Abstract
The RNA-binding protein, HuR, is involved in the stabilization of AU-rich element-containing mRNAs with products that are involved in cell-cycle progression, cell differentiation and inflammation. We show that there are multiple polyadenylation variants of HuR mRNA that differ in their abundance, using both bioinformatics and experimental approaches. A polyadenylation variant with distal poly(A) signal is a rare transcript that harbors functional AU-rich elements (ARE) in the 3′UTR. A minimal 60-nt region, but not a mutant form, fused to reporter-3′UTR constructs was able to downregulate the reporter activity. The most predominant and alternatively polyadenylated mature transcript does not contain the ARE. HuR itself binds HuR mRNA, and upregulated the activity of reporter from constructs fused with ARE-isoform and the HuR ARE. Wild-type tristetraprolin (TTP), but not the zinc finger mutant TTP, competes for HuR binding and upregulation of HuR mRNA. The study shows that the HuR gene codes for several polyadenylation variants differentially regulated by AU-rich elements, and demonstrates an auto-regulatory role of HuR.
INTRODUCTION
Transcriptional and post-transcriptional regulation mechanisms ensure tight control of gene expression in many physiological and cellular processes, for example, during responses to external stimuli and stress. Post-transcriptional regulation contributes significantly to the rapid and transient nature of these biological processes, and comprises multiple steps including RNA processing, export, localization, degradation and translation. The RNA-binding proteins play key roles in each of these processes. The stability of the mRNA, which is an important regulatory step, can be modulated by inactivation of RNA decay-promoting proteins. Examples of those are the zinc finger protein, tristetraprolin (TTP) (1), K-homology splicing-regulatory protein (KSRP) (2), butyrate response factor 1 (3) and certain gene products of the AU-rich element (ARE)/poly(U)-binding/degradation factor 1 (AUF1) (4). The mRNA stability can be enhanced by the activity of mRNA stabilization-promoting proteins such as human antigen R (HuR).
HuR is a member of the mammalian homologs of embryonic lethal abnormal vision (ELAV) proteins comprising a group of RNA-binding proteins first described in Drosophila (5). The HuR gene is localized to human chromosome 19p13.2 (6) and according to the GenBank RefSeq database, the reference HuR transcript encodes a 6-kb mRNA derived from six exons, evidence of other variants is presented in this paper. The HuR protein is composed of 326 amino acids. It is predominantly nuclear, but translocates to the cytoplasm upon cellular activation, binds selected mRNAs and causes their stabilization (7). HuR and other members of ELAV proteins possess three RNA-recognition motifs through which they bind with high affinity to specific mRNAs bearing AU- and U-rich sequences affecting their stability and/or translation (8–12). HuR is implicated in the stabilization of a number of ARE- and U-rich mRNAs such as those involved in cancer and inflammation, for example, COX-2, urokinase activator (uPA), certain chemokines such as IL-8, Cyclins A, B, D and p21 (10,12–15). Large-scale analysis involving profiling of mRNAs that are bound to HuR protein, reveals that the repertoire of HuR mRNA targets are not limited and contain many previously unrecognized mRNAs (11,16,17).
Using the whole transcriptome bioinformatics approach, we and others have previously found that there are many genes that code for alternative polyadenylation variants in which they exist as long 3′ untranslated region (3′UTR) comprising ARE isoforms and shorter non-ARE isoforms (18,19). The AREs which largely exist in the 3′UTR comprise different classes, ranging from highly homogeneous overlapping repeats of AUUUA to heterogeneous AU- and U-rich sequences (20). They are among the most important mRNA decay determinants and promote deadenylation of several ARE-mRNAs both in vivo and in vitro (66) and also promote decapping (67). Following deadenylation, the main degradation pathway seems to involve 3′ to 5′ exonuclease activity (68). Thus, the presence of ARE alternative forms allows differential regulation of mRNA decay.
The sequence-functional characteristics of the HuR 3′UTR which harbors different polyadenylation signals and their role in alternative polyadenylation are not known. This study provides evidence that the RNA binding protein, HuR, gene codes for several alternative polyadenylation variants that results in their differential expression patterns, ARE involvement, and response to HuR itself. We found that the 6-kb alternative polyadenylation transcript is a rare transcript, and determined a functional ARE mRNA decay element that is subject to binding and auto-regulation by HuR. Moreover, we found that TTP can compete for HuR binding to its own HuR mRNA. Several cancers, including breast, colon, gastric, prostate and ovarian cancers, have been linked to over-expression of HuR and increased cytoplasmic localization, when compared to normal tissues (21–25). Thus, understanding HuR regulatory pathways may lead to further understanding of the role of HuR in disease, particularly in cancer and inflammation.
MATERIALS AND METHODS
Cell lines and transfection
HEK293 and HeLa cell lines were obtained from American Type Culture Collection (ATCC; Rockville, MD, USA) and cultured in RPMI 1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS and antibiotics. Huh-7 cell line was obtained from Dr. Stephen Polyak (University of Washington, Seattle, WA, USA) and was propagated in DMEM medium with 10% FBS and antibiotics. HEK 293 cells were transfected with pcDNA3.1 vector, pcDNA3.1 containing full-length human cDNA for HuR (kindly provided by Dr. Christopher Moroni, Basel, Switzerland). The wild type TTP and C124R mutant of CMV.hTTP.tag vectors were obtained from Dr. Perry Blackshear (NIH) and subcloned into pCR3.1 vector (pcDNA 3.1 equivalent vector for TA overhang cloning). Transfections were performed in medium without serum using Lipofectamine 2000 (Invitrogen) for 6 h, followed by replacing the medium with serum-supplemented medium. Transient transfections were continued for 18 h.
RNA preparation and northern blot analysis
Total RNA was extracted using Tri Reagent (Molecular Research Center, Cincinnati, OH, USA) and 15 µg of each sample was separated by electrophoresis through a 1.2% agarose/2.2 M formaldehyde gel. Northern blotting was performed with cDNA probes specific for HuR or β-actin mRNA. The probes were labeled with [α-32P]dCTP (Amersham) using a nick translation kit (Invitrogen) and used for the standard northern blotting technique, also previously described (26). RNA signals were measured with a densitometer (Bio-Rad) and Image analysis software (Amersham).
In later experiments, we switched to non-radioactive labeling for the northern blotting. The EGFP sequence was amplified using specific primers to EGFP in which the reverse primer has T7 promoter sequence. The sequence for the PCR primers are: forward 5′ CTACTCAGACAATGCGA TGCA 3′ and the Reverse primer is 5′ CCGAATTAACCCTCACTAAAGGG AGAGCGCAAGAAATGG CTAG 3′. The PCR product was used to generate the DIG-labeled single-stranded antisense RNA probe by in vitro transcription using T7 RNA polymerase (Roche diagnostics, Germany). The DIG label is detected by anti-DIG-AP conjugate with chemiluminescent substrate CSPD (Roche) on northern membranes as recommended by the manufacturer.
EST clustering
Clustering of ESTs found in ELAVL1 Unigene record, Hs.184492, was performed using SEQMAN II (DNASTAR, Inc., Madison, WI, USA). Initially, the ESTs were pre-processed to remove sequences less than 100 bases, vector sequences (word size = 10 bp, at least 90% identity), and to trim low-quality ends by removing ends based on a minimum number of two ambiguous residues (Ns) within a sliding window of 50 bases. Fragment assembly in SEQMANII was used to cluster the ESTs using the following parameters (match size of 50 consecutive bases bp and at least 90% identity), maximum added gap per kilobase cluster is 50 bp, maximum register is 30 bp, gap penalty is 0.5 and gap length penalty is 1.0). No chimeras’ contigs were observed. Consensus calling of the clusters was with 75% as the minimum percentage of identical residues at each position required to identify the consensus base at this position using Trapezoidal Weights.
