Abstract
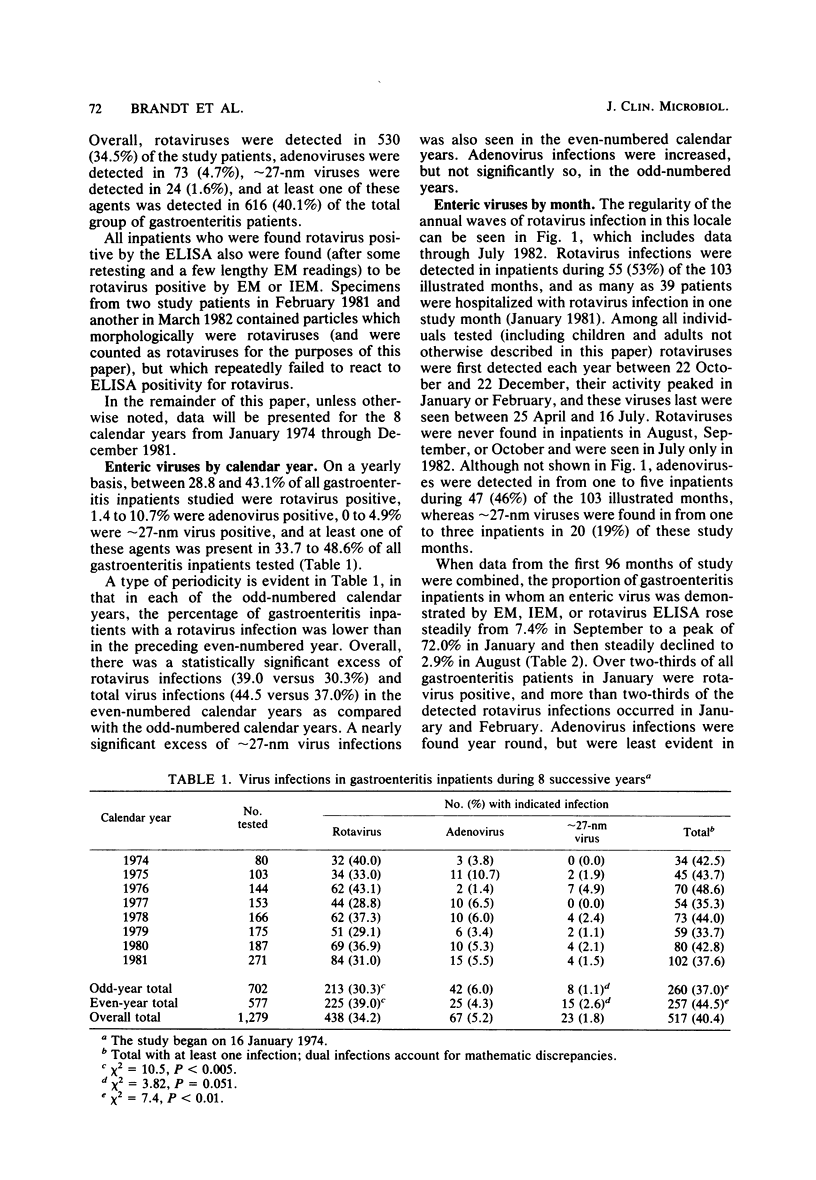
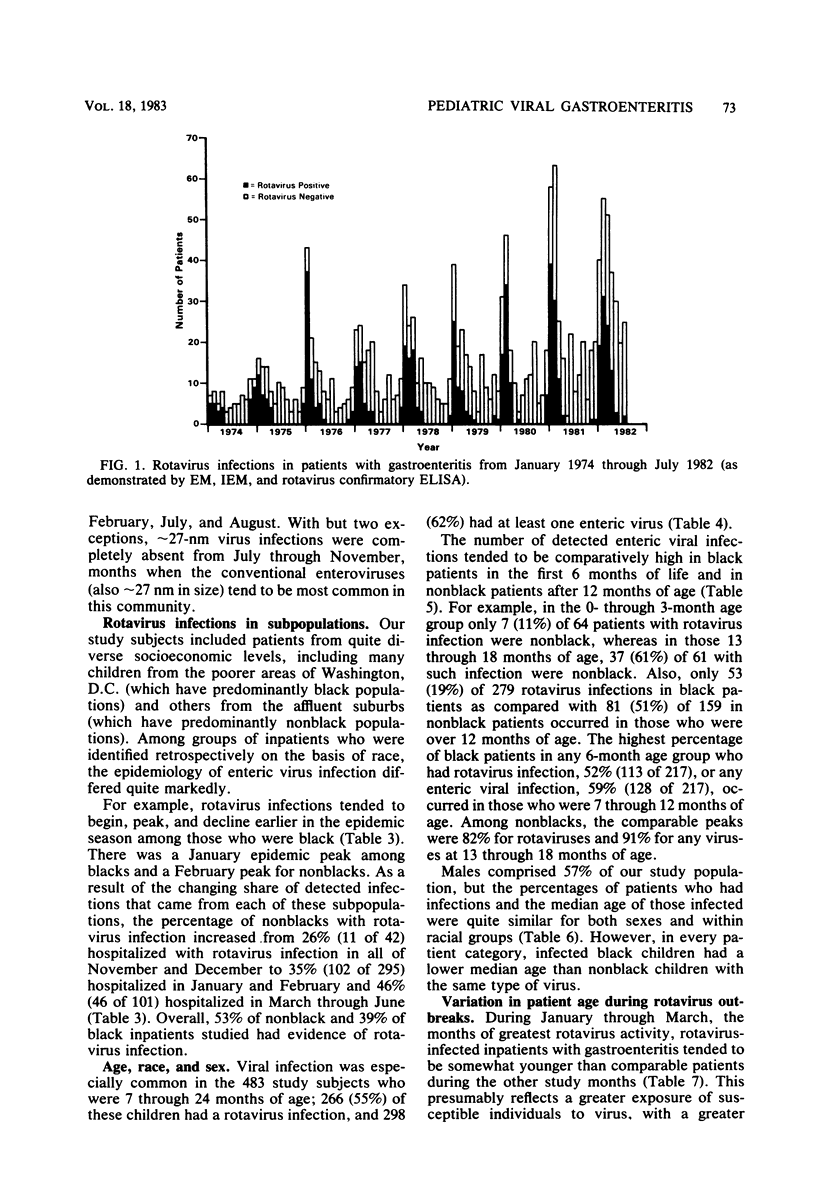
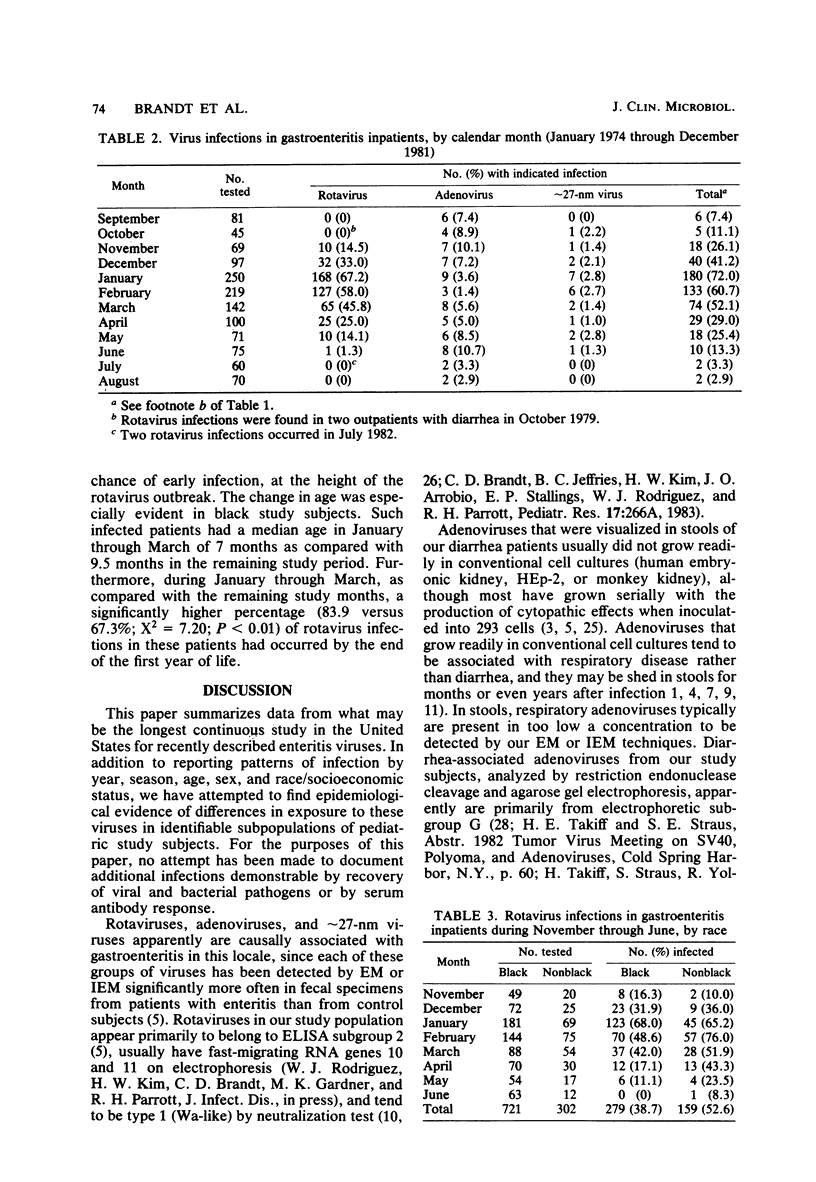
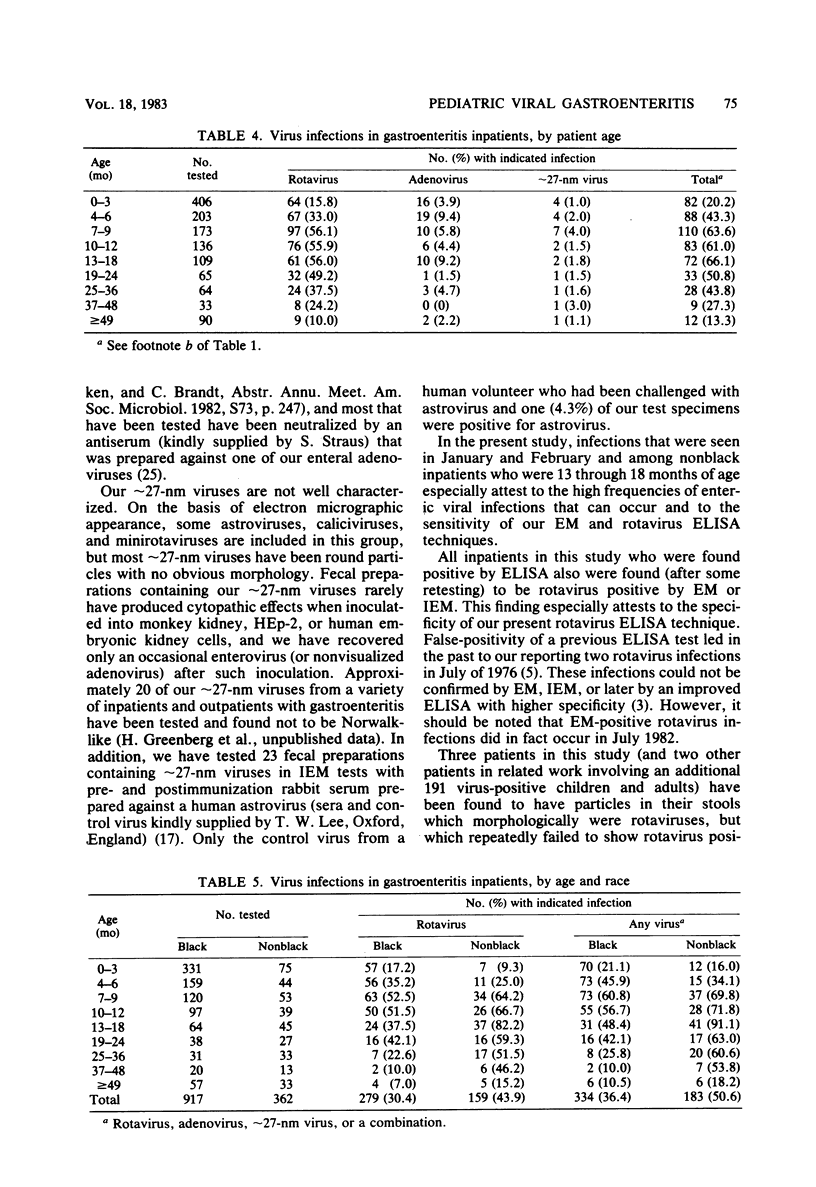
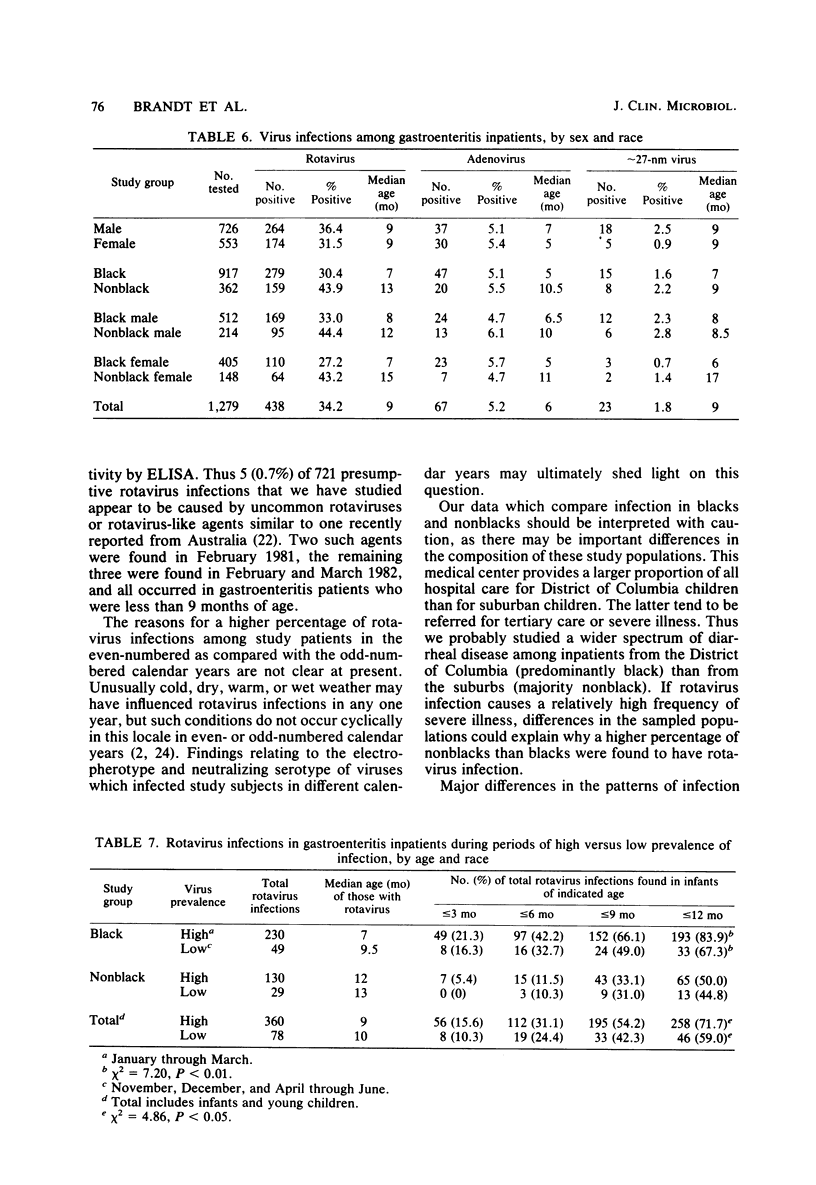
During the period January 1974 through July 1982, fecal samples from 1,537 pediatric inpatients with gastroenteritis were tested for enteric viruses by electron microscopic and rotavirus enzyme-linked immunosorbent assay techniques. Rotaviruses were detected in 34.5% of these patients, enteric adenoviruses were detected in 4.7%, approximately 27-nm viruses were detected in 1.6%, and at least one of these agents was found in 40.1% of the study subjects. Three infections were by an apparently new agent which morphologically is a rotavirus, but which failed to react in the rotavirus enzyme-linked immunosorbent assay. During the first 8 calendar years of study, rotaviruses were detected in 39.0% of 577 patients in the even-numbered years and 30.3% of 702 patients in the odd-numbered years. Adenoviruses were found in all calendar months. Rotaviruses were found in inpatients in November through July, whereas approximately 27-nm viruses were found in October through June. The percentage of patients who had a demonstrated viral infection rose steadily from 7.4% in September to 72.0% in January and then steadily declined to 2.9% in August. Viral infection was especially common in study subjects who were 7 through 24 months of age; 61% of such children had one or more enteric