Abstract
In bone, osteoblasts and chondrocytes synthesize matrix vesicles (MVs) that interact with collagen to initiate calcification. MVs have been identified in human calcified arteries but are poorly characterized. The objective of this study is to determine the role of annexins and fetuin-A in MV formation and activity during calcification in bovine vascular smooth muscle cells (BVSMCs). BVSMCs were treated with control or calcification (high phosphorus) media, and cellular MVs were isolated by collagenase digestion and secreted MVs were isolated from cultured media by ultracentrifugation. The results showed that alkaline phosphatase (ALP) activity was significantly increased in MVs from calcified BVSMCs compared with noncalcified BVSMCs, as was annexin II and VI content and 45Ca uptake. We also determined that MVs from calcifying BVSMCs could mineralize type I collagen but not type II collagen in the absence of cells in a dose- and time-dependent manner. Blockade of annexin calcium channel activity by K201 significantly decreased ALP activity and reduced the ability of the MVs to subsequently calcify on collagen, whether the K201 was added during or after MV formation. Furthermore, cellular MVs had significantly increased ability to calcify on collagen compared with secreted MVs, likely because of their increased ALP activity and annexin II content but low fetuin-A content. In conclusion, our results suggest that mineralization in VSMCs requires both active MVs and an interaction of the MVs with type I collagen, and both steps require annexin activity.
Key words: matrix vesicles, annexins, vascular smooth muscle cells, calcification, fetuin-A
INTRODUCTION
Vascular calcification is a prominent finding in aging, diabetes, chronic kidney disease (CKD), and inflammatory diseases. Both coronary artery and peripheral artery calcification are associated with increased morbidity and mortality in the general population,(1) diabetes,(2) and in patients with CKD.(3,4) The pathophysiology of vascular calcification is complex but seems to be similar to normal bone osteogenesis. Vascular smooth muscle cells (VSMCs) are derived from the same pluripotential mesenchymal cell as osteoblasts and can dedifferentiate or transform to an osteoblast like phenotype. This transformation is characterized by the ability of VSMCs to express “bone” transcription factors (e.g., RUNX2 [Cbfa1]) and matrix proteins (e.g., collagen and osteopontin) and by their ability to mineralize in vitro in the presence of phosphorus.(5) The mechanism by which these cells mineralize is not currently known but is believed to parallel the mechanism of mineralization in chondrocytes and osteoblasts, where matrix vesicles (MVs) initiate mineralization by interacting with the extracellular matrix.
MVs have been identified in nearly all forms of normal mineralization/calcification including bone, cartilage, and tendon.(6,7) The current belief is that mineralization is initiated when MVs are released into the extracellular space from the cell surface through polarized budding, with subsequent attachment to extracellular matrix proteins to initiate mineralization.(8) MVs are characterized by both their appearance as small (50–200 nm) electron dense spherical particles on electron microscopy and the biochemical presence of calcium and phosphorus, alkaline phosphatase (ALP), and the membrane proteins annexins.(9) We and others have identified MVs in areas of vascular calcification in human arteries by electron microscopy.(10,11) MVs have also been isolated from the media of cultured VSMCs incubated with elevated concentrations of calcium and phosphate.(12) In addition, fetuin-A, the circulating inhibitor of mineralization, has been identified in these secreted MVs, and its presence decreases calcium uptake.(12) In vitro, fetuin-A inhibits calcification of both VSMCs and osteoblasts,(13–15) and fetuin-A can bind to calcium in serum to create small particles containing calcium, phosphorus, and fetuin.(16)
We recently found that annexin II binds to fetuin-A at the cell membrane of bovine VSMCs (BVSMCs) in the presence of high calcium, leading to endocytosis of fetuin-A. In addition, blocking annexin Ca2+ channel activity with K201 significantly decreased fetuin-A uptake in BVSMCs.(17) In chondrocytes, extracellular calcium upregulates annexin II, V, and VI, and calcium influx into MV; conversely, inhibition of annexin activity decreases chondrocyte mineralization.(18–20) Thus, annexins may play a similar regulatory role in vascular calcification. The objective of this study was to characterize the role of annexins and fetuin-A in MV formation and activity during calcification in BVSMCs and to examine differences in secreted, compared with cellular, MVs.
MATERIALS AND METHODS
Cell culture
BVSMCs were isolated from the descending thoracic aorta by the explant method as previously described.(21) The BVSMCs were grown in DMEM (Sigma, St Louis, MO, USA), with 10% FBS until confluent, at which time they were replated for specific experiments. Only cells between passages 2 and 8 were used in the experiments. To induce calcification, BVSMCs were treated with 10 mM β-glycerophosphate, 10−7 M insulin and 50 μg/ml ascorbic acid in the presence of 15% serum.(21) Control or noncalcifying BVSMCs were cultured in identical conditions but without the β-glycerophosphate. We have found a consistent 18-fold increase in calcification of BVSMCs with these two conditions (calcification versus control) by 7 days, similar to the results of Wada et al.(22) In some experiments, BVSMCs were also treated with K201,(23) a specific annexin calcium channel blocker (kindly provided by Aetas Pharma Co.).
MVs isolation
MVs were isolated by two techniques. For cellular MVs, a collagenase digestion protocol was used.(24) Cells were incubated with crude collagenase (500 U/ml, type IA; Sigma) in a solution of 0.25 M sucrose, 0.12 M NaCl, 0.01 M KCl, and 0.02 M Tris buffer, pH 7.45, at 37°C for 3 h The digests were centrifuged at 800g and 30,000g to remove cell debris and microsomes, respectively. The supernatant were centrifuged at 250,000g to pellet the MV, followed by resuspension in TBS (pH 7.6) with 0.25 M sucrose. In addition to these cellular MVs, secreted MVs were isolated according to the protocol by Wuthier et al.(6) Briefly, after culture, the medium was decanted and spun at 2500 rpm to remove apoptotic bodies. MVs were harvested from the supernatant by centrifugation at 35,000 rpm (100,000g) for 30 min at 4°C. The MV amount was determined by protein concentration (Bio-Rad).