3′UTR PCR, construction and cloning of reporter plasmids
Regions that contain putative ARE and ARE-like sequences which correspond to the partial 3′UTRs of HuR [NM_00419; 1146–2359 nt for the 2.7-kb mature transcript and 1146–2920 nt for ARE-containing 6-kb transcript and urokinase activator (NM_002658; 2161–2301 nt)] were obtained by RT-PCR. Briefly, total RNA was extracted by Tri Reagent from LPS-induced THP-1 cell line. The cDNAs were amplified by PCR in which the forward primer contains BamHI site (underlined) and the reverse primer with XbaI site (underlined) as follows: The HuR 3′UTR mature mRNA region (2.7-kb mRNA) was amplified by: forward primer: 5′ CAGCAGGGATCC TAACTCGCTCATGCTTTTTTTTG 3′. Reverse primer: 5′ CGACCTCTAGAATTCGAGCAAA ACAAAATC 3′. The HuR 3′UTR of 6-kb transcript was amplified using the following primers: Forward primer: 5′ CAGCAGGGATCCTAACTCGCTCATGCTTTTTTTTG 3′ and the reverse primer: 5′ CGACCTCTAGACACAGCCCCTCA GTAAAAGA 3′. The uPA 3′UTR forward and reverse primers are as follows: CAGCAGGGATCCCACTGTCTCAGTTTCACTTT 3′ and 5′ CGACCTCTAGACATCAGAAAAATCACATTTTATTG 3′. The IL-8 3′UTR forward and reverse primers are as follows: 5′ GCACCGGATCCGATGTTGTGAGGACATGTG 3′ and the reverse primer with XbaI site (underlined): 5′ GCCAGTCTAGAACCCTGATTGAAATTTAT 3′. The PCR products were purified, precipitated, and cut by BamHI and XbaI sequentially, and subsequently ligated into EGFP plasmid (Gene Therapy Systems, Inc., San Diego, CA, USA) which is under CMV/Intron A constitutive promoter. The 60-nt ARE spanning region of HuR and uPA and their mutant forms were cloned in the expression vectors as duplex with BamHI and XbaI overhangs. The COX-2 3′UTR 130-nt region (NM_000963; 1954–2083) was made as duplex oligonucleotide. These short double-stranded DNA were made by annealing two synthetic complementary oligonucleotides. After ligation and transformation, DNA from recombinant colonies was verified by PCR using a forward vector specific primer and 3′UTR or ARE reverse primer.
Reporter transfection and activity assessment
Reporter constructs containing GFP-3′UTR/ARE were used in transient transfection at 25 ng per 2 × 104 cell/well in 96-well microplates. Transfection was performed with Lipofectamine 2000. In co-transfection experiments the expression plasmids of HuR or TTP and mock (pcDNA 3.1/pCR3.1) were kept constant as indicated. Transfection efficiency and normalization to control was achieved using GFP reporter fused with stable BGH 3′UTR. The intra-well variance of any replicate groups in fluorescence is generally <6%, which does not warrant intra-well normalization of transfection (27). Fluorescence was quantitated using a sensitive bottom read instrument ZENYTH 3100 (Anthos Labtec, Eugendorf, Austria). Data are presented as mean value ± SEM of fluorescence intensity. For comparison between two groups (columns on figures) the student paired t-test was used. Two-tailed probabilities were reported. ANOVA was used to comparison with more than two groups of data. Densitometry, band detection, background subtraction and normalization of images were performed using ImageMaster Software (Amersham).
Real-time RT-PCR, and half-life determinations
For real-time measurements of half-lives of EGFP reporters generated from constructs with HuR 3′UTRs, primers and TaqMan probe specific to EGFP were custom synthesized by PE Applied Biosystems. The primers span the CMV promoter intron A to control DNA contamination. The 6-carboxyfluorescein (6FAM)-labeled TaqMan probe that target CMV exon 1-EGFP (exon 2) junction sequence was used. The probe design allowed further control of DNA contamination. The control RPL0 mRNA probe is labeled with a 5′ reporter VIC dye (Applied Biosystems). Cell-culture experiments for the mRNA half-life determination were incubated with increasing duration of actinomycin D treatment (5 μg/ml) followed by total RNA extraction and cDNA synthesis. The real-time PCR was run on Chroma 4 thermocycler instrument (BioRad). Standard curves for each gene were generated to determine the relative concentrations of amplified transcripts. The concentration of each transcript was then normalized to RPL0 mRNA levels and the normalized values were used to calculate the half-lives.
Immunoprecipitation of HuR mRNA species
Pre-swollen protein A/G–Sepharose beads (Santa Cruz) were pre-coated with 30 ug of goat IgG (Santa Cruz), mouse IgG (Santa Cruz), anti-HuR (Santa Cruz) or anti-RNase L monoclonal antibody (Novus Biologics) in NT2 buffer [50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1 mM MgCl2 and 0.05% NP-40]. The beads were washed with NT2 buffer and incubated (2 h, 4°C) with cell lysates in 1 ml of NT2 buffer supplemented with DTT and RNAseOut (Invitrogen). The beads were washed and further treated with 100 µl NT2 buffer that contains 10 U RNase-free DNase I to remove genomic DNA contamination, if any, for 15 min at 30°C, washed with NT2 buffer and further incubated in 100 µl NT2 buffer containing 0.1% SDS and 0.5 mg/ml proteinase K (55°C, 20 min). RNA was isolated and used for semi-quantitative RT-PCR. For real-time quantitative RT-PCR for HuR mRNA, primer pair/TaqMan probes (Applied Biosysems) were used in the same manner as above.
Semi-quantitative RT-PCR
RT reaction was performed using 200 ng total RNA, 500 ng oligo dT(18–23), 500 mM dNTP mixture, 20 U RNAsin (Pharmacia), 200 U of SuperScript II (Invitrogen). Hot start PCR amplification was performed using Taq DNA polymerase (Qiagen). cDNA was amplified according to an amplification curve, for example, β-actin mRNA and HuR mRNA were amplified at 28 cycles and 36 cycles, respectively. The amplification curve was determined for each cDNA of interest by plotting increasing cycle numbers against ethidium bromide stained gel intensity of the amplified products. The optimum cycle number chosen is within the exponential phase of the curve. Cycling was 94°C for 60 s, 60°C for 60 s and 72°C for 60 s using primers specific to, GFP, β-actin, HuR intron, HuR exon or the last portion of terminal exon 6 (6-kb transcript). The GFP forward primer is 5′ GAAGCGTTCAACTAGCAGACC A3′ and GFP reverse primer is 5′ CCATGCCATGTGTAATCCCAG 3′. The β-actin forward primer is 5′ ATCTGGC ACCACACCTTCTACAATGAGCTGCG 3′; β-actin reverse primer: 5′ CGTCATACTCCTGCT TGCTGATCCACA 3′. The β-actin primers were designed to span short intronic sequences so that the larger PCR products would be shown in case there was genomic DNA contamination. The sequence of HuR exon 3/4 specific primers are as follows: forward primer, 5′ AACACGCTGAACGGCTTGAGGCT 3′ and reverse primer, CCGCCCAAACCGAGAGA ACAT 3′. The PCR using the exon 3/4 primers allows the specific amplification of short PCR products (158 bases) representing the HuR mRNA transcript but not the pre-mRNA, since the latter would give 7.4 kb that is not amplified with the PCR conditions that use an extension time of 60 s. In other words, the PCR is not a long-distance PCR. The forward primer for HuR intron (intron 3) is: 5′ CAGCGGCAGGTGACTTGAGGAA and the reverse primer is: 5′ GCCCACGAACCCAGGAGCCAGAA 3′. The intron specific primers allow monitoring of DNA contamination in absence of RT. The PCR for the distal portion of exon 6, which exists also in the 6-kb transcript variant, utilized the following primers: The forward primer is: 5′ CCC AGAGCAGGTCAGCGTCTC and the reverse primer is: 5′ GGTGCTACAAGCCCG TCATCA 3′. In all of the above, PCR conditions allowed at least semi-quantitative comparisons of signal strength on agarose gels as the cycle number was chosen in the exponential range which has not reached the plateau. These comparisons were validated by generating PCR products using different cycles in which the signal strength was within linearity up to 32 cycles for both GFP and β-actin control and up to 38 cycles for HuR mRNA following IP-RNA.
RNA EMSA, supershift and in vitro HuR translation
The sense RNA oligonucleotides corresponding to HuR ARE and mutant ARE (Figure 3D) were custom synthesized (Metabion) which were 5′end-labeled with biotin and HPLC purified. Unlabeled competitor oligonucleotides were also synthesized. Ten femtomoles of the biotin-labeled sense probe was incubated with 5 µg of total cell lysate for 30 min at room temperature in 20 µl of binding buffer containing 10 mM Tris–HCl (pH 7.5), 50 mM KCl, 1 mM DTT, 1 µg of tRNA. Five microliters of 5× loading buffer was added to the mixtures. Following nondenaturing polyacrylamide gel electrophoresis, gel was transferred onto positively charged nylon membrane (Amersham), and UV-cross linked. The membrane was then subjected to detection by chemiluminescent EMSA kit (Pierce) following the manufacturer's protocol. Competition assays were performed in the same manner, except that 1000-fold excess (10 pmol) of mutant or unlabeled competitor ARE RNA were pre-incubated with protein extract for 30 min at room temperature before the addition of the 5′-biotinylated probe. The RNA EMSA was also verified by recombinant HuR. Briefly, HuR cDNA clone was transcribed and translated using T7 RNA polymerase and the TNT-coupled reticulocyte lysate system (Promega, USA) according to the manufacturer's protocol. Reaction components included (0.5 µg) human HuR cDNA plasmid and 24 µl TNT reticulocyte T7 master mix in a final volume of 25 µl. The reaction mix was incubated at 30°C for 1.5 h. The supershift assay was carried in the same manner, except that 2 µg of anti-HuR antibody (sc-5483) (Santa cruz) was pre-incubated with HEK293 protein extract for 30 min before the addition of the labeled probe.
