Abstract
Context: Although women with anorexia nervosa (AN) have severe depletion of body fat, a paradoxical increase in bone marrow fat has been described. Recent data suggest that marrow fat measured by 1H-magnetic resonance spectroscopy (MRS) in combination with bone mineral density (BMD) may be more valuable than either parameter alone in detecting bone weakness.
Objective: The objective of the study was to investigate the effect of AN on accumulation of marrow fat in spine and femur using 1H-MRS and the relationship between marrow fat, BMD, and body composition in subjects with AN and normal-weight controls.
Design: This was a cross-sectional study.
Setting: The study was conducted at a referral center.
Patients: Patients included 10 women with AN (29.8 ± 7.6 yr) and 10 normal-weight age-matched women (29.2 ± 5.2 yr).
Interventions: There were no interventions.
Main Outcomes Measure: Marrow fat content of the fourth lumbar vertebra and femur measured by 1H-MRS. BMD of spine and hip measured by dual-energy x-ray absorptiometry.
Results: Subjects with AN had higher marrow fat at the fourth lumbar vertebra and femur compared with controls (P = 0.004–0.01). There was an inverse correlation between marrow fat of L4 and femur and BMD of the spine and hip (r = −0.56 to −0.71, P = 0.01–0.0002) and body mass index and sc adipose tissue of the thigh (r = −0.49 to −0.71, P = 0.03–0.0007). There was an inverse correlation between femur marrow fat and sc and total abdominal adipose tissue (r = −0.53 to −0.67, P = 0.003–0.03).
Conclusion: Women with AN have greater lumbar and femoral marrow fat than controls, and marrow fat correlates inversely with BMD. This paradoxical increase in marrow fat at a time when sc and visceral fat are markedly reduced raises important questions about functional consequences of this process.
Patients with anorexia nervosa have increased lumbar and femoral bone marrow fat compared to normal weight controls.
Anorexia nervosa (AN) is a prevalent eating disorder that is associated with significant loss of weight and severe depletion of body fat (1). Among the many comorbidities of this disease, bone loss is an almost universal feature in adult women such that the majority of women have osteopenia and a significant number meet the criteria for osteoporosis (2). Bone loss in patients with AN is associated with a 7-fold increased fracture risk, which may persist despite recovery (3,4). Recent studies have demonstrated an important physiologic link between bone and fat (5,6,7). Bone and fat cells arise from the same mesenchymal precursor cell within the bone marrow, capable of differentiation into osteoblasts and adipocytes under the control of transcription factors such as peroxisomal proliferator-activated receptor (PPAR)-γ (7,8). The relationship between bone and fat formation within the bone marrow in patients with AN is complex. During growth and acquisition of peak bone mass, surges in growth hormone, and gonadal steroid secretion lead to skeletal expansion and stem cell recruitment into osteoblasts, and states of mal- or undernutrition lead to a decreased rate of bone mass accrual and a reduction in peak bone mass (9). Bone mass is also influenced by the fat-derived hormone leptin, which has been found to be low in patients with AN and associated with low bone mineral density (BMD) (10,11,12,13).
In patients with AN, histopathological studies have shown hypoplastic and aplastic bone marrow with an increase in bone marrow adipocytes (14). In severe cases, partial or complete gelatinous degeneration of bone marrow can be seen (15,16). However, these studies did not evaluate the relationship between bone marrow fat content and skeletal integrity. Fatty infiltration of bone marrow is usually a characteristic feature of older individuals and is inversely related to BMD and skeletal integrity (6,17). We hypothesize that women with AN have increased vertebral and femoral marrow fat content compared with normal weight controls, despite marked diminution of body fat.
Bone marrow fat content can be assessed noninvasively, using proton magnetic resonance spectroscopy (1H-MRS), and it has been suggested that bone marrow fat content measured by 1H-MRS in combination with BMD is of significance in evaluating skeletal integrity, more valuable than either parameter alone (18,19). We hypothesize, that women with AN have decreased BMD with increased bone marrow fat.
Given the potential importance of bone marrow fat content on bone strength, we investigated the effects of AN on bone marrow fat accumulation in the spine and femur using 1H-MRS. In addition, we examined the relationship between bone marrow fat content, BMD, and body composition in subjects with AN and normal-weight controls.
Subjects and Methods
The study was approved by Partners Healthcare Institutional Review Board and complied with Health Insurance Portability and Accountability Act guidelines. Written informed consent was obtained from all subjects after the nature of the procedure had been fully explained.
Between July 2008 and November 2008, we studied a total of 20 premenopausal women aged 20–42 yr, with a mean age 29.5 ± 6.4 (sd) yr. These included 10 women with AN (mean age 29.8 ± 7.6 yr) and 10 normal-weight, age-matched controls (mean age 29.2 ± 5.2 yr). All control subjects had regular menses and no history of amenorrhea. All AN study subjects met weight and all psychiatric diagnostic criteria of AN. AN subjects were referred to the study by eating disorder providers in the area and healthy controls were recruited from the community through advertisements.
Exclusion criteria included pregnancy, presence of chronic disease known to affect bone density, abnormal TSH or a high FSH, hypothalamic or pituitary disorders, diabetes mellitus, or estrogen or glucocorticoid use. No control subject had a present or past history of an eating disorder. None of the subjects in the AN or control group were receiving oral contraceptives.
