Abstract
The Asian Pacific Association for the Study of the Liver (APASL) set up a working party on acute-on-chronic liver failure (ACLF) in 2004, with a mandate to develop consensus guidelines on various aspects of ACLF relevant to disease patterns and clinical practice in the Asia-Pacific region. Experts predominantly from the Asia–Pacific region constituted this working party and were requested to identify different issues of ACLF and develop the consensus guidelines. A 2-day meeting of the working party was held on January 22–23, 2008, at New Delhi, India, to discuss and finalize the consensus statements. Only those statements that were unanimously approved by the experts were accepted. These statements were circulated to all the experts and subsequently presented at the Annual Conference of the APASL at Seoul, Korea, in March 2008. The consensus statements along with relevant background information are presented in this review.
Keywords: Acute-on-chronic liver failure, Acute-on-chronic liver disease, Liver failure, Hepatic failure, Hepatitis, Cirrhosis
Introduction
Liver failure can develop as acute liver failure (ALF) (in the absence of any pre-existing liver disease), acute-on-chronic liver failure (ACLF) (an acute deterioration of known or unknown chronic liver disease), or a chronic decompensation of an end-stage liver disease. There is limited data on the entity of ACLF and there are no consensus guidelines on its definition, diagnosis, and management. The Asian Pacific Association for the Study of the Liver (APASL) set up a working party on ACLF in 2004 with a mandate to study and analyze the various aspects of this clinical entity and to develop consensus guidelines on ACLF relevant to the disease pattern and clinical practice. Experts from all over the globe, especially from the Asia–Pacific region, constituted this working party and were requested to identify different issues of ACLF and develop the consensus guidelines. The process for the development of these consensus guidelines contained the following steps: review of all available published literature on ACLF; an effort to define the acute hepatic insults, the underlying chronic liver disease, and the liver failure of ACLF; a survey of the current approaches for the diagnosis and management of ACLF; discussion on contentious issues; and deliberations to prepare the consensus statement by the experts of the working party. A 2-day meeting was held on January 22–23, 2008, at New Delhi, India, to discuss and finalize the recommendations and guidelines. Only those statements that were unanimously approved by the experts were accepted. These statements were circulated to all the experts, posted on the ACLF Web site (www.aclf.in), and subsequently finalized. The working party adopted the Oxford System [1] of evidence-based approach for developing the consensus statements. The group assessed the level of existing evidence and accordingly ranked the recommendations (i.e., level of evidence from 1 (highest) to 5 (lowest); grade of recommendation from A (strongest) to D (weakest)).
The consensus statements are presented in this review. A brief background note has been added to explain in more detail the genesis of the consensus statements.
The concept of ACLF and need for a definition
Acute liver failure is a well-defined and understood entity and connotes a poor outcome. Acute-on-chronic liver failure is also a serious condition with varied etiology and manifestations, as well as high mortality. This term was first used in 1995 to describe a condition in which two insults to liver are operating simultaneously, one of them being ongoing and chronic and the other acute [2]. However, a clear definition of ACLF is lacking, and this term is being used to mean different entities by different clinicians. Any patient who had underlying chronic liver disease with superimposed acute insult is being labeled as having ACLF. Most people raised the concern that this would lead to overlap with decompensated liver disease. The main emphasis of the APASL Working Party was to identify from this large group of patients a subset of patients who have a homogenous presentation and similar outcome and restrict the use of the label “acute on chronic liver failure” to this subset.
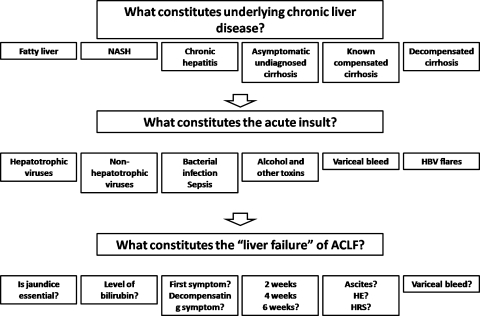
There is a lack of uniformity in diagnostic criteria of ACLF with many unresolved and contentious issues as well (Fig. 1); for example, what constitutes the chronic liver disease in ACLF. The spectrum of underlying chronic liver disease can range from bland steatosis to hepatitis to compensated cirrhosis to decompensated cirrhosis. Similarly, what constitutes the acute insult: hepatotropic viruses, toxins, sepsis, or even a variceal bleed? Moreover, the definition of liver failure in ACLF has been imprecise in terms of which criteria to include: level of bilirubin and the time period of deterioration from the onset, initiating event to be accepted as jaundice, or any symptom pertaining to hepatic dysfunction. The experts in the working party deliberated on these issues at length.
Fig. 1.
Contentious issues in acute-on-chronic liver failure
Definition of ACLF
The aim of the APASL Working Party was to carefully analyze the existing terminologies and first of all identify whether there is any need for a new terminology. The main emphasis was whether one could identify a subset of patients who have a relatively homogenous presentation and likely similar outcome.
There is no consistent definition of ACLF in literature. Each study done previously on ACLF has used its own definition, and there is no unanimity in these definitions in terms of criteria for liver failure, the acute event precipitating ACLF, and the diagnosis of underlying chronic liver disease. Since most of these studies were on patients who required liver support devices or liver transplantation, these studies were biased toward including sicker patients in the definition and patients having a milder course were generally excluded from this definition.
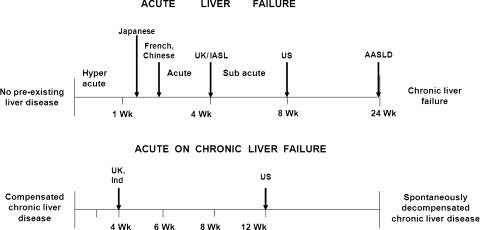
The definitions of the hyperacute, acute, and subacute liver failure [3]; fulminant and subfulminant liver failure [4]; and late-onset hepatic failure [5] were carefully reviewed (Fig. 2). The recent guidelines of American Association for the Study of Liver Disease to include liver failure up to 24 weeks as a revised definition of ALF [6] was also critically analyzed in the wake of the etiology, diagnosis, transplant need, and outcome.
Fig. 2.
Various definitions of acute and acute-on-chronic liver failure
After reviewing all the published literature on the subject and sharing live cases from nearly 20 countries, a working definition of ACLF was unanimously agreed upon by the working party to define patients who belonged to this subgroup. Special emphasis was given to the fact that a homogenous population of patients is identified so that the natural history and interventions to improve the outcome could be applied universally. The experts also felt that the primary precipitating event, the acute hepatic insult, should be hepatic in origin. This may not always be easy to discern; however, the theme should be adhered to in identifying the patient group with ACLF. There was unanimity that any new entity, if christened, should be clinically distinct from ALF and decompensated liver disease, terms that are clearly understood and defined.
Recommendation
Definition of ACLF: Acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease. (2a, B)
What constitutes the acute event?
While chronic decompensation of the end-stage liver disease usually results in an irreversible deterioration, with liver transplantation being the only realistic therapeutic option, both ALF and ACLF result due to acute episodes, which are potentially reversible. The reversibility depends on the severity and nature of the acute insult and the degree of underlying chronic liver disease.
The acute episodes vary depending on the geographic region and the population under study. They include both infectious and noninfectious causes. It was also appreciated that the major etiologic agents responsible for precipitating ACLF are quite distinct in the East and the West. Alcohol and drugs constitute the majority of acute insults in the West, whereas infectious etiologies predominate in the East. The difference in the etiologies of ACLF between the East and the West reflects the differences in the etiology of the underlying chronic liver disease in the different geographic regions as well.