Figure 3.
Differential Involvement of AU-rich elements in HuR polyadenylation variants. (A) Schematic diagram of exon 6 of HuR that contains ARE and reporter constructs. Sequences from the EEF1A1 3′UTR (Control, construct 1), 2.7 kb mRNA 3′UTR (construct 2), 6-kb transcript 3′UTR (construct 3), uPA 3′UTR (construct 4), COX-2 3′UTR (construct 5) and IL-8 3′UTR (construct 6) were inserted in BamHI/XbaI sites as described in ‘Materials and Methods’ section. (B) Analysis of GFP reporter activity generated from GFP mRNA fused with the indicated 3′UTR. HEK293 cells were transfected with the different 3′UTR constructs in 96-well microplates. Reporter activity was assessed in 96-well black clear-bottom plates by measuring bottom fluorescence using bottom-read fluorometer. The EEF1A1 3′UTR is the control construct in which its fluorescence activity was taken as 100% and data are presented as mean ± SEM (n = 4) of % of the control. ***Denote P-values of <0.01 and <0.005, respectively (Student's t-test). Percent transfection efficiency in the control was 80% with a coefficient of variation of <6%, thus, normalization with another vector was not needed (27). (C) Analysis of GFP mRNA levels by quantitative real-time RT-PCR. HEK293 cells were transfected with the different 3′UTR constructs in 6-well plates. Total RNA was extracted and real-time quantitative RT-PCR was performed using primers and Taqman probe specific to GFP reporter sequence as described in ‘Materials and methods’ section. The fluorescence activity from control (EEF1A1) 3′UTR-fused construct was taken as 100%. Data (mean ± SEM) was presented as percentage of control. (D) A 60-nt double-stranded oligonucleotide corresponding to the ARE regions from HuR 6-kb 3′UTR, uPA 3′UTR and control stable growth hormone (GH1) 3′UTR were fused with EGFP reporter construct. Furthermore, 60-nt double-stranded oligonucleotides as mutant forms of the HuR ARE and uPA were fused with GFP reporter construct (sequences of ARE and their mutants are shown). These constructs were used for HEK293 transfection. Percentage of control 3′UTR (EEF1A1)-linked construct fluorescence activity (100%) are presented as mean ± SEM (n = 4). ***Denote P-values of <0.005 (Student t-test).
RESULTS
Bioinformatics evaluation of alternative polyadenylation of HuR mRNA
The HuR gene structure and the polyadenylation variants are depicted in Figure 1A. The HuR gene is composed of five introns and six exons. The coding region is in exons three, four and five, and part of exon 6 (324 nt of the total 5234-nt 3′UTR), while the majority of exon 6 is comprised of long 3′UTR (42163–47073). The detailed sequence information is found in Supplementary Figure 1. According to the EST data (below) and NCBI assembly, there are no alternative spliced transcripts derived from the HuR gene. However, the presence of multiple putative poly(A) signals in the 3′UTR, which is encoded in exon 6 (Figure 1A), suggests the presence of alternative polyadenylation variants. Thus, we have carried both bioinformatics and experimental analysis to investigate HuR alternative polyadenylation.
Figure 1.
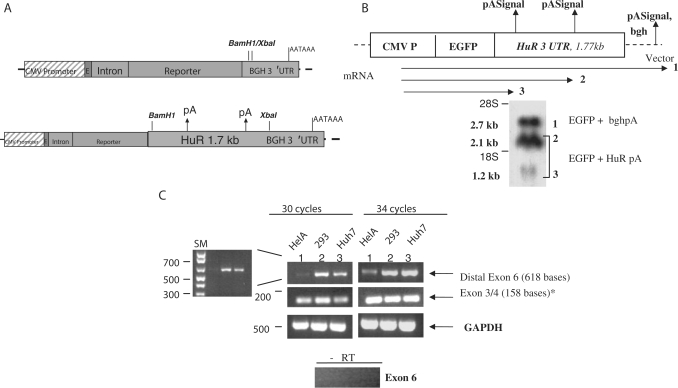
Bioinformatics and mRNA species assessment of HuR gene. (A) A gene structure for HuR (ELAVL1). Exons are denoted by the green boxes while the red box denotes the terminal exon that harbors the entire 3′UTR of HuR and their polyadenylation variant signals. Positions for the specific primers used in this study are shown: for the pre-mRNA (intron; blue arrows), terminal sequences in exon 6 (6-kb transcript, black arrows) and exon 3/4 (green arrows). Positions of putative pA signals leading to alternative polyadenylation are shown (vertical arrows). pA denotes polyadenylation signal. (B) EST clustering strategy view of the ESTs belonging to the HuR. The top cluster represents ∼70% of the 460 ESTs from Unigene Cluster (Hs.184492). The dark inner lines represent individual ESTs in the EST assembly. The bottom cluster represents 23% of the total ESTs in the assembly (individual ESTs are not shown). (C) Total RNA was extracted from HeLa cells and used for northern blotting with cDNA probes to HuR and β-actin mRNA. Arrows show the two different mRNA species as assessed by size markers. Lower panel showed the three mRNA variants with over-exposed autoradiography.
We first evaluated the HuR mRNA species by bioinformatics. Using a refined EST clustering approach (18), we clustered 460 ESTs. This resulted in a predominant transcript of 2711 bases (68% of all the ESTs; Figure 1B) and a smaller transcript of 1463 bases of lesser abundance (23% of all ESTs). Both match the size of the mRNA species in northern blot from HeLa cell line (Figure 1C). Both of the transcripts (2.7- and 1.5-kb variants) have ATTAAA as proximal poly(A) signal and, as middle poly(A) signals in the 2.7-kb variant (Supplementary Figure 1), these signals terminate at the 3′ends within the last 20 nt of the poly(A) site (Figure 1B). Thus, the two mRNAs are likely alternative polyadenylation variants. There was no EST cluster for the reported 6-kb RefSeq mRNA that may represent the longest and rare polyadenylation variant. The lack of an EST contig consensus for the 6-kb mRNA is likely due to the nonmerging of the long 6-kb mRNA 3′UTR with the 5′ends of the EST. Thus, we examined the individual EST that shows the presence of exon six terminal sequence to be only 4.3% of the 3′EST records (163 records; Supplementary Table 1) in which the distal poly(A) signal, AATAAA is within the last 20 nt. The rare long ARE-positive 6 kb can only be seen in northern blot only after using a radioactively labeled cDNA probe against HuR mRNA with extended exposure (Figure 1C, lower panel). Furthermore, a weakly expressed 6-kb transcript in brain tissues was previously documented in northern blots by another group (7).
Experimental evidence of HuR mRNA alternative polyadenylation variants
The polyadenylation variants were further validated by expressing enhanced green fluorescence protein (EGFP) coding region fused with 1.77-kb 3′UTR of HuR that contains the two putative poly(A) signals, i.e. the proximal and the middle poly(A) signals (Figure 2A). The EGFP-HuR 3′UTR construct produced two alternative polyadenylation variants that corresponded to the expected sizes of the bioinformatics-derived transcripts and northern blotting (Figure 2B). Furthermore, the variants also corresponded to similar abundance levels as determined by bioinformatics and viewed in the northern blots (Figure 1). The most predominant band is the EGFP mRNA with the middle poly(A) signal (2.1 kb of GFP mRNA), which corresponds to the natural HuR alternative transcript of 2.7 kb.
RT-PCR experiments with primers specific to the 6-kb form (distal part of exon 6) showed the presence of the 6-kb form in addition to other mRNAs (exons 3 and 4) in three different cell lines (Figure 2C). RT-PCR using exons 3 and 4 primers do not amplify genomic DNA because the PCR conditions do not favor the long genomic region of >7.2 kb. In accordance with the northern blotting, the semi-quantitative RT-PCR experiments with two different cycle numbers within exponential phase indicate that the 6-kb mRNA levels are lower than those of all HuR mRNA variants (Figure 2C). Based on all of above bioinformatics and experimental analyses, it is concluded that the mature mRNA of 2.7 kb is the most predominant transcript which is also the most abundant alternative polyadenylation variant and 6-kb transcript is the rarest variant.