ALP activity
ALP activity was measured using p-nitrophenyl substrate supplied in an ALP assay kit (Pointe Scientific). ALP activity is normalized by protein content.(21)
Western blotting
Western blotting was performed as previously described.(21) The blots were incubated with antibody against annexin II,VI (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or fetuin-A (1:2000; a gift from Dr Willi Jahnen-Dechent, University Hospital, Aachen, Germany) overnight at 4°C followed by incubating with peroxidase-conjugated secondary antibody (1:5000 dilution) and immunodetection with the Enhanced Chemiluminescence Kit (Amersham, Piscataway, NJ, USA). The band intensity was analyzed by scanning densitometry (Quantity One; Bio-Rad, Richmond, CA, USA).
Calcium uptake by MVs isolated from BVSMCs
The calcium uptake was assessed using an adaptation of a protocol by Hsu et al.(24) Briefly, aliquots of MVs (10 μg of protein) were incubated in 100 μl reaction media of 50 mM Tris, pH 7.65, 85 mM NaCl, 15 mM KCl, 1 mM MgCl2, 30 mM NaHCO3, 1.45 mM CaCl2, and 2.3 mM Pi, with 1 × 106 cpm 45Ca at 37°C for 5 h; 100-μl aliquots were filtered through 0.1-μm filters, and after washing, the radioactivity associated with the filters was determined by scintillation counting. The background 45Ca uptake was defined as the radioactivity nonspecifically bound to the filters under the identical conditions in the absence of the MVs.
MV-collagen calcification assay
Glass coverslips were coated with or without type I or type II collagen (Sigma) in a 0.01% solution in 0.1 M acetic acid at room temperature for 4 h, which yields ∼8–10 μg/cm2 coating. Some of the experiments were also performed on commercially available, precoated type I collagen glass bottom culture dishes (MatTek, Ashland, MA, USA) to verify our type I collagen coating. MVs isolated as above were added in equal concentrations (5–20 μg/dish) to type I or II collagen-coated and noncoated coverslips in the presence of calcification media (DMEM with 15% FBS and 10 mM β-glycerophosphate) to yield an acellular MV-collagen culture and incubated at 37°C for 24–72 h. To determine the magnitude of calcification of this MV-collagen culture, the media were removed, and the MV-extracellular matrix (ECM) complex on the coverslips was incubated in 0.6 N HCl for 24 h. The calcium content of HCl supernatants was determined colorimetrically by the o-cresolphthalein complex 1 method (Calcium kit; Pointe Scientific) as previously described.(21) This assay allowed us to directly test the interaction and biologic activity of MVs in the absence of VSMCs. Importantly, the source of phosphate was β-glycerophosphate, which requires cleavage by membrane ALP to release free phosphate. Thus, calcification in this assay presents active MV-mediated calcification and not spontaneous precipitation.
RESULTS
ALP activity, annexin II and VI content, and 45Ca uptake by MVs is significantly increased during calcification
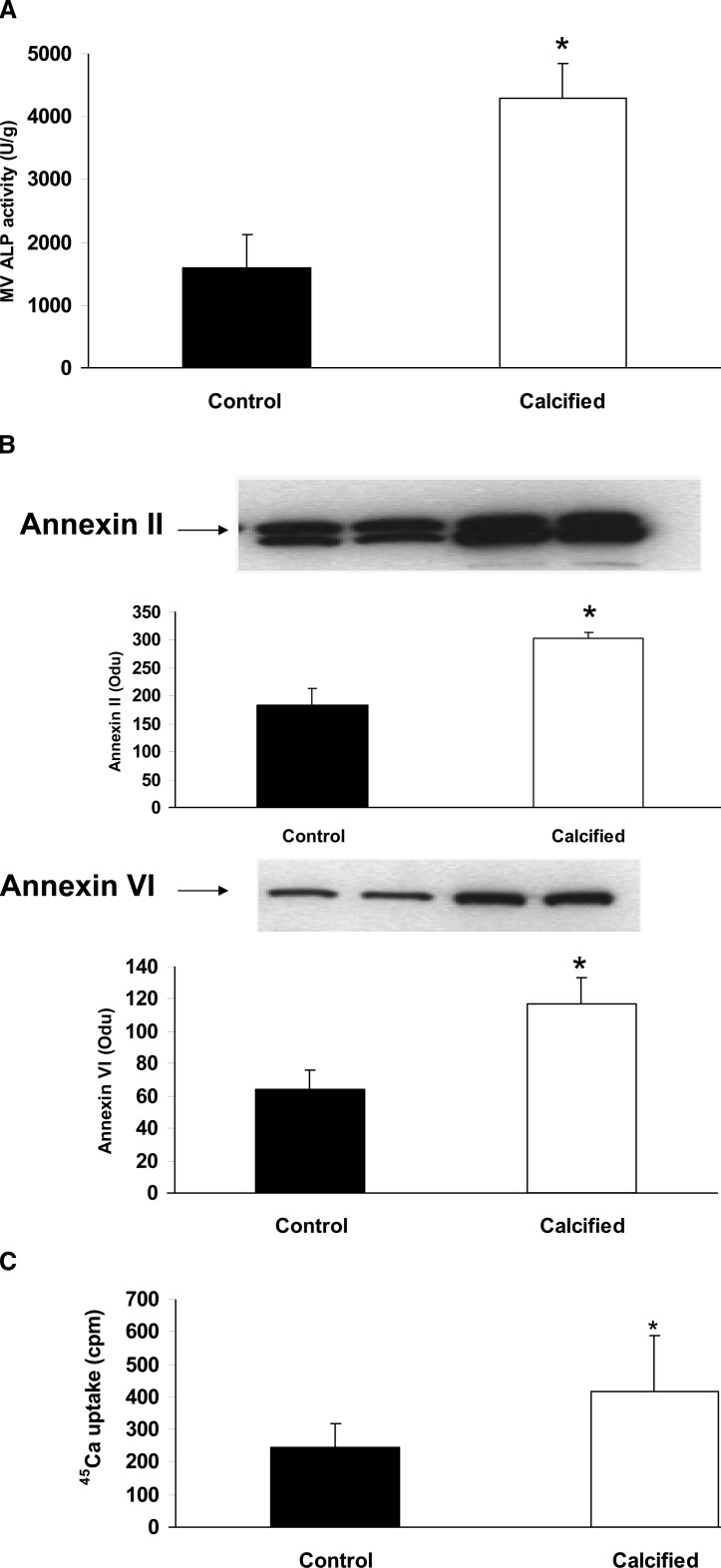
