Summary
Embryonic stem cells form descendants of all three germ layers when differentiated as aggregates, termed embryoid bodies. In vivo, differentiation of cells depends on signals and morphogen gradients that provide instructive and positional cues, but do such gradients exist in embryoid bodies? We report here the establishment of anteroposterior polarity and the formation of a primitive streak-like region in the embryoid body, dependent on local activation of the Wnt pathway. In this region, cells undergo an epithelial-to-mesenchymal transition and differentiate into mesendodermal progenitors. Exogenous Wnt3a protein posteriorizes the embryoid body, resulting in predominantly mesendodermal differentiation. Conversely, inhibiting Wnt signaling promotes anterior character and results in neurectodermal differentiation. The activation of Wnt signaling and primitive streak formation requires external signals but is self-reinforcing after initiation. Our findings show that the Wnt pathway mediates the local execution of a gastrulation-like process in the embryoid body, which displays an unexpected degree of self-organization.
Introduction
A major goal of ES cell research is to direct the differentiation of stem cells into specific developmental lineages. Descendants from all three germ layers can be produced by allowing ES cells to differentiate as an aggregate, called an embryoid body (Burkert et al., 1991; Doetschman et al., 1985). Under these conditions, ES cells can differentiate into a wide variety of cell types, including hematopoietic, vascular, pancreatic, hepatic and neural lineages (Murry and Keller, 2008), and even into germ cells (Geijsen et al., 2004). It is thought that tissue differentiation in embryoid bodies occurs in a disorganized fashion (Murry and Keller, 2008). In vivo however, morphogen gradients impart positional information and influence cell lineage decisions, and such cues can also promote the formation of specific cell types in embryoid bodies. For example, adding retinoic acid to embryoid bodies caudalizes cells, a treatment that together with the ventralizing activity of Sonic hedgehog leads to motor neuron differentiation (Wichterle et al., 2002). Upon transplantation, these cells segregate and innervate tissues according to their positional specification (Wichterle et al., 2002). This demonstrates the importance of providing differentiating cells not only with instructive but also positional signals. In this study, we sought to determine to what extent cells in the supposedly unorganized embryoid body are exposed to morphogen gradients and positional signals.
ES cells are derived from the inner cell mass of blastocyst stage embryos. Following implantation, the inner cell mass forms a solid mass of pluripotent unpolarized embryonic ectoderm, separated by a basement membrane from an outer layer of primitive endoderm. The central cells of the embryonic ectoderm undergo programmed cell death and disappear in a process called cavitation, whereas the surrounding embryonic ectoderm differentiates into a pseudostratified columnar epithelium. The embryonic ectoderm, or epiblast, is further characterized by expression of the pluripotency marker Oct4 (Rosner et al., 1990). Upon further development, the primary germ layers are established and patterned during gastrulation, when the primitive streak forms on the posterior side of the embryo. Epiblast cells ingressing through the primitive streak are the source of definitive endoderm and mesoderm, whereas the anterior epiblast differentiates into ectodermal derivatives (reviewed in (Tam and Loebel, 2007)). The posterior expression of several Wnt ligands and Wnt signaling components is essential for the establishment of the primitive streak and anteroposterior polarity, the epithelial-to-mesenchymal transition of epiblast cells in the primitive streak, and the formation of mesoderm (Galceran et al., 1999; Greco et al., 1996; Haegel et al., 1995; Huelsken et al., 2000; Kelly et al., 2004; Kemler et al., 2004; Liu et al., 1999; Takada et al., 1994; Yoshikawa et al., 1997). In vitro, manipulation of the Wnt signaling pathway during embryoid body formation greatly influences the direction of cell differentiation (Aubert et al., 2002; Gadue et al., 2006; Lindsley et al., 2006).
Wnt signaling can be visualized through the activity of several reporter constructs, including 7xTCF-eGFP (Brugmann et al., 2007), and the targeted mouse mutant Axin2LacZ/+, which expresses a LacZ reporter from the locus of the Wnt target gene Axin2 (Aulehla et al., 2003; Jho et al., 2002; Lustig et al., 2002). Using these reporters, we find that endogenous Wnt signals polarize the embryoid body and mediate the local execution of a gastrulation-like process.
Results
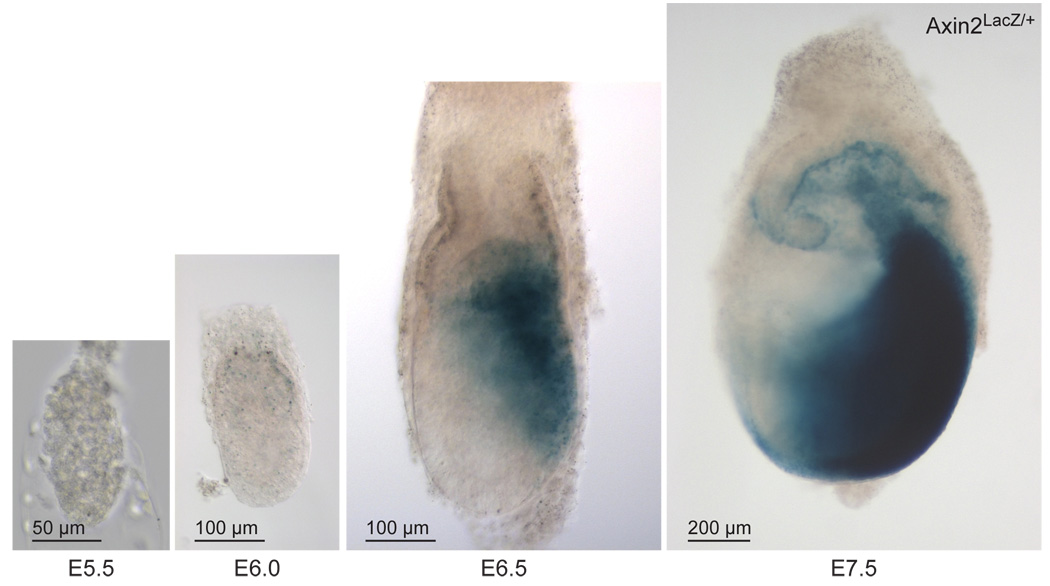
Wnt signaling is required for establishment of the primitive streak, and therefore we asked whether activation of the Wnt pathway could serve to visualize the location of primitive streak formation. Using Axin2LacZ/+ reporter mice, we found no evidence of Wnt signaling in E5.5 and E6.0 embryos, but reporter activity became visible around E6.5 in the posterior region of the embryo (Fig. 1). At this stage the primitive streak begins to form, and reporter activity is visible in the newly formed mesodermal cells that migrate anteriorly and proximally (Jho et al., 2002; Morkel et al., 2003). At late streak stage (E7.5), reporter activity was visible in the entire posterior half of the embryo, and in the extra-embryonic allantois and chorion (Fig. 1). The first activation of the Axin2LacZ/+ reporter in the post-implantation embryo therefore coincides with the establishment of the primitive streak and its derived cell populations.
Figure 1. Axin2LacZ/+ reporter is active in the region of the primitive streak and derived cell populations.
No expression is visible in E5.5 and E6.0 embryos, before primitive streak formation. The E6.5 and E7.5 embryos are oriented with their posterior pointing right.