Figure 2.
Experimental validation of the HuR polyadenylation variants. (A) Cloning of 1774 nt of 3′UTR of HuR mRNA that contains the putative two poly(A) signals into EGFP expression vector that is under the control of CMV IE promoter and stable bovine growth hormone 3′UTR/poly(A) signal. (B) HEK393 cells were transfected with the EGFP construct in (A) using Lipofectamine 2000 for 16 h. Northern blotting was performed using antisense probe to EGFP. Numbers denote the expected length of the polyadenylation variants of EGFP-HuR 3′UTR mRNAs (lanes 2 and 3) and EGFP-BGH 3′UTR transcripts (lane 1)—denote positions for the 18S (∼2 kb) and 28S rRNA (∼5 kb) bands. (C) DNase-treated total RNA extracted from three different cell lines (1, HeLa; 2, HEK293; and 3, Huh7) were used. RT-PCRs were performed for HuR gene products encoding the 6-kb transcript (distal polyadenylation variant; black-colored arrows in Figure 1A) and exon 3/4 containing forms (all polyadenylation variants, green-colored arrows in Figure 1A) using specific primers as outlined above. *These primers do not amplify intervening introns between exons 3 and 4 (7.2 kb) since RT-PCR conditions were performed with short extension times. RT-PCR control without RT to monitor genomic contamination was used with intron-specific primers.
Analysis, cloning and functional validation of HuR 3′UTR AU-rich elements
Analysis of 3′UTR (exon 6) of the HuR mRNA transcripts indicated that the mature predominant mRNA (2.7 kb), which represents the middle polyadenylation variant, lacks typical ARE that act as mRNA decay determinants, although they have potential U-rich stretches (dotted lines, Figure 3A). There is a typical ARE stretch (Figure 3A; Supplementary Figure 1) that exists in the rare 6-kb transcript variant, which has distal poly(A) signal; this ARE may be responsible for the rare presence of the transcript. The ARE is a typical class 2 ARE or cluster II (28) having four overlapping pentamers with only one mismatch. Accordingly, we carried out experiments that aim to understand the role of HuR 3′UTR and these AREs in HuR regulation.
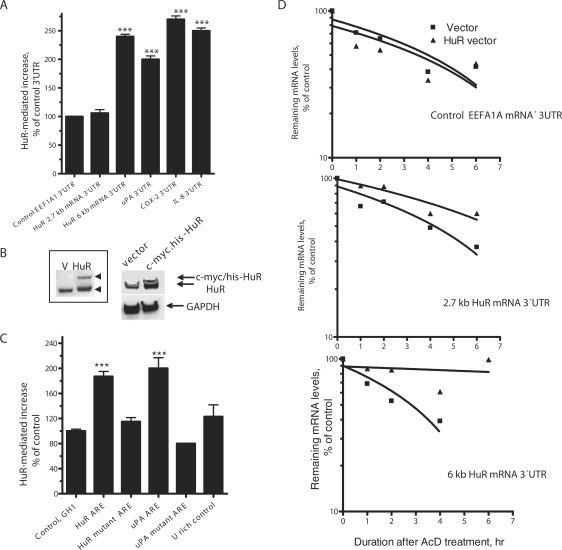
We have cloned two 3′UTR sequence regions, one sequence region (1213 nt) contains the proximal and middle poly(A) signals and does not harbor the ARE, and thus represents the most abundant 2.7-kb transcript variant. The second region (1774 nt) includes further downstream sequences that contain the typical ARE stretch which only exists in the 6-kb transcript (Figure 3A and Supplementary Figure 1). We fused the 3′UTR to an EGFP coding region as a reporter expression system. This system in our hands is sensitive, with minimal (mostly, <6%) variation in transfection efficiency, has a large dynamic linear range (27); and can be performed on live cells. Several known ARE-bearing 3′UTRs were also used in these experiments. The ARE-bearing 3′UTR sequences of the 6-kb transcript, but not that of the control EEFA1A 3′UTR or the short HuR 3′UTR of the 2.7-kb transcript that lacks the ARE, led to reduction of GFP reporter (70% reduction) from the fused constructs (Figure 3B). Likewise, the ARE-bearing 3′UTR of known mRNAs (uPA, COX-2 and IL-8) led to significant reduction (60–70%) of the reporter activity (Figure 3B). The EEFA1A 3′UTR sequence used (210 nt) acts as a control for the uPA, COX-2 and IL-8 3′UTR regions that were cloned; all are within close size range of 130–220 bases. We considered the HuR non-ARE 3′UTR (1.2 kb) as a control for the ARE-bearing transcript HuR 3′UTR (1.7 kb) for two reasons; first, housekeeping stable 3′UTRs tend to be of smaller sizes, average length of 650 ± 30 (27,29,30), and thus cannot be used as control for long 3′UTRs; and second, the reporter activity expressed from 1.2-kb non-ARE HuR 3′UTR construct did not change from the EEFA1A control (Figure 3B).
The 3′UTR sequence of the 6-kb transcript that contains the ARE strongly destabilized the reporter mRNA; there was 80% reduction in the mRNA levels when compared to the control activity (Figure 3C). In contrast, the 3′UTR of the 2.7-kb transcript (Figure 3C) did not lead to an appreciable or statistically significant reduction either in the reporter activity or in mRNA levels. Thus, class 2 ARE-containing 3′UTR of the 6-kb transcript, but not the 3′UTR of the 2.7-kb abundant transcript, was functional in regard to the mRNA destabilization activity. As a control, the uPA 3′UTR caused significant reduction of the reporter mRNA levels.
In order to confirm the genuine involvement of the AREs themselves, rather than the 3′UTR as a large region in the 6-kb transcript, we fused the reporter with a 60-nt region that spans the putative ARE in the 6-kb transcript (Supplementary Figure 1), and also with the same sequence but with discrete mutation of the pentamers (Figure 3D, insert table). Many functional ARE regions that respond to both destabilization and stabilization signals, particularly those of class 2 ARE, were shown within the length of 60 nt (31). The AREs were able to cause significant reduction in the reporter activity, down to 20% of the control 3′UTR that has 60 nt of human growth hormone GH1 non-ARE sequence (Figure 3D). As a control, the mutant ARE of the same 60-nt length inserted in the wild-type 3′UTR was not able to trigger reduction in the reporter activity (Figure 3D). As a positive ARE control, a 60-nt region of uPA, 3′UTR caused significant reduction in the reporter activity.
HuR regulation of HuR 3′UTR and ARE
We next investigated the effect of transient transfection of HuR-c-myc-his cDNA expression vector into HEK293 cells on the EGFP reporter expression from the reporter constructs fused with the different HuR 3′UTR and other control 3′UTR sequences (Figure 4A). Western blotting confirmed the expression of the transfected c-myc-his HuR cDNA, which has larger weight compared to the endogenous HuR (Figure 4B). We found that HuR over-expression can preferentially increase the activity of the reporter gene fused with the ARE-bearing 6-kb transcript 3′UTR sequence (2.3-fold; P < 0.01), compared to the activity of reporter fused with abundant 2.7-kb transcript 3′UTR (1.4-fold) or control EEFA1A 3′UTR sequence (Figure 4A). The uPA (13), COX-2 and IL-8 3′UTR, as positive controls were also responsive to HuR in a similar degree (Figure 4A). To determine if the minimal 60-nt region was responsible for HuR-induced upregulation of the reporter activity, we used the reporter constructs in which the 3′UTR contains only the 60-nt ARE region. Over-expression of HuR caused a specific increase (1.9-fold, P < 0.001) in the activity of the reporter fused with HuR 60-nt ARE derived from the 6-kb poly(A) variant transcript, but not with the mutant sequence (Figure 4C). In addition, as a positive control, HuR caused increase of the reporter activity from the construct fused with uPA 60 nt but not the mutant sequence (Figure 4C). Use of a U-rich 60-nt control, randomly selected from a library of U-rich sequences (our unpublished data) that lacks an HuR recognition site (17), was unresponsive to HuR (Figure 4C). As a negative control, GH1 60-nt sequence was not responsive to HuR over-expression.
Figure 4.
