Abstract
The specific pharmacological response evoked by a nicotinic acetylcholine receptor (nAChR) agonist is governed by the anatomical distribution and expression of each receptor subtype and by the stoichiometry of subunits comprising each subtype. Contributing to this complexity is the ability of agonists that bind to the orthosteric site of the receptor to alter the affinity state of the receptor and induce desensitization and the observation that, at low doses, some nAChR antagonists evoke agonist-like nicotinic responses. Brain concentrations of nicotine rarely increase to the low-mid micromolar concentrations that have been reported to evoke direct agonist-like responses, such as calcium influx or neurotransmitter release. Low microgram per kilogram doses of nicotine administered to humans or to nonhuman primates to improve cognition and working memory probably result only in low nanomolar brain concentrations—more in line with the ability of nicotine to induce receptor desensitization. Here we review data illustrating that nicotine, its major metabolite cotinine, and two novel analogs of choline, JWB1-84-1 [2-(4-(pyridin-3-ylmethyl)piperazin-1-yl)ethanol] and JAY2-22-33, JWB1-84-1 [2-(methyl(pyridine-3-ylmethyl)amino)-ethanol], improve working memory in macaques. The effectiveness of these four compounds in the task was linearly related to their effectiveness in producing desensitization of the pressor response to ganglionic stimulation evoked by a nAChR agonist in rats. Only nicotine evoked an agonist-like action (increased resting blood pressure). Therefore, it is possible to develop new chemical entities that have the ability to desensitize nAChRs without an antecedent agonist action. Because these “silent desensitizers” are probably acting allosterically, an additional degree of subtype specificity could be attained.
Nicotine Agonist or Antagonist?
The exploration of the actions of the tobacco alkaloid nicotine and the initial concept of nicotinic receptors has continued for well over 100 years. Despite the wealth of published literature that is now available, the pharmacology of nicotine remains to be fully elucidated, and depending on the system studied, the drug can evoke responses that are complex, unpredictable, and difficult to interpret. Although traditionally described as a receptor agonist, the net effect of nicotine (i.e., agonist or functional antagonist) could depend on several factors, such as the drug dose or concentration, the length of the time of exposure, and the affinity state of the receptor (reviewed in Rowell and Duggan, 1998). The concept that the central nervous system effects of nicotine are due to its “agonist” effects at nicotinic acetylcholine receptors (nAChRs) and the subsequent increases in the release of neurotransmitters (as has been suggested many times) is probably overly simplistic, because in a number of settings, nicotine and nAChR antagonists can have very similar physiologic effects. For example, the nAChR antagonists d-tubocurarine and α-bungarotoxin have been observed to produce neuronal excitatory responses (i.e., increased population spikes) in rodent hippocampal slices that were quantitatively similar to those produced by nicotine (Ropert and Krnjević, 1982). In addition, both nicotine and the α7 nAChR antagonist methyllycaconitine enhanced long-term potentiation after their application to GABA-containing neurons in the CA1 region of the hippocampus (Fujii et al., 2000). Intrahypothalamic injection of d-tubocurarine was reported to evoke excitatory behavioral responses (i.e., fear and escape reactions) in rats (Decsi and Karmos-Varszegi, 1969; Buccafusco and Brezenoff, 1980) in a fashion similar to carbachol. Moreover, both nicotine and mecamylamine increased serotonin release in rat dorsal hippocampal slices (Kenny et al., 2000), and in guinea pig striatum, mecamylamine and the high-affinity antagonist, dihydro-β-erythroidin, exerted effects similar to nicotine on dopamine release under conditions of phasic and tonic activity (Rice and Cragg, 2004). In studies of receptor regulation, the chronic exposure to either nicotine or to nAChR antagonists in vivo has revealed similarities of action. For example, chronic administration of nicotine or mecamylamine to rats increased the expression of [3H]nicotine binding sites in frontal cortex (Abdulla et al., 1996), and chronic exposure to nicotine or mecamylamine increased the expression of cell surface nerve growth factor receptors in PC-12 cells (Terry and Clarke, 1994).