Polarized activation of the Wnt pathway in embryoid bodies
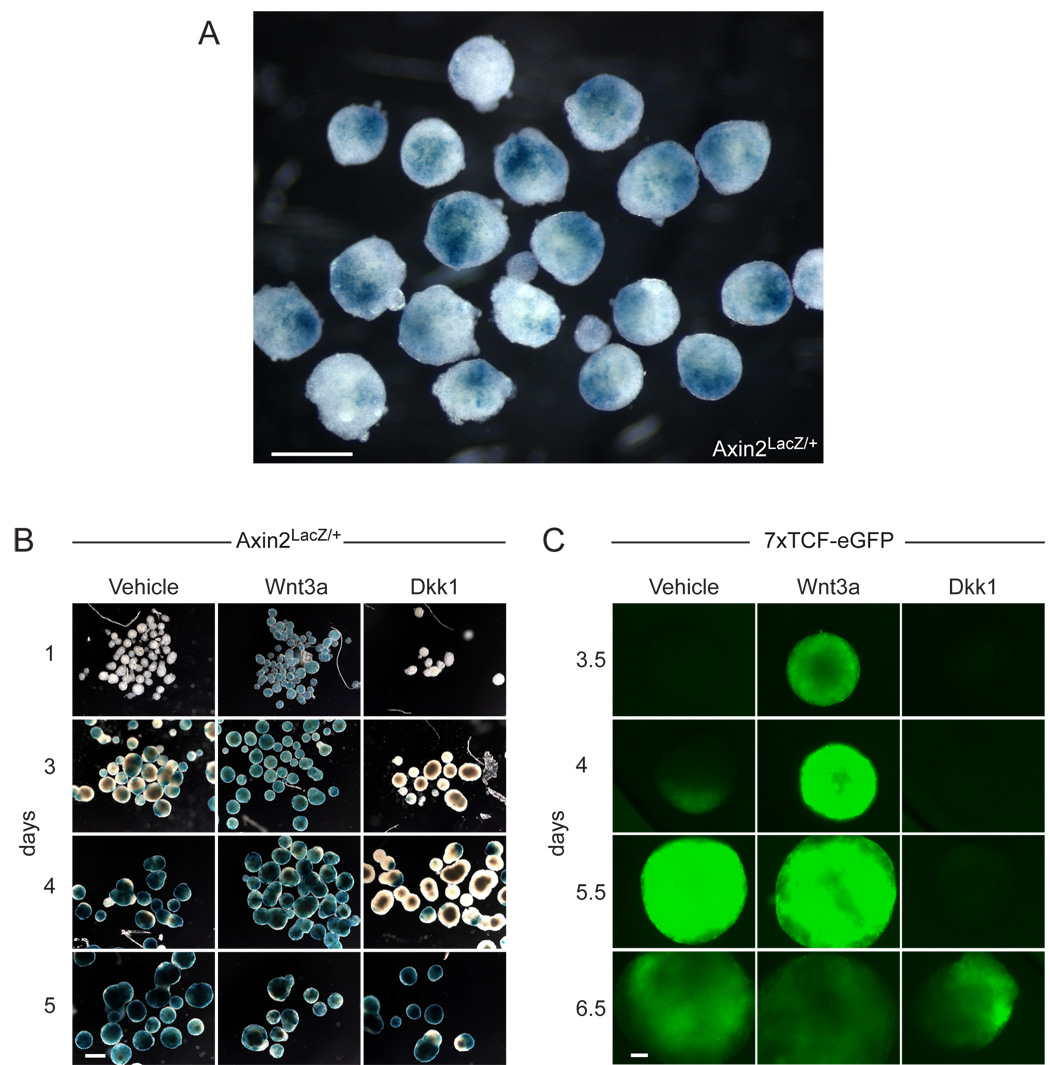
When we generated embryoid bodies from Axin2LacZ/+ ES cells, we found evidence of polarization of the embryoid bodies in the form of local Wnt pathway activation. After 3 days, reporter activity became visible as one or two highly localized spots on each embryoid body (Fig. 2A). These spots slowly expanded and after 5 days encompassed most of the embryoid body (Fig. 2B, vehicle). Similar results were obtained with an ES cell line that expressed the 7xTCF-eGFP Wnt reporter (Fig. 2C, vehicle). Addition of Wnt3a protein led to rapid activation of both the Axin2LacZ/+ and 7xTCF-eGFP reporters throughout the embryoid bodies, whereas in presence of the Wnt antagonist, Dkk1 protein, reporter activation was delayed by 1.5-2 days (Fig. 2B and Fig. 2C). Even stronger effects were seen using the antagonist Fz8CRD, which prevented activation of the reporter altogether (not shown, n>50). Since both Dkk1 and Fz8CRD act extracellularly by inhibiting Wnt proteins from activating their receptors, these data demonstrate that a localized source of Wnt signals polarizes the embryoid body.
Figure 2. Localized Wnt signaling in embryoid bodies.
(A) Axin2LacZ/+ embryoid bodies spontaneously activated the reporter in a localized fashion after 3 days in culture (n>200). (B) Wnt signaling expanded overtime throughout the entire Axin2LacZ/+ embryoid body. Addition of recombinant Wnt3a (200 ng/ml) led to accelerated activation of the reporter, whereas Dkk1 (80 ng/ml) delayed reporter activation by 2 days (n>200). (C) 7xTCFeGFP embryoid bodies displayed similar expression of the reporter as Axin2LacZ/+ embryoid bodies, but with a delay of approximately one day. The delay is probably due to the larger size of the embryoid bodies, and time required for GFP maturation (n>200). Scale bar 500 µm (A, B), 100 µm (C).
Wnt signaling controls local mesendodermal differentiation
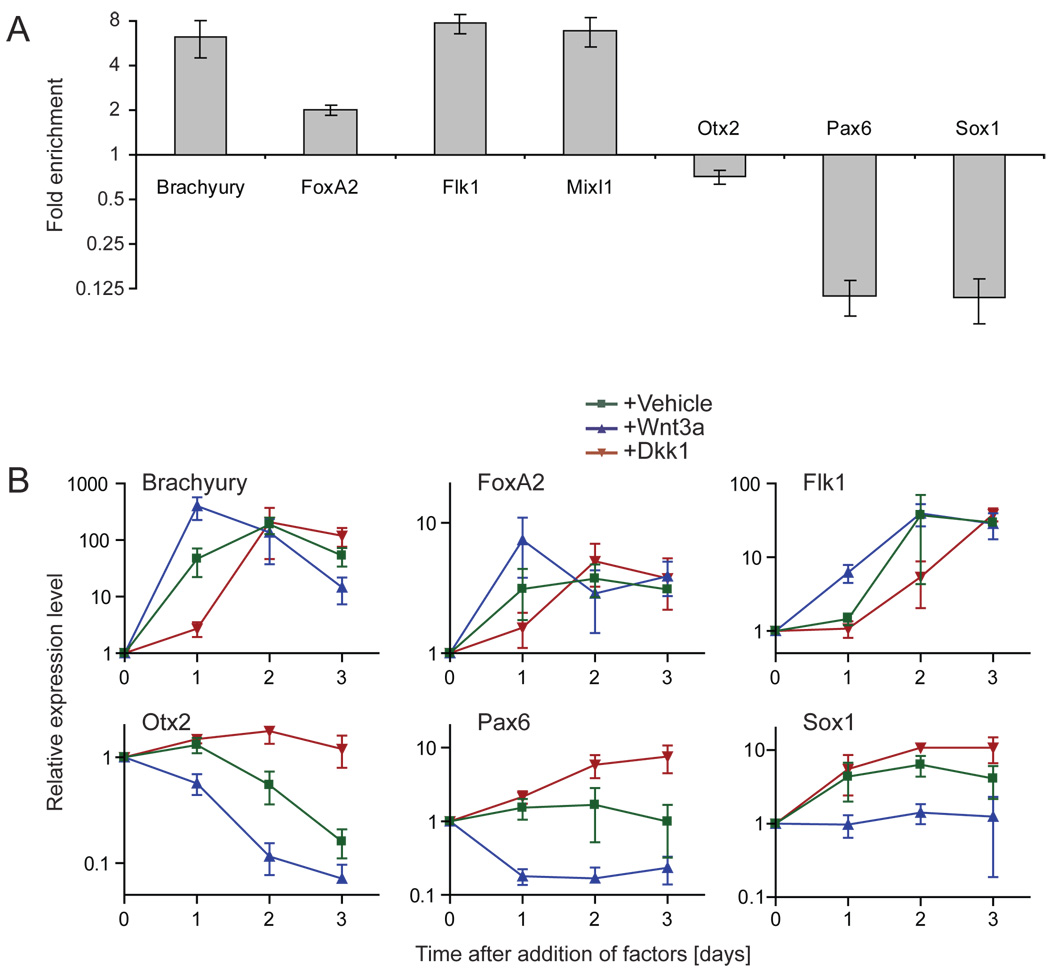
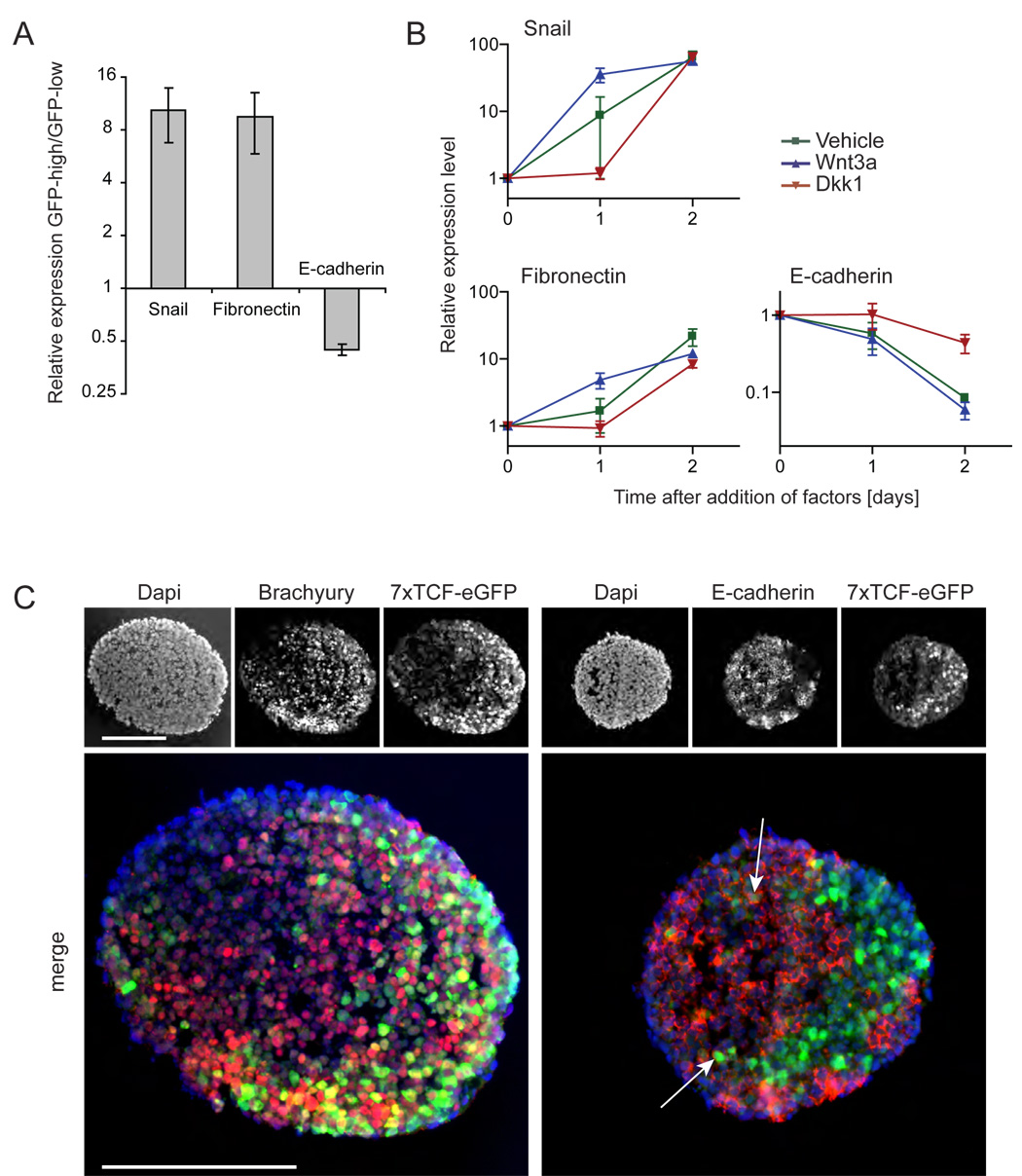
Since activation of Wnt signaling in the embryo overlaps with differentiation of the primitive ectoderm into mesendodermal precursors, we tested whether the Wntresponsive cell population in embryoid bodies consists of such precursors. Using a cell sorter, we separated GFP-positive cells and GFP-negative cells from 7xTCF-eGFP embryoid bodies cultured for 4 days, and analyzed the two populations by real-time PCR. The mesendodermal markers Brachyury, which is expressed throughout the primitive streak and nascent mesoderm (Herrmann and Kispert, 1994; Kispert and Herrmann, 1994) and is a direct Wnt target (Arnold et al., 2000; Yamaguchi et al., 1999), FoxA2 (Ang et al., 