All subjects were examined at a single study visit at our General Clinical Research Center, where testing was performed. Body mass index (BMI) was calculated using the following formula: weight (kilograms)/height (square meters).
1H-MR spectroscopy
All subjects underwent 1H-MRS of bone marrow to determine lipid content using a 3.0T magnetic resonance imaging system (Siemens Trio; Siemens Medical Systems, Erlangen, Germany). For lumbar 1H-MRS, subjects were positioned feet first in the magnet bore in the prone position. A body matrix phased array coil was positioned over the lumbar region. A triplane gradient echo localizer pulse sequences with echo time (TE) of 5 msec and repetition time (TR) of 15 msec, slice thickness of 3 mm of the lumbar spine was obtained to localize the L4 vertebral body. A voxel measuring 15 × 15 × 15 mm (3.4 ml) was placed within the L4 vertebral body. Single-voxel 1H-MRS data were acquired using point-resolved spatially localized spectroscopy (PRESS) pulse sequence without water suppression with the following parameters: TE of 30 msec, TR of 3,000 msec, eight acquisitions, 1024 data points, and receiver bandwidth of 2000 Hz. The PRESS acquisition time was 36 sec. Subsequently the patient was placed supine and a triplane localizer of the left hip and thigh was obtained. A voxel measuring 12 × 12 × 12 mm (1.7 ml) was positioned within the proximal femoral epiphysis and single-voxel 1H-MRS using the same nonwater suppressed PRESS pulse sequence was performed. This process was repeated with voxel placement in the metaphysis at the intertrochanteric region and the middiaphysis. Oblique voxels and manual spatial presaturation bands were not used. Automated procedures for optimization of gradient shimming and transmit and receive gain were used.
To evaluate for interscan variability, 1-MRS of L4 was performed in five volunteers before and after repositioning. Interscan variability for 1H-MRS to determine vertebral marrow fat content was low. The mean difference between scans was −0.029 arbitrary units with a 95% confidence interval between 0.024 and −0.082 AU. The mean difference represents 4.5% of the mean value between measures.
1H-MR spectroscopy data analysis
Fitting of all 1H-MRS data were performed using LCModel (version 6.1–4A; Stephen Provencher, Oakville, Canada) (20). Data were transferred from the scanner to a Linux workstation, and metabolite quantification was performed using eddy current correction and water scaling. A customized fitting algorithm for bone marrow analysis provided estimates for all lipid signals combined (0.9, 1.3, and 2.3 ppm). LCModel bone marrow lipid estimates were automatically scaled to unsuppressed water peak (4.7 ppm) and expressed as lipid to water ratio.
Magnetic resonance imaging
Single axial magnetic resonance imaging slice through the abdomen at the level of L4 and a single slice through the midthigh were obtained (Trio, 3T, Siemens Medical Systems). A triplane gradient echo localizer pulse sequence with TE of 1.6 ms and TR of 49.0 ms was performed. For the abdomen, an axial T1-weighted image (fast spin-echo pulse sequence, 10 mm slice thickness, 40 cm field of view, TR 300 msec, TE 12 msec, echo train of 4, 512 × 512 matrix, 1 number of acquisitions) was prescribed through the midportion of L4. A single slice through the midthigh was also performed, equidistant to femoral head and medial femoral condyle. Visceral and sc fat areas as well as midthigh sc fat and muscle areas were determined based on offline analysis of tracings obtained using commercial software (VITRAK; Merge/eFilm, Milwaukee, WI).
Dual-energy x-ray-absorptiometry (DXA)
We used DXA to measure BMD using a QDR 4500 scanner (Hologic Inc., Waltham, MA). The following parameters were obtained: BMD of the spine (L1-L4) and BMD of the distal radius, hip, and total body. Coefficients of variation of DXA have been reported as less than 1% (21).
Statistical analysis
Statistical analysis was performed using JMP software (SAS Institute, Cary, NC). The means and sds of bone marrow fat content and body composition were calculated for the AN and control groups. Groups were compared using the Student’s t test.
Linear regression analysis of marrow fat content (as determined by 1-H MRS) on BMD measurements and marrow fat content on body composition parameters was performed. Multivariate standard least squares regression modeling was performed to control for BMI and menstrual status when assessing the associations between BMD and marrow fat. The Bonferroni correction was used to adjust for multiple comparisons.
The Bland Altman analysis was used to assess interscan variability of 1H-MRS to determine bone marrow fat content.
Results
Clinical characteristics
Subjects with AN did not differ from healthy controls in age. Subjects with AN had lower weight, BMI, percent ideal body weight, fat mass (total, sc, and visceral), percent body fat, and lean mass compared with normal-weight controls. Five of the AN subjects were amenorrheic (duration of amenorrhea 6–36 months). Healthy controls had regular menses (Table 1).
Table 1.
