Abstract
TLRs activate immune responses by sensing microbial structures such as bacterial LPS, viral RNA, and endogenous “danger” molecules released by damaged host cells. MyD88 is an adapter protein that mediates signal transduction for most TLRs and leads to activation of NF-κB and MAPKs and production of proinflammatory cytokines. TLR4-mediated signaling also leads to rapid activation of PI3K, one of a family of kinases involved in regulation of cell growth, apoptosis, and motility. LPS stimulates phosphorylation of Akt, a downstream target of PI3K, in wild-type (WT) mouse macrophages. LPS-induced phosphorylation of Akt serine 473 was blunted in MyD88−/− macrophages and was completely TLR4-dependent. MyD88 and p85 were shown previously to co-immunoprecipitate, and a YXXM motif within the Toll-IL-1 resistance (TIR) domain of MyD88 was suggested to be important for this interaction. To test this hypothesis, we compared expressed MyD88 variants with mutations within the YXXM motif or lacking the TIR domain or death domain and measured their capacities to bind PI3K p85, MyD88, and TLR4 by co-immunoprecipitation analyses. The YXXM → YXXA mutant MyD88 bound more strongly to p85, TLR4, and WT MyD88 than the other variants, yet was significantly less active than WT MyD88, suggesting that sustained interaction of MyD88/PI3K with the TLR4 intracellular “signaling platform” negatively regulates signaling. We propose a hypothetical model in which sustained PI3K activity at the membrane limits the availability of the PI3K substrate, thereby negatively regulating signaling.
Keywords: Toll-like receptors, co-immunoprecipitation, lipopolysaccharide Akt
INTRODUCTION
TLRs are a family of closely related type I transmembrane receptors that enable the innate immune system to recognize pathogen-associated molecular patterns or endogenous “danger” molecules and respond with signal transduction. One of the most extensively studied TLRs, TLR4, recognizes Gram-negative bacterial LPS as its prototype agonist. In response to LPS, two pairs of intracellular adapter proteins—MyD88 and Toll-IL-1 resistance (TIR) domain-containing adapter protein [TIRAP; also known as MyD88 adapter-like (MAL)] and TIR domain-containing adapter-inducing IFN-β (TRIF) and a TRIF-related adapter molecule (TRAM)—interact with TLR4 to activate two main signaling pathways, i.e., “MyD88-dependent” and “MyD88-independent.” Both pathways require receptor dimerization, adapter recruitment, and the activation of specific kinases and transcription factors and result in inflammatory gene expression.
MyD88 is used by all known TLRs, with the exception of TLR3, and also serves as a signaling adapter for IL-1R and IL-18R. MyD88 is comprised of a death domain (DD) at its N terminus, a TIR domain at its C terminus, and an intermediate domain [1, 2]. MyD88-dependent TLR4 signaling is initiated when LPS binds to myeloid differentiation protein 2 (MD-2), a non-membrane-spanning protein that binds to the TLR4 ectodomain, TLR4 molecules dimerize, and TIRAP is recruited to the membrane by binding to phosphatidylinositol (4,5)-bisphosphate (PIP2) [3]. A mutation of the TIRAP PIP2-binding motif results in the inability of this mutant to complement TIRAP−/− macrophages for TLR4-mediated signaling [3]. Membrane-localized TIRAP recruits cytosolic MyD88 to the TLR4 signaling complex, where MyD88 is thought to bind TLR4 through TIR–TIR interactions. Although the P712H mutation in murine TLR4 TIR leads to a complete loss of LPS signaling [4], its role in MyD88 recruitment remains controversial. Although one report suggested that the proline at position 712 in mouse TLR4 is required for the interaction of TLR4 with MyD88 [5], another observed the interaction of MyD88 with P712H TLR4 [6]. After MyD88 binds to TLR4, it recruits IL-1R-associated kinase 4 (IRAK4) to the intermediate domain between the TIR domain and DD [7] and IRAK1 through a DD–DD interaction [8, 9]. Macrophages from MyD88−/− mice are defective in many TLR4-mediated responses, such as LPS-induced secretion of cytokines (e.g., IL-6, TNF-α, and IL-1β), indicating the importance of MyD88 in TLR4-mediated signaling [10]. The phenotype of MyD88−/− mice is essentially identical to that of TIRAP−/− mice, supporting the concept that TIRAP and MyD88 are essential for the MyD88-dependent arm of TLR4 signaling [11].
PI3K is involved in many important cellular pathways including cell growth, migration, and apoptosis (reviewed in ref. [12]). The PI3K family consists of three classes of enzymes. Classes I and II enzymes are primarily involved in transmitting signals from membrane receptors, whereas Class III enzymes contribute to intracellular trafficking (reviewed in ref. [12]). Class I enzymes are subdivided further into Classes IA and IB based on structural and functional differences. Class IA PI3Ks are heterodimeric lipid kinases comprised of the regulatory domain subunit, p85, and the catalytic domain subunit, p110 (reviewed in refs. [12, 13]) and are activated after stimulation through TLR2, TLR3, TLR4, TLR5, TLR9, and IL-1R [14,15,16,17,18,19,20,21]. Although the p85 subunit has multiple roles that include binding to proteins, mediating the location of the kinase, protecting p110 from degradation, and conferring an activating conformation to the p110 subunit (reviewed in refs. [12, 13]), the p110 subunit is constitutively associated with the p85 subunit and mediates downstream signaling by phosphorylating phosphoinositol lipids [12]. Full activation of PI3K is a two-step process. First, when the p85 subunit binds to a phosphorylated tyrosine within a YXXM motif of another protein, a conformational change in p85 occurs that allows for partial activation of the pre-associated p110 subunit [22]. Binding of p85 to such proteins and the subsequent activation of PI3K can be blocked by mutating the YXXM motif [14, 15, 21, 23,24,25,26]. Second, phosphorylation of the p85 subunit, mediated by src kinases [27], facilitates full activation of the p110 catalytic subunit. Once activated, PI3K converts PIP2 into phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which in turn, activates phosphoinositide-dependent protein kinase 1 (PDK1), which in turn, phosphorylates and activates the serine/threonine-specific protein kinase Akt [28]. Phosphorylation of Akt on serine 473 is often used as a surrogate marker of PI3K activity.
In the RAW 264.7 murine macrophage cell line, Akt is phosphorylated on serine 473 after stimulation of cells with LPS via TLR4 or with a synthetic lipopeptide S-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-(R)-Cys-(S)-Ser-Lys4-OH trihydrochloride (P3C) via TLR2 [18, 29,30,31]. Overexpression of the MyD88 TIR domain blocked IL-1β- and LPS-induced Akt activation in endothelial cells, suggesting a connection between MyD88 and PI3K signaling pathways [32]. A dominant-negative p85 construct inhibited NF-κB activation in response to IL-1β and LPS, further supporting a role for PI3K in IL-1R- and TLR4-mediated, MyD88-dependent signaling [32]. Whether PI3K plays a positive or negative role in TLR4 signaling has been a controversy for many years. A negative role for PI3K on TLR4-mediated signaling is supported by the observation that activation of NF-κB [33, 34], ERK1/2, p38, and JNK [34] as well as induction of cyclooxygenase-2 (COX-2) [35], inducible NO synthetase [20, 36], TNF-α, IL-6, IL-10, and IL-12 [37] are increased upon PI3K inhibition. Conversely, PI3K has also been reported to contribute to TLR4-mediated activation of NF-κB and COX-2, as well as IL-6, MCP-1, IFN-inducible protein 10, IL-12, and IL-10 [32, 38, 39]. Recently, Luyendyk et al. [40] used Pik3r1−/− mice and PTEN−/− (PTEN, phosphatase and tensin homolog) mice to study the role of PI3K in TLR4 signaling. Pik3r1−/− mice lack all of the PI3K regulatory subunits (p85, p55, p50) and cannot convert PIP2 to PIP3, and PTEN−/− mice lack PTEN, a phosphatase that converts PIP3 to PIP2. Without PTEN, the PI3K pathway leading to Akt phosphorylation is constitutively active. Compared with wild-type (WT) macrophages, Pik3r1−/− macrophages expressed little phosphorylated Akt (P-Akt) after LPS stimulation, and PTEN−/− macrophages exhibited much higher levels of basal and LPS-induced P-Akt. LPS-induced MAPK activation was lower in the PTEN−/− macrophages but higher in the Pik3r1−/−. In addition, levels of the transcription factors, activating transcription factor 2 (ATF-2) and early growth response 1 (EGR-1), and the cytokines, TNF-α and IL-6, were lower in the PTEN−/− macrophages but higher in the Pik3r1−/− macrophages. This genetic approach clearly supports the hypothesis that PI3K plays a negative role in TLR4 signaling.
