Abstract
The failure of axons to regenerate after spinal cord injury remains one of the greatest challenges facing both medicine and neuroscience, but in the last twenty years there have been tremendous advances in the field of spinal cord injury repair. One of the most important of these has been the identification of inhibitory proteins in CNS myelin, and this has led to the development of strategies that will enable axons to overcome myelin inhibition. Elevation of intracellular cyclic AMP (cAMP) has been one of the most successful of these strategies, and in this review we examine how cAMP signaling promotes axonal regeneration in the CNS. Intracellular cAMP levels can be increased through a peripheral conditioning lesion, administration of cAMP analogues, priming with neurotrophins, or treatment with the phosphodiesterase inhibitor rolipram, and each of these methods has been shown to overcome myelin inhibition both in vitro and in vivo. It is now known that the effects of cAMP are transcription-dependent, and that cAMP-mediated activation of CREB leads to upregulated expression of genes such as arginase I and interleukin-6. The products of these genes have been shown to directly promote axonal regeneration, which raises the possibility that other cAMP-regulated genes could yield additional agents that would be beneficial in the treatment of spinal cord injury. Further study of these genes, in combination with human clinical trials of existing agents such as rolipram, would allow the therapeutic potential of cAMP to be fully realized.
Keywords: Cyclic AMP, regeneration, myelin, MAG, rolipram, spinal cord injury, axon
I. Mechanisms of myelin signal transduction
A. Myelin-associated inhibitors
Cajal was the first to propose that the central nervous system (CNS) environment limits axonal regeneration after injury, but only recently has CNS myelin been identified as a major factor contributing to regenerative failure. In 1988, the laboratory of Marin Schwab provided the first direct evidence that CNS myelin contains proteins that inhibit axonal growth. In their first study, 35 and 250 kD protein fractions were isolated from CNS myelin and these were subsequently shown to inhibit neurite outgrowth in vitro (Caroni and Schwab, 1988a). Monoclonal antibodies were then raised against these proteins and it was found that the IN-1 antibody blocked inhibition by myelin in vitro, and enhanced regeneration of corticospinal axons after spinal cord injury (Caroni and Schwab, 1988b; Schnell and Schwab, 1990; 1993; Bregman et al., 1995). A protein named Nogo was eventually identified as the antigen of the IN-1 antibody, but of the three Nogo isoforms (A, B and C) that are expressed in the CNS, only Nogo-A is enriched in oligodendrocytes (Chen et al., 2000; GrandPre et al., 2000; Prinjha et al., 2000). Nogo-A is a member of the Reticulon family of proteins and inhibits neurite outgrowth through two distinct domains: a 66-residue extracellular domain (Nogo-66) that is shared by all three Nogo isoforms, and amino-Nogo, which is unique to Nogo-A (Chen et al., 2000; GrandPre et al., 2000; Prinjha et al., 2000).
Six years earlier, myelin-associated glycoprotein (MAG) became the first myelin-associated inhibitor to be identified. MAG was already a well-characterized component of both PNS and CNS myelin, but its role in axonal plasticity was unknown. To address this issue, our laboratory developed a unique neurite outgrowth assay in which monolayers of MAG-expressing Chinese hamster ovary (CHO) cells were used as a substrate (Mukhopadhyay et al., 1994). When dorsal root ganglion (DRG) and cerebellar neurons were plated on these monolayers, neurite outgrowth was strongly inhibited (Mukhopadhyay et al., 1994). A parallel study demonstrated that recombinant MAG ectodomain could inhibit neurite outgrowth in a similar manner and that immunodepletion of MAG from CNS myelin reversed this effect (McKerracher et al., 1994). Sequencing experiments had previously established that MAG is a member of the immunoglobulin (Ig) superfamily, containing five extracellular Ig-like domains (Salzer et al., 1987; 1990). It is also a sialic acid-binding protein and its first four Ig-like domains are homologous to the Siglec family of sialic acid binding Ig-like lectins (Kelm et al., 1994). The sialic acid binding site of MAG is localized to arginine 118 (R118) in the fifth Ig domain and mutation of this residue abolishes inhibition by soluble MAG (Tang et al., 1997). A requirement for sialic acid binding in MAG inhibition is unlikely however, because inhibition by membrane-bound MAG is unaffected by R118 mutation (Tang et al., 1997). It has therefore been proposed that inhibition and sialic acid binding are mediated by two distinct sites on the MAG protein.
In the PNS, MAG contributes to the initiation of myelination by Schwann cells (Owens and Bunge, 1989) and later maintains the interaction between the axon and its myelin sheath (Martini and Schachner, 1986). Studies of MAG null mutant mice later determined that the latter function was crucial to sustain normal axonal morphology. Myelination of peripheral axons by Schwann cells occurs normally in the absence of MAG, but eventually, the disruption of axon-myelin interactions in these animals leads to abnormalities in the periaxonal space and loss of myelin compaction (Li et al., 1994; Montag et al., 1994). In addition, MAG null mutants older than 8 months display widespread degeneration of axons and myelin, which indicates that MAG is necessary for long-term survival of axons (Fruttiger et al., 1995).
Recently published findings suggest that MAG may also play a role in axonal guidance. During development, proprioceptive axons are repelled from the dorsal horn of the spinal cord through semaphorin 6C and 6D signaling through the plexin A1 receptor, but in plexin A1 null mutant mice, aberrant growth of these axons into the medial dorsal horn is observed (Yoshida et al., 2006). Conversely, sensory axons expressing IB4 lectin normally terminate within the medial dorsal horn; however, in plexin A1 mutants, IB4-positive axons were absent from this area. Interestingly, the number of MAG-expressing oligodendrocytes within the medial dorsal horn increased dramatically from P4 onward in plexin A1 mutants, and these oligodendrocytes were intimately associated with the ingrowing proprioceptive axons (Yoshida et al., 2006). It was therefore proposed that the oligodendrocytes co-migrate with the axons as they grow into the dorsal horn, and that this creates ectopic foci of MAG expression that later cause IB4-positive axons to be repelled (Yoshida et al., 2006).
Oligodendrocyte myelin glycoprotein (OMgp) and ephrin B3 are the latest proteins that have been shown to function as myelin inhibitors. OMgp is a glycosyl-phosphatidylinositol (GPI)-linked protein with a leucine-rich repeat (LRR) domain and is expressed in both neurons and oligodendrocytes (Mikol and Stefansson, 1988; Mikol et al., 1990; Habib et al., 1998, Wang et al., 2002a). It is a potent inhibitor of neurite outgrowth in vitro (Wang et al., 2002a), and in the spinal cord, OMgp is expressed at the nodes of Ranvier, where it maintains the normal morphology of these structures by inhibiting collateral axon sprouting (Huang et al., 2005). Ephrin B3 is an axonal guidance cue that repels corticospinal axons from the midline of the spinal cord during development and this effect is mediated by binding to the EphA4 receptor (Yokoyama et al., 2001). It was subsequently found that ephrin B3 is expressed in mature oligodendrocytes and that neurite outgrowth for cortical neurons was inhibited by treatment with ephrin B3-Fc (Benson et al., 2005).
B. Receptors and intracellular signaling
MAG, Nogo and OMgp have no sequence similarity or structural homology, yet surprisingly they all bind to a common receptor complex to mediate inhibition. The Nogo receptor (NgR1) was cloned from a mouse expression library using a soluble form of Nogo-66, and it was shown that binding of Nogo-66 to NgR1 was necessary to induce growth cone collapse (Fournier et al., 2001). NgR1 can be precipitated from primary neurons using soluble MAG and it was shown that this binding was independent of sialic acid (Domeniconi et al., 2002). Neurite outgrowth was inhibited by MAG binding to NgR1, and this inhibition could be blocked by neutralization of NgR1 function through the addition of NgR1 antibody, soluble NgR1, or dominant-negative NgR1 (Domeniconi et al., 2002; Liu et al., 2002). MAG is the only myelin inhibitor that can also mediate inhibition through a structurally related receptor known as NgR2; however, binding to this receptor is sialic acid-dependent (Venkatesh et al., 2005). Expression cloning and co-immunoprecipitation experiments revealed that OMgp is a third high-affinity ligand for NgR1 (Wang et al., 2002a). It was also shown that enzymatic removal of NgR1 and all other glycosyl-phosphatidylinositol (GPI)-linked proteins caused DRG neurons to become insensitive to OMgp (Wang et al., 2002a). Conversely, ectopic expression of NgR1 conferred responsiveness to OMgp and inhibited neurite outgrowth in embryonic retinal ganglion neurons that are normally unresponsive to myelin (Wang et al., 2002a).