Among the infectious etiologies, reactivation of hepatitis B virus (HBV) infection is one of the major causes of ACLF in the Asian region [7–13]. Reactivation may be either spontaneous or due to intensive chemotherapy or immunosuppressive therapy [7, 8], immune restoration after highly active antiretroviral therapy for HIV [9, 10], treatment related [11], or reactivation of the occult HBV infection by rituximab (anti-CD20)-based chemotherapy [12, 13]. Similarly, reactivation of hepatitis C virus infection has also been reported, especially after immunosuppressive therapy [14, 15]. The other very important infectious etiology of the acute event is superinfection with hepatitis E virus, predominantly in patients in the Indian subcontinent [16–20]. Various bacterial, parasitic, and fungal infections may affect the liver. Spirochetal, protozoal, helminthic, or fungal organisms may directly infect the liver, whereas bacterial or parasitic infection may spread to the liver from other sites [21]. These infections may lead to liver failure in patients with underlying chronic liver disease.
Among the noninfectious etiologies, alcoholic hepatitis is the major cause of acute deterioration in stable known or unknown chronic liver diseases, more often in the western countries [22–24]. Hepatotoxic drugs and herbal indigenous medicines are important causes for liver failure in the Asia–Pacific region [25, 26].
Acute variceal bleeding has been included as one of the events to define hepatic decompensation in the natural history of cirrhosis [27]. Variceal bleeding has also been taken as an acute insult of ACLF in some western trials of ACLF. It was extensively debated whether to consider variceal bleed as an acute event of ACLF. It was discussed that for a patient with chronic liver disease who presents for the first time with variceal bleed without any previous or present signs or symptoms of chronic liver disease, it would not constitute an acute insult. This is especially relevant if such a patient does not develop any jaundice. On the other hand, the definition of ACLF includes hepatic decompensation in the form of jaundice. Most experts considered variceal bleed as an expression of elevated portal pressure and a form of decompensation of underlying chronic liver disease but not as an acute event leading to ACLF. However, no unanimous consensus could be reached to label acute variceal bleeding as an acute event for ACLF.
Sepsis plays an important role in the progression and management decisions of ACLF, but whether it itself acts as an initial precipitating event was debatable. The existing literature from the United Kingdom and the United States has included sepsis as an integral cause for the development of ACLF. However, it was argued that sepsis alone might not directly cause an acute hepatic insult but could result in worsening of the condition of the patient. Furthermore, sepsis per se can cause organ failure in cirrhotic patients without direct hepatic derangements. It was therefore not considered as a cause of acute insult. To bring homogeneity of the population under consideration of the hepatorenal syndrome of ACLF, it was proposed that any infectious agent directly afflicting the liver leading to acute derangement in its function should be included.
Recent publications from the West have shown that major surgical procedures could pose an acute insult to liver [28, 29]. There was conflicting opinion among the experts whether surgery would qualify for a direct hepatic insult or not. However, it was finally agreed that if the outcome of surgery would result in a clinical syndrome befitting the current accepted definition of ACLF, it could be considered as a precipitating event.
It was also agreed that in spite of the best evaluation to detect the etiology of the acute event, in some patients it could not be clearly defined.
Following etiologies were finally agreed upon by the working party to be included as acute events leading to the development of ACLF.
Recommendations
- Defining the acute event in ACLF:
- Infectious etiology:
- Hepatotropic and nonhepatotropic viruses. (1a, A)
- Reactivation of hepatitis B (overt or occult) or hepatitis C. (2b, B)
- Other infectious agents afflicting the liver. (5, D)
- Noninfectious etiology:
- Alcohol: active drinking within the last 4 weeks. (1a, A)
- Use of hepatotoxic drugs, herbs. (2b, B)
- Flare of autoimmune hepatitis or Wilson’s disease. (3b, B)
- Surgical intervention. (3b, B)
- Variceal bleed.1 (4, C)
- Unknown hepatotoxic etiology. (5, D)
Defining the underlying chronic liver disease
Etiologic profile of cirrhosis in ACLF is similar to etiology of cirrhosis in general in the respective countries (Table 1) [19, 20, 30–39]. Alcoholic cirrhosis constitutes 50–70% of all the underlying liver diseases of ACLF in the western countries, whereas hepatitis-related cirrhosis constitutes about 10–15% of all the cases. However, in most of the Asian countries, hepatitis B constitutes 70% and alcohol only about 15% of all the etiologies of ACLF (Table 1). Autoimmune liver disease, Wilson’s disease, metabolic liver disease, and chronic cholestatic liver disease constitute only minority of patients (Table 1).
Table 1.
Etiology of underlying chronic liver disease in ACLFa
| Alcohol | Hepatitis B/C | Cryptogenic | Others | |
|---|---|---|---|---|
| UK (n = 312) [30] | 65 | 17 | 5 | 13 |
| Germany (n = 143) [31] | 75 | 14 | 4 | 7 |
| Germany (n = 27) [32] | 52 | 33 | 7 | 8 |
| Germany (n = 13) [33] | 54 | 23 | – | 23 |
| Germany (n = 24) [34] | 79 | 8 | 13 | |
| Austria (n = 196) [35] | 71 | 10 | 10 | 9 |
| Korea (n = 10) [36] | 80 | 20 | ||
| Singapore (n = 9) [37] | 78 | 22 | ||
| Singapore (n = 26) [38] | 15 | 42 | 19 | 24 |
| China (n = 338) [39] | 13 | 82 | 5 | |
| India (n = 42) [20] | 17 | 67 | 9 | 7 |
| India (n = 43) [19] | 26 | 52 | 17 | 5 |
aValues given are percentages
Nonalcoholic steatohepatitis (NASH), irrespective of stage of fibrosis, has been included as an underlying chronic liver disease in ACLF. There was unanimity that patients with NASH do behave differently compared with subjects with a healthy liver. Nonalcoholic steatohepatitis could be anticipated in an obese, diabetic subject especially if he or she is older than 40 years. There could, however, be a difficult situation in which due to an acute onset the underlying NASH cannot be diagnosed. In such subjects, a liver biopsy through percutaneous or transjugular route with additional hepatic venous-pressure gradient (HVPG) measurements could be of help. It was debated whether to consider benign fatty liver, steatosis, as chronic liver disease as well. Several experts brought to notice the data from transplant centers that live donors or cadaver livers with steatosis do not constitute the best organs for liver transplantation. However, since simple steatosis may not always be progressive, it was decided not to include this as an underlying chronic liver disease for ACLF.
Recommendations
- Defining the underlying CLD: diseases qualified as underlying CLD:
- Included:
- Compensated cirrhosis of any etiology. (1a, A)
- Chronic hepatitis. (5, D)
- Nonalcoholic steatohepatitis. (5, D)
- Cholestatic liver disease. (2b, B)
- Metabolic liver disease. (2b, B)
- Not included:
- Steatosis. (5, D)
Defining the liver failure in ACLF
Acute liver failure has widely accepted definition that includes evidence of coagulation abnormality, usually an INR >1.5, and any degree of mental alteration (encephalopathy) in a patient without pre-existing cirrhosis and with an illness of less than 26 weeks’ duration [40]. Beyond 26 weeks, this is considered as chronic liver failure. There is, however, a great degree of heterogeneity in the current definition of ALF. The outcome of various entities included in ALF would indeed be different. There have been several attempts in the past to define the timeline of liver failure from the time of onset of the jaundice or symptoms. Terms such as “hyperacute liver failure” (<7 days), “acute liver failure” (8–28 days), and “subacute liver failure” (>28 days) have been proposed [3]. On the other hand, the timeline for fulminant and subfulminant hepatic failure (<2 weeks) [4] and late-onset hepatic failure (>8 weeks) has also been reported [5].
On the other extreme, the group of patients with decompensated liver disease has been well defined and acute decompensation of liver disease has also been documented. Development of jaundice, ascites, hepatic encephalopathy, or variceal bleed is known to constitute hepatic decompensation.
Patients with ACLF manifest in varied forms owing to the heterogeneity in the patient population. In the published reports, patients included as having ACLF had severe jaundice associated with organ failure manifested as either hepatic encephalopathy or hepatorenal syndrome (HRS) [41, 42]. Systemic hemodynamic changes generally accompany the development of ACLF. Defining the liver failure in ACLF, therefore, required a detailed consideration of all the existing terminologies and to analyze first the need for a new term. In the only available previous attempt to define the entity, ACLF has been described as deterioration of liver function in cirrhotic patients over a period of 2–4 weeks, associated with progressive jaundice, hepatic encephalopathy and/or HRS, and signs of multiorgan dysfunction [41, 42]. However, a precise definition of the level of jaundice and the seminal defining feature of liver failure—the coagulopathy—have not been given attention.
