Abstract
E-selectin and its ligands are essential for extravasation of leukocytes in inflammation. Here, we report that basigin (Bsg)/CD147 is a ligand for E-selectin that promotes renal inflammation in ischemia/reperfusion. Compared with wild-type mice, Bsg-deficient (Bsg−/−) mice demonstrated striking suppression of neutrophil infiltration in the kidney after renal ischemia/reperfusion. Although E-selectin expression increased similarly between the two genotypes, Bsg−/− mice exhibited less renal damage, suggesting that Bsg on neutrophils contribute to renal injury in this model. Neutrophils expressed Bsg with N-linked polylactosamine chains and Bsg−/− neutrophils showed reduced binding to E-selectin. Bsg isolated from HL-60 cells bound to E-selectin, and tunicamycin treatment to abolish N-linked glycans from Bsg abrogated this binding. Furthermore, Bsg−/− neutrophils exhibited reduced E-selectin-dependent adherence to human umbilical vein endothelial cells in vitro. Injection of labeled neutrophils into mice showed that Bsg−/− neutrophils were less readily recruited to the kidney after renal ischemia/reperfusion than Bsg+/+ neutrophils, regardless of the recipient's genotype. Taken together, these results indicate that Bsg is a physiologic ligand for E-selectin that plays a critical role in the renal damage induced by ischemia/reperfusion.
The selectins and their ligands are essential for leukocyte tethering/rolling on endothelial cells and the initiation of inflammatory response. The selectins are C-type lectins and consist of three members, i.e., P-, L-, and E-selectin.1,2 P-selectin is expressed upon inflammatory stimulation in platelets and endothelial cells. L-selectin is constitutively expressed on the tip of leukocyte microvilli and implicated in lymphocyte homing to lymph nodes.3 E-selectin is specifically induced in the endothelium upon inflammatory stimulation. Thus, E- and P-selectin closely collaborate with one another and play a major role in leukocyte recruitment to inflammatory sites.4–6 Among the several glycoproteins reported to bind to E-selectin, three have been identified as representative physiologic E-selectin ligands on neutrophils. There are P-selectin glycoprotein ligand-1 (PSGL-1), E-selectin ligand-1, and CD44, and all three play distinct roles during tethering and slow rolling of neutrophils on the endothelium.7 A minimal recognition motif for all selectins is sialylated and fucosylated glycan determinants, such as sialyl Lewis X, that decorate the terminal extensions of carbohydrates of these molecules.8,9 However, because of the poor immunogenicity of highly glycosylated epitopes, it has proven difficult to identify selectin ligands.
Basigin (Bsg)/CD147 (Bsg is the name of the mouse gene) is a membrane glycoprotein that belongs to the Ig superfamily. Bsg was discovered in embryonal carcinoma cells as a receptor for Lotus tetragonolobus agglutinin10 and was determined to have the structure Galβ1→4(Fucα1→3)GlcNAc, which is known as the Lewis X structure. But it has been unclear whether Bsg has sialyl Lewis X structure and whether Bsg serves as a selectin ligand. Bsg is expressed in many cell types, e.g., blood cells, epithelial cells, endothelial cells, and germ cells. We previously generated Bsg-deficient (Bsg−/−) mice and found several abnormalities that included male and female sterility, progressive retinal degeneration, increased cell proliferation upon mixed lymphocyte culture, decreased memory function, and abnormal sensory function.11–13 In addition to these functions deduced through the study of Bsg−/− mice, two additional Bsg functions have recently been highlighted. First, Bsg activates matrix metaloproteases (MMPs), thereby promoting cancer invasion.14 Second, Bsg functions like a chaperone for monocarboxylate transporters (MCTs).15,16 In the present study, we found an additional and unexpected role of Bsg; namely, its glycosylation was crucial for inflammation.
Acute kidney injury (AKI) is a common complication that occurs in approximately 5% of hospitalized patients and in approximately 30% of patients in intensive care units. As the mortality of AKI is still unacceptably high, between 40% and 60%,17 this disease is being intensively studied. Renal ischemia/reperfusion injury is characteristic of acute renal inflammation involving marked infiltration of inflammatory cells, such as neutrophils, and is the most widely used model for human AKI.18 We used this model to investigate the role of Bsg in inflammation in the present study. Bsg−/− mice exhibited less renal damage after ischemia/reperfusion. To our surprise, this phenotype was attributable to Bsg on neutrophils, rather than Bsg on other cells in the inflammation area. We found that highly glycosylated Bsg on neutrophils bound to E-selectin on endothelial cells and led to neutrophil infiltration to the inflammatory lesion. Our results may shed light on the mechanisms underlying the pathogenesis of AKI.
RESULTS
Bsg Deficiency Preserves Renal Function and Decreases Renal Injury after Ischemia/Reperfusion
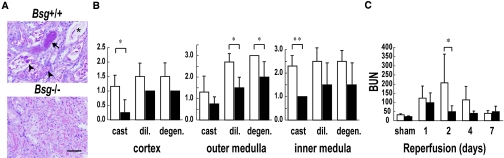
We subjected Bsg+/+ and Bsg−/− mice to renal ischemia/reperfusion injury. As shown in Figure 1, the renal damage was less pronounced in Bsg−/− mice than in Bsg+/+ mice. Thus, 2 d after ischemia/reperfusion, Bsg−/− mice showed less tubulointerstitial injury by all three criteria examined, i.e., tubular cast formation, dilation, and degeneration (Figure 1, A and B). The increase of serum urea nitrogen levels was also significantly suppressed in Bsg−/− mice (Figure 1C). The serum urea nitrogen reached the maximum level on day 1 after ischemia in Bsg−/− mice versus day 2 in Bsg+/+ mice. Thus, postischemic renal injury was quickly terminated in Bsg−/− mice.
Figure 1.
Renal injury is less severe in Bsg−/− mice after renal ischemia/reperfusion. (A) A representative image of tubular lesions 2 d after ischemia/reperfusion injury. Less renal damage was observed in Bsg−/− mice than in Bsg+/+ mice. Arrow, tubular cast; arrowhead, degeneration of the tubule; asterisk, dilation of the tubule. Scale bar, 50 μm. Periodic acid-Schiff staining. (B) Semiquantitative analysis of tubulointerstitial damage 2 d after ischemia/reperfusion injury. The degree of tubular cast formation, tubular dilation, and tubular degeneration were comparatively rated as described in the Concise Methods section. High values indicate more severe damage. White columns, Bsg+/+ mice; black columns, Bsg−/− mice. cast, cast formation. dil., dilation. degen., degeneration. Data are means (columns) and SEM; bars). *P < 0.05; **P < 0.01; n = 6. (C) Blood urea nitrogen (BUN) levels in Bsg+/+ and Bsg−/− mice after ischemia/reperfusion. Renal function was better preserved in Bsg−/− than Bsg+/+ mice. White columns, Bsg+/+ mice; black columns, Bsg−/− mice. BUN is shown as mg/dl. Data are means (columns) and SEM (bars). *P < 0.05; n = 6.