At relatively high doses, mecamylamine is well documented to impair cognition across multiple domains. In the lower dose range, mecamylamine has been reported to improve memory-related task performance similar to nicotine. For example, microgram per kilogram doses of nicotine and mecamylamine each have been observed to enhance delayed matching-to-sample (DMTS) accuracy in monkeys (Buccafusco and Jackson, 1991; Terry et al., 1999) and to improve the performance by rats of a delayed stimulus discrimination task (Terry et al., 1999). In addition, in rats, mecamylamine dose-dependently improved working memory in a T-maze alternation task when 30-s intervals were imposed between stimulus and response (Moran, 1993). Supporting data also are derived from studies in which chronic administration of either nicotine or mecamylamine was reported to improve performance in a radial arm maze task and in a T-maze alternation task (see Levin et al., 1997).
The Case for Nicotinic Receptor Desensitization
The complex pharmacology of nicotine and the perplexing pharmacological similarities between nicotine and low doses/concentrations of the nAChR antagonists described above could be due to the ability of nicotine to both activate and desensitize its receptors over a relatively short time course. nAChRs can exist in various conformational states that are rapidly interconvertible. Agonist binding stabilizes the desensitized state, which is characterized by the high-affinity binding of agonists. Despite the conversion to a high-affinity state, desensitization results in decreased responsiveness of the receptor for a subsequent stimulus. Therefore, over time, there is a compensatory increase in the expression of receptor protein (up-regulation). As discussed below, the kinetic properties of the various subtypes of nicotinic receptors help explain the pharmacological responses produced by nAChR agonists and antagonists. More relevant to this discussion are new compounds that alter nAChR function by binding to allosteric sites on the ion channel. Some of these compounds impart interesting kinetic properties to the receptor protein, particularly the newly classified “silent desensitizers” that induce nAChR desensitization without an antecedent agonist-like response.
Similar to nicotine, the ability of cotinine, the primary metabolite of nicotine, to induce the release of dopamine in vitro occurs at much higher concentrations than those present in the brains of smokers (O'Leary et al., 2008). Cotinine has relevance to the actions of nicotine because the metabolite has been reported to induce a variety of behavioral responses in animals, including positive effects on information processing and cognitive function (Terry et al., 2005). Because brain nicotine and cotinine concentrations after smoking are not sufficient to induce neurotransmitter release, both compounds could mediate their behavioral actions through nAChR desensitization. nAChR desensitization, rather than receptor activation, is rapidly gaining attention as a major contributor to the behavioral actions of nicotine (Picciotto et al., 2008). The large disconnect between high concentrations of nicotinic drugs used to activate nicotinic responses in vitro and the lower doses used to induce various behavioral responses in vivo suggests that the latter are related more to the desensitized state as occurs in binding studies. However, even during periods of receptor desensitization, nicotinic receptor-mediated activity is far from being completely eliminated (Grady et al., 1997). This situation could help explain the need for continuous exposure to nicotine by cells in culture in order for the drug to induce a cytoprotective action (Jonnala et al., 2002). It is clear that in such situations, nAChRs exist primarily in the desensitized-high-affinity state. This situation helps regulate calcium influx through nAChR channels, thus maintaining intracellular calcium levels that are prosurvival. In tissue culture, the question arises how incubation of cells with low concentrations of nAChR antagonists such as mecamylamine results in cytoprotection. One possibility is that incomplete antagonism could mimic agonist-induced desensitization, which is also a form of incomplete antagonism. Low concentrations of mecamylamine could limit the endogenous toxicity mediated through α7 nAChRs (see Lukas et al., 2001). Thus, it is possible to envision a mechanism characterized by partial inhibition of nAChR function that could explain the ability of agonists and antagonists of the receptor to evoke largely similar pharmacological responses.