Clinical characteristics of AN and control group
| Variable | AN (n = 10) | Control group (n = 10) | P |
|---|---|---|---|
| Age (yr) | 29.8 ± 7.6 | 29.2 ± 5.2 | 0.84 |
| Ideal body weight (%) | 78.5 ± 4.9 | 99.5 ± 5.6 | <0.0001 |
| BMI (kg/m2) | 17.6 ± 1.0 | 21.9 ± 1.7 | <0.0001 |
| Weight (kg) | 48.0 ± 4.4 | 61.7 ± 6.9 | <0.0001 |
| Duration of disease (months) | 45.0 ± 30.6 | ||
| Duration of amenorrhea (months) | 19.4 ± 15.3 | ||
| VAT (cm2) | 23.8 ± 4.9 | 47.8 ± 18.6 | 0.001 |
| SAT (abdomen) (cm2) | 77.2 ± 21.6 | 170.2 ± 74.5 | 0.002 |
| TAT (abdomen) (cm2) | 101.0 ± 25.9 | 218.0 ± 89.4 | 0.001 |
| SAT (thigh) (cm2) | 46.2 ± 16.3 | 83.6 ± 26.9 | 0.001 |
| Muscle (thigh) (cm2) | 90.8 ± 13.4 | 114.3 ± 12.1 | 0.001 |
Data presented as mean± sd. VAT, Visceral adipose tissue; SAT: sc adipose tissue; TAT: total adipose tissue.
Bone marrow fat content
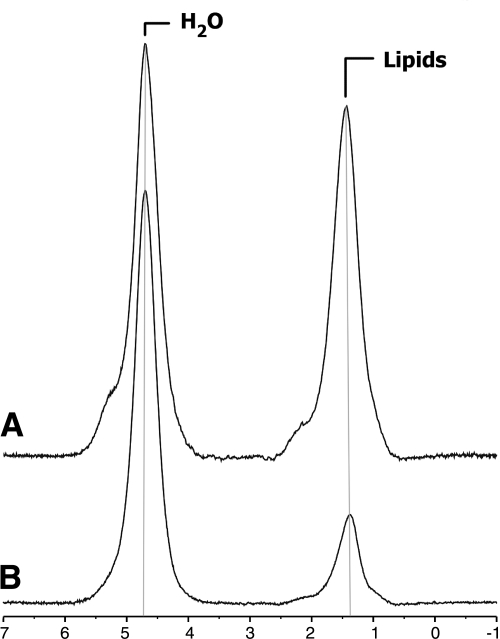
1H-MRS was able to quantify bone marrow fat content in subjects with AN and normal-weight controls (Fig. 1). There was a significant difference in bone marrow fat content between AN and normal-weight groups. Subjects with AN had significantly higher bone marrow fat content in L4 and the femoral metaphysis and diaphysis compared with normal-weight controls. There was no significant difference in marrow fat content of the femoral epiphysis (Table 2).
Figure 1.
1H-MRS of L4 marrow obtained at 3.0 T from a subject with AN (A) and an age-matched healthy control (B). The prominent lipid resonance in A indicates a relatively higher marrow fat content.
Table 2.
Bone marrow fat content as expressed in lipid to water ratio of AN and control group
| Variable | AN (n = 10) | Control group (n = 10) | P |
|---|---|---|---|
| L4 | 0.97 ± 0.39 | 0.52 ± 0.18 | 0.004 |
| Femoral epiphysis | 7.67 ± 2.35 | 7.63 ± 1.59 | 0.9 |
| Femoral metaphysis | 4.55 ± 2.27 | 2.07 ± 0.89 | 0.007 |
| Femoral diaphysis | 7.11 ± 1.99 | 4.05 ± 2.89 | 0.01 |
AN subjects with amenorrhea (n = 5) had increased lumbar marrow fat compared with AN subjects without amenorrhea (n = 5). L4 lipid to water ratio in subjects with AN with amenorrhea vs. AN subjects without amenorrhea was 1.2 ± 0.36 vs. 0.73 ± 0.28, P = 0.05. There was no significant difference between femoral marrow fat between AN subjects with and without amenorrhea (femoral epiphysis lipid to water ratio in AN subjects with amenorrhea vs. AN subjects without amenorrhea 7.4 ± 2.9 vs. 8.0 ± 1.9, P = 0.8, femoral metaphysis lipid to water ratio in AN subjects with amenorrhea vs. AN subjects without amenorrhea 4.1 ± 1.1 vs. 5.0 ± 1.1, P = 0.5, femoral diaphysis lipid to water ratio in AN subjects with amenorrhea vs. AN subjects without amenorrhea 6.5 ± 2.7 vs. 7.7 ± 0.9, P = 0.3).
BMD
Subjects with AN had lower total BMD and BMD of the hip and spine compared with normal-weight controls (Table 3). There was no significant difference between BMD of the whole body, lumbar spine, and hip between AN subjects with and without amenorrhea (P = 0.3–0.5).
Table 3.
BMD parameters measured by DXA of AN and control group
| Variable | AN (n = 10) | Control group (n = 10) | P |
|---|---|---|---|
| AP L-spine BMD (g/cm2) | 0.82 ± 0.09 | 1.03 ± 0.09 | 0.0002 |
| AP L-spine BMD T-score | −1.96 ± 0.87 | 0.05 ± 0.83 | <0.0001 |
| Lateral L-spine BMD (g/cm2) | 0.67 ± 0.08 | 0.79 ± 0.07 | 0.002 |
| Lateral L-spine BMD T-score | −1.85 ± 0.97 | −0.27 ± 0.86 | 0.002 |
| Hip BMD (g/cm2) | 0.77 ± 0.14 | 0.94 ± 0.10 | 0.008 |
| Hip BMD T-score | −1.37 ± 1.11 | 0.02 ± 0.83 | 0.007 |
| Total BMD (g/cm2) | 0.97 ± 0.03 | 1.14 ± 0.03 | 0.002 |
| Total BMD T-score | −1.46 ± 1.00 | 0.45 ± 1.40 | 0.002 |
Data presented as mean± sd. AP, Anterior-posterior; L-spine, lumbar spine.
