Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is the etiological agent of Kaposi's sarcoma, primary effusion lymphoma (PEL), and multicentric Castleman's disease. Like other herpesviruses, KSHV establishes life-long latency in the human host with intermittent periods of reactivation. Physiological triggers of herpesviral reactivation are poorly defined. Toll-like receptors (TLRs) recognize pathogens and are vital for the host innate immune response. We screened multiple TLR agonists for their ability to initiate KSHV replication in latently infected PEL. Agonists specific for TLR7/8 reactivated latent KSHV and induced viral lytic gene transcription and replication. Furthermore, vesicular stomatitis virus (VSV), a bonafide physiological activator of TLR7/8, also reactivated KSHV from latency. This demonstrates that secondary pathogen infection of latently infected cells can reactivate KSHV. Human herpesviruses establish life-long latency in the host, and it is plausible that a latently infected cell will encounter multiple pathogens during its lifetime and that these encounters lead to episodic reactivation. Our findings have broad implications for physiological triggers of latent viral infections, such as herpesviral reactivation and persistence in the host.
Keywords: TLR7, TLR8
Kaposi's sarcoma-associated herpesvirus (KSHV/HHV8) is a member of the gammaherpesviridae. KSHV is the etiological agent of Kaposi's sarcoma (KS), the most common AIDS-associated malignancy, and is causally linked to two B cell lymphoproliferative disorders, primary effusion lymphoma (PEL) and multicentric Castleman's disease (MCD) (1, 2). All herpesviruses, including KSHV, exhibit both latent and lytic phases during their lifecycle. B lymphocytes are the primary latent reservoir of KSHV, although KSHV can persist latently in multiple cell types (3). KSHV reactivates and replicates in response to chemical compounds such as 12-O-tetradecanoyl-phorbol-13-acetate (TPA/PMA) or butyrate. Demethylation of the promoter for the KSHV replication and transcription activator (RTA), and activation of the protein kinase C pathways occur in response to treatment with these agents.
Toll-like receptors (TLRs) mediate innate immunity and recognize molecular patterns unique to pathogens, for example, bacteria, fungi, or viruses (4). Several TLRs reside within endosomes and can recognize either RNA (TLRs 3, 7, and 8) or DNA (TLR9) (4). The TLR pathway can also be activated by endogenous host stimuli, such as components of dying cells. Thus, cellular RNA released from apoptotic or necrotic cells can activate TLR7 and TLR8 as well (5). PEL are considered post-germinal center B cells (2). Normal germinal center B cells express TLR1, as well as TLR6 through TLR10 (6). Therefore, endosomal TLRs constitute the majority of TLRs expressed in B cells, and the TLRs expressed in PEL were hitherto unknown.
TLR activation stimulates the transcription of IFN regulatory factors (IRF) and NF-κB transcription factors. We have previously reported that KSHV infection of monocytes leads to the up-regulation of TLR3 and its downstream targets, IFN-β1 and CXCL10 (7). Moreover, TLR4 has been shown to mediate innate immune responses against KSHV in endothelial cells (8).
A central question regarding the KSHV viral lifecycle relates to the physiological trigger that governs the switch from viral latency to lytic replication. We hypothesized that during its lifetime, a KSHV latently infected B cell would encounter additional secondary pathogens or other stimuli, for example, nucleic acids released from necrotic cells, which would trigger TLR signaling in latently infected cells. We speculated that these would be physiological triggers for viral reactivation of KSHV from latency.
To test this hypothesis, we screened several latently infected PEL cell lines with agonists specific for different human TLRs. Stimulation of KSHV infected latent B lymphocytes with the TLR8 ligand, single-stranded polyuridine (ssPoly-U), a synthetic ssRNA homolog, strongly reactivated KSHV from latency. Stimulation with synthetic TLR7 ligands also reactivated the virus, albeit to a lesser extent than ssPoly-U. To test the hypothesis that TLR7/8 activation resulted in authentic viral reactivation, we analyzed KSHV transcription in four different PEL cell lines that were stimulated with TLR7/8 ligands. In all cases, TLR7/8 activation triggered the signature lytic reactivation pattern of transcription. In contrast, stimulation with LPS, a ligand for TLR4, did not reactivate KSHV from latently infected B lymphocytes and did not induce significant changes in viral transcription. Additionally, we determined whether a physiological trigger of TLR7/8 could also reactivate KSHV. We infected PEL with a secondary pathogen, VSV, a known inducer of TLR7/8 signaling, and found that VSV infection also induced KSHV reactivation from latency. Thus, our findings demonstrate a link between innate immune activation and viral reactivation from latency and suggest that single-stranded RNA can serve as a biological trigger for KSHV reactivation from B lymphocytes.
Results
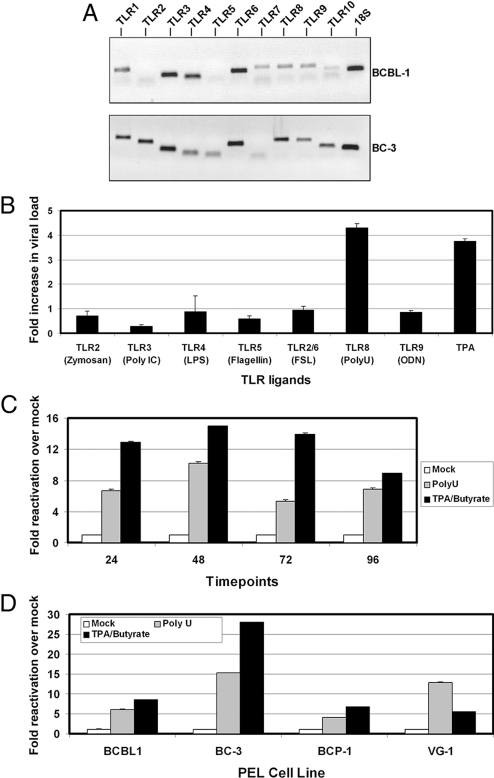
We first screened latently infected PEL for TLR gene expression. BCBL-1 and BC-3 cells were harvested and expression of TLRs 1 through 10 were analyzed by qPCR with specific TLR primers. TLR profiling revealed that these cells express multiple TLRs, including TLR7 and TLR8 (Fig. 1A).