1993; Dufort et al., 1998; Weinstein et al., 1994) and Mixl1 (Hart et al., 2002; Mohn et al., 2003; Pearce and Evans, 1999; Robb et al., 2000), and the mesodermal marker Flk1 (Kdr, VEGFR2) (Dumont et al., 1995; Kataoka et al., 1997; Palis et al., 1995; Yamaguchi et al., 1993) were all from 2- to 10-fold enriched in the GFP-positive pool, compared to the GFP-negative pool (Fig. 3A). In contrast, expression of the neurectodermal markers Pax6 (Walther and Gruss, 1991) and Sox1 (Pevny et al., 1998; Wood and Episkopou, 1999) were repressed in the GFP-positive population. Expression of Otx2, which is a marker of epiblast, delaminating mesoderm, and anterior neurectoderm (Ang et al., 1994; Simeone et al., 1993) showed little change (Fig. 3A). This suggests that endogenous Wnt signals mediate the formation of mesendodermal precursors from epiblast cells in embryoid bodies, and prevent the formation of neurectoderm. To test this hypothesis we determined whether addition of Wnt3a protein to 2.5 days old embryoid bodies would induce mesendodermal markers and repress neurectodermal markers. As observed before for Brachyury (Robertson et al., 2000; Ueno et al., 2007), the mesendodermal markers displayed a dynamic expression profile, with expression levels increasing for 2 days and then falling off (Fig. 3B, vehicle). Wnt3a protein consistently accelerated and increased this induction of Brachyury, FoxA2 and Flk1 in embryoid bodies, whereas it repressed Otx2, Pax6, and Sox1 (Fig. 3B). In contrast, Dkk1 protein delayed the induction of Brachyury, FoxA2 and Flk1 (Fig. 3B), just as it delayed Wnt-pathway activation (Fig. 2B and Fig 2C), and promoted expression of Otx2, Pax6 and Sox1 (Fig. 3B).
Figure 3. Wnt signaling regulates the local expression of markers for the primary germ layers in embryoid bodies.
(A) 7xTCF-eGFP embryoid bodies were cultured for 4 days, separated into GFP-positive and GFP-negative cell pools using a cell sorter, and the pools were analyzed by real-time PCR. Expression of Brachyury, FoxA2, Flk1 and Mixl1 was increased in the GFP-positive cells compared to the GFP-negative cells, whereas Otx2, Pax6 and Sox1 were repressed (mean +/− s.e.m., n=3). (B) Real time PCR analysis of RNA collected from embryoid bodies cultured in presence of the indicated factors. Factors were added 2.5 days after the start of aggregation. Addition of Wnt3a (200 ng/ml) resulted in early peaking levels of Brachyury, Foxa2 and Flk1, whereas the neurectodermal markers Otx2 and Pax6 became rapidly down regulated, and induction of Sox1 was prevented. Addition of Dkk1 (80ng/ml) resulted in delayed induction of Brachyury, Foxa2 and Flk1, and promoted expression of Otx2, Pax6 and Sox1 (mean +/− s.e.m., n=3).
