Abstract
In response to various environmental stresses, the stress-responsive MAPKs p38 and JNK are activated and phosphorylate ATF2 and c-Jun transcription factors, thereby affecting cell-fate decision. Targeted gene disruption studies have established that JNK-c-Jun signaling plays a vital role in stress-induced apoptosis. The oncogenic phosphatase Wip1 acts as an important regulator in DNA damage pathway by dephosphorylating a spectrum of proteins including p53, p38, Chk1, Chk2, and ATM. In this study we show that Wip1 negatively regulates the activation of MKK4-JNK-c-Jun signaling during stress-induced apoptosis. The loss of Wip1 function sensitizes mouse embryonic fibroblasts to stress-induced apoptosis via the activation of both p38-ATF2 and JNK-c-Jun signaling. Here we reveal that Wip1 has dual roles in alternatively regulating stress- and DNA damage-induced apoptosis through p38/JNK MAPKs and p38/p53-dependent pathways, respectively. Our results point to Wip1 as a general regulator of apoptosis, which further supports its role in tumorigenesis.
Introduction
Wip1 (wild-type p53-induced-phosphatase 1) is a relatively new member of the PP2C family and possesses oncogenic properties (1, 2). It was initially found that the expression of Wip1 phosphatase is induced upon ionizing radiation and UV exposure in a p53-dependent manner (1, 2). Wip1 inhibits UV-induced p38 activation by dephosphorylating the conserved Thr(P)180 residue in p38, thereby suppressing the activation of its downstream target p53 (2). Moreover, Wip1 dephosphorylates p53 at the Ser15 residue, which is targeted by the ATM2/ATR (ATM and RAD3-related) pathway (3). Wip1 also mediates the base excision pathway by negatively regulating the phosphorylation of UNG2 (4). In addition, Wip1 serves as a homoeostatic regulator of checkpoint kinases Chk1 and Chk2 (3, 5). The phosphorylation of ATM at the Ser1981 residue is also negatively regulated by Wip1 (6). Recently, Mdm2 was identified as a novel substrate of Wip1, which dephosphorylates Mdm2 at the Ser395 residue and increases its stability, thus further destabilizing p53 (7). Accumulating evidence has implicated that Wip1 can be an oncogene and plays critical roles in regulating DNA damage pathways.
Wip1 is encoded by the Ppm1d (protein phosphatase magnesium-dependent 1 delta) gene, which maps to chromosome 17q22-q23 (8). This locus is a hot spot for gene amplification in multiple primary human cancers, including breast cancer, neuroblastomas, and ovarian clear cell adenocarcinomas (8–10). The overexpression of Wip1 acts in concert with other oncogenes such as HRas1, ErbB2, and Myc to transform mouse embryonic fibroblasts (11). In contrast, disruption of Wip1 can suppress HRas1- or ErbB2-induced transformation by activating p38, which activates p53 and Ink4a/ARF pathways consequently (12). A mouse model study further demonstrated that activation of MKK6-p38 is critical in rendering Wip1−/− mice resistant to mammary gland tumors driven by the ErbB2 and HRas1 oncogenes (13). As a negative regulator of ATM, Wip1 controls the magnitude and duration of ATM phosphorylation and activity. Wip1−/− mice display a dramatic delay in the onset of lymphomas induced by the Myc oncogene in an ATM- and p53-dependent but not a p38- or ARF-dependent manner (14). These findings suggest that Wip1 is an indispensible regulator of tumorigenesis.
The biological function of Wip1 confers its oncogenic properties. It has been well established that Wip1 plays a vital role in controlling cell proliferation by dephosphorylating Chk1 and Chk2, thereby negatively regulating DNA damage-induced cell cycle checkpoints (3, 5). Several groups have suggested that Wip1 is a negative regulator of apoptosis in response to DNA damage. Bulavin et al. (12) have shown that tumors derived from Wip1−/>− MMTV-ErbB2 mice possess a reduced mitotic index and increased levels of apoptosis compared with tumors derived from wild-type mice. In addition, Takekawa et al. (2) have reported that Wip1 suppresses UV-induced apoptosis by negatively regulating p38/p53 signaling. Ectopic expression of Wip1 results in the inhibition of Chk2-mediated apoptosis following ionizing radiation (5). On the contrary, the depletion of Wip1 by siRNA prolongs the E2F1-induced activation of p38 signaling pathway, resulting in an enhancement of E2F1-induced apoptosis (15). Recently, it was found that the loss of Wip1 suppresses APCMin-driven polyposis by lowering the threshold for p53-dependent apoptosis of stem cells, thus preventing their conversion into tumor-initiating stem cells (16). Taken together, these results suggest that Wip1 acts as an important regulator of apoptosis upon environmental stresses. However, it is still obscure whether the regulation of apoptosis by Wip1 is induced by stresses other than DNA damage stress.
Our study shows that Wip1−/>− MEFs are more sensitive to apoptosis induced by various environmental stresses, including ribotoxic, oxidative, and DNA damage stresses. In Wip1−/>− MEFs, the external stresses elicit more significant activation of p38 and JNK compared with wild-type MEFs. Herein, we demonstrate that in addition to p38 and p53 signaling, Wip1 negatively regulates the MKK4-JNK-c-Jun pathway. Upon stress induction, the loss of Wip1 function in cells causes an accumulation of MKK4 phosphorylation at the Thr261 residue, resulting in the transcriptional activation of c-Jun and subsequently leading to the up-regulation of its downstream gene FasL (17, 18). We therefore propose that Wip1 acts as a negative regulator of both stress-induced and DNA damage-induced apoptosis.
EXPERIMENTAL PROCEDURES
Mammalian Cell Culture and Drug Treatments
HEK293T, U2OS, and MCF-7 cells were obtained from ATCC. Mouse embryonic fibroblasts derived from wild-type and Wip1−/>− mice were kindly provided by Bulavin and co-workers (19). MEFs were immortalized with SV40. The cells were maintained in Dulbecco's modified Eagle's medium, supplemented with 10% (v/v) fetal bovine serum. The cells were seeded and cultured for 36 h prior to treatments. Anisomycin and etoposide were obtained from Sigma. H2O2 was obtained from AppliChem. Staurosporine was purchased from Alexis Biochemicals. UV irradiations were performed at a dose rate of 50 J/m2 using an XL-1500 UV cross-linker.
siRNA and Transfection
Two siRNAs designed to knock down Wip1 and a scrambled negative control siRNA were purchased from Invitrogen. The sequences of Wip1 siRNAs are as follows: Wip1 siRNA 1, 5′-UUG UGA GUG AGU CGA GGU CGU UUC C-3′; and Wip1 siRNA 2, 5′-UAU CCU UAA AGU CAG GGC UUU AGC G-3′. The siRNA oligonucleotides for Wip1 were transfected into U2OS and MCF-7 cells using oligofectamine (Invitrogen).