In cultured cells, particularly those that are differentiated, both acetylcholine and choline can serve as agonists for nAChRs. Choline is a low-potency but full agonist of α7 nAChRs. Standard culture media often contain fetal bovine or calf serum and, along with contributions from the turnover and degradation of cell membranes, contribute free choline to the cell environment. Free choline in the medium of PC-12 cells grown and maintained in Dulbecco's modified Eagle's medium/nutrient mixture F-12 containing 10% fetal bovine serum is approximately 70 μM (Yen et al., 2002). During long incubation periods, free choline could also be derived from cell membrane injury and degradation and result in activation of α7 nAChRs. Likewise, in primary cell cultures, during medium changes, glutamate-mediated cytotoxicity occurs (Buccafusco et al., 2007a). Both the prolonged activation of α7 nAChRs and medium change toxicity have the potential for increasing intracellular calcium to levels associated with cytotoxicity. Low concentrations of mecamylamine or desensitizing concentrations of nicotine could limit high intracellular calcium levels induced by growth factor withdrawal or by chemical insults, such as β-amyloid or glutamate. Further studies will be needed to confirm this possibility.
It is perhaps easier to conceive that desensitization of α7 nAChRs in vivo could induce an agonist-like behavioral response (e.g., improvement in working memory) through the process of disinhibition. The α7 subtype is well expressed in the hippocampus, which receives considerable innervation from cholinergic afferents arising from the medial septum. Presynaptic and postsynaptic nAChRs have been identified in layer CA1 where they are expressed by local circuit neurons. Activation of these cells by nAChRs can result both in inhibition and disinhibition of pyramidal cells (Frazier et al., 2003). Specifically, nAChR activation has been reported to activate GABAergic activity in hippocampal CA1 stratum radiatum interneurons (Mok and Kew, 2006). Whereas brief exposure to a very high concentration of agonist can produce excitatory currents in hippocampal interneurons, prolonged application leads to desensitization. Moreover, desensitization of nAChRs expressed on hippocampal hilar neurons is produced by 250-fold lower concentrations of choline than is required for receptor activation (Frazier et al., 2003). Therefore, under conditions in which relatively low doses of nicotine or other agonists are administered, e.g., to produce an improvement in working memory, populations of hippocampal interneurons expressing α7 nAChRs could exist in the desensitized state. A similar situation could exist after the administration of low doses of nAChR antagonists, although the amnestic actions produced in the higher dose range of antagonists might be more difficult to explain with this simplistic model. The ability of high doses of mecamylamine to enhance GABA release (a potential mechanism for its amnestic actions) has not been reported; however, the antagonist has been reported to induce the release of serotonin from hippocampal synaptosomes in vitro (Kenny et al., 2000). Alternatively, nicotinic agonists also induce the release of glutamate, facilitating long-term potentiation (Welsby et al., 2006), and the higher dose range of antagonists such as mecamylamine could reduce glutamatergic transmission (O'Dell and Christensen, 1988). One example involves the finding that α7 nAChR agonists induce a pattern of opposing responses mediated by GABAergic stratum radiatum interneurons in the CA1 region of the hippocampus (Wanaverbecq et al., 2007). Agonists were shown to induce GABA release from target cells, but simultaneously they depressed inhibitory currents mediated postsynaptically by GABAA receptors. It remains to be determined whether nAChR antagonists differentially inhibit these two cellular events; however, very low concentrations (maximally 10 nM) of methyllycaconitine blocked nAChR agonist-induced inhibition of GABAA-mediated currents (Wanaverbecq et al., 2007). This degree of sensitivity to α7 nAChR blockade could explain the agonist-like action of low doses of mecamylamine.
In summary, the discussion thus far makes a case for focusing new drug development on compounds that desensitize nAChRs. In fact, almost all of the new agonists developed in the past two decades have the potential to desensitize nAChRs; however, most of these accomplish this action by first activating the receptor at the orthosteric site. The problem with this approach is that it has not generated very highly subtype-selective ligands, and many compounds share side-effect profiles with nicotine. Targeting allosteric sites on nAChRs could be one approach to potentially improve subtype selectivity. However, if the idea is to induce desensitization without activation, a new type of compound is warranted.
