Abstract
The effects of vascular endothelial growth factor (VEGF) blockade on the vascular biology of human tumors are not known. Here we show here that a single infusion of the VEGF-specific antibody bevacizumab decreases tumor perfusion, vascular volume, microvascular density, interstitial fluid pressure and the number of viable, circulating endothelial and progenitor cells, and increases the fraction of vessels with pericyte coverage in rectal carcinoma patients. These data indicate that VEGF blockade has a direct and rapid antivascular effect in human tumors.
VEGF has a crucial role in physiological and pathological angiogenesis1–3. Although VEGF blockade, alone or in combination with cytotoxic therapies, is being tested in a number of clinical trials4, including the first successful phase 3 clinical trial5, the effects of anti-VEGF treatment on the vascular biology of human tumors are not known. To this end, we recently initiated a National Cancer Institute–sponsored phase 1 clinical trial that integrates the VEGF-specific antibody bevacizumab (Avastin; Genentech) into a contemporary treatment program of preoperative chemotherapy and radiation therapy followed by surgery, for patients with primary and nonmetastatic rectal cancer. To gain insight into the mechanisms of action of bevacizumab, we designed the trial to evaluate the effects of bevacizumab alone on (i) tumor physiology (blood perfusion, blood volume, permeability–surface area product, microvascular density (MVD), perivascular coverage, interstitial fluid pressure (IFP) and 18-fluorodeoxyglucose (FDG) uptake); (ii) systemic response (VEGF level in blood, number of circulating endothelial cells (CECs) and progenitor cells); and (iii) tumor response (see Supplementary Note online for methods).
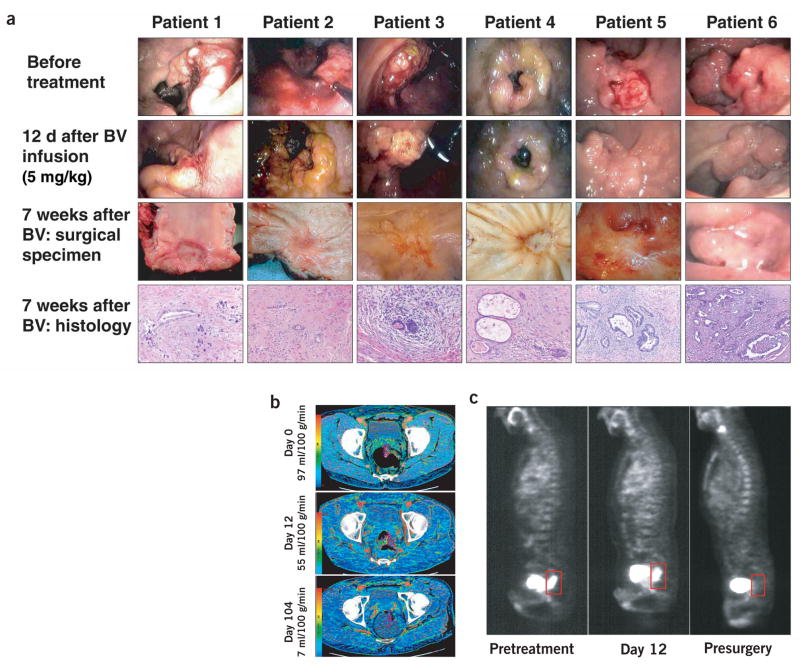
Six patients with primary and locally advanced adenocarcinoma of the rectum have been enrolled in a preoperative treatment protocol of bevacizumab administration alone (5 mg/kg intravenously), followed after 2 weeks—the approximate half-life of bevacizumab in circulation—by concurrent administration of bevacizumab with 5-fluorouracil and external beam radiation therapy to the pelvis and surgery, 7 weeks after treatment completion. Twelve days after bevacizumab infusion, flexible sigmoidoscopy (Fig. 1) revealed that bevacizumab induced tumor regression of >30% in patient 1, and no change in tumor size in the remaining five patients. Functional computed tomography (CT) scans at this time point indicated significant decreases in tumor blood perfusion (40–44%; P < 0.05) and blood volume (16–39% in four of five patients analyzed; P < 0.05; Figs. 1b and 2; see Supplementary Table 1 online for group statistics). This was accompanied by a significant decrease in tumor MVD (29–59% in five patients analyzed; P < 0.05; Fig. 2d). These three sets of data provide direct evidence of the antivascular effects of bevacizumab in human tumors, which is in line with preclinical findings6,7.
Figure 1.