Relationship between bone marrow fat content and body composition
There was an inverse correlation between bone marrow fat content (of L4, the femoral metaphysis and diaphysis), BMI, and sc adipose tissue of the thigh. There was an inverse correlation between bone marrow fat content (of the metaphysis and diaphysis) and abdominal sc and total abdominal adipose tissue. There was no significant correlation between bone marrow fat content of the epiphysis and body composition (Table 4). There was no significant correlation between disease duration and bone marrow fat content of L4 (r = −0.3, P = 0.42) and the femur (r = 0.06–0.46, P = 0.87–0.24).
Table 4.
Correlation between bone marrow fat content measured by 1H-MRS with BMI and body composition measured by magnetic resonance imaging in subjects with AN and normal-weight controls
| L4
|
Epi
|
Meta
|
Dia
|
|||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| BMI | −0.51 | 0.02 | −0.005 | 0.9 | −0.63 | 0.004a | −0.71 | 0.0007a |
| SAT abd. | −0.31 | 0.2 | 0.05 | 0.86 | −0.54 | 0.03 | −0.67 | 0.003a |
| VAT | −0.22 | 0.36 | 0.17 | 0.54 | −0.44 | 0.08 | −0.44 | 0.08 |
| TAT abd. | −0.30 | 0.22 | 0.07 | 0.79 | −0.53 | 0.03 | −0.63 | 0.006a |
| SAT thigh | −0.49 | 0.03 | 0.02 | 0.93 | −0.52 | 0.03 | −0.50 | 0.03 |
| Muscle thigh | −0.55 | 0.01 | 0.04 | 0.89 | −0.53 | 0.03 | −0.30 | 0.21 |
Epi, Femoral epiphysis; Meta, femoral metaphysis; Dia, femoral diaphysis; abd., abdominal; SAT, sc adipose tissue; VAT, visceral adipose tissue; TAT, total adipose tissue.
Significant after adjusting for multiple comparisons using Bonferroni method.
Relationship between bone marrow fat content and BMD
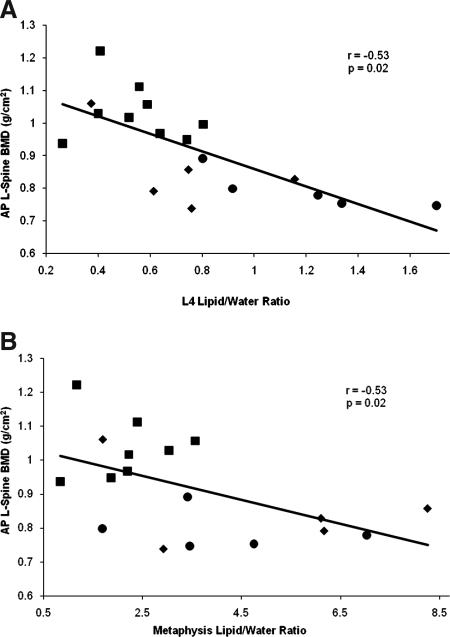
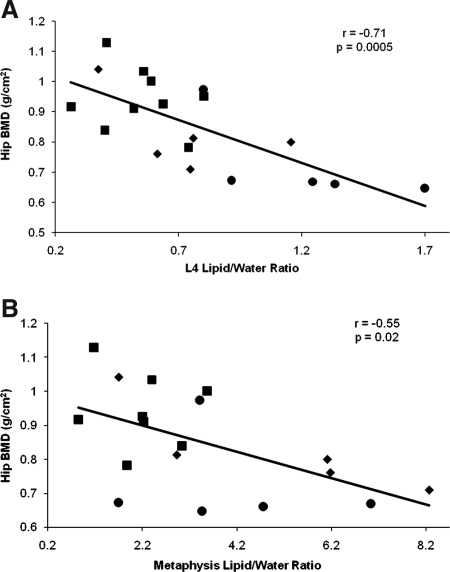
There was an inverse correlation between bone marrow fat content (of L4 and the metaphysis) and total BMD and BMD of the spine (Fig. 2) and hip (Fig. 3). Because BMD is related to BMI, we controlled for BMI using multivariate analysis. After controlling for BMI, there remained a significant correlation between bone marrow fat content of L4 and total BMD and BMD of the lumbar spine and hip (P = 0.003–0.05) as well as bone marrow fat content of the metaphysis and BMD of the lumbar spine and hip (P = 0.03–0.08). After controlling for amenorrhea, there remained a significant correlation between bone marrow fat content of L4 and BMD of the lumbar spine and hip (P = 0.02–0.05) and between bone marrow fat of the femoral metaphysis and BMD of the lumbar spine and hip (P = 0.03–0.005). There was no significant correlation between bone marrow fat content of the epiphysis and diaphysis with any of the BMD parameters (Table 5).
Figure 2.