viruses. Rotavirus-infected patients tended to be younger during the months of greatest rotavirus activity than at the beginning and end of the rotavirus season, presumably because of a greater exposure to virus at the height of the rotavirus outbreak.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt C. D., Kim H. W., Jeffries B. C., Pyles G., Christmas E. E., Reid J. L., Chanock R. M., Parrott R. H. Infections in 18,000 infants and children in a controlled study of respiratory tract disease. II. Variation in adenovirus infections by year and season. Am J Epidemiol. 1972 Mar;95(3):218–227. doi: 10.1093/oxfordjournals.aje.a121389. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Arrobio J. O., Jeffries B. C., Parrott R. H. Rotavirus gastroenteritis and weather. J Clin Microbiol. 1982 Sep;16(3):478–482. doi: 10.1128/jcm.16.3.478-482.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Thomas L., Yolken R. H., Arrobio J. O., Kapikian A. Z., Parrott R. H., Chanock R. M. Comparison of direct electron microscopy, immune electron microscopy, and rotavirus enzyme-linked immunosorbent assay for detection of gastroenteritis viruses in children. J Clin Microbiol. 1981 May;13(5):976–981. doi: 10.1128/jcm.13.5.976-981.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Vargosko A. J., Jeffries B. C., Arrobio J. O., Rindge B., Parrott R. H., Chanock R. M. Infections in 18,000 infants and children in a controlled study of respiratory tract disease. I. Adenovirus pathogenicity in relation to serologic type and illness syndrome. Am J Epidemiol. 