochemotherapy (responders and nonresponders). Patients whose tumors decreased in size after treatment (partial response and complete response) are grouped as responders (n = 24), whereas those that had progressive disease are grouped as nonresponders (n = 24). Two patients had stable disease and were removed from the final statistical analysis. The biochemotherapy regimen was administered in 5-day periods at 21-day intervals and included the administration of multiagent chemotherapy, consisting of dacarbazine, cisplatin, vinblastine, and tamoxifen, with the addition of the biological response modifiers, interleukin 2 and IFNα-2b. Patients were accrued through both the John Wayne Cancer Institute and The Angeles Clinic and Research Institute. Human Subjects Institutional Review Board approval was obtained for the purposes of this study at the participating institutions. Signed informed consent was obtained from all patients. Serum samples from 18 healthy donors, which served as controls, were also analyzed.
Fourteen melanoma cell lines established and characterized at the John Wayne Cancer Institute, as previously described, were assessed for B-RAFmt (V600E; refs. 24–26). The cell lines were grown in 10% heat-inactivated FCS (Gemini, Calabasas, CA). RPMI 1640 plus penicillin and streptomycin, as previously described, and assessed at early passages (24). DNA was extracted from cells when cultures reached 70% to 80% confluency.
DNA extraction
Blood was collected from patients in TigerTop separation tubes (Fisher Scientific). Serum was immediately separated from blood cells by differential centrifugation at 1,000 × g for 15 min, filtered through a 13-mm serum filter (Fisher Scientific, Pittsburgh PA), and cryopreserved at −80°C. DNA was isolated from the serum using Qiagen mini-columns (Valencia, CA) according to the manufacturer’s instructions, with modifications. DNA was precipitated with 1 µL of Pellet Paint NF coprecipitant (Novagen, Madison, WI) before the proteinase-digested samples were centrifuged. DNA from cell lines was extracted using DNAzol (Molecular Research Center, Cincinnati, OH), as previously described (27). All serum specimens were shown to have DNA.
Oligo design
Briefly, primers were designed to amplify exon 15 of the B-RAF gene, including the mutation hotspot (V600E). PNA (Applied Biosystems, Foster City, CA) was designed to clamp the hotspot on the wild-type (wt) template and block the wt template from being amplified by PCR. A fluorescence resonance energy transfer (FRET) dual-labeled LNA probe was designed and synthesized (Proligo, Boulder, CO) to recognize and hybridize at V600E, specifically the T-to-A mutation, as this mutation is the most frequently seen mutation for B-RAF at this hotspot (5). A second FRET DNA probe was purchased from Biosource (Camarillo, CA) and synthesized using the adjacent sequences to the LNA probe, avoiding the hotspot, to amplify and estimate the total number of DNA templates, both wild type (V600E) and mutant (V600E), in the PCR reaction. Real-time quantitative PCR for mutation using both the PNA clamp and FRET LNA probe was done in a separate reaction from the quantitative PCR for total number of templates using the FRET DNA probe.
Real-time quantitative PCR and quantification of B-RAFmt
PCR was done using the following primers and probe: B-RAF, 5′-CCTCACAGTAAAAATAGGTG-3′ (forward), 5′-ATAGCCTCAATTCTTACCA-3′ (reverse), 5′-CTACAGAGAAATCTCGAT-BHQ-1-3′ (LNA), CTACAGTGAAATCTCG (PNA). The PCR assay was done with the iCycler iQ real-time PCR Detection System (Bio-Rad Laboratories, Hercules, CA; Fig. 1). Genomic DNA (20 ng) from serum was amplified using real-time PCR (iCycler) in a 20-µL reaction containing each PCR primer, LNA, PNA, deoxynucleotide triphosphate, MgCl2, PCR buffer, and AmpliTaq Gold Polymerase (Applied Biosystems, Branchburg, NJ). Each PCR reaction was subjected to 55 cycles at 94°C for 60 s, 72°C for 50 s, 53°C for 50 s, and 72°C for 60 s. Each sample was assayed in triplicate with appropriate positive and negative cell line and reagent controls.