Fig. 1.
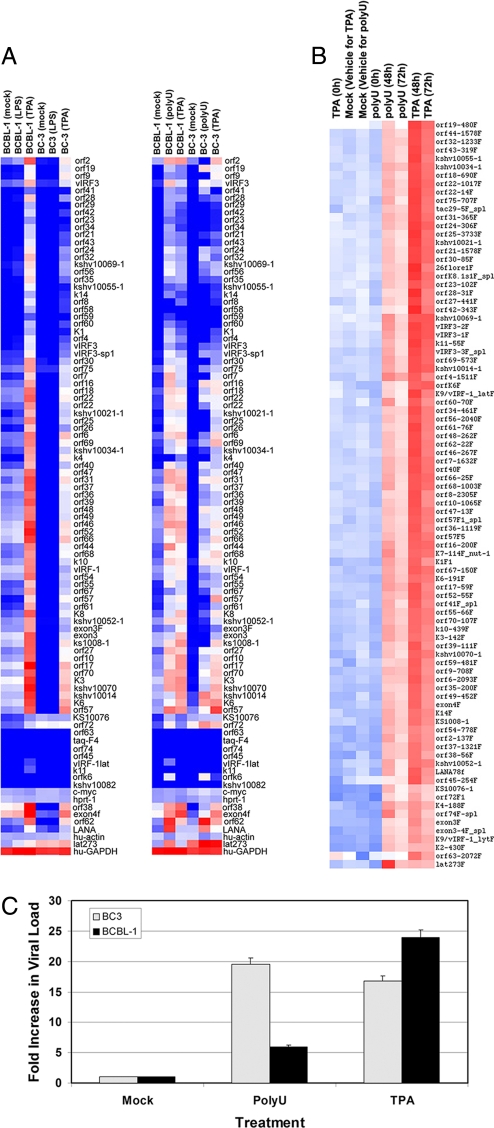
TLR stimulation and KSHV reactivation. (A) TLR expression in PEL cells. Gene expression of the ten TLRs was determined in BCBL-1 and BC-3 by quantitative RT-PCR using primers specific to each human TLR gene. 18S gene expression was used as a loading control. (B) KSHV reactivation in response to TLR agonists. BCBL-1 cells were treated with 10 μg/mL of the indicated TLR ligands and 20 ng/mL positive control (TPA) for 48 h. DNA was isolated and KSHV replication was determined by qPCR for KSHV ORF49 or ORF57 gene expression compared to vehicle control. (C) Time course of KSHV reactivation after TLR8 stimulation. BCBL-1 cells were treated with vehicle control, ssPoly-U, or a combination of TPA and Na-Butyrate. RNA was isolated at the indicated time points, and KSHV ORF49 and ORF57 gene expression was measured by qRT-PCR. (D) Single-stranded RNA reactivates KSHV from 4 different PEL. BCBL-1, BC-3, BCP-1, and VG-1 PEL cell lines were treated with vehicle control, 50 μg/mL ssPoly-U, or a combination of TPA and NaB for 48 h. Fold reactivation was calculated using real-time PCR values of the TLR agonist treated samples versus the vehicle control treated samples. All values were normalized to GAPDH.
To determine whether TLR activation could induce reactivation of KSHV, we stimulated BCBL-1 and BC-3 cells with individual cognate TLR ligands for 24 h, as well as a positive control, TPA (Fig. 1B). Intracellular DNA viral load was determined by real-time PCR and normalized for input using GAPDH copy number. Fold activation for each sample was determined relative to the vehicle control using the ΔΔCT method. TLR2 stimulation with zymosan or TLR4 stimulation with LPS derived from Escherichia coli strain 0111:B4 showed no significant difference in lytic gene expression. Treatment of BCBL-1 cells with FSL-1 (TLR2/6 ligand) also had no significant effect on replication compared with vehicle control and neither did TLR9 stimulation with CpG DNA. We observed a decrease in viral DNA copy number in response to 1 μg/mL recombinant flagellin (TLR5 ligand) or 10 μg/mL polyI:C (TLR3 ligand). This was in contrast to stimulation with single-stranded RNA, a molecular pattern associated with viral infection that is recognized by TLR7 and TLR8 (9). ssPoly-U is a synthetic homolog of single-stranded RNA that specifically activates TLR8 (10). Stimulation with 10 μg/mL ssPoly-U resulted in a 4-fold increase in viral genome copy number compared to control (Fig. 1B). Moreover, ssPoly-U reactivated KSHV to a greater extent than the positive control, TPA. In summary, ssPoly-U was the only TLR agonist that up-regulated KSHV lytic gene expression, suggesting TLR8 activation reactivates KSHV from latency.