Regression analysis between lumbar spine BMD and bone marrow fat content expressed as lipid to water ratio. There is an inverse correlation between anterior-posterior lumbar spine BMD and L4 marrow fat (A) and anterior-posterior lumbar spine BMD and metaphyseal marrow fat (B). Square, Normal-weight control; circle; AN with amenorrhea; diamond, AN without amenorrhea.
Figure 3.
Regression analysis between hip BMD and bone marrow fat content expressed as lipid to water ratio. There is an inverse correlation between hip BMD and L4 marrow fat (A) and hip BMD and metaphyseal marrow fat (B). Square, Normal-weight control; circle, AN with amenorrhea; diamond, AN without amenorrhea.
Table 5.
Correlation between bone marrow fat content measured by 1H-MRS and BMD in subjects with AN and normal-weight controls
| L4
|
Epi
|
Meta
|
Dia
|
|||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| AP L-spine BMD | −0.71 | 0.0006a | −0.1 | 0.69 | −0.53 | 0.02 | −0.29 | 0.25 |
| AP L-spine BMD T-score | −0.7 | 0.001a | −0.16 | 0.54 | −0.52 | 0.03 | −0.37 | 0.12 |
| Lateral L-spine BMD | −0.58 | 0.009 | −0.28 | 0.28 | −0.63 | 0.005a | −0.33 | 0.18 |
| Lateral L-spine T-score | −0.58 | 0.009 | −0.29 | 0.26 | −0.64 | 0.005a | −0.33 | 0.17 |
| Hip BMD | −0.71 | 0.0005a | −0.006 | 0.98 | −0.55 | 0.02 | −0.13 | 0.6 |
| Hip T-score | −0.71 | 0.0006a | −0.005 | 0.99 | −0.55 | 0.02 | −0.13 | 0.6 |
| Total BMD | −0.58 | 0.008 | −0.05 | 0.86 | −0.47 | 0.04 | −0.25 | 0.3 |
| Total BMD T-score | −0.57 | 0.009 | −0.04 | 0.87 | −0.46 | 0.04 | −0.24 | 0.3 |
Epi, Femoral epiphysis; Meta, femoral metaphysis; Dia, femoral diaphysis; AP, anterior-posterior; L-spine, lumbar spine.
Significant after adjusting for multiple comparisons using Bonferroni method.
Discussion
We have demonstrated that women with AN have increased bone marrow fat content in the lumbar spine as well as in the femoral metaphysis and diaphysis compared with normal-weight controls as determined by 1H-MRS. We have also shown a highly significant inverse correlation between bone marrow fat content in both L4 and the femoral metaphysis and BMD at multiple skeletal sites including lumbar spine, hip, and total body. We did not find a difference in bone marrow fat content of the epiphysis between subjects with AN and normal-weight controls. This might be due to the large amount of marrow fat content of the epiphysis. The epiphysis is the first part of the femur to convert from hematopoietic to yellow marrow and the last to convert to hematopoietic marrow in disease states that are associated with marrow reconversion (such as chronic disease, malignancy). Because none of our patients had severe AN associated with severe marrow reconversions, the fat in the epiphyses of both AN patients and normal-weight controls remained high.
AN is a primary psychiatric disorder characterized by low body weight due to a state of nutritional deprivation affecting 0.5–1% of college-aged women in the United States (22,23,24). This disease is associated with multiple medical comorbidities including significant and often permanent bone loss despite recovery. Studies have demonstrated that 51–90% of these women have osteopenia and 34–38% of them have osteoporosis (25,26). Fracture risk is significantly greater in this population compared with healthy controls. A prospective study following up 27 women with AN for 2 yr demonstrated a 7-fold increased risk of nonvertebral fractures compared with healthy controls (27). Similarly, a population based retrospective cohort study by Lucas et al. (28) demonstrated a nearly 3-fold increased risk of fractures in women, even many years after the initial diagnosis of AN; the long-term cumulative incidence of any fracture in this cohort after diagnosis was 57%. Therefore, understanding the mechanisms of bone loss and fracture risk is of particular importance in this population.
AN is associated with low body fat and adipokine levels have been shown to be abnormally decreased or increased in this population. Leptin, a hormone highly correlated with body fat percentage, is low in women with AN (29), whereas ghrelin, an orexigenic hormone, and peptide YY, an anorexigenic hormone secreted in the intestine in response to caloric intake, have been shown to be elevated in AN (12,30,31). Recent evidence suggests that adipokines and appetite regulatory hormones may affect BMD. For example, peptide YY levels have been shown to be negatively correlated with BMD at the spine, total hip, femoral neck, and radius in women with AN (32). Whether adipokines are secreted by bone marrow fat and therefore influence the formation of bone in a paracrine manner is currently unknown, and further studies evaluating hormonal parameters and the relationship to marrow adiposity are needed.