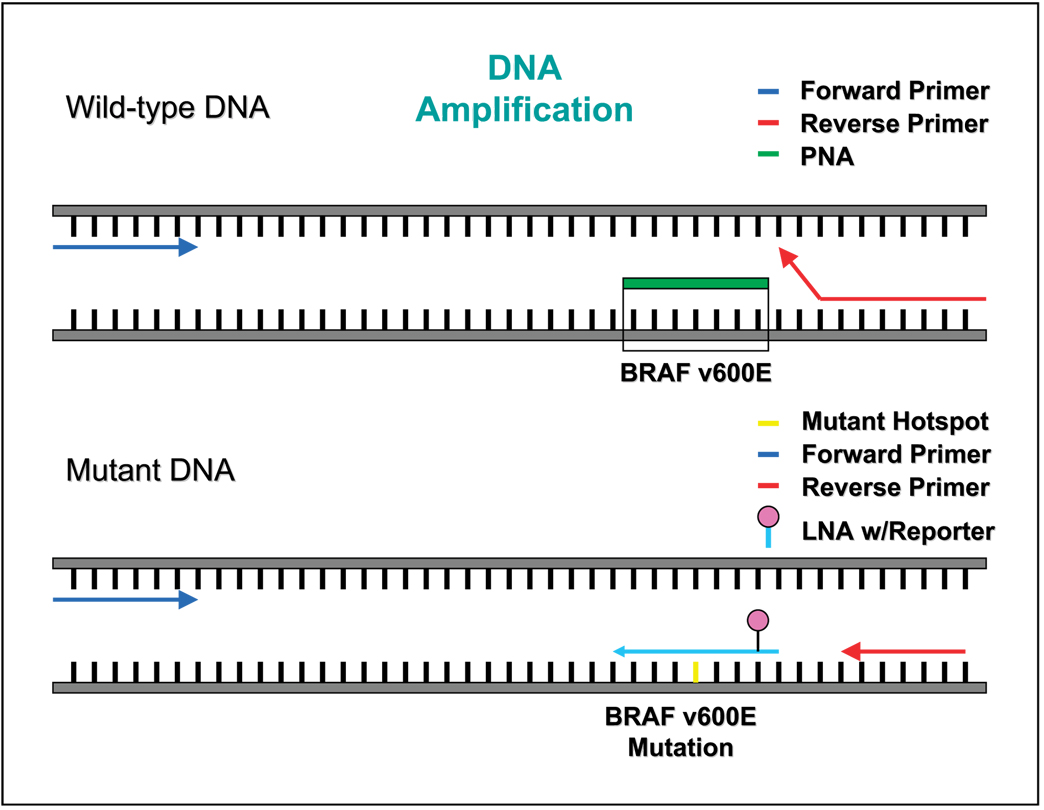
Fig. 1.
Schematic of PNA/LNA clamp directed PCR.Top, PNA/wt DNA complex, with no amplification. Bottom, amplification of DNA template containing B-RAFmt using the dual-labeled LNA probe that recognizes and hybridizes toV600E.
We established the MA cell line DNA as the standard for measuring units of V600E B-RAFmt target DNA (heterozygous); the amount of target mutant DNA contained in 1 µg/mL MA genomic DNA was arbitrarily established to be 1 unit of B-RAFmt. Quantitative PCR results of the samples, generated by iCycler, were compared with this standard to quantify the relative units of B-RAFmt in all the samples. All PCR assays for mutant sequence analysis were done in triplicate, and the median was used for data analysis.
Representative B-RAFmt V600E and B-RAFwt V600E tumors (n = 4) were sequenced to confirm the accuracy of the PCR assay, as previously described (5). PCR amplification was done using the following primers for B-RAF: 5′-TGTTTTCCTTTACTTACTACACCTCA-3′ (forward) and 5′-AGCATCTCAGGGCCAAAAAT-3′ (reverse). The PCR products were purified with QIAquick PCR Purification kit (Qiagen) and subsequently direct-sequenced at 58°C using Dye Terminator Cycle Sequence Quick Start kit (Beckman Coulter, Fullerton, CA) according to the manufacturer’s instructions. Dye-terminated products were assessed by capillary array electrophoresis on a CEQ8000XL Genetic Analysis System (Beckman Coulter).
Biochemotherapy response: evaluation of B-RAFsmt
For data analysis, we used the ratio of B-RAFsmt DNA copies (in units equivalent to V600E mutation copies in µg/mL MA DNA) to total B-RAFwt DNA templates (in units equivalent to V600E wt copies in 1 µg/mL DNA) in the reaction as reported results. Mutant DNA copies were calculated by quantitative PCR using a V600E mutant-specific FRET LNA probe with dilution series of MA DNA for the standard curve; total B-RAFwt DNA template copies were measured by quantitative PCR with the FRET DNA probe to the V600E region. If the ratio for the post-biochemotherapy serum decreased by one tenth or more when compared with the pre-biochemotherapy serum sample, we determined that the patient “decreased” in B-RAFsmt; if the ratio increased by ≥10-fold, it was designated as “increased.”