Jaundice is considered an essential criterion for the diagnosis of ACLF. Various authors have used different cutoff levels of jaundice, varying from a serum bilirubin of 6–20 mg/dl [37–39, 43]. All the experts unanimously agreed to take a lower cutoff level of serum bilirubin (i.e., >5 mg/dl) to enroll a larger group of patients for the evaluation of the natural history of these patients. All agreed on the concept of coagulopathy as mandatory for defining liver failure. As in ALF, INR >1.5 was considered an essential criterion for the diagnosis of coagulopathy. Measurement of INR is easy and widely available in every country. However, in some countries where prothrombin index is widely used as a marker of coagulopathy, prothrombin activity of less than 40% can be used to define coagulopathy [44]. Development of clinical ascites and/or encephalopathy was also taken as a marker of decompensation and included in defining the liver failure as in the previous studies.
Recommendations
- Defining the liver failure in ACLF:
- Jaundice (serum bilirubin ≥5 mg/dl [85 μmol/l]) and coagulopathy (INR ≥1.5 or prothrombin activity <40%) are mandatory. (2a, B)
- Ascites and/or encephalopathy as determined by physical examination. (2b, B)
Pathophysiology of ACLF
Systemic inflammatory response, characterized by a predominantly proinflammatory cytokine profile, causes the transition from stable cirrhosis to ACLF. Proinflammatory cytokines are believed to mediate hepatic inflammation, apoptosis, and necrosis of liver cells; cholestasis; and fibrosis [32, 45]. Wasmuth et al. [32] demonstrate that patients with ACLF have immunologic “defects” that are comparable with those in patients with sepsis. The clinical picture of both ACLF and septic shock is strikingly similar, characterized by progressive vasodilatory shock and multiple organ failure. ACLF is a state of severe functional failure of neutrophils in a proportion of patients with cirrhosis and alcoholic hepatitis and that these defects are associated with increased risk of infection, organ failure, and mortality. The ex vivo studies support the notion that this neutrophil dysfunction is contributed by endotoxins and is reversible if the plasma is treated with endotoxin-removal strategies. The clinical importance of these neutrophil abnormalities, identified by a high resting oxidative burst of 55% or more and a reduced phagocytic capacity (relative geometric mean fluorescence intensity <42%), is highlighted by the observation of increased risk of infection and the association with organ failure and mortality in these patients [46].
Inflammation and oxidative stress also induce production of nitric oxide (NO), which appears to cause the circulatory and renal disturbances of liver failure. There is increasing evidence that the mediators of inflammation (e.g., proinflammatory cytokines, NO, and oxidative stress) could modulate the effect of hyperammonemia in precipitating encephalopathy. The liver plays a prominent role in the metabolism of asymmetric dimethyl-l-arginine (ADMA), an endogenous inhibitor of NO synthase. Hepatocellular damage is a main determinant of elevated ADMA concentration in advanced alcoholic cirrhosis [47–51]. By inhibiting NO release from vascular endothelium, ADMA might oppose the peripheral vasodilation caused by excessive NO production in severe cirrhosis [49]. Plasma ADMA and stereoisomer symmetric dimethylarginine (SDMA) are significantly high in patients with alcoholic hepatitis and nonsurvivors [50].
Recommendations
- Major pathophysiologic events of ACLF:
- There is a central role of inflammation and neutrophil dysfunction in organ failure. (2a)
- Systemic inflammatory response syndrome as a marker of prognosis in predicting mortality in patients with ACLF needs further validation. (3a, C)
- High ADMA and SDMA concentrations are markers of poor prognosis in patients with ACLF. Dimethyl arginine score of >1.23 indicates higher mortality. The role of ischemia-modified albumin (IMA) needs assessment in ACLF. (3b, C)
Role of sepsis and cytokines in ACLF
Cytokines are believed to play an important role in ACLF. Elevated serum levels of several cytokines, including TNF-α, sTNF-αR1, sTNF-αR2, IL-2, IL-2R, IL-4, IL-6, IL-8, IL-10, and interferon-γ, have been described in patients with ACLF [52]. Elevated levels of circulating cytokines in ACLF may be the result of increased production due to endotoxemia, cytokine release by necrotic liver cells, and/or reduced hepatic removal. TNF-α can induce apoptosis of hepatocytes, especially in alcoholic liver disease when hepatocytes are sensitized to TNF-α-induced apoptosis [53, 54]. Therefore, removal of proinflammatory cytokines such as TNF-α from plasma might be considered beneficial. However, cytokines such as TNF-α and IL-6 may also promote liver regeneration by inducing acute-phase proteins and hepatic proliferation and exhibiting antiapoptotic effects [52]. Because cytokines represent not only endocrine but also autocrine and paracrine effector molecules, it should be pointed out that elevated systemic levels are not representative of their role in the pathophysiology of liver failure [52–54]. Recent studies suggest that the transition from a stable cirrhotic condition to the burst of an acute decompensation leading to liver failure is based on an acute systemic inflammatory response, mainly mediated by cytokines [54].
Fibrin deposition and thrombosis within the microvasculature is now appreciated to play a pivotal role in the hepatocellular injury observed in viral hepatitis. Importantly, the pathways by which fibrin generation is elicited in viral hepatitis may be mechanistically distinct from the classical pathways of coagulation induced by mechanical trauma or bacterial lipopolysaccharide. Activated endothelial cells and macrophages express distinct cell-surface procoagulants, including a novel prothrombinase, fgl2/fibroleukin, which are important for both the initiation and the localization of fibrin deposition. Fgl2/fibroleukin plays a critical role in the pathophysiology of viral hepatitis [55]. Human fgl2 (hfgl2) was detected in 21 of 23 patients (91%) with severe ACLF due to HBV infection [56]. There was a positive correlation between hfgl2 expression and the severity of the liver disease as indicated by the levels of bilirubin. The measurement of hfgl2/fibroleukin expression in peripheral blood mononuclear cell may serve as a useful marker to monitor the severity of acute-on-chronic hepatitis B and a target for therapeutic intervention [56].
Recommendations
- Role of sepsis and cytokines in ACLF:
- It is likely that cytokines influence the development and course of ACLF. (3b)
- Inhibition of the inflammatory cytokine responses might offer a novel approach for reducing the morbidity and mortality in patients with ACLF. (3b, C)
- Circulating toxins in the setting of ACLF cause secondary liver damage, and liver regeneration is impaired despite circulating growth factors. (2b)
- TNF-α and IL-6 probably have dual action, induce hepatocyte death on one hand and promote hepatocyte proliferation on the other through differential interactions with Kupffer cells and hepatocytes. (3b)
Hemodynamics in ACLF
Characteristic hemodynamic alterations in cirrhotic patients include increased portal pressure, increased cardiac output, a dilated and hyporesponsive peripheral circulation, increased portosystemic shunting, and a reduced renal blood flow [57, 58]. These phenomena are thought to be secondary to a reduction in vascular responsiveness and downregulation of receptors leading to hyporesponsive vasoconstrictors. As these alterations are reversible with liver transplantation, it seems likely that they are related to the liver dysfunction and a common mechanism probably underlies their development.
ACLF is an acute event on an underlying liver disease and hence the portal hemodynamics of such patients is likely to be different from patients with compensated and decompensated liver diseases. Hyperdynamic circulation and portal hypertension characterize ACLF, partially because of circulating mediators. While patients with ACLF with small varices had HVPG values (13.2 (±5.5) mm Hg) comparable with those of compensated cirrhotic patients, those with large varices had HVPG values comparable with those of decompensated cirrhotic patients (18.2 (±6.5) mm Hg) [59]. The data clearly showed that patients with small varices and lower HVPG levels have a higher chance of recovery after the acute insult settles down.