Recent attention has focused on the emerging role of bone marrow fat and its relationship to bone lineage and osteoporosis risk. The clinical significance of bone marrow fat has been demonstrated in a number of studies. Intravertebral fat has been shown to affect assessment of BMD, an important determinant of fracture risk. A study comparing various measurements of bone mineral content in postmenopausal women demonstrated that single-energy quantitative computed tomography (QCT) underestimates the bone mineral content compared with dual-energy QCT due to intravertebral fat content (33). More recently pelvic bone mineral adipose tissue as measured by magnetic resonance imaging has been shown to have a significant negative correlation with whole body BMD and pelvic BMD as assessed by DXA (34), and studies have suggested that alterations in bone marrow fat could explain bone fragility and fracture risk independent of BMD in osteoporosis (18,19). Increased bone marrow fat affects biomechanical strength of bone as yellow marrow is a weaker biomechanical support medium than red bone marrow (18). Schellinger et al. (19) found a 45% higher vertebral marrow fat content in subjects with morphologic evidence of bone weakness, such as Schmorl’s nodes, endplate depression, or compression fractures. Therefore, an understanding of the factors that influence the development of bone marrow fat is of great clinical importance in normal as well as abnormal states.
Given the extreme changes in body fat, which characterize this disorder, patients with AN are an interesting model in which to examine the relationship between bone density and marrow fat. A prior study, using dual energy QCT, demonstrated that despite having significantly lower levels of body fat and sc fat, the intravertebral fat content of women and adolescents with AN was significantly greater than a slightly older population of healthy volunteers (35). Unlike our study, this prior study did not demonstrate a correlation between intravertebral fat and bone mineral density. In addition to differences in technique, one reason for this difference may be the greater heterogeneity of their population compared with ours due to their inclusion of adolescents (35). The prior population consisted of women aged 15–33 yr, whereas our population consists of adult women with age-matched controls. Because age and pubertal status are significant positive determinants of marrow fat, a heterogeneous population will make it more difficult to accurately assess significant correlations.
Bone marrow biopsies in individuals with AN support the finding of increased marrow adiposity. Forty-eight percent of iliac crest bone marrow biopsies in 44 individuals with AN demonstrated an increased proportion of adipocytes (14). Only five of the marrow specimens (11%) were classified as normal, with the remaining specimens demonstrating hypoplasia, aplasia, or gelatinous degeneration (14). Interestingly, duration of illness did not correlate with bone marrow pattern, whereas weight loss demonstrated a significant correlation and weight recovery was associated with bone marrow recovery, suggesting reversibility (14). Also, in our study, there was no correlation between disease duration and bone marrow fat content of L4 and the femur. It appears more likely that changes in marrow fat may be more directly related to the nutritional state of the subject over a narrower time frame. We were able to quantify bone marrow fat content noninvasively using 1H-MRS. In addition, we demonstrated an inverse correlation with bone marrow fat content of the spine and femoral metaphysis with BMD and body composition. We did not find a significant correlation between BMD and bone marrow fat of the epiphysis or diaphysis. A potential explanation could be the significantly higher fat content of the epiphysis and diaphysis compared with the metaphysis and L4. Because DXA measurements were performed at the hip (which included the metaphysis) and lumbar spine, the lack of correlation between the femoral diaphysis and epiphysis adiposity and BMD measurements of the spine and hip might be due to the marked increase in fatty marrow and relative lack of trabeculae at these sites. Schellinger et al. (19) showed no correlation between lumbar bone marrow fat content and BMD in 26 subjects. One explanation for their findings might be the heterogeneity of their study population (both men and women were included). Also, in contrast to our study, subjects included in that study had a large age range (32–70 yr). In addition, no data on BMI are provided in that report and the BMI range might have been too narrow to see a significant correlation. A final explanation for detecting a significant negative correlation in our study population compared with that reported by Schellinger et al. might also be improved technique. We performed MRS at 3.0 Tesla, and spectral analysis was performed with software that took into consideration the line width values for all measured peaks which increases the precision of resonance estimates for lipids and water.
In a prior study evaluating bone marrow content of the femoral epiphysis, diaphysis, and the lumbar spine by means of 1H-MRS, individuals with AN did not have increased bone marrow adiposity (15). In fact, an increase in marrow adiposity was seen with weight recovery in individuals with AN (15). Our disparate findings are likely due to the heterogeneity of the population in the prior study. Their AN subjects ranged in age from 15–56 with a BMI range of 9.7–16.4 kg/m2. As age is a significant positive determinant of bone marrow fat, the more homogeneous the study population the greater likelihood of finding a true effect. Similarly, their average BMI was quite low and as demonstrated by the biopsy data, individuals with the greatest weight loss had a significantly greater likelihood of having gelatinous degeneration of the bone marrow, a state of low marrow adiposity. Our study is the first to use 1H-MRS in a homogeneous population and demonstrates a significant increase in marrow adiposity in the AN population compared with the controls, consistent with the biopsy data (14).
Bone marrow adipocytes are derived from mesenchymal stem cells, which also have the potential to differentiate into osteoblasts. Whether the process of differentiation is mutually exclusive remains an area of great debate. The idea of a mutually exclusive differentiation process is supported by in vitro and in vivo studies demonstrating stimulators of adipocyte formation, such as PPAR-γ, leading to increased expression of adipocyte marker genes and concomitant decreases in the expression of osteoblast marker genes (36,37). Our study, demonstrating a correlation between bone marrow adiposity and BMD with the use of 1H-MRS supports this hypothesis. Yet the interaction between osteoblasts and adipocytes is clearly more complex as there are examples of periods during development, such as puberty, in which both bone marrow adipocytes and osteoblasts increase, thereby suggesting the differentiation processes can occur contemporaneously.