Biostatistical analysis
All clinicopathologic factors and B-RAFsmt frequency were compared by Student’s t test and Fisher’s exact test. Kaplan-Meier survival curve analysis was used to assess overall and disease-free survival. Univariate analysis of prognostic factors, including age, gender, Eastern Cooperative Oncology Group status, the number of metastatic sites, the site of metastases (soft tissue, lymph nodes, and lung versus other organs), lactate dehydrogenase (LDH) levels, and prior previous treatment (vaccine, chemotherapy, and/or IFN versus no treatment) was assessed. A multivariate analysis using the Cox proportional hazard regression model was also done to evaluate the prognostic significance of B-RAFsmt when clinical prognostic factors were adjusted. All analyses were done using SAS (SAS/STAT User’s Guide, version 8; SAS Institute, Inc., Cary, NC), and tests were two sided with a significance level of <0.05.
Results
B-RAFmt assay sensitivity
Using the melanoma cell line MA, shown to have B-RAFmt V600E, we did several serial dilution studies to determine the sensitivity of the PNA clamp with the LNA hybridizing probe assay using real-time quantitative PCR. MA DNA in µg/mL was diluted in lymphocyte DNA from normal individuals to simulate an in vivo model. The B-RAFmt could be detected in 1 × 10−4 unit of MA DNA diluted in 10 units of lymphocyte DNA. However, no B-RAFmt (V600E) was detected when 1 × 10−5 unit of MA DNA was diluted in 10 units of lymphocyte DNA. In this series of dilutions, we observed that at >10 units of DNA as a template, the assay will detect amplification of the B-RAFwt (V600E) gene as there is only a limited quantity of PNA in each reaction to block amplification of wt DNA. In keeping with this observation, we assessed each sample to estimate the quantity of nonspecific copies to ensure that the DNA templates did not exceed an amount that would result in depletion of the PNA in the reaction based on comparisons with the threshold cycle of the MA DNA dilution series with known units of DNA.
The PNA/LNA PCR assay was subsequently optimized in melanoma cell lines. Fourteen melanoma cell lines were assessed for B-RAFmt (V600E), of which 8 (57%) were found to have the B-RAFmt. The detection of B-RAFmt (V600E) was further validated by sequencing the genomic DNA of the cell lines.
B-RAFsmt of melanoma patients’ sera
Of 103 melanoma patients in the study, including patients treated with biochemotherapy, 38 (37%) patients had B-RAFsmt detected in their serum. Furthermore, when the patients were divided based on early and advanced stages of disease, B-RAFsmt was detected in 11 of 34 (32%) early-stage patients (American Joint Committee on Cancer stage I/II) and in 27 of 69 (39%) with metastatic disease (American Joint Committee on Cancer stage III/IV). B-RAFsmt was not detected in any of the 18 healthy normal donor serum samples.
B-RAFsmt in stage IV melanoma patients
The frequency of the B-RAFsmt in 50 stage IV melanoma patients before biochemotherapy was compared with known prognostic factors in melanoma (Table 1). These factors included age, gender, Eastern Cooperative Oncology Group status, the number of metastatic sites, the site of metastases (soft tissue, lymph nodes, and lung versus other organs), LDH levels, and prior previous treatment (vaccine, chemotherapy, and/or IFN versus no treatment). B-RAFsmt DNA was detected in 20 (42%) patients. The frequency of B-RAFmt and B-RAFwt DNA was compared with known prognostic factors. Of the factors considered, significant differences were seen in patients who had metastases in soft tissue, lymph nodes, and lung versus other organs (P < 0.021) and patients who presented with higher LDH levels (P < 0.027; Table 1).
Table 1.
Frequency of B-RAFsmt when compared with known clinical prognostic factors in stage IV melanoma patients receiving biochemotherapy
| Clinical factors | B-RAFsmt (N = 20) | B-RAFswt (N = 30) | P |
|---|---|---|---|
| Age | |||
| Mean ± SD | 43.4 ± 10.8 | 45.9 ± 11.8 | 0.629 |
| ≤50 | 15 | 19 | 0.386 |
| >50 | 5 | 11 | |
| Gender | |||
| Female | 4 | 8 | 0.740 |
| Male | 16 | 22 | |
| ECOG | |||
| 0–1 | 8 | 18 | 0.166 |
| 2 | 12 | 12 | |
| No. Met sites | |||
| 1–2 | 10 | 20 | 0.239 |
| ≥3 | 10 | 10 | |
| Met sites | |||
| ST/LN/lung only | 2 | 12 | 0.021 |
| Other | 18 | 18 | |
| CNS Met | 15 | 28 | 0.100 |
| LDH | |||
| Mean ± SD | 500.4 ± 857.2 | 295.2 ± 280.7 | 0.037 |
| ≤190 | 5 | 17 | 0.027 |
| >190 | 15 | 13 |
NOTE: B-RAFwt at V600E.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ST, soft tissue; LN, lymph node; CNS, central nervous system.