The Relevance of Allosteric Binding Targets (Silent Desensitizers)
Positive allosteric modulators, defined as compounds that facilitate endogenous neurotransmission without directly stimulating the target receptors, have been known for ligand-gated ion channel receptors, including nAChRs, for several years (see Albuquerque et al., 2001). Very few such compounds have been characterized in vivo. In addition to its property as an inhibitor of acetylcholinesterase, the Alzheimer's drug galantamine has been suggested to act as a positive allosteric modulator of nAChRs (see Coyle et al., 2007). However, the galantamine in vivo will always be overshadowed by its anticholinesterase activity. A more relevant proof of concept is derived from studies with the compound PNU-120596, which has been demonstrated to act as a positive allosteric modulator of nAChRs both in vitro and in vivo (Hurst et al., 2005). PNU-120596 inhibited the ability of amphetamine to suppress auditory gating as measured by auditory-evoked potential in anesthetized rats. This property of the compound suggests its potential for use in schizophrenia, a disorder characterized by auditory gating deficits. The in vitro effects of PNU-120596 were shown to be mediated by α7 nAChRs. However, positive allosteric modulators such as PNU-120596 enable nicotinic cholinergic neurotransmission in large part by preventing desensitization, and on their own, they cannot desensitize nAChRs.
If desensitization of nAChRs is to be considered a viable drug target, compounds will need to be developed that simply desensitize the receptor without activation—so-called “silent desensitizers.” It is possible that the first compound so characterized was sazetidine-A [6-(5-(((S)-azetidin-2-yl)methoxy)-pyridine-3-yl)hex-5-yn-1-ol] (Xiao et al., 2006). Sazetidine-A exhibited high affinity and selectivity in equilibrium binding assays for the α4β2 subtype of nAChRs. Despite its affinity for the orthosteric site of the receptor, the compound evoked no agonist-like effect; however, pretreatment for as little as 10 min with the drug inhibited the subsequent response (86Rb+ release) evoked by nicotine. In this regard, the compound was 50-fold more potent than the competitive antagonist dihydro-β-erythroidin. The concept is that a silent desensitizer traps the receptor in a high-affinity desensitized state. Sazetidine-A also exhibits certain nicotinic-like pharmacological responses. For example, the compound can substitute in a nicotine/saline discrimination task, and it exhibits epibatidine-like antinociceptive activity (see Zwart et al., 2008). Based on the premise put forth in this review, we would surmise that these agonist-like responses were a direct result of desensitization. However, a more recent study with the compound indicates that sazetidine-A induces the release of dopamine from striatal slices, an effect mediated through α4β2- and α6-containing subtypes of nAChRs (Zwart et al., 2008). Of particular interest was the finding that the precise stoichiometry of the α4β2 receptor was important for determining the potency of sazetidine-A. Thus, it remains to be determined which, if any, of the compound's actions could be attributed to silent desensitization.