Many factors have been shown to influence the differentiation of the mesenchymal stem cells into either osteoblasts or adipocytes. PPAR-γ agonists and glucocorticoids have both been shown to induce mesenchymal stem cell differentiation into adipocytes (37,38). However, in vitro studies have shown that estrogen leads to osteoblastogenesis with concomitant inhibition of adipogenesis (39). Because both hypercortisolemia (40) and hypoestrogenism are characteristic of AN, it is likely that these factors may contribute to the increase in bone marrow fat observed in this study, and further studies are needed to evaluate the relationship of these hormonal parameters and marrow adiposity. We found increased lumbar bone marrow fat in AN subjects with amenorrhea compared with AN subjects without amenorrhea, whereas there was no significant difference in BMD. After controlling for amenorrhea, there remained a significant correlation between marrow fat of L4 and the femoral metaphysis and BMD of the lumbar spine and hip. However, a limitation of these data are our small sample size. The impact of gonadal function on fat mass will be an important area to pursue in these subjects. Yet hypercortisolemia is unlikely to be the only contributor to the increase in bone marrow fat observed in individuals with AN. Intravertebral fat was not significantly different in hypercortisolemic individuals with Cushing’s syndrome compared with controls when evaluated by dual-energy QCT despite the significantly greater percentage of body fat and computed tomography-measured sc fat in the individuals with Cushing’s (35).
Our data demonstrate that women with AN have significantly greater bone marrow fat in their L4 vertebra and femoral metaphysis and diaphysis as assessed by 1H-MRS. Importantly, the degree of bone marrow fat is inversely correlated with BMD at multiple skeletal sites. Interestingly, although there was a trend toward a negative correlation, L4 bone marrow fat content did not significantly correlate with fat mass, whereas bone marrow fat content in the metaphysis and diaphysis did demonstrate a significant inverse correlation. This suggests there may be greater adipocyte accumulation within trabecular bone compartments, sites that remodel more rapidly, than cortical bone. However, we cannot determine whether the increased marrow fat content in the lumbar spine is just a reflection of decreased trabecular bone. Notwithstanding those differences, we found strong evidence that marrow adiposity was a prominent feature of AN. This paradoxical increase in marrow fat at a time when sc and visceral fat are markedly reduced raises important questions about the functional consequences of this process. Further studies are needed to determine whether these changes represent a compensatory mechanism, or a default into the fat lineage as a result of impaired osteoblastogenesis.
Footnotes
This work was supported by the following National Institutes of Health Grants: RO1 DK052625, MO1 RR01066, UL1 RR025758.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 24, 2009
Abbreviations: AN, Anorexia nervosa; BMD, bone mineral density; BMI, body mass index; DXA, dual-energy x-ray-absorptiometry; 1H-MRS, 1H-magnetic resonance spectroscopy; PPAR, peroxisomal proliferator-activated receptor; PRESS, point-resolved spatially localized spectroscopy; QCT, quantitative computed tomography; TE, echo time; TR, repetition time.
References
- Misra M, Soyka LA, Miller KK, Grinspoon S, Levitsky LL, Klibanski A 2003 Regional body composition in adolescents with anorexia nervosa and changes with weight recovery. Am J Clin Nutr 77:1361–1367 [DOI] [PubMed] [Google Scholar]
- Legroux-Gerot I, Vignau J, D'Herbomez M, Collier F, Marchandise X, Duquesnoy B, Cortet B 2007 Evaluation of bone loss and its mechanisms in anorexia nervosa. Calcif Tissue Int 81:174–182 [DOI] [PubMed] [Google Scholar]
- Misra M, Klibanski A 2006 Anorexia nervosa and osteoporosis. Rev Endocr Metab Disord 7:91–99 [DOI] [PubMed] [Google Scholar]
- Vestergaard P, Emborg C, Stoving RK, Hagen C, Mosekilde L, Brixen K 2002 Fractures in patients with anorexia nervosa, bulimia nervosa, and other eating disorders—a nationwide register study. Int J Eat Disord 32:301–308 [DOI] [PubMed] [Google Scholar]
- Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V 2008 Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab 93:2281–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B 2004 Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 3:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada I, Suzawa M, Matsumoto K, Kato S 2007 Suppression of PPAR transactivation switches cell fate of bone marrow stem cells from adipocytes into osteoblasts. Ann NY Acad Sci 1116:182–195 [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Bouxsein ML 2006 Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2:35–43 [DOI] [PubMed] [Google Scholar]
- Recker RR, Heaney RP 1993 Peak bone mineral density in young women. JAMA 270:2926–2927 [PubMed] [Google Scholar]
- Grinspoon S, Gulick T, Askari H, Landt M, Lee K, Anderson E, Ma Z, Vignati L, Bowsher R, Herzog D, Klibanski A 1996 Serum leptin levels in women with anorexia nervosa. J Clin Endocrinol Metab 81:3861–3863 [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Miller K, Coyle C, Krempin J, Armstrong C, Pitts S, Herzog D, Klibanski A 1999 Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. J Clin Endocrinol Metab 84:2049–2055 [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A 2005 Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab 289:E373–E381 [DOI] [PubMed] [Google Scholar]