PI3K p85 has been reported to associate with TLR2, TLR3, TLR4, and TLR5 in cells [14, 15, 18, 21]. TLR2, TLR3, and TLR5 have YXXM motifs in their cytosolic domains, and in the case of TLR2 and TLR3, mutation of these motifs has been reported to prevent binding and activation of PI3K [14, 15]. In addition, Ojaniemi et al. [18] showed by co-immunoprecipitation that p85 associates with WT TLR4 upon LPS stimulation of RAW 264.7 cells and that MyD88 constitutively binds p85; however, binding of MyD88 and p85 increased further upon LPS stimulation. More recently, Gelman et al. [26] showed this association in the context of TLR9 in T cells and demonstrated that MyD88−/− T cells, transduced with mutant MyD88 that lacks the tyrosine residue of the YXXM motif (Y257F MyD88), exhibited decreased Akt phosphorylation. Rhee et al. [21] also reported an association of MyD88 with p85 in the context of TLR5 signaling in intestinal epithelial cells and observed decreased TLR5-induced phosphorylation of Akt, NF-κB activation, and IL-8 production in the absence of MyD88 [26]. Although these two papers reported that the YXXM motif in MyD88 is necessary for p85 binding to MyD88, the necessity of this motif for TLR4 signaling, as well as the details of the interactions among TLR4, PI3K, and MyD88, are largely unknown. When we carried out sequence analyses of TLR4 and its four adapters, only MyD88 was found to contain a putative YXXM PI3K-binding motif. Therefore, we sought to characterize the interactions of TLR4, MyD88, and PI3K (p85), as well as the functional consequences of YXXM MyD88 mutations on MyD88-mediated “autoactivation” (receptor-independent signaling as a result of overexpression of MyD88) and on LPS-induced TLR4 signaling. Our data reveal three major findings: The YXXM motif in MyD88 is not obligatory for its interaction with p85; mutation of the methionine to alanine in the MyD88 YXXM motif enhanced binding of this mutant protein to TLR4, p85, and MyD88 WT, but decreased signaling capability; and, all three proteins have the ability to interact with each other, suggesting that the coordinated assembly of a “signaling platform,” dependent on multiple protein–protein interactions, is required for TLR4 signaling. Importantly, the observation that sustained interaction of PI3K with the TLR4 signaling platform leads to decreased signaling suggests a mechanism by which PI3K exerts a negative regulatory effect on TLR4 signaling, which is caused by depletion of PI3K substrates in the cell membrane.
MATERIALS AND METHODS
Mice and cell culture
LPS-unresponsive C3H/HeJ (Lpsd) and WT C3H/OuJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). MyD88−/− mice were generously provided by Dr. Shuzio Akira (University of Osaka Medical School, Osaka, Japan) and have been described previously [41]. These mice were backcrossed onto a C57BL/6 background for greater than or equal to eight generations prior to use and genotyped to insure their genetic status. C57BL/6 mice from The Jackson Laboratory were used as WT controls for MyD88−/− mice. Primary peritoneal macrophages were prepared from mice following thioglycollate injection as described previously [42]. Human embryonic kidney (HEK)293T cells were maintained in DMEM supplemented with 10% FCS, 5 mM-glutamine, and streptomycin-penicillin in air supplemented with 5% CO2. The murine macrophage RAW 264.7 cell line (ATCC TIB-71, American Type Culture Collection, Manassas, VA, USA) was cultured in RPMI-1640 medium supplemented with 10% FCS, 5 mM glutamine, and streptomycin-penicillin in air supplemented with 5% CO2. All experiments were carried out with institutional approval.
Reagents
Protein-free (<0.008%) LPS was prepared from Escherichia coli K235 by a modification of the hot phenol-water extraction method [43]. P3C was purchased from EMC Microcollections GmbH (Tübingen, Germany). The PI3K inhibitor LY294002 was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Superfect transfection reagent was purchased from Qiagen (Valencia, CA, USA). Rabbit polyclonal antibodies against Akt, phospho-serine 473 Akt, and P-p65 were from Cell Signaling Technology (Beverly, MA, USA). Mouse anti-AU1 mAb was purchased from Covance Research Products (Berkeley, CA, USA), affinity-purified rabbit anti-AU1 (AU1 peptide sequence-DTYRYI) antibody from Immunology Consultants Laboratory, Inc. (Nerberg, OR, USA), and the rabbit polyclonal anti-MyD88 antibody from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse anti-hemagglutinin (HA) mAb was from Roche-Boehringer Mannheim (Indianapolis, IN, USA), and mouse anti-Flag mAb was from Sigma Chemical Co. Rabbit polyclonal anti-GFP antibody was from Invitrogen (Carlsbad, CA, USA). Restriction enzymes BamHI and EcoRI were from Fermentas (Ontario, Canada), and BsgI was from New England Biolabs (NEB; Ipswich, MA, USA). T4 DNA polymerase and T4 DNA ligase were also from NEB.
Plasmids
The constitutively active form of murine TLR4, HA-ΔTLR4, and inactive TLR4 plasmid, HA-ΔTLR4 (P712H), was described previously [5]. The Flag-tagged PI3K p85α subunit plasmid was a gift from Dr. David Fruman (University of California, Irvine, CA, USA). Dr. Katherine Fitzgerald (University of Massachusetts Medical School, Worcester, MA, USA) generously provided the pcDNA3-AU1-MyD88 vector encoding AU1-tagged human (hu)MyD88. The Flag-CMV1-TLR4 expression plasmid, pcDNA3-huCD14 expression plasmid, pEFBOS-HA-huMD-2 expression plasmid, endothelial leukocyte adhesion molecule 1 (ELAM-1) luciferase NF-κB reporter plasmid (pELAM-luc), pcDNA3-yellow fluorescent protein (YFP)-TLR4 (WT), and pCMV1-β-galactosidase reporter plasmid (pCMV1-β-gal) were provided by Dr. Douglas Golenbock (University of Massachusetts Medical School) and have been described elsewhere [44, 45]. The IL-8 promoter luciferase reporter plasmid was kindly provided by Dr. Naofumi Mukaida (Cancer Research Institute, Kanazawa University, Kanazawa, Japan). The AP-1-specific and CREB-specific luciferase promoter constructs were purchased from Stratagene (La Jolla, CA, USA). The plasmid encoding the P714H YFP-TLR4 mutant was generated by site-directed mutagenesis, using the Quick Change site-directed mutagenesis kit (Stratagene) as described [46]. The MyD88 Y257A construct was generated using sequential PCR steps. A 5′ product was generated by PCR using the 5′ primer 5′ gatatggatccgccatggac 3′ containing the 5′ BamHI site and the 3′ primer 5′ attgccttggccttgatgggg 3′, which introduce the Y-to-A mutation. The 3′ product was generated using the primers 5′ ccccatcaaggccaaggcaat 3′, also, which introduces the Y-to-A mutation, and 5′ gacgagaattctcagggcag 3′, which contains the 3′ EcoRI site. The MyD88 M260A construct was generated using sequential PCR steps. A 5′ product was generated by PCR using the 5′ primer 5′ gatatggatccgccatggac 3′ containing the 5′ BamHI site and the 3′ primer 5′ actctttcttcgctgccttgtag 3′, which introduce the M-to-A mutation. The 3′ product was generated using the primers 5′ gtacaaggcagcgaagaaagagt 3′, also, which introduces the M-to-A mutation, and 5′ gacgagaattctcagggcag 3′, which contains the 3′ EcoRI site. All PCR products were subjected to electropheresis on a 1% agarose gel and were gel-purified (QIAquick gel extraction kit). These two PCR products for each construct were combined in the same reaction, and the full-length MyD88 with the desired mutation was amplified using the primers 5′ gatatggatccgccatggac 3′ and 5′ gacgagaattctcagggcag 3′. The ΔDD MyD88 expression vector was generated by PCR amplification of the MyD88 TIR domain using the primers 5′ ggatccgccatggacacataccgctacatccccctggggcatatgcctg 3′, which contains a BamHI site and the AU1 tag, and 5′ gacgagaattctcagggcag 3′, which contains the EcoRI site. The ΔTIR MyD88 construct, which consists of the DD, intermediate domain, and 30 aa of the TIR domain, was generated by restriction digest of the AU1-MyD88 WT plasmid with BsgI. After restriction digest, the larger 6074-bp fragment was treated with T4 polymerase to remove 3′ overhangs that allowed for blunt-end ligation, generating the final ΔTIR MyD88 construct. The ΔTIRΔ30 MyD88 expression vector, which includes only the DD and an intermediate domain, was generated by PCR amplification of the MyD88 TIR domain using the primers 5′ gatatggatccgccatggac 3′, which contains a BamHI site and the AU1 tag, and 5′ gacgagaattcgtcatcaagtgtggtgatgcc 3′, which contains the EcoRI site. All mutant MyD88s were then ligated into pcDNA3.1(+) (Invitrogen) with BamHI and EcoRI and sequenced to verify the mutation was present. All plasmids were prepared using the EndoFree plasmid kit as recommended by the manufacturer (Qiagen) and were sequenced to confirm that the modifications were present.
Western analysis
Cells were harvested and washed once with PBS, pH 7.5, and then lysed in lysis buffer (20 mM HEPES, 0.5% Triton X-100, 150 mM NaCl, 12.5 mM β-glycerolphosphate, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 2 mM DTT, 1 mM sodium orthovanadate, 1 mM PMSF, 5 mM 4 nitrophenyl phosphate disodium salt hexahydrate) with a protease inhibitor tablet (Roche Applied Science, Indianapolis, IN, USA) at 4°C for 2–3 h, and then cell lysates were clarified by centrifugation at 4°C for 10 min at 14,000 g. Protein concentrations of lysates were measured by the Bradford method (BioRad, Hercules, CA, USA), and equal amounts of total protein/lane were fractionated on a 4–20% SDS-polyacrylamide gel (BioRad) using 4× SDS sample buffer (Novagen, San Diego, CA, USA). Gels were transferred to Immobilon-O (Millipore, Billerica, MA, USA). The membranes were blocked with TBS containing 0.1% Tween-20 with 5% nonfat dry milk or BSA, depending on the antibody manufacturer’s suggestion, and then incubated with the indicated primary antibodies overnight. The membrane was then washed three times with TBS containing 0.1% Tween-20 and incubated with the appropriate anti-mouse or HRP-coupled anti-rabbit IgG antibody (Cell Signaling Technology). Bound antibodies were visualized using the enhanced Phototope-HRP Western blot detection system, according to the manufacturer’s instructions (Cell Signaling Technology).