Bsg Deficiency Reduces Neutrophil Infiltration into the Tubulointerstitium
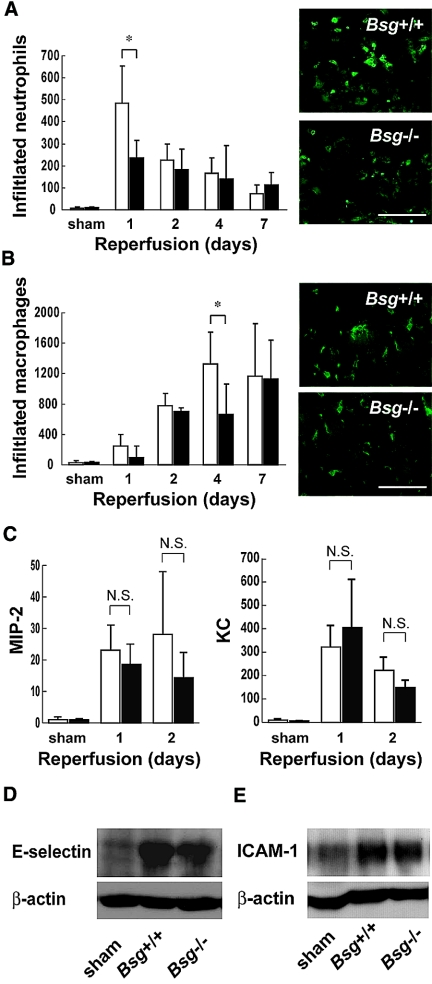
Renal ischemia/reperfusion injury is characterized by a massive influx of neutrophils early after reperfusion, which plays a crucial role in the pathogenesis of postischemic renal failure through the release of cytotoxic proteases and oxygen-derived radicals.19 We next compared the amount of neutrophil influx and renal function. Bsg deficiency greatly reduced the influx of neutrophils to the kidney 1 d after ischemia/reperfusion (Figure 2A). The number of infiltrated neutrophils reached the maximum level 1 d after ischemia/reperfusion (Figure 2A). This was in contrast to the macrophage influx, which peaked 4 d after ischemia/reperfusion (Figure 2B). As the difference in renal function between Bsg+/+ and Bsg−/− mice became apparent 2 d after ischemia/reperfusion (Figure 1C), it is conceivable that neutrophil influx, rather than macrophage influx, was crucial for the role of Bsg in renal damage in our model.
Figure 2.
Reduced neutrophil influx to postischemic kidneys in Bsg−/− mice. (A) Neutrophil influx in postischemic kidneys. The number of infiltrated neutrophils to the kidneys after ischemia/reperfusion was counted in a blind manner. Immunostaining for neutrophils revealed suppressed influx of neutrophils into the kidneys of Bsg−/− compared with Bsg+/+ mice 1 d after ischemia/reperfusion. White columns, Bsg+/+ mice; black columns, Bsg−/− mice. Data are means (columns) and SEM (bars). *P < 0.05; n = 6. Representative immunostainings for neutrophils 1 d after ischemia/reperfusion are presented on the right. Scale bar, 50 μm. (B) Macrophage influx in postischemic kidneys. White columns, Bsg+/+ mice; black columns, Bsg−/− mice. Data are means (columns) and SEM (bars). *P < 0.05; n = 6. Representative immunostainings for macrophages 4 d after ischemia/reperfusion are presented on the right. Scale bar, 50 μm. (C) Representative chemokine levels for neutrophil migration in the postischemic kidneys. MIP-2 and KC levels were determined by ELISA for renal homogenates and corrected for the quantity of protein. No significant differences in local MIP-2 and KC were observed between Bsg+/+ (white columns) and Bsg−/− (black columns) mice. Amounts of MIP-2 and KC are presented as pg/mg protein. Data are means (columns) and SEM (bars). n = 6. (D) Western blot analysis of E-selectin expression in the kidney after ischemia/reperfusion. Lysates of the kidney at 12 h after ischemia/reperfusion or sham operation were subjected to immunoblot analysis of E-selectin expression. After ischemia/reperfusion injury, E-selectin expression was elevated, but the expression was comparable between the Bsg+/+ and Bsg−/− mouse kidneys. (E) Western blot analysis of ICAM-1 expression in the kidney after ischemia/reperfusion. Lysates of the kidney at 24 h after ischemia/reperfusion or sham operation were subjected to immunoblot analysis. After ischemia/reperfusion injury, ICAM-1 expression was also elevated, but the expression was comparable between the Bsg+/+ and Bsg−/− mouse kidneys.
It is widely accepted that chemokines are generated by ischemic tubular epithelial cells in the early phase of renal ischemic injury.20 In addition, the tubular epithelial cells express Bsg.21 Accordingly, we next examined whether the difference in influx of neutrophils was mediated through differences in chemokine levels. Macrophage inflammatory protein-2 (MIP-2) and keratinocyte-derived chemokine (KC) are representative CXC chemokines that are known to be induced after ischemia/reperfusion injury and to attract neutrophils.22 MIP-2 and KC levels were elevated in Bsg+/+ and Bsg−/− mice at days 1 and 2 after ischemia/reperfusion, but there was no difference in the degree of elevation between the two genotypes (Figure 2C).
Neutrophil recruitment in the postischemia/reperfusion kidney requires adhesion molecules. E-selectin and intercellular adhesion molecule-1 (ICAM-1) on peritubular capillary cells play particularly crucial roles in this model.23 In the present study, western blot analysis showed upregulation of E-selectin in the kidney 12 h after ischemia/reperfusion in both Bsg+/+ and Bsg−/− mice, but the expression was not significantly different between the two genotypes (Figure 2D). The expression of ICAM-1 in the kidney at 24 h postischemia was also comparable between the two genotypes (Figure 2E). E-selectin and ICAM-1 expression was localized along the length of the peritubular capillaries (Supplementary Figure S1).
Bsg Expression on the Surface of Neutrophils
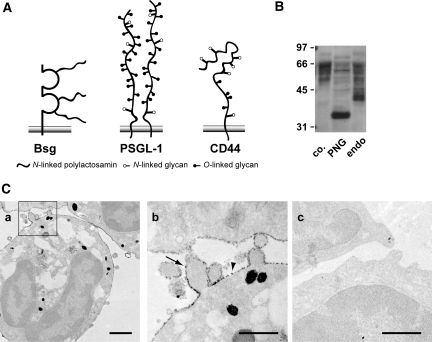
Figure 3A shows the schematic molecular structures of Bsg and two major E-selectin ligands, PSGL-1 and CD44. Bsg is a type 1 integral membrane protein with a predicted molecular mass of 28 kD, but its glycosylated form on various cells is between 35 and 66 kD, with the actual molecular mass being dependent on the cell type.24 Thus, highly glycosylated Bsg harbors long sugar chains. This is in contrast to PSGL-1 (approximately 120 kD) and CD44 (approximately 85 kD), which have relatively short glycans. Determinants for E-selectin-binding exist on O-glycans of PSGL-1 and N-glycans of CD44.25,26
Figure 3.
Bsg expression on the surface of neutrophils. (A) Structural diagrams representing Bsg, PSGL-1, and CD44. (B) Extracts from mouse neutrophils were treated with N-glycosidase F or endo-β-galactosidase and then subjected to western blot analysis. Molecular mass markers (in kD) are indicated on the left. co, untreated control; PNG, N-glycosidase F; endo, endo-β-galactosidase. (C) Immunoelectron micrograph showing the subcellular distribution of Bsg on the surface of neutrophils. Neutrophils were stained with anti-mouse Bsg antibody (a and b) or control antibody (c). The photo shown in (b) represents a magnification of the framed area in (a). Bsg was distributed both on microvilli (arrow) and on the planar surface of the neutrophils (arrowhead). Scale bars indicate 1 μm in (a) and 500 nm in (b and c).