To determine the prognostic significance of B-RAFsmt in patients after treatment with biochemotherapy, clinicopathologic variables were compared by a univariate analysis (Table 2). Age and gender were not significant predictors of response to treatment. However, Eastern Cooperative Oncology Group status (P = 0.049), the site of metastases (P = 0.019), and LDH levels (P = 0.041) significantly predicted treatment response. The presence of pre-biochemotherapy B-RAFsmt was not a significant predictor of response to treatment.
Table 2.
Univariate analysis of response of biochemotherapy patients
| Clinical factors | CR, PR, SD (N = 26) | PD (N = 24) | P (χ2 test) |
|---|---|---|---|
| Age | |||
| Mean ± SD | 46.8 ± 11.9 | 42.9 ± 10.6 | 0.341 |
| ≤50 | 15 | 19 | 0.104 |
| >50 | 11 | 5 | |
| Gender | |||
| Female | 5 | 7 | 0.411 |
| Male | 21 | 17 | |
| ECOG | |||
| 0–1 | 17 | 9 | 0.049 |
| 2 | 9 | 15 | |
| Met sites | |||
| ST/LN/Lung only | 11 | 3 | 0.019 |
| Other | 15 | 21 | |
| No. Met sites | |||
| 1–2 | 17 | 13 | 0.419 |
| ≥3 | 9 | 11 | |
| LDH | |||
| Mean ± SD | 264.0 ± 259.0 | 500.0 ± 790.7 | 0.041 |
| ≤190 | 14 | 8 | 0.144 |
| >190 | 12 | 16 |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ECOG, Eastern Cooperative Oncology Group; ST, soft tissue; LN, lymph node.
Variables found to affect response to biochemotherapy (P < 0.05) upon univariate analysis were analyzed by Cox multivariate regression analysis. These variables included age, gender, Eastern Cooperative Oncology Group status, number of metastatic sites, site of metastases (soft tissue, lymph nodes, and lung versus other organs), LDH levels, and prior previous treatment (vaccine, chemotherapy, and/or IFN versus no treatment). Of the factors considered, only Eastern Cooperative Oncology Group status (hazard ratio, 0.24, 95% confidence interval, 0.06–0.98; P = 0.047), site of metastases (hazard ratio, 11.5, 95% confidence interval, 1.62–82.5; P = 0.015), and previous treatment (chemotherapy and/or IFN versus no treatment: hazard ratio, 0.12, 95% confidence interval, 0.02–0.92; P = 0.041) were significant predictors of tumor response to biochemotherapy. However, the presence of pre-biochemotherapy B-RAFsmt (hazard ratio, 2.2; 95% confidence interval, 0.49–9.80; P = 0.30) did not significantly correlate with tumor response to biochemotherapy.