Immunoprecipitation
After 2 days in culture, transfected cells were put on ice and washed once with ice-cold PBS. Cells were scraped and centrifuged at 1200 rpm at 4°C for 10 min. The pellet was resuspended in 0.75 mL lysis buffer and rotated for at least 1 h at 4°C to insure lysis. Lysates were centrifuged at 14,000 rpm, 4°C, for 20 min. Supernatants were transferred to fresh Eppendorf tubes. Lysate protein (500–1000 μg; in a volume of 1 mL) was precleared with 20 μL EZview Red Protein A affinity gel (Sigma Chemical Co.) for 1 h. Separately, 40 μL of the gel was preloaded with the manufacturer’s recommended amount of antibody for at least 2 h. Preloaded gel was washed once with lysis buffer, and precleared lysates were added to the antibody-bound gel and rotated overnight at 4°C. The next day, the gel was washed three times in lysis buffer and resuspended in 15–20 μL 1× Laemmli buffer (BioRad). Samples were boiled for 10 min and electropheresed on 4–20% SDS-polyacrylamide gels.
Transfection and luciferase reporter assays
HEK293T cells were plated and transfected with the indicated plasmid DNA, including pCMV1-β-gal as an internal control, using SuperFect transfect reagent (Qiagen), according to the manufacturer’s instruction. One day after transfection, cells were treated and then lysed in 1× reporter assay lysis buffer (Promega, Madison, WI, USA) and β-gal (Galacto-Light system, Tropix, Bedford, MA, USA) and luciferase (Promega; luciferase assay system) activities analyzed using an LB 9507 luminometer (Berthold Technologies, Oak Ridge, TN, USA). Relative luciferase activity (RLU) was calculated by normalizing each sample’s luciferase activity for constitutive β-gal activity measured within the same sample. All individual assays were performed in triplicate, and a single representative experiment is shown.
RESULTS
LPS stimulation leads to serine phosphorylation of Akt that is dependent on TLR4 and MyD88
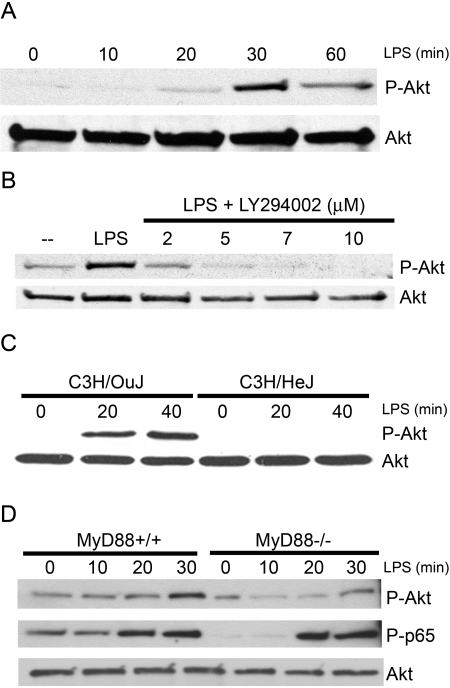
Previous reports have shown that TLR4 signaling leads to activation of P-I3K, that in turn, leads to activation of the kinase Akt [18,29,30,31]. To confirm and extend this finding, LPS-inducible activation of PI3K was first examined in the RAW 264.7 murine macrophage cell line, as measured by phosphorylation of Akt on serine 473 (P-Akt). Western analyses of whole cell lysates with specific antibodies that discriminate between P-Akt versus total Akt showed LPS-mediated phosphorylation of Akt was measurable at 20 min, peaked at 30 min, and declined by 1 h (Fig. 1A).
Fig. 1.
LPS stimulation leads to serine phosphorylation of Akt, which is dependent on TLR4 and MyD88. (A) Western blot analysis of phosphorylated serine 473 Akt (P-Akt) and total Akt in lysates from RAW 264.7 cells stimulated with LPS (100 ng/mL). (B) Western blot analysis of RAW 264.7 cells stimulated with LPS (100 ng/mL) and increasing amounts of LY294002. Blots were probed for P-Akt and total Akt antibodies. (C) Western blot analysis of P-Akt and total Akt in peritoneal macrophages from C3H/OuJ and C3H/HeJ, which were stimulated with LPS (100 ng/mL). (D) Western blot of P-Akt, total Akt, and P-p65 in peritoneal macrophages from MyD88+/+ and MyD88−/− mice, which were stimulated with LPS (100 ng/mL). Each blot is derived from a representative experiment (n=3).
To insure that LPS-induced phosphorylation of Akt on serine 473 was PI3K-dependent, RAW 264.7 macrophages were stimulated with LPS in the absence or presence of the specific PI3K inhibitor, LY294002. Akt phosphorylation induced by LPS was inhibited by LY294002 in a dose-dependent manner (Fig. 1B), indicating LPS-induced Akt phosphorylation is PI3K-dependent. To determine if TLR4 is necessary for activation of Akt by LPS, peritoneal macrophages from C3H/OuJ mice (TLR4+/+) and C3H/HeJ mice (which express the P712H mutation that renders TLR4 nonfunctional) [4, 47] were stimulated in vitro with LPS. Phosphorylation of Akt on serine 473 was strongly induced in the fully LPS-responsive C3H/OuJ macrophages (Fig. 1C). In contrast, no detectable phosphorylation of Akt was observed in C3H/HeJ macrophages, indicating that activation of Akt by LPS is fully TLR4-dependent.
To determine if MyD88 is necessary for PI3K activation by LPS, peritoneal macrophages from MyD88+/+ (WT) and MyD88−/− mice were stimulated in vitro with LPS. Western analysis for detection of the P-p65 subunit of NF-κB (P-p65) was included as a positive control, as it has been shown that p65 phosphorylation, as well as NF-κB activation, are delayed in MyD88−/− macrophages [10]. This was confirmed in Figure 1D. Also shown in Figure 1D, phosphorylation of Akt on serine 473 was strongly induced in MyD88+/+ macrophages, while a less-robust and delayed induction of Akt phosphorylation at serine 473 was observed in MyD88−/− macrophages. These data suggest that LPS-induced activation of PI3K is partially MyD88-dependent.
The p85 subunit of PI3K associates with WT and signal-incompetent TLR4
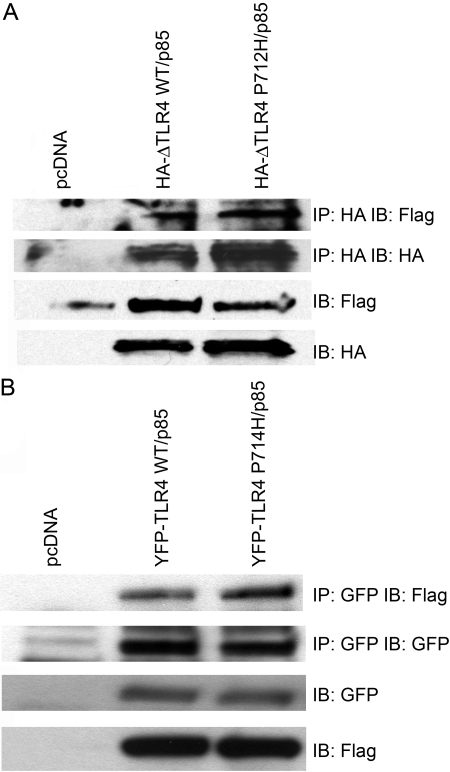
It has been shown previously that TLR2, TLR3, TLR4, and TLR5 associate with the p85 regulatory subunit of PI3K in cells [14, 15, 18, 21]. To investigate the details of this interaction, HEK293T cells were co-transfected with a vector encoding Flag-tagged p85α (p85) and a construct encoding HA-tagged TLR4, rendered constitutively active by deletion of a large part of the ectodomain (HA-ΔTLR4 WT), or an inactive, HA-tagged, signaling-deficient P712H TLR4 mutant (HA-ΔTLR4 P712H) [5]. Whole cell lysates were prepared, and WT and mutant HA-ΔTLR4s were immunoprecipitated with anti-HA antibody. Immunoprecipitates were then immunoblotted with an anti-Flag antibody to detect TLR4-associated p85. Control experiments were performed in which each construct was transfected individually, and the lysates were immunoprecipitated to insure that the beads did not nonspecifically trap the plasmid-encoded proteins during immunoprecipitation. No nonspecific trapping was observed (data not shown). Figure 2A shows that Flag-p85 PI3K co-immunoprecipitated with the active HA-ΔTLR4 WT and the inactive HA-ΔTLR4 P712H proteins. This same experiment was also carried out using a YFP-tagged, full-length huTLR4 and the corresponding, inactive P714H mutant. Like the HA-ΔTLR4 WT, overexpression of WT YFP-TLR4 also results in agonist-independent autoactivation. Again, Flag-p85 was found in association with WT and P714H TLR4 proteins in the absence of LPS (Fig. 2B). Together, these data suggest that PI3K p85 associates with active TLR4 and that the proline at position 712 in murine TLR4 or at position 714 in huTLR4 is not required for this association.
Fig. 2.
The p85 subunit of PI3K associates with TLR4. (A) HEK293T cells were co-transfected with expression plasmids encoding Flag-p85 and HA-ΔTLR4 WT or HA-ΔTLR4 P712H. Cell lysates were immunoprecipitated (IP) with anti-HA mAb (IP: HA) and immunoblotted (IB) using anti-Flag mAb (IB: Flag). The same lysates were immunoblotted directly using anti-Flag mAb (IB: Flag) or anti-HA mAb (IB: HA). (B) HEK293T cells were co-transfected with expression plasmids encoding Flag-p85 and YFP-TLR4 WT or YFP-TLR4 P714H. Cell lysates were immunoprecipitated with anti-GFP antibody (IP: GFP) and immunoblotted using anti-Flag mAb (IB: Flag). The same lysates were immunoblotted directly using anti-Flag mAb (IB: Flag) or anti-GFP Ab (IB: GFP). This figure is representative of at least three independent analyses.