1969 Dec;90(6):484–500. doi: 10.1093/oxfordjournals.aje.a121094. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Yolken R. H., Kapikian A. Z., Arrobio J. O., Rodriguez W. J., Wyatt R. G., Chanock R. M., Parrott R. H. Comparative epidemiology of two rotavirus serotypes and other viral agents associated with pediatric gastroenteritis. Am J Epidemiol. 1979 Sep;110(3):243–254. doi: 10.1093/oxfordjournals.aje.a112809. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Wassermann F. E., Fox J. P. The Virus Watch program. IV. Recovery and comparison of two serological varieties of adenovirus type 5. Proc Soc Exp Biol Med. 1966 Nov;123(2):513–518. doi: 10.3181/00379727-123-31530. [DOI] [PubMed] [Google Scholar]
- Engleberg N. C., Holburt E. N., Barrett T. J., Gary G. W., Jr, Trujillo M. H., Feldman R. A., Hughes J. M. Epidemiology of diarrhea due to rotavirus on an Indian reservation: risk factors in the home environment. J Infect Dis. 1982 Jun;145(6):894–898. doi: 10.1093/infdis/145.6.894. [DOI] [PubMed] [Google Scholar]
- Fox J. P., Brandt C. D., Wassermann F. E., Hall C. E., Spigland I., Kogon A., Elveback L. R. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol. 1969 Jan;89(1):25–50. doi: 10.1093/oxfordjournals.aje.a120913. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Wyatt R. G., Kapikian A. Z., Kalica A. R., Flores J., Jones R. Rescue and serotypic characterization of noncultivable human rotavirus by gene reassortment. Infect Immun. 