Effect of HuR over-expression on HuR 3′UTR and HuR ARE. (A) HEK293 cells in 96-well plates were co-transfected with 37 ng pcDNA 3.1 empty vector or HuR-c-myc-his expressing pcDNA 3.1 vector and 25 ng of each of EGFP-3′UTR reporter vectors as shown. The reporter 3′UTR constructs were described in Figure 3A. Data mean ± SEM of quadruplicates presented as % of reporter fluorescence with control EEF1A1 3′UTR taken as 100%. Percent transfection efficiency in the control was 80% with a coefficient of variation of <6%, thus, normalization with another vector was not needed (27). (B) Representative western blotting confirming expression of the transfected c-myc-his-HuR cDNA is shown. Inset is a longer gel run for larger separation of endogenous and transfected HuR. (C) HEK293 cells in 96-well plates were co-transfected with reporter vectors and either pcDNA3.1 (control) or HuR expression pcDNA3.1. The reporter vector is EGFP fused with 3′UTR that contains a 60 nt corresponding to the ARE regions from HuR the 6-kb variant 3′UTR, uPA 3′UTR, control GH1 sequence, a 60 nt as mutant form of the HuR 6-kb ARE (Figure 3D, inset table) and another 60 nt representing U-rich control that lacks HuR binding site (17). Fluorescence data of mean ± SEM of quadruplicates were obtained. The fluorescence levels obtained from cells transfected with the pcDNA3.1 and the reporter with control GH1 3′UTR were taken as 100%. The y-axis numbers represent percentages of this control. ***Denotes P-values of <0.001 (Student t-test) when compared to control. (D) HEK293 cells were transfected with the EEF1A1 3′UTR (Control), 2.7-kb mRNA 3′UTR, 6-kb transcript 3′UTR (constructs 1–3, Figure 3A) overnight and then treated with AcD (5 μg/ml). Total RNA was extracted at the indicated time points and subjected to RT-PCR using primer pair/TaqMan probe specific to EGFP mRNA. The TaqMan primer/probe spans an intron–exon-junction sequence for efficient control of DNA contamination. Half-life mRNA determinations were obtained by plotting the changes in RPL0-normalized EGFP mRNA levels expressed as a percentage of that seen at time 0 (taken as 100%; y-axis) against AcD treatment duration (x-axis). RPL0 is a housekeeping gene. Data are from one representative experiment of at least two independent experiments. Details are given in ‘Materials and Methods’ section.
Further, we performed half-life experiments using real-time RT PCR to measure the effects of HuR over-expression. The half-life of the reporter mRNA was similar in case of the control EEFA1A 3′UTR in the presence or absence of HuR over-expression (∼4 h), whereas, the half-life of the reporter mRNA released from the 6-kb HuR mRNA sequence (2.5 h) increased and become stabilized due to HuR over-expression (>8 h; Figure 4D). The half-life of the short HuR mRNA transcript sequence did not significantly change due to HuR over-expression (Figure 4D).
HuR binding to HuR mRNA
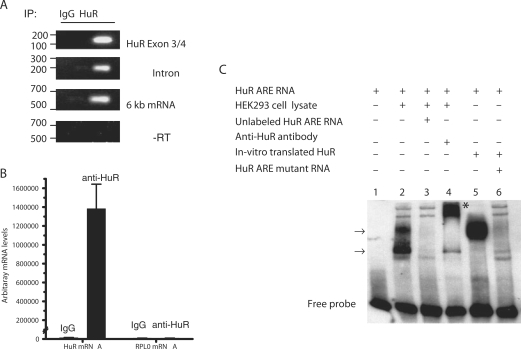
Since HuR is known to bind and stabilize ARE-mRNAs, including their pre-mRNA forms, we examined the binding of HuR to HuR mRNA and the ARE-containing polyadenylation 6-kb transcript. An immunoprecipitation (IP) assay was performed under conditions that preserved endogenous ribonuclear protein associations (11). An RT-PCR of the IP RNA material obtained after anti-HuR or IgG control was performed using primers specific for exon 3/4. The exon 3/4 amplicon (green-colored arrows in Figure 1A,) represents all transcripts of HuR mRNA. HuR interacted with at least one isoform of the HuR mRNA (Figure 5A, top panel). To pinpoint specifically whether the 6 kb or the pre-mRNA was bound to HuR, we performed RT-PCRs of the IP RNA material obtained after anti-HuR or IgG control, using primers specific to the 6-kb transcript or HuR pre-mRNA (intron). We found that HuR interacted with the 6-kb transcript and pre-mRNA forms (Figure 5A, middle and lower panels). The RT-PCR specific bands are not due to genomic contamination, since RT-PCR without RT did not yield any bands.
Figure 5.
Binding of HuR protein to HuR mRNA and consequences of HuR over-expression. (A) The binding of endogenous HuR to endogenous target mRNA was detected by RT-PCR of RNA material obtained by precipitation with either anti-HuR antibody or goat IgG control antibody. Primers specific to the intron (pre-mRNA), distal part of exon 6 (6-kb transcript) or all mRNA (exons 3 and 4) were used in the RT-PCR reaction with a cycle number that allows at least semi-quantitative comparison as indicated in ‘Materials and Methods’ section. The positions and types/size of PCR products are shown in Figures 1A and 2C. All RNA samples were treated with DNase to eliminate genomic DNA contamination. In addition, RT-PCR (intron) was performed without RT to further monitor DNA contamination (lower panel). This protocol is performed according to a standard protocol (11,58,59). (B) Real-time RT-PCR monitoring of HuR mRNA levels following precipitation of endogenous HuR with either anti-HuR antibody or goat IgG control as described above. The real-time RT-PCR was performed with primers/TaqMan probe specific to HuR mRNA and RPL0 (background control); the TaqMan primer pair and probe span intron and exon–intron junction, respectively, in HuR mRNA to eliminate signal quantitation due to DNA contamination. Lower panel shows data in terms of % specific binding (anti-HuR/IgG). The data are one representative experiments of two with mean ± SEM of triplicates. (C) RNA-binding activity of endogenous and exogenous HuR. The HuR ARE 60-nt probe (lane 1) was incubated with 5 µg HEK293 protein lysate (lanes 2–4). Competition assay was carried in the presence (+) of 1000-fold excess of unlabeled RNA competitor (lane 3). In vitro translated HuR protein was incubated with either the HuR ARE (lane 5) or the mutant ARE (lane 6) oligonucleotide. Arrows indicate RNA–protein complexes formation in the presence of protein lysate. The supershift assay was carried out in the same manner, except that protein lysate was pre-incubated with anti-HuR antibody for 30 min at room temperature before the addition of biotinylated probe. Asterisk Indicates the super-shifted position.
Moreover, real-time quantitative RT-PCR experiments were performed in order to further verify the HuR-bound mRNA levels in relation to a housekeeping mRNA, RPL0, as background control. There were extremely low background levels of RPL0 mRNA levels bound to both IgG and anti-HuR (Figure 5B). However, significant HuR-bound HuR mRNA levels were seen in comparison to IgG control.
To further confirm that HuR can bind to the HuR ARE region, gel mobility shift assay was performed using biotinylated HuR ARE RNA probes. Two prominent ARE–protein complexes could be seen to interact with the HuR ARE but not with unlabeled competitor (Figure 5C, lanes 2 and 3, respectively). A band is shifted with an anti-HuR monoclonal antibody (Figure 5C, lane 4). To verify the direct binding of HuR to the HuR ARE, in vitro translated HuR was found to bind to the HuR ARE but not the mutant ARE (Figure 5C, lanes 5 and 6, respectively).
Functional TTP competition with HuR auto-regulation
Since it is known that RNA-binding proteins that promote mRNA decay can compete for HuR, we over-expressed TTP and the zinc finger mutant (C124R) and subsequently measure the HuR mRNA levels bound to HuR using the IP-RNA approach-with normalization of RNA levels using housekeeping/background control (RPL0). TTP but not the mutant TTP competed for HuR binding to HuR mRNA (Figure 6A). When normalized to RPL0 mRNA levels, there was a 28-fold increase in HuR binding to HuR mRNA in vector-transfected cells, whereas wild-type TTP reduced the HuR binding by ∼2-fold. The zinc finger TTP mutant has been suggested as dominant-negative (32) and indeed increased the binding of HuR to HuR mRNA by 2-fold (Figure 6A). This is also reflected in the reporter assays; the mutant TTP increased, nearly by 2-fold (P < 0.01), the activity from the reporter expressed from the construct with the ARE-bearing 6-kb HuR mRNA 3′UTR when compared to the vector control (pCR3.1). Whereas the wild-type TTP decreased the reporter activity from the construct with 6-kb HuR mRNA 3′UTR (Figure 6B), there was no effect of the wild-type or mutant TTP on the reporter activity generated from the construct with the 2.7-kb HuR 3′UTR or the control EEF1A1 3′UTR (Figure 6B).
Figure 6.