PI3K p85 differentially associates with MyD88 WT and mutant proteins
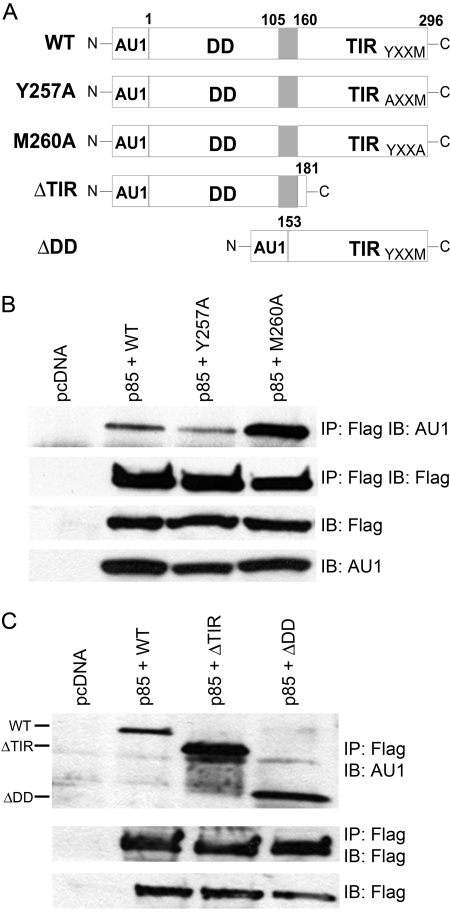
It has been reported previously that MyD88 binds constitutively to p85 in RAW 264.7 cells [18]. To confirm this observation and to identify domain(s) within MyD88 that interact with p85, four mutant MyD88 constructs were generated (Fig. 3A). Two contained mutations in the MyD88 YXXM motif were located in the TIR domain, i.e., Y257A and M260A. In addition, two MyD88 deletion mutants, ΔDD and ΔTIR, were constructed. Others have shown that ΔDD, that lacks the N-terminal DD region of MyD88, can act as a dominant-negative inhibitor of TLR4-mediated signaling [8, 9,48, 49] and that ΔTIR, that lacks most of the TIR domain, is constitutively active when overexpressed [48, 50]. All of the MyD88 constructs expressed an N-terminal AU1 epitope tag.
Fig. 3.
The PI3K p85 subunit differentially associates with MyD88 WT and mutant proteins. (A) Schematic illustrations of the MyD88 mutant proteins. N, N-terminal; C, C-terminal. (B) HEK293T cells were co-transfected with expression plasmids encoding Flag-p85 and WT, Y257A, or M260A MyD88. Cell lysates were immunoprecipitated with anti-Flag mAb (IP: Flag) and immunoblotted using anti-AU1 mAb (IB: AU1). The same lysates were immunoblotted directly using anti-Flag mAb (IB: Flag) and using anti-AU1 mAb (IB: AU1). (C) HEK293T cells were co-transfected with expression plasmids encoding Flag-p85 and WT, ΔTIR, or ΔDD MyD88. The same immunoprecipitation and Western blot analyses were performed as described in B, except a polyclonal anti-AU1 antibody was used. This figure is representative of at least three independent analyses.
MyD88 constructs were co-transfected individually, together with the Flag-tagged p85 construct, into HEK293T cells. Whole cell lysates were subjected to IP with anti-Flag antibody to immunoprecipitate the p85 subunit of PI3K. Immunoprecipitated proteins were separated by SDS-PAGE and subjected to Western analysis (IB) with anti-AU1 antibody to detect MyD88 associated with the Flag-p85. Figure 3B illustrates that Y257A and M260A mutant proteins retained the capacity to bind Flag-p85. However, we consistently observed a much stronger association between the M260A MyD88 mutant protein and p85 than observed for WT or Y257A MyD88 proteins under conditions where there was equal expression of the MyD88 proteins, as evidenced by comparable detection of the AU1 in the lysates. The converse immunoprecipitation was also performed (IP: anti-AU1; IB: anti-Flag) with similar results (data not shown). Surprisingly, the two deletion mutants, ΔDD and ΔTIR, were also found to bind Flag-p85 (Fig. 3C), indicating that Flag-tagged p85 associates with both domains of MyD88. Moreover, as Flag-p85 binds to the MyD88 ΔTIR (which lacks the YXXM site) and both YXXM mutant proteins, our data strongly suggest that the YXXM motif in the TIR domain of MyD88 is not essential for p85 binding to MyD88.
MyD88 proteins differentially associate with WT and P712H TLR4
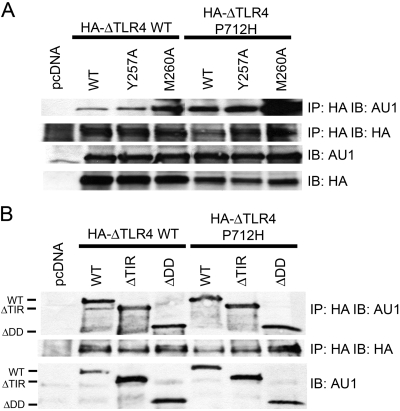
Although an interaction between WT MyD88 and TLR4 has been reported [5, 6, 16, 49, 51, 52], the effect that point mutations and deletions in MyD88 have on its capacity to interact with TLR4 has not been studied rigorously. Therefore, we next compared the ability of WT and mutant MyD88 proteins to interact with TLR4. We first used the constitutively active HA-ΔTLR4 and the inactive P712H mutant of HA-ΔTLR4 to discern whether mutations in the YXXM motif of MyD88 would affect its association with TLR4 or if deficiencies in the TIR domain and DD of MyD88 would alter its ability to interact with the TLR4 signaling complex. The MyD88 constructs were co-transfected individually into HEK293T cells with the constitutively active HA-ΔTLR4 WT or the inactive HA-ΔTLR4 P712H. Whole cell lysates were immunoprecipitated with anti-HA antibody, separated by SDS-PAGE, and subjected to Western analysis with an anti-AU1 antibody to detect the MyD88 WT and mutant proteins. Figure 4A shows that WT, Y257A, and M260A MyD88 proteins differentially associated with HA-ΔTLR4 WT and HA-ΔTLR4 P712H proteins: Again, the M260A MyD88 protein associated to a greater extent with the HA-ΔTLR4 WT and HA-ΔTLR4 P712H proteins than did the WT and Y257A MyD88 proteins. Interestingly, we consistently observed that the inactive HA-ΔTLR4 associated with WT and mutant MyD88 proteins to a greater extent than the constitutively active HA-ΔTLR4 protein. The MyD88 ΔDD and ΔTIR deletion mutants also associated with HA-ΔTLR4 WT and HA-ΔTLR4 P712H proteins (Fig. 4B). Together, these data reveal that the M260A MyD88 mutant protein interacts with TLR4 more strongly than the WT or Y257A mutant, mirroring the same binding pattern seen with p85. Also, these data show that the MyD88-TLR4 association is not dependent on the proline residue located in the BB loop of TLR4, a loop that connects the second β-strand and the second helix of the TIR domain [6, 65].
Fig. 4.
MyD88 proteins differentially associate with WT and P712H TLR4. (A) HEK293T cells were co-transfected with expression plasmids containing HA-ΔTLRWT or HA-ΔTLR4 P712H along with WT, Y257A, or M260A MyD88. Cell lysates were immunoprecipitated with anti-HA mAb (IP: HA) and immunoblotted using anti-AU1 mAb (IB: AU1). The same lysates were immunoblotted directly using anti-HA mAb (IB: HA) and using anti-AU1 mAb (IB: AU1). (B) HEK293T cells were co-transfected with expression plasmids containing HA-ΔTLRWT or HA-ΔTLR4 P712H along with WT, ΔTIR, or ΔDD MyD88. The same immunoprecipitation and Western blot analysis were performed as decribed in A, except a polyclonal AU1 antibody was used. This figure is representative of at least three independent analyses.
MyD88 proteins differentially interact to form dimers with WT MyD88
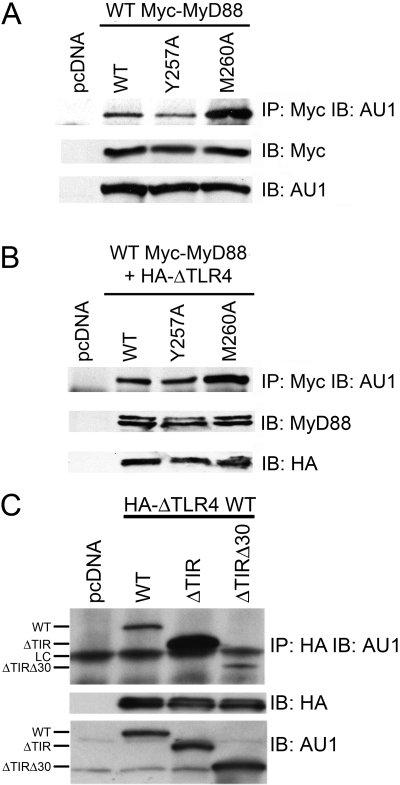
Several experimental approaches have been used to show that overexpression of MyD88 results in the formation of functional homodimers, thereby bypassing the need for ligand-mediated TLR4 oligomerization and recruitment of MyD88 to the TLR4 complex [48, 53]. Cells treated with cell-permeable peptides consisting of amino acids of the MyD88 TIR domain BB loop exhibited decreased LPS-induced responses by disrupting MyD88 homodimers [53]. We examined the ability of our WT or mutant MyD88 proteins to dimerize with WT MyD88. Each AU1-tagged MyD88 vector (WT or mutant) was transfected individually into HEK293T cells with a WT Myc-tagged MyD88 and dimerization assessed by co-immunoprecipitation using anti-Myc antibody and Western analysis to detect dimers comprised of AU1-MyD88 and Myc-MyD88. As shown in Figure 5A, the relative capacities of the mutant MyD88 proteins to dimerize with WT MyD88 mirrored their abilities to bind p85 and TLR4 in that the M260A protein was observed repeatedly to associate to a greater extent with the Myc-MyD88 than the other two MyD88 species.