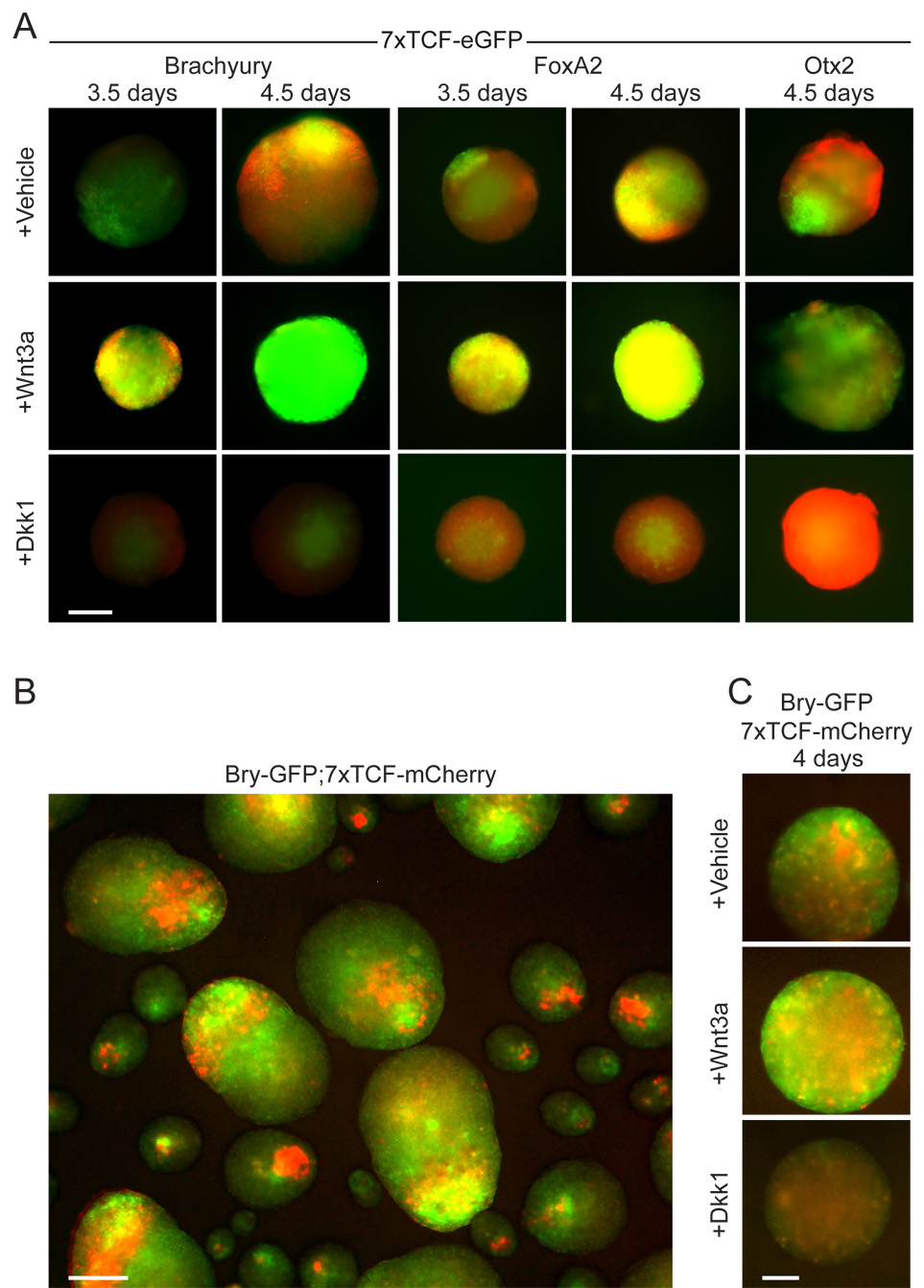
We then directly compared Wnt reporter activity with the expression of Brachyury, FoxA2, or Otx2 by immunostaining of 7xTCF-eGFP embryoid bodies (Fig. 4A). Brachyury and FoxA2 were both expressed in the Wnt-responsive domain, whereas Otx2 was expressed throughout the embryoid body, but was not observed where the Wnt reporter was active (Fig. 4A, vehicle). Treatment with Wnt3a resulted in rapid induction of the Wnt reporter, Brachyury, and FoxA2 throughout the embryoid bodies, whereas Otx2 was completely repressed (Fig. 4A, Wnt3a). Conversely, in the presence of Dkk1 the Wnt reporter, Brachyury, and FoxA2 were not induced, and Otx2 remained expressed throughout the embryoid body (Fig. 4A, Dkk1). We confirmed the local co-regulation of Brachyury and Wnt-signaling using an ES cell line in which a GFP reporter is expressed from the Brachyury locus (Fehling et al., 2003). We derived 2 clones from this cell line transduced with a 7xTCF-mCherry Wnt reporter, in which Wnt signaling is visualized by expression of the red fluorescent protein mCherry. Embryoid bodies derived from either clone displayed local co-expression of GFP and mCherry (Fig. 4B). Treatment with Wnt3a resulted in ubiquitous expression of both reporters throughout the embryoid bodies, whereas treatment with Dkk1 prevented expression of the reporters (Fig. 4C). Combined, these data show that Wnt signaling regulates the local formation of mesendoderm at the expense of neurectoderm in embryoid bodies.
Figure 4. Wnt signaling regulates the local formation of mesendoderm in embryoid bodies.
(A) Whole mount immunostaining of 7xTCF-eGFP embryoid bodies showed co-localization (yellow) of primitive streak markers Brachyury and Foxa2 (red) with the Wnt reporter (green). Expression of Otx2 (red) was mutually exclusive with the Wnt reporter. Treatment with Wnt3a (200 ng/ml) accelerated the upregulation of the Wnt reporter, Brachyury, and FoxA2, whereas it repressed Otx2. Dkk1 treatment (80 ng/ml) had the opposite effect, repressing Wnt signaling, Brachyury, and FoxA2 expression, and promoting Otx2 expression throughout the embryoid body. Factors were added 2.5 days after the start of aggregation. (B) In embryoid bodies derived from a Brachyury-GFP;7xTCF-mCherry cell line, both reporters expressed in close proximity or overlapping domains (GFP-green, mCherry-red; 80%, n>120). The side population of small embryoid bodies that formed during aggregation was excluded from subsequent analyses. (C) Wnt3a treatment induced expression of both reporters throughout Brachyury-GFP;7xTCF-mCherry embryoid bodies (50%, n>60), whereas Dkk1 repressed expression of both reporters (>90%, n>60). Scale bar 100 µm (A, C), 200 µm (B).
Wnt signaling controls local epithelial to mesenchymal transition
One of the hallmarks of gastrulation is the epithelial to mesenchymal transition (EMT) that epiblast cells undergo while they ingress through the primitive streak. During EMT, their adhesive interactions change from cell-cell adhesion, mediated by E-cadherin, to cell-substratum interactions, mediated by integrins and fibronectin (Burdsal et al., 1993). The transcriptional repressor Snail triggers this process (Carver et al., 2001), in part by repressing E-cadherin (Cano et al., 2000). Thus, gastrulating cells express Snail (Nieto et al., 1992; Smith et al., 1992) and fibronectin (Franke et al., 1983; Jackson et al., 1981), and cease to express E-cadherin (Damjanov et al., 1986). To test whether the Wnt-responsive cell population in 7xTCF-eGFP embryoid bodies is undergoing EMT, we separated GFP-positive from GFP-negative cells using a cell sorter, and analyzed the two populations by real-time PCR. This analysis revealed that expression of Snail and fibronectin was upregulated in the GFP-positive population, relative to the GFP-negative population, whereas E-cadherin was repressed (Fig. 5A). To test whether Wnt signaling could regulate this EMT signature, we treated embryoid bodies with Wnt3a and found that this indeed resulted in the accelerated upregulation of Snail and fibronectin, and in the repression of E-cadherin (Fig. 5B). Conversely, Dkk1 treatment repressed the upregulation of Snail and fibronectin, and maintained E-cadherin expression (Fig. 5B). We then compared Wnt reporter activity with the expression of Brachyury or E-cadherin by immunostaining cryosections from 7xTCF-eGFP embryoid bodies. Whereas the Wnt reporter and Brachyury expression always overlapped to a great extent, E-cadherin was expressed throughout the embryoid body, except where strong Wnt reporter activity was visible (Fig. 5C). E-cadherin and GFP expression were visible in the same cells at the edges of the GFP-positive domain, where presumably the epithelial-to-mesenchymal transition was still underway (Fig. 5C, arrows).
Figure 5. Wnt signaling controls epithelial-to-mesenchymal transition in embryoid bodies.
(A) 7xTCF-eGFP embryoid bodies were cultured for 4 days, separated into GFP-positive and GFP-negative cell pools using a cell sorter, and the pools were analyzed by real-time PCR. Expression of Snail and fibronectin was enriched in the GFP-positive cells compared to the GFP-negative cells, whereas E-cadherin was repressed (mean +/− s.e.m., n=3). (B) Real time PCR analysis of RNA collected from embryoid bodies cultured in presence of the indicated factors. Addition of Wnt3a (200 ng/ml) resulted in accelerated induction of Snail and fibronectin, whereas E-cadherin was rapidly down regulated. Addition of Dkk1 (80ng/ml) resulted in delayed induction of Snail and fibronectin, and maintained expression of E-cadherin (mean +/− s.e.m., n=3). (C) Cryosections through 7xTCF-eGFP (green) embryoid bodies were immunostained for Brachyury or E-cadherin (red). Brachyury and GFP expression overlap to a large extent (80%, n>25), whereas E-cadherin and GFP were mutually exclusive except at the edges of the GFP domain (arrows) (>90%, n>25). Scale bar 100 µm.