- Soyka LA, Misra M, Frenchman A, Miller KK, Grinspoon S, Schoenfeld DA, Klibanski A 2002 Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 87:4177–4185 [DOI] [PubMed] [Google Scholar]
- Abella E, Feliu E, Granada I, Milla F, Oriol A, Ribera JM, Sanchez-Planell L, Berga LI, Reverter JC, Rozman C 2002 Bone marrow changes in anorexia nervosa are correlated with the amount of weight loss and not with other clinical findings. Am J Clin Pathol 118:582–588 [DOI] [PubMed] [Google Scholar]
- Geiser F, Murtz P, Lutterbey G, Traber F, Block W, Imbierowicz K, Schilling G, Schild H, Liedtke R 2001 Magnetic resonance spectroscopic and relaxometric determination of bone marrow changes in anorexia nervosa. Psychosom Med 63:631–637 [DOI] [PubMed] [Google Scholar]
- Sicard D, Casadevall N, Wyplosz B, Picart F, Blanene P 1994 Anorexia nervosa and gelatinous transformation of bone marrow. Nouv Rev Fr Hematol 36(Suppl 1):S85–S86 [PubMed] [Google Scholar]
- Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M 2001 Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2:165–171 [DOI] [PubMed] [Google Scholar]
- Schellinger D, Lin CS, Hatipoglu HG, Fertikh D 2001 Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol 22:1620–1627 [PMC free article] [PubMed] [Google Scholar]
- Schellinger D, Lin CS, Lim J, Hatipoglu HG, Pezzullo JC, Singer AJ 2004 Bone marrow fat and bone mineral density on proton MR spectroscopy and dual-energy X-ray absorptiometry: their ratio as a new indicator of bone weakening. AJR Am J Roentgenol 183:1761–1765 [DOI] [PubMed] [Google Scholar]
- Provencher SW 1993 Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679 [DOI] [PubMed] [Google Scholar]
- Barthe N, Braillon P, Ducassou D, Basse-Cathalinat B 1997 Comparison of two Hologic DXA systems (QDR 1000 and QDR 4500/A). Br J Radiol 70:728–739 [DOI] [PubMed] [Google Scholar]
- 2000 Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association [Google Scholar]
- Lucas AR, Beard CM, O'Fallon WM, Kurland LT 1991 50-year trends in the incidence of anorexia nervosa in Rochester, Minn.: a population-based study. Am J Psychiatry 148:917–922 [DOI] [PubMed] [Google Scholar]
- Pope HG, Hudson JI, Yurgelun-Todd D, Hudson MS 1984 Prevalence of anorexia nervosa and bulimia in three student populations. International J Eating Disord 3:45–51 [Google Scholar]
- Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, Herzog D, Klibanski A 2000 Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med 133:790–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A 2005 Medical findings in outpatients with anorexia nervosa. Arch Intern Med 165:561–566 [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR 1991 The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA 265:1133–1138 [PubMed] [Google Scholar]
- Lucas AR, Melton 3rd LJ, Crowson CS, O'Fallon WM 1999 Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc 74:972–977 [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Blum WF, Barth N, Coners H, Englaro P, Juul A, Ziegler A, Warnke A, Rascher W, Remschmidt H 1997 Leptin levels in patients with anorexia nervosa are reduced in the acute stage and elevated upon short-term weight restoration. Mol Psychiatry 2:330–334 [DOI] [PubMed] [Google Scholar]
- Pfluger PT, Kampe J, Castaneda TR, Vahl T, D'Alessio DA, Kruthaupt T, Benoit SC, Cuntz U, Rochlitz HJ, Moehlig M, Pfeiffer AF, Koebnick C, Weickert MO, Otto B, Spranger J, Tschop MH 2007 Effect of human body weight changes on circulating levels of peptide YY and peptide YY3–36. J Clin Endocrinol Metab 92:583–588 [DOI] [PubMed] [Google Scholar]
- Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A 2005 Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab 289:E347–E356 [DOI] [PubMed] [Google Scholar]
- Utz AL, Lawson EA, Misra M, Mickley D, Gleysteen S, Herzog DB, Klibanski A, Miller KK 2008 Peptide YY (PYY) levels and bone mineral density (BMD) in women with anorexia nervosa. Bone 43:135–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbold WD, Genant HK, Reiser UJ, Harris ST, Ettinger B 1986 Bone mineral content in early-postmenopausal and postmenopausal osteoporotic women: comparison of measurement methods. Radiology 160:469–478 [DOI] [PubMed] [Google Scholar]
- Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB 2007 MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int 18:641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo-Smith W, Rosenthal DI, Goodsitt MM, Klibanski A 1989 Intravertebral fat measurement with quantitative CT in patients with Cushing disease and anorexia nervosa. Radiology 170:835–838 [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL 1999 Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARγ2. J Cell Biochem 74:357–371 [PubMed] [Google Scholar]
- Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B 2004 Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotti G, Angeli A, Bilezikian JP, Canalis E, Giustina A 2006 Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab 17: 144–149 [DOI] [PubMed] [Google Scholar]
- Dang ZC, van Bezooijen RL, Karperien M, Papapoulos SE, Lowik CW 2002 Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J Bone Miner Res 17:394–405 [DOI] [PubMed] [Google Scholar]
- Putignano P, Dubini A, Toja P, Invitti C, Bonfanti S, Redaelli G, Zappulli D, Cavagnini F 2001 Salivary cortisol measurement in normal-weight, obese and anorexic women: comparison with plasma cortisol. Eur J Endocrinol 145:165–171 [DOI] [PubMed] [Google Scholar]