The molecular mass of Bsg from peritoneal-elicited neutrophils was reduced by N-glycosidase F digestion as well as endo-β-glycosidase digestion, indicating that Bsg harbored N-linked polylactosamine chains (Figure 3B). This was consistent with a previous report.27 We then analyzed the subcellular distribution of Bsg in neutrophils. At the electron microscope level, neutrophils display a complex surface architecture with prominent microvillus-like membrane protrusions. These microvilli represent principal sites of initial contact with the vascular endothelium. As shown in Figure 3C, a and b, Bsg was widely distributed on both the planar cell surface and the microvilli of peritoneal-elicited neutrophils. A negative control experiment was performed with an isotype-matched antibody, but no signals were observed (Figure 3Cc). The microvillous presentation argues for the participation of Bsg in the early interaction between neutrophils and endothelial cells during extravasation. Bsg may also be expressed on the surface of unstimulated neutrophils as well, since Bsg is readily detected by FACS analysis on leukocytes obtained from peripheral blood.28
Bsg Binds to E-Selectin
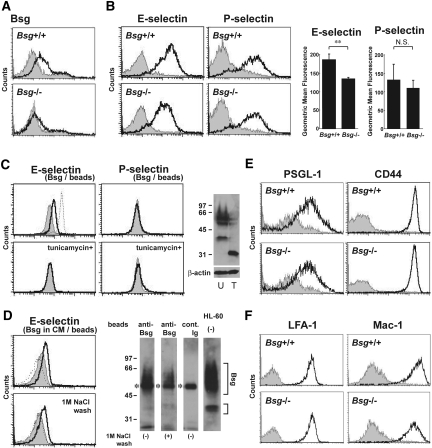
Consistent with Figure 3C, Bsg expression on the cell surface of peritoneal-elicited neutrophils was confirmed by FACS analysis, but the expression was lost in neutrophils from Bsg-/- mice (Figure 4A). Peritoneal-elicited neutrophils from wild-type mice bound to soluble mouse E-selectin (E-selectin/Fc) and P-selectin (P-selectin/Fc) (Figure 4B). Notably, Bsg-/- neutrophils showed less binding to E-selectin (Figure 4B). On the other hand, there was no difference in binding to P-selectin between Bsg+/+ and Bsg−/− neutrophils (Figure 4B).
Figure 4.
Neutrophil Bsg binds to E-selectin. (A) Bsg expression on mouse neutrophils. Peritoneal-elicited neutrophils were stained with anti-mouse Bsg antibodies (open histogram) or isotype-matched control antibodies (gray-filled histogram). Analysis gates were set on the granulocytic population using forward/side scatter distributions. The upper panel shows the data for Bsg+/+ mice, and the lower one the data for Bsg−/− mice. (B) E-selectin and P-selectin ligands on Bsg+/+ and Bsg−/− mouse neutrophils. Peritoneal-elicited neutrophils were stained with E-selectin/Fc or P-selectin/Fc. Gray-filled histograms represent EDTA treatment. The geometric mean fluorescence of E-selectin and P-selectin binding is indicated next to the histograms. Data are means (columns) and SEM (bars). **P < 0.01; n = 3. (C) Binding of E-selectin and P-selectin to Bsg immunopurified from HL-60 cells. The solid lines indicate the binding of human E- or P-selectin/Fc to the beads. The dotted line indicates that of mouse E-selectin/Fc to the beads. Gray-filled histograms represent EDTA treatment. The lower panels show binding to Bsg, which was immunopurified from HL-60 cells pretreated with tunicamycin to inhibit N-glycosylation. Tunicamycin treatment abolished the binding between Bsg and E-selectin. The right panel shows western blot analysis results for Bsg and β-actin expression in tunicamycin-treated (T) or untreated (U) HL-60 cells. Molecular mass markers (in kDa) are indicated on the left. (D) Binding of E-selectin to Bsg immunopurified from HL-60 culture medium supernatant (upper panel). In the lower panel, the immunopurified beads were further washed with 1 M NaCl-PBS and then subjected to this binding assay. The solid lines indicate the binding of human E-selectin/Fc to the Bsg-coated beads. The dotted line indicates the binding to the control beads. Gray-filled histograms represent EDTA treatment. The protein(s) on the beads used in this FACS analysis were detached from the beads, and subjected to western blot analysis for Bsg (right panel). The smear bands of Bsg are indicated. Asterisks indicate the position of the heavy chain of IgG. Anti-Bsg, the beads were coated with anti-human Bsg antibody before incubating with HL-60 culture medium supernatant; cont. Ig, the beads were coated with normal mouse IgG; HL60, the lysate of HL-60 cells. Molecular mass markers (in kDa) are indicated on the left. (E) PSGL-1 and CD44 expression on Bsg+/+ and Bsg−/− neutrophils. Gray-filled histograms represent the isotype-matched control. (F) Integrin expression on mouse neutrophils. Peritoneal-elicited neutrophils were stained with anti-CD11a (LFA-1) or anti-CD11b (Mac-1) antibodies (open histogram). Gray-filled histograms represent the isotype-matched control.
Since this result suggested that Bsg might bind to E-selectin, we next addressed this question. We examined Bsg isolated from HL-60 cells, a human promyelocytic cell line, that were pretreated with or without tunicamycin. Protein extracts from HL-60 cells were incubated with immunomagnetic beads coated with mouse anti-human Bsg antibody. Immobilized Bsg beads were then incubated with soluble human E-selectin and P-selectin with or without EDTA. Soluble E-selectin bound to the Bsg beads, and this binding was abrogated under a Ca2+ chelate condition (Figure 4C, left upper panel, solid line). Because it has been reported that mouse E-selectin/Fc has a higher level of binding activity to human leukocytes than human E-selectin/Fc,29 we also used mouse E-selectin/Fc. Mouse E-selectin/Fc bound to the Bsg beads (Figure 4C, left upper panel, dotted line). On the other hand, human P-selectin/Fc did not bind to the Bsg beads (Figure 4C). Tunicamycin treatment abolished N-linked glycans from Bsg (Figure 4C, right) and consequently diminished the binding between E-selectin and isolated Bsg (Figure 4C, left lower panel).
A recent study revealed that Bsg is released from the cell surface via microvesicle shedding, which is promoted by PMA.30 As these microvesicles are unstable and rapidly broken down, Bsg can be recovered from the supernatant fraction of the culture medium after centrifugation.30 In the present study, we treated HL-60 cells with 100 nM PMA for 24 h and detected Bsg secretion in the culture medium by western blot (data not shown). We prepared Bsg beads from this culture medium supernatant. Human E-selectin bound to these Bsg beads (Figure 4D, upper panel, solid line). Even after washing with 1 M NaCl, the beads retained the ability to bind to E-selectin, suggesting that soluble factors, if any, binding to Bsg on the beads do not affect the binding ability of the beads (Figure 4D, lower panel).
Expression of Other Adhesion-Related Molecules in Bsg-Deficient Neutrophils
The level of expression of the major E-selectin ligands PSGL-1 and CD44 on neutrophils was similar between Bsg+/+ and Bsg−/− neutrophils (Figure 4E). In the renal ischemia/reperfusion injury model, integrins on the neutrophils are another factor to be considered.31 However, we found that the expressions of lymphocyte function antigen 1 (LFA-1: CD11a/CD18) and macrophage-1 antigen (Mac-1: CD11b/CD18) on neutrophils from Bsg+/+ mice were comparable to those on neutrophils from Bsg−/− mice (Figure 4F).
In addition to PSGL-1 and CD44, CD43 is known to function as an E-selectin ligand.32 However, there was no difference of CD43 expression between Bsg+/+ and Bsg−/− neutrophils (Supplementary Figure S2A). Furthermore, the beads used for Figure 4C did not contain PSGL-1, CD44, or CD43 (Supplementary Figure S2B). Together with the data shown in Figure 4D, these data exclude the possibility that the Bsg used for the bead experiments was co-isolated with associated molecules that exert an E-selectin-binding activity.