Functional TTP-mediated antagonism of HuR autoregulation. (A) HEK293 cells were transfected with pCR3.1 vector, wild-type TTP vector or the zinc finger C124R mutant for overnight. Cell lysates were obtained, and RNA materials obtained by precipitation with either anti-HuR antibody or goat IgG control antibody were used for real-time quantitative PCR. The real-time RT-PCR was performed with primers/TaqMan probe specific to HuR mRNA and RPL0 (background control). Data show RPL0-normalized anti-HuR/PRL0-normalized anti-IgG ratios. Data are from one representative experiment (mean ± SEM of triplicate) of two. ***Denotes P-values of <0.005 one-way ANOVA test for the three group comparisons. (B) HEK293 cells in 96-well plates were co-transfected with 30-ng pCR3.1 empty vector, TTP or C124R TTP and 25 ng of each of EGFP-3′UTR reporter vectors as indicated. The reporter 3′UTR constructs are described in Figure 3A. The fluorescence levels obtained from cells transfected with the pCR3.1 and the reporter construct with control 3′UTR were taken as 100%. The y-axis numbers represent percentages of this control. ***Denotes P-values of <0.001 two-way ANOVA test when compared to the control group. (C) HEK293 cells in 96-well plates were co-transfected with 30-ng pCR3.1 empty vector, TTP or C124R TTP and 25 ng of each of EGFP-3′UTR reporter vectors as indicated. The reporter vector is EGFP fused with 3′UTR that contains a 60 nt corresponding to the ARE regions from HuR 6-kb variant 3′UTR, uPA 3′UTR, their mutant forms, and control GH1 sequence (Figure 3D, inset table). Fluorescence data of mean ± SEM of quadruplicates were obtained. The fluorescence levels obtained from cells transfected with the pCR3.1 and the reporter with control 3′UTR were taken as 100%. The y-axis numbers represent percentages of this control. **Denotes P-values of <0.01 two-way ANOVA test when compared to the control group.
Further, we used the minimal 60-nt regions of the HuR ARE and uPA ARE (as a positive control), and GH1 (non-ARE control sequence) to study the effects of wild-type TTP and mutant TTP. The effect of mutant TTP (∼1.6-fold, P < 0.01) was seen with the HuR ARE and uPA ARE but not with the mutant sequences or GH1 control (Figure 6C). The effect of wild-type TTP was minimal in contrast to the larger 3′UTRs. However, there was significant statistical difference in the case of HuR ARE and uPA ARE column groups in contrast to other column groups when the two-away ANOVA test was used (Figure 6C).
DISCUSSION
We show that HuR mRNA has long 3′untranslated region with multiple polyadenylation sites that results in alternative variants with differential ARE involvement and differential stability. Moreover, the different alternative variants have differential response to HuR-induced stabilization and TTP-induced destabilization. The long ARE-bearing transcript of HuR is labile and it constitutes the rare alternative form of HuR mRNA species. The low abundance of HuR can be explained by its lability to RNA destabilizing proteins, such as TTP. The HuR mRNA, particularly the ARE-bearing transcript binds and responds to HuR protein itself, thus, an auto-regulatory role of HuR is revealed in this paper.
The finding of alternative polyadenylation variants, such as the ones in this study, is facilitated by bioinformatics, specifically, alignment and clustering/assembly of cDNA and EST sequences (19,33,34). We used the Unigene record of ELAVL1 gene (HuR), which contains both the cDNA and EST sequences. The EST records (35), which are now in millions of records, are a rich source for the 3′end ESTs that constitute the 3′UTR harboring putative alternative polyadenylation variants. Alternative polyadenylation occurs mostly as a result of usage of multiple poly(A) sites in the 3′UTR. It appears that this is widespread and can affect tissue-specific expression, mRNA localization, mRNA decay and translation efficiency (36–40). Additionally, alternative polyadenylation may have a role in certain disease situations (20).
Alternative polyadenylation that leads to ARE-containing and non-ARE-containing transcripts can cause differential expression patterns. A notable example is COX-2 mRNA, which has been shown to have both ARE-deficient and ARE-proficient transcripts that differ in their abundance of expression in cancer due to alternative polyadenylation (41,42) and that TTP binds only the most distal AREs and promotes the decay of the longer isoform (42). In our study, we have shown that the distal ARE can destabilize the HuR 6-kb alternative polyadenylation variant, while the proximal region does not affect the 2.7-kb HuR mRNA that lacks the typical ARE, leading to their differential abundance. In addition, the distal ARE-containing 6-kb variant binds HuR protein itself and it is responsive to HuR-mediated upregulation of the activity of the reporter fused with the 6-kb 3′UTR sequence. Additionally, these observations were confirmed with a minimal region of ARE in the HuR 6-kb transcript. RNA-immunoprecipitation experiments indicated that the 6-kb transcripts are bound to HuR and also to intron containing sequence (pre-mRNA). In general, this is the case with many RNA-binding proteins, which predominantly associate with the pre-mRNAs of their targets (9,11).
The activities of the RNA-stabilizing activity of HuR and the RNA decay-promoting proteins, such as TTP, KSRP and AUF1, can compete for both the binding and the activity on the target mRNA. In normal cells or in the absence of external stimulus, ARE-mRNAs tend to be of low abundance due to the activity of RNA destabilizing proteins. In tumor cells or during cell cycle, also in response to stimuli, HuR may stabilize mRNA by the displacement or inhibition of factors that specifically cleave or deadenylate these mRNAs, such as TTP, KSRP or AUF1 (43,44). In our results, and in the absence of HuR ectopic expression, the long HuR polyadenylation mRNA variant is kept low because of the action of RNA destabilizing proteins, such as TTP, since over-expression of TTP reduced HuR binding to its HuR mRNA. In contrast, the mutant zinc finger TTP, which acts a dominant negative (32), increased the binding of HuR to HuR mRNA and HuR activity. The mutant TTP used here has a single-point mutation (Cys to Arg) in first of the two zinc finger domains of TTP, which leads to non-binding to the AREs (32,45). The dominant negative effect of the mutant TTP is likely due to the non-binding to the ARE and thus lack of TTP decay-promoting action, leading to further permission of HuR action. Another explanation is that mutant TTP inhibits the activity of other proteins involved in the degradation of the mRNA (45). During HuR over-expression, HuR can displace TTP, AUF1 or others, leading to stabilization of HuR mRNA. It has been shown that HuR over-expression led to displacement of AUF1 from c-fos AREs (46). It has been also shown that siRNA-mediated knockdown of HuR led to larger AUF1 association with cyclin D and p21 mRNAs (11). Ectopic expression of HuR overcomes TTP-mediated destabilization of IL-3 mRNA in ARE-dependent manner (47). Ectopic expression of TTP was also found to be competing for HuR binding toward IL-8 and VEGF mRNA. These results support our findings that TTP and HuR are competing with each other for HuR mRNA.
Both the expression and cytoplasmic localization of HuR protein has been linked in many types of cancer including colon, breast and others (21–25,48–52). Because of the involvement of HuR in human pathology, particularly, in cancer, further understanding of post-transcriptional regulation of HuR gene is needed. Alternative polyadenylation is an important gene-expression-tuning mechanism. It is estimated that half of mammalian genes encode alternative polyadenylation variants (33). Many of these genes have multiple poly(A) sites that may be differentially used during development, proliferation and differentiation, and can be tissue-specific regulated (37,38,40). These alternative polyadenylation variants have 3′ untranslated regions (3′UTR) with various lengths and may harbor differential sequence regulatory elements such as the ARE. The existence of two transcripts generated by the use of alternative polyadenylation sequences that differ in their 3′UTRs and the differential presence of AREs have been documented in a number of genes. Notably, these are cyclooxygenase 2 (COX-2), cyclin D1, vascular endothelial factor, fibroblast growth factor 2 and rat receptor for advanced glycation end products (41,53–57).
In this study, we used bioinformatics and experimental approaches to find and assess the functional role of HuR polyadenylation variants in mRNA abundance, ARE involvement and response to HuR and TTP. The auto-regulatory role of HuR may amplify the pathological role of HuR, particularly, in cancer; and small molecule drugs that disturb the binding of HuR to HuR 3′UTR can be used as therapeutics
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
ACKNOWLEDGEMENTS
The authors thank Dr. Myriam Gorospe (NIH) for critical reading of the manuscript. The wild-type TTP and C124R mutant TTP vectors were kindly provided by Dr. P. Blackshear (NIH). We also thank Dr. Christopher Moroni for supplying HuR expression vector. The assistance of Dr. P. Mohideen, Mr. Fahad Al-Zoghaibi, and Ms. Nora al-Suhaibani in cloning is appreciated. We are also indebted to the King Faisal Specialist Hospital and Research Center (KFSHRC) Subsidiary in Maryland, USA for managing and expediting reagent and supplies purchases for this study. Funding for open access charge: King Faisal Specialist Hospital and Research Center (KFSHRC).