Circulating B-RAFsmt and survival
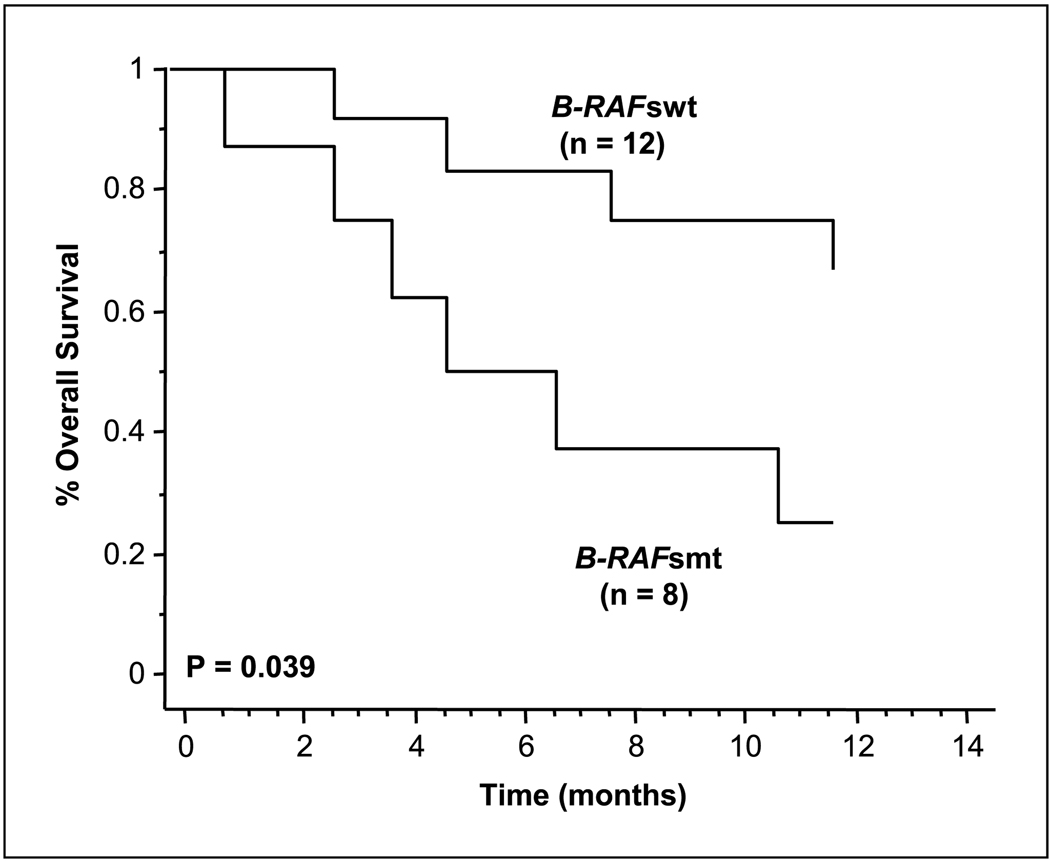
Kaplan-Meier curves were developed to determine whether the B-RAFsmt correlated with overall survival (Fig. 2). A significant difference in overall survival was present between the 20 patients with the B-RAFsmt before biochemotherapy compared with those that did not have the B-RAFsmt (median, 13 versus 30.6 months, respectively; log-rank, P = 0.039).
Fig. 2.
Kaplan-Meier survival curves of biochemotherapy patients. Correlation of post-biochemotherapy serum B-RAF status: B-RAFsmt and B-RAFswt with overall survival (log-rank test, P = 0.039).
B-RAFsmt in response to biochemotherapy
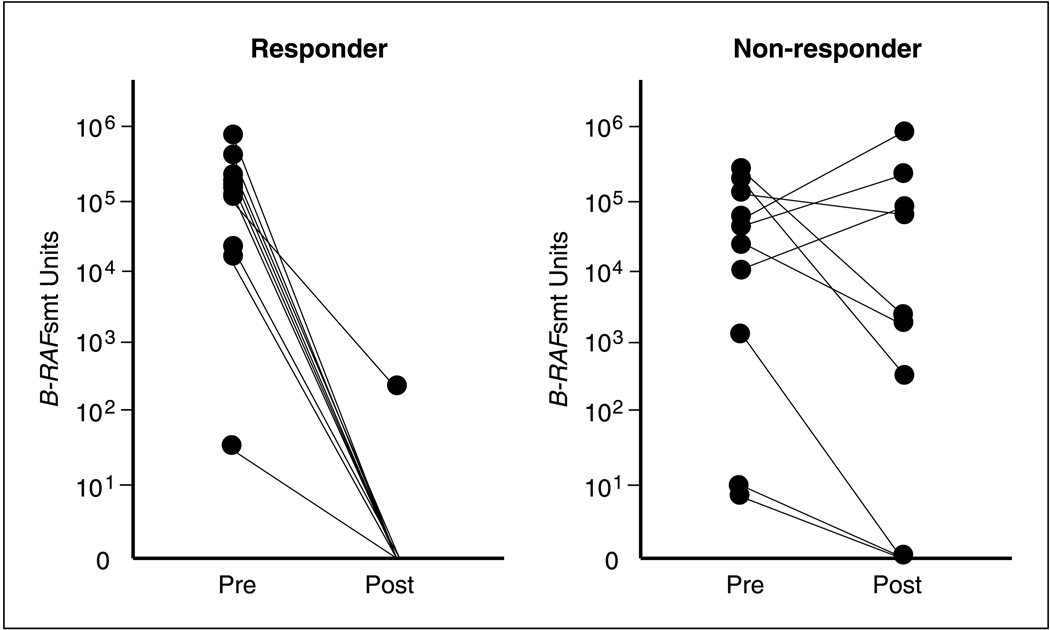
In assessing the change in the detection of circulating B-RAFsmt in response to biochemotherapy (N = 48), serum from 10 of 24 (42%) patients were detected positive for the presence of the B-RAFsmt in the responder group and 10 of 24 (42%) in the nonresponder group (Fig. 3). However, post-biochemotherapy treatment, circulating B-RAFsmt was detected in only 1 of the 10 (10%) patients found to have circulating B-RAFsmt in their pre-biochemotherapy treatment serum in the responder group. In contrast, for the nonresponder group, a statistically significant number of patients (7 of 10, 70%; P = 0.02) continued to have circulating B-RAFsmt in their post-biochemotherapy treatment serum (Table 3). In 30 of the patients, we were able to assess respective melanoma paraffin embedded tissues (primary, metastasis), as previously described (5). Ten of these patients were pre-biochemotherapy B-RAFsmt positive and had 100% concordance in having B-RAFmt (V600E).