1982 Jul;37(1):104–109. doi: 10.1128/iai.37.1.104-109.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. E., Brandt C. D., Frothingham T. E., Spigland I., Cooney M. K., Fox J. P. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. IX. A comparison of infections with several respiratory pathogens in New York and New Orleans families. Am J Epidemiol. 1971 Oct;94(4):367–385. doi: 10.1093/oxfordjournals.aje.a121332. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Kim H. W., Wyatt R. G., Cline W. L., Arrobio J. O., Brandt C. D., Rodriguez W. J., Sack D. A., Chanock R. M., Parrott R. H. Human reovirus-like agent as the major pathogen associated with "winter" gastroenteritis in hospitalized infants and young children. N Engl J Med. 1976 Apr 29;294(18):965–972. doi: 10.1056/NEJM197604292941801. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Brandt C. D., Jeffries B. C., Pyles G., Reid J. L., Chanock R. M., Parrott R. H. Epidemiology of respiratory syncytial virus infection in Washington, D.C. I. Importance of the virus in different respiratory tract disease syndromes and temporal distribution of infection. Am J Epidemiol. 1973 Sep;98(3):216–225. doi: 10.1093/oxfordjournals.aje.a121550. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Brandt C. D., Arrobio J. O., Murphy B., Chanock R. M., Parrott R. H. Influenza A and B virus infection in infants and young children during the years 1957-1976. Am J Epidemiol. 1979 Apr;109(4):464–479. doi: 10.1093/oxfordjournals.aje.a112704. [DOI] [PubMed] [Google Scholar]