Immunoblotting
The cells were solubilized in lysis buffer (50 mm Tris-HCl, pH 7.4, 10% glycerol, 1% (v/v) Triton X-100, 100 mm NaCl, 0.5 mm MgCl2, 1 mm Na3VO4, 10 μg/ml aprotinin, 1 mm phenylmethylsulfonyl fluoride, 1 μm pepstatin, and leupeptin), and the resulting lysates were prepared by centrifugation at 16,000 × g for 30 min at 4 °C. Protein concentrations of whole cell lysates were detected using protein assay reagent (Bio-Rad) prior to immunoblotting. The samples were separated on SDS-PAGE, and transferred onto polyvinylidene difluoride membrane filters. The immunoblots were probed with the primary antibodies listed in the supplementary information. The secondary antibodies were either horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (Santa Cruz Biotechnology).
MAPK Inhibitors and Cytotoxicity Assay
The p38 inhibitor SB203580 and JNK inhibitor SP600125 were purchased from Sigma. Equal numbers of wild-type and Wip1−/>− MEFs were seeded and cultured for 36 h before treatment with anisomycin, etoposide, H2O2, UV, and staurosporine, respectively. 5 μm SB203580 and/or SP600125 were added to the MEFs and incubated for 30 min prior to stress treatment. After treatment with different types of stress stimuli for 12 h, MTS (inner salt) (Promega) was applied to MEFs according to the manufacturer's instructions. Absorption at 495 nm was recorded at the 4-h time point. The data were expressed as the means ± S.D. from three independent experiments.
FACS Analysis for Live/Dead Assay and Apoptosis
The live/dead assay was used to simultaneously investigate both the live and death ratios of cells by measuring two parameters of cell viability, namely intracellular esterase activity and plasma membrane integrity. Higher esterase activity (labeled with fluorescein isothiocyanate) indicated live cells, represented by the dots located in region 4. Higher membrane permeability (labeled with Texas Red) indicated dead cells, represented by the dots located in region 1 (supplemental Fig. S1). The dots located in region 3 indicated unstained cells, and the dots located in region 2 indicated double-stained cells. For the live/dead assay, wild-type and Wip1−/>− MEFs were seeded and cultured for 36 h before treatment. At 18 h after treatment, both the floating and attached cells were harvested and subjected to labeling with a live/dead assay kit (l-3224; Molecular Probes) according to the manufacturer's instructions. After labeling, analyses were performed using Dako Cytomation, and the results were analyzed with Summit 4.3. In each experiment, 30,000 events were recorded. For propidium iodide labeling, wild-type and Wip1−/>− MEFs were seeded and cultured for 36 h before treatment. At 18 h after treatment, both floating and attached cells were harvested, washed twice with PBS, and fixed in 70% ethanol. DNA was stained with 50 μg/ml propidium iodide (Sigma) in PBS containing 100 μg/ml RNaseA (Qiagen). Analyses were then performed using Dako Cytomation, and the results were analyzed with Summit 4.3. In each experiment, 10,000 events were recorded, and the results were representative of three independent experiments.
Hoechst Staining and Fluorescence Imaging
The cells grown on coverslips were fixed in 4% paraformaldehyde/PBS for 15 min and then permeabilized with PBS containing 0.2% Triton X-100 for 15 min at room temperature. Fixed cells were incubated with 10 μg/ml Hoechst 33342 (Molecular probe) for 40 min. The cells were washed twice in PBS and then mounted with FluorSaveTM reagent (Calbiochem) and analyzed under a fluorescence microscope.
Detection of Gene Expression by Reverse Transcription-PCR
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen), and was reverse-transcribed using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer's recommendations. The sequences of primers are shown in the supplementary information.
RESULTS
Effects of Wip1 on Cell Death by Various Types of Stress Stimuli
To elucidate whether Wip1 is involved in cell death, we treated wild-type and Wip1−/>− MEFs with different external stimuli such as anisomycin, etoposide, UV radiation, H2O2, and staurosporine. The cytotoxic responses of MEFs were assessed by measuring the metabolic activities of viable cells using MTS. As shown in Fig. 1A, in response to anisomycin treatment, Wip1−/>− MEFs displayed a significantly lower live cell ratio compared with wild-type MEFs (29.6% of Wip1−/>− MEFs compared with 56.1% of wild-type MEFs). Upon etoposide treatment, wild-type MEFs displayed a live cell ratio as high as 97.4%, whereas in Wip1−/>− MEFs this ratio was much lower at 28.4%. Similarly, Wip1−/>− MEFs showed a lower live cell ratio than wild-type MEFs in response to UV radiation, H2O2, and staurosporine treatments (Fig. 1A). To exclude factors other than cell death as a cause of lower metabolic activity in Wip1−/>− MEFs, we performed a live/dead assay. As shown in Fig. 1B, in response to anisomycin treatment, wild-type MEFs displayed a dead cell ratio of 3.57% and a live cell ratio of 87.49%. However, Wip1−/>− MEFs displayed a higher dead cell ratio of 37.15% and a lower live cell ratio of 34.56%. In agreement with the results of the cytotoxicity assay, Wip1−/>− MEFs displayed a markedly higher dead cell ratio than wild-type MEFs upon exposure to all five tested stimuli (Fig. 1B). Our results indicated that the loss of Wip1 sensitizes MEFs to cell death response resulting from different types of stress stimuli.
FIGURE 1.
Antagonistic function of Wip1 during stress-induced cell death. Both wild-type and Wip1−/>− MEFs were mock treated or treated with 10 μm anisomycin, 10 μg/ml etoposide, 15 μm H2O2, 50 J/m2 UV, and 1 μm staurosporine, respectively. A, after 12 h of treatment, MTS was applied to the cells for cytotoxicity assay. Live cell ratios were then determined by normalization of the readings for treated cells to the readings for mock treated cells. The data are expressed as the means ± S.D. from three independent experiments. B, after 18 h of treatment, the cells were harvested live and stained with 0.1 μm calcein AM and 1 μm EthD-1. The labeled cells were then subjected to FACS analysis immediately. The results are representative of three independent experiments. WT, wild type; KO, knock-out.