Fig. 3.
DNA concentration of circulating B-RAFsmt in serum of responders and nonresponders after biochemotherapy treatment.
Table 3.
B-RAFsmt status in response to bio-chemotherapy
| Response, n (%) |
Nonresponse, n (%) |
|
|---|---|---|
| Pre-B-RAFsmt/post-B-RAFsmt | 1 (10) | 7 (70) |
| Pre-B-RAFsmt/post-B-RAFswt | 9 (90) | 3(30) |
| Total | 10 (100) | 10 (100) |
NOTE: Comparison of response to nonresponse (P = 0.02).
In the single patient for the responder group with circulating B-RAFsmt found in posttreatment serum DNA, we observed that the ratio of the B-RAFsmt copy number to total serum B-RAFwt in serum (B-RAFswt) copy number was significantly reduced from that of the pre-biochemotherapy treatment serum sample: 0.11 (pretreatment) to 0.0021 (posttreatment). However, the three patients in the nonresponder group whose post-biochemotherapy treatment serum showed absence of circulating B-RAFsmt had low mutant to B-RAFswt ratios in their pre-biochemotherapy treatment serum. The pretreatment B-RAFsmt to B-RAFswt DNA ratio ranged from 1.5 × 10−3 to 9.0 × 10−6 in these three patients, whereas the ratio was substantially higher in the other pre-biochemotherapy treatment serum samples.
In comparing the pre-biochemotherapy treatment sera to the post-biochemotherapy treatment sera, we observed that the B-RAFsmt DNA ratio decreased in all 24 patients in the responder group and 20 of 24 patients in the nonresponder group. Of the remaining patients in the nonresponder group, one patient was found to have increased, and three patients had “no remarkable changes” in the B-RAFsmt DNA ratio. This observation may have been related to the possibility that circulating B-RAFsmt was not detected due to insufficient amounts of DNA.
Discussion
The frequency of B-RAFmt (V600E and other sites) in patients with metastatic melanoma has been reported to be >55% (28–31). B-RAFmt has been suggested to contribute to the development of melanoma; however, this topic has been under debate. Primary melanomas of different types vary in B-RAFmt frequency. In the biochemotherapy group studied, 43 of the 50 patient (93%) primaries identified were of cutaneous origin. The importance of mutations in both the N-RAS and B-RAF genes in dysplastic nevi and melanomas has been of considerable interest in that deregulation of the RAS-RAF-MEK-ERK pathway may be important in melanoma progression (2, 14). Because the B-RAFmt (V600E) has been shown to occur frequently in metastatic melanoma, it is important to determine if B-RAFmt V600E can be used to detect patients with metastatic melanoma and identify which patients would potentially be more responsive to specific adjuvant therapies.
In this study, we used a highly specific assay that recognizes a single base pair mismatch to detect the B-RAF mutation at V600E. This is the first study showing ability to detect B-RAFsmt in melanoma patients and potential clinical utility of predicting response to biochemotherapy. In stage IV patients who underwent biochemotherapy, a significant number of patients (P = 0.02) who did not respond to biochemotherapy continued to have circulating B-RAFsmt after the completion of treatment. Moreover, only one patient with a clinical response to biochemotherapy was found to have circulating B-RAFsmt. The presence of B-RAFsmt in these patients indicated a lack of clinical response. The explanation for the lack of B-RAFsmt in responding patients is that tumors responding to biochemotherapy undergo apoptosis, thus inducing DNA to breakdown into small fragments, which, when shed into body fluids, get rapidly cleared away. In nonresponding patients, DNA can be released by tumor cell turnover, physical disruption of circulating tumor cells, and/or from tumor necrosis. The DNA released from these processes may not have gone through apoptosis processes, thus maintaining the DNA integrity and is released as longer sized fragments.