Fig. 5.
MyD88 proteins differentially interact to form dimers and the interaction of MyD88 ΔTIR mutants with TLR4. (A) HEK293T cells were co-transfected with expression plasmids containing Myc-MyD88 WT and WT, Y257A, or M260A MyD88. Cell lysates were immunoprecipitated with anti-Myc mAb (IP: Myc) and immunoblotted using polyclonal anti-AU1 antibody (IB: AU1). The same lysates were immunoblotted directly using anti-Myc mAb (IB: Myc) and using polyclonal anti-AU1 antibody (IB: AU1). (B) HEK293T cells were co-transfected with expression plasmids containing Myc-MyD88 WT and HA-ΔTLR WT along with WT, Y257A, or M260A MyD88. Cell lysates were immunoprecipitated with anti-Myc mAb (IP: Myc) and immunoblotted using polyclonal anti-AU1 antibody (IB: AU1). The same lysates were immunoblotted directly using polyclonal anti-MyD88 antibody (IB: MyD), which detects Myc-tagged and AU1-tagged MyD88, and anti-HA mAb (IB: HA). (C) HEK293T cells were co-transfected with expression plasmids containing HA-ΔTLR WT along with WT, ΔTIR, or ΔTIRΔ30 MyD88. Cell lysates were immunoprecipitated with anti-HA mAb (IP: HA) and immunoblotted using anti-AU1 mAb (IB: AU1). The same lysates were immunoblotted directly using anti-HA mAb (IB: HA) and anti-AU1 mAb (IB: AU1). LC, Light chain. These figures are representative of at least three different analyses.
The same experiment was repeated in the presence of the constitutively active HA-ΔTLR4 (Fig. 5B), and we again observed that the M260A mutant bound more strongly to WT MyD88 (Fig. 5B). Thus, the presence of activated TLR4 neither contributes to nor alters dimerization of MyD88. The ΔDD and ΔTIR mutants associated with WT MyD88, consistent with a previous report [48], and this association is not altered by the presence of activated TLR4 (data not shown). Together, these data support the conclusion that the TIR domain and DD of MyD88 are involved in MyD88 dimerization.
The first 30 aa of the MyD88 TIR domain increase its binding to TLR4
The MyD88 ΔTIR construct used in the previous experiments was generated using restriction digestion. This construct encodes a protein that retains the first N-terminal 30 aa of the MyD88 TIR domain in addition to the DD and intermediate domain. To determine if these 30 aa contributed to the differences in binding observed thus far, a new construct, ΔTIRΔ30, was engineered to generate a mutant that retained only the DD and the intermediate domain and no portion of the TIR domain. When transfected into HEK293T cells with HA-ΔTLR4 WT (Fig. 5C), the new ΔTIRΔ30 protein bound to HA-ΔTLR4 to a lesser extent than the ΔTIR MyD88. The relative capacity of ΔTIRΔ30 to interact with WT MyD88 and Flag-p85 proteins was similar to that of ΔTIR (data not shown). These data indicate that the 30 aa of the N-terminal end of the MyD88 TIR domain strengthen the interaction of MyD88 with TLR4.
Differential signaling capacities of MyD88 proteins
Our data indicate that the M260A mutant of MyD88 binds more strongly to p85, TLR4, and MyD88. Therefore, we sought to determine whether mutations in MyD88 and differences in binding of these mutants to p85 or TLR4 could be correlated with functional differences in MyD88-dependent cellular activation. For the next series of experiments, we evaluated the relative activities of the MyD88 variant proteins with respect to cellular activation induced in a receptor-independent manner by overexpression of MyD88 variants and by ligand-mediated TLR4 signaling under conditions of reduced MyD88 overexpression.
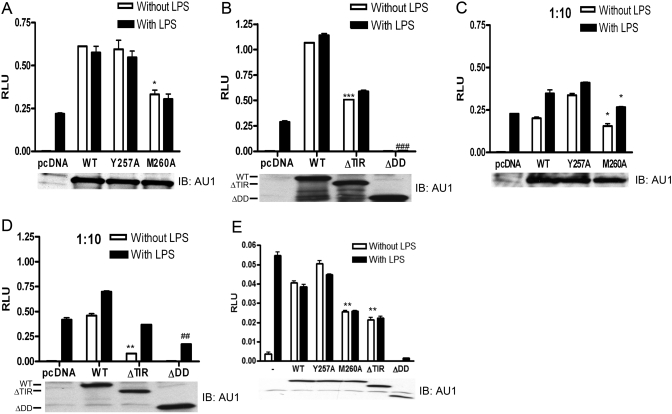
Overexpression of WT MyD88 or the MyD88 ΔTIR results in the activation of NF-κB in a receptor-independent manner, i.e., autoactivation [48, 49]. The pcDNA3.1 control vector (pcDNA) or each of the MyD88 constructs was co-transfected individually into HEK293T cells along with TLR4, MD-2, CD14, and the NF-κB-dependent luciferase reporter (ELAM). Figure 6A shows that cells transfected with the control vector (pcDNA) did not exhibit autoactivation but responded to LPS, indicating an intact TLR4 signalosome. Under conditions where the various MyD88 proteins were expressed equally (see Western blot for AU1 below each graph), the Y257A protein activated NF-κB luciferase reporter activity to the same degree as the WT MyD88, and M260A and ΔTIR proteins resulted in levels of autoactivation that were ∼50% of WT MyD88 (open bars, Fig. 6, A and B). The ΔDD protein failed to elicit any autoactivation (open bars, Fig. 6B) as previously reported [8, 9]. It is important to note that the single M260A mutation in the TIR domain of MyD88 exhibited the same phenotype as if the TIR domain were essentially deleted, i.e., ΔTIR.
Fig. 6.
Differential autoactivation of MyD88 proteins and the effects of MyD88 proteins on TLR4 signaling. (A and B) HEK293T cells were co-transfected with expression plasmids containing Flag-CMV1-TLR4, pcDNA3-huCD14, pEFBOS-HA-huMD-2, pELAM-luc, pCMV1-β-gal, and the indicated MyD88 plasmids (WT, 0.4 μg; Y257A, 0.4 μg; M260A, 0.350 μg; ΔTIR, 0.075 μg; and ΔDD, 1.4 μg DNA). Cells were cultured for 24 h and then stimulated with LPS (100 ng/mL) for 6 h. Cells were lysed, and luciferase activity was determined as described in Materials and Methods. In addition, Western blot analysis was performed on cell lysates from these transfected cells. Blots were probed with polyclonal anti-AU1 antibody. (C and D) The same experiment was performed as in A and B except one-tenth the amount of the MyD88 plasmids was used to transfect cells. (E) HEK293T cells were co-transfected with expression plasmids containing Flag-CMV1-TLR4, pcDNA3-huCD14, pEFBOS-HA-huMD-2, IL-8 luciferase reporter plasmid, pCMV1-β-gal, and the indicated MyD88 plasmids (WT, 0.4 μg; Y257A, 0.4 μg; M260A, 0.350 μg; ΔTIR, 0.075 μg; and ΔDD, 1.4 μg DNA). Cells were cultured for 24 h and then stimulated with LPS (100 ng/mL) for 6 h. Cells were lysed, and luciferase activity was determined as described in Materials and Methods. In addition, Western blot analysis was performed on cell lysates from these transfected cells. Blots were probed with polyclonal anti-AU1 antibody. This figure is representative of at least three independent analyses. Values are mean ± se (n=3). (A) *, P < 0.05, relative to WT (without LPS); (B) ***, P < 0.001, relative to WT (without LPS); ###, P < 0.001, relative to pcDNA (with LPS); (C) * over open bar, P < 0.05, relative to WT (without LPS); * over solid bar, P < 0.05, relative to WT (with LPS); (D) **, P < 0.01, relative to WT (without LPS); ##, P < 0.01, relative to pcDNA (with LPS); (E) **, P < 0.01, relative to WT (without or with LPS).
Under these conditions of MyD88 overexpression, stimulation of transfectants with LPS induced no further increase in luciferase reporter activity (solid bars, Fig. 6, A and B), suggesting that the strength of the autoactivation induced by overexpression of MyD88 variants exceeded that induced by ligand-dependent signaling. Therefore, the same experiment was repeated at one-tenth the MyD88 vector input used in Figure 6, A and B, in an effort to reduce the contribution of MyD88-driven autoactivation, yet still enable detection of the AU1-tagged MyD88 proteins so that we could insure comparable expression. When the vector input was reduced ten-fold, autoactivation induced by each MyD88 construct was decreased by more than half (compare A with C), yet now permitted detection of LPS-induced signaling (Fig. 6, C and D). Consistent with the diminished autoactivation capacity of the M260A MyD88 mutant, this mutant was also less capable of responding to LPS-induced signaling than the WT or Y257A mutants (solid bars, Fig. 6C). It has been shown previously that the ΔDD construct exerts dominant-negative activity on TLR4 signaling [8, 9]. The ΔDD mutant, but none of the others, exerted a dominant-negative effect on LPS-induced signaling, as evidenced by a significant decrease in NF-κB reporter activity (ΔDD, solid bar) compared with that induced in the control cells (pcDNA, solid bar) in Figure 6, B and D.