Loss of Wip1 Sensitizes MEFs to Stress-induced Apoptosis
To further elucidate the possible role of Wip1 in apoptosis, we examined the apoptotic responses of wild-type and Wip1−/>− MEFs to anisomycin and etoposide, respectively. As shown in Fig. 2A, after 12 h of treatment with these stimuli, Wip1−/>− MEFs displayed more malformed nuclei with condensed DNA (white arrows) compared with wild-type MEFs. Propidium iodide staining was also performed to reveal the ratio of apoptotic cells in wild-type and Wip1−/>− MEFs upon stresses. As shown in Fig. 2B, after 12 h of treatment with anisomycin, the ratio of sub-G1 apoptotic cells increased from 3.24 to 11.48% in Wip1−/>− MEFs. Similarly, in response to etoposide, Wip1−/>− MEFs displayed a substantial increase in the apoptotic cell ratio (from 3.24 to 16.51%; Fig. 2B). In contrast, no significant changes were observed in wild-type MEFs treated with anisomycin as compared with the mock treated control. However, wild-type MEFs exhibited a strong G2/M arrest response and less apoptotic cells upon etoposide treatment, in agreement with previous reports (20, 21).
FIGURE 2.
Sensitization of Wip1−/− MEFs to apoptosis induced by anisomycin and etoposide. Wild-type and Wip1−/>− MEFs were mock treated or treated with 10 μm anisomycin and 10 μg/ml etoposide, respectively, A, after 18 h of treatment, the cells were fixed and stained with 10 μg/ml Hoechst 33342 to reveal nuclei. B, after 12 h of treatment, the cells were harvested, labeled with propidium iodide, and subjected to FACS analysis. The results are representative of three independent experiments. C and D, wild-type and Wip1−/>− MEFs were mock treated or treated with 10 μm anisomycin and 10 μg/ml etoposide, respectively. Extracts prepared at different time points after treatment were analyzed with anti-cleaved PARP and anti-cleaved caspase-3 antibodies. α-Tubulin was used as a protein loading control.
Two apoptotic markers, cleaved caspase-3 and cleaved poly(ADP-ribose) polymerase (PARP), were examined in wild-type and Wip1−/>− MEFs, upon anisomycin or etoposide treatment. As shown in Fig. 2 (C and D), after 6 h of treatment, both compounds evoked a more dramatic accumulation of cleaved caspase-3 and cleaved PARP in Wip1−/>− MEFs compared with wild type. UV radiation and H2O2 treatments also stimulated a more significant increase in the protein levels of cleaved caspase-3 and cleaved PARP in Wip1−/>− MEFs compared with wild type (supplemental Fig. S2). Taken together, our results thus indicated that Wip1-deficient MEFs are more sensitive to external stress-induced apoptosis, suggesting that Wip1 may act as a negative regulator of apoptosis and regulates apoptosis induced by other stresses in addition to DNA damage.
Loss of Wip1 Sustains the Activation of p38 and JNK MAPKs
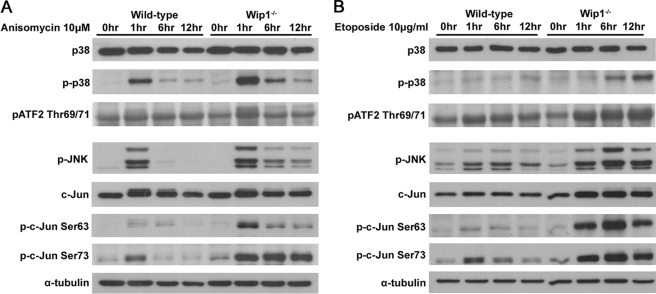
To investigate the role of Wip1 in regulating stress apoptotic pathways, we utilized anisomycin because it is a protein synthesis inhibitor, and its involvement in the p38 and JNK MAPK pathways has been well characterized (22). Consistent with previous reports, anisomycin induced a more considerable increase of the phospho-p38 protein levels but not the total p38 protein levels in Wip1−/>− MEFs compared with wild-type cells (Fig. 3A). Accordingly, Wip1−/>− MEFs displayed higher levels of phospho-ATF2, an important downstream target of p38, upon anisomycin treatment (Fig. 3A). In addition, although the protein levels of phospho-JNK were induced in both wild-type and Wip1−/>− MEFs, the phosphorylation of JNK was found to be sustained for a longer time in the Wip1−/>− MEFs (Fig. 3A, lower panel). Similarly, although the total protein levels of c-Jun were slightly increased in both wild-type and Wip1−/>− MEFs, the latter showed a substantially faster increase in the phosphorylation of c-Jun at Ser63 and Ser73 residues compared with wild-type MEFs in response to anisomycin treatment (Fig. 3A). Interestingly, comparable results were found for etoposide (Fig. 3B), UV (supplemental Fig. S3), and H2O2 treatments (supplemental Fig. S3). The activation of p38 and JNK MAPKs might happen within a short time period in response to extracellular stress. We therefore proceeded to examine the earlier response of wild-type and Wip1−/>− MEFs to anisomycin and etoposide treatments. Within 1 h of treatment, anisomycin stimulated a more substantial activation of p38 and JNK signaling in Wip1−/>− MEFs than in wild-type MEFs (supplemental Fig. S4). In addition, the treatment of etoposide induced activated p38 and JNK at relatively later time points and sustained for a longer time in the Wip1−/>− MEFs (supplemental Fig. S4). Hence, various stresses can cause a more significant up-regulation of p38 and JNK in Wip1−/>− MEFs as compared with wild-type MEFs, suggesting that Wip1 acts as a negative regulator not only in the p38 pathway but also in the JNK pathway.
FIGURE 3.