Although presence of the B-RAFsmt did not significantly correlate with treatment response when compared with other known prognostic factors, such as location of metastases, LDH levels, and prior treatment, the presence of post-biochemotherapy circulating B-RAFsmt in patients did correlate with significantly poorer outcomes, such as decreased overall survival.
This pilot study shows the potential clinical utility of monitoring patients with metastatic melanoma receiving therapy. Because studies have shown the frequency of B-RAFmt (V600E) in metastatic melanoma tissue to be higher than in primary tumors, the serum assay may also be useful in patient follow-up for monitoring disease progression (13, 32–34). There are reports suggesting that B-RAFmt (V600E) may be important in disease progression and may potentially be of prognostic utility (29, 32, 34). Our current findings showed no significant correlation with known clinical variables that have been shown to affect outcome.
In conclusion, our findings confirm that the presence of the B-RAFsmt in circulating DNA in serum may have clinical utility in predicting tumor response and disease outcome. Although B-RAFsmt was not associated with other markers of disease progression, our study did show that the presence of the mutation confers poor outcomes with significantly lower overall survival. The raf kinase inhibitor sorafenib (BAY 43-9006), which inhibits melanoma and other cancers by targeting the RAF/MEK/ERK pathway (3, 35, 36), has been Food and Drug Administration approved for renal cell carcinoma. BAY 43-9006 used alone has been disappointing in melanoma patients. The combination of BAY 43-9006 with other drugs may have benefits to melanoma patients. The detection of circulating B-RAFsmt before initiation of therapy may be very useful in monitoring treatment response to RAF/MEK/ERK pathway–targeted drugs.
Acknowledgments
Grant support: National Cancer Institute/NIH grants POCA029605, POCA012582, and R33-CA100314.
References
- 1.Martinez SR, Takeuchi H, Hoon DS. Clinical utility of RNA and DNA molecular markers as prognostic indicators of disease outcome and response to therapy in malignant melanoma. In: Hearing VJ, Leong SP, editors. Melanocytes to melanoma: the progression to malignancy. Totowa (NJ): Humana Press; 2006. [Google Scholar]
- 2.Gray-Schopfer VC, da Rocha Dias S, Marais R. The role of B-RAF in melanoma. Cancer Metastasis Rev. 2005;24:165–183. doi: 10.1007/s10555-005-5865-1. [DOI] [PubMed] [Google Scholar]
- 3.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 4.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Giuliano AE, Turner RR. Lymphatic mapping establishes the role of BRAF mutation in papillary thyroid cancer. Ann Surg. 2006;244:799–804. doi: 10.1097/01.sla.0000224751.80858.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naoki K, Chen TH, Richards WG, Sugarbaker DJ, Meyerson M. Missense mutations of the BRAF gene in human lung adenocarcinoma. Cancer Res. 2002;62:7001–7003. [PubMed] [Google Scholar]
- 7.Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 8.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 9.Mercer KE, Pritchard CA. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim Biophys Acta. 2003;1653:25–40. doi: 10.1016/s0304-419x(03)00016-7. [DOI] [PubMed] [Google Scholar]
- 10.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 11.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- 12.Weber A, Hengge UR, Urbanik D, et al. Absence of mutations of the BRAF gene and constitutive activation of extracellular-regulated kinase in malignant melanomas of the uvea. Lab Invest. 2003;83:1771–1776. doi: 10.1097/01.lab.0000101732.89463.29. [DOI] [PubMed] [Google Scholar]
- 13.Shinozaki M, Fujimoto A, Morton DL, Hoon DS. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753–1757. doi: 10.1158/1078-0432.ccr-1169-3. [DOI] [PubMed] [Google Scholar]
- 14.Smalley KS. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int J Cancer. 2003;104:527–532. doi: 10.1002/ijc.10978. [DOI] [PubMed] [Google Scholar]
- 15.Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–759. [PubMed] [Google Scholar]