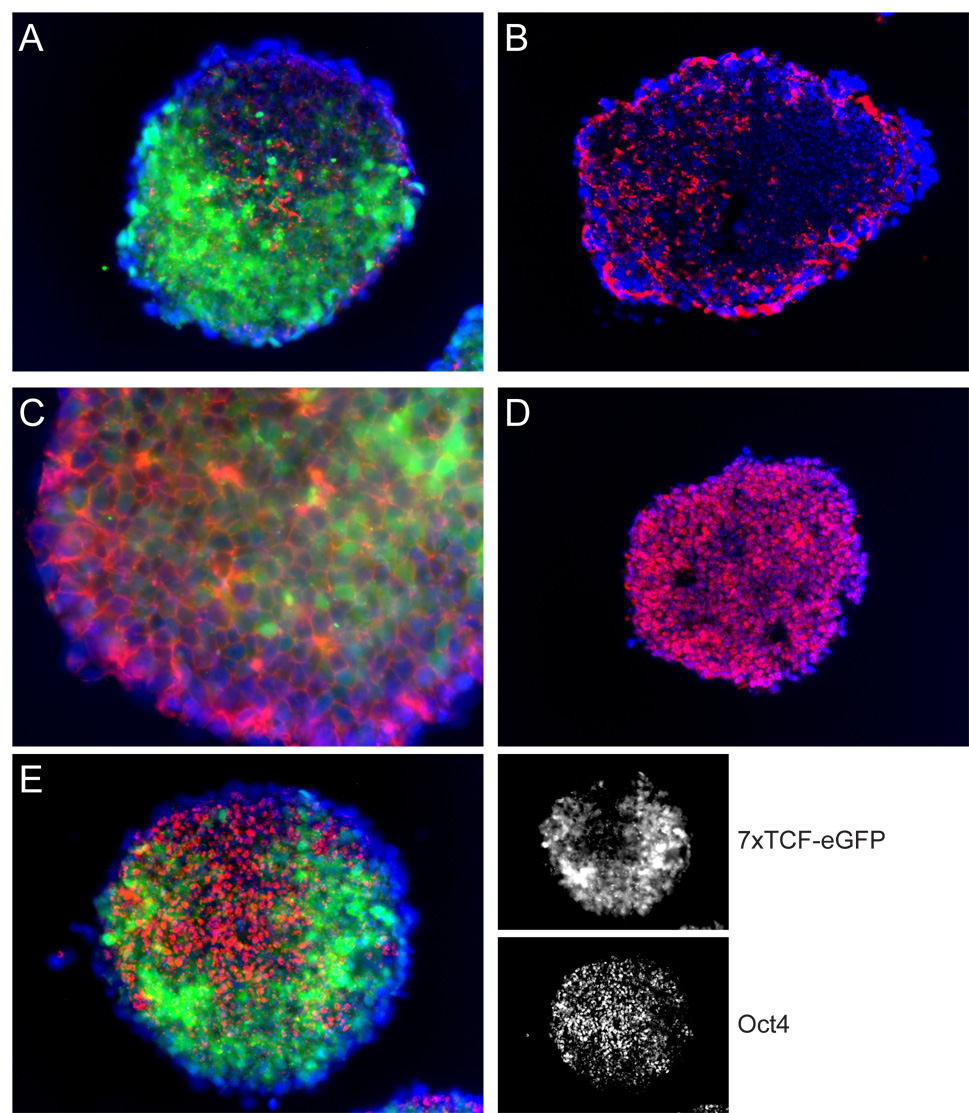
In vivo, the epiblast that undergoes the epithelial-to-mesenchymal transition is a polarized columnar epithelium that is attached to a basement membrane and expresses Oct4. What is the nature of the epithelium on which Wnt signals act in embryoid bodies? Using immunostaining for laminin, we detected no basement membrane at the stage that the Wnt reporter was active (Fig. 6A) or in earlier stages (2.5 and 3.5 days, not shown), whereas we were able to detect basement membranes surrounding more advanced (7 days) embryoid bodies (Fig 6B). Similar results were obtained with an antibody against collagen IV, another marker for basement membranes (not shown). Also, staining of the actin cytoskeleton with phalloidin did not reveal epithelial polarity (Fig. 6C). The epithelia present in 7 day embryoid bodies were not representative of epiblast, as they did not express the pluripotency marker Oct4 (not shown), whereas Oct4 expression was detected throughout 2.5 and 3.5 day embryoid bodies (Fig. 6D). Upon activation of Wnt signaling, Oct4 became down regulated in the Wnt-responsive cells (Fig. 6E), consistent with their differentiation to mesendoderm (Rosner et al., 1990). This data suggest that differentiation of the ES cells to a polarized epithelium is not required for their differentiation into mesendodermal precursors. This is consistent with earlier studies showing that polarization and differentiation of ES cells in embryoid bodies can occur independently from each other (Li et al., 2001; Murray and Edgar, 2001).
Figure 6. Tissue organization in 7xTCF-eGFP embryoid bodies.
Cryosections through 7xTCF-eGFP (green in A, C and E) embryoid bodies stained for laminin (A,B), phalloidin (C) or Oct4 (D,E) (red). Nuclei were stained with dapi (blue). (A) No basal lamina could be detected in 4 day old embryoid bodies (n>60). (B) Basal lamina were readily detectable around the periphery of 7 day old embryoid bodies. (C) Phalloidin staining (red) revealed no epithelial polarity in cells prior to activating 7xTCF-eGFP. (D) Oct4 was expressed throughout 3.5 day (shown) and earlier (not shown) embryoid bodies, before expression of the Wnt reporter became apparent. (E) Oct4 expression was down regulated in cells activating 7xTCF-eGFP (green). No GFP was imaged in (B) and (D).
Combined, our data show that Wnt signaling is required and sufficient for the generation of a domain with primitive streak characteristics in the embryoid body. In this domain, cells undergo EMT and acquire mesendodermal character. When Wnt signaling is suppressed, this primitive streak-like structure does not form and differentiation towards the neurectodermal lineage prevails. Thus, at this stage the embryoid body displays an organization and polarity reminiscent of that seen in gastrulating embryos, where neurectodermal character defines the anterior, and the posterior is characterized by the conversion of epiblast into mesendoderm. By manipulating Wnt signaling we are able to shift the embryoid body between these two fates.
Polarized Wnt signaling is activated by external signals
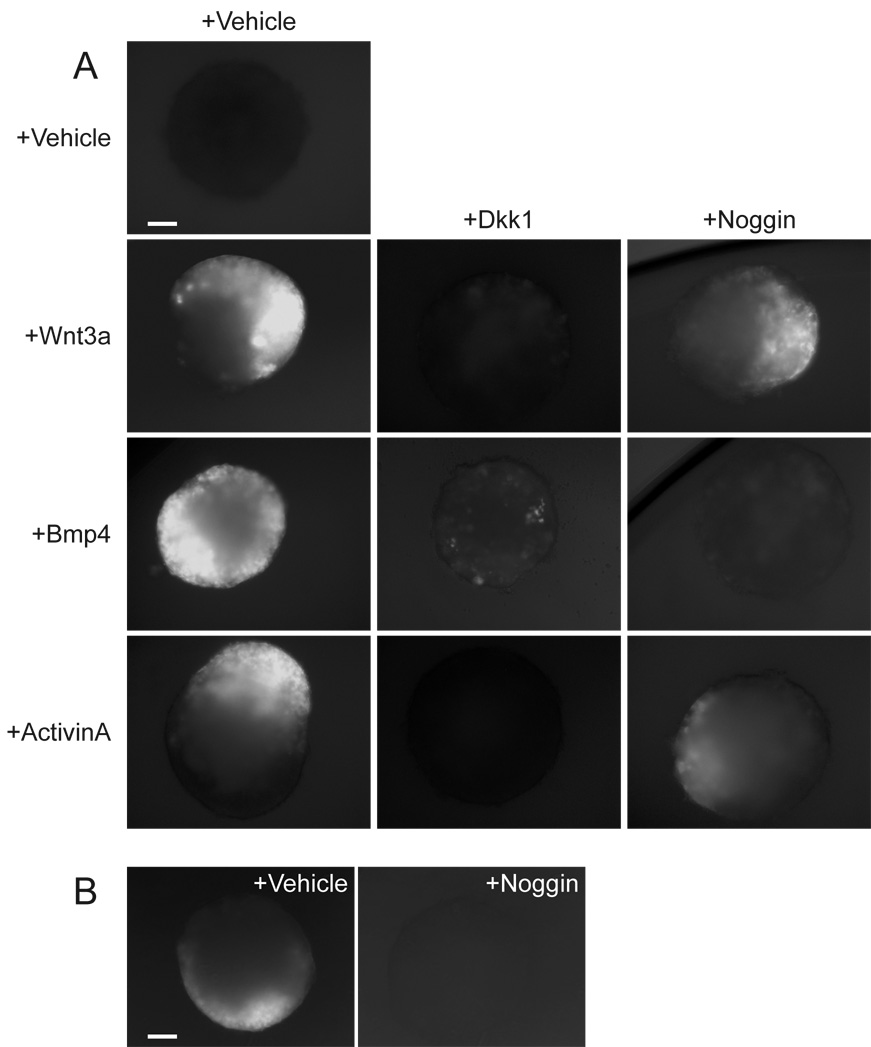
In the mouse embryo, establishment of the primitive streak involves reciprocal and mutually reinforcing interactions between epiblast and extra-embryonic ectoderm that are mediated by nodal, Bmp4, and Wnt3 (Ben-Haim et al., 2006; Brennan et al., 2001). We addressed the role and interdependency of these factors in our in vitro model of primitive streak formation. The extra-embryonic ectoderm is an important source of signals in vivo, but since ES cells differentiate poorly into extra-embryonic ectoderm (Beddington and Robertson, 1989), we asked instead whether activation of the Wnt pathway required external signals from the culture medium. When embryoid bodies were cultured in serum-free defined medium devoid of all signaling proteins, we could not detect Wnt reporter expression over the course of 8 days of culture (Fig. 7A). However, supplementing the medium with low concentrations of Wnt3a, Bmp4, or ActivinA (which activates the nodal pathway (Schier and Shen, 2000)) all resulted in local 7xTCF-eGFP activation (Fig. 7A). In all cases, Dkk1 blocked reporter activation, showing that Bmp4 and ActivinA induced the endogenous production of Wnt signals (Fig. 7A). Since the low Wnt3a stimulus was insufficient to induce widespread reporter activity, it too likely induced local endogenous production of Wnt signals, similar to Bmp4 and ActivinA. An external signal is therefore required to start Wnt signaling and primitive streak formation in embryoid bodies. Our observation that Wnt3a, Bmp4, and ActivinA can each individually perform this function confirms the mutually reinforcing nature of the signaling interactions that start gastrulation. Interestingly, while the Bmp antagonist Noggin blocked local reporter activation by Bmp4, it was unable to block the activation by ActivinA or Wnt3a (Fig. 7A). Thus, local activation of the reporter by ActivinA and Wnt3a is independent of Bmp signaling, and although Bmp signaling is sufficient to start this process, it is not required.