Enhanced activation of p38 and JNK MAPK pathways in Wip1−/− MEFs. Wild-type and Wip1−/>− MEFs were mock treated or treated with 10 μm anisomycin (A) or 10 μg/ml etoposide (B), respectively. Extracts were prepared at different time points after treatments. The proteins were separated on SDS-PAGE and probed with anti-p38, anti-p-p38 Thr180/Tyr182, anti-p-ATF2 Thr69/71, anti-p-JNK Thr183/Tyr185, anti-c-Jun, anti-p-c-Jun Ser63, and anti-p-c-Jun Ser73 antibodies. α-Tubulin was used as a protein loading control.
Inhibition of Wip1 in Cancer Cells Reconstitutes Stress-induced Apoptosis
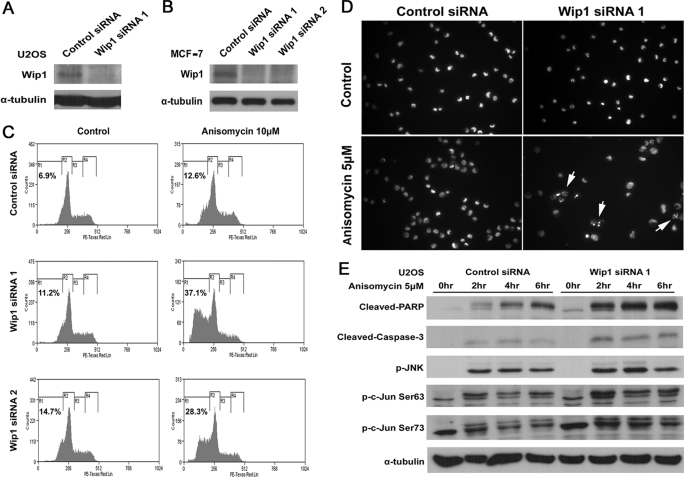
A previous study has shown that tumors derived from Wip1−/>− MMTV-ErbB2 mice displayed an increased level of apoptosis compared with tumors derived from wild-type mice (12). To verify whether Wip1 negatively regulates apoptosis in cancer cells, we depleted Wip1 protein in U2OS and MCF-7 cells by two different siRNA oligonucleotides specific for Wip1 and monitored their effects on anisomycin-induced apoptosis (Fig. 4, A and B). Knockdown of Wip1 protein significantly sensitized MCF-7 cells to anisomycin-induced apoptosis (Fig. 4C). MCF-7 cells transfected with Wip1 siRNA 1 displayed 37.1% sub-G1 apoptotic cells in response to 12 h of 10 μm anisomycin treatment; whereas cells transfected with negative control siRNA only displayed 12.6% of apoptotic cells (Fig. 4C). Similar phenomena were also observed in U2OS cells. As shown in Fig. 4D, Hoechst staining revealed that Wip1 siRNA 1-transfected U2OS cells had normal nuclei, which were comparable with those transfected with negative control siRNA. However, after 5 h of 5 μm anisomycin treatment, U2OS cells transfected with Wip1 siRNA 1 exhibited more apoptotic cells than those transfected with negative control siRNA (Fig. 4D, white arrows). Accordingly, in Wip1 knocked down U2OS cells, the protein levels of both cleaved caspase-3 and cleaved PARP showed a significant increase in response to anisomycin treatment (Fig. 4E). More importantly, anisomycin induced a considerably stronger activation of JNK-c-Jun signaling in Wip1 siRNA 1-transfected U2OS cells than in negative control siRNA-transected cells (Fig. 4E). Consistently, in MCF-7 cells, knockdown of Wip1 using both siRNA oligonucleotides caused a moderately higher activation of JNK-c-Jun signaling upon anisomycin treatment (supplemental Fig. S5), as seen in Wip1−/>− MEFs. These results demonstrate that Wip1 inhibits stress-induced apoptosis not only in normal cells but also in cancer cells, suggesting that Wip1 has a role in preventing cells from apoptosis via the inhibition of JNK-c-Jun signaling.
FIGURE 4.
Sensitization of Wip1 siRNA expressing cells to anisomycin-induced apoptosis. U2OS and MCF-7 cells were transfected with scrambled negative control siRNA or Wip1 siRNAs for 72 h. A and B, after 72 h of transfection, extracts were separated on SDS-PAGE and probed with anti-Wip1 antibody. α-Tubulin was used as a protein loading control. C, MCF-7 cells were subjected to 10 μm anisomycin treatment. After 12 h of treatment, the cells were harvested, labeled with propidium iodide, and subjected to FACS analysis. D, U2OS cells were subjected to 5 μm anisomycin treatment. After 5 h of treatment, the cells were fixed and stained with 10 μg/ml Hoechst 33342 to reveal nuclei. E, U2OS cell were subjected to 5 μm anisomycin treatment, and extracts were prepared at different time points after treatment. The proteins were separated on SDS-PAGE and probed with anti-cleaved PARP, anti-cleaved caspase-3, anti-p-JNK Thr183/Tyr185, anti-p-c-Jun Ser63, and anti-p-c-Jun Ser73 antibodies. α-Tubulin was used as a protein loading control.
Loss of Wip1 in MEFs Leads to FasL-induced Apoptosis
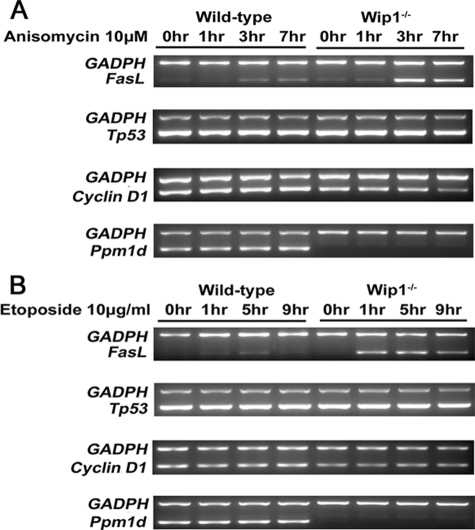
To elucidate whether Wip1 regulates the transcription of genes that function downstream of c-Jun, we stimulated wild-type and Wip1−/>− MEFs with anisomycin or etoposide and examined the transcription of several c-Jun downstream genes, such as Tp53, cyclin D1, and FasL (23). As shown in Fig. 5, the mRNA levels of FasL were significantly up-regulated in Wip1−/>− MEFs, but not in wild-type MEFs at 3 h post-anisomycin treatment or 1 h post-etoposide induction. However, the mRNA levels of Tp53 did not change in both wild-type and Wip1−/>− MEFs upon these two treatments (Fig. 5). Initially, we expected that the transcription of cyclin D1 would be up-regulated in parallel with the activation of c-Jun, because cyclin D1 is a well established downstream gene of c-Jun (24). However, there were no significant change in the mRNA levels of cyclin D1 in both the wild-type and Wip1−/>− MEFs in response to stress, which suggests that other factors may counteract the transcription of cyclin D1 in this circumstance. Hence, our results demonstrated that the loss of Wip1 specifically stimulates FasL transcription upon external stresses.