Conflict of interest statement. None declared.
REFERENCES
- 1.Blackshear PJ. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem. Soc. Trans. 2002;30:945–952. doi: 10.1042/bst0300945. [DOI] [PubMed] [Google Scholar]
- 2.Chou CF, Mulky A, Maitra S, Lin WJ, Gherzi R, Kappes J, Chen CY. Tethering KSRP, a decay-promoting AU-rich element-binding protein, to mRNAs elicits mRNA decay. Mol. Cell Biol. 2006;26:3695–3706. doi: 10.1128/MCB.26.10.3695-3706.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidlin M, Lu M, Leuenberger SA, Stoecklin G, Mallaun M, Gross B, Gherzi R, Hess D, Hemmings BA, Moroni C. The ARE-dependent mRNA-destabilizing activity of BRF1 is regulated by protein kinase B. EMBO J. 2004;23:4760–4769. doi: 10.1038/sj.emboj.7600477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barreau C, Paillard L, Osborne HB. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res. 2005;33:7138–7150. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma WJ, Cheng S, Campbell C, Wright A, Furneaux H. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J. Biol. Chem. 1996;271:8144–8151. doi: 10.1074/jbc.271.14.8144. [DOI] [PubMed] [Google Scholar]
- 6.Van Tine BA, Knops JF, Butler A, Deloukas P, Shaw GM, King PH. Localization of HuC (ELAVL3) to chromosome 19p13.2 by fluorescence in situ hybridization utilizing a novel tyramide labeling technique. Genomics. 1998;53:296–299. doi: 10.1006/geno.1998.5468. [DOI] [PubMed] [Google Scholar]
- 7.Atasoy U, Watson J, Patel D, Keene JD. ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J. Cell Sci. 1998;111:3145–3156. doi: 10.1242/jcs.111.21.3145. [DOI] [PubMed] [Google Scholar]
- 8.Levine TD, Gao F, King PH, Andrews LG, Keene JD. Hel-N1: an autoimmune RNA-binding protein with specificity for 3′ uridylate-rich untranslated regions of growth factor mRNAs. Mol. Cell Biol. 1993;13:3494–3504. doi: 10.1128/mcb.13.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keene JD. Why is Hu where? Shuttling of early-response-gene messenger RNA subsets. Proc. Natl Acad. Sci. USA. 1999;96:5–7. doi: 10.1073/pnas.96.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Furneaux H, Cheng H, Caldwell MC, Hutter D, Liu Y, Holbrook N, Gorospe M. HuR regulates p21 mRNA stabilization by UV light. Mol. Cell Biol. 2000;20:760–769. doi: 10.1128/mcb.20.3.760-769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J. 2004;23:3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casolaro V, Fang X, Tancowny B, Fan J, Wu F, Srikantan S, Asaki SY, De Fanis U, Huang SK, Gorospe M, et al. Posttranscriptional regulation of IL-13 in T cells: role of the RNA-binding protein HuR. J. Allergy Clin. Immunol. 2008;121:853–859. doi: 10.1016/j.jaci.2007.12.1166. e854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran H, Maurer F, Nagamine Y. Stabilization of urokinase and urokinase receptor mRNAs by HuR is linked to its cytoplasmic accumulation induced by activated mitogen-activated protein kinase-activated protein kinase 2. Mol. Cell Biol. 2003;23:7177–7188. doi: 10.1128/MCB.23.20.7177-7188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Yang X, Cristofalo VJ, Holbrook NJ, Gorospe M. Loss of HuR is linked to reduced expression of proliferative genes during replicative senescence. Mol. Cell Biol. 2001;21:5889–5898. doi: 10.1128/MCB.21.17.5889-5898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subbaramaiah K, Marmao TP, Dixon DA, Dannenberg AJ. Regulation of cyclooxygenase-2 mRNA stability by taxanes. Evidence for involvement of p38, MAPKAPK-2 and HuR. J. Biol. Chem. 2003;25:25. doi: 10.1074/jbc.W119.012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez de Silanes I, Fan J, Galban CJ, Spencer RG, Becker KG, Gorospe M. Global analysis of HuR-regulated gene expression in colon cancer systems of reducing complexity. Gene Expr. 2004;12:49–59. doi: 10.3727/000000004783992215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez de Silanes I, Zhan M, Lal A, Yang X, Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proc. Natl Acad. Sci. USA. 2004;101:2987–2992. doi: 10.1073/pnas.0306453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khabar KS, Bakheet T, Williams BR. AU-rich transient response transcripts in the human genome: expressed sequence tag clustering and gene discovery approach. Genomics. 2005;85:165–175. doi: 10.1016/j.ygeno.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Moucadel V, Lopez F, Ara T, Benech P, Gautheret D. Beyond the 3′ end: experimental validation of extended transcript isoforms. Nucleic Acids Res. 2007;35:1947–1957. doi: 10.1093/nar/gkm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khabar KS. The AU-rich transcriptome: more than interferons and cytokines, and its role in disease. J. Interferon Cytokine Res. 2005;25:1–10. doi: 10.1089/jir.2005.25.1. [DOI] [PubMed] [Google Scholar]
- 21.Lopez de Silanes I, Fan J, Yang X, Zonderman AB, Potapova O, Pizer ES, Gorospe M. Role of the RNA-binding protein HuR in colon carcinogenesis. Oncogene. 2003;22:7146–7154. doi: 10.1038/sj.onc.1206862. [DOI] [PubMed] [Google Scholar]
- 22.Denkert C, Weichert W, Pest S, Koch I, Licht D, Kobel M, Reles A, Sehouli J, Dietel M, Hauptmann S. Overexpression of the embryonic-lethal abnormal vision-like protein HuR in ovarian carcinoma is a prognostic factor and is associated with increased cyclooxygenase 2 expression. Cancer Res. 2004;64:189–195. doi: 10.1158/0008-5472.can-03-1987. [DOI] [PubMed] [Google Scholar]
- 23.Mrena J, Wiksten JP, Thiel A, Kokkola A, Pohjola L, Lundin J, Nordling S, Ristimaki A, Haglund C. Cyclooxygenase-2 is an independent prognostic factor in gastric cancer and its expression is regulated by the messenger RNA stability factor HuR. Clin. Cancer Res. 2005;11:7362–7368. doi: 10.1158/1078-0432.CCR-05-0764. [DOI] [PubMed] [Google Scholar]
- 24.Heinonen M, Bono P, Narko K, Chang SH, Lundin J, Joensuu H, Furneaux H, Hla T, Haglund C, Ristimaki A. Cytoplasmic HuR expression is a prognostic factor in invasive ductal breast carcinoma. Cancer Res. 2005;65:2157–2161. doi: 10.1158/0008-5472.CAN-04-3765. [DOI] [PubMed] [Google Scholar]
- 25.Niesporek S, Kristiansen G, Thoma A, Weichert W, Noske A, Buckendahl AC, Jung K, Stephan C, Dietel M, Denkert C. Expression of the ELAV-like protein HuR in human prostate carcinoma is an indicator of disease relapse and linked to COX-2 expression. Int. J. Oncol. 2008;32:341–347. [PubMed] [Google Scholar]
- 26.Khabar KS, Al-Haj L, Al-Zoghaibi F, Marie M, Dhalla M, Polyak SJ, Williams BR. Expressed gene clusters associated with cellular sensitivity and resistance towards anti-viral and anti-proliferative actions of interferon. J. Mol. Biol. 2004;342:833–846. doi: 10.1016/j.jmb.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 27.Al-Zoghaibi F, Ashour T, Al-Ahmadi W, Abulleef H, Demirkaya O, Khabar KS. Bioinformatics and experimental derivation of an efficient hybrid 3′ untranslated region and use in expression active linear DNA with minimum poly(A) region. Gene. 2007;391:130–139. doi: 10.1016/j.gene.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Bakheet T, Frevel M, Williams BRG, Greer W, Khabar KSA. ARED: Human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional reportiore of encoded proteins. Nucleic Acids Res. 2001;29:246–254. doi: 10.1093/nar/29.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenberg E, Levanon EY. Human housekeeping genes are compact. Trends Genet. 2003;19:362–365. doi: 10.1016/S0168-9525(03)00140-9. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Zhang C, Zhou Y. Uneven size distribution of mammalian genes in the number of tissues expressed and in the number of co-expressed genes. Hum. Mol. Genet. 2006;15:1313–1318. doi: 10.1093/hmg/ddl051. [DOI] [PubMed] [Google Scholar]