To determine the role of MVs in calcification of BVSMCs, cells were incubated with control (noncalcified, no β-glycerophosphate) or calcification media (10 mM β-glycerophosphate) for 7 days, and MVs were isolated from cell lysate by collagenase digestion. The amount of MV protein, ALP activity, and the expression of annexin II and VI were examined. The results showed that, although the total amount of MV proteins did not significantly change during calcification, ALP activity (Fig. 1A) was increased in MVs from calcified BVSMCs compared with control (noncalcified) BVSMCs (4303 ± 540 versus 1596 ± 538 U/g, p < 0.01, calcifying versus control conditions). Western blot analysis showed that the content of annexin II and VI was also significantly increased in MVs from calcified BVSMCs (Fig. 1B; p < 0.003, calcified versus control conditions). In addition, MVs from calcified BVSMCs had a 70% increase in 45Ca uptake compared with that from control BVSMCs (Fig. 1C; p < 0.05, calcifying versus control conditions). These results indicated that MVs from calcified BVSMCs had a increased ALP activity, annexin II and VI content, and enhanced ability to take up calcium compared with MVs from noncalcified BVSMCs. However, as detailed later, the MVs isolated by collagenase digestion did not contain measurable fetuin-A by Western blot analyses.
FIG. 1.
ALP activity, annexin II and VI content, and 45Ca uptake by MVs from calcified and noncalcified BVSMCs. MVs were isolated from calcified and noncalcified (control) BVSMCs by collagenase digestion. (A) MV ALP activity was measured and normalized by total MV protein. (B) Annexin II (top) and annexin VI (bottom) content in MVs was determined by Western blot analysis and band density was quantified. (C) Equal amounts of MVs (10 μg of protein) were incubated in 100 μl reaction media with 1 × 106 cpm 45Ca at 37°C for 5 h, and uptake was determined by scintillation counting. The results show that MVs from calcified BVSMCs had increased ALP activity, increased annexin II and VI content, and enhanced ability to take up calcium. Data are shown as mean ± SD from three to four separate experiments. *p < 0.001, calcified vs. control.
MVs from calcified BVSMCs have increased ability to calcify type I collagen
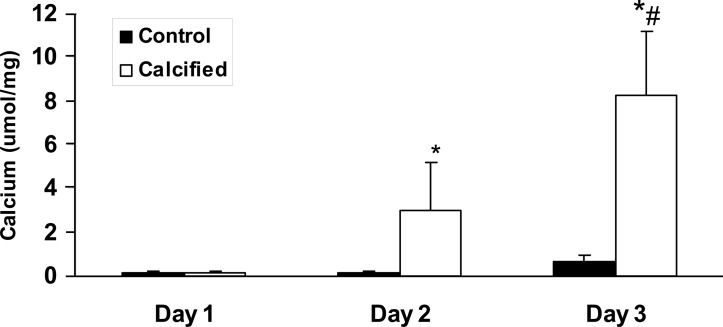
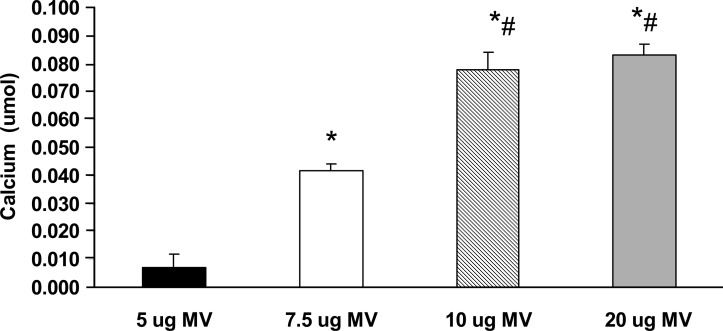
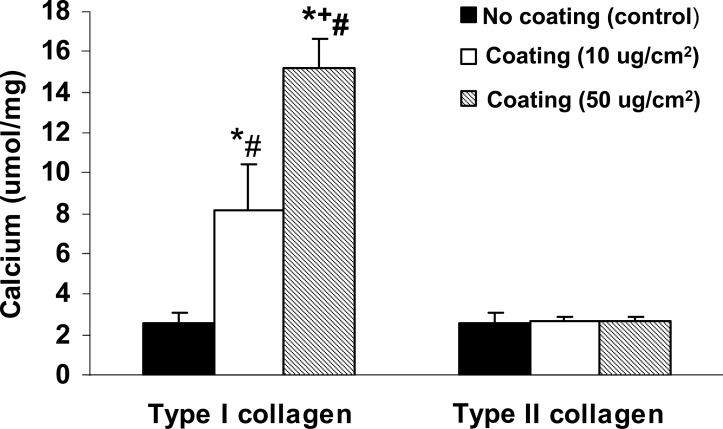
To determine the ability of MVs to calcify collagen, we developed a MV-collagen calcification assay to measure the ability of MVs to mineralize matrix in the absence of cells. The results showed that MVs from mineralizing BVSMCs calcify in type I collagen that increases in a time-dependent manner. In contrast, MVs from nonmineralizing BVMSCs failed to calcify in type I collagen over time (Fig. 2). The results also showed a dose-dependent increase in the amount of calcification with increasing quantity of MVs added (Fig. 3). To determine the specificity of MV calcification on collagen, MVs were isolated and incubated for 3 days with calcification media on coverslips coated with increasing concentrations of either type I or type II collagen, and calcification was determined by HCL extraction. As shown in Fig. 4, there is a dose-dependent increase in MV calcification with type I collagen, whereas MVs failed to calcify type II collagen. These findings suggest that mineralization of VSMCs requires both bioactive MVs and an interaction of the MVs with a specific ECM protein such as type I collagen.