Analogs of Choline
We synthesized a series of approximately 50 analogs of choline with the expectation that these compounds would serve as selective agonists for the α7 subtype of nAChRs (Buccafusco 2004). Most of the compounds were created as tertiary amine analogs so that they would have drug-like characteristics without the need to serve as substrates for the choline transporters. Our initial examination of these compounds demonstrated varying potencies and efficacies in an assay for cytoprotection (Buccafusco et al., 2005). The compounds did not interact with choline transporters, and with a few notable exceptions, they were not inhibitors of acetylcholinesterase. Two lead compounds, JWB1-84-1 and JAY2-22-33 were studied for other pharmacological properties. JWB1-84-1 improved accuracy in a spatial working memory task by transgenic mice bearing the amyloid precursor protein-Swedish mutation/PS1 mutations related to Alzheimer's disease. The compound also improved DMTS task accuracy by aged monkeys and significantly reversed distractor-impaired accuracies in an attention deficit model in young macaques (Sood et al., 2007). JAY2-22-33 exhibited similar properties in this model but perhaps not as effectively as JWB1-84-1 (unpublished data). Therefore, it was quite surprising to determine that neither compound nor any of the other analogs that was tested exhibited any ability to displace α7 ligands in competition binding assays. In fact, the compounds were ineffective in displacing other labeled nAChR ligands such as [3H]epibatidine from binding sites on rat brain membranes. JWB1-84-1 and JAY2-22-33 (10 μM) also were submitted for activity in a limited neurotransmitter screen (National Institute of Mental Health Psychoactive Drug Screening Program, University of North Carolina at Chapel Hill). There were no significant interactions in the screen of 40 potential drug targets, with the exception of JAY2-22-33 and H3 histamine receptor interaction; however, this interaction has not been confirmed. Thus, we were left with a series of interesting compounds, with significant therapeutic potential but no mechanism of action to consider.
Fortunately, we were able to derive some insight from our parallel studies of cotinine, which seemed to present the same conundrums as did the choline analogs. As mentioned above, cotinine exhibits several nicotine-like properties (Buccafusco and Terry, 2003). 1) In the rat, the motor response to acoustic startle can be inhibited by the presentation of a low-level acoustic prepulse presented just in advance of the high-level acoustic pulse, thereby providing a measure of sensory gating. Treatment with cotinine significantly reversed the effects of apomorphine on acoustic startle. 2) Cotinine was effective in preventing the cytotoxicity associated with growth factor withdrawal in differentiated PC12 cells. In this regard, cotinine was slightly more potent than nicotine. 3) Cotinine produced a dose-dependent increase in accuracy in an automated DMTS task by rhesus monkeys. Cotinine has been characterized as a weak nicotinic agonist capable of evoking partial nAChR desensitization (Briggs et al., 1999; Vainio and Tuominen, 2001). We recently confirmed the ability of cotinine to partially desensitize ganglionic nicotinic receptors in vivo, with no apparent activation of the receptor. In this paradigm, both nicotine and cotinine readily desensitized nAChRs to the stimulus properties of the ganglionic, nicotinic agonist dimethylphenylpiperazinium (DMPP). Acute injection of DMPP induced a dose-related increase mean arterial blood pressure in freely moving rats. In this regard, cotinine produced no significant effects on blood pressure of its own at up to 13 times the dose of nicotine (Buccafusco et el., 2007b).
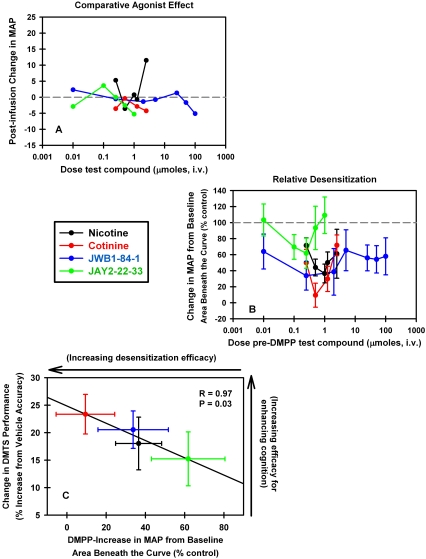
Thus, it was no great leap of insight to then evaluate JWB1-84-1 and JWB2-22-33 in the DMPP paradigm. Adult rats were instrumented to allow the continuous recording of blood pressure via previously implanted aortic catheters and for the intravenous infusion of drug solutions through a catheter implanted in the jugular vein (for experimental details see Buccafusco et al., 2007b). Test compound or vehicle (sterile, heparinized saline) was infused over a 20-min period followed by a 5-min rest interval, during which preinjection MAPs were obtained. At the end of the interval, 40 μg/kg DMPP was injected as a bolus to produce a short-lived (approximately 10 min) but dramatic (up to 40 mm Hg) increase in MAP. Figure 1A shows the potential agonist effect of four compounds: nicotine, cotinine, JWB1-84-1, and JWB2-22-33, i.e., the ability of each compound to increase MAP during the infusion period. Only nicotine exhibited agonist-like activity at the highest dose. Figure 1B depicts the DMPP-induced increase in MAP measured after the 20-min infusion of test compound determined as the area beneath the MAP time curve for the first 10 min after DMPP injection. Data are presented as percentage of vehicle control; the smaller the mean value, the greater the degree of desensitization of ganglionic nAChRs. Although cotinine seemed to be most efficacious in this regard, JWB1-84-1 evoked a response that was nearly as effective but was observable across a wider dose range.