- 31.Winzen R, Gowrishankar G, Bollig F, Redich N, Resch K, Holtmann H. Distinct domains of AU-rich elements exert different functions in mRNA destabilization and stabilization by p38 mitogen-activated protein kinase or HuR. Mol. Cell Biol. 2004;24:4835–4847. doi: 10.1128/MCB.24.11.4835-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai WS, Kennington EA, Blackshear PJ. Interactions of CCCH zinc finger proteins with mRNA: non-binding tristetraprolin mutants exert an inhibitory effect on degradation of AU-rich element-containing mRNAs. J. Biol. Chem. 2002;277:9606–9613. doi: 10.1074/jbc.M110395200. [DOI] [PubMed] [Google Scholar]
- 33.Tian B, Hu J, Zhang H, Lutz CS. A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res. 2005;33:201–212. doi: 10.1093/nar/gki158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Texier V, Riethoven JJ, Kumanduri V, Gopalakrishnan C, Lopez F, Gautheret D, Thanaraj TA. AltTrans: transcript pattern variants annotated for both alternative splicing and alternative polyadenylation. BMC Bioinformatics. 2006;7:169. doi: 10.1186/1471-2105-7-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boguski MS, Lowe TM, Tolstoshev CM. dbEST–database for “expressed sequence tags”. Nat Genet. 1993;4:332–333. doi: 10.1038/ng0893-332. [DOI] [PubMed] [Google Scholar]
- 36.Hesketh J. 3′-Untranslated regions are important in mRNA localization and translation: lessons from selenium and metallothionein. Biochem. Soc. Trans. 2004;32:990–993. doi: 10.1042/BST0320990. [DOI] [PubMed] [Google Scholar]
- 37.Edwalds-Gilbert G, Veraldi KL, Milcarek C. Alternative poly(A) site selection in complex transcription units: means to an end? Nucleic Acids Res. 1997;25:2547–2561. doi: 10.1093/nar/25.13.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Lee JY, Tian B. Biased alternative polyadenylation in human tissues. Genome Biol. 2005;6:R100. doi: 10.1186/gb-2005-6-12-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ara T, Lopez F, Ritchie W, Benech P, Gautheret D. Conservation of alternative polyadenylation patterns in mammalian genes. BMC Genomics. 2006;7:189. doi: 10.1186/1471-2164-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science. 2008;320:1643–1647. doi: 10.1126/science.1155390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ristimaki A, Narko K, Hla T. Down-regulation of cytokine-induced cyclo-oxygenase-2 transcript isoforms by dexamethasone: evidence for post-transcriptional regulation. Biochem. J. 1996;318:325–331. doi: 10.1042/bj3180325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawaoka H, Dixon DA, Oates JA, Boutaud O. Tristetraprolin binds to the 3′-untranslated region of cyclooxygenase-2 mRNA. A polyadenylation variant in a cancer cell line lacks the binding site. J. Biol. Chem. 2003;278:13928–13935. doi: 10.1074/jbc.M300016200. [DOI] [PubMed] [Google Scholar]
- 43.Chen CY, Gherzi R, Ong SE, Chan EL, Raijmakers R, Pruijn GJ, Stoecklin G, Moroni C, Mann M, Karin M. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell. 2001;107:451–464. doi: 10.1016/s0092-8674(01)00578-5. [DOI] [PubMed] [Google Scholar]
- 44.Suswam E, Li Y, Zhang X, Gillespie GY, Li X, Shacka JJ, Lu L, Zheng L, King PH. Tristetraprolin down-regulates interleukin-8 and vascular endothelial growth factor in malignant glioma cells. Cancer Res. 2008;68:674–682. doi: 10.1158/0008-5472.CAN-07-2751. [DOI] [PubMed] [Google Scholar]
- 45.Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol. Cell Biol. 1999;19:4311–4323. doi: 10.1128/mcb.19.6.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen CY, Xu N, Shyu AB. Highly selective actions of HuR in antagonizing AU-rich element-mediated mRNA destabilization. Mol. Cell Biol. 2002;22:7268–7278. doi: 10.1128/MCB.22.20.7268-7278.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ming XF, Stoecklin G, Lu M, Looser R, Moroni C. Parallel and independent regulation of interleukin-3 mRNA turnover by phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase. Mol. Cell Biol. 2001;21:5778–5789. doi: 10.1128/MCB.21.17.5778-5789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dixon DA, Tolley ND, King PH, Nabors LB, McIntyre TM, Zimmerman GA, Prescott SM. Altered expression of the mRNA stability factor HuR promotes cyclooxygenase-2 expression in colon cancer cells. J. Clin. Invest. 2001;108:1657–1665. doi: 10.1172/JCI12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nabors LB, Gillespie GY, Harkins L, King PH. HuR, a RNA stability factor, is expressed in malignant brain tumors and binds to adenine- and uridine-rich elements within the 3′ untranslated regions of cytokine and angiogenic factor mRNAs. Cancer Res. 2001;61:2154–2161. [PubMed] [Google Scholar]
- 50.Heinonen M, Fagerholm R, Aaltonen K, Kilpivaara O, Aittomaki K, Blomqvist C, Heikkila P, Haglund C, Nevanlinna H, Ristimaki A. Prognostic role of HuR in hereditary breast cancer. Clin. Cancer Res. 2007;13:6959–6963. doi: 10.1158/1078-0432.CCR-07-1432. [DOI] [PubMed] [Google Scholar]
- 51.Mazan-Mamczarz K, Hagner PR, Corl S, Srikantan S, Wood WH, Becker KG, Gorospe M, Keene JD, Levenson AS, Gartenhaus RB. Post-transcriptional gene regulation by HuR promotes a more tumorigenic phenotype. Oncogene. 2008;27:6151–6163. doi: 10.1038/onc.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ortega AD, Sala S, Espinosa E, Gonzalez-Baron M, Cuezva JM. HuR and the bioenergetic signature of breast cancer: a low tumor expression of the RNA-binding protein predicts a higher risk of disease recurrence. Carcinogenesis. 2008;29:2053–2061. doi: 10.1093/carcin/bgn185. [DOI] [PubMed] [Google Scholar]
- 53.Sawaoka H, Dixon DA, Oates JA, Boutaud O. Tristetrapolin binds to the 3chi untranslated region of cyclooxygenase-2mRNA: A polyadenylation variant in a cancer cell line lacks the binding site. J. Biol. Chem. 2003 doi: 10.1074/jbc.M300016200. M300016200. [DOI] [PubMed] [Google Scholar]
- 54.Rimokh R, Berger F, Bastard C, Klein B, French M, Archimbaud E, Rouault JP, Santa Lucia B, Duret L, Vuillaume M, et al. Rearrangement of CCND1 (BCL1/PRAD1) 3′ untranslated region in mantle-cell lymphomas and t(11q13)-associated leukemias. Blood. 1994;83:3689–3696. [PubMed] [Google Scholar]
- 55.Touriol C, Morillon A, Gensac MC, Prats H, Prats AC. Expression of human fibroblast growth factor 2 mRNA is post- transcriptionally controlled by a unique destabilizing element present in the 3′-untranslated region between alternative polyadenylation sites. J. Biol. Chem. 1999;274:21402–21408. doi: 10.1074/jbc.274.30.21402. [DOI] [PubMed] [Google Scholar]
- 56.Caballero JJ, Girón MD, Vargas AM, Sevillano N, Suárez MD, Salto R. AU-rich elements in the mRNA 3′-untranslated region of the rat receptor for advanced glycation end products and their relevance to mRNA stability. Biochem. Biophys. Res. Commun. 2004;319:247–255. doi: 10.1016/j.bbrc.2004.04.178. [DOI] [PubMed] [Google Scholar]
- 57.Hall-Pogar T, Zhang H, Tian B, Lutz CS. Alternative polyadenylation of cyclooxygenase-2. Nucleic Acids Res. 2005;33:2565–2579. doi: 10.1093/nar/gki544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lopez de Silanes I, Galban S, Martindale JL, Yang X, Mazan-Mamczarz K, Indig FE, Falco G, Zhan M, Gorospe M. Identification and functional outcome of mRNAs associated with RNA-binding protein TIA-1. Mol. Cell Biol. 2005;25:9520–9531. doi: 10.1128/MCB.25.21.9520-9531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keene JD, Komisarow JM, Friedersdorf MB. RIP-Chip: the isolation and identification of mRNAs, microRNAs and protein components of ribonucleoprotein complexes from cell extracts. Nature Protoc. 2006;1:302–307. doi: 10.1038/nprot.2006.47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.