Impaired Bsg−/− Neutrophil Adhesion to Cytokine-Activated Endothelial Cells
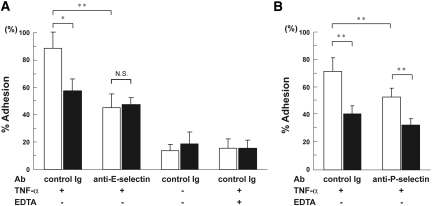
We next investigated the biologic significance of Bsg on neutrophils using an in vitro adhesion assay. It is known that human umbilical vein endothelial cells (HUVECs) express E- and P-selectin upon stimulation with TNF-α. We found that, compared with Bsg+/+ neutrophils, Bsg−/− neutrophils were less adherent to HUVECs at 4 h after TNF-α stimulation (Figure 5, A and B). This result was primarily due to the interaction between E-selectin and its ligands, since an E-selectin-blocking antibody significantly suppressed the adhesion, and the difference in adherence to HUVECs between Bsg+/+ and Bsg−/− neutrophils was abolished (Figure 5A). The adhesion was further suppressed when Ca2+ was chelated with EDTA, suggesting that, in addition to E-selectin, other components of the Ca2+-dependent adhesion machinery, e.g., other selectins, also played a role in this adhesion (Figure 5A). Indeed, P-selectin blocking antibody suppressed the neutrophil adhesion to HUVECs, but the difference in adhesion between the two genotypes remained intact (Figure 5B). It is noteworthy that the adhesion between HUVECs and Bsg+/+ neutrophils was suppressed by approximately 50% by the E-selectin-blocking antibody, whereas the adhesion between HUVECs and Bsg−/− neutrophils was only suppressed by about 10% by this antibody (Figure 5A). Therefore, the results strongly suggested that Bsg on neutrophils played an indispensable role in adhesion to HUVECs through E-selectin.
Figure 5.
Adhesion assay of mouse neutrophils to HUVECs. (A) Fluorescence-labeled mouse neutrophils were incubated on TNF-α-stimulated HUVECs. Relative adhesion (% input) is shown. Control Ig, HUVECs were pretreated with isotype-matched control antibody; anti-E-selectin, HUVECs were pretreated with anti-E-selectin antibody. White columns, neutrophils from Bsg+/+ mice; black columns, neutrophils from Bsg−/− mice. (B) Experiments were performed as in panel A, using anti-P-selectin antibody. anti-P-selectin, HUVECs were pretreated with anti-P-selectin antibody. Data are means (columns) and SEM (bars). *P < 0.05; **P < 0.01; n = 5.
Few Neutrophils from Bsg−/− Mice Infiltrated into the Postischemic Kidney
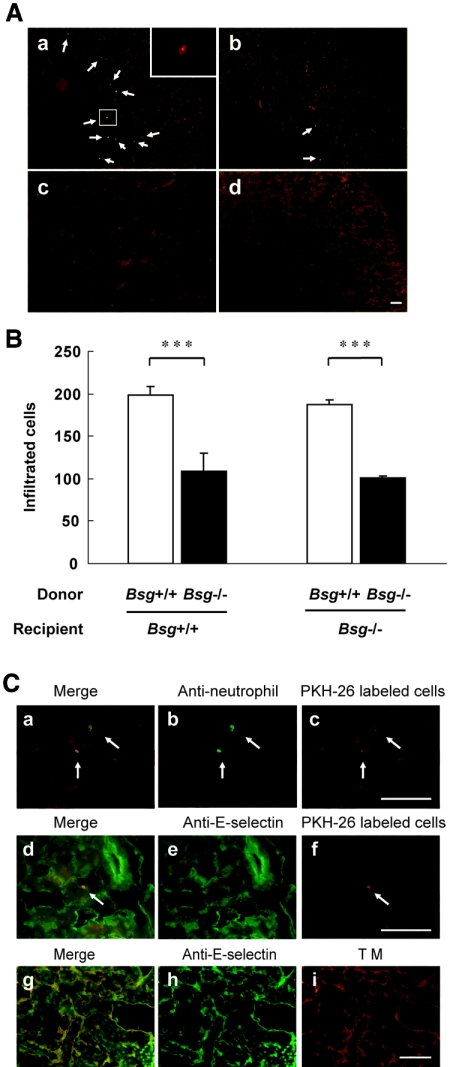
The suppressed infiltration of neutrophils in Bsg−/− mice after renal ischemia/reperfusion could be interpreted in two ways. First, Bsg on infiltrating neutrophils may have played a role. Second, Bsg on other cells in the inflammatory tissues may have been important. To examine these possibilities, fluorescence-labeled neutrophils were adoptively transferred into mice 5 min after renal ischemia/reperfusion surgery. After 6 h, the mice were sacrificed, and the postischemic kidneys were examined. The fluorescence-labeled neutrophils predominantly infiltrated around the vasa recta in the outer medulla, which was one of the main damaged areas after ischemia/reperfusion injury (Figure 6Aa). Much less infiltration was observed when Bsg−/− neutrophils were injected, compared with Bsg+/+ neutrophils (Figure 6A, a and b, neutrophils are indicated by arrows). Labeled cells were barely detectable in the right kidney, which was not subjected to ischemia/reperfusion surgery (Figure 6Ac). Labeled cells were also not observed when saline was injected instead of labeled neutrophils (Figure 6Ad). The numbers of labeled cells infiltrating into the postischemic kidney are summarized in Figure 6B. Regardless of the genotypes of recipients, fewer adopted Bsg−/− neutrophils infiltrated than adopted Bsg+/+ neutrophils. These data clearly indicated that Bsg on neutrophils, rather than Bsg on other cells at inflammatory sites, was responsible for the reduced infiltration of neutrophils into the postischemic kidney in Bsg−/− mice (Figure 2A). We confirmed that the infiltrating labeled cells (PKH26; Figure 6Cc, red) were indeed neutrophils by staining with anti-mouse neutrophil antibody (Figure 6Cb, green). Thus, the red and green spots were completely merged (Figure 6Ca). We also confirmed that infiltrating cells were located on the portions of the endothelium that were positive for E-selectin and thrombomodulin, a vascular endothelial marker (Figure 6C, d through i). Thus, E-selectin expression was found along the peritubular capillary blood vessels of the postischemic kidney, and the PKH26-labeled cells were in close proximity to E-selectin (Figure 6C, d through f). As the majority of the PKH26 staining in Figure 6C, c and f, represents autofluorescence, the PKH26-labeled cells are indicated by arrows in these figures.
Figure 6.
Adoptive transfer of neutrophils. (A) Adoptive transfer of labeled neutrophils just after renal ischemia/reperfusion. (a) Bsg+/+ neutrophils were adoptively transferred to Bsg+/+ recipient mice. Insert, a magnified image of the framed field. (b) Bsg−/− neutrophils were adoptively transferred to Bsg+/+ recipient mice. (c) A contralateral kidney that did not receive ischemia/reperfusion. (d) A kidney that was injected with saline instead of labeled neutrophils. Arrows show labeled neutrophils. All specimens were obtained 6 h after ischemia/reperfusion. Scale bar, 100 μm. (B) The numbers of labeled (adopted) neutrophils infiltrated into the kidneys after ischemia/reperfusion. Cell counting was performed as described in the Concise Methods section. Data are means (columns) and SEM (bars). ***P < 0.001; n = 3. (C) Immunostaining of mouse neutrophils and E-selectin after adoptive transfer of labeled cells. PKH26-labeled Bsg+/+ neutrophils (red) were adoptively transferred to Bsg+/+ recipient mice after ischemia/reperfusion. Kidney specimens were then immunostained with anti-neutrophil antibody followed by FITC-conjugated secondary antibody (green in a through c) or by anti-E-selectin antibody followed by FITC-conjugated secondary antibody (green in d through f). The arrows indicate fluorescence-positive cells. The capillary endothelium was also stained with anti-thrombomodulin (TM) antibody followed by rhodamine-conjugated secondary antibody (g through i). Scale bar, 50 μm.