Since KSHV does not replicate to high titers in PEL, quantification of DNA replication is not very sensitive. We therefore tested TLR8-mediated reactivation of KSHV transcription in BCBL-1 cells. BCBL-1 cells were treated with ssPoly-U for various timepoints. ORF57 and ORF49 mRNA levels were subsequently analyzed by qPCR as a marker of viral reactivation. ssPoly-U treatment resulted in >10-fold reactivation compared to the vehicle control. In response to ssPoly-U, the levels of KSHV replication peaked at 48 h and declined after 72 h (Fig. 1C). For this experiment, we used a combination of both TPA and sodium butyrate (NaB) as a more potent positive control for KSHV reactivation, due to the variability in responses of individual PEL lines to TPA alone. This combination gives considerably higher reactivation levels than either agent alone. To establish that TLR8-mediated reactivation was common to PEL in general, we screened additional PEL cells using ssPoly-U. BCBL-1, BC-3, BCP-1, or VG-1 cells were treated with ssPoly-U for 48 h. This resulted in lytic reaction of KSHV in all samples (Fig. 1D). This demonstrates that the activation of specific TLR signaling pathways can lead to KSHV reactivation.
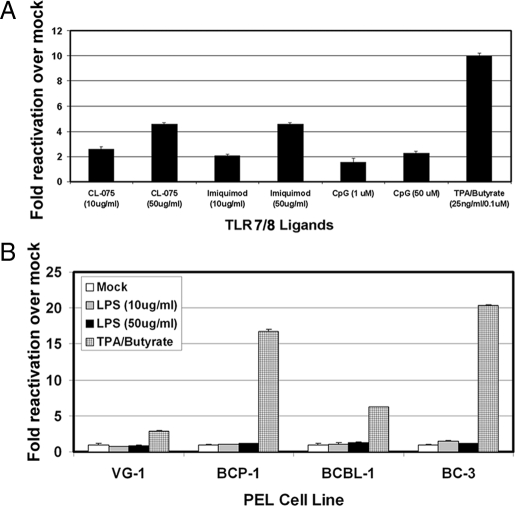
We next queried the specificity of TLR7 and TLR8 for mediating KSHV lytic replication since both receptors recognize single-stranded RNA. Both TLR7 and TLR8 reside within the endosome, have similar structures, and are often functionally redundant (10, 11). We used several validated TLR7 and TLR8 ligands to test for KSHV reactivation in BCBL-1 cells. CL-075 (3M-002) predominantly activates TLR8, and TLR7 to a lesser degree (10). Imiquimod has been shown to be specific for TLR7 (12). We stimulated BCBL-1 cells with increasing concentrations of CL-075, Imiquimod, and CpG DNA (TLR9 ligand) and measured lytic gene transcription. Increasing concentrations of CL-075 reactivated KSHV approximately 2.5- and 4.5-fold over mock (Fig. 2A). Interestingly, the TLR7 ligand, Imiquimod, reactivated KSHV to similar levels as CL-075. In contrast, increasing concentrations of TLR9 agonist (CpG DNA) had no effect. In summary, stimulation of TLR7 and TLR8 led to reactivation of latent KSHV. We used the TLR4 agonist, LPS, as an additional negative control. We treated BCBL-1, BC-3, BCP-1 and VG-1 PEL cells with 10 and 50 μg/mL LPS derived from E. coli strain 0111:B4. Increasing concentrations of LPS did not result in KSHV reactivation from latency (Fig. 2B), whereas the positive control (TPA and NaB) did reactivate the virus. Another TLR4 agonist, LPS from E. coli strain K12, also failed to induce KSHV lytic replication.
Fig. 2.
TLR7/8, and not TLR4, reactivates latent KSHV. (A) TLR7 and TLR8 ligands reactivate KSHV. BCBL-1 cells were treated with increasing concentrations of CL-075, Imiquimod, CpG DNA, or a combination of TPA and NaB for 48 h. (B) LPS does not activate KSHV replication. BCBL-1, VG-1, BCP-1, and BC-3 cells were treated with either LPS for 48 h or a combination of TPA and NaB. ORF57 transcription was measured by qRT-PCR using GAPDH as an endogenous control.