The ELAM-luciferase promoter used for all experiments thus far also contains AP-1- and CREB-sensitive sites in addition to three NF-κB sites [54]. Therefore, we also used an IL-8 luciferase reporter construct that includes three copies of the NF-κB-binding site of the endogenous IL-8 promoter but no AP-1 or CREB sites [55]. Figure 6E shows similar results to the original NF-κB-dependent ELAM promoter, in that the M260A and ΔTIR MyD88 activated the reporter ∼50% less than WT MyD88. As the ELAM promoter also contains an AP-1 and a CREB site, in addition to its three NF-κB sites, we also tested AP-1- and CREB-specific promoter constructs. There was no difference in the ability of WT MyD88 and M260A MyD88 to activate these reporter constructs (data not shown). Collectively, these data indicate that despite the increased capacity of the M260A protein to interact with p85, TLR4, and MyD88, this mutation results in a decreased capacity to signal via NF-κB.
DISCUSSION
Previous studies have suggested that certain TLRs possess a YXXM motif that enables direct interaction with the p85 subunit of PI3K [14, 15, 18, 21]. As TLR4 activates PI3K (Fig. 1), our failure to identify a YXXM motif in TLR4 by sequence analysis suggested that its interaction with p85 may be indirect, possibly through another molecule that interacts with TLR4. In the present study, we identified a conserved YXXM motif in MyD88, but not in any of the other known adapter molecules. Therefore, we sought to study the possible role of this motif in mediating the interactions among MyD88, the p85 subunit of PI3K, and TLR4. Our experimental approach examined pair-wise interactions using a p85 construct; TLR4 constructs that were constitutively active, LPS-inducible, or inactive as a result of the presence of the P → H mutation in the BB loop of the TIR domain; and MyD88 vectors with mutations in this YXXM motif. The results from this study are summarized in Table 1.
TABLE 1.
Summary of Coimmunoprecipitation and NF-κB Activation Data
| WT MyD88 | p85 | HA-ΔTLR4 WT | HA-ΔTLR4 P712H | Activation | |
|---|---|---|---|---|---|
| WT | + | + | + | ++ | +++ |
| Y257A | + | + | + | ++ | +++ |
| M260A | +++ | +++ | +++ | ++++ | + |
| ΔTIR | + | + | + | + | + |
| ΔDD | + | + | + | + | – |
| p85 | + | NA | + | + | NA |
Protein–protein interactions and NF-κB luciferase activity were graded as “–,” no interaction/activity detected, to “++++,” maximum interaction/activity detected. NA, Not applicable; interaction/activity was not examined.
We first confirmed the role of PI3K in TLR4 signaling [18, 29,30,31] by showing that Akt was phosphorylated rapidly in response to LPS and that this response was suppressed by a specific PI3K inhibitor, LY294002. Also, LPS-induced Akt phosphorylation was TLR4-dependent and considerably delayed and diminished in MyD88-deficient macrophages. These findings support and extend the findings of previous studies that implicate PI3K in the regulation of many LPS-inducible gene products.
We also demonstrated an association between TLR4 and p85, which was not dependent on signal competence. In the absence of a YXXM motif on TLR4, we hypothesized that binding of p85 to TLR4 required an intermediate molecule, possibly MyD88. To test this hypothesis, we studied the effect of mutations of the YXXM motif found within the C-terminal TIR domain of MyD88. The Y → A mutation had no effect on the interaction between MyD88 and p85. In the case of the M → A mutation, however, the association of MyD88 with p85 was increased significantly. We also examined the association between MyD88 and TLR4, addressing the possibility that the deletion of the TIR domain and DD of MyD88 or mutations within the YXXM motif of MyD88 would alter its ability to complex with TLR4. Interestingly, the TLR4 P712H mutation increased the overall association between TLR4 and WT or mutant MyD88 molecules but did not affect the relative capacities of the different mutant MyD88 molecules to associate with TLR4. Similar to the pattern seen with MyD88 and p85, the M260A MyD88 mutant protein associated most strongly with WT or P712H TLR4. Although a previous study concluded that this conserved BB loop proline is required for TLR4 interaction with MyD88 [5], our data support another study in which the P → H mutation in TLR4 was found not to affect this interaction [6]. As MyD88 dimerization is required for signaling [53], we also examined the effect of YXXM mutations on the capacity to form complexes with WT MyD88. Again, the binding pattern mirrored that seen with p85 and TLR4; the M → A MyD88 mutant protein associated more strongly with WT MyD88 than the WT or Y → A mutant proteins.
The observation that the association patterns of the MyD88 proteins with WT MyD88, TLR4, and p85 were so similar strongly suggests that each of these proteins has the capacity to interact simultaneously with the other two. Thus, the mutation of amino acids in one protein (MyD88) likely affects its ability to interact with the other two (i.e., TLR4 and p85) and itself (dimerization). Based on this hypothesis, we developed the hypothetical model shown in Figure 7, in which we propose that MyD88 dimerizes in the absence of TLR4 activation. This supports and extends data reported by others using overexpression and yeast two-hybrid approaches [48, 53]. Also, the observation that MyD88 can interact with itself in the yeast two-hybrid system indicates that p85 is not required for dimerization; however, it is possible that MyD88 dimer formation is facilitated and/or stabilized by its association with p85. This complex, consisting of the MyD88 dimer, p85, and p110 (p110 constitutively interacts with p85 [12]), can then together interact with TLR4 in response to ligand-mediated receptor activation.
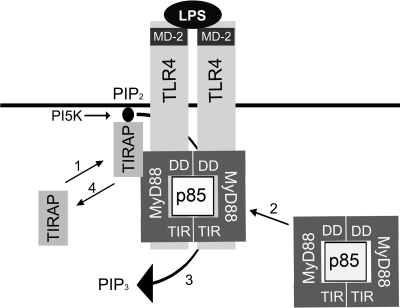
Fig. 7.
Hypothetical model of TLR4, MyD88, and p85 interactions. 1. TIRAP is recruited to TLR4 membrane receptors by interacting with PIP2 [3]. 2. TIRAP facilitates the recruitment of the MyD88 dimer/p85 (PI3K) complex to TLR4 [18, 48, 53]. 3. PI3K converts PIP2 into PIP3 [56], thereby locally decreasing membrane stores of PIP2. 4. As a result of consumption of PIP2, TIRAP dissociates from the membrane, resulting in diminished signaling.
It is generally accepted that MyD88 interacts with TLR4 through TIR–TIR interactions. Kagan and Medzhitov [3] recently described membrane-associated TIRAP as a “bridging adapter” that facilitates this interaction. The data presented in Figure 5C demonstrate that the MyD88 ΔDD and ΔTIR proteins associated with TLR4 (WT or P712H). At first glance, this would suggest that both domains contribute to the interaction with TLR4. However, as the ΔTIR construct was made by restriction digestion and not by PCR amplification, 30 aa into the N terminus of the TIR domain were encoded in the ΔTIR construct. Therefore, the possibility existed that these 30 remaining amino acids may contribute to the interaction between MyD88 and TLR4. When these experiments were repeated with ΔTIRΔ30, a construct that lacks these 30 aa, they revealed that although TLR4 and p85 still bind the MyD88 mutant protein that contains only the DD/intermediate domain, binding of the protein encoded by ΔTIRΔ30 to TLR4 was consistently less than that seen between the original ΔTIR protein and TLR4. Thus, it appears that the presence of these 30 aa, when not constrained within the intact MyD88 protein, allows for increased interaction of the truncated MyD88 protein with TLR4. These 30 aa are located mainly within the βA strand of the TIR domain, which is a hydrophobic region [57]. This hydrophobicity may facilitate the observed, increased binding of ΔTIR, as hydrophobic regions play a major role in protein folding and structure.
Finally, we demonstrate herein that the M → A mutation in MyD88 has functional consequences for signaling, as evidenced by a diminished capacity for autoactivation and in ligand-dependent, TLR4-dependent signaling. The Y257A MyD88 mutant mediated NF-κB reporter activation to the same extent as WT MyD88 and showed a similar degree of association with TLR4, WT MyD88, and p85. In contrast to its increased capacity to associate with TLR4, MyD88, or p85, M260A overexpression induced only ∼50% of WT NF-κB luciferase activity shown by using two different NF-κB reporters. In addition, we have shown that unlike the ΔDD MyD88 protein, neither of our YXXM mutants nor the ΔTIR MyD88 protein could act as dominant-negative inhibitors of TLR4-mediated signaling.
Another interesting observation obtained from the binding and functional data was that the WT and point-mutant MyD88 proteins bound more strongly to the inactive P712H mutant TLR4 when compared with WT TLR4 (Fig. 4). In addition, we observed that the less signaling-competent M260A MyD88 bound more strongly to MyD88, TLR4, and p85. This trend, i.e., proteins with reduced signaling ability binding more strongly to other proteins within the signalosome, was also observed recently by Medvedev et al. [46] in experiments showing that their less-active TLR4 tyrosine mutants bound more strongly to MyD88. This suggests that mutations that alter the normal interaction of MyD88 with other components of the TLR4 signaling complex may diminish its dissociation from the complex and thereby, decrease signaling.