FIG. 2.
Time course of collagen calcification of MVs from control or calcified BVSMCs in MV-collagen calcification assay. Glass coverslips were coated with type I collagen (10 μg/cm2), and MVs (10 μg) isolated from calcified and noncalcified (control) BVSMCs were added in the presence of calcification media (DMEM with 15% FBS and 10 mM β-glycerophosphate) and incubated at 37°C for 1, 2, or 3 days to yield an acellular MV-collagen culture. The calcium content was determined by HCL extraction. The results show MVs from calcified BVSMCs calcify type I collagen in a time-dependent manner, but MVs from control BVSMC fail to calcify. Data are shown as mean ± SD from three separate experiments. p < 0.0001, calcified vs. control; same day, # p < 0.0002, day 3 vs. day 2, control or calcified.
FIG. 3.
MV calcification on type I collagen in MV-collagen calcification assay. Glass coverslips were coated with type I collagen (10 μg/cm2). Increasing concentrations (5, 7.5, 10, and 20 μg) of MVs isolated from calcified BVSMCs were added in the presence of calcification media (DMEM with 15% FBS and 10 mM β-glycerophosphate) and incubated at 37°C for 72 h to yield an acellular MV-collagen culture. The calcium content was determined by HCL extraction. The results show a dose-dependent increase in the amount of calcification with the addition of increasing concentrations of MVs added. Data are shown as mean ± SD from three separate experiments. *p < 0.005 compared with 5 μg MVs; # p < 0.05 compared with 7.5 μg MVs.
FIG. 4.
Effect of extracellular matrix in MV-collagen calcification assay. Glass coverslips were coated with various amounts of type I or type II collagen (0, 10, 50 μg/cm2), and MVs were added in the presence of calcification media (DMEM with 15% FBS and 10 mM β-glycerophosphate) and incubated at 37°C for 72 h to yield an acellular MV-collagen culture. The calcium content was determined by HCL extraction. The results show a dose-dependent increase in MV calcification with type I collagen, whereas MVs fail to calcify type II collagen. Data are shown as mean ± SD from three separate experiments. *p < 0.0001, coating vs. noncoating control; + p < 0.005, 50 vs. 10 μg/cm2; # p < 0.0001, type I vs. type II collagen coated, 50 or 10 μg/cm2.
Blocking annexin calcium channel activity significantly inhibits ALP activity and 45Ca uptake in MVs from calcified BVSMCs and inhibits the ability of MVs to calcify collagen
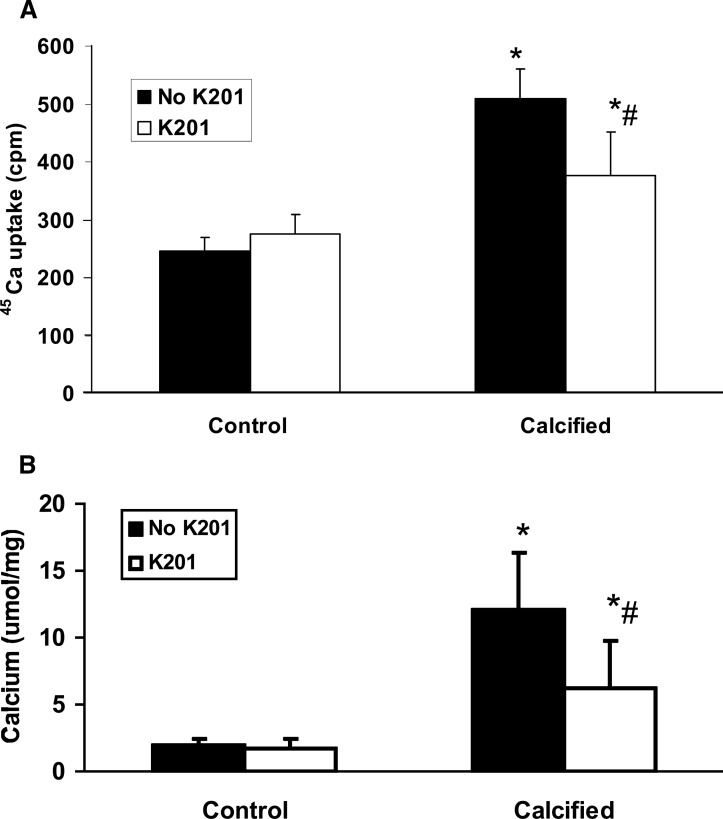
To determine the role of annexins in MV activity, BVSMCs were incubated in DMEM in calcification media (with 10 mM β-glycerophosphate) or noncalcifying media (no β-glycerophosphate) for 7 days in the presence or absence of K201, a broad inhibitor of annexin calcium channel activity,(23) and MVs were isolated. The results showed that blockade of annexin calcium channel activity by K201 during MV synthesis significantly decreased ALP activity in MVs isolated from calcified BVSMCs (calcified = 4302 ± 540 U/g protein; calcified + K201 = 3127 ± 625 U/g protein, p < 0.0001) but had no effect on ALP activity in MVs from control (noncalcified) BVSMCs (control = 1596 ± 538 U/g protein; control + K201 = 1465 ± 388 U/g protein). In addition, blockade of annexin calcium channel activity by K201 significantly decreased 45Ca uptake by MV isolated from calcified BVSMCs but had no effect on MVs from control (noncalcified) BVSMCs (Fig. 5A; *p < 0.01, calcified versus control; # p < 0.005, K201 versus no K201). To determine the role of annexins in MV calcification in ECM, BVSMCs were incubated in calcifying or noncalcifying conditions in the presence or absence of K201 for 7 days, and MVs were isolated, placed on type I collagen-coated dishes, and incubated with calcification media (10 mM β-glycerophosphate) for 3 days for the MV-collagen calcification assay. The results showed that MVs isolated from BVSMCs incubated with K201 decreased the ability of the MVs to subsequently calcify collagen (Fig. 5B; *p < 0.01, calcified versus control; # p < 0.001, K201 versus no K201). To determine whether annexins were also important during MV calcification on collagen, we isolated MVs from BVSMCs incubated in calcified or noncalcified (control) conditions but without K201, and subsequently cultured the MVs on type I collagen in the presence or absence of K201. The data showed identical results, suggesting that annexin activity was also critical after MV formation in the subsequent mineralization of MV with type I collagen. These data showed that annexin activity is critical during MV formation and in the subsequent ability of MVs to adhere to type I collagen.