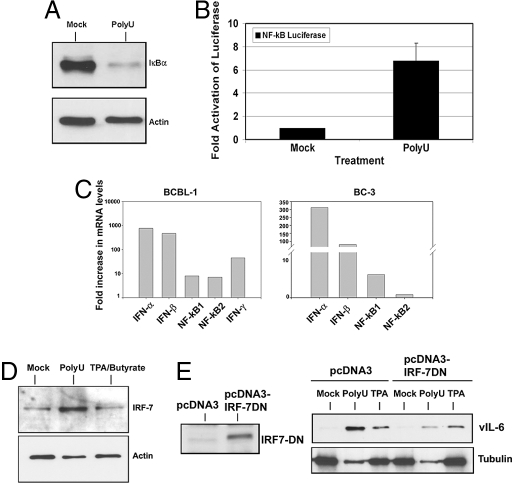
TLRs 7 and 8 are known to signal through the NF-κB and IRF7 pathways, leading to the up-regulation of pro-inflammatory cytokines and type I IFN, respectively. First, we looked at NF-κB activation following ssPoly-U stimulation by probing for IκB-α, an inhibitor of NF-κB nuclear translocation and transactivation. Upon activation of TLR7/8, IκB-α is ubiquitinated and targeted for proteasome-mediated degradation. Treatment of BCBL-1 cells with ssPoly-U for 5 h induced IκB-α degradation (Fig. 3A), indicating that the canonical NF-κB pathway was activated by TLR7/8 stimulation in PEL. Next, we tested whether the NF-κB promoter was activated in response to ssPoly-U stimulation. BCBL-1 cells were nucleofected with 200 ng NF-κB-luciferase plasmid. Cells were pooled after 48 h, and either treated with ssPoly-U or vehicle control. Sixteen hours post-treatment, cells were harvested and analyzed for luciferase activity. ssPoly-U treatment induced an approximate 7-fold activation of the NF-κB reporter compared to vehicle treated cells (Fig. 3B). Therefore, ssPoly-U potently activates NF-κB in BCBL-1 cells. ssPoly-U stimulation also induced the up-regulation of IFN-α, IFN-β and IFN-γ, as well as NF-κB transcription in PEL (Fig. 3C). Induction of NF-κB and IFN-α, β, and γ transcription serve as validated markers for the activation of TLR signaling in many different cell types (4). Moreover, activation of IFN-α/β induces IRFs (including IRF-7) in a positive-feedback loop necessary for amplification of the initial innate immune response. Therefore, we analyzed IRF-7 protein expression 24 h after ssPoly-U stimulation of BCBL-1 cells and observed IRF-7 up-regulation as expected (Fig. 3D). To determine whether IRF-7 was critical for TLR7/8 induced lytic replication, we transfected an IRF-7 dominant-negative (IRF-7DN) construct (13) into BCBL-1 cells (Fig. 3E, Left). Forty-eight hours later, the cells were split into 3 samples and treated with equivalent amounts of either ssPoly-U, vehicle, or TPA. Twenty-four hours post-treatment, cells were harvested and lysates were analyzed for vIL-6 as a marker for lytic replication by western blot. We found that IRF-7DN expression resulted in a marked reduction of KSHV lytic reactivation compared to vector control (Fig. 3E, Right). Taken together, our data demonstrate that the TLR7/8 pathway is activated in response to ssPoly-U stimulation of PEL and imply that activation of this pathway can lead to KSHV reactivation from latency in B lymphocytes. IRF-7 is a critical component of this signaling pathway, whose activation by TLR7/8 is required for viral reactivation.
Fig. 3.
Single-stranded RNA activates an innate immune response. (A) Single-stranded RNA activates the canonical NF-κB pathway. IkB-α protein expression was determined by western blot 5 h post-treatment with ssPoly-U. (B) Single-stranded RNA activates the NF-κB promoter in PEL. BCBL-1 cells were nucleofected with 200 ng NF-κB promoter-luciferase plasmid. After 48 h, cells were pooled and treated with ssPoly-U for an additional 16 h. Cells were then harvested and luciferase assays were performed. (C) TLR7/8 stimulation activates IFN and NF-κB transcription. BC-3 and BCBL-1 cells were treated with 50 μg/mL ssPoly-U for 48 h. IFN-α,β,γ, NF-kB1, and NF-kB2 transcription levels were determined by qRT-PCR. All values were normalized to GAPDH as the endogenous control. (D) IRF-7 expression is up-regulated by single-stranded RNA. Cells were treated with ssPoly-U for 24 h, lysed, and IRF-7 protein expression was analyzed by western blot. (E) Inhibition of IRF-7 function blocks ssPoly-U-induced KSHV reactivation. Flag-tagged IRF-7DN was analyzed by western blot 48 h after nucleofection of BCBL-1 cells (Left). pcDNA3-IRF-7DN or pcDNA3 control transfected cells were treated with 50 μg/mL ssPoly-U, equivalent amount of vehicle (mock sample), or 20 ng/mL TPA. Cells were analyzed for viral reactivation 24 h post-treatment by performing a western blot for vIL-6 protein expression (Right).
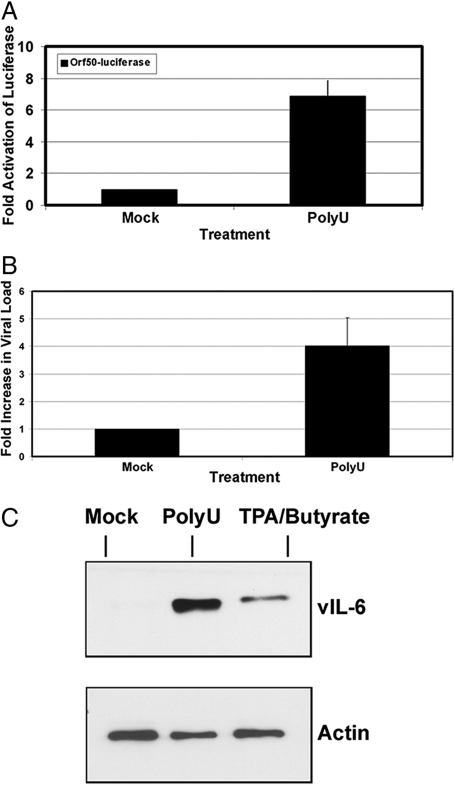
RTA/ORF50 is the key lytic switch protein of KSHV. It is necessary and sufficient to activate KSHV replication (14). We therefore queried whether TLR7/8 signaling in PEL activated the KSHV RTA/ORF50 promoter. BCBL-1 cells were nucleofected with 5 μg ORF50 promoter-luciferase reporter plasmid, and 48 h post-transfection, cells were pooled and treated with ssPoly-U RNA or vehicle. After 16 h, luciferase activity was quantified as previously described (7). ssPoly-U treatment resulted in a 7-fold increase in luciferase activity compared with vehicle control (Fig. 4A). Hence, ssPoly-U-induced TLR7/8 signaling can activate the RTA promoter.
Fig. 4.