Alcoholic hepatitis over underlying alcoholic cirrhosis is a prototype of ACLF. TNF-α plays an important role in the development of portal hypertension in alcoholic hepatitis. TNF-α has been reported to be elevated in alcoholic liver disease, and, in particular, high levels of TNF-α are found in alcoholic hepatitis.
Catalina et al. [60] evaluated the portal pressure and systemic hemodynamic in patients with ACLF. All patients had severe portal hypertension (HVPG = 23 ± 7 mm Hg) and pronounced hyperdynamic circulation (mean arterial pressure (MAP) = 77.8 ± 11.7 mm Hg; cardiac output = 11.2 ± 1.6 l/min; SVRI = 478.5 ± 105 dynes/cm5).
Recommendations
- Hemodynamics in ACLF:
- HVPG of patients with ACLF ranges between those with compensated and decompensated chronic liver diseases. (3b)
- Large varices in patients with ACLF reflect high HVPG resulting in poor prognosis. (3b)
- Higher liver blood flow levels in patients with ACLF correlate with higher mortality. (3b, C)
Liver histology in ACLF
There is scarcity of histologic data on ACLF. Prognosis and treatment depend upon the etiology along with the extent of parenchymal collapse and stage of fibrosis. There was unanimity among the experts that liver histopathology is very helpful for the assessment and diagnosis of ACLF. Moreover, liver biopsy is a good predictor of outcome in patients with ACLF [61]. Ballooning and/or eosinophilic degeneration of hepatocytes, cholestasis, and other features of acute hepatitis, parenchymal necrosis, or collapse with features of underlying chronic liver disease, especially fibrosis, are main biopsy findings. Differentiating ALF and chronic hepatitis with flare is based on findings of fibrous bands (spurs and bridges) and ductular proliferation. Features of cholestasis and bile duct proliferation are more common in patients with acute injury (classical features of acute hepatitis along with cellular and ductular cholestasis are indicative of acute injury). Differentiation between cirrhosis with acute deterioration and compensated cirrhosis is based on the presence of necrosis and features of acute hepatitis in the former group of patients. It was proposed that the diagnostic stains for fibrosis and necrosis be mentioned. It was also proposed that connective tissue stains (especially Shikata’s orcein) should be done in all such cases for differentiating necrosis from fibrosis.
Since it is not easy and practical to obtain a liver biopsy in patients with ACLF who are relatively sick, it was agreed that the need for liver biopsy should be individualized in patients with ACLF, considering the clinical condition of the patients. It not only helps in the assessment of the underlying cirrhosis or severe fibrosis but can also be useful in identifying the etiology of the chronic liver disease. Transjugular liver biopsy is relatively safe and the best approach to get a liver biopsy. None of the histologic features, however, was accepted to be pathognomonic of ACLF. Single-center data from India [62] on 40 patients with ACLF suggested two patterns of liver histology in patients with ACLF: pattern I: hepatocyte ballooning, rosette formation, cellular cholestasis, variable interface activity, and fibrosis; pattern II: marked ductular proliferation, coarse, inspissated bile plugs, foci of confluent necrosis/bridging necrosis, eosinophilic degeneration of hepatocytes, higher stage of fibrosis, and variable activity. Pattern II was associated with a much worse prognosis. These findings were found to be quite convincing and consistent with the observations of other experts.
Recommendations
- Liver histology in ACLF:
- Liver histology is quite helpful in assessing the presence and degree of hepatic fibrosis and/or cirrhosis. (1a, A)
- Two distinct histologic patterns are seen: (3b, C)
- Pattern I: Hepatocyte ballooning, rosette formation, cellular cholestasis, variable interface activity, and fibrosis
- Pattern II: Marked ductular proliferation, coarse, inspissated bile plugs, foci of confluent necrosis/bridging necrosis, eosinophilic degeneration of hepatocytes, higher stage of fibrosis, and variable activity
- The need of liver biopsy in ACLF should be individualized. (2a, B)
Prognostic scores for ACLF
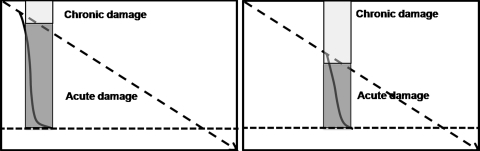
ACLF constitutes an illness condition in which two simultaneous insults are operating: acute and chronic. The degree of each insult would defer quantitatively, nevertheless resulting in the same level of decompensation (Fig. 3). Whether the prognosis of the patient depends on the degree of acute insult, chronic insult, or the combination of the two, is still not well defined.
Fig. 3.
Degree of acute and chronic insult in acute-on-chronic liver failure (ACLF): Two scenarios. The first artwork shows a patient with mild chronic liver disease but severe acute liver insult leading to ACLF. The second artwork shows a patient with moderate chronic liver disease and less severe acute insult. The resulting severity of ACLF is same in these two situations
Two categories of prognostic models are commonly used: first, those evaluating the severity of illness: Acute Physiology and Chronic Health Evaluation (APACHE) II and III, Simplified Acute Physiology Score (SAPS) II, and Mortality Prediction Model II, which are most commonly used, and, second, models quantifying organ dysfunction and failure: Logistic Organ Dysfunction System, Multiple Organ Dysfunction Score, Organ System Failure (OSF), and Sequential Organ Failure Assessment (SOFA). Several prognostic scoring systems have been developed for patients with chronic liver disease as well as patients admitted to an intensive care unit (ICU). Liver-specific scoring systems (Mayo Risk Score, Combined Clinical and Laboratory Index) are adequate, but the APACHE II and III proved to be more powerful, because they include additional physiologic parameters and therefore also take into account additional complications associated with this liver disorder [63, 64]. It was considered that both SOFA and APACHE were not primarily developed for liver failure and APACHE III is better than APACHE II. Sakka et al. [65] suggested that measurement of the indocyanine green plasma disappearance rate has a sensitivity and specificity comparable with that of APACHE II and SAPS II scores for estimating survival on ICU admission. Concept of pre-ACLF was raised, and it was agreed that all these scores are helpful only when patients are admitted in the ICU and may not be useful when they are in a pre-ACLF state, that is, not meeting the criteria of ACLF. Thabut et al. [66] concluded that the presence of SIRS, with or without infection, is a major independent prognostic factor in patients with cirrhosis and acute functional renal failure. This suggests that preventing and treating SIRS could decrease mortality in patients with cirrhosis and acute renal failure. In a meta-analysis by Cholongitas et al. [30], general ICU models had better performance in cirrhotic populations than those with Child-Turcotte-Pugh (CTP) score; both OSF and SOFA had the best predictive ability. Recently, dimethylarginine-dimethylamino-hydrolase protein expression was reduced and that of protein-arginine-methyltransferase-1 increased in alcoholic hepatitis livers. Asymmetric dimethylarginine, SDMA, and their combined sum, which is termed as dimethylarginine score, were reported as better predictors of outcome than CTP score, Mayo end-stage liver disease (MELD), and Maddrey’s discriminant function. Elevated dimethylarginines may serve as important biologic markers of deleterious outcome in alcoholic hepatitis [51]. Role of serum levels of Gc globulin, a hepatically synthesized component of the extracellular actin scavenger system responsible for complexing circulating actin and attenuating intravascular microthrombus formation, is associated with poor outcome in ALF [67], and IMA levels predict mortality in patients with end-stage renal disease and acute coronary event needs evaluation for predicting mortality in ACLF [68, 69].