Our data support a hypothetical model of MyD88 dimer/p85 recruitment to the TLR4 complex as shown in Figure 7. It has been demonstrated by us herein and others [18, 43, 53] that MyD88 and PI3K constitutively bind and that MyD88 functions as a dimer. It has also been shown that upon LPS stimulation, MyD88 and PI3K are recruited to TLR4 with the same kinetics [18]. The binding of TIRAP to membrane-bound PIP2 is required for TLR4 signaling, and this event is required for subsequent recruitment of MyD88 [3]. Lastly, it is well documented that PI3K generates PIP3 by phosphorylating PIP2 [56]. We propose that dimeric MyD88, complexed with PI3K, is recruited to TLR4 via TIRAP. Our hypothetical model suggests that once recruited to TLR4, MyD88 and PI3K become phosphorylated by a src kinase(s) present in the TLR4 signaling complex [18, 46, 58, 59], leading to activation of PI3K. Activated PI3K, in turn, consumes PIP2 and thereby, down-regulates TLR4 signaling by preventing subsequent recruitment of TIRAP to the membrane. Therefore, the stronger association of PI3K and the MyD88 YXXA mutant with TLR4 may diminish signaling by facilitating a more sustained consumption of PIP2 at the membrane and in so doing, leads to diminished signaling by precluding more TIRAP from docking at the membrane or releasing already-bound TIRAP. An alternative possibility that should be acknowledged is that the decreased capacity of the YXXA mutant to signal may be the consequence of a structural change in MyD88 that is independent of PI3K but precludes the assembly of an active signalosome. Studies are under way to test these hypotheses using lentiviral vectors to introduce these MyD88 variants in primary cell types.
In the context of previous studies, our data support a negative regulatory role for PI3K in LPS signaling and support work by Luyendyk et al. [40] in which Pik3r1−/− mice and PTEN−/− mice were used to demonstrate that PI3K plays a negative role in many TLR4-mediated signaling events. PI3K is activated upon stimulation through TLR2, TLR5, TLR9, and IL-1R [14, 19, 21, 60,61,62], all of which use the MyD88-dependent pathway exclusively, and in the case of TLR5 and TLR9, a direct role for MyD88 in the activation of PI3K has been demonstrated by loss of activity upon mutagenesis [21, 26]. Furthermore, in support of our findings with TLR4/MyD88, there have been several additional studies that have suggested a negative role for PI3K in TLR2, TLR5, and IL-1 signaling [60,61,62]. These studies, taken together, support a general role of PI3K activation in TLR signaling, and our data, specifically, support a direct role for MyD88 in the activation of PI3K by TLR4 engagement. Relatively little has been done to examine the contribution of PI3K to the MyD88-independent pathway; however, it has been demonstrated that inhibition of PI3K activity increases LPS-induced IFN-β synthesis elicited by TLR3 or TLR4 ligands [16, 63]. In addition, we have shown previously that in macrophages from IFN-β−/− mice, LPS-induced P-Akt is markedly diminished [64].
Overall, these data provide novel insights into the molecular interactions among MyD88, PI3K, and TLR4 and strongly suggest that signaling is achieved upon the simultaneous interaction of multiple proteins that create a signaling platform, such that disruption of any one interaction has a universal effect on the other protein–protein interactions involved. This is supported by the findings of Toshchakov et al. [65], who used cell-permeable peptides corresponding to the BB loops of TLR4 and the adapter proteins and showed all of these peptides blocked TLR4 signaling. It was suggested that these peptides interfere with correct formation of a TLR4 signaling platform [65]. This correlates well with our data, in that dysregulation of any protein–protein interaction by mutations in MyD88 or by the cell-permeable BB loop adapter peptides would be predicted to result in altered platform assembly, leading to a disruption of signaling. Given that TLR4 signaling plays an important role in Gram-negative bacterial infections, sepsis, and atherosclerosis [66], the ability to manipulate this signaling and characterize the signaling platform is important for therapeutic treatments.
Acknowledgments
This work was supported by National Institutes of Health grants AI057490 and AI18797 (to S. N. V.) and AI-059524 (to A. E. M.).
References
- Hardiman G, Rock F L, Balasubramanian S, Kastelein R A, Bazan J F. Molecular characterization and modular analysis of human MyD88. Oncogene. 1996;13:2467–2475. [PubMed] [Google Scholar]
- Bonnert T P, Garka K E, Parnet P, Sonoda G, Testa J R, Sims J E. The cloning and characterization of human MyD88: a member of an IL-1 receptor related family. FEBS Lett. 1997;402:81–84. doi: 10.1016/s0014-5793(96)01506-2. [DOI] [PubMed] [Google Scholar]
- Kagan J C, Medzhitov R. Phosphoinositide-mediated adapter recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu M Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Rhee S H, Hwang D. Murine Toll-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF κ B and expression of the inducible cyclooxygenase. J Biol Chem. 2000;275:34035–34040. doi: 10.1074/jbc.M007386200. [DOI] [PubMed] [Google Scholar]
- Dunne A, Ejdeback M, Ludidi P L, O'Neill L A, Gay N J. Structural complementarity of Toll/interleukin-1 receptor domains in Toll-like receptors and the adapters Mal and MyD88. J Biol Chem. 2003;278:41443–41451. doi: 10.1074/jbc.M301742200. [DOI] [PubMed] [Google Scholar]
- Burns K, Janssens S, Brissoni B, Olivos N, Beyaert R, Tschopp J. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med. 2003;197:263–268. doi: 10.1084/jem.20021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M, Ni J, Feng P, Dixit V M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- Wesche H, Henzel W J, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S. Essential role for TIRAP in activation of the signaling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield M D. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003;4:313–319. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- Arbibe L, Mira J P, Teusch N, Kline L, Guha M, Mackman N, Godowski P J, Ulevitch R J, Knaus U G. Toll-like receptor 2-mediated NF-κ B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- Sarkar S N, Peters K L, Elco C P, Sakamoto S, Pal S, Sen G C. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat Struct Mol Biol. 2004;11:1060–1067. doi: 10.1038/nsmb847. [DOI] [PubMed] [Google Scholar]
- Aksoy E, Vanden Berghe W, Detienne S, Amraoui Z, Fitzgerald K A, Haegeman G, Goldman M, Willems F. Inhibition of phosphoinositide 3-kinase enhances TRIF-dependent NF-κ B activation and IFN-β synthesis downstream of Toll-like receptor 3 and 4. Eur J Immunol. 2005;35:2200–2209. doi: 10.1002/eji.200425801. [DOI] [PubMed] [Google Scholar]
- Reddy S A, Huang J H, Liao W S. Phosphatidylinositol 3-kinase in interleukin 1 signaling. Physical interaction with the interleukin 1 receptor and requirement in NFκB and AP-1 activation. J Biol Chem. 1997;272:29167–29173. doi: 10.1074/jbc.272.46.29167. [DOI] [PubMed] [Google Scholar]
- Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- Ishii K J, Takeshita F, Gursel I, Gursel M, Conover J, Nussenzweig A, Klinman D M. Potential role of phosphatidylinositol 3 kinase, rather than DNA-dependent protein kinase, in CpG DNA-induced immune activation. J Exp Med. 2002;196:269–274. doi: 10.1084/jem.20020773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y C, Lee C H, Kang H S, Chung H T, Kim H D. Wortmannin, a specific inhibitor of phosphatidylinositol-3-kinase, enhances LPS-induced NO production from murine peritoneal macrophages. Biochem Biophys Res Commun. 1997;240:692–696. doi: 10.1006/bbrc.1997.7722. [DOI] [PubMed] [Google Scholar]
- Rhee S H, Kim H, Moyer M P, Pothoulakis C. Role of MyD88 in phosphatidylinositol 3-kinase activation by flagellin/Toll-like receptor 5 engagement in colonic epithelial cells. J Biol Chem. 2006;281:18560–18568. doi: 10.1074/jbc.M513861200. [DOI] [PubMed] [Google Scholar]
- Backer J M, Myers M G, Jr, Shoelson S E, Chin D J, Sun X J, Miralpeix M, Hu P, Margolis B, Skolnik E Y, Schlessinger, J. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Windmiller D A, Wang L, Backer J M. YXXM motifs in the PDGF-β receptor serve dual roles as phosphoinositide 3-kinase binding motifs and tyrosine-based endocytic sorting signals. J Biol Chem. 2003;278:40425–40428. doi: 10.1074/jbc.C300225200. [DOI] [PubMed] [Google Scholar]
- Hellyer N J, Cheng K, Koland J G. ErbB3 (HER3) interaction with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochem J. 1998;333:757–763. doi: 10.1042/bj3330757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellyer N J, Kim M S, Koland J G. Heregulin-dependent activation of phosphoinositide 3-kinase and Akt via the ErbB2/ErbB3 co-receptor. J Biol Chem. 2001;276:42153–42161. doi: 10.1074/jbc.M102079200. [DOI] [PubMed] [Google Scholar]