The functions of NgR1 and NgR2 are not limited to inhibition, as a newly published study describes a role for NgR1 and NgR2 in macrophage clearance. Recruitment of macrophages to the injury site is an important component of peripheral nerve regeneration, as they phagocytose the axonal and myelin debris generated by Wallerian degeneration (Mueller et al., 2003). These macrophages migrate out of the nerve once Wallerian degeneration is complete, but the signals that regulate this efflux are unknown. Fry and colleagues (2007) present evidence that NgR binding to newly synthesized myelin is responsible for this phenomenon. Ultrastructural analysis of crushed sciatic nerves revealed that the onset of macrophage efflux is correlated with the remyelination of regenerated axons, and it was also shown that activated macrophages upregulate expression of NgR1 and NgR2 as they accumulate in the injured sciatic nerve (Fry et al., 2007). It was therefore proposed that remyelination serves as the stimulus for NgR-mediated macrophage efflux. This hypothesis was supported by the observation that macrophage migration was impaired in sciatic nerves from NgR1 and MAG null mutant mice, which suggested that MAG binding to NgR1 is required to expel macrophages from peripheral nerve (Fry et al., 2007).
Both NgR1 and NgR2 are both GPI-linked proteins (Fournier et al., 2001; Venkatesh et al., 2005), which means that they are incapable of intracellular signaling and must therefore rely on co-receptors to mediate inhibition. The first co-receptor to be identified was the p75 neurotrophin receptor (p75NTR), a member of the tumor necrosis factor receptor superfamily (Roux and Barker, 2002). Yamashita and colleagues (2002) presented the first evidence of p75NTR's role in MAG signaling by showing that DRG and cerebellar neurons from p75NTR null mutant mice were not inhibited by MAG. Immunoprecipitation studies then demonstrated that there was a physical association between NgR1 and p75NTR, as MAG, Nogo-66 and OMgp were each able to precipitate receptor complexes containing NgR1 and p75NTR (Wang et al., 2002b; Wong et al., 2002). Binding of myelin inhibitors to the NgR1-p75NTR receptor complex activates protein kinase C (PKC) and causes the small GTPase Rho to assume its active, GTP-bound state (Yamashita et al., 2002; Yamashita and Tohyama, 2003; Sivasankaran et al., 2004). Rho-mediated activation of downstream effectors such as Rho-associated kinase (ROCK) then induces actin polymerization, which leads to growth cone collapse and inhibition of neurite outgrowth (Dergham et al., 2002; Yamashita et al., 2002). Predictably, pharmacological inhibitors of PKC, Rho, and ROCK have been highly effective in overcoming myelin inhibitors in vitro and promoting axonal regeneration in the injured spinal cord (Lehmann et al., 1999; Dergham et al., 2002; Fournier et al., 2003; Sivasankaran et al., 2004).
A recent study by our laboratory provided further insight into MAG signaling mechanisms by showing that regulated intramembrane proteolysis of p75NTR is required for MAG-mediated activation of Rho (Domeniconi et al., 2005). In this study, cerebellar neurons were treated with soluble MAG and this induced cleavage of the p75NTR extracellular domain by α-secretase. This was followed by a PKC-dependent γ-secretase cleavage within the transmembrane domain of p75NTR, which released the intracellular domain into the cytoplasm. Cleavage of p75NTR was necessary for both activation of Rho and inhibition of neurite outgrowth as pharmacological inhibitors of α- and γ-secretase blocked these events and led to increased neurite outgrowth on MAG (Domeniconi et al., 2005). Conversely, expression of the cytoplasmic domain of p75NTR was sufficient to induce activation of Rho and inhibit neurite outgrowth on a permissive substrate (Yamashita et al., 1999; Domeniconi et al., 2005).
Neurons in many regions of the CNS do not express p75NTR, and so, it was postulated that another receptor was transducing myelin signals in these areas. This was confirmed when two groups independently discovered that TROY (also known as TAJ), another member of the TNF receptor family, could form a receptor complex with NgR1 and inhibit neurite outgrowth through activation of Rho (Park et al., 2005; Shao et al., 2005). Further evidence of TROY's role in myelin inhibition was provided by experiments in which TROY function was suppressed by addition of soluble TROY, expression of dominant negative TROY, or generation of TROY/TAJ-deficient mice. In each case, cerebellar and DRG neurons were less inhibited by OMgp and Nogo-66 (Park et al., 2005; Shao et al., 2005).
LINGO-1 is a leucine rich repeat protein that is exclusively expressed in the nervous system and serves as a third component of the NgR1 receptor complex (Mi et al., 2004). Coexpression of LINGO-1 with NgR1 and p75NTR in COS cells confers responsiveness to MAG, Nogo-66 and OMgp and activates RhoA, and this is mediated by the signaling through the cytoplasmic domain (Mi et al., 2004). It is not known how LINGO-1 signaling leads to RhoA activation, but neurite outgrowth on myelin was significantly increased when dominant negative LINGO-1 was expressed in cerebellar neurons, suggesting that LINGO-1 is essential for inhibition (Mi et al., 2004).
Lastly, epidermal growth factor receptor (EGFR) can now be counted among the transmembrane proteins that have a role in myelin signal transduction. EGFR is phosphorylated in response to Nogo-66 and OMgp, and this activation is dependent on both calcium and the NgR1 receptor complex (Koprivica et al., 2005). Treatment with epidermal growth factor does not lead to inhibition and myelin inhibitors do not bind to EGFR, and so, it is unlikely that direct activation of the receptor mediates this effect. Furthermore, EGFR could not be immunoprecipitated with NgR1 or p75NTR, and this led the authors to conclude that phosphorylation of the receptor occurs through transactivation (Koprivica et al., 2005). Neurite outgrowth on myelin, MAG and Nogo-66 was enhanced following inhibition of EGFR kinase activity, as was regeneration of retinal ganglion cell axons in the optic nerve (Koprivica et al., 2005). These findings suggested that like p75NTR, TROY, and LINGO-1, EGFR is required for myelin inhibition. This not only illustrates the growing complexity of myelin signaling, but also indicates that there may be redundancy in many of these pathways.
II. Reversal of myelin inhibition through cyclic AMP signaling
A. Cyclic AMP analogues
Because MAG, Nogo and OMgp signal through a common pathway, pharmacological inhibition of PKC, Rho and ROCK is a logical strategy to promote axonal regeneration. However, it is also possible to manipulate the neuron at the molecular level so that it no longer responds to myelin inhibitors. This approach is derived from the observation that MAG is initially permissive to neurite outgrowth and only becomes inhibitory beyond a specific developmental timepoint. When P1 DRG neurons are plated on CHO cells expressing MAG, neurite outgrowth is promoted, whereas growth from DRG neurons that are P5 and older is inhibited (Johnson et al., 1989, Mukhopadhyay et al., 1994; Cai et al., 2001). This switch from growth promotion to inhibition has been observed in all neuronal subtypes that have been tested to date. For some neurons the switch occurs during embryonic development, while in others it occurs post-natally (Mukhopadhyay et al., 1994; de Bellard et al., 1996; Turnley and Bartlett, 1998). The MAG protein remains unaltered during development, and so, it was hypothesized that molecular changes within the neurons must be responsible for triggering inhibition.