Effect of treatment on tumors in patients who completed entire combined treatment regimen, and surgery. (a) Endoscopic and pathological evaluation of rectal tumors. Surgical specimens showed grade II tumor regression in patients 1–5 and grade III in patient 6, by Mandard criteria (see Supplementary Note). Endoscopic image (instead of surgical specimen) was taken for patient 6, 3.5 weeks before surgery. BV, bevacizumab. (b) Representative functional CT images of blood perfusion before treatment (day 0), after bevacizumab (day 12) and after completion of treatment (day 104) in patient 5. (c) Tumor FDG uptake before treatment (pretreatment), 12 d after bevacizumab treatment and 6–7 weeks after completion of all neoadjuvant therapy (presurgery). Sagittal projections of FDG-PET scans for patient 1 are shown. Tumor is outlined in box, posterior to bladder.
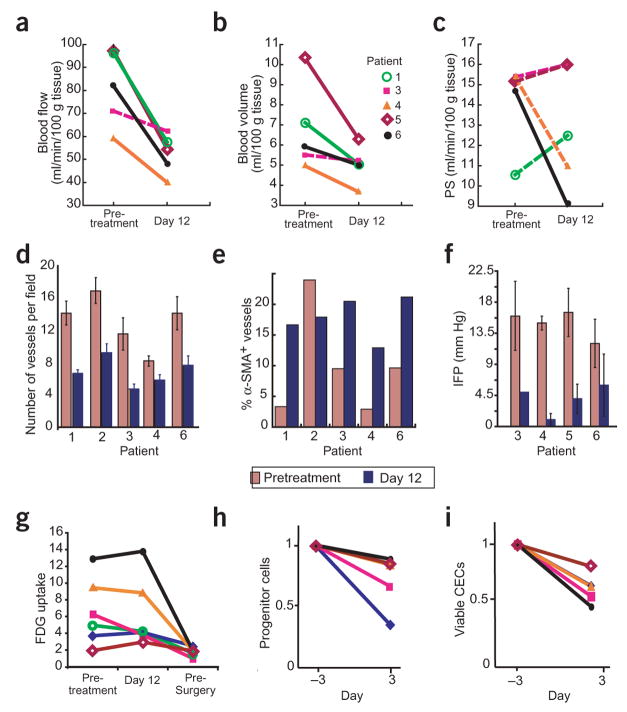
Figure 2.
Effect of a single injection of bevacizumab on tumor vasculature and FDG uptake. Parameters were obtained pretreatment and after one bevacizumab infusion. (a–c) Blood perfusion (a), blood volume (b) and permeability–surface area product (PS; c). Significant decreases after treatment are indicated by solid lines (P < 0.05 by t-test). Blood flow and blood volume decreased significantly in four of the patients. (d) Microvascular density. All patients showed significant decreases after treatment (P < 0.05 by t-test). (e) Fraction of vessels with pericyte coverage. The difference in the fraction of vessels positive for α-smooth muscle actin (α-SMA) in patient 2 was identified as an outlier by the Extreme Studentized Deviate test. Paired t-test analyses of the mean values that included and excluded the data of patient 2 had P < 0.09 and 0.001, respectively. (f) Mean tumor IFP decreased significantly after bevacizumab (P < 0.01 by paired t-test). (g) Tumor FDG uptake before treatment, on day 12 and presurgery (day 93), normalized for muscle values. On day 12 after bevacizumab treatment, a 40% decrease was observed in patient 3, and no change in the other patients. Lower levels were found in all patients before surgery except for patient 5, who had low levels throughout the treatment. In comparison to pretreatment and day 12 values, the median standard uptake value was significantly lower on day 93 (P < 0.01; Supplementary Table 1). (h) Circulating progenitor/stem cells (AC133+; left) and viable CECs (right) in peripheral blood. Samples were run to acquire 50,000 events in the mononuclear/lymphocyte gate. For both cell populations, bevacizumab induced a significant decrease in mean values (P < 0.05 by Wilcoxon signed-rank test). Key in b applies to a,c, g–i.
Twelve days after bevacizumab treatment, IFP was reduced in four of four patients (Fig. 2f) and overall mean IFP decreased significantly from 15.0 ± 2.0 to 4.0 ± 2.2 mm Hg (P < 0.01). The decrease in IFP is in concert with our preclinical findings6. Elevated IFP, a hallmark of solid tumors, is a result of abnormalities in tumor vessels (such as abnormal structure of the vessel wall). The decrease in IFP after anti-VEGF treatment may be a result of ‘normalization’; that is, the resumption of normal function in the tumor vasculature8. The increased fraction of vessels positive for α-smooth muscle actin in four of five patients (Fig. 2e and Supplementary Fig. 1 online) is supportive of vascular normalization9. As a result of the decrease in vascular volume and MVD, one would expect a reduction in vascular surface area, and hence a lowering of the permeability–surface area product. Surprisingly, four of five patients had no significant changes in permeability–surface area product (Fig. 2c and Supplementary Table 1), providing indirect evidence for improved extravasation of the CT contrast agent from the normalized vasculature. Finally, on day 12 the follow-up positron emission tomography (PET) scans indicated no change in tumor FDG uptake in five patients, and showed a 40% decrease in patient 3 (Fig. 2g). Collectively, these data suggest that the efficiency of blood vessels after bevacizumab treatment is improved. These clinical findings are consistent with previous preclinical data on tumor oxygenation6 and drug uptake10 after anti-VEGF treatment.