- 16.Mori T, O’Day SJ, Umetani N, et al. Predictive utility of circulating methylated DNA in serum of melanoma patients receiving biochemotherapy. J Clin Oncol. 2005;23:9351–9358. doi: 10.1200/JCO.2005.02.9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taback B, O’Day SJ, Boasberg PD, et al. Circulating DNA microsatellites: molecular determinants of response to biochemotherapy in patients with metastatic melanoma. J Natl Cancer Inst. 2004;96:152–156. doi: 10.1093/jnci/djh011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoon DS, Spugnardi M, Kuo C, Huang SK, Morton DL, Taback B. Profiling epigenetic inactivation of tumor suppressor genes in tumors and plasma from cutaneous melanoma patients. Oncogene. 2004;23:4014–4022. doi: 10.1038/sj.onc.1207505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori T, O’Day SJ, Martinez SR, et al. Estrogen receptor-alpha methylation predicts melanoma progression. Cancer Res. 2006;66:6692–6698. doi: 10.1158/0008-5472.CAN-06-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulasova P, Pellestor F. The peptide nucleic acids (PNAs): a new generation of probes for genetic and cytogenetic analyses. Ann Genet. 2004;47:349–358. doi: 10.1016/j.anngen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Taback B, Bilchik AJ, Saha S, et al. Peptide nucleic acid clamp PCR: a novel K-ras mutation detection assay for colorectal cancer micrometastases in lymph nodes. Int J Cancer. 2004;111:409–414. doi: 10.1002/ijc.20268. [DOI] [PubMed] [Google Scholar]
- 22.O’Day SJ, Gammon G, Boasberg PD, et al. Advantages of concurrent biochemotherapy modified by decrescendo interleukin-2, granulocyte colony-stimulating factor, and tamoxifen for patients with metastatic melanoma. J Clin Oncol. 1999;17:2752–2761. doi: 10.1200/JCO.1999.17.9.2752. [DOI] [PubMed] [Google Scholar]
- 23.O’Day SJ, Boasberg PD, Piro L, et al. Maintenance biotherapy for metastatic melanoma with interleukin-2 and granulocyte macrophage-colony stimulating factor improves survival for patients responding to induction concurrent biochemotherapy. Clin Cancer Res. 2002;8:2775–2781. [PubMed] [Google Scholar]
- 24.Sarantou T, Chi DD, Garrison DA, et al. Melanoma-associated antigens as messenger RNA detection markers for melanoma. Cancer Res. 1997;57:1371–1376. [PubMed] [Google Scholar]
- 25.Bostick PJ, Chatterjee S, Chi DD, et al. Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol. 1998;16:2632–2640. doi: 10.1200/JCO.1998.16.8.2632. [DOI] [PubMed] [Google Scholar]
- 26.Hoon DS, Wang Y, Dale PS, et al. Detection of occult melanoma cells in blood with a multiple-marker polymerase chain reaction assay. J Clin Oncol. 1995;13:2109–2116. doi: 10.1200/JCO.1995.13.8.2109. [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara Y, Chi DD, Wang H, et al. Plasma DNA microsatellites as tumor-specific markers and indicators of tumor progression in melanoma patients. Cancer Res. 1999;59:1567–1571. [PubMed] [Google Scholar]
- 28.Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95:1878–1890. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- 29.Dong J, Phelps RG, Qiao R, et al. BRAF oncogenic mutations correlate with progression rather than initiation of human melanoma. Cancer Res. 2003;63:3883–3885. [PubMed] [Google Scholar]
- 30.Chang DZ, Panageas KS, Osman I, Polsky D, Busam K, Chapman PB. Clinical significance of BRAF mutations in metastatic melanoma. J Transl Med. 2004;2:46. doi: 10.1186/1479-5876-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chudnovsky Y, Khavari PA, Adams AE. Melanoma genetics and the development of rational therapeutics. J Clin Invest. 2005;115:813–824. doi: 10.1172/JCI24808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omholt K, Platz A, Kanter L, Ringborg U, Hansson J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483–6488. [PubMed] [Google Scholar]
- 33.Akslen LA, Angelini S, Straume O, et al. BRAF and NRAS mutations are frequent in nodular melanoma but are not associated with tumor cell proliferation or patient survival. J Invest Dermatol. 2005;125:312–317. doi: 10.1111/j.0022-202X.2005.23788.x. [DOI] [PubMed] [Google Scholar]
- 34.Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6. doi: 10.1186/1477-3163-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flaherty KT. Chemotherapy and targeted therapy combinations in advanced melanoma. Clin Cancer Res. 2006;12:2366s–2370s. doi: 10.1158/1078-0432.CCR-05-2505. [DOI] [PubMed] [Google Scholar]
- 36.Stadler WM. Targeted agents for the treatment of advanced renal cell carcinoma. Cancer. 2005;104:2323–2333. doi: 10.1002/cncr.21453. [DOI] [PubMed] [Google Scholar]