FIGURE 5.
Higher FasL responsiveness of Wip1−/− MEFs upon anisomycin and etoposide treatment. Wild-type and Wip1−/>− MEFs were mock treated or treated with 10 μm anisomycin (A) and 10 μg/ml etoposide (B), respectively. RNAs were prepared at different time points and reverse transcribed. Expression levels of FasL, Tp53, Cyclin D1, Ppm1d, and GADPH were determined by semi-quantitative reverse transcription-PCR.
Inhibition of p38 and JNK MAPKs Rescues the Apoptotic Susceptibility of Wip1−/− MEFs
To address whether Wip1 functions through p38 and JNK in apoptosis, we inhibited p38 and JNK with specific kinase inhibitors. The p38 inhibitor SB203580 inhibited the phosphorylation of ATF-2 induced by anisomycin and etoposide (Fig. 6, A and B, upper panels). In addition, the JNK inhibitor SP600125 effectively inhibited the phosphorylation of c-Jun induced by anisomycin and etoposide (Fig. 6, A and B, lower panels), suggesting that SB203580 and SP600125 specifically inhibited the activity of p38 and JNK kinases, respectively. Importantly, as shown in Fig. 6C, the inhibition of either p38 or JNK MAPK partially rescued the enhanced apoptosis phenotype in Wip1−/>− MEFs upon anisomycin treatment. Simultaneous inhibition of both p38 and JNK MAPKs effectively rescued the susceptibility of Wip1−/>− MEFs to anisomycin-induced apoptosis, indicating that Wip1 regulates this response specifically via the p38 and JNK MAPK pathways (Fig. 6C). To further confirm the results derived from the cytotoxicity assay, we also examined the protein levels of both uncleaved and cleaved caspase-3 in Wip1−/>− MEFs; consistently, inhibition of p38 and/or JNK kinases significantly suppressed the induction of cleaved caspase-3 protein levels in Wip1−/>− cells upon anisomycin treatment (supplemental Fig. S6). On the other hand, the inhibition of both p38 and JNK MAPKs failed to rescue the higher susceptibility of Wip1−/>− MEFs to etoposide-induced apoptosis, suggesting that the p53-dependent, but not p38/JNK-dependent, apoptotic pathway may play a major role in etoposide-induced apoptosis (Fig. 6D).
FIGURE 6.
Inhibition of anisomycin-induced but not etoposide-induced apoptosis by suppression of both the p38 and JNK. A, C, and E, anisomycin; B, D, and F, etoposide. A and B, wild-type and Wip1−/>− MEFs were subjected to treatment as indicated in the figure. Extracts prepared after treatment were separated on SDS-PAGE and probed with anti-p-ATF2 Thr69/71, anti-p-c-Jun Ser63, and anti-p-c-Jun Ser73 antibodies. α-Tubulin was used as a protein loading control. C and D, equal numbers of wild-type and Wip1−/>− MEFs were seeded and cultured for 36 h prior to treatment. The cells were subjected to treatment as indicated in the figure. After 12 h of treatment, MTS was applied to the cells for cytotoxicity assay. Live cell ratios were then determined by normalization of the readings for treated cells to the control cell measurements. The data are expressed as the means ± S.D. from three independent experiments. **, p < 0.01; *, p < 0.05. E and F, wild-type and Wip1−/>− MEFs were mock treated or treated with 10 μm anisomycin and 10 μg/ml etoposide, respectively. Extracts were prepared at different time points after treatment. The proteins were separated on SDS-PAGE and probed with anti-p-MKK4 Thr261 and anti-p-MKK7 Ser271/Thr275 antibodies. α-Tubulin was used as a protein loading control.
Classic MAPK signaling pathways are comprised of three components that are sequentially activated upon extracellular signals. We therefore could not exclude the possibility that the upstream kinases of p38 and JNK are responsible for the activation of downstream apoptotic signal in the absence of Wip1. To further address the molecular mechanism, we examined the activation of upstream kinases such as MKK4 and MKK7 upon anisomycin and etoposide treatments, respectively. As shown in supplemental Fig. S6, phospho-MKK4 increased significantly within 1 h in response to anisomycin treatment, whereas phospho-MKK7 increased at later time points. Compared with anisomycin, etoposide induced a modest accumulation of phosphorylation in both MKK4 and MKK7 at relatively late time points (supplemental Fig. S6). Markedly, both anisomycin and etoposide treatments stimulated a more substantial increase of phospho-MKK4 protein levels in Wip1−/>− MEFs than in wild type (Fig. 6, E and F). However, the induction of phospho-MKK7 protein levels had no significant difference between wild-type and Wip1−/>− MEFs. Our results demonstrated that both anisomycin and etoposide selectively stimulated substantial phosphorylation of MKK4 but not MKK7 in Wip1−/>− MEFs than in wild-type MEFs, indicating that MKK4 may be preferentially regulated by Wip1 upon anisomycin and etoposide stimulations.
It was reported that the activation of MKK4 kinase is dependent on the phosphorylation at the Ser257 and Thr261 residues (25, 26). To further investigate how Wip1 regulates the phosphorylation of MKK4, we performed an in vitro phosphatase assay using phospho-peptides derived from MKK4. Our results showed that Wip1 preferentially dephosphorylated the peptide containing the Thr(P)261 residue of MKK4 but had little effect on the peptide containing the Ser(P)257 residue (supplemental Fig. S7). To verify this result, we further examined the phosphorylation at Ser257 in MEFs upon anisomycin treatment, using an antibody that specifically recognizes the Ser(P)257 in MKK4. As shown in supplemental Fig. S7, there was no distinguishable induction of phosphorylation at Ser257 of MKK4 in response to anisomycin treatment between wild-type and Wip1−/>− MEFs. Taken together, these results indicate that Wip1 may preferentially target the Thr(P)261 residue of MKK4 in vivo as a potential substrate of Wip1. More importantly, Wip1 may exert dual roles in regulating stress-induced and DNA damage-induced apoptosis through the MKK4-p38/JNK MAPK pathway and p38/p53-dependent pathway, respectively.