In 2001, we demonstrated that there is a direct correlation between neuronal cAMP levels and inhibition of neurite outgrowth by MAG and myelin. Levels of endogenous cAMP are high in P1 DRG neurons and substantial neurite outgrowth is observed on MAG and myelin at this timepoint; however, at P3−4 there is a sudden decrease in neuronal cAMP, which coincides with the onset of myelin inhibition (Cai et al., 2001). These findings raised the possibility that pharmacological agents could be used to elevate cAMP levels in neurons and thereby increase their ability to regenerate.
Intracellular cAMP can be increased through indirect methods such as stimulating adenylate cyclase with forskolin, which has been shown to increase the rate of regeneration for transected axons in the sciatic nerve (Kilmer and Carlsen, 1984). Cell-permeable cAMP analogues, however, are a more effective means of directly elevating intracellular cAMP levels, and dibutyryl cAMP (dbcAMP), a non-hydrolyzable cAMP analogue, is one of the most widely used of these agents. Administration of dbcAMP overcomes inhibition by MAG and myelin for several neuronal subtypes, including DRG, cerebellar, cortical, and hippocampal neurons (Cai et al., 1999; Hannila and Filbin, unpublished observations). This phenomenon is not limited to mammalian neurons, as cAMP analogues have been found to have similar effects in lower vertebrates. For example, administration of dbcAMP can induce regeneration of transected spinal axons in zebrafish (Bhatt et al., 2004). In culture, the spinal neurons of the African clawed frog (Xenopus laevis) display prominent growth cones that are also highly motile. This feature led to the development of growth cone turning assays that have been used to test neuronal responses to MAG and cAMP. Microscopic gradients of the chemorepellent semaphorin III/D induce repulsive turning responses in Xenopus growth cones, and these can be converted to attraction by addition of the cAMP analogue Sp-cAMPS (Song et al., 1998). In a similar study, MAG induced a low-level gradient of intracellular calcium within the growth cone and this led to a repulsive turning response (Henley et al., 2004). Interestingly, calcium levels were further increased by treatment with Sp-cAMPS and this converted MAG-mediated repulsion to attraction. These findings demonstrated that growth cone turning responses are regulated by calcium flux and it was proposed that cAMP mediates attraction by stimulating additional calcium release from intracellular stores (Henley et al., 2004).
B. The conditioning lesion effect
Unlike their counterparts in the CNS, PNS axons, such as those in the sciatic nerve, are capable of regenerating after injury and this has prompted researchers to explore the molecular changes that occur following a peripheral nerve lesion. DRG neurons are an ideal model system for these studies, as they have both peripheral and central processes that project into peripheral nerves and the spinal cord, respectively. When DRG central processes are lesioned there is no upregulation of regeneration-associated genes such as growth-associated protein 43 (GAP-43) (Schreyer and Skene, 1993), and this is an indication of impending regenerative failure. By contrast, expression of GAP-43 is strongly upregulated following transection of DRG peripheral processes, and this enhances the intrinsic growth state of the neurons (Schreyer and Skene, 1993). It was therefore proposed that regeneration of DRG central processes could be improved by performing a peripheral nerve lesion. When this hypothesis was tested in studies of axonal regeneration, it was found that concomitant lesioning of DRG central and peripheral processes enhanced regeneration of DRG central processes into a peripheral nerve graft, and that optimal regeneration was obtained when the peripheral lesion preceded the central lesion by one week (Richardson and Issa, 1984; Oudega et al., 1994; Chong et al., 1996). This procedure is now known as a conditioning lesion for its ability to increase axonal regeneration by “conditioning” DRG neurons.
At this point it should be noted that there is a tremendous difference between enhancing the growth capacity of axons and overcoming inhibition by myelin. For example, overexpression of GAP-43 is not sufficient to promote axonal regeneration after CNS injury, even in the presence of permissive embryonic tissue grafts (Buffo et al., 1997; Neumann and Woolf, 1999; Bomze et al., 2001). A recent study of activating transcription factor-3 (ATF-3) further illustrates the importance of this distinction. ATF-3 is strongly upregulated after a conditioning lesion, and neurite outgrowth on a permissive substrate is significantly increased when ATF-3 is exogenously expressed in DRG neurons (Seijffers et al., 2006). It is clear from these observations that ATF-3 plays a role in stimulating axonal growth, but it is unknown if this effect is limited to general growth, or if ATF-3 can also overcome inhibition by myelin. Neurite outgrowth on a permissive substrate such as L1 can be abolished by the addition of soluble MAG (Tang et al., 1997), and so, observation of enhanced neurite outgrowth on a permissive substrate does not establish that an agent is capable of overcoming inhibition and promoting regeneration in vivo. Transcription factors and other signaling molecules identified through the conditioning lesion model must therefore be carefully assayed to determine exactly how they influence axonal growth.
In 1999, Neumann and Woolf used the conditioning lesion model to determine whether axonal regeneration in the spinal cord could be improved in the absence of a peripheral nerve graft. Axons failed to regenerate into the lesion site in adult rats that received only a dorsal column lesion, and this observation remained consistent when animals were examined at 2 months and 1 year after injury. However, when animals received simultaneous dorsal column and sciatic nerve lesions, substantial growth of axons into the lesion site was observed. This response was even more robust when the conditioning lesion was performed one week prior to the dorsal column transection, producing axonal regeneration that extended several millimeters beyond the site of injury. Many regenerating axons were observed in myelinated regions of the spinal cord, and this demonstrated that the conditioning lesion not only increases intrinsic growth, but also induces molecular changes that allow axons to overcome myelin inhibition in vivo.
The nature of these molecular changes remained unknown until it was discovered that cAMP levels become elevated following a conditioning lesion. Twenty-four hours after a sciatic nerve lesion, cAMP levels in DRG neurons were increased two-fold, and these neurons were able to overcome inhibition by myelin (Qiu et al., 2002). This effect was blocked by treatment with protein kinase A (PKA) inhibitors, which indicated that the conditioning lesion effect is initially dependent on PKA activity. By one week after the lesion, this response becomes PKA-independent and cAMP levels have returned to baseline. Intriguingly, however, neurite outgrowth on myelin at 7 days post-lesion greatly exceeds that observed at 24 hours post-lesion (Qiu et al., 2002). To demonstrate that elevation of intracellular cAMP was sufficient to mimic the conditioning lesion effect, dbcAMP was injected into the DRG 1, 2 or 7 days prior to plating the neurons on myelin, and neurite outgrowth was significantly increased (Qiu et al., 2002; Neumann et al., 2002). More importantly, intraganglionic injections of dbcAMP 7 days prior to a dorsal column lesion produced extensive regeneration of dorsal column axons in vivo (Qiu et al., 2002; Neumann et al., 2002). These studies demonstrated that cAMP can mimic the conditioning lesion effect both in vivo and in vitro, and confirmed that cAMP directly influences the extent of axonal regeneration by regulating neuronal responses to myelin inhibitors. This finding has proven to be extremely valuable because prophylactic measures such as a conditioning lesion cannot be used to treat spinal cord injury in humans. Recent studies have therefore focused on identifying the cAMP-regulated signaling pathways and intermediates that mediate the conditioning lesion effect.
C. Cyclic AMP-induced transcription and downstream effectors
One of the most important findings of the study by Qiu and colleagues (2002) was that cAMP mediates its effects in sequential PKA-dependent and PKA-independent phases. Upon becoming PKA-independent, the effects of cAMP become transcription dependent, and evidence of this was provided by experiments that used 5,6-dichloro-1-b-D-ribo-furanosyl-benzimidazole (DRB) to inhibit transcription. Cerebellar neurons that received DRB in conjunction with dbcAMP were unable to overcome inhibition by MAG, thereby confirming that transcription is an essential component of cAMP signaling (Cai et al., 2002).