DISCUSSION
In this study, we demonstrated that Bsg isolated from HL-60 bound to E-selectin, but not after N-linked polylactosamine chains of Bsg were ablated by tunicamycin treatment. The massive neutrophil infiltration into the kidney after renal ischemia/reperfusion was partly attributable to Bsg on neutrophils, because (i) the level of neutrophil infiltration was lower in Bsg−/− mice and (ii) regardless of the recipients’ genotypes, exogenously injected Bsg−/− neutrophils were less readily recruited to the inflammatory kidney tissue. In vitro E-selectin-dependent adherence to HUVECs was also reduced in Bsg−/− neutrophils. Therefore, our data strongly suggest that Bsg is a physiologic ligand for E-selectin.
The selectins are required for leukocyte adhesion during inflammation. E-selectin but not P-selectin controls slow leukocyte rolling on inflamed venules, and this rolling may enhance an efficient transition to firm adhesion and extravasation.33–35 Although blocking of P-selectin ameliorates ischemia/reperfusion-induced AKI, platelet P-selectin, but not endothelial P-selectin, is the key component in P-selectin-mediated AKI.36,37 Therefore, we focused on endothelial E-selectin and its ligand in these renal ischemia/reperfusion experiments. Several glycoproteins have been found to bind to E-selectin in vitro, and the topographic distribution of the ligands on the surface of neutrophils is a major determinant of their ability to mediate initial contacts to the endothelium under flow. For example, PSGL-1 distributes on the very tip of neutrophil microvilli and contributes to the primary interaction between neutrophils and the endothelium.38 On the other hand, CD44 is exclusively distributed on the planar surface of the neutrophils and mediates steady slow rolling.39 In this context, it is of note that Bsg is equally distributed on the planar surface and microvilli of the neutrophils (Figure 3C). The localization of Bsg on microvilli is an indication of its possible role in the early steps of leukocyte endothelial contact formation.
Bsg is known to induce MMPs and thus, is referred to as an extracellular matrix metalloproteinase inducer (EMMPRIN). To confirm the effects of MMP activity in the postischemic kidneys on this model, we performed gelatin zymography. Both MMP-2 and MMP-9 activities in Bsg+/+ mice were comparable to those in Bsg−/− mice at both 1 and 2 d postischemia/reperfusion (data not shown). Another molecule we considered was cyclophilin. It has been reported that extracellular cyclophilins can induce leukocyte chemotaxis, and Bsg is a signaling receptor for these proteins. Blocking the cyclophilin-Bsg interaction pharmacologically or by means of an antibody reduces the inflammation responses in a LPS-induced acute lung injury model and a bronchial asthma model in mice.28,40 Dear et al.41 found that cyclophilin is upregulated in the liver in a sepsis model, and inhibition of Bsg attenuates sepsis-induced AKI.
However, the expression of cyclophilin A, the most representative cyclophilin, did not increase after ischemia in the kidney, and there was no difference in the expression of cyclophilin A between the two genotypes in the present study (data not shown). Thus, it is not likely that the function of Bsg is always exerted through cyclophilin in AKI, although further studies are needed to fully understand the involvement of cyclophilin in AKI. Furthermore, Bsg−/− neutrophils expressed normal levels of PSGL-1, CD44, and integrins (LFA-1 and Mac-1). We also observed that the expression of E-selectin on renal microvessels and chemokines in the kidney was comparable between Bsg+/+ and Bsg−/− mice. These data support the idea that the difference in renal damage between Bsg+/+ and Bsg−/− mice was due to the difference in neutrophil infiltration mediated by Bsg on neutrophils.
Bsg on neutrophils has long sugar chains that are N-linked polylactosamines (Figure 3A). The biologic significance of these long sugar chains has long been obscure. In this context, it is noteworthy that O-linked glycans on PSGL-1 contribute to binding to its receptors, E- and P-selectins, while N-linked glycans on CD44 are responsible for the binding to E-selectin (Figure 3A).8,25 Based on the present results, Bsg has unique sugar chains, i.e., N-linked polylactosamines, that are responsible for its binding to E-selectin.
Renal ischemia/reperfusion leads to increased endothelial expression of a variety of adhesion molecules that promote endothelial-leukocyte interaction. Gene knockout, antibody, and pharmacologic inhibitor studies have suggested a role for E-selectin in ischemia/reperfusion injury.42 In particular, E-selectin-deficient mice show a 75% reduction in myeloperoxidase activity (an indicator of neutrophil infiltration) in the postischemic kidneys at 24 h compared with wild-type mice.23 Our Bsg−/− mice showed a 50% reduction in neutrophil counts in the kidney compared with wild-type mice. Therefore, Bsg may not fully account for the function of E-selectin. Our in vitro binding assay between neutrophils and HUVECs also supports this idea. Therefore, other E-selectin ligands on neutrophils, such as PSGL-1 and CD44, may also be important for neutrophil recruitment and the subsequent renal damages induced by ischemia/reperfusion. Infiltrating neutrophils produce cytokines, growth factors, proteases, and reactive oxygen species, all of which can injure renal cells. Injured renal cells in turn produce factors that stimulate neutrophils. This chain reaction may contribute to the establishment of renal dysfunction. If neutrophil infiltration is moderately suppressed as in the case of Bsg−/− mice, the suppression of renal dysfunction may be delayed. Therefore, the delayed effect of Bsg deficiency on BUN as compared with E-selectin knockout may not necessarily indicate that Bsg is not an early-acting E-selectin ligand. Rather, the localization of Bsg on the microvilli (Figure 3) may suggest that it participates in initial capture (tethering) on the endothelium, as in the case of PSGL-1.7
Finally, our study has shed light on the mechanisms underlying AKI. There is no specific therapy for AKI except for supportive care, and AKI is associated with unacceptably high mortality that has been reported to range from 40% to 60%.17,43 The overwhelming majority of studies have exclusively looked at neutrophils as the most important prevalent leukocytes during AKI. Our study thus introduces a novel player in the pathogenesis of AKI. Bsg might be a good candidate target for intervention of AKI.
CONCISE METHODS
Bsg-Deficient Mice
Mice deficient in the Bsg gene were generated as described previously.44 All experiments were performed with Bsg+/+ and Bsg−/− littermates. The mice used were 8- to 12-wk-old females weighing 20 to 25 g. The mice were housed under controlled environmental conditions and maintained with standard food and water.
The experiments described above were conducted according to The Animal Experimentation Guide of Nagoya University School of Medicine.
Renal Ischemia/Reperfusion Injury Model
We used a previously characterized model of renal ischemia/reperfusion injury in mice.45 Briefly, we anesthetized the mice by intraperitoneal administration of 40 mg/kg sodium pentobarbital. We placed the animals on a heating pad to maintain a constant body temperature of 37°C. Under general anesthesia, we removed the right kidney. This heminephrectomy procedure was omitted in the experiment of adoptive transfer of labeled neutrophils. After 7 d, we anesthetized the mice as described above and exposed the left kidney. We occluded the renal artery for 45 min with nontraumatic microvascular clamps. The animals received 30 ml/kg warm saline instilled into the peritoneal cavity after the procedure and were allowed to recover with free access to food and water. Sham-operated mice underwent the same procedure without clamping of the artery and were killed 1 d after surgery. Groups of mice (n = 6) were killed 1, 2, 4, and 7 d after surgery.