Single-stranded polyU RNA activates KSHV replication. (A) ssPoly-U activates the KSHV RTA/ORF50 promoter. BCBL-1 cells were nucleofected with 5 μg RTAp-Luciferase reporter plasmid and 48 h later cells were stimulated with ssPoly-U or vehicle control for 16 h. Luciferase activity of both samples was measured. (B) KSHV viral load analysis. KSHV viral DNA was isolated 48 h after treatment of BCBL-1 cells with ssPoly-U or vehicle control. QPCR was performed with vIL-6 primers to determine viral DNA loads. (C) Viral Reactivation. Western blot for KSHV vIL-6 and actin (loading control) after treatment of BCBL-1 cells with the indicated agonists.
Next, we measured KSHV viral DNA in the ssPoly-U treated BCBL-1 cells (Fig. 4B), and were able to detect a 4-fold increase in viral DNA levels. To corroborate these results, KSHV early lytic protein (ORF59) expression was analyzed by immunofluoresence assays (IFA). KSHV ORF59 was detected in ssPoly-U treated BCBL-1 cells as well as the positive control, but not in mock-treated cells (Fig. S1). Similar results were obtained when we analyzed the expression of KSHV vIL-6 in ssPoly-U-treated cells by western blot analysis (Fig. 4C). In summary, our data demonstrate that ssPoly-U stimulates the KSHV RTA promoter and induces KSHV early lytic gene transcription, replication, and the expression of early lytic proteins, vIL-6 and ORF59.
Although the above data suggested that KSHV lytic gene transcription and protein expression was activated by ssPoly-U treatment of PEL, it remained possible that lytic replication was incomplete. Therefore, we profiled genome-wide KSHV transcription using our KSHV qPCR array (15, 16). We treated BCBL-1 and BC-3 cells with either ssPoly-U or TPA (positive control) for 48 h and analyzed KSHV transcription. Two independent cell lines, BCBL-1 and BC-3, showed up-regulation of KSHV viral gene expression 48 h after ssPoly-U treatment (Fig. 5A). Their expression profile resembled that of the positive control, TPA. As a negative control, we treated BCBL-1 and BC-3 cells with 50 μg/mL LPS for 48 h, but did not observe any KSHV reactivation (Fig. 5A). LPS treated BCBL-1 cells displayed the same transcription profile as the vehicle control-treated cells.
Fig. 5.
Whole genome profiling of KSHV after ssPoly-U treatment. (A) ssPoly-U induces KSHV replication. KSHV viral arrays were performed after treatment of BCBL-1 and BC-3 with 50 μg/mL LPS, 50 μg/mL ssPoly-U, or a combination of TPA and NaB. Heat maps for each array are shown. (B) Time course of KSHV replication. Heat map of KSHV viral arrays after 0 h, 48 h, and 72 h treatment with 50 μg/mL ssPoly-U. Relative gene expression levels are represented by blue, white, and red, which indicate no detectable, intermediate, or high KSHV gene expression, respectively. (C) ssPoly-U reactivation of PEL generates infectious KSHV virions. BCBL-1 and BC-3 cells were treated with 50 μg/mL ssPoly-U, an equivalent amount of vehicle, or 20 ng/mL TPA. Forty-eight hours post-treatment, supernatants were harvested and used to infect Vero cells. Ninety-six hours postinfection of Vero cells, intracellular DNA was isolated and KSHV viral loads were quantified by qPCR. Values were normalized to GAPDH. Fold increase in KSHV viral loads from ssPoly-U treated PEL, compared to mock-treated PEL is shown.
We also performed a time course experiment. BCBL-1 and BC-3 cells treated for 0 h, 48 h, and 72 h with ssPoly-U or TPA displayed lytic replication at the 48 h and 72 h time points, with peak activation at 48 h (Fig. 5B). These results indicate that complete KSHV lytic gene transcription occurs in response to TLR7/8 stimulation of PEL.
To further verify that infectious virus was produced by ssPoly-U mediated reactivation of PEL, we treated BCBL-1 and BC-3 cells for 48 h with 50 μg/mL ssPoly-U. Supernatants were subsequently harvested and used to infect uninfected Vero cells. Ninety-six hours postinfection, viral DNA was isolated and KSHV viral loads were analyzed using qPCR with ORF57 primers. As shown in Fig. 5C, treatment of BCBL-1 and BC-3 cells resulted in approximate 5- and 20-fold increases in production of infectious KSHV virions, respectively. Of note, the endogenous levels of TLR8 in these 2 cell lines may correlate with differences in lytic replication between PEL cells. BCBL-1 cells have reduced levels of TLR8 expression compared to BC-3 (Fig. 1A), which may explain observed differences in infectious virus produced upon ssPoly-U stimulation of different PEL.
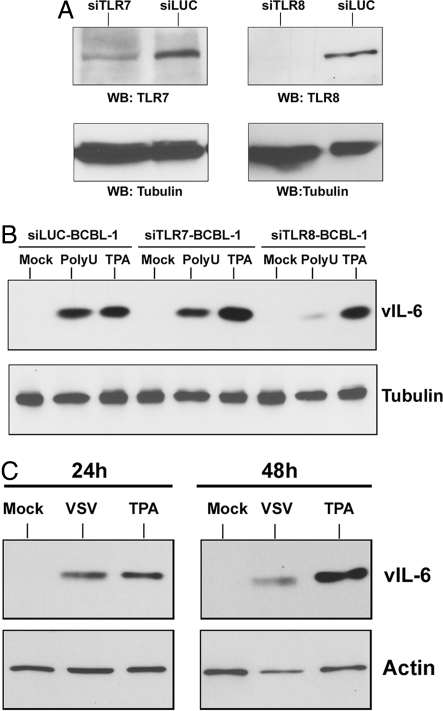
To demonstrate that TLR7/8 stimulation was necessary for ssPoly-U induced KSHV lytic replication, we used TLR7- and TLR8-specific shRNA vectors to create stable BCBL-1 cells containing knock-down of either TLR7 or TLR8. shRNA directed against luciferase was used as a control. We achieved moderate knock-down of TLR7 and complete loss of TLR8 in our knock-down stable cells (Fig. 6A).