DISCUSSION
Apoptosis is triggered by a variety of extrinsic and intrinsic signals and is tightly regulated by many upstream regulators. Among these regulators, p53 is of great importance especially in DNA damage-induced apoptosis (27–30). The wild-type p53-induced phosphatase 1, Wip1, acts as a homeostatic regulator of the DNA damage response by dephosphorylating a spectrum of proteins, including p38, p53, ATM, Chk1, Chk2, UNG2, and Mdm2 (2–7). In the current study, we have found that the apoptotic response regulated by Wip1 is induced not only by DNA damage but also by ribotoxic and oxidative stresses. Wip1 negatively regulates stress-induced apoptosis in both normal cells (Fig. 2) and cancer cells (Fig. 4). In the absence of Wip1, both p38 and JNK display an abnormally elevated activation upon stress exposure (Fig. 3 and supplemental Fig. S3). Moreover, the elevated activation of MKK4-JNK-c-Jun signaling in Wip1−/>− MEFs stimulates the transcription of FasL, which confers a higher sensitivity to stress-induced apoptosis.
Anisomycin is a protein synthesis inhibitor and a well studied activator specific for p38 and JNK MAPK pathways (31, 32). In agreement with previous studies, the loss of Wip1 leads to an enhancement of p38 activation as indicated by higher levels of p-p38 and p-ATF2 in Wip1−/>− MEFs (Fig. 3 and supplemental Fig. S3). It was reported that overexpression of Wip1 does not directly reduce the phosphorylation and activation of JNK1 (2). However, we have observed a sustained augmentation of JNK phosphorylation upon exposure to stresses in Wip1−/>− MEFs (Fig. 3 and supplemental Fig. S3). The JNK kinases have three isoforms, JNK1–3. Studies in mouse genetic models imply that the ubiquitously expressed JNK1 and JNK2 have proapoptotic functions (33, 34). Moreover, both JNK1 and JNK2 play a positive role in regulating the phosphorylation and transcription activity of c-Jun (35). JNK3 is predominantly expressed in the mouse central nervous system and heart and also acts as a proapoptotic regulator (36). Although Wip1 may not regulate JNK1, we could not exclude the possibility that Wip1 regulates other JNK isoforms. In addition, it has already been shown that p38 and JNK kinases are activated by Gadd45α, a p53 downstream target that is an important inhibitor of breast tumors (37). Moreover, the loss of Wip1 can also up-regulate p53 activity (2, 3). Thus, in the absence of Wip1, the enhanced activation of JNK could also indirectly result from p53 activation and subsequent activation of its target Gadd45α.
We have initially observed that various stresses lead to an abnormally elevated phosphorylation of c-Jun at Ser63 and Ser73 in Wip1-deficient MEFs (Fig. 3 and supplemental Fig. S3), resulting in an activation of FasL and apoptosis (Fig. 5). Similar induction of c-Jun phosphorylation was also observed in both Wip1 siRNA-transfected U2OS and MCF-7 cells (Fig. 4 and supplemental Fig. S5), suggesting a possible involvement for Wip1 in the regulation of c-Jun transcription activity. In addition to c-Jun-FasL-induced apoptosis, the activation of JNK might stimulate apoptosis via phosphorylation and regulation of Bcl-2 family (38). Because c-Jun does not possess typical TXY or SQ/TQ Wip1 substrate motifs, the abnormally elevated phosphorylation of c-Jun raised the possibility that Wip1 may regulate its phosphorylation through the activation of JNK family members or its upstream kinases, such as MKK4 and MKK7 (39). MKK4 is an important component in MAPK signal cascade. It can directly phosphorylate and activate JNK and p38 kinases in response to environmental stresses (40, 41). Compared with MKK4, MKK7 is more specific for the phosphorylation and activation of JNK (42). Our results show that the phosphorylation of MKK4 but not MKK7 is differentially regulated in wild-type and Wip1−/>− MEFs upon stresses (Fig. 6, E and F). The possible regulation of Wip1 on MKK4 explains the simultaneous hyperactivation of both p38 and JNK in Wip1−/>− MEFs in response to environmental stresses. It was reported that the phosphorylation of MKK4 is regulated by PP2Cα and PP2Cβ1 (43). As a member of the PP2C family, Wip1 possesses similar characteristics with other PP2Cs. The in vitro phosphatase assay utilizing phospho-peptides derived from MKK4 indicates that Wip1 preferentially dephosphorylates the Thr(P)261 residue but not the Ser(P)257 residue in MKK4 (supplemental Fig. S7). These results raise the possibility that Wip1 may target MKK4 for dephosphorylation and inactivation in vivo. Thus, Wip1 may be involved in a multi-step and complex mechanism in regulating MAPK pathways.
Tp53 and cyclin D1 are two important downstream genes in the JNK-c-Jun pathway. Upon survival signaling, c-Jun suppresses the transcription of Tp53, whereas it induces the transcription of cyclin D1 (24, 44). In this study, we have found that in response to anisomycin and etoposide treatments, the mRNA levels of Tp53 and cyclin D1 do not change in cultured MEFs (Fig. 5). Because the AP-1 transcription factor is comprised of two subunits, c-Jun needs to form a dimer with a spectrum of binding partners such as Fos and ATF2, in response to different kinds of environmental stimuli (23, 45, 46). The activities of AP-1 are modulated by the differential expression of its components and dimer composition (23). Thus, the expression levels of AP-1 target genes are tightly regulated by specific environmental stimuli. It would be interesting to study whether Wip1 regulates other AP-1 mediators and its downstream genes.