We determined serum urea by a standard diagnostic procedure using a kit from KAINOS Laboratories (Tokyo, Japan).
We measured the cytokine MIP-2 and KC in renal homogenates as described previously46 by specific ELISA according to the manufacturer's instructions (MIP-2: R&D Systems; KC: Immuno-Biologic Laboratories Ltd., Gunma, Japan). We normalized the results for the total protein concentration.
Histology
We fixed renal tissues in 4% paraformaldehyde, embedded them in paraffin, and then cut them into 2-μm sections. We stained the sections with periodic acid-Schiff reagent. Using semiquantitative indices, we analyzed the sections to evaluate tubulointerstitial damage in each region by light microscopy, as described previously.45 Briefly, the extent of cast formation, tubular dilation, and tubular degeneration in the cortex, outer medulla, and inner medulla were scored according to the following criteria by two observers in a blind manner: 0, normal; 1, below 30% of the pertinent area; 2, 30% to 70% of the pertinent area; 3, over 70% of the pertinent area.
Parts of the kidney tissues were snap-frozen in liquid nitrogen. We cut 2-μm-thick sections with a cryostat and then fixed them in acetone. We stained the cryosections with rat anti-mouse neutrophil antibody (dilution, 1:200; clone 7/4; Serotec, Oxford, UK), rat anti-mouse macrophage antibody (dilution, 1:50; clone F4/80; Serotec), rat anti-mouse E-selectin antibody (dilution, 1:50; clone 96419; R&D Systems, Minneapolis, MN), or goat anti-mouse ICAM-1 antibody (dilution, 1:50; R&D Systems), followed by detection with FITC-conjugated rabbit anti-rat IgG (dilution, 1:100; Zymed Laboratories, San Francisco, CA) or FITC-conjugated rabbit anti-goat IgG (dilution, 1:100; Sigma-Aldrich, St. Louis, MO). We counted leukocytes positive for 7/4 and F4/80 in all renal regions (cortex, outer medulla, and inner medulla) under a microscope at ×200 magnification in a blind manner.
For immunoelectron microscopy, we washed peritoneal-elicited mouse neutrophils twice and then stained them with rat anti-mouse Bsg antibody (dilution, 1:25; clone OX114; Abcam Ltd., Cambridge, UK) or control rat IgG followed by HRP-conjugated goat F(ab′)2 fragment anti-rat IgG (Histofine; Nichirei Corporation, Tokyo, Japan). After fixation with 1% glutaraldehyde, we incubated the cells with 3,3′ diaminobenzidine (Dako, Carpinteria, CA) for 30 min, then washed them twice. The cells were postfixed in osmium tetroxide, dehydrated in alcohol, and embedded in epoxy resin (Quetol-812; Nissin EM Corporation, Tokyo, Japan). We examined ultrathin sections with a JEM-1400 electron microscope (JOEL Ltd., Tokyo, Japan).
Preparation of Mouse Peritoneal-Elicited Neutrophils
Mouse peritoneal neutrophils were elicited by intraperitoneal injection of 2 ml 3% thioglycollate medium (Wako, Osaka, Japan), which induced aseptic peritoneal inflammation. After 5 h, we collected peritoneal exudate fluid with 5 ml ice-cold PBS.47 We washed the isolated peritoneal cells three times. The purity of neutrophils was approximately 90% as confirmed by FACS analysis (anti-GR-1-positive cells) and May-Giemsa staining. The cell viability was more than 98% checked by trypan blue staining.
Cells
HUVECs (Cell Applications, San Diego, CA) were cultured using an EGM-2 BulletKit (Takara Bio, Shiga, Japan) at 37°C in 5% CO2 and used between the second and fifth passages. We obtained the human promyelocytic cell line HL-60 from the American Type Culture Collection (ATCC; accession no. CCL-240; Manassas, VA) and cultured them in RPMI 1640 (Sigma-Aldrich) containing 10% fetal bovine serum (Life Technologies BRL, Gaithersburg, MD) at 37°C in 5% CO2.
Deglycosylation
To remove N-glycans in the cell lysate, we treated the lysate of mouse peritoneal-elicited neutrophils with 10 U N-glycosidase F (Roche Diagnostics, Mannheim, Germany) at 37°C overnight in a buffer containing 50 mM sodium phosphate, pH 7.5, and 1% Nonidet-P 40. To remove polylactosamine chains in the cell lysate, we treated the cell lysate with 5 mU endo-β-galactosidase (Seikagaku Corporation, Tokyo, Japan) at 37°C overnight in a buffer containing 10 mM sodium acetate, pH 6.0.
For the inhibition of N-glycosylation in cells, we cultured HL-60 cells in the presence of 15 μg/ml tunicamycin (Calbiochem, San Diego, CA) for 48 h.
Flow Cytometry E- and P-Selectin-Binding Assay
First, we prepared immunomagnetic beads. Lysates were prepared by incubation of HL-60 in lysis buffer (1% Triton-X 100 in PBS with EDTA-free Protease Inhibitor Cocktail; Nakalai Tesque, Kyoto, Japan) for 30 min on ice, and cell debris was removed by centrifugation at 17,000 × g for 10 min at 4°C. We incubated anti-mouse IgG-coated beads (M-280 Dynabeads; Dynal Biotech ASA, Oslo, Norway) with mouse anti-human Bsg antibody (clone MEM-M6/1; Abcam Ltd.) and control mouse IgG for 4 h under rotation. We then washed the beads twice with lysis buffer and incubated them overnight at 4°C under rotation with the prepared HL-60 cell lysate (2 × 106 cells/106 beads) or culture medium supernatant obtained after ultracentrifuge. We washed the beads three times with PBS or 1 M NaCl-PBS before the binding assay. To detach the protein on the prepared immunomagnetic beads, we boiled the beads with sample buffer for 5 min. We then subjected the supernatants to western blot analysis.
Mouse peritoneal-elicited neutrophils (5 × 105) were stained by incubation with antibody against Bsg (dilution, 1:50; clone OX114; Abcam Ltd.), PSGL-1 (dilution, 1:50; clone 4RA10; Becton Dickinson, Franklin Lakes, NJ), CD44 (dilution, 1:50; IM7; BioLegend, San Diego, CA), CD11a (dilution, 1:50; clone I21/7; SouternBiotech, Birmingham, AL), CD11b (dilution, 1:50; clone M1/70; Cedarlane, ON, Canada), Gr-1 (dilution, 1:100; clone RB6–8C5; Cedarlane), and CD43 (dilution, 1:50; clone 1B11; Biolegend) or control antibodies. Subsequently, we washed these cells with PBS and then incubated them with FITC-conjugated anti-rat IgG (dilution, 1:100; Zymed Laboratories). Cells were washed three times before flow cytometry analysis. To assess the E- and P-selectin-binding property, we incubated mouse peritoneal-elicited neutrophils or prepared Bsg-coated beads with 10 μg/ml recombinant human E-selectin/Fc chimera, human P-selectin/Fc chimera, mouse E-selectin/Fc chimera, and mouse P-selectin/Fc chimera (R&D Systems) at 4°C with gentle shaking for 30 min in the presence or absence of 5 mM EDTA. We then incubated the cells with FITC-conjugated goat F(ab′)2 fragment anti-human IgG (Fcγ; Beckman Coulter, Fullerton, CA). In the analysis of mouse neutrophils, the gates were set on the granulocytic population using forward/side scatter distributions. All samples were analyzed using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson).