The transcription factor cAMP response element binding protein (CREB) is activated by elevated levels of cAMP, and therefore serves as the primary mediator of cAMP-induced transcription (Lonze and Ginty, 2002). When cerebellar neurons are treated with dbcAMP, CREB phosphorylation occurs within 5 minutes, and peak phosphorylation is observed 1 hour after treatment (Gao et al., 2004). This activation occurs via the PKA and MEK/Erk pathways, as CREB phosphorylation is reduced when pharmacological inhibitors of PKA and MEK are used in conjunction with dbcAMP (Gao et al., 2004). In the same study we showed that CREB activity is essential for overcoming inhibition by MAG and myelin. Expression of dominant negative CREB in DRG and cerebellar neurons blocked the ability of dbcAMP to overcome inhibition, whereas constitutively active CREB promoted neurite outgrowth on MAG in the absence of elevated cAMP (Gao et al., 2004). To investigate the role of CREB in axonal regeneration, adenoviruses expressing constitutively active CREB were injected into the L4 DRG of adult rats and a dorsal column lesion was performed 4 days later. Animals that received control adenovirus displayed little regeneration at 4 weeks after injury, but there was significant regeneration of axons into the lesion site in animals expressing constitutively active CREB (Gao et al., 2004). These findings demonstrated that activation of CREB is sufficient to promote axonal regeneration and led us to hypothesize that activation of CREB by cAMP leads to the transcription of genes that are involved in overcoming myelin inhibition.
We have now identified several cAMP-regulated genes that play a role in overcoming inhibition by myelin and the first of these was arginase I (Arg I). During post-natal development, ArgI expression in DRG neurons declines dramatically between P3 and P5, which coincides with decreases in intracellular cAMP and the onset of myelin inhibition (Cai et al., 2002). This striking correlation between cAMP levels and ArgI expression suggested that ArgI was the product of CREB-mediated transcription, and this was later confirmed by experiments showing that the expression of ArgI is PKA- and CREB-dependent (Gao et al., 2004). ArgI expression is increased in cerebellar neurons in response to dbcAMP or brain-derived neurotrophic factor (BDNF), and overexpression of ArgI is sufficient to overcome inhibition by MAG (Cai et al., 2002). ArgI stimulates the synthesis of polyamines by hydrolyzing arginine to ornithine and urea (Lange et al., 2004), and we have shown that polyamines are also capable of overcoming myelin inhibitors. Priming with the polyamine putrescine enhances neurite outgrowth on MAG, and this effect was lost when pharmacological inhibitors of polyamine synthesis were administered in conjunction with dbcAMP and BDNF (Cai et al., 2002). To build on these results we are now conducting in vivo studies that will evaluate the ability of polyamines to promote axonal regeneration after spinal cord injury.
Microarray analysis of DRG neurons that received a conditioning lesion or treatment with dbcAMP revealed increased expression of the cytokine interleukin-6 (IL-6; Cao et al., 2006), making it a second potential target for therapeutic intervention in spinal cord injury. The efficacy of IL-6 was assessed using a series of three tests, and the first of these was to administer IL-6 in vitro and measure neurite outgrowth on MAG and myelin. For both DRG and hippocampal neurons, recombinant IL-6 overcame inhibition by MAG and myelin in a dose-dependent manner and this effect was also dependent on transcription (Cao et al., 2006). In the second test, IL-6 was delivered intrathecally to adult rats for 24 hours. The L4 and L5 DRG were then removed and the neurons were plated on MAG-expressing CHO cells. This approach of in vivo delivery and in vitro assessment is used by our laboratory to determine whether an agent can overcome inhibition when delivered in vivo, thus providing an indication of its ability to promote axonal regeneration. Neurite outgrowth on MAG was significantly increased for DRG neurons that received intrathecal delivery of IL-6, which suggests that IL-6 induces molecular changes that allow neurons to overcome inhibition (Cao et al., 2006). For the final test, IL-6 was delivered intrathecally to adult rats after a dorsal column lesion and axonal regeneration was assessed by retrograde tracing with horseradish peroxidase (HRP). In control animals, no axonal regeneration was observed and there was substantial retraction of axons from the lesion site. By contrast, numerous HRP-labeled axons were present within the lesion site in animals that received IL-6, and in some cases regenerating axons extended beyond the site of injury. These observations provided the first evidence that a protein produced from a cAMP-regulated gene could directly promote axonal regeneration after spinal cord injury.
IL-6 signaling is initiated by binding to the IL-6 receptor (IL-6R) and its co-receptor gp130, which in turn leads to activation of Janus kinase (JAK), and induction of transcription by signal transducers and activators of transcription (STAT; Heinrich et al., 1998). Administration of soluble gp130 or pharmacological inhibition of JAK activity abolished the ability of IL-6 to promote neurite outgrowth on MAG, demonstrating that this effect is mediated by signaling through the IL-6 pathway (Cao et al., 2006). Conversely, blocking IL-6R or JAK did not affect the ability of dbcAMP to promote neurite outgrowth on MAG, which suggested that IL-6 signaling is not required to overcome inhibition (Cao et al., 2006). This finding was supported by studies of the conditioning lesion effect in IL-6 null mutant mice. DRG neurons from IL-6 mutants retained the ability to extend neurites on MAG following a conditioning lesion, and when the conditioning lesion was followed by dorsal column transection, IL-6 mutants and wild-type mice displayed comparable regeneration of dorsal column axons (Cao et al., 2006). It was therefore proposed that IL-6 is sufficient but not necessary to overcome myelin inhibition (Cao et al., 2006). Curiously, these findings were the exact opposite of those obtained in a similar study by Cafferty and colleagues (2004). They observed no regeneration of dorsal column axons in IL-6 mutant mice following a conditioning lesion, and thus, concluded that IL-6 expression is required to promote axonal growth (Cafferty et al., 2004). While the role of IL-6 in the conditioning lesion effect remains unclear, our study has provided strong evidence that administration of IL-6 promotes axonal regeneration after a dorsal column lesion. Additional studies using other models of spinal cord injury should therefore be undertaken to more thoroughly investigate the therapeutic potential of IL-6.
This potential is tempered, however, by the fact that IL-6 is a potentially harmful pro-inflammatory cytokine. IL-6 is strongly upregulated following peripheral axotomy, and this has been correlated with accelerated peripheral nerve regeneration (Hirohata et al., 1996; Gadient and Otten, 1997). IL-6 expression is also increased after CNS injury (Gadient and Otten, 1997), but in this case, high levels of IL-6 could exacerbate the inflammation that occurs after spinal cord injury and lead to increased cell death. This possibility is supported by observations made in IL-6 transgenic mice, which display extensive astrogliosis, neurodegeneration, and breakdown of the blood-brain barrier (Campbell et al., 1993; Brett et al., 1995; Chiang et al., 1996). Clearly, additional work is needed to determine whether IL-6 is a viable option for the treatment of human spinal cord injury, and it would be particularly beneficial to elucidate the mechanism underlying the growth-promoting properties of IL-6. Identification of the genes and signaling pathways activated by IL-6 could lead to the development of agents that promote axonal regeneration without stimulating an inflammatory response.