Recommendations
Prognostic scores for ACLF: CPT, MELD, SOFA, and APACHE scores are generally not different in patients with different etiologies of ACLF. (3b, C)
Use of antivirals in ACLF
Spontaneous or treatment-induced flares of inflammation are frequently observed in chronic hepatitis B. These abrupt elevations in serum ALT levels are the result of an increase in intrahepatic necroinflammation associated with expanded numbers of intrahepatic lymphocytes, in particular cytotoxic T lymphocytes. Cytotoxic T lymphocytes are important to control HBV but can also induce liver damage, depending on the environment and functional capability. The reactivation of HBV is common (14–50%) following chemotherapy and is associated with a high mortality (5%–12%) despite prompt antiviral treatment. The reactivation HBV may necessitate interruption of chemotherapy, with adverse prognostic consequences for the hematologic disease. Chemotherapy-induced immune suppression may lead to increased HBV replication. Immune reconstitution within the weeks and months following recovery from chemotherapy may be associated with a flare of hepatitis B manifested by hepatocellular injury. Risk factors associated with HBV reactivation include treatment with corticosteroids, young age, male gender, and drug resistance. While both acute hepatitis B and reactivation of chronic hepatitis B may present similarly, HBV DNA levels are high in the later group of patients. Lamivudine has been shown to be effective during HBV reactivation due to immune suppression [70–72]. Despite the potential benefits of the prophylactic approach, careful clinical monitoring is still required. In patients with chronic HBV infection, prolonged lamivudine therapy that exceeds 6 months is reportedly associated with an increased risk of treatment-induced HBV variants with YMDD mutations [73]; so alternative treatments with adefovir, tenofovir, or entecavir should be tested [74].
Recommendations
- Use of antivirals in ACLF:
- Antiviral therapy should be initiated in patients with ACLF due to hepatitis B. (3b, C)
- Lamivudine may be used for a short-term period, but other potent drugs such as entecavir or tenofovir may be preferred in view of the long-term need for viral suppression with low frequency of drug resistance. (3b, C)
- Prophylactic therapy is recommended for HBsAg-positive patients undergoing chemotherapy. (3b, C)
- There is insufficient data to recommend antiviral therapy for HBsAg-negative and anti-HBc-positive patients. (3b, C)
Use of liver support devices in ACLF
Molecular adsorbent recirculating system (MARS) is an important option for patients with liver failure either to give them additional time for recovery or to serve as a “bridge” to transplantation. This therapy offers a valid therapeutic option in patients with ACLF by removing toxins generated during liver failure, as well as lowering the levels of proinflammatory cytokines TNF-α, IL-10, and IL-6 that may perpetuate the liver damage and extend the inflammatory cascade to other organs [23, 52]. Moreover, a recent article published by Guo et al. [75] demonstrated that among patients affected by severe liver failure associated with multiple organ dysfunction syndrome, MARS treatment efficiently removed TNF-α, IL-6, IL-8, interleukin-γ, and IL-4. Plasma levels of these cytokines in nonsurvivors were significantly higher than those in survivors and that the removal rates of these molecules were lower than those in the survivors. Therefore, lowering of the concentrations of these cytokines may represent useful markers to assess the effectiveness of MARS therapy.
However, in a meta-analysis [76], MARS treatment did not appear to reduce mortality significantly compared with standard medical treatment (relative risk, 0.56; 95% confidence interval, 0.28–1.14; P = 0.11). Subgroup analysis of two trials for ACLF did not reveal any benefit to survival with MARS treatment. In contrast, explorative analysis of two nonrandomized trials showed a significant survival benefit with MARS treatment (relative risk, 0.36; 95% confidence interval, 0.17–0.76; P = 0.007). This was possibly related to bias in the selection of patients in the nonrandomized trials. In conclusion, MARS treatment had no significant survival benefit on patients with liver failure when compared with standard medical therapy.
In a report from China, plasma exchange was found to significantly improve survival [77]; however, it needs further validation.
Recommendations
- Use of liver support devices for treatment of ACLF:
- Molecular adsorbent recirculating system does not offer any survival benefit to patients with ACLF. (1a, A)
- Role of MARS as a bridge to transplantation in patients with ACLF is still to be defined. (2b, B)
- Molecular adsorbent recirculating system may improve hepatic encephalopathy in patients with ACLF. (1a, A)
- Plasma exchange needs further validation for the treatment of ACLF. (3b, C)
Liver transplant in ACLF
There is scarcity of data on liver transplant in ACLF. Orthotopic liver transplantation remains the only definitive therapy for patients who do not improve with supportive measures to sustain life. Although post-transplant survival rates for ALF have been reported to be as high as 80–90%, accurate long-term outcome data are not yet available for ACLF. Developing effective methods of liver support or other alternatives for transplantation and better prognostic scoring systems remains key goals to further improve overall survival rates for the condition. Long-term results from chronic hepatitis B-related liver disease were satisfactory [78–81]. In a study by Liu et al. [82], patients received liver grafts that were 52 ± 2% of the estimated standard liver weight. At a median follow-up of 23 months, both patient and graft survival rates were 88%. They concluded that live donor liver transplantation (LDLT) using right lobe is an effective therapeutic option for patients with ACLF due to hepatitis B. It results in satisfactory survival outcomes comparable with those in patients undergoing LDLT for elective conditions.
The experts agreed to the use of standard King’s college hospital criteria for liver transplant in patients with ACLF and the need for earlier intervention if HRS develops. The most ominous complications in these patients are spontaneous bacterial peritonitis and rapid-onset (type I) HRS. Less than half of those in whom spontaneous bacterial peritonitis develops are expected to survive for more than 1 year, and the median survival among patients with type I HRS is less than 2 weeks [83, 84]. All patients with liver failure are at risk for acquisition of bacterial or fungal infection or sepsis, which may preclude transplantation or complicate the post-operative course [85, 86]. While adequate fluid replacement and treatment of potential infection and sepsis may help correct hypotension, inotropic or pressor support may be required to maintain mean arterial pressures (MAPs) of at least 50–60 mm Hg. Hemodynamic instability and need for high-dose inotropes make the patients unsuitable for liver transplant. Similarly, raised intracranial pressure (ICP) and reductions in cerebral perfusion pressure (calculated as MAP minus ICP) are considered relative contraindications to liver transplantation in many centers.
Recommendations
- Liver transplant in patients with ACLF:
- Criteria when to transplant:
- Liver transplantation should be performed according to prognosis scores suggesting death within the next 3 months. (2b, B)
- King’s College Hospital criteria need further validation for patients with ACLF. (2b, B)
- Earlier intervention if HRS develops. (2b, B)
- However, liver transplantation should not be performed when there is HRS with anuria. (3b, C)
- Results of liver transplantation are better when HRS has been partially controlled by terlipressin. (2b, B)
- Criteria when not to transplant:
- Hemodynamic instability and high-dose inotrope requirement (sepsis, bleeding). (2a, B)
- Severe bacterial infection. (2a, B)
- Fungal infection. (2a, B)
- Cerebral edema or intracranial bleeding. (1a, A)
- Living donor liver transplantation for patients with ACLF:
- The use of liver graft of sufficient graft weight for the recipient and with uniform venous outflow is preferred. (3b, C)
Conclusion
In summary, ACLF is a distinct clinical entity. There is now sufficient data on the presentation and course of patients with this profile of liver failure. The recommendations made by the global experts are likely to help the readers improve the identification and management of these patients. There is every possibility that with the availability of newer information, especially correlated with liver histology, would help further improve our understanding of this disease entity in the future.
Footnotes
No consensus.
Contributor Information
Shiv Kumar Sarin, Email: sksarin@nda.vsnl.net.in.
Ashish Kumar, Email: ashishk10@yahoo.com.
John A. Almeida, priyanair@ozemail.com.au
Yogesh Kumar Chawla, Email: ykchawla@gmail.com.
Sheung Tat Fan, Email: stfan@hku.hk.
Hitendra Garg, Email: drhitendragarg@yahoo.com.
H. Janaka de Silva, Email: hjdes@sltnet.lk.
Saeed Sadiq Hamid, Email: saeed.hamid@aku.edu.
Rajiv Jalan, Email: r.jalan@ucl.ac.uk.
Piyawat Komolmit, Email: pkomolmit@yahoo.co.uk.
George K. Lau, Email: gkklau@netvigator.com
Qing Liu, Email: liuqing7@yahoo.com.
Kaushal Madan, Email: k_madan_2000@yahoo.com.
Rosmawati Mohamed, Email: ros@ummc.edu.my.