Fig. 6.
TLR7/8 stimulation mediates KSHV reactivation in response to single-stranded RNA and VSV infection. (A) Knock-down of TLRs 7 and 8 in BCBL-1 cells. Western blot for TLR7 and TLR8 protein expression in BCBL-1 cells stably transfected with the psiRNA-luciferase control, psiRNA-TLR7, or psiRNA-TLR8 plasmids. Tubulin is shown as a loading control. (B) TLR7/8 mediate KSHV reactivation. ssPoly-U treatment of psiRNA-luciferase, psiRNA-TLR7, and psiRNA-TLR8 stable BCBL-1 cell lines was performed. Expression of the lytic protein, vIL6, was determined by western blot 24 h after ssPoly-U treatment of each cell line. Tubulin is shown as a loading control. (C) VSV infection of PEL mediates KSHV reactivation. BCBL-1 cells were infected with VSV at an MOI of 1. KSHV vIL-6 protein expression was determined by western blot analysis.
BCBL-1 cells knocked-down for TLR7 or for TLR8 were used in ssPoly-U reactivation assays. Viral reactivation was measured by western blot analysis for the lytic protein, vIL-6. The control cell line, psiLuciferase-BCBL-1, showed considerable reactivation after treatment with ssPoly-U (Fig. 6B). Treatment of psiTLR7-BCBL-1 with ssPoly-U resulted in slightly reduced vIL-6 expression, compared to the psiLuciferase-BCBL-1 treated cells, demonstrating a potential role for TLR7 in KSHV reactivation. However, ssPoly-U treatment of the psiTLR8-BCBL-1 cells displayed substantially reduced vIL-6 expression compared to the psiLuciferase-BCBL-1 treated cells. Importantly, all of the knock-down cell lines remained similarly responsive to lytic reactivation by TPA, and showed considerable reactivation (Fig. 6B). In summary, while TLR7 may play a role in KSHV reactivation, TLR8 activation is more important for viral reactivation since the TLR8 knock-down cells showed a significant reduction in ssPoly-U-stimulated viral reactivation.
To translate our results to a physiologically relevant setting, we used the single-stranded RNA virus, vesicular stomatitis virus (VSV), for infection of PEL. VSV is endocytosed in a clathrin-dependent manner (17, 18) and is known to activate TLR7/8 in mouse and human cells (19). BCBL-1 cells were infected with VSV at an MOI of 1, or treated with TPA as a positive control. KSHV vIL-6 protein expression was determined 24 h and 48 h postinfection. Infection with VSV resulted in KSHV reactivation at both time points (Fig. 6C). Similar results were seen with VSV infection at 0.1 MOI. VSV-induced reactivation was also accompanied by increased NF-κB, IFN-α, β, and γ transcription (Fig. S2) and cell death was visually observed. Thus, single-stranded RNA, specifically viral RNA, is capable of inducing KSHV reactivation.
Discussion
In summary, we report evidence for KSHV reactivation through TLR signaling. TLR7/8 mediated reactivation from latency offers a paradigm for how KSHV may initiate lytic replication in vivo. Periodic reactivation of herpesviruses is important for the establishment of new latent reservoirs of virus, viral transmission, and persistence in the host. Since human herpesviruses establish life-long latency in their hosts, it is very plausible that a latently infected cell is exposed to infection by a secondary pathogen, be it a virus, bacterium, or fungus. We report that secondary viral infection that is dependent on endosomal entry and stimulation of TLR7/8 signaling can lead to reactivation of latent KSHV from B lymphocytes. It is well-established that VSV activates TLR7/8. Although VSV is a rhabdovirus and a natural pathogen of livestock, human infections have been documented (20, 21), and VSV can infect human B lymphocytes (22). Other RNA viruses that activate TLR7/8, such as dengue virus, also infect B lymphocytes (23). Of note, it was previously reported that infection of PEL with VSV-pseudotyped HIV particles reactivated KSHV (24), although the exact mechanism was not deduced. Since HIV RNA is known to activate TLR7 and TLR8 (9, 25), the observed outcome in this earlier study may be attributable to HIV RNA-induced activation of TLR7/8 in PEL.
In immunosuppressed KSHV-positive individuals, KSHV reactivation rates may be increased by secondary infections that trigger TLR7/8 activation. Since lytic replication of KSHV is linked to an increased risk of KSHV-associated cancers (26), one can speculate that KSHV-positive individuals exposed to TLR7/8-activating pathogens are at a higher risk for developing KSHV-associated malignancies. Endogenous RNAs from necrotic cells also activates TLR7/8 (27). Hence, it is equally plausible that KSHV reactivation is triggered by endosomal trafficking of endogenous RNA from necrotic cells. A recent publication indicates that MHV-68 can also be reactivated in vivo by TLR signaling (28), although it was shown that multiple TLR ligands could reactivate MHV-68 from B lymphocytes. This may reflect a biological difference between the two viruses, or the difference in TLR signaling between normal and malignant B lymphocytes.