FIG. 5.
K201 inhibits the ability of MV to calcify on type I collagen. To determine the role of annexins in MV activity, BVSMCs were incubated in DMEM in calcification media (calcified, with 10 mM β-glycerophosphate) or noncalcifying media (control, no β-glycerophosphate) for 7 days in the presence or absence of K201, a broad inhibitor of annexin calcium channel activity and MVs were isolated. (A) Equal amounts of MVs (10 μg of protein) were incubated in 100 μl reaction media with 1 × 106 cpm 45Ca at 37°C for 5 h and determined by scintillation counting. (B) MVs were placed on type I collagen-coated coverslips in culture dishes and incubated with calcification media (10 mM β-glycerophosphate) for 3 days, and the calcification was examined by HCL extraction. The results show that blockade of annexin calcium channel activity by K201 during MV synthesis significantly decreases ALP activity and decreases the ability of the MV to subsequently calcify collagen in MVs isolated from calcified BVSMCs. Data are shown as mean ± SD from four separate experiments. *p < 0.01, calcified vs. control; same treatment; # p < 0.001, K201 vs. no K201, control or calcified.
Characterization of secreted (media) versus cellular (collagenase-released) MVs in BVSMCs during calcification
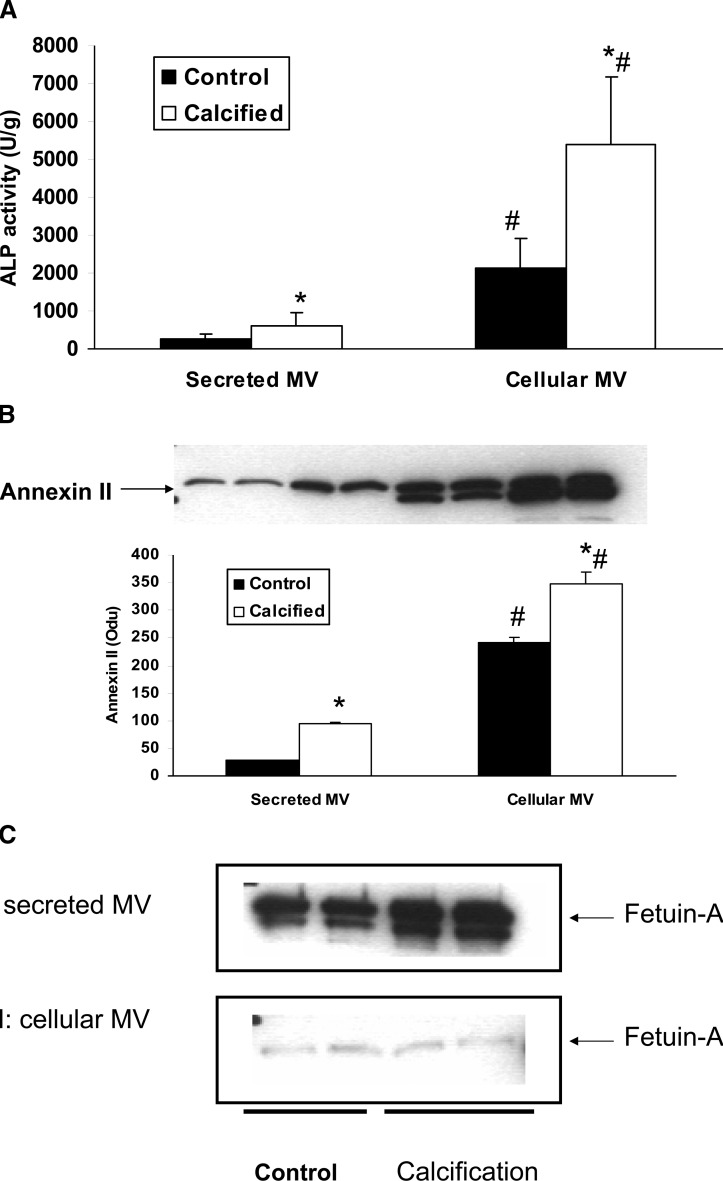
As described above, we did not find evidence for fetuin-A in MVs using collagenase digestion in contrast to previously published results that examined MVs isolated from the media.(12) Therefore, we directly compared secreted (media) and cellular (collagenase released) MV formation, content, and activity in BVSMCs during calcification. BVSMCs were incubated in DMEM in calcification media (with 10 mM β-glycerophosphate) or control media (no β-glycerophosphate) for 7 days. Secreted MVs were isolated from media in cultured cells by ultracentrifugation, and cellular MVs were isolated from collagenase digestion of these cultured cells followed by centrifugation from the same cultures. The amount of MVs, as determined by protein content, was markedly greater when isolated from collagenase digestion than the media (767 ± 26 versus 106 ± 18 μg). Thus, MV ALP activity and the contents of annexin II and fetuin-A were all normalized by MV protein content. The results showed that ALP activity was significantly increased in MVs from calcified BVSMCs compared with noncalcified BVSMCs in both cellular and secreted MVs (Fig. 6A). However, there was an 8.6-fold increase in ALP activity in cellular MV compared with secreted MV (Fig. 6A). Western blot showed that the content of annexin II was also increased by 320% in cellular MVs compared with secreted MVs from calcified BVSMCs (Fig. 6B). Interestingly, whereas fetuin-A content was barely detectable in cellular MVs isolated from both control or calcified BVSMCs, there was a 26- to 34-fold increase in fetuin-A content in secreted MVs from both control (208 ± 3 versus 8 ± 2 Odu, p < 0.001) and calcified (300 ± 31 versus 9 ± 1 Odu, p < 0.001) BVSMCs (Fig. 6C).