Figure 7. Induction of Wnt signaling in embryoid bodies requires external signals.
(A) When 7xTCF-eGFP embryoid bodies were cultured in serum- and growth factor-free medium (initial aggregation was performed in serum-containing medium for 2.5 days), they failed to induce Wnt signaling (white) (>90%, n=48). Addition of Wnt3a (10 ng/ml), Bmp4 (12.5 ng/ml) or ActivinA (12.5 ng/ml) activated the reporter (83%, 50%, and 79%, respectively, n=24). This could be repressed by Dkk1 (60 ng/ml), showing that it is the result of the induction of endogenous Wnt proteins (100%, n=24). Noggin (12.5 µg/ml) was unable to inhibit induction of the reporter by Wnt3a or ActivinA (n=8). (B) The Bmp antagonist noggin (12.5 µg/ml) inhibited activation of the Wnt-reporter in 7xTCF-eGFP embryoid bodies cultured in serum-containing medium (83%, n=12). Scale bar 100 µm (A, B).
Finally, we addressed how initiation of Wnt signaling in the embryoid body is regulated by serum-containing medium. When we cultured embryoid bodies in presence of serum and the Bmp antagonist Noggin, virtually no activation of the Wnt reporter occurred (Fig. 7B). Since Noggin inhibits local activation of the Wnt reporter by Bmp4, but not by Wnt3a or ActivinA, the role of serum in embryoid body differentiation is likely to provide a Bmp activity that activates Wnt signaling and starts primitive streak formation.
Discussion
Almost 50 years ago, it was realized that aggregates of pluripotent embryocarcinoma cells recapitulated aspects of early embryonic development (Pierce and Dixon, 1959; Stevens, 1959). This was quickly recognized as a model system for the understanding of normal embryonic cell differentiation. These aggregates—termed embryoid bodies because of their superficial resemblance to mouse blastocysts—generally show few morphological signs of gastrulation and display unorganized tissue differentiation (Martin et al., 1977; Wiley et al., 1978). However, this issue has not been readdressed since the advent of ES cells with their superior ability to generate embryonic tissues (Evans and Kaufman, 1981; Martin, 1981). Our data show that embryoid bodies derived from ES cells display a large degree of self-organization: they establish anteroposterior polarity and develop a domain with characteristics of the primitive streak, where cells undergo EMT and form mesendoderm precursors in a process that is dependent on local activation of the Wnt pathway. Embryoid body development therefore resembles normal embryonic development much closer than previously thought, and provides an easily accessible model for the formation of anteroposterior polarity and the establishment of the primitive streak. Moreover, the presence of self-organization and polarity suggests that embryoid bodies can establish morphogen gradients controlling cell differentiation. This would not only explain the wide repertoire of developmental activities present in the embryoid body, but would provide a new tool for investigating the establishment and mode of action of such gradients.
In vivo, primitive streak formation and the establishment of anteroposterior polarity depend on interactions between the epiblast and two extraembryonic tissues, the visceral endoderm and the extraembryonic ectoderm (reviewed in (Tam and Loebel, 2007)). Primitive-streak formation requires specification of the posterior epiblast by Wnt3 (Huelsken et al., 2000; Liu et al., 1999). It is thought that expression of Wnt3 is activated by nodal, through BMP4 signaling in the extraembryonic ectoderm (Ben-Haim et al., 2006; Brennan et al., 2001). In turn, Wnt3 activates a feedback loop that maintains nodal expression in the epiblast (Ben-Haim et al., 2006). Since all three factors can start the feedback loop, this explains why they were all able to initiate self-organization in the embryoid body. However, our results indicate that Bmp4 is not required for this process, and that nodal/ActivinA can activate Wnt signaling in absence of a functional Bmp pathway. This conclusion is in agreement with a recent study that found that Bmp4 has a posteriorizing effect on mesoderm, but is not required for generation of mesoderm in embryoid bodies (Nostro et al., 2008). In vivo, loss of Bmp4 severely affects gastrulation and establishment of the primitive streak (Winnier et al., 1995). Combined, these results suggest that Bmp4 is necessary for induction of Wnt3 and nodal signaling in the embryo, but is not longer required once these pathways are active. This also implies that Bmp4, not nodal, is the signal that starts the feedback loop in vivo.
During embryogenesis, the visceral endoderm promotes anterior patterning by producing nodal and Wnt antagonists, including Cerl, Lefty1, and Dkk1, in the anterior region of the embryo (Glinka et al., 1998; Kimura-Yoshida et al., 2005; Perea-Gomez et al., 2002; Yamamoto et al., 2004). Establishment of anteroposterior polarity is therefore the result of a balance between posteriorizing signals and their antagonists. Using Wnt3a and Dkk1 we could manipulate this balance in embryoid bodies to favor either posterior or anterior character. By providing exogenous Wnt proteins we posteriorized the embryoid body and promoted mesendodermal fate. Conversely, Wnt antagonists promoted anterior character and neurectodermal differentiation. The status of the Wnt signaling pathway is therefore a critical parameter in protocols for directed ES cell differentiation.
How is the initial polarity of the embryoid body established? A deterministic mechanism seems unlikely, since the embryoid body consists of cells that cannot be distinguished by virtue of lineage or inductive environment. In a stochastic mechanism, differences in cells arise from developmental noise, which can have multiple sources. These differences are amplified and stabilized, and can lead to asymmetric cell fate decisions in a uniform cellular assembly (Losick and Desplan, 2008). In the great majority of the embryoid bodies we studied, a single domain of Wnt signaling slowly expanded throughout the embryoid body. This suggests a cell-nonautonomous decision-making system, in which cells nearby the original inducing cell are recruited into the polarizing center, whereas an inhibiting signal, acting over a longer range, prevents formation of multiple polarizing centers. Indeed, in larger embryoid bodies, produced by aggregating more cells, we found more instances of embryoid bodies with two domains of Wnt signaling (not shown and Fig. 2B, 3 days vehicle). This suggests that the range of the putative inhibitory signal was insufficient in these larger embryoid bodies. Nodal and the Wnt signal itself are candidates for the recruiting signal, since a single, low intensity pulse of these signals suffices to induce a polarizing center. A better understanding of this phenomenon could have implications for the in vivo establishment of anteroposterior polarity.
A persistent problem in understanding the regulation of cell differentiation in ES cell cultures is the presence of fetal calf serum, which consists of undefined mixtures of growth factors and inhibitors. We show that an important role of serum in embryoid body differentiation is to provide a Bmp activity that activates Wnt signaling and starts primitive streak formation. For this function, we could replace serum-containing medium with purified growth factors in chemically defined medium. The ability to differentiate ES cells in defined conditions should facilitate the derivation of pure cell populations from ES cells.