Lastly, our data have implications for oncolytic therapy of viral cancers. There is a growing interest in using VSV oncolysis to treat tumors (29, 30). Our data suggests that VSV infection of KSHV-infected lymphomas results in oncolysis, but also reactivates KSHV. This may be detrimental to KSHV-positive cancer patients since VSV oncolysis would inadvertently aid viral reactivation and dissemination. Thus, for some viral cancers it may be advisable to use VSV oncolytic treatment in combination with viral replication inhibitors, such as ganciclovir, to prevent reactivation and spread of the virus to naïve cells in the host.
Defining the physiological stimuli of reactivation from latency is a fundamental question in herpesvirus biology. We report evidence of innate immune receptors mediating KSHV reactivation. Moreover, TLR7/8 signaling mediated by secondary infection of KSHV-infected B lymphocytes not only induces KSHV reactivation and lytic replication, but also allows the virus to propagate itself and escape a cell that is fated to die. This ensures KSHV survival and persistence for the lifetime of the infected host.
Materials and Methods
Cell Culture.
PEL cells were maintained in RPMI medium 1640 (Cellgro) containing 10% FBS, 1% penicillin-streptomycin (PS), and 0.05 M beta-mercaptoethanol. Vero, HeLa, and BHK cells were maintained in DMEM media containing 10% FBS and 1% PS. Cells were maintained at 37 °C in 5% carbon dioxide.
TLR Expression.
Toll-like receptor screening of PEL cell lines was carried out by isolating RNA, generating cDNA, and analyzing gene expression by quantitative real-time PCR using TLR and 18S specific primers (SA Biosciences) according to the manufacturer's instructions. qPCR products were resolved on a 1% agarose gel.
TLR Stimulations.
PEL cells (1 × 105/mL) were resuspended in 500 μL of 2% FBS/RPMI. TLR agonists (Invivogen) FSL-1 (TLR2/6), zymogen (TLR2), poly I:C (TLR3), lipopolysaccharide (TLR4), recombinant flagellin (TLR5), imiquimod (TLR7), gardiquimod (TLRs 7/8), CL-097 (TLR7), single-stranded poly U (TLR 8), and CpG DNA (TLR9) were reconstituted in water according to the manufacturer's recommendations and TLR stimulations performed at the indicated concentrations. For positive controls, 20 ng/mL TPA (Sigma) and 0.1 mM NaB (Sigma) in DMSO was used. Cells were harvested and viral load assays were performed as described below. Additionally, western blots, promoter reporter assays, and immunofluorescence assays were also performed and are detailed in SI Materials and Methods.
Viral Load Assay.
For intracellular viral load assays, DNA was isolated using DNeasy kit according to manufacturer's instructions (Qiagen). For KSHV transcription, cells were harvested and RNA isolated using RNeasy Plus RNA isolation kit (Qiagen) followed by treatment with 1 unit RQ1 DNase (Promega). cDNA was reverse transcribed from a minimum of 100 ng RNA using MMLV reverse transcriptase (Invitrogen) and random hexamer primers (Invitrogen). Fold gene expression compared to mock treated samples was determined by quantitative PCR (qPCR) using Sybr GREEN PCR master mix (Applied Biosystems). Primers used are described in SI Materials and Methods. The qPCR reactions were run on an ABI 7000 or ABI 7300 machine (Applied Biosystems). Relative fold calculations were determined by the ΔΔCT method (15).
IRF-7 Dominant-Negative (IRF-7DN) Assays.
BCBL-1 cells (1 × 106) were nucleofected using 2 μg Flag-tagged pcDNA3-IRF-7 dominant-negative plasmid (a kind gift from Drs. Shunbin Ning and Joseph Pagano) or pcDNA3 vector control. Forty-eight h postnucleofection, cells were split into 3 samples, and each sample was stimulated with either ssPoly-U, vehicle, or TPA for 24 h. Expression of IRF-7DN was verified by western blot with an anti-Flag antibody.
KSHV Viral Arrays.
BCBL-1 cells were treated with TLR agonists for 48 h unless otherwise noted. RNA was isolated and cDNA generated as described previously (15). Real-time qPCR profiling of KSHV genes was performed followed by hierarchical clustering using ArrayMinerTM software (Optimal Design) as previously described (15).
KSHV Infection Assay.
BCBL-1 or BC-3 cells were treated with ssPoly-U, vehicle, or TPA as described for 48 h. Supernatants were harvested and added to confluent monolayers of uninfected Vero cells in a 12-well dish. Polybrene (4 μg/mL) was added to each well and the plate was spinoculated at 2,500 rpm for 1.5 h at 30 °C as previously described (7). Ninety-six hours postinfection, intracellular KSHV viral loads were determined by real-time PCR.
Stable Knock-Down of TLR7 and TLR8 in BCBL-1 Cells.
BCBL-1 cells (1 × 106) were transfected by either nucleofection (Amaxa) or Hyperfect reagent (Qiagen) with plasmids psiRNA-Luciferase, psiRNA-TLR7, or psiRNA-TLR8 (Invivogen). Cells were selected in 100 μg/mL Zeocin 48 h postnucleofection. BCBL-1 cells stably expressing the individual shRNAs were verified by GFP expression and TLR7/TLR8 protein expression as described above.
Vesicular Stomatitis Virus Infections.
Vesicular stomatitis virus (Orsay strain; a kind gift from Dr. Doug Lyles) was grown on HeLa cells. BCBL-1 (1 × 105) were infected with 0.1 and 1.0 MOI for 24 and 48 h in 2% RPMI, in a 48-well dish at 37 °C. Cell lysates were subjected to western blot analysis for vIL-6 expression as described above.
Supplementary Material
Acknowledgments.