- Gelman A E, LaRosa D F, Zhang J, Walsh P T, Choi Y, Sunyer J O, Turka L A. The adapter molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006;25:783–793. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas B D, Lu Y, Mao M, Zhang J, LaPushin R, Siminovitch K, Mills G B. Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:27455–27461. doi: 10.1074/jbc.M100556200. [DOI] [PubMed] [Google Scholar]
- Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Monick M M, Carter A B, Robeff P K, Flaherty D M, Peterson M W, Hunninghake G W. Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of β-catenin. J Immunol. 2001;166:4713–4720. doi: 10.4049/jimmunol.166.7.4713. [DOI] [PubMed] [Google Scholar]
- Jones B W, Heldwein K A, Means T K, Saukkonen J J, Fenton M J. Differential roles of Toll-like receptors in the elicitation of proinflammatory responses by macrophages. Ann Rheum Dis. 2001;60:iii6–12. doi: 10.1136/ard.60.90003.iii6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Hoshino K. Myeloid differentiation factor 88-dependent and -independent pathways in Toll-like receptor signaling. J Infect Dis. 2003;187:S356–S363. doi: 10.1086/374749. [DOI] [PubMed] [Google Scholar]
- Li X, Tupper J C, Bannerman D D, Winn R K, Rhodes C J, Harlan J M. Phosphoinositide 3 kinase mediates Toll-like receptor 4-induced activation of NF-κ B in endothelial cells. Infect Immun. 2003;71:4414–4420. doi: 10.1128/IAI.71.8.4414-4420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Guerra M J, Castrillo A, Martin-Sanz P, Bosca L. Negative regulation by phosphatidylinositol 3-kinase of inducible nitric oxide synthase expression in macrophages. J Immunol. 1999;162:6184–6190. [PubMed] [Google Scholar]
- Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- Monick M M, Robeff P K, Butler N S, Flaherty D M, Carter A B, Peterson M W, Hunninghake G W. Phosphatidylinositol 3-kinase activity negatively regulates stability of cyclooxygenase 2 mRNA. J Biol Chem. 2002;277:32992–33000. doi: 10.1074/jbc.M203218200. [DOI] [PubMed] [Google Scholar]
- Pahan K, Raymond J R, Singh I. Inhibition of phosphatidylinositol 3-kinase induces nitric-oxide synthase in lipopolysaccharide- or cytokine-stimulated C6 glial cells. J Biol Chem. 1999;274:7528–7536. doi: 10.1074/jbc.274.11.7528. [DOI] [PubMed] [Google Scholar]
- Dennehy K M, Ferwerda G, Faro-Trindade I, Pyz E, Willment J A, Taylor P R, Kerrigan A, Tsoni S V, Gordon S, Meyer-Wentrup F, Adema G J, Kullberg B J, Schweighoffer E, Tybulewicz V, Mora-Montes H M, Gow N A, Williams D L, Netea M G, Brown G D. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F, Strominger J L. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- Lee J Y, Ye J, Gao Z, Youn H S, Lee W H, Zhao L, Sizemore N, Hwang D H. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- Luyendyk J P, Schabbauer G A, Tencati M, Holscher T, Pawlinski R, Mackman N. Genetic analysis of the role of the PI3K-Akt pathway in lipopolysaccharide-induced cytokine and tissue factor gene expression in monocytes/macrophages. J Immunol. 2008;180:4218–4226. doi: 10.4049/jimmunol.180.6.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigo Y, Isomura M, Nishiwaki T, Tamari M, Ishikawa S, Kai M, Murata Y, Takeuchi K, Yamane Y, Hayashi R, Minami M, Fujino M A, Hojo Y, Uchiyama I, Takagi T, Nakamura Y. Characterization of a 1200-kb genomic segment of chromosome 3p22–p21.3. DNA Res. 1999;6:37–44. doi: 10.1093/dnares/6.1.37. [DOI] [PubMed] [Google Scholar]
- Means T K, Lien E, Yoshimura A, Wang S, Golenbock D T, Fenton M J. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999;163:6748–6755. [PubMed] [Google Scholar]
- McIntire F C, Sievert H W, Barlow G H, Finley R A, Lee A Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967;6:2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Chow J C, Young D W, Golenbock D T, Christ W J, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia M A, Medvedev A E, Thomas K E, Cuesta N, Toshchakov V, Ren T, Cody M J, Michalek S M, Rice N R, Vogel S N. Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: effects of TLR “homotolerance” versus “heterotolerance” on NF-κ B signaling pathway components. J Immunol. 2003;170:508–519. doi: 10.4049/jimmunol.170.1.508. [DOI] [PubMed] [Google Scholar]
- Medvedev A E, Piao W, Shoenfelt J, Rhee S H, Chen H, Basu S, Wahl L M, Fenton M J, Vogel S N. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem. 2007;282:16042–16053. doi: 10.1074/jbc.M606781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi S T, Gros P, Malo D. The Lps locus: genetic regulation of host responses to bacterial lipopolysaccharide. Inflamm Res. 1999;48:613–620. doi: 10.1007/s000110050511. [DOI] [PubMed] [Google Scholar]
- Burns K, Martinon F, Esslinger C, Pahl H, Schneider P, Bodmer J L, Di Marco F, French L, Tschopp J. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–12209. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway. C A., Jr MyD88 is an adapter protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K A, Palsson-McDermott E M, Bowie A G, Jefferies C A, Mansell A S, Brady G, Brint E, Dunne A, Gray P, Harte M T, McMurray D, Smith D E, Sims J E, Bird T A, O'Neill L A. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- Akazawa T, Masuda H, Saeki Y, Matsumoto M, Takeda K, Tsujimura K, Kuzushima K, Takahashi T, Azuma I, Akira S, Toyoshima K, Seya T. Adjuvant-mediated tumor regression and tumor-specific cytotoxic response are impaired in MyD88-deficient mice. Cancer Res. 2004;64:757–764. doi: 10.1158/0008-5472.can-03-1518. [DOI] [PubMed] [Google Scholar]
- Bjorkbacka H, Fitzgerald K A, Huet F, Li X, Gregory J A, Lee M A, Ordija C M, Dowley N E, Golenbock D T, Freeman M W. The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiol Genomics. 2004;19:319–330. doi: 10.1152/physiolgenomics.00128.2004. [DOI] [PubMed] [Google Scholar]
- Loiarro M, Sette C, Gallo G, Ciacci A, Fanto N, Mastroianni D, Carminati P, Ruggiero V. Peptide-mediated interference of TIR domain dimerization in MyD88 inhibits interleukin-1-dependent activation of NF-{κ}B. J Biol Chem. 2005;280:15809–15814. doi: 10.1074/jbc.C400613200. [DOI] [PubMed] [Google Scholar]
- Jensen L E, Whitehead A S. ELAM-1/E-selectin promoter contains an inducible AP-1/CREB site and is not NF-κB-specific. Biotechniques. 2003;35:54–56. doi: 10.2144/03351bm05. [DOI] [PubMed] [Google Scholar]
- Mukaida N, Morita M, Ishikawa Y, Rice N, Okamoto S, Kasahara T, Matsushima K. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-κ B is target for glucocorticoid-mediated interleukin 8 gene repression. J Biol Chem. 1994;269:13289–13295. [PubMed] [Google Scholar]
- Hawkins P T, Jackson T R, Stephens L R. Platelet-derived growth factor stimulates synthesis of PtdIns(3,4,5)P3 by activating a PtdIns(4,5)P2 3-OH kinase. Nature. 1992;358:157–159. doi: 10.1038/358157a0. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tao X, Shen B, Horng T, Medzhitov R, Manley J L, Tong L. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature. 2000;408:111–115. doi: 10.1038/35040600. [DOI] [PubMed] [Google Scholar]
- Cuschieri J, Billgren J, Maier R V. Phosphatidylcholine-specific phospholipase C (PC-PLC) is required for LPS-mediated macrophage activation through CD14. J Leukoc Biol. 2006;80:407–414. doi: 10.1189/jlb.1105622. [DOI] [PubMed] [Google Scholar]
- Gray P, Dunne A, Brikos C, Jefferies C A, Doyle S L, O'Neill L A. MyD88 adapter-like (Mal) is phosphorylated by Bruton’s tyrosine kinase during TLR2 and TLR4 signal transduction. J Biol Chem. 2006;281:10489–10495. doi: 10.1074/jbc.M508892200. [DOI] [PubMed] [Google Scholar]
- Yu Y, Nagai S, Wu H, Neish A S, Koyasu S, Gewirtz A T. TLR5-mediated phosphoinositide 3-kinase activation negatively regulates flagellin-induced proinflammatory gene expression. J Immunol. 2006;176:6194–6201. doi: 10.4049/jimmunol.176.10.6194. [DOI] [PubMed] [Google Scholar]
- Martin M, Schifferle R E, Cuesta N, Vogel S N, Katz J, Michalek S M. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J Immunol. 2003;171:717–725. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- Choi E K, Jang H C, Kim J H, Kim H J, Kang H C, Paek Y W, Lee H C, Lee S H, Oh W M, Kang I C. Enhancement of cytokine-mediated NF-κB activation by phosphatidylinositol 3-kinase inhibitors in monocytic cells. Int Immunopharmacol. 2006;6:908–915. doi: 10.1016/j.intimp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Wang H, Garcia C A, Rehani K, Cekic C, Alard P, Kinane D F, Mitchell T, Martin M. IFN-β production by TLR4-stimulated innate immune cells is negatively regulated by GSK3-β. J Immunol. 2008;181:6797–6802. doi: 10.4049/jimmunol.181.10.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K E, Galligan C L, Newman R D, Fish E N, Vogel S N. Contribution of interferon-β to the murine macrophage response to the Toll-like receptor 4 agonist, lipopolysaccharide. J Biol Chem. 2006;281:31119–31130. doi: 10.1074/jbc.M604958200. [DOI] [PubMed] [Google Scholar]
- Toshchakov V U, Basu S, Fenton M J, Vogel S N. Differential involvement of BB loops of Toll-IL-1 resistance (TIR) domain-containing adapter proteins in TLR4- versus TLR2-mediated signal transduction. J Immunol. 2005;175:494–500. doi: 10.4049/jimmunol.175.1.494. [DOI] [PubMed] [Google Scholar]
- Cook D N, Pisetsky D S, Schwartz D A. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]