Experimental Procedures
Cell culture
Axin2LacZ/+ ES cells (kindly provided by Dr. W. Birchmeier) and R1 mouse ES cells (Stanford Transgenic Facility) were cultured using standard conditions on irradiated primary mouse embryo fibroblasts and DMEM containing 15% fetal bovine serum (Hyclone) and 1,000 U/ml LIF (Chemicon). For the Axin2LacZ/+ embryoid bodies shown in Fig. 2B, 200,000 feeder-depleted Axin2LacZ/+ ES cells were aggregated in 6-well low attachment plates (Corning) in ES medium without LIF (differentiation medium). Embryoid bodies were collected at the indicated time points, fixed in 4% paraformaldehyde and stained with X-gal. We found that embryoid bodies generated using aggregation displayed a large size variation, and that this affected the time course of differentiation, with smaller embryoid bodies differentiating quicker. For all other experiments, and for the Axin2LacZ/+ embryoid bodies shown in Fig. 2A, we therefore generated embryoid bodies of reproducible size using hanging drop cultures containing 2,000 feeder-depleted ES cells per drop in differentiation medium. After 60 hours, embryoid bodies were transferred to a low attachment 96-well plate (Corning) and cultured individually in differentiation medium or serum-free basal medium (SF002-100, Chemicon). Occasionally a side population of small embryoid bodies formed in the hanging drops, these were excluded from subsequent analyses. 7xTCF-eGFP ES cells were produced by infecting R1 cells with a 7xTCF-eGFP lentivirus (Brugmann et al., 2007), and selecting a clone that activated the reporter upon stimulation with Wnt3a protein. Brachyury-GFP;7xTCF-mCherry ES cells were produced by infecting Brachyury-GFP ES cells (Fehling et al., 2003) with a 7xTCF-mCherry lentivirus, and selecting clones that activated mCherry upon stimulation with Wnt3a protein. To create the 7xTcf-mCherry lentiviral vector, mCherry (Shaner et al., 2004) was amplified by PCR from pECE-mCherry (kindly provided by J. Sage, Stanford University) and inserted in place of eGFP in the 7xTcf-eGFP lentiviral vector. Fz8CRD, Dkk1, and Wnt3a proteins were purified as described (Hsieh et al., 1999; Kuhnert et al., 2004; Willert et al., 2003). Recombinant Bmp4, ActivinA, and Noggin were obtained from R&D Systems.
Quantitative real time PCR
RNA was prepared using a Qiagen RNeasy mini kit with on-column DNAse digestion, followed by reverse transcription using a Thermoscript RT-PCR kit with random hexamer primers (Invitrogen). Quantitative real-time PCR was performed on a Roche Lightcycler using a Faststart DNA Masterplus SYBR green I kit (Roche), and quantification performed using HPRT as a reference gene. All PCRs were performed on 3 biological replicates and data plotted as means +/− s.e.m. Primer sequences were designed using Lightcycler Probe Design Software 2.0 (Roche) such that they spanned splice junctions, and are available upon request.
Flow cytometry
Embryoid bodies were dissociated with 0.25% Trypsin-EDTA (Invitrogen), resuspended in PBS with 1% serum and 0.05% propidium iodide, and sorted using a Facstar cell sorter (Becton-Dickinson). RNA of both populations was collected using a Qiagen RNeasy mini kit.
Immunohistochemistry
Embryoid bodies were fixed in 4% paraformaldehyde (pfa) for 1 hour on ice, washed 3 times for 30 min with PBS/0.5% Triton X-100 (PBT), and blocked with 10% normal donkey serum (NDS)/PBT for 2 hours. The fixed embryoid bodies were incubated overnight with primary antibodies in NDS/PBT at 4°C, washed 3 times with PBT for 30 min and blocked again, incubated with biotinylated secondary antibodies overnight at 4°C, washed 3 times with PBT and incubated another night with streptavadin-Cy3 at 4°C. After a final 3 washes with PBT for 30 min, the stained embryoid bodies were mounted in Vectashield in a depression slide and imaged using a Zeiss Axioplan2 imaging station. For cryosections, embryoid bodies were fixed in 1% pfa for 1 hour on ice, followed by incubation in PBS/30% sucrose overnight at 4°C, and frozen in OCT (Tissue-Tek). Cryosections (10 µm) were fixed in acetone at −20°C for 5 min, blocked with avidin, biotin, and finally 5% NDS/PBT, each for 15 min, and incubated with primary antibodies in 5% NDS/PBT overnight at 4°C. The slides were then washed 3 times with PBT for 5 min, incubated with secondary antibodies (Alexa488-conjugated anti-rabbit for the GFP antibody, and biotin-conjugated anti-goat/rat for anti-Brachyury/E-cadherin, Jackson immunoresearch, 1:2,000) in 5% NDS/PBT for 1 hr at room temperature, washed 3 times 5 min with PBT, incubated with streptavidin-Cy3 (Jackson immunoresearch 1:1,000) for 30 min at room temperature, washed 3 times 5 min with PBT, and coverslipped with Vectashield. For GFP and laminin, phalloidin, or Oct4 co-imaging, cryosections were first imaged for GFP and dapi, followed by removal of the coverslips and immunostaining for Oct4 or laminin, or Alexa568-phalloidin staining. The slides were then re-imaged and the 2 sets of images overlaid to produce a single merged image. Antibodies and concentrations: GFP, Otx2 (ab290-50, 1:2,000; ab21990 0.5 µg/ml; Abcam), Brachyury, Foxa2 (sc-17743 1 µg/ml, sc-6554 2 µg/ml; Santa Cruz Biotechnology), E-cadherin (205604 1:20,000; Calbiochem), Laminin (AB2034 1:80, Millipore), Collagen IV (T40263R 1:500, Biodesign (Saco, Maine)), Alexa568-phalloidin (2 U/ml, Invitrogen).
Acknowledgments
These studies were supported by the Howard Hughes Medical Institute, a grant from the California Institute of Regenerative Medicine (RC1-00133-1), a grant from the NIH (DK67834-01), and the Swiss National Science Foundation (C. Fuerer). Axin2LacZ/+ ES cells and Brachyury-GFP ES cells were kindly provided by Drs W. Birchmeier and G. Keller, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ang SL, Conlon RA, Jin O, Rossant J. Positive and negative signals from mesoderm regulate the expression of mouse Otx2 in ectoderm explants. Development. 1994;120:2979–2989. doi: 10.1242/dev.120.10.2979. [DOI] [PubMed] [Google Scholar]
- Ang SL, Wierda A, Wong D, Stevens KA, Cascio S, Rossant J, Zaret KS. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119:1301–1315. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Stappert J, Bauer A, Kispert A, Herrmann BG, Kemler R. Brachyury is a target gene of the Wnt/beta-catenin signaling pathway. Mech Dev. 2000;91:249–258. doi: 10.1016/s0925-4773(99)00309-3. [DOI] [PubMed] [Google Scholar]
- Aubert J, Dunstan H, Chambers I, Smith A. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotech. 2002;20:1240. doi: 10.1038/nbt763. [DOI] [PubMed] [Google Scholar]
- Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, Herrmann BG. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395–406. doi: 10.1016/s1534-5807(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989;105:733–737. doi: 10.1242/dev.105.4.733. [DOI] [PubMed] [Google Scholar]
- Ben-Haimx N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. The Nodal Precursor Acting via Activin Receptors Induces Mesoderm by Maintaining a Source of Its Convertases and BMP4. Developmental Cell. 2006;11:313. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, Robertson EJ. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–969. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Goodnough LH, Gregorieff A, Leucht P, ten Berge D, Fuerer C, Clevers H, Nusse R, Helms JA. Wnt signaling mediates regional specification in the vertebrate face. Development. 2007;134:3283–3295. doi: 10.1242/dev.005132. [DOI] [PubMed] [Google Scholar]
- Burdsal CA, Damsky CH, Pedersen RA. The role of E-cadherin and integrins in mesoderm differentiation and migration at the mammalian primitive streak. Development. 1993;118:829–844. doi: 10.1242/dev.118.3.829. [DOI] [PubMed] [Google Scholar]
- Burkert U, von Ruden T, Wagner EF. Early fetal hematopoietic development from in vitro differentiated embryonic stem cells. New Biol. 1991;3:698–708. [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature cell biology. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanov I, Damjanov A, Damsky CH. Developmentally regulated expression of the cell-cell adhesion glycoprotein cell-CAM 120/80 in peri-implantation mouse embryos and extraembryonic membranes. Developmental Biology. 1986;116:194. doi: 10.1016/0012-1606(86)90056-4. [DOI] [PubMed] [Google Scholar]
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- Dufort D, Schwartz L, Harpal K, Rossant J. The transcription factor HNF3beta is required in visceral endoderm for normal primitive streak morphogenesis. Development. 1998;125:3015–3025. doi: 10.1242/dev.125.16.3015. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Fong GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn. 1995;203:80–92. doi: 10.1002/aja.1002030109. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, Kouskoff V. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- Franke WW, Grund C, Jackson BW, Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. IV. Ultrastructure of primary mesenchymal cells and their cell-cell interactions. Differentiation. 1983;25:121–141. [PubMed] [Google Scholar]
- Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. PNAS. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 1999;13:709–717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Greco TL, Takada S, Newhouse MM, McMahon JA, McMahon AP, Camper SA. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 1996;10:313–324. doi: 10.1101/gad.10.3.313. [DOI] [PubMed] [Google Scholar]
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- Hart AH, Hartley L, Sourris K, Stadler ES, Li R, Stanley EG, Tam PPL, Elefanty AG, Robb L. Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development. 2002;129:3597–3608. doi: 10.1242/dev.129.15.3597. [DOI] [PubMed] [Google Scholar]
- Herrmann BG, Kispert A. The T genes in embryogenesis. Trends in Genetics. 1994;10:280. doi: 10.1016/0168-9525(90)90011-t. [DOI] [PubMed] [Google Scholar]
- Hsieh J-C, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt-Frizzled interactions using a soluble, biologically active vertebrate Wnt protein. PNAS. 1999;96:3546–3551. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for {beta}-Catenin in Anterior-Posterior Axis Formation in Mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson BW, Grund C, Winter S, Franke WW, Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. II. Epithelial differentiation and intermediate-sized filaments in early postimplantation embryos. Differentiation. 1981;20:203–216. doi: 10.1111/j.1432-0436.1981.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka H, Takakura N, Nishikawa S, Tsuchida K, Kodama H, Kunisada T, Risau W, Kita T, Nishikawa SI. Expressions of PDGF receptor alpha, c-Kit and Flk1 genes clustering in mouse chromosome 5 define distinct subsets of nascent mesodermal cells. Dev Growth Differ. 1997;39:729–740. doi: 10.1046/j.1440-169x.1997.t01-5-00009.x. [DOI] [PubMed] [Google Scholar]
- Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- Kemler R, Hierholzer A, Kanzler B, Kuppig S, Hansen K, Taketo MM, de Vries WN, Knowles BB, Solter D. Stabilization of beta-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development. 2004;131:5817–5824. doi: 10.1242/dev.01458. [DOI] [PubMed] [Google Scholar]
- Kimura-Yoshida C, Nakano H, Okamura D, Nakao K, Yonemura S, Belo JA, Aizawa S, Matsui Y, Matsuo I. Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm. Dev Cell. 2005;9:639–650. doi: 10.1016/j.devcel.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Kispert A, Herrmann BG. Immunohistochemical Analysis of the Brachyury Protein in Wild-Type and Mutant Mouse Embryos. Developmental Biology. 1994;161:179. doi: 10.1006/dbio.1994.1019. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang H-T, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. PNAS. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen Y, Scheele S, Arman E, Haffner-Krausz R, Ekblom P, Lonai P. Fibroblast growth factor signaling and basement membrane assembly are connected during epithelial morphogenesis of the embryoid body. J Cell Biol. 2001;153:811–822. doi: 10.1083/jcb.153.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3767–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Losick R, Desplan C. Stochasticity and cell fate. Science. 2008;320:65–68. doi: 10.1126/science.1147888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, et al. Negative Feedback Loop of Wnt Signaling through Upregulation of Conductin/Axin2 in Colorectal and Liver Tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR, Wiley LM, Damjanov I. The development of cystic embryoid bodies in vitro from clonal teratocarcinoma stem cells. Dev Biol. 1977;61:230–244. doi: 10.1016/0012-1606(77)90294-9. [DOI] [PubMed] [Google Scholar]
- Mohn D, Chen SW, Dias DC, Weinstein DC, Dyer MA, Sahr K, Ducker CE, Zahradka E, Keller G, Zaret KS, et al. Mouse Mix gene is activated early during differentiation of ES and F9 stem cells and induces endoderm in frog embryos. Dev Dyn. 2003;226:446–459. doi: 10.1002/dvdy.10263. [DOI] [PubMed] [Google Scholar]
- Morkel M, Huelsken J, Wakamiya M, Ding J, van de Wetering M, Clevers H, Taketo MM, Behringer RR, Shen MM, Birchmeier W. Beta-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development. 2003;130:6283–6294. doi: 10.1242/dev.00859. [DOI] [PubMed] [Google Scholar]
- Murray P, Edgar D. The regulation of embryonic stem cell differentiation by leukaemia inhibitory factor (LIF) Differentiation. 2001;68:227–234. doi: 10.1046/j.1432-0436.2001.680410.x. [DOI] [PubMed] [Google Scholar]
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Bennett MF, Sargent MG, Wilkinson DG. Cloning and developmental expression of Sna, a murine homologue of the Drosophila snail gene. Development. 1992;116:227–237. doi: 10.1242/dev.116.1.227. [DOI] [PubMed] [Google Scholar]
- Nostro MC, Cheng X, Keller GM, Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell stem cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J, McGrath KE, Kingsley PD. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood. 1995;86:156–163. [PubMed] [Google Scholar]
- Pearce JJH, Evans MJ. Mml, a mouse Mix-like gene expressed in the primitive streak. Mechanisms of Development. 1999;87:189. doi: 10.1016/s0925-4773(99)00135-5. [DOI] [PubMed] [Google Scholar]
- Perea-Gomez A, Vella FD, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, Pfister V, Chen L, Robertson E, Hamada H, et al. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell. 2002;3:745–756. doi: 10.1016/s1534-5807(02)00321-0. [DOI] [PubMed] [Google Scholar]
- Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125:1967–1978. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- Pierce GB, Dixon FJ., Jr Testicular teratomas. I. Demonstration of teratogenesis by metamorphosis of multipotential cells. Cancer. 1959;12:573–583. doi: 10.1002/1097-0142(195905/06)12:3<573::aid-cncr2820120316>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Robb L, Hartley L, Begley CG, Brodnicki TC, Copeland NG, Gilbert DJ, Jenkins NA, Elefanty AG. Cloning, expression analysis, and chromosomal localization of murine and human homologues of a Xenopus mix gene. Dev Dyn. 2000;219:497–504. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1070>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Robertson SM, Kennedy M, Shannon JM, Keller G. A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/tal-1. Development. 2000;127:2447–2459. doi: 10.1242/dev.127.11.2447. [DOI] [PubMed] [Google Scholar]
- Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW, Staudt LM. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345:686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nature biotechnology. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Simeone A, Acampora D, Mallamaci A, Stornaiuolo A, D'Apice MR, Nigro V, Boncinelli E. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. Embo J. 1993;12:2735–2747. doi: 10.1002/j.1460-2075.1993.tb05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Franco del Amo F, Gridley T. Isolation of Sna, a mouse gene homologous to the Drosophila genes snail and escargot: its expression pattern suggests multiple roles during postimplantation development. Development. 1992;116:1033–1039. doi: 10.1242/dev.116.4.1033. [DOI] [PubMed] [Google Scholar]
- Stevens LC. Embryology of testicular teratomas in strain 129 mice. J Natl Cancer Inst. 1959;23:1249–1295. [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Tam PP, Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nature reviews. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Weinstein DC, Ruiz i Altaba A, Chen WS, Hoodless P, Prezioso VR, Jessell TM, Darnell JE. The winged-helix transcription factor HNF-3[beta] is required for notochord development in the mouse embryo. Cell. 1994;78:575. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Wiley LM, Spindle AI, Pedersen RA. Morphology of isolated mouse inner cell masses developing in vitro. Developmental Biology. 1978;63:1. doi: 10.1016/0012-1606(78)90108-2. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Wood HB, Episkopou V. Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mechanisms of Development. 1999;86:197. doi: 10.1016/s0925-4773(99)00116-1. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999;13:3185–3190. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Saijoh Y, Perea-Gomez A, Shawlot W, Behringer RR, Ang SL, Hamada H, Meno C. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature. 2004;428:387–392. doi: 10.1038/nature02418. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Fujimori T, McMahon AP, Takada S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol. 1997;183:234. doi: 10.1006/dbio.1997.8502. [DOI] [PubMed] [Google Scholar]