In addition to being a mitogen and survival factor for endothelial cells, VEGF mobilizes progenitor cells from the bone marrow into the circulation11. VEGF blockade decreased the number of progenitor cells circulating on day 3 (Fig. 2h). No decrease in circulating VEGF (plasma, serum and urine) was detected with the current assay (see Methods; data not shown). After bevacizumab treatment, the number of CD31brightCD45− cells, but not the total number of CD31+CD45− cells, decreased in all patients (Fig. 2h). Further analysis indicated that CD31brightCD45− cells represented viable CECs (Supplementary Fig. 2 online). An increase in the number and viability of CECs was recently found in lymphoma and breast cancer patients12. In addition, preclinical studies indicate that CEC kinetics might serve as a surrogate marker of response to treatment13. Based on these data, we suggest that the kinetics of progenitor cells or viable CECs in peripheral blood should be explored as an early indicator of tumor response to anti-VEGF agents.
Six weeks after completion of the bevacizumab, radiation therapy and chemotherapy regimen, follow-up PET scans (Fig. 1c) showed decreased tumor FDG uptake compared with pretreatment values in five patients (Fig. 2g). Tumor FDG uptake in patient 5 was low, and similar before and at the end of therapy (Fig. 2g). Notably, all six patients completed the combined treatment without dose-limiting toxicity, and underwent surgery without perioperative or postoperative complications. Macroscopic and histologic analysis of the surgical specimens revealed a marked response in all six patients, with only microscopic disease in five of the patients (Fig. 1a and Supplementary Note).
High doses of bevacizumab are more effective when used as monotherapy for highly VEGF-dependent tumors such as renal-cell carcinoma14. Our data from six consecutive patients show for the first time that even at low doses, bevacizumab alone can decrease perfusion, MVD and IFP in a solid tumor, and decrease the number of CECs. The decrease in IFP and increase in the fraction of vessels with pericyte coverage support the normalization hypothesis8. This normalization process may retard the shedding of metastatic cells in the circulation and improve the delivery of therapeutic agents in tumors. Bevacizumab may also sensitize the endothelium to cytotoxic agents. Collectively, these mechanisms may explain the unprecedented efficacy of bevacizumab in recent clinical trials, as well as the possible synergistic or additive interaction between antiangiogenic and cytotoxic therapies that has been observed in preclinical settings for more than a decade4,15. The identification of valid surrogate markers for antiangiogenic therapy, alone or combined with cytotoxic therapies, has been elusive. The results of this phase 1 study will hopefully facilitate and stimulate future research in this area.
Supplementary Material
Acknowledgments
This study was supported by two National Cancer Institute grants (R21 CA099237 to C.G.W. and PO1 CA80124 to R.K.J.). D.G.D. is a Cancer Research Institute fellow. R.T.T. is a fellow of the Susan G. Komen Breast Cancer Foundation. We thank T. Lee for his contribution to the CT analysis, M. Ancukiewicz, T.P. Padera and W. Strauss for helpful comments, and J. Tooredman for the ELISAs.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Carmeliet P, Jain RK. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Dvorak HF. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Gerber HP, LeCouter J. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 4.Kerbel R, Folkman J. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy M. Lancet. 2003;361:1959. doi: 10.1016/S0140-6736(03)13603-3. [DOI] [PubMed] [Google Scholar]
- 6.Lee C, et al. Cancer Res. 2000;60:5565–5570. [PubMed] [Google Scholar]
- 7.Yuan F, et al. Proc Natl Acad Sci USA. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain RK. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 9.Jain RK. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 10.Wildiers H, et al. Br J Cancer. 2003;88:1979–1986. doi: 10.1038/sj.bjc.6601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafii S, et al. Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 12.Mancuso P, et al. Blood. 2001;97:3658–3661. doi: 10.1182/blood.v97.11.3658. [DOI] [PubMed] [Google Scholar]
- 13.Monestiroli S, et al. Cancer Res. 2001;61:4341–4344. [PubMed] [Google Scholar]
- 14.Yang JC, et al. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teicher BA. Cancer Metastasis Rev. 1996;15:247–272. doi: 10.1007/BF00437479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.