In many cases, the p38 and JNK MAPK pathways are simultaneously activated by environmental stresses (31, 32). Inhibition of either p38 or JNK MAPK was able to rescue the apoptosis phenotype of Wip1−/>− MEFs to a lesser extent (Fig. 6C). Moreover, by inhibiting both p38 and JNK MAPKs, we have successfully rescued the enhanced apoptotic response in Wip1−/>− MEFs upon anisomycin treatment, which is known to induce apoptosis through activating both p38 and JNK kinases. However, inhibition of both p38 and JNK MAPKs was not able to rescue the enhanced apoptotic response induced by etoposide in Wip1−/>− MEFs (Fig. 6D), suggesting that etoposide induces apoptosis via a different mechanism from anisomycin. Unlike anisomycin, etoposide is a drug that stabilizes DNA-topo II complexes, thereby blocking DNA replication and causing direct DNA strand break (47). Although etoposide can also induce apoptosis via the activation of JNK pathway, it is widely accepted that etoposide induces apoptosis mainly via DNA damage pathways in a p53-dependent manner (48, 49). On the other hand, the dramatic effects induced by the protein synthesis inhibitor anisomycin on MKK4-p38/JNK signaling in Wip1-deficient MEFs indicate that these activations may not be directly related to the p53-dependent DNA damage pathway. Taken together, we speculate that Wip1 has a role in preventing cells from apoptosis via multiple pathways.
In conclusion, our results described herein further validate the function of Wip1 in regulating apoptosis. As a negative regulator of apoptosis, Wip1 exerts its activity in response to various types of environmental stresses. In addition to p38/p53 and ATM-Chk2-p53 signaling, Wip1 acts as a negative regulator of the MKK4-JNK-c-Jun signaling. In response to different environmental stresses, Wip1 negatively regulates the activation of MKK4-JNK and the transcriptional activation of c-Jun. Subsequent suppression of FasL transcription inhibits stress-induced apoptosis. Together with the previous findings showing that Wip1 negatively regulates DNA damage-induced apoptosis, our current study provides novel evidence that Wip1 acts as a general “inhibitor” of apoptosis, implying that apoptosis could be reconstituted in cancers harboring Wip1 gene amplifications.
Supplementary Material
Acknowledgments
We are grateful to D. V. Bulavin for providing Wip1 wild-type and knock-out MEF cells and several plasmids and helpful discussion. We also thank H. M. Shen for kindly providing antibodies and the members of Y. C. Liou laboratory for support and helpful discussion. We are thankful to K. Perrem, B. C. Low, H. Yu, and C. Y. He for critical reading of the manuscript.
This work was supported, in whole or in part, by Grant 06/1/21/19/473 from the Biomedical Research Council, the Agency for Science, Research and Technology, Singapore (to Y. C. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7.
- ATM
- ataxia-telangiectasia mutated
- siRNA
- small interfering RNA
- MAPK
- mitogen-activated protein kinase
- JNK
- c-Jun N-terminal kinase
- MEF
- mouse embryonic fibroblast
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- FACS
- fluorescence-activated cell sorter
- PBS
- phosphate-buffered saline
- PARP
- poly(ADP-ribose) polymerase.
REFERENCES
- 1.Fiscella M., Zhang H., Fan S., Sakaguchi K., Shen S., Mercer W. E., Vande Woude G. F., O'Connor P. M., Appella E. ( 1997) Proc. Natl. Acad. Sci. U.S.A. 94, 6048– 6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takekawa M., Adachi M., Nakahata A., Nakayama I., Itoh F., Tsukuda H., Taya Y., Imai K. ( 2000) EMBO J. 19, 6517– 6526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu X., Nannenga B., Donehower L. A. ( 2005) Genes Dev. 19, 1162– 1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu X., Bocangel D., Nannenga B., Yamaguchi H., Appella E., Donehower L. A. ( 2004) Mol. Cell 15, 621– 634 [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto H., Onishi N., Kato N., Takekawa M., Xu X. Z., Kosugi A., Kondo T., Imamura M., Oishi I., Yoda A., Minami Y. ( 2006) Cell Death Differ. 13, 1170– 1180 [DOI] [PubMed] [Google Scholar]
- 6.Shreeram S., Demidov O. N., Hee W. K., Yamaguchi H., Onishi N., Kek C., Timofeev O. N., Dudgeon C., Fornace A. J., Anderson C. W., Minami Y., Appella E., Bulavin D. V. ( 2006) Mol. Cell 23, 757– 764 [DOI] [PubMed] [Google Scholar]
- 7.Lu X., Ma O., Nguyen T. A., Jones S. N., Oren M., Donehower L. A. ( 2007) Cancer Cell 12, 342– 354 [DOI] [PubMed] [Google Scholar]
- 8.Li J., Yang Y., Peng Y., Austin R. J., van Eyndhoven W. G., Nguyen K. C., Gabriele T., McCurrach M. E., Marks J. R., Hoey T., Lowe S. W., Powers S. ( 2002) Nat. Genet. 31, 133– 134 [DOI] [PubMed] [Google Scholar]
- 9.Hirasawa A., Saito-Ohara F., Inoue J., Aoki D., Susumu N., Yokoyama T., Nozawa S., Inazawa J., Imoto I. ( 2003) Clin. Cancer Res. 9, 1995– 2004 [PubMed] [Google Scholar]
- 10.Saito-Ohara F., Imoto I., Inoue J., Hosoi H., Nakagawara A., Sugimoto T., Inazawa J. ( 2003) Cancer Res. 63, 1876– 1883 [PubMed] [Google Scholar]
- 11.Harrison M., Li J., Degenhardt Y., Hoey T., Powers S. ( 2004) Trends Mol. Med. 10, 359– 361 [DOI] [PubMed] [Google Scholar]
- 12.Bulavin D. V., Phillips C., Nannenga B., Timofeev O., Donehower L. A., Anderson C. W., Appella E., Fornace A. J., Jr. ( 2004) Nat. Genet. 36, 343– 350 [DOI] [PubMed] [Google Scholar]
- 13.Demidov O. N., Kek C., Shreeram S., Timofeev O., Fornace A. J., Appella E., Bulavin D. V. ( 2007) Oncogene 26, 2502– 2506 [DOI] [PubMed] [Google Scholar]
- 14.Shreeram S., Hee W. K., Demidov O. N., Kek C., Yamaguchi H., Fornace A. J., Jr., Anderson C. W., Appella E., Bulavin D. V. ( 2006) J. Exp. Med. 203, 2793– 2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hershko T., Korotayev K., Polager S., Ginsberg D. ( 2006) J. Biol. Chem. 281, 31309– 31316 [DOI] [PubMed] [Google Scholar]
- 16.Demidov O. N., Timofeev O., Lwin H. N., Kek C., Appella E., Bulavin D. V. ( 2007) Cell Stem Cell 1, 180– 190 [DOI] [PubMed] [Google Scholar]
- 17.Kasibhatla S., Brunner T., Genestier L., Echeverri F., Mahboubi A., Green D. R. ( 1998) Mol. Cell 1, 543– 551 [DOI] [PubMed] [Google Scholar]
- 18.Kolbus A., Herr I., Schreiber M., Debatin K. M., Wagner E. F., Angel P. ( 2000) Mol. Cell. Biol. 20, 575– 582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi J., Nannenga B., Demidov O. N., Bulavin D. V., Cooney A., Brayton C., Zhang Y., Mbawuike I. N., Bradley A., Appella E., Donehower L. A. ( 2002) Mol. Cell. Biol. 22, 1094– 1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cliby W. A., Lewis K. A., Lilly K. K., Kaufmann S. H. ( 2002) J. Biol. Chem. 277, 1599– 1606 [DOI] [PubMed] [Google Scholar]
- 21.Rossi R., Lidonnici M. R., Soza S., Biamonti G., Montecucco A. ( 2006) Cancer Res. 66, 1675– 1683 [DOI] [PubMed] [Google Scholar]
- 22.Morton S., Davis R. J., Cohen P. ( 2004) FEBS Lett. 572, 177– 183 [DOI] [PubMed] [Google Scholar]
- 23.Eferl R., Wagner E. F. ( 2003) Nat. Rev. Cancer 3, 859– 868 [DOI] [PubMed] [Google Scholar]
- 24.Bakiri L., Lallemand D., Bossy-Wetzel E., Yaniv M. ( 2000) EMBO J. 19, 2056– 2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyriakis J. M., Avruch J. ( 2001) Physiol. Rev. 81, 807– 869 [DOI] [PubMed] [Google Scholar]
- 26.Herskowitz I. ( 1995) Cell 80, 187– 197 [DOI] [PubMed] [Google Scholar]
- 27.Meek D. W. ( 2004) DNA Repair 3, 1049– 1056 [DOI] [PubMed] [Google Scholar]
- 28.Motoyama N., Naka K. ( 2004) Curr. Opin. Genet. Dev. 14, 11– 16 [DOI] [PubMed] [Google Scholar]
- 29.Manfredi J. J. ( 2003) Mol. Cell 11, 552– 554 [DOI] [PubMed] [Google Scholar]
- 30.Liu G., Chen X. ( 2006) J. Cell. Biochem. 97, 448– 458 [DOI] [PubMed] [Google Scholar]
- 31.Hazzalin C. A., Cano E., Cuenda A., Barratt M. J., Cohen P., Mahadevan L. C. ( 1996) Curr. Biol. 6, 1028– 1031 [DOI] [PubMed] [Google Scholar]
- 32.Hazzalin C. A., Le Panse R., Cano E., Mahadevan L. C. ( 1998) Mol. Cell. Biol. 18, 1844– 1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuan C. Y., Yang D. D., Samanta Roy D. R., Davis R. J., Rakic P., Flavell R. A. ( 1999) Neuron 22, 667– 676 [DOI] [PubMed] [Google Scholar]
- 34.Tournier C., Dong C., Turner T. K., Jones S. N., Flavell R. A., Davis R. J. ( 2001) Genes Dev. 15, 1419– 1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaeschke A., Karasarides M., Ventura J. J., Ehrhardt A., Zhang C., Flavell R. A., Shokat K. M., Davis R. J. ( 2006) Mol. Cell 23, 899– 911 [DOI] [PubMed] [Google Scholar]
- 36.Yang D. D., Kuan C. Y., Whitmarsh A. J., Rincón M., Zheng T. S., Davis R. J., Rakic P., Flavell R. A. ( 1997) Nature 389, 865– 870 [DOI] [PubMed] [Google Scholar]
- 37.Hildesheim J., Bulavin D. V., Anver M. R., Alvord W. G., Hollander M. C., Vardanian L., Fornace A. J., Jr. ( 2002) Cancer Res. 62, 7305– 7315 [PubMed] [Google Scholar]
- 38.Lei K., Nimnual A., Zong W. X., Kennedy N. J., Flavell R. A., Thompson C. B., Bar-Sagi D., Davis R. J. ( 2002) Mol. Cell. Biol. 22, 4929– 4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitmarsh A. J., Davis R. J. ( 2007) Oncogene 26, 3172– 3184 [DOI] [PubMed] [Google Scholar]
- 40.Dérijard B., Raingeaud J., Barrett T., Wu I. H., Han J., Ulevitch R. J., Davis R. J. ( 1995) Science 267, 682– 685 [DOI] [PubMed] [Google Scholar]
- 41.Lin A., Minden A., Martinetto H., Claret F. X., Lange-Carter C., Mercurio F., Johnson G. L., Karin M. ( 1995) Science 268, 286– 290 [DOI] [PubMed] [Google Scholar]
- 42.Lawler S., Fleming Y., Goedert M., Cohen P. ( 1998) Curr. Biol. 8, 1387– 1390 [DOI] [PubMed] [Google Scholar]
- 43.Hanada M., Kobayashi T., Ohnishi M., Ikeda S., Wang H., Katsura K., Yanagawa Y., Hiraga A., Kanamaru R., Tamura S. ( 1998) FEBS Lett. 437, 172– 176 [DOI] [PubMed] [Google Scholar]
- 44.Schreiber M., Kolbus A., Piu F., Szabowski A., Möhle-Steinlein U., Tian J., Karin M., Angel P., Wagner E. F. ( 1999) Genes Dev. 13, 607– 619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaulian E., Karin M. ( 2002) Nat. Cell Biol. 4, E131– 136 [DOI] [PubMed] [Google Scholar]
- 46.Wagner E. F. ( 2001) Oncogene 20, 2334– 2335 [DOI] [PubMed] [Google Scholar]
- 47.Baldwin E. L., Osheroff N. ( 2005) Curr. Med. Chem. Anticancer Agents 5, 363– 372 [DOI] [PubMed] [Google Scholar]
- 48.Chresta C. M., Masters J. R., Hickman J. A. ( 1996) Cancer Res. 56, 1834– 1841 [PubMed] [Google Scholar]
- 49.Anderson S. M., Reyland M. E., Hunter S., Deisher L. M., Barzen K. A., Quissell D. O. ( 1999) Cell Death Differ. 6, 454– 462 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.