Western Blot Analysis
We performed western blot analysis as described previously.48 Briefly, we separated the samples by 10% SDS-PAGE and then transferred the gels to a nitrocellulose membrane (Whatman, Florham Park, NJ). We blocked the membranes with 5% (wt/vol) dry fat-free milk in PBS with 0.1% Tween for 60 min at room temperature. We then incubated the membranes with rat anti-mouse E-selectin antibody (dilution, 1:1000; clone 96419; R&D Systems), goat anti-mouse ICAM-1 antibody (dilution, 1:1000; R&D Systems), goat anti-mouse Bsg antibody (dilution, 1:1000; R&D Systems), mouse anti-human Bsg antibody (dilution, 1:1000; clone HIM6; Biolegend), mouse anti-human PSGL-1 antibody (dilution, 1:1000; clone KPL-1; BD Biosciences), mouse anti-human CD44 antibody (dilution, 1:1000; clone 2C5; R&D Systems), mouse anti-human CD43 antibody (dilution, 1:1000; clone MEM-59; Biolegend), rabbit anti-mouse cyclophilin A antibody (dilution, 1:1000; Upstate, Lake Placid, NY), or mouse anti-β actin antibody (dilution, 1:1000; Sigma-Aldrich). Each primary antibody was incubated at 4°C overnight. After washing with PBS containing 0.1% Tween, we incubated these membranes with peroxidase-conjugated anti-rat IgG, anti-goat IgG, or anti-mouse IgG (dilution, 1:5000; Jackson Immunoresearch Laboratories, West Grove, PA), respectively, for 60 min at room temperature. We visualized the proteins with an enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia, Amersham Biosciences, Piscataway, NJ).
Adhesion Assay of Mouse Neutrophils to HUVECs
We performed the adhesion assay on HUVECs as described previously.49 Briefly, we washed the mouse peritoneal-elicited neutrophils in HBSS without Ca2+ and Mg2+ and concentrated them to 107 cells/ml. We then incubated the suspensions with 5 μM calcein-AM (Molecular Probes, Eugene, OR) at 37°C for 15 min.50 We stopped the labeling by adding RPMI 1640 with 10% fetal bovine serum and washing three times. HUVECs were plated at 5 × 104 cells/well in a 96-well microplate (MICROTEST 96; Becton Dickinson). After overnight culture, we obtained confluent monolayers. To stimulate the expression of endothelial adhesion molecules, including E-selectin, we pretreated HUVECs with 10 ng/ml recombinant human TNF-α (R&D Systems) for 4 h at 37°C. Afterwards, HUVECs were washed twice and then treated with 30 μg/ml rat anti-human CD62E (E-selectin) antibody (clone UZ4; Abcam Ltd.), 20 μg/ml sheep anti-human CD62P (P-selectin) antibody (R&D Systems), control rat antibody (clone RTK2118; Abcam Ltd.), or control sheep antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h. After washing, we allowed 5 × 105 fluorescence-labeled mouse neutrophils in RPMI 1640 medium to adhere for 30 min with or without EDTA. This procedure was examined at 4°C, since this temperature is known to be optimal for the investigation of the selectin-mediated adhesion mechanism and does not activate integrins.51We then inverted the plates for 30 min to eliminate unadherent cells and washed carefully. We then added an equal number of labeled cells (5 × 105) to the well to measure total fluorescence (Ft). The remaining fluorescence (Fx) and the fluorescence of a blank well (Fb) were measured.50 We measured all fluorescences on a Fluoroskan AscentCF (Labsystems, Helsinki, Finland). Calcein-AM-labeled cells were excited at 485 nm and evaluated at 530 nm. We calculated the percentage adhesion using the following formula:
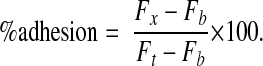 |
Adoptive Transfer of Labeled Neutrophils
We washed peritoneal-elicited neutrophils from Bsg+/+ and Bsg−/− mice with PBS and stained them with a PKH26 red fluorescence cell linker kit (Sigma-Aldrich) according to the manufacturer's instructions. The viability of neutrophils was more than 98% after labeling with trypan blue exclusion. Five million neutrophils from Bsg+/+ and Bsg−/− mice were injected intravenously into Bsg+/+ and Bsg−/− mice, respectively, at 5 min after renal ischemia/reperfusion surgery. After 6 h of reperfusion, we harvested the postischemic kidney. We prepared frozen sections of the kidney at 4-μm thickness. We counted the number of transferred neutrophils (PKH26-labeled cells) by examining all renal regions in 20 continuous sections of the short axis hilum of the kidney. Then we fixed other sections as described above and stained them with rat anti-mouse neutrophil antibody (clone 7/4; Serotec), followed by FITC-conjugated rabbit anti-rat IgG (Zymed Laboratories) to confirm that the infiltrated PKH26-labeled cells were neutrophils. We incubated other sections with chicken anti-mouse E-selectin antibody (dilution, 1:50; R&D Systems), followed by FITC-conjugated rabbit anti-chicken IgG (dilution, 1:50; Zymed Laboratories) to check the positional relations of transferred neutrophils in the kidney. For double-immunofluorescence staining of E-selectin and the endothelium, we first stained the section with E-selectin as described above. It was then incubated with rabbit anti-rat thrombomodulin antibody52 (dilution, 1:1000) followed by incubation with rhodamine-conjugated goat anti-rabbit IgG (dilution, 1:100; Zymed Laboratories).
Statistical Analysis
We expressed all values as means ± SEM. We performed statistical analysis with unpaired, two-tailed t test for single comparisons. Values of P < 0.05 were considered to indicate statistically significant differences.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We thank Kenji Uchimura for his helpful comments on this manuscript and Norihiko Suzuki, Naoko Asano, and Yuriko Sawa for their excellent technical assistance. This work was supported by a Grant-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan (no. 19590947 to Y.Y.)