D. Neurotrophins and cyclic AMP
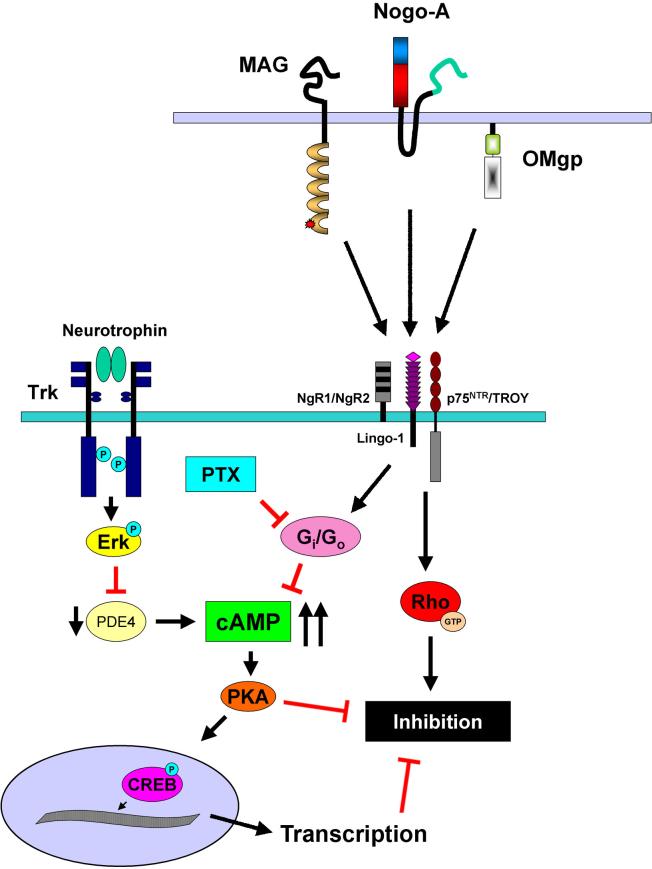
Priming neurons with neurotrophins such as nerve growth factor (NGF) and BDNF is yet another means of stimulating cAMP production. The term priming is used to describe experiments where neurons are treated overnight with neurotrophic factors and subsequently transferred to inhibitory substrates such as myelin or MAG-expressing CHO cells. Cerebellar neurons primed with BDNF or glial-derived neurotrophic factor (GDNF) were able to overcome MAG inhibition, while BDNF, NGF and GDNF each enhanced neurite outgrowth for DRG neurons (Cai et al., 1999). Competitive cAMP immunoassays revealed that cAMP levels were increased two-fold in response to NGF, BDNF, and GDNF, thereby confirming the role of cAMP in this effect. Direct addition of BDNF and GDNF to cerebellar neurons failed to overcome inhibition by MAG or myelin, but when these neurotrophic factors were added in conjunction with pertussis toxin (PTX), an inhibitor of the GTP-binding proteins Gi and Go, inhibition was blocked without priming (Figure1; Cai et al., 1999). PTX alone did not reverse inhibition by MAG, discounting the possibility that G proteins directly mediate inhibition (Cai et al., 1999), but Gi/Go protein is known to inhibit adenylate cyclase, which would prevent neurotrophin-induced production of cAMP. This provides a possible explanation for why priming with neurotrophins is necessary to overcome inhibition (Figure 1). When neurons are exposed to neurotrophins and myelin simultaneously, inhibition of adenylate cyclase by Gi/Go blocks increases in cAMP levels, leading to inhibition of neurite outgrowth (Figure 1). However, when neurons are primed, cAMP accumulates in response to neurotrophins and reaches a level that is sufficient to override inhibitory signals when the neurons are subsequently plated on myelin (Figure 1).
Figure 1.
Schematic representation of intracellular signaling pathways activated by neurotrophins and their role in overcoming inhibition by CNS myelin. Priming with neurotrophins activates Trk receptors and Erk, which in turn produces a transient inhibition of PDE4 activity. This causes intracellular cAMP levels to rise and upon reaching a threshold level, cAMP will activate PKA and initiate transcription by CREB. These events allow primed neurons to overcome inhibition mediated by MAG, Nogo-A, and OMgp binding to the receptor complex consisting of NgR1 or NgR2, p75NTR or TROY, and LINGO-1. Myelin inhibitors also activate Gi/Go, which inactivates adenylate cyclase and inhibits cAMP synthesis. When neurotrophins are added directly to neurons in the presence of myelin, intracellular cAMP rises, but the threshold level cannot be reached due to the activity of Gi/Go, and as a result, inhibition persists. Administration of PTX in conjunction with neurotrophins blocks the effects of Gi/Go and allows neurons to overcome inhibition in the absence of priming.
Neurotrophins are among the most potent neural growth factors known, but the fact that they cannot directly overcome inhibition by myelin makes them largely ineffective as a treatment for spinal cord injury. NGF, BDNF, and neurotrophin-3 (NT-3) have all been used in studies of spinal cord injury, but in most cases regeneration and functional recovery have been modest. For example, infusion of BDNF after rubrospinal tract transection produced only moderate axonal growth, and corticospinal axons only regenerated short distances when NT-3 was administered after spinal cord injury (Schnell et al., 1994; Kobayashi et al., 1997). Fibroblasts that have been genetically modified to secrete NGF, BDNF, or NT-3 provide both physical and trophic support for transected axons, and this approach has produced extensive axonal regeneration, but only small improvements in functional recovery (Grill et al., 1997; Tobias et al., 2003; Tuszynski et al., 2003). As shown by Cai and colleagues (1999), PTX allows neurotrophin-treated neurons to overcome inhibition by myelin, and thereby enhances their efficacy. PTX and other G protein inhibitors therefore merit further study and it is possible that using these agents in combination with neurotrophins could greatly improve axonal regeneration and functional recovery after spinal cord injury.
Neurotrophins do not elevate cAMP levels by inducing cAMP synthesis, but rather by initiating an intracellular signaling cascade that prevents cAMP degradation. Neurotrophin binding to Trk tyrosine kinase receptors leads to autophosphorylation of the receptor and activation of extracellular signal-regulated kinase (Erk; Kaplan and Miller, 2000), and we have shown that these events are required for BDNF and NGF to overcome inhibition by MAG (Figure 1; Gao et al., 2003). It was also found that BDNF-mediated activation of Erk produced a transient inhibition of phosphodiesterase 4 (PDE4), the main enzyme responsible for cAMP hydrolysis, and this caused intracellular cAMP to rise (Figure 1; Gao et al., 2003). These results suggested that a threshold level of cAMP was required to overcome inhibition, and this hypothesis was supported by the observation that Erk-mediated inactivation of PDE4 and dbcAMP work synergistically to attain the required levels of cAMP (Gao et al., 2003). The regulation of PDE4 activity by Erk therefore represents a unique mechanism in which cross talk between the neurotrophin and cAMP signaling pathways allows primed neurons to overcome inhibition by myelin.
III. cAMP-mediated axonal regeneration in models of spinal cord injury
The therapeutic potential of cAMP was recently assessed in three independent studies of spinal cord injury, each using different injury models and methods of increasing cAMP. In two of these studies, cAMP elevation was accomplished by inhibiting PDE4 activity with rolipram. PDE4 is the major source of phosphodiesterase activity in the CNS (Iona et al., 1998), making it a logical target for therapeutic intervention, and rolipram is a specific PDE4 inhibitor (Krause and Kuhne, 1988). It also has the added advantage of being able to cross the blood-brain barrier, which makes subcutaneous and oral administration possible (Krause and Kuhne, 1988). Rolipram was initially developed as an antidepressant (Horowski and Sastre y Hernandez, 1985), but has now been shown to enhance neurite outgrowth and axonal regeneration in the presence of myelin inhibitors.