FIG. 6.
Characterization of secreted (media) vs. cellular (collagenase-released) MVs in BVSMCs during calcification. BVSMCs were incubated in DMEM in calcification media (with 10 mM β-glycerophosphate) or control media (no β-glycerophosphate) for 7 days. Secreted MVs were isolated from media in cultured cells by ultracentrifugation, and cellular MVs were isolated from collagenase digestion of these cultured cells from the same experiments. (A) Secreted and cellular MV ALP activity was determined and normalized by total MV proteins. (B) Annexin II and (C) fetuin-A content in secreted and cellular MVs was determined by Western blot and quantified. The results show that there was an significant increase in ALP activity and annexin II content but a decrease in fetuin-A content in cellular MVs compared with secreted MVs. Data are shown as mean ± SD from four separate experiments. Also shown are representative Western blot from the experiments. *p < 0.005, calcified vs. control, secreted, or cellular MVs; # p < 0.001, cellular MVs vs. secreted MVs, control, or calcified.
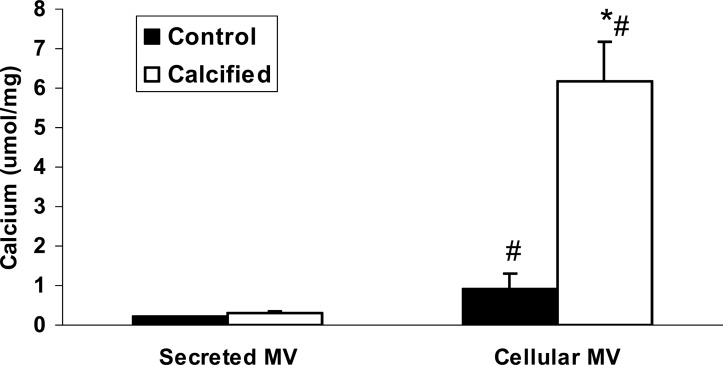
To determine whether these differences in secreted and cellular MVs affect the ability to calcify, MVs were incubated without cells in calcification media on coverslips coated with type I collagen. The results showed that cellular MVs isolated from calcified BVSMCs significantly increased calcification of collagen compared with that from control BVSMCs (6.2 ± 1.0 versus 0.9 ± 0.4 μmol/mg, p < 0.0001; Fig. 7). However, secreted MVs isolated from either calcified or control BVSMCs did not calcify (Fig. 7). These results showed that cellular MVs have a greater ability to calcify type I collagen than secreted MVs.
FIG. 7.
Calcification of secreted vs. cellular MVs in M-collagen calcification assay. BVSMCs were incubated in DMEM in calcification media (with 10 mM β-glycerophosphate) or control media (no β-glycerophosphate) for 7 days. Secreted MVs were isolated from media in cultured cells by ultracentrifugation and cellular MVs were isolated from collagenase digestion of these cultured cells followed by centrifugation from the same experiments. MVs (10 μg) were added to type I collagen-coated coverslips in the presence of calcification media (DMEM with 15% FBS and 10 mM β-glycerophosphate) and incubated for 3 days. The calcium content was determined by HCL extraction. The results show that cellular MVs isolated from calcified BVSMCs significantly increases calcification of collagen but secreted MVs do not calcify. Data are shown as mean ± SD from four separate experiments. *p < 0.0001, calcified vs. control, cellular, or secreted; # p < 0.0001, cellular vs. secreted MVs, control, or calcified.
DISCUSSION
MVs have a critical role in the initiation of mineral deposition in skeletal tissues. MVs produced by mineralizing chondrocytes, unlike control or nonmineralizing MVs, have very strong ALP activity and contain annexin II, V, and VI, the membrane-associated proteins known to mediate Ca2+ influx into matrix vesicles. In this study, we characterized MVs isolated from BVSMCs, showing that ALP activity and annexin II and VI content were significantly increased, and the 45Ca uptake was greater, in MVs from calcified BVSMCs compared with noncalcified BVSMCs. These results indicate that, although MVs are produced by both nonmineralizing and mineralizing BVSMCs, their components and activity are different, similar to observations of normal chondrocytic mineralization. Thus, although vascular calcification is an unwanted phenomenon, the initiation step of MV synthesis is similar to desirable calcification.
The MVs produced by the mineralizing BVSMCs, similar to mineralizing chondrocytes, have key features rendering them capable of mineralization. First, we showed that MVs from BVSMCs were extremely rich in ALP activity and thus capable of hydrolyzing a variety of organic phosphate substrates to increase the local availability of free phosphate ions to use for apatite crystallization. The presence of ALP activity also removes putative inhibitors of mineralization, including pyrophosphate.(25,26) Second, the vesicles from BVMSCs contained a large amount of annexin II and VI, which can mediate the influx of Ca2+ into matrix vesicles, enabling intralumenal crystal growth.(27) Last, using a novel assay, we showed the MVs from calcified BVSMCs have an ability to interact with type I collagen to mineralize this extracellular matrix protein, even in an acellular environment. Calcification was increased with increasing amounts of MVs and virtually absent without the presence of type I collagen. Importantly, it should be noted that the β-glycerophosphate used in calcification media in this latter assay can only be converted to phosphorus if the MVs have biologically active membrane ALP activity. Thus, calcification in this assay represents MV-mediated calcification and not spontaneous precipitation nor calcification from adjacent cells. Overall, our data suggest that mineralization in VSMCs requires not only bioactive MVs but a source of phosphorus and an interaction of the MVs with the ECM.