- Konno T., Suzuki H., Katsushima N., Imai A., Tazawa F., Kutsuzawa T., Kitaoka S., Sakamoto M., Yazaki N., Ishida N. Influence of temperature and relative humidity on human rotavirus infection in Japan. J Infect Dis. 1983 Jan;147(1):125–128. doi: 10.1093/infdis/147.1.125. [DOI] [PubMed] [Google Scholar]
- Kurtz J. B., Lee T. W., Craig J. W., Reed S. E. Astrovirus infection in volunteers. J Med Virol. 1979;3(3):221–230. doi: 10.1002/jmv.1890030308. [DOI] [PubMed] [Google Scholar]
- Marier R., Rodriguez W., Chloupek R. J., Brandt C. D., Kim H. W., Baltimore R. S., Parker C. L., Artenstein M. S. Coxsackievirus B5 infection and aseptic meningitis in neonates and children. Am J Dis Child. 1975 Mar;129(3):321–325. doi: 10.1001/archpedi.1975.02120400031007. [DOI] [PubMed] [Google Scholar]
- Middleton P. J., Szymanski M. T., Petric M. Viruses associated with acute gastroenteritis in young children. Am J Dis Child. 1977 Jul;131(7):733–737. doi: 10.1001/archpedi.1977.02120200015004. [DOI] [PubMed] [Google Scholar]
- Parrott R. H., Kim H. W., Arrobio J. O., Hodes D. S., Murphy B. R., Brandt C. D., Camargo E., Chanock R. M. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973 Oct;98(4):289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- Paul M. O., Erinle E. A. Influence of humidity on rotavirus prevalence among Nigerian infants and young children with gastroenteritis. J Clin Microbiol. 1982 Feb;15(2):212–215. doi: 10.1128/jcm.15.2.212-215.