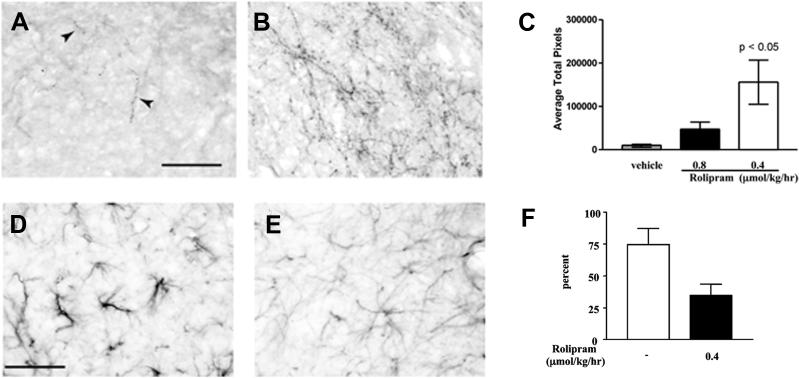
In the first of these studies, rolipram was administered through priming or subcutaneous delivery and neurite outgrowth on MAG-expressing CHO cells or CNS myelin was significantly increased (Nikulina et al., 2004). To test the efficacy of rolipram in vivo, spinal cord hemisections were performed in adult rats and this was followed by transplantation of embryonic spinal cord tissue into the lesion site and subcutaneous delivery of rolipram. Axonal regeneration was assessed 6−8 weeks after injury and few axons were observed within the transplant for vehicle treated animals. In animals that received rolipram, however, there was significantly more growth of serotonergic fibers into the transplant (Figure 2; Nikulina et al., 2004). Rolipram-treated animals also had greater functional recovery as measured by forelimb paw placement, which suggests that the regenerating axons may have formed synaptic connections (Figure 2; Nikulina et al., 2004).
Figure 2.
Rolipram promotes growth of serotonergic axons, reduces astrogliosis, and improves functional recovery after spinal cord injury. Few serotonergic fibers (arrowheads) are present within the embryonic spinal cord tissue grafts of vehicle-treated animals (A) at 6−8 weeks after spinal cord hemisection, but in animals that received rolipram, growth of serotonergic axons is significantly increased (B, C). Animals treated with rolipram (E) also show significantly less GFAP staining within the tissue grafts when compared to animals that received vehicle alone (D), and this is indicative of a reduction in astrogliosis. Lastly, animals treated with rolipram show improved functional recovery, as demonstrated by a significant decrease in paw placement errors (F). Scale bars = 50 μm. (Copyright 2004 National Academy of Sciences, U.S.A)
Somewhat unexpectedly, decreased expression of glial fibrillary acidic protein (GFAP) was also observed in animals that received rolipram, and this is indicative of reduced glial scarring (Figure 2; Nikulina et al., 2004). The glial scar is another major contributor to regenerative failure, forming both a physical and biochemical barrier to regenerating axons. It is the product of injury-induced astrogliosis, which is characterized by astrocyte proliferation and elevated expression of GFAP and extracellular matrix molecules such as chondroitin sulfate proteoglycans (CSPGs), keratin sulfate proteoglycan, and cytotactin/tenascin (McKeon et al., 1991; Jones et al., 2002; Silver and Miller, 2004). CSPGs are major components of the glial scar and are believed to inhibit axonal regeneration after spinal cord injury (Jones et al., 2002; 2003; Tang et al., 2003). Our results demonstrated that astrogliosis can be reduced through elevation of cAMP, and this could enhance axonal regeneration by rendering the CNS environment more permissive. It is not known how cAMP signaling mediates this effect, but inhibition of reactive astrocyte proliferation and downregulation of CSPG expression are two possibilities.
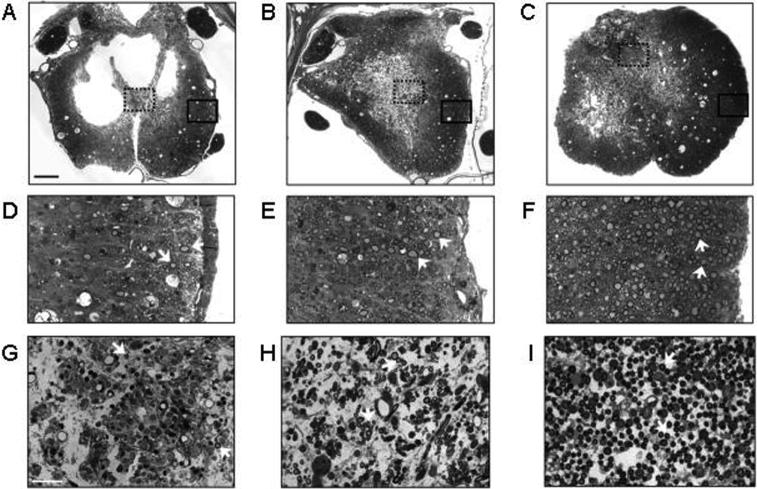
Rolipram has also been very effective when used in combination with dbcAMP and cell transplantation. In a study by Pearse and colleagues (2004), adult rats received Schwann cell grafts and intraspinal injections of dbcAMP one week after a moderate spinal cord contusion. Some animals were also treated with rolipram beginning at the time of injury (acute). The animals that received acute rolipram, dbcAMP, and Schwann cells displayed enhanced sparing of myelinated axons, greater myelination of spinal cord axons by the engrafted Schwann cells, and an overall increase in the number of axons within the grafts (Figure 3; Pearse et al., 2004). These observations indicated that this treatment strategy was highly neuroprotective. Most importantly, there was also significant regeneration of serotonergic axons across the lesion site and recovery of hindlimb locomotor function as measured on the Basso-Beattie-Bresnahan scale (Basso et al., 1995). These findings demonstrated that elevation of cAMP could increase the regenerative capacity of injured axons following spinal cord contusion.
Figure 3.
Rolipram in combination with Schwann cell transplantation and dbcAMP promotes axonal sparing and myelination after spinal cord contusion injury. Transverse sections of control spinal cords (A) showed extensive cavitation at 11 weeks after injury, but in animals that received Schwann cell grafts (B) or Schwann cells plus dbcAMP and rolipram (C) there was greater tissue preservation. Analysis at higher magnification revealed that densities of myelinated axons were significantly increased in animals that received Schwann cells, dbcAMP and rolipram (F, I) or Schwann cells alone (E, H) compared to control animals (D, G). (Adapted by permission from Macmillan Publishers Ltd.: Pearse, D. D., Pereira, F. C., Marcillo, A. E., Bates, M. L., Berrocal, Y. A., Filbin, M. T., and Bunge, M. B., cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nature Medicine 10, 610−616, copyright 2004.)
The third study combined administration of dbcAMP and NT-3 with transplantation of bone marrow stromal cells (MSCs), which have been shown to physically support axonal regeneration in the spinal cord (Hofstetter et al., 2002; Lu et al., 2004; 2005). Prior to injury, dbcAMP was injected into the L4 DRG, and NT-3 was injected into the injury site and caudal spinal cord immediately after dorsal column lesion (Lu et al., 2004). MSCs were also transplanted after injury. The goal of this approach was to replicate the conditioning lesion effect in vivo by stimulating neuronal cell bodies with dbcAMP, while simultaneously providing trophic support to the transected axons. Like a conditioning lesion, the injections of dbcAMP made prior to injury are prophylactic, and so, this approach should be considered a means of providing proof of principle rather than a clinically applicable treatment strategy. Animals that received a combination of dbcAMP, NT-3, and MSCs were the only treatment group that displayed extensive axonal regeneration beyond the lesion site, more than ever reported before in this lesion paradigm (Lu et al., 2004). The exact contribution of NT-3 to this response is unknown, but it is undoubtedly acting as a chemoattractant for the regenerating dorsal column axons. NT-3 may also be working synergistically with dbcAMP to overcome inhibition by myelin, but it is important to bear in mind that neurotrophins can only overcome inhibition with priming or administration of PTX (Cai et al., 1999), neither of which were used in this study. It is therefore possible that myelin signaling may override the effects of NT-3 in this model. Functional recovery was not observed in animals that received dbcAMP, NT-3, and MSCs, but this study still provides another clear example of axonal regeneration mediated by dbcAMP, and also emphasizes the benefits of combinatorial approaches to spinal cord injury repair.