Collagen fibrils are believed to play an important role in regulating the ensuing biomineralization cascade once the initial mineral is formed by MVs.(7) It has been shown in cartilage that type II and X collagens are bound to the outer surfaces of MVs and may serve as a bridge for crystal propagation out in the extravesicular matrix.(28) Indeed, selective removal of type II and X collagen from the chondrocyte MV surface drastically reduced the ability of MVs to take up Ca2+.(29) The addition of purified type II and X collagen to these collagen-depleted MVs restored their Ca2+ uptake ability. Whereas type II and X collagen are the main collagens that interact with MVs in cartilage, the majority of arterial collagen is type I and III collagen.(30) Type I collagen expression is upregulated in human atherosclerotic plaques and medial calcification.(31,32) Our data clearly shows that MVs can increase calcification on type I collagen, but failed to calcify on type II collagen. These data suggest that MVs from VSMCs may interact only with a specific ECM present in arteries. Such MV–ECM interaction specificity may be one mechanism by which some tissues calcify and others do not.
In this study, we showed that K201, a specific annexin channel inhibitor,(23) significantly decreased ALP activity in MVs from calcified BVSMCs but had no effect on MVs from noncalcified BVSMCs. Furthermore, K201 decreased the ability of MVs to calcify collagen when the MVs were formed in the presence of K201 or if the MVs were subsequently incubated with K201 after formation. These findings establish that annexin-mediated calcium uptake is critical for MV formation and calcification of BVSMCs. Annexins are also important in cartilage calcification because only posthypertrophic chondrocytes that contain annexin II, V, and VI are mineralization-competent. Gillete et al.(33) also overexpressed annexin II in osteoblasts, showing a dramatic increase in ALP activity and mineralization. In this study, we confirmed the importance of annexin calcium channel activity by using the inhibitor K201. However, calcium channel activity is a function of multiple annexins. Thus, which annexin, or combination of annexins, is responsible for the mineralizing activity of MVs on collagen will require further studies. Nonetheless, annexins seem critical for both skeletal and extraskeletal mineralization.
We have previously found that annexins are also critical in calcium-mediated BVSMC fetuin-A endocytosis and that annexin II and fetuin-A bind at the cell membrane in the presence of elevated extracellular calcium.(34) Fetuin-A is a known inhibitor of VSMC and osteoblast mineralization in vitro(13–15) and, when present in secreted matrix vesicles, renders them incapable of calcium uptake.(14) We found clear differences in the fetuin-A and annexin content in secreted versus cellular MVs. In secreted MVs, the annexin content and ALP activity was decreased, whereas fetuin-A was increased compared with cellular (collagenase digested) MVs. These differences also resulted in a decreased ability of secreted, compared with cellular, MVs to mineralize type I collagen in an acellular environment. Given our previous data showing that annexins mediate fetuin-A uptake in the presence of extracellular calcium, we hypothesize that the interaction of these two proteins at the cell membrane somehow regulates the fate and function of MVs. Importantly, Xiao et al.,(35) using a proteomic approach, also found many other differences in secreted and cellular MVs from osteoblasts.
In conclusion, our results showed that mineralization in VSMCs requires both bioactive MVs and an interaction of the MVs with specific ECM proteins. Annexin activity plays an important role in both the generation of bioactive MVs and in their ability to mineralize type I collagen. Thus, whether a MV stays associated with the cell or is secreted into the media away from the cell may be determined by the protein content of the MVs. The cellular mechanisms by which these proteins are incorporated into the MV may therefore be a key regulatory step. Our data presented here, together with our previous study,(34) suggest that the annexin–fetuin-A interaction at the cell membrane may be one such regulatory mechanism. Unraveling how this regulation occurs will require additional studies, but these mechanisms may result in novel therapeutics to prevent unwanted extraosseous calcification.