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Bishop R. F., Holmes I. H. Detection of a rotavirus-like agent associated with diarrhea in an infant. J Clin Microbiol. 1982 Oct;16(4):724–726. doi: 10.1128/jcm.16.4.724-726.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez W. J., Kim H. W., Brandt C. D., Bise B., Kapikian A. Z., Chanock R. M., Curlin G., Parrott R. H. Rotavirus gastroenteritis in the Washington, DC, area: incidence of cases resulting in admission to the hospital. Am J Dis Child. 1980 Aug;134(8):777–779. doi: 10.1001/archpedi.1980.02130200047015. [DOI] [PubMed] [Google Scholar]
- Takiff H. E., Straus S. E., Garon C. F. Propagation and in vitro studies of previously non-cultivable enteral adenoviruses in 293 cells. Lancet. 1981 Oct 17;2(8251):832–834. doi: 10.1016/s0140-6736(81)91104-1. [DOI] [PubMed] [Google Scholar]
- Thouless M. E., Beards G. M., Flewett T. H. Serotyping and subgrouping of rotavirus strains by the ELISA test. Arch Virol. 1982;73(3-4):219–230. doi: 10.1007/BF01318076. [DOI] [PubMed] [Google Scholar]
- Wadell G., Hammarskjöld M. L., Winberg G., Varsanyi T. M., Sundell G. Genetic variability of adenoviruses. Ann N Y Acad Sci. 1980;354:16–42. doi: 10.1111/j.1749-6632.1980.tb27955.x. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Lawrence F., Leister F., Takiff H. E., Strauss S. E. Gastroenteritis associated with enteric type adenovirus in hospitalized infants. J Pediatr. 1982 Jul;101(1):21–26. doi: 10.1016/s0022-3476(82)80173-x. [DOI] [PubMed] [Google Scholar]