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Ley K: The role of selectins in inflammation and disease. Trends Mol Med 9: 263–268, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Vestweber D, Blanks JE: Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev 79: 181–213, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Sperandio M, Smith ML, Forlow SB, Olson TS, Xia L, McEver RP, Ley K: P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J Exp Med 197: 1355–1363, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullard DC, Kunkel EJ, Kubo H, Hicks MJ, Lorenzo I, Doyle NA, Doerschuk CM, Ley K, Beaudet AL: Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J Exp Med 183: 2329–2336, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD: Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell 84: 563–574, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Labow MA, Norton CR, Rumberger JM, Lombard-Gillooly KM, Shuster DJ, Hubbard J, Bertko R, Knaack PA, Terry RW, Harbison ML, Kontgen F, Stewart CL, McIntyre KW, Will PC, Burns DK, Wolitzky BA: Characterization of E-selectin-deficient mice: Demonstration of overlapping function of the endothelial selectins. Immunity 1: 709–720, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS: Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity 26: 477–489, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowe JB: Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr Opin Cell Biol 15: 531–538, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Zarbock A, Ley K: Mechanisms and consequences of neutrophil interaction with the endothelium. Am J Pathol 172: 1–7, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyauchi T, Kanekura T, Yamaoka A, Ozawa M, Miyazawa S, Muramatsu T: Basigin, a new, broadly distributed member of the immunoglobulin superfamily, has strong homology with both the immunoglobulin V domain and the beta-chain of major histocompatibility complex class II antigen. J Biochem 107: 316–323, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Igakura T, Kadomatsu K, Kaname T, Muramatsu H, Fan QW, Miyauchi T, Toyama Y, Kuno N, Yuasa S, Takahashi M, Senda T, Taguchi O, Yamamura K, Arimura K, Muramatsu T: A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol 194: 152–165, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Igakura T, Kadomatsu K, Taguchi O, Muramatsu H, Kaname T, Miyauchi T, Yamamura K, Arimura K, Muramatsu T: Roles of basigin, a member of the immunoglobulin superfamily, in behavior as to an irritating odor, lymphocyte response, and blood-brain barrier. Biochem Biophys Res Commun 224: 33–36, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Naruhashi K, Kadomatsu K, Igakura T, Fan QW, Kuno N, Muramatsu H, Miyauchi T, Hasegawa T, Itoh A, Muramatsu T, Nabeshima T: Abnormalities of sensory and memory functions in mice lacking Bsg gene. Biochem Biophys Res Commun 236: 733–737, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K: The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res 55: 434–439, 1995 [PubMed] [Google Scholar]
- 15.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP: CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J 19: 3896–3904, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philp NJ, Wang D, Yoon H, Hjelmeland LM: Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophthalmol Vis Sci 44: 1716–1721, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Ympa YP, Sakr Y, Reinhart K, Vincent JL: Has mortality from acute renal failure decreased? A systematic review of the literature. Am J Med 118: 827–832, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Singbartl K, Ley K: Leukocyte recruitment and acute renal failure. J Mol Med 82: 91–101, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Johnson KJ, Weinberg JM: Postischemic renal injury due to oxygen radicals. Curr Opin Nephrol Hypertens 2: 625–635, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Abuelo JG: Normotensive ischemic acute renal failure. N Engl J Med 357: 797–805, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Deora AA, Philp N, Hu J, Bok D, Rodriguez-Boulan E: Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proc Natl Acad Sci U S A 102: 16245–16250, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL: Neutralization of Gro alpha and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol 159: 2137–2145, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singbartl K, Ley K: Protection from ischemia-reperfusion induced severe acute renal failure by blocking E-selectin. Crit Care Med 28: 2507–2514, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Muramatsu T, Miyauchi T: Basigin (CD147): A multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histol Histopathol 18: 981–987, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Katayama Y, Hidalgo A, Chang J, Peired A, Frenette PS: CD44 is a physiological E-selectin ligand on neutrophils. J Exp Med 201: 1183–1189, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou X, Shinde Patil VR, Dagia NM, Smith LA, Wargo MJ, Interliggi KA, Lloyd CM, Tees DF, Walcheck B, Lawrence MB, Goetz DJ: PSGL-1 derived from human neutrophils is a high-efficiency ligand for endothelium-expressed E-selectin under flow. Am J Physiol Cell Physiol 289: C415–C424, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Tang W, Chang SB, Hemler ME: Links between CD147 function, glycosylation, and caveolin-1. Mol Biol Cell 15: 4043–4050, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arora K, Gwinn WM, Bower MA, Watson A, Okwumabua I, MacDonald HR, Bukrinsky MI, Constant SL: Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol 175: 517–522, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni Z, Walcheck B: Varied levels of reactivity by different E-selectin/Fc constructs with cutaneous lymphocyte-associated antigen (CLA)(+) CD4(+) T cells. Immunol Lett 108: 179–182, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum, C: The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene 23: 956–963, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Rabb H, Mendiola CC, Dietz J, Saba SR, Issekutz TB, Abanilla F, Bonventre JV, Ramirez G: Role of CD11a and CD11b in ischemic acute renal failure in rats. Am J Physiol 267: F1052–F1058, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Khunkaewla P, Schiller HB, Paster W, Leksa V, Cermak L, Andera L, Horejsi V, Stockinger H: LFA-1-mediated leukocyte adhesion regulated by interaction of CD43 with LFA-1 and CD147. Mol Immunol 45: 1703–1711, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Jung U, Norman KE, Scharffetter-Kochanek K, Beaudet AL, Ley K: Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J Clin Invest 102: 1526–1533, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunkel EJ, Ley K: Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ Res 79: 1196–1204, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Ley K, Allietta M, Bullard DC, Morgan S: Importance of E-selectin for firm leukocyte adhesion in vivo. Circ Res 83: 287–294, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Singbartl K, Forlow SB, Ley, K: Platelet, but not endothelial, P-selectin is critical for neutrophil-mediated acute postischemic renal failure. FASEB J 15: 2337–2344, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Singbartl K, Green SA, Ley K: Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J 14: 48–54, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Moore KL, Patel KD, Bruehl RE, Li F, Johnson DA, Lichenstein HS, Cummings RD, Bainton DF, McEver RP: P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol 128: 661–671, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Andrian UH, Hasslen SR, Nelson RD, Erlandsen SL, Butcher EC: A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell 82: 989–999, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Gwinn WM, Damsker JM, Falahati R, Okwumabua I, Kelly-Welch A, Keegan AD, Vanpouille C, Lee JJ, Dent LA, Leitenberg D, Bukrinsky MI, Constant SL: Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J Immunol 177: 4870–4879, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dear JW, Leelahavanichkul A, Aponte A, Hu X, Constant SL, Hewitt SM, Yuen PS, Star RA: Liver proteomics for therapeutic drug discovery: Inhibition of the cyclophilin receptor CD147 attenuates sepsis-induced acute renal failure. Crit Care Med 35: 2319–2328, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nemoto T, Burne MJ, Daniels F, O'Donnell MP, Crosson J, Berens K, Issekutz A, Kasiske BL, Keane WF, Rabb H: Small molecule selectin ligand inhibition improves outcome in ischemic acute renal failure. Kidney Int 60: 2205–2214, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Star RA: Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Chen S, Kadomatsu K, Kondo M, Toyama Y, Toshimori K, Ueno S, Miyake Y, Muramatsu T: Effects of flanking genes on the phenotypes of mice deficient in basigin/CD147. Biochem Biophys Res Commun 324: 147–153, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Sato W, Kadomatsu K, Yuzawa Y, Muramatsu H, Hotta N, Matsuo S, Muramatsu T: Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J Immunol 167: 3463–3469, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Rouschop KM, Sewnath ME, Claessen N, Roelofs JJ, Hoedemaeker I, van der Neut R, Aten J, Pals ST, Weening JJ, Florquin S: CD44 deficiency increases tubular damage but reduces renal fibrosis in obstructive nephropathy. J Am Soc Nephrol 15: 674–686, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Lagasse E, Weissman IL: Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods 197: 139–150, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Kadomatsu K, Hagihara M, Akhter S, Fan QW, Muramatsu H, Muramatsu T: Midkine induces the transformation of NIH3T3 cells. Br J Cancer 75: 354–359, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hisano T, Namba T, Hashiguchi-Ikeda M, Ito T, Hirota K, Fukuda K: Inhibition of E-selectin-mediated leukocyte adhesion by volatile anesthetics in a static condition. J Anesth 19: 1–6, 2005 [DOI] [PubMed] [Google Scholar]
- 50.De Clerck LS, Bridts CH, Mertens AM, Moens MM, Stevens WJ: Use of fluorescent dyes in the determination of adherence of human leucocytes to endothelial cells and the effect of fluorochromes on cellular function. J Immunol Methods 172: 115–124, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Hammel M, Weitz-Schmidt G, Krause A, Moll T, Vestweber D, Zerwes HG, Hallmann R: Species-specific and conserved epitopes on mouse and human E-selectin important for leukocyte adhesion. Exp Cell Res 269: 266–274, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Yuzawa Y, Brentjens JR, Brett J, Caldwell PR, Esposito C, Fukatsu A, Godman G, Stern D, Andres G: Antibody-mediated redistribution and shedding of endothelial antigens in the rabbit. J Immunol 150: 5633–5646, 1993 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.