Fig. 1.
Relationship between ganglionic nAChR desensitization and the enhancement of working memory performance in two animal models. A, the potential agonist effect of four compounds: nicotine, cotinine, JWB1-84-1 and JAY2-22-33, as determined from their ability to increase MAP during a 20-min infusion period in freely moving rats. B, the ganglionic stimulant (DMPP)-induced increase in MAP measured after the infusion of test compound determined as the area beneath the MAP time curve for the first 10 min after DMPP injection. Data are presented as percentage vehicle control; the smaller the mean value, the greater the degree of desensitization of ganglionic nAChRs. C, the increase in DMTS task accuracy by macaques produced by each of the four compounds represented as the percentage increase from vehicle as a function of the ability to desensitize ganglionic nAChRs in the rat DMPP study. The 96-trial DMTS session includes four randomly and equally represented memory retention intervals of increasing durations, termed zero, short, medium, and long delay intervals. DMTS values plotted in the figure were the averaged best doses derived from dose-response series for each compound. Best doses were selected as the maximal increases in task accuracy associated with drug treatment across short, medium, and long delay intervals (there is little room for improvement during zero-delay trials) for each subject (n = 8–13) and calculated as the percentage of vehicle baseline accuracies. These values were plotted against the average maximal decreases in MAP for each compound derived from B. The solid line is the linear regression though the mean values.
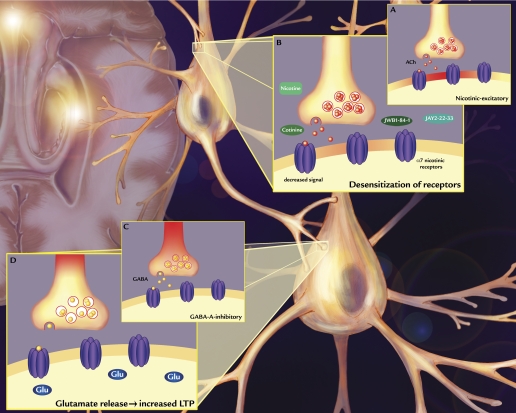
All four compounds were studied in the same DMTS task in macaques (Buccafusco et al., 1999; Terry et al., 2005; Sood et al., 2007) (data for JWB2-22-33 are unpublished). All four compounds significantly improved task accuracy, with predominant effects during long delay intervals (representing retention/retrieval components of working memory). Figure 1C presents the increase in task accuracy represented as the percentage increase from vehicle produced by each compound in the primate task plotted as a function of the ability to desensitize ganglionic nAChRs in the rat DMPP study (i.e., the data derived from Fig. 1B). It is surprising that there was a very high degree of correlation between the two. Recognizing that we are working only with four data points, the high degree of correlation between these two disparate responses to our test compounds at least suggests the possibility that efficacy for desensitization plays a role in the positive mnemonic responses produced by these compounds. The hypothesis is modeled in Fig. 2. It presumes that there is a direct relationship between the three systems; acetylcholine activates nicotinic receptors on GABA interneurons in the hippocampus, which in turn leads to inhibition of primary hippocampal glutamate neurons. This situation would keep activation of glutamate neurons under check