IV. Future directions
Many promising treatments have resulted from the identification of cAMP as a modulator of axonal regeneration, but of all the agents that have been tested to date, rolipram has shown the greatest potential. Arguably the most significant finding of the study by Pearse and colleagues (2004) was that acute administration of rolipram alone significantly improved axonal integrity and functional outcome. The fact that a single agent could so dramatically impact functional recovery after spinal cord injury is remarkable, and attests to the tremendous therapeutic potential of this drug. Rolipram's ability to cross the blood-brain barrier is equally important, as this allows it to be delivered subcutaneously rather than to the lesion site. From a clinical perspective, this feature is invaluable because it eliminates the need for invasive surgery that could cause further damage to the spinal cord. In addition, methylprednisolone is no longer considered the standard of care for spinal cord injury (Hurlbert, 2000; Short, 2001; Hugenholtz, 2003) and new agents are urgently needed to fill this void. Because of its efficacy in animal models and ease of administration, rolipram should be considered a leading candidate for this role and its use in human clinical trials should be expedited. Rolipram has already been approved by the Food and Drug Administration for use in humans, which greatly facilitates the organization of clinical trials, and so long as adequate funding is available, this process should go forward. It is very possible that rolipram would only produce modest functional recovery following spinal cord injury in humans, but even small improvements in sensory or motor function would make a tremendous difference in a patient's quality of life.
Another exciting possibility is that further study of cAMP-regulated genes may yield additional agents capable of promoting axonal regeneration. Cao and colleagues (2006) noted that 11 genes were upregulated in response to dbcAMP, and these included neuropeptide Y, CREM (cAMP response element modulator), and VGF (nerve growth factor-inducible growth factor). These and other genes should be studied to determine their role in axonal regeneration, and it is possible that a combination of cAMP-induced factors could prove to be a useful therapeutic strategy for the treatment of spinal cord injury. One potential advantage of targeting downstream effectors of cAMP is that they may be more specific, and therefore more effective in promoting regeneration. For example, dbcAMP or rolipram induce expression of a range of genes that promote regeneration, but some of these genes, such as IL-6, may also promote inflammation or other adverse effects. By using specific gene products, it may be possible to directly affect the cytoskeleton while avoiding detrimental side effects. This approach could greatly facilitate the development of new therapies and ultimately, better serve the needs of spinal cord injury patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc. Natl. Acad. Sci. USA. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DH, Otto SJ, Depoister B, Fetcho JR. Cyclic AMP-induced repair of zebrafish spinal circuits. Science. 2004;305:254–258. doi: 10.1126/science.1098439. [DOI] [PubMed] [Google Scholar]
- Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Skene JH. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat. Neurosci. 2001;4:38–43. doi: 10.1038/82881. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- Brett FM, Mizisin AP, Powell HC, Campbell IL. Evolution of neuropathologic abnormalities associated with blood-brain barrier breakdown in transgenic mice expressing interleukin-6 in astrocytes. J. Neuropathol. Expl. Neurol. 1995;54:766–775. doi: 10.1097/00005072-199511000-00003. [DOI] [PubMed] [Google Scholar]
- Buffo A, Holtmaat AJ, Savio T, Verbeek JS, Oberdick J, Oestreicher AB, Gispen WH, Verhaagen J, Rossi F, Strata P. Targeted overexpression of the neurite growth-associated protein B-50/GAP-43 in cerebellar Purkinje cells induces sprouting after axotomy but not axon regeneration into growth-permissive transplants. J. Neurosci. 1997;17:8778–8791. doi: 10.1523/JNEUROSCI.17-22-08778.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Gardiner NJ, Das P, Qiu J, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J. Neurosci. 2004;24:4432–4443. doi: 10.1523/JNEUROSCI.2245-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J. Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Deng K, Mellado W, Lee J, Ratan RR, Filbin MT. Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. Neuron. 2002;35:711–719. doi: 10.1016/s0896-6273(02)00826-7. [DOI] [PubMed] [Google Scholar]
- Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MBA, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc. Natl. Acad. Sci. USA. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Gao Y, Bryson JB, Hou J, Chaudry N, Siddiq M, Martinez J, Spencer T, Carmel J, Hart RB, Filbin MT. The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J. Neurosci. 2006;26:5565–5573. doi: 10.1523/JNEUROSCI.0815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J. Cell Biol. 1988a;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988b;1:85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Chiang C, Stalder A, Samimi A, Campbell IL. Reactive gliosis as a consequence of interleukin-6 expression in the brain: studies in transgenic mice. Dev. Neurosci. 1994;16:212–221. doi: 10.1159/000112109. [DOI] [PubMed] [Google Scholar]
- Chong MS, Woolf CJ, Turmaine M, Emson PC, Anderson PN. Intrinsic versus extrinsic factors in determining the regeneration of the central processes of rat dorsal root ganglion neurons: the influence of a peripheral nerve graft. J. Comp. Neurol. 1996;370:97–104. doi: 10.1002/(SICI)1096-9861(19960617)370:1<97::AID-CNE9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- De Bellard ME, Tang S, Mukhopadhyay G, Shen YJ, Filbin MT. Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol. Cell. Neurosci. 1996;7:89–101. doi: 10.1006/mcne.1996.0007. [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, He Z, Filbin MT. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Zampieri N, Spencer T, Hilaire M, Mellado W, Chao MV, Filbin MT. MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron. 2005;46:849–855. doi: 10.1016/j.neuron.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruttiger M, Montag D, Schachner M, Martini R. Crucial role for the myelin-associated glycoprotein in the maintenance of axon-myelin integrity. Eur. J. Neurosci. 1995;7:511–515. doi: 10.1111/j.1460-9568.1995.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Fry EJ, Ho C, David S. A role for Nogo receptor in macrophage clearance from injured peripheral nerve. Neuron. 2007;53:649–662. doi: 10.1016/j.neuron.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten UH. Interleukin-6 (IL-6)--a molecule with both beneficial and destructive potentials. Prog. Neurobiol. 1997;52:379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- Gao Y, Nikulina E, Mellado W, Filbin MT. Neurotrophins elevate cAMP to reach a threshold required to overcome inhibition by MAG through extracellular signal-regulated kinase-dependent inhibition of phosphodiesterase. J. Neurosci. 2003;23:11770–11777. doi: 10.1523/JNEUROSCI.23-37-11770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, Spencer T, Mellado W, Kandel ER, Filbin MT. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Grill RJ, Blesch A, Tuszynski MH. Robust growth of chronically injured spinal cord axons induced by grafts of genetically modified NGF-secreting cells. Exp. Neurol. 1997;148:444–452. doi: 10.1006/exnr.1997.6704. [DOI] [PubMed] [Google Scholar]
- Habib AA, Marton LS, Allwardt B, Gulcher JR, Mikol DD, Hognason T, Chattopadhyay N, Stefansson K. Expression of the oligodendrocyte-myelin glycoprotein by neurons in the mouse central nervous system. J. Neurochem. 1998;70:1704–1711. doi: 10.1046/j.1471-4159.1998.70041704.x. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JR, Huang KH, Wang D, Poo MM. Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron. 2004;44:909–916. doi: 10.1016/j.neuron.2004.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohata H, Kiyama H, Kishimoto T, Taga T. Accelerated nerve regeneration in mice by upregulated expression of interleukin (IL) 6 and IL-6 receptor after trauma. J. Expl. Med. 1996;183:2627–2634. doi: 10.1084/jem.183.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowski R, Sastre y Hernandez M. Clinical effects of the neurotrophic selective cAMP phosphodiesterase inhibitor rolipram in depressed patients: global evaluation of the preliminary reports. Curr. Ther. Res. 1985;38:23–29. [Google Scholar]
- Huang JK, Phillips GR, Roth AD, Pedraza L, Shan W, Belkaid W, Mi S, Fex-Svenningsen A, Florens L, Yates JR, III, Colman DR. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science. 2005;310:1813–1817. doi: 10.1126/science.1118313. [DOI] [PubMed] [Google Scholar]