We thank Dr. Doug Lyles (Wake Forest University, Winston-Salem, NC) for providing us with the VSV Orsay strain and Drs. Shunbin Ning and Joseph Pagano (University of North Carolina, Chapel Hill, NC) for the pcDNA3-IRF-7DN plasmid. We thank Stuart Krall for assistance with tissue culture and members of the Damania and Dittmer laboratories for helpful discussions. This work was supported by National Institutes of Health Grants DE018281 and CA096500 and a Burroughs Wellcome Fund grant (to B.D.); National Institutes of Health Grants DE018304 and CA109232 (to D.P.D.); and National Institutes of Health Training Grants T32-AI007419 (to S.G.), F32-AI78735 (to J.A.W.), and T32-CA009156 (P.D.). B.D. is a Leukemia and Lymphoma Society Scholar and Burroughs Wellcome Fund Investigator in Infectious Disease.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905316106/DCSupplemental.
References
- 1.Chang Y, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 3.Whitby D, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet. 1995;346:799–802. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 5.Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: Inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–963. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 7.West J, Damania B. Upregulation of the TLR3 pathway by Kaposi's sarcoma-associated herpesvirus during primary infection. J Virol. 2008;82:5440–5449. doi: 10.1128/JVI.02590-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagos D, et al. Toll-like receptor 4 mediates innate immunity to Kaposi sarcoma herpesvirus. Cell Host Microbe. 2008;4:470–483. doi: 10.1016/j.chom.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heil F, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 10.Gorden KK, et al. Oligodeoxynucleotides differentially modulate activation of TLR7 and TLR8 by imidazoquinolines. J Immunol. 2006;177:8164–8170. doi: 10.4049/jimmunol.177.11.8164. [DOI] [PubMed] [Google Scholar]
- 11.Du X, Poltorak A, Wei Y, Beutler B. Three novel mammalian toll-like receptors: gene structure, expression, and evolution. Eur Cytokine Netw. 2000;11:362–371. [PubMed] [Google Scholar]
- 12.Hemmi H, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 13.Ning S, Huye LE, Pagano JS. Regulation of the transcriptional activity of the IRF7 promoter by a pathway independent of interferon signaling. J Biol Chem. 2005;280:12262–12270. doi: 10.1074/jbc.M404260200. [DOI] [PubMed] [Google Scholar]
- 14.Sun R, et al. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dittmer DP. Transcription profile of Kaposi's sarcoma-associated herpesvirus in primary Kaposi's sarcoma lesions as determined by real-time PCR arrays. Cancer Res. 2003;63:2010–2015. [PubMed] [Google Scholar]
- 16.Fakhari FD, Dittmer DP. Charting latency transcripts in Kaposi's sarcoma-associated herpesvirus by whole-genome real-time quantitative PCR. J Virol. 2002;76:6213–6223. doi: 10.1128/JVI.76.12.6213-6223.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johannsdottir HK, Mancini R, Kartenbeck J, Amato L, Helenius A. Host cell factors and functions involved in vesicular stomatitis virus entry. J Virol. 2009;83:440–453. doi: 10.1128/JVI.01864-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Blanc I, et al. Endosome-to-cytosol transport of viral nucleocapsids. Nat Cell Biol. 2005;7:653–664. doi: 10.1038/ncb1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund JM, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson KM, Vogel JE, Peralta PH. Clinical and serological response to laboratory-acquired human infection by Indiana type vesicular stomatitis virus (VSV) Am J Trop Med Hyg. 1966;15:244–246. doi: 10.4269/ajtmh.1966.15.244. [DOI] [PubMed] [Google Scholar]
- 21.Tesh RB, Peralta PH, Johnson KM. Ecologic studies of vesicular stomatitis virus. I. Prevalence of infection among animals and humans living in an area of endemic VSV activity. Am J Epidemiol. 1969;90:255–261. doi: 10.1093/oxfordjournals.aje.a121068. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt MR, Gravel KA, Woodland RT. Progression of a vesicular stomatitis virus infection in primary lymphocytes is restricted at multiple levels during B cell activation. J Immunol. 1995;155:2533–2544. [PubMed] [Google Scholar]
- 23.Lin YW, et al. Virus replication and cytokine production in dengue virus-infected human B lymphocytes. J Virol. 2002;76:12242–12249. doi: 10.1128/JVI.76.23.12242-12249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varthakavi V, Browning PJ, Spearman P. Human immunodeficiency virus replication in a primary effusion lymphoma cell line stimulates lytic-phase replication of Kaposi's sarcoma-associated herpesvirus. J Virol. 1999;73:10329–10338. doi: 10.1128/jvi.73.12.10329-10338.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 26.Decker LL, et al. The Kaposi sarcoma-associated herpesvirus (KSHV) is present as an intact latent genome in KS tissue but replicates in the peripheral blood mononuclear cells of KS patients. J Exp Med. 1996;184:283–288. doi: 10.1084/jem.184.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sioud M. Innate sensing of self and non-self RNAs by Toll-like receptors. Trends Mol Med. 2006;12:167–176. doi: 10.1016/j.molmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Gargano LM, Forrest JC, Speck SH. Signaling through Toll-like receptors induces murine gammaherpesvirus 68 reactivation in vivo. J Virol. 2009;83:1474–1482. doi: 10.1128/JVI.01717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barber GN. VSV-tumor selective replication and protein translation. Oncogene. 2005;24:7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- 30.Cesaire R, et al. Oncolytic activity of vesicular stomatitis virus in primary adult T-cell leukemia. Oncogene. 2006;25:349–358. doi: 10.1038/sj.onc.1209055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.