ACKNOWLEDGMENTS
This work was supported by an unrestricted research grant from Genzyme Corporation (NXC), a K01 Grant from the NIH/NIDDK (NXC), and a VA Merit Award (SMM). We thank Michelle Murray for excellent secretarial assistance.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events the st. Francis heart study. J Am Coll Cardiol. 2005;46:158–165. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 2.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 4.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 5.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 6.Wuthier RE, Chin JE, Hale JE, Register TC, Hale LV, Ishikawa Y. Isolation and characterization of calcium-accumulating matrix vesicles from chondrocytes of chicken epiphyseal growth plate cartilage in primary culture. J Biol Chem. 1985;260:15972–15979. [PubMed] [Google Scholar]
- 7.Arsenault AL, Frankland BW, Ottensmeyer FP. Vectorial sequence of mineralization in the turkey leg tendon determined by electron microscopic imaging. Calcif Tissue Int. 1991;48:46–55. doi: 10.1007/BF02555795. [DOI] [PubMed] [Google Scholar]
- 8.Anderson HC, Garimella R, Tague SE. The role of matrix vesicles in growth plate development and biomineralization. Front Biosci. 2005;10:822–837. doi: 10.2741/1576. [DOI] [PubMed] [Google Scholar]
- 9.Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003;5:222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed S, O'Neill KD, Hood AF, Evan AP, Moe SM. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:1267–1276. doi: 10.1053/ajkd.2001.24533. [DOI] [PubMed] [Google Scholar]
- 11.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/Chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–494. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 13.Moe SM, Reslerova M, Ketteler M, O'Neill K, Duan D, Koczman J, Westenfeld R, Jahnen-Dechent W, Chen NX. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD) Kidney Int. 2005;67:2295–2304. doi: 10.1111/j.1523-1755.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds JL, Skepper JN, McNair R, Kasama T, Gupta K, Weissberg PL, Jahnen-Dechent W, Shanahan CM. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol. 2005;16:2920–2930. doi: 10.1681/ASN.2004100895. [DOI] [PubMed] [Google Scholar]
- 15.Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, Jahnen-Dechent W. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J Biol Chem. 1996;271:20789–20796. doi: 10.1074/jbc.271.34.20789. [DOI] [PubMed] [Google Scholar]
- 16.Heiss A, DuChesne A, Denecke B, Grotzinger J, Yamamoto K, Renne T, Jahnen-Dechent W. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J Biol Chem. 2003;278:13333–13341. doi: 10.1074/jbc.M210868200. [DOI] [PubMed] [Google Scholar]
- 17.Chen NX, O'Neill KD, Chen X, Duan D, Wang E, Sturek MS, Edwards JM, Moe SM. Fetuin-A uptake in bovine vascular smooth muscle cells is calcium dependent and mediated by annexins. Am J Physiol Renal Physiol. 2007;292:F599–F606. doi: 10.1152/ajprenal.00303.2006. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Xu J, Kirsch T. Annexin-mediated Ca2+ influx regulates growth plate chondrocyte maturation and apoptosis. J Biol Chem. 2003;278:3762–3769. doi: 10.1074/jbc.M208868200. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Kirsch T. Retinoic acid stimulates annexin-mediated growth plate chondrocyte mineralization. J Cell Biol. 2002;157:1061–1069. doi: 10.1083/jcb.200203014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirsch T, Wang W, Pfander D. Functional differences between growth plate apoptotic bodies and matrix vesicles. J Bone Miner Res. 2003;18:1872–1881. doi: 10.1359/jbmr.2003.18.10.1872. [DOI] [PubMed] [Google Scholar]
- 21.Chen NX, O'Neill KD, Duan D, Moe SM. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002;62:1724–1731. doi: 10.1046/j.1523-1755.2002.00625.x. [DOI] [PubMed] [Google Scholar]
- 22.Wada T, McKee MD, Steitz S, Giachelli CM. Calcification of vascular smooth muscle cell cultures: Inhibition by osteopontin. Circ Res. 1999;84:166–178. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko N. New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac cell death and intracellular calcium blocking action. Drug Dev Res. 1994;33:429–438. [Google Scholar]
- 24.Hsu HH, Camacho NP. Isolation of calcifiable vesicles from human atherosclerotic aortas. Atherosclerosis. 1999;143:353–362. doi: 10.1016/s0021-9150(98)00322-0. [DOI] [PubMed] [Google Scholar]
- 25.Anderson HC, Reynolds JJ. Pyrophosphate stimulation of calcium uptake into cultured embryonic bones. Fine structure of matrix vesicles and their role in calcification. Dev Biol. 1973;34:211–227. doi: 10.1016/0012-1606(73)90351-5. [DOI] [PubMed] [Google Scholar]
- 26.O'Neill WC. Pyrophosphate, alkaline phosphatase, and vascular calcification. Circ Res. 2006;99:e2. doi: 10.1161/01.RES.0000234909.24367.a9. [DOI] [PubMed] [Google Scholar]
- 27.Kirsch T, Harrison G, Golub EE, Nah HD. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J Biol Chem. 2000;275:35577–35583. doi: 10.1074/jbc.M005648200. [DOI] [PubMed] [Google Scholar]
- 28.Wu LN, Genge BR, Lloyd GC, Wuthier RE. Collagen-binding proteins in collagenase-released matrix vesicles from cartilage. Interaction between matrix vesicle proteins and different types of collagen. J Biol Chem. 1991;266:1195–1203. [PubMed] [Google Scholar]
- 29.Kirsch T, Wuthier RE. Stimulation of calcification of growth plate cartilage matrix vesicles by binding to type II and X collagens. J Biol Chem. 1994;269:11462–11469. [PubMed] [Google Scholar]
- 30.Mayne R. Collagenous proteins of blood vessels. Arteriosclerosis. 1986;6:585–593. doi: 10.1161/01.atv.6.6.585. [DOI] [PubMed] [Google Scholar]
- 31.Rekhter MD, Zhang K, Narayanan AS, Phan S, Schork MA, Gordon D. Type I collagen gene expression in human atherosclerosis. Localization to specific plaque regions. Am J Pathol. 1993;143:1634–1648. [PMC free article] [PubMed] [Google Scholar]
- 32.Moe SM, O'Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, Fineberg N, Kopecky K. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61:638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 33.Gillette JM, Nielsen-Preiss SM. The role of annexin 2 in osteoblastic mineralization. J Cell Sci. 2004;117:441–449. doi: 10.1242/jcs.00909. [DOI] [PubMed] [Google Scholar]
- 34.Chen NX, O'Neill KD, Chen X, Duan D, Wang E, Sturek MS, Edwards JM, Moe SM. Fetuin-A uptake in bovine vascular smooth muscle cells is calcium dependent and mediated by annexins. Am J Physiol Renal Physiol. 2007;292:F599–F606. doi: 10.1152/ajprenal.00303.2006. [DOI] [PubMed] [Google Scholar]
- 35.Xiao Z, Camalier CE, Nagashima K, Chan KC, Lucas DA, de la Cruz MJ, Gignac M, Lockett S, Issaq HJ, Veenstra TD, Conrads TP, Beck GR., Jr Analysis of the extracellular matrix vesicle proteome in mineralizing osteoblasts. J Cell Physiol. 2007;210:325–335. doi: 10.1002/jcp.20826. [DOI] [PubMed] [Google Scholar]