- Hugenholtz H. Methylprednisolone for acute spinal cord injury: not a standard of care. CMAJ. 2003;168:1145–1146. [PMC free article] [PubMed] [Google Scholar]
- Hurlbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J. Neurosurg. 2000;93:1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- Iona S, Cuomo M, Bushnik T, Naro F, Sette C, Hess M, Shelton ER, Conti M. Characterization of the rolipram-sensitive, cyclic AMP-specific phosphodiesterases: identification and differential expression of immunologically distinct forms in the rat brain. Mol. Pharmacol. 1998;53:23–32. doi: 10.1124/mol.53.1.23. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Ann. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Johnson PW, Abramow-Newerly W, Seilheimer B, Sadoul R, Tropak MB, Arquint M, Dunn RJ, Schachner M, Roder JC. Recombinant myelin-associated glycoprotein confers neural adhesion and neurite outgrowth function. Neuron. 1989;3:377–385. doi: 10.1016/0896-6273(89)90262-6. [DOI] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J. Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan and versican are differentially regulated following spinal cord injury. Exp. Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kelm S, Pelz A, Schauer R, Filbin MT, Tang S, de Bellard ME, Schnaar RL, Mahoney JA, Hartnell A, Bradfield P, Crocker PR. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr. Biol. 1994;4:965–972. doi: 10.1016/s0960-9822(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Kilmer SL, Carlsen RC. Forskolin activation of adenylate cyclase in vivo stimulates nerve regeneration. Nature. 1984;307:455–457. doi: 10.1038/307455a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi NR, Fan DP, Giehl KM, Bedard AM, Wiegand SJ, Tetzlaff W. BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha1-tubulin mRNA expression, and promote axonal regeneration. J. Neurosci. 1997;17:9583–9595. doi: 10.1523/JNEUROSCI.17-24-09583.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivica V, Cho KS, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E, Tessier-Lavigne M, Chen DF, He Z. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- Krause W, Kuhne G. Pharmacokinetics of rolipram in the rhesus and cynomolgus monkeys, the rat and the rabbit. Studies on species differences. Xenobiotica. 1988;18:561–571. doi: 10.3109/00498258809041693. [DOI] [PubMed] [Google Scholar]
- Lange PS, Langley B, Lu P, Ratan RR. Novel roles for arginase in cell survival, regeneration, and translation in the central nervous system. J. Nutr. 2004;134:2812S–2817S. doi: 10.1093/jn/134.10.2812S. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Tropak MB, Gerlai R, Clapoff S, Abramow-Newerly W, Trapp B, Peterson A, Roder J. Myelination in the absence of myelin-associated glycoprotein. Nature. 1994;369:747–750. doi: 10.1038/369747a0. [DOI] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J. Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp. Neurol. 2005;191:344–360. doi: 10.1016/j.expneurol.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Martini R, Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and MAG) and their shared carbohydrate epitope and myelin basic protein in developing sciatic nerve. J. Cell Biol. 1986;103:2439–2448. doi: 10.1083/jcb.103.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, Crowell T, Cate RL, McCoy JM, Pepinsky RB. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat. Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Mikol DD, Stefansson K. A phosphatidylinositol-linked peanut agglutinin-binding glycoprotein in central nervous system myelin and on oligodendrocytes. J. Cell Biol. 1988;106:1273–1279. doi: 10.1083/jcb.106.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikol DD, Gulcher JR, Stefansson K. The oligodendrocyte-myelin glycoprotein belongs to a distinct family of proteins and contains the HNK-1 carbohydrate. J. Cell Biol. 1990;110:471–479. doi: 10.1083/jcb.110.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag D, Giese KP, Bartsch U, Martini R, Lang Y, Bluthmann H, Karthigasan J, Kirschner DA, Wintergerst ES, Nave KA, Zielasek J, Toyka KV, Lipp H, Schachner M. Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron. 1994;13:229–246. doi: 10.1016/0896-6273(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Mueller M, Leonhard C, Wacker K, Ringelstein EB, Okabe M, Hickey WF, Kiefer R. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab. Invest. 2003;83:175–185. doi: 10.1097/01.lab.0000056993.28149.bf. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc. Natl. Acad. Sci. USA. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega M, Varon S, Hagg T. Regeneration of adult rat sensory axons into intraspinal nerve grafts: promoting effects of conditioning lesion and graft predegeneration. Exp. Neurol. 1994;129:194–206. doi: 10.1006/exnr.1994.1161. [DOI] [PubMed] [Google Scholar]
- Owens GC, Bunge RP. Evidence for an early role for myelin-associated glycoprotein in the process of myelination. Glia. 1989;2:119–128. doi: 10.1002/glia.440020208. [DOI] [PubMed] [Google Scholar]
- Park JB, Yiu G, Kaneko S, Wang J, Chang J, He XL, Garcia KC, He Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog. Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Salzer JL, Holmes WP, Colman DR. The amino acid sequences of the myelin-associated glycoproteins: homology to the immunoglobulin gene superfamily. J. Cell Biol. 1987;104:957–965. doi: 10.1083/jcb.104.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL, Pedraza L, Brown M, Struyk A, Afar D, Bell J. Structure and function of the myelin-associated glycoproteins. Ann. NY Acad. Sci. 1990;605:302–312. doi: 10.1111/j.1749-6632.1990.tb42404.x. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Sprouting and regeneration of lesioned corticospinal tract fibres in the adult rat spinal cord. Eur. J. Neurosci. 1993;5:1156–1171. doi: 10.1111/j.1460-9568.1993.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Schreyer DJ, Skene JH. Injury-associated induction of GAP-43 expression displays axon branch specificity in rat dorsal root ganglion neurons. J. Neurobiol. 1993;24:959–970. doi: 10.1002/neu.480240709. [DOI] [PubMed] [Google Scholar]
- Seijffers R, Allchorne AJ, Woolf CJ. The transcription factor ATF-3 promotes neurite outgrowth. Mol. Cell. Neurosci. 2006;32:143–154. doi: 10.1016/j.mcn.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, Murray B, Jung V, Pepinsky RB, Mi S. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Short D. Is the role of steroids in acute spinal cord injury now resolved? Curr. Opin. Neurol. 2001;14:759–763. doi: 10.1097/00019052-200112000-00013. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat. Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Tang S, Shen YJ, DeBellard ME, Mukhopadhyay G, Salzer JL, Crocker PR, Filbin MT. Myelin-associated glycoprotein interacts with neurons via a sialic acid binding site at ARG118 and a distinct neurite inhibition site. J. Cell Biol. 1997;138:1355–1366. doi: 10.1083/jcb.138.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Davies JE, Davies SJ. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J. Neurosci. Res. 2003;71:427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- Tobias CA, Shumsky JS, Shibata M, Tuszynski MH, Fischer I, Tessler A, Murray M. Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp. Neurol. 2003;184:97–113. doi: 10.1016/s0014-4886(03)00394-7. [DOI] [PubMed] [Google Scholar]
- Turnley AM, Bartlett PF. MAG and MOG enhance neurite outgrowth of embryonic mouse spinal cord neurons. Neuroreport. 1998;9:1987–1990. doi: 10.1097/00001756-199806220-00013. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Grill R, Jones LL, Brant A, Blesch A, Low K, Lacroix S, Lu P. NT-3 gene delivery elicits growth of chronically injured corticospinal axons and modestly improves functional deficits after chronic scar resection. Exp. Neurol. 2003;181:47–56. doi: 10.1016/s0014-4886(02)00055-9. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, Mage R, Rader C, Giger RJ. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J. Neurosci. 2005;25:808–822. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002a;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002b;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, Poo MM. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat. Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J. Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat. Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- Yokoyama N, Romero MI, Cowan CA, Galvan P, Helmbacher F, Charnay P, Parada LF, Henkemeyer M. Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron. 2001;29:85–97. doi: 10.1016/s0896-6273(01)00182-9. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Han B, Mendelsohn M, Jessell TM. PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron. 2006;52:775–788. doi: 10.1016/j.neuron.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]