Qin Ning, Email: qning@tjh.tjmu.edu.cn.
Salimur Rahman, Email: hbd@dhaka.net.
Archana Rastogi, Email: dr_archanarastogi@yahoo.co.in.
Stephen M. Riordan, sriordan@ozemail.com.au
Puja Sakhuja, Email: pujasak@hotmail.com.
Didier Samuel, Email: didier.samuel@pbr.ap-hopparis.fr.
Samir Shah, Email: drshahsamir@gmail.com.
Barjesh Chander Sharma, Email: drbcsharma@hotmail.com.
Praveen Sharma, Email: drpraveen_sharma@yahoo.com.
Yasuhiro Takikawa, Email: ytakikaw@iwate-med.ac.jp.
Babu Ram Thapa, Email: brthapa1@yahoo.co.in.
Chun-Tao Wai, Email: dr_desmond_wai@yahoo.com.sg.
Man-Fung Yuen, Email: mfyuen@hkucc.hku.hk.
References
- 1.Centre for evidence-based medicine. Levels of evidence. 2001. http://www.cebm.net/index.aspx?o=1047. Accessed 12 Oct 2008
- 2.Ohnishi H, Sugihara J, Moriwaki H, Muto Y. Acute-on-chronic liver failure. Ryoikibetsu Shokogun Shirizu 1995;(7):217–219 [PubMed]
- 3.O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273–275. doi: 10.1016/0140-6736(93)91818-7. [DOI] [PubMed] [Google Scholar]
- 4.Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: definitions and causes. Semin Liver Dis. 1986;6:97–106. doi: 10.1055/s-2008-1040593. [DOI] [PubMed] [Google Scholar]
- 5.Gimson AE, O’Grady J, Ede RJ, Portmann B, Williams R. Late onset hepatic failure: clinical, serological and histological features. Hepatology. 1986;6:288–294. doi: 10.1002/hep.1840060222. [DOI] [PubMed] [Google Scholar]
- 6.Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohrt HE, Ouyang DL, Keeffe EB. Antiviral prophylaxis for chemotherapy-induced reactivation of chronic hepatitis B virus infection. Clin Liver Dis. 2007;11:965–991. doi: 10.1016/j.cld.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Millonig G, Kern M, Ludwiczek O, Nachbaur K, Vogel W. Subfulminant hepatitis B after infliximab in Crohn’s disease: need for HBV-screening? World J Gastroenterol. 2006;12:974–976. doi: 10.3748/wjg.v12.i6.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheng WH, Kao JH, Chen PJ, Huang LM, Chang SY, Sun HY, et al. Evolution of hepatitis B serological markers in HIV-infected patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;45:1221–1229. doi: 10.1086/522173. [DOI] [PubMed] [Google Scholar]
- 10.Paitoonpong L, Suankratay C. Immunological response to hepatitis B vaccination in patients with AIDS and virological response to highly active antiretroviral therapy. Scand J Infect Dis. 2008;40:54–58. doi: 10.1080/00365540701522975. [DOI] [PubMed] [Google Scholar]
- 11.Flink HJ, Sprengers D, Hansen BE, Zonneveld M, Man RA, Schalm SW, HBV 99–01 Study Group et al. Flares in chronic hepatitis B patients induced by the host or the virus? Relation to treatment response during Peg-interferon {alpha}-2b therapy. Gut. 2005;54:1604–1609. doi: 10.1136/gut.2004.062208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perceau G, Diris N, Estines O, Derancourt C, Lévy S, Bernard P. Late lethal hepatitis B virus reactivation after rituximab treatment of low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2006;155:1053–1056. doi: 10.1111/j.1365-2133.2006.07451.x. [DOI] [PubMed] [Google Scholar]
- 13.Sera T, Hiasa Y, Michitaka K, Konishi I, Matsuura K, Tokumoto Y, et al. Anti-HBs-positive liver failure due to hepatitis B virus reactivation induced by rituximab. Intern Med. 2006;45:721–724. doi: 10.2169/internalmedicine.45.1590. [DOI] [PubMed] [Google Scholar]
- 14.Locasciulli A, Bruno B, Alessandrino EP, Meloni G, Arcese W, Bandini G, Italian Cooperative Group for Blood and Marrow Transplantation et al. Hepatitis reactivation and liver failure in haemopoietic stem cell transplants for hepatitis B virus (HBV)/hepatitis C virus (HCV) positive recipients: a retrospective study by the Italian group for blood and marrow transplantation. Bone Marrow Transplant. 2003;31:295–300. doi: 10.1038/sj.bmt.1703826. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh CY, Huang HH, Lin CY, Chung LW, Liao YM, Bai LY, et al. Rituximab-induced hepatitis C virus reactivation after spontaneous remission in diffuse large B-cell lymphoma. J Clin Oncol. 2008;26:2584–2586. doi: 10.1200/JCO.2007.15.4807. [DOI] [PubMed] [Google Scholar]
- 16.Hamid SS, Atiq M, Shehzad F, Yasmeen A, Nissa T, Salam A, et al. Hepatitis E virus superinfection in patients with chronic liver disease. Hepatology. 2002;36:474–478. doi: 10.1053/jhep.2002.34856. [DOI] [PubMed] [Google Scholar]
- 17.Ramachandran J, Eapen CE, Kang G, Abraham P, Hubert DD, Kurian G, et al. Hepatitis E superinfection produces severe decompensation in patients with chronic liver disease. J Gastroenterol Hepatol. 2004;19:134–138. doi: 10.1111/j.1440-1746.2004.03188.x. [DOI] [PubMed] [Google Scholar]
- 18.Monga R, Garg S, Tyagi P, Kumar N. Superimposed acute hepatitis E infection in patients with chronic liver disease. Indian J Gastroenterol. 2004;23:50–52. [PubMed] [Google Scholar]
- 19.Kumar M, Sharma BC, Sarin SK. Hepatitis E virus as an etiology of acute exacerbation of previously unrecognized asymptomatic patients with hepatitis B virus-related chronic liver disease. J Gastroenterol Hepatol. 2008;23(6):83–87. doi: 10.1111/j.1440-1746.2007.05243.x. [DOI] [PubMed] [Google Scholar]
- 20.Acharya SK, Sharma PK, Singh R, Mohanty SK, Madan K, Jha JK, et al. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46:387–394. doi: 10.1016/j.jhep.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Chung RT, Friedman LS. Bacterial, parasitic, and fungal infections of the liver, including liver abscess. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 8. Philadelphia: WB Saunders; 2006. p. 1731. [Google Scholar]
- 22.Laleman W, Wilmer A, Evenepoel P, Elst IV, Zeegers M, Zaman Z, et al. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit Care. 2006;10:R108. doi: 10.1186/cc4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sen S, Davies NA, Mookerjee RP, Cheshire LM, Hodges SJ, Williams R, et al. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: a randomized controlled study. Liver Transpl. 2004;10:1109–1119. doi: 10.1002/lt.20236. [DOI] [PubMed] [Google Scholar]
- 24.Hessel FP, Mitzner SR, Rief J, Guellstorff B, Steiner S, Wasem J. Economic evaluation and 1-year survival analysis of MARS in patients with alcoholic liver disease. Liver Int. 2003;23(Suppl 3):66–72. doi: 10.1034/j.1478-3231.23.s.3.5.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee KH, Lee MK, Sutedja DS, Lim SG. Outcome from molecular adsorbent recycling system (MARS) liver dialysis following drug-induced liver failure. Liver Int. 2005;25:973–977. doi: 10.1111/j.1478-3231.2005.01091.x. [DOI] [PubMed] [Google Scholar]
- 26.Mattéi A, Rucay P, Samuel D, Feray C, Reynes M, Bismuth H. Liver transplantation for severe acute liver failure after herbal medicine (Teucrium polium) administration. J Hepatol. 1995;22:597. doi: 10.1016/0168-8278(95)80458-7. [DOI] [PubMed] [Google Scholar]
- 27.Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Portal Hypertension Collaborative Group et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Olmo JA, Flor-Lorente B, Flor-Civera B, Rodriguez F, Serra MA, Escudero A, et al. Risk factors for nonhepatic surgery in patients with cirrhosis. World J Surg. 2003;27:647–652. doi: 10.1007/s00268-003-6794-1. [DOI] [PubMed] [Google Scholar]
- 29.Rice HE, O’Keefe GE, Helton WS, Johansen K. Morbid prognostic features in patients with chronic liver failure undergoing nonhepatic surgery. Arch Surg. 1997;132:880–884. doi: 10.1001/archsurg.1997.01430320082013. [DOI] [PubMed] [Google Scholar]
- 30.Cholongitas E, Senzolo M, Patch D, Kwong K, Nikolopoulou V, Leandro G, et al. Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther. 2006;23:883–893. doi: 10.1111/j.1365-2036.2006.02842.x. [DOI] [PubMed] [Google Scholar]
- 31.Wehler M, Kokoska J, Reulbach U, Hahn EG, Strauss R. Short-term prognosis in critically ill patients with cirrhosis assessed by prognostic scoring systems. Hepatology. 2001;34:255–261. doi: 10.1053/jhep.2001.26522. [DOI] [PubMed] [Google Scholar]
- 32.Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E, et al. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol. 2005;42:195–201. doi: 10.1016/j.jhep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, Bader BD, et al. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6:277–286. doi: 10.1053/lv.2000.6355. [DOI] [PubMed] [Google Scholar]
- 34.Heemann U, Treichel U, Loock J, Philipp T, Gerken G, Malago M, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology. 2002;36:949–958. doi: 10.1053/jhep.2002.36130. [DOI] [PubMed] [Google Scholar]
- 35.Zauner C, Schneeweiss B, Schneider B, Madl C, Klos H, Kranz A, et al. Short-term prognosis in critically ill patients with liver cirrhosis: an evaluation of a new scoring system. Eur J Gastroenterol Hepatol. 2000;12:517–522. doi: 10.1097/00042737-200012050-00007. [DOI] [PubMed] [Google Scholar]
- 36.Choi JY, Bae SH, Yoon SK, Cho SH, Yang JM, Han JY, et al. Preconditioning by extracorporeal liver support (MARS) of patients with cirrhosis and severe liver failure evaluated for living donor liver transplantation—a pilot study. Liver Int. 2005;25:740–745. doi: 10.1111/j.1478-3231.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- 37.Wagholikar GD, Lee KH, Pandey D, Leong SO, Singh R, Tan KC. Pre-transplant optimization by molecular adsorbent recirculating system in patients with severely decompensated chronic liver disease. Indian J Gastroenterol. 2007;26:110–112. [PubMed] [Google Scholar]
- 38.Wai CT, Lim SG, Aung MO, Lee YM, Sutedja DS, Dan YY, et al. MARS: a futile tool in centres without active liver transplant support. Liver Int. 2007;27:69–75. doi: 10.1111/j.1478-3231.2006.01388.x. [DOI] [PubMed] [Google Scholar]
- 39.Du WB, Li LJ, Huang JR, Yang Q, Liu XL, Li J, et al. Effects of artificial liver support system on patients with acute or chronic liver failure. Transplant Proc. 2005;37:4359–4364. doi: 10.1016/j.transproceed.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 40.Polson J, Lee WM, American Association for the Study of Liver Disease AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 41.Sen S, Williams R, Jalan R. The pathophysiological basis of acute-on-chronic liver failure. Liver. 2002;22(Suppl 2):5–13. doi: 10.1034/j.1600-0676.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 42.Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252–261. doi: 10.1159/000047017. [DOI] [PubMed] [Google Scholar]
- 43.Stadlbauer V, Krisper P, Aigner R, Haditsch B, Jung A, Lackner C, et al. Effect of extracorporeal liver support by MARS and Prometheus on serum cytokines in acute-on-chronic liver failure. Crit Care. 2006;10:R169. doi: 10.1186/cc5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q, Liu Z, Wang T, Wang Q, Shi X, Dao W. Characteristics of acute and sub-acute liver failure in China: nomination, classification and interval. J Gastroenterol Hepatol. 2007;22:2101–2106. doi: 10.1111/j.1440-1746.2006.04362.x. [DOI] [PubMed] [Google Scholar]
- 45.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 46.Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, et al. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831–840. doi: 10.1002/hep.21737. [DOI] [PubMed] [Google Scholar]
- 47.Mookerjee RP, Dalton RN, Davies NA, Hodges SJ, Turner C, Williams R, et al. Inflammation is an important determinant of levels of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine (ADMA) in acute liver failure. Liver Transpl. 2007;13:400–405. doi: 10.1002/lt.21053. [DOI] [PubMed] [Google Scholar]
- 48.Lluch P, Mauricio MD, Vila JM, Segarra G, Medina P, Del Olmo JA, et al. Accumulation of symmetric dimethylarginine in hepatorenal syndrome. Exp Biol Med (Maywood) 2006;231:70–75. doi: 10.1177/153537020623100108. [DOI] [PubMed] [Google Scholar]
- 49.Vizzutti F, Romanelli RG, Arena U, Rega L, Brogi M, Calabresi C, et al. ADMA correlates with portal pressure in patients with compensated cirrhosis. Eur J Clin Invest. 2007;37:509–515. doi: 10.1111/j.1365-2362.2007.01814.x. [DOI] [PubMed] [Google Scholar]
- 50.Lluch P, Torondel B, Medina P, Segarra G, Del Olmo JA, Serra MA, et al. Plasma concentrations of nitric oxide and asymmetric dimethylarginine in human alcoholic cirrhosis. J Hepatol. 2004;41:55–59. doi: 10.1016/j.jhep.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Mookerjee RP, Malaki M, Davies NA, Hodges SJ, Dalton RN, Turner C, et al. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology. 2007;45:62–71. doi: 10.1002/hep.21491. [DOI] [PubMed] [Google Scholar]
- 52.Ambrosino G, Naso A, Feltracco P, Carraro P, Basso SM, Varotto S, et al. Cytokines and liver failure: modification of TNF-α and IL–6 in patients with acute on chronic liver decompensation treated with molecular adsorbent recycling system (MARS) Acta Biomed. 2003;74(Suppl 2):7–9. [PubMed] [Google Scholar]
- 53.Auth MK, Kim HS, Beste M, Bonzel KE, Baumann U, Ballauff A, et al. Removal of metabolites, cytokines and hepatic growth factors by extracorporeal liver support in children. J Pediatr Gastroenterol Nutr. 2005;40:54–59. doi: 10.1097/00005176-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734–739. doi: 10.1053/jhep.2000.17687. [DOI] [PubMed] [Google Scholar]
- 55.Marsden PA, Ning Q, Fung LS, Luo X, Chen Y, Mendicino M, et al. The Fgl2/fibroleukin prothrombinase contributes to immunologically mediated thrombosis in experimental and human viral hepatitis. J Clin Invest. 2003;112:58–66. doi: 10.1172/JCI18114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu CL, Yan WM, Zhu F, Zhu YF, Xi D, Tian DY, et al. Fibrinogen-like protein 2 fibroleukin expression and its correlation with disease progression in murine hepatitis virus type 3-induced fulminant hepatitis and in patients with severe viral hepatitis B. World J Gastroenterol. 2005;11:6936–6940. doi: 10.3748/wjg.v11.i44.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bosch J, García-Pagán JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32(1 Suppl):141–156. doi: 10.1016/S0168-8278(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 58.Newby DE, Jalan R, Masumori S, Hayes PC, Boon NA, Webb DJ. Peripheral vascular tone in patients with cirrhosis: role of the renin-angiotensin and sympathetic nervous systems. Cardiovasc Res. 1998;38:221–228. doi: 10.1016/S0008-6363(98)00008-X. [DOI] [PubMed] [Google Scholar]
- 59.Kumar A, Das K, Sharma P, Mehta V, Sharma BC, Sarin SK. Hemodynamic studies in acute-on-chronic liver failure. Dig Dis Sci 2008 Aug 9. [Epub ahead of print] [DOI] [PubMed]
- 60.Catalina MV, Barrio J, Anaya F, Salcedo M, Rincón D, Clemente G, et al. Hepatic and systemic haemodynamic changes after MARS in patients with acute on chronic liver failure. Liver Int. 2003;23(Suppl 3):39–43. doi: 10.1034/j.1478-3231.23.s.3.10.x. [DOI] [PubMed] [Google Scholar]
- 61.Rastogi A, Sakhuja P, Gondal R, Garg H, Hissar SS, Sarin SK. Liver biopsy is a good predictor of the outcome in patients with acute on chronic liver failure [abstract]. Hepatology 2008;48(Suppl):446A [DOI] [PubMed]
- 62.Sakhuja P, Rastogi A, Gondal R, Garg H, Sarin SK. Acute on chronic liver failure—analysis of two distinct liver histological patterns [abstract] J Hepatol. 2008;48(Suppl 2):S95. doi: 10.1016/S0168-8278(08)60233-0. [DOI] [Google Scholar]
- 63.Zauner CA, Apsner RC, Kranz A, Kramer L, Madl C, Schneider B, et al. Outcome prediction for patients with cirrhosis of the liver in a medical ICU: a comparison of the APACHE scores and liver-specific scoring systems. Intensive Care Med. 1996;22:559–563. doi: 10.1007/BF01708096. [DOI] [PubMed] [Google Scholar]
- 64.Sarin SK, Kumar A, Garg HK. Clinical profile of acute on chronic liver failure (ACLF) and predictors of mortality: a study of 64 patients [abstract]. Hepatology 2008;48(Suppl):450A
- 65.Sakka SG, Reinhart K, Meier-Hellmann A. Prognostic value of the indocyanine green plasma disappearance rate in critically ill patients. Chest. 2002;122:1715–1720. doi: 10.1378/chest.122.5.1715. [DOI] [PubMed] [Google Scholar]
- 66.Thabut D, Massard J, Gangloff A, Carbonell N, Francoz C, Nguyen-Khac E, et al. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872–1882. doi: 10.1002/hep.21920. [DOI] [PubMed] [Google Scholar]
- 67.Antoniades CG, Berry PA, Bruce M, Cross TJ, Portal AJ, Hussain MJ, et al. Actin-free Gc globulin: a rapidly assessed biomarker of organ dysfunction in acute liver failure and cirrhosis. Liver Transpl. 2007;13:1254–1261. doi: 10.1002/lt.21196. [DOI] [PubMed] [Google Scholar]
- 68.Sharma R, Gaze DC, Pellerin D, Mehta RL, Gregson H, Streather CP, et al. Ischemia-modified albumin predicts mortality in ESRD. Am J Kidney Dis. 2006;47:493–502. doi: 10.1053/j.ajkd.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 69.Collinson PO, Gaze DC, Bainbridge K, Morris F, Morris B, Price A, et al. Utility of admission cardiac troponin and “ischemia modified albumin” measurements for rapid evaluation and rule out of suspected acute myocardial infarction in the emergency department. Emerg Med J. 2006;23:256–261. doi: 10.1136/emj.2005.028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inoue T, Fuke H, Yamamoto N, Ito K, Yutaka KY, Yamanaka SK. Lamivudine for treatment of spontaneous exacerbation and reactivation after immunosuppressive therapy in patients with hepatitis B virus infection. Hepatogastroenterology. 2007;54:889–891. [PubMed] [Google Scholar]
- 71.Liao CA, Lee CM, Wu HC, Wang MC, Lu SN, Eng HL. Lamivudine for the treatment of hepatitis B virus reactivation following chemotherapy for non-Hodgkin’s lymphoma. Br J Haematol. 2002;116:166–169. doi: 10.1046/j.1365-2141.2002.03239.x. [DOI] [PubMed] [Google Scholar]
- 72.Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: a randomized trial. Hepatology. 2008;47:844–853. doi: 10.1002/hep.22106. [DOI] [PubMed] [Google Scholar]
- 73.Ling R, Mutimer D, Ahmed M, Boxall EH, Elias E, Dusheiko GM, et al. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology. 1996;24:711–713. doi: 10.1002/hep.510240339. [DOI] [PubMed] [Google Scholar]
- 74.Zoulim F, Perrillo R. Hepatitis B: reflections on the current approach to antiviral therapy. J Hepatol. 2008;48(Suppl 1):S2–S19. doi: 10.1016/j.jhep.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Guo LM, Liu JY, Xu DZ, Li BS, Han H, Wang LH, et al. Application of molecular adsorbents recirculating system to remove NO and cytokines in severe liver failure patients with multiple organ dysfunction syndrome. Liver Int. 2003;23(Suppl 3):16–20. doi: 10.1034/j.1478-3231.23.s.3.7.x. [DOI] [PubMed] [Google Scholar]
- 76.Khuroo MS, Khuroo MS, Farahat KL. Molecular adsorbent recirculating system for acute and acute-on-chronic liver failure: a meta-analysis. Liver Transpl. 2004;10:1099–1106. doi: 10.1002/lt.20139. [DOI] [PubMed] [Google Scholar]
- 77.Li LJ, Yang Q, Huang JR, Xu XW, Chen YM, Fu SZ. Effect of artificial liver support system on patients with severe viral hepatitis: a study of four hundred cases. World J Gastroenterol. 2004;10:2984–2988. doi: 10.3748/wjg.v10.i20.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wai CT, Da Costa M, Sutedja D, Lee YM, Lee KH, Tan KC, et al. Long-term results of liver transplant in patients with chronic viral hepatitis-related liver disease in Singapore. Singapore Med J. 2006;47:588–591. [PubMed] [Google Scholar]
- 79.Stärkel P, Horsmans Y, Geubel A, Ciccarelli O, Goubau P, Rahier J, et al. Favorable outcome of orthotopic liver transplantation in a patient with subacute liver failure due to the emergence of a hepatitis B YMDD escape mutant virus. J Hepatol. 2001;35:679–681. doi: 10.1016/S0168-8278(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 80.Saab S, Kim M, Wright TL, Han SH, Martin P, Busuttil RW. Successful orthotopic liver transplantation for lamivudine-associated YMDD mutant hepatitis B virus. Gastroenterology. 2000;119:1382–1384. doi: 10.1053/gast.2000.19279. [DOI] [PubMed] [Google Scholar]
- 81.Osborn MK, Han SH, Regev A, Bzowej NH, Ishitani MB, Tran TT, NIH HBV-OLT Study Group et al. Outcomes of patients with hepatitis B who developed antiviral resistance while on the liver transplant waiting list. Clin Gastroenterol Hepatol. 2007;5:1454–1461. doi: 10.1016/j.cgh.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu CL, Fan ST, Lo CM, Wei WI, Yong BH, Lai CL, et al. Live-donor liver transplantation for acute-on-chronic hepatitis B liver failure. Transplantation. 2003;76:1174–1179. doi: 10.1097/01.TP.0000087341.88471.E5. [DOI] [PubMed] [Google Scholar]
- 83.Andreu M, Sola R, Sitges-Serra A, Alia C, Gallen M, Vila MC, et al. Risk factors for spontaneous bacterial peritonitis in cirrhotic patients with ascites. Gastroenterology. 1993;104:1133–1138. doi: 10.1016/0016-5085(93)90284-j. [DOI] [PubMed] [Google Scholar]
- 84.Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 85.Rolando N, Harvey F, Brahm J, Philpott-Howard J, Alexander G, Gimson A, et al. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology. 1990;11:49–53. doi: 10.1002/hep.1840110110. [DOI] [PubMed] [Google Scholar]
- 86.Rolando N, Harvey F, Brahm J, Philpott-Howard J, Alexander G, Casewell M, et al. Fungal infection: a common, unrecognised complication of acute liver failure. J Hepatol. 1991;12:1–9. doi: 10.1016/0168-8278(91)90900-V. [DOI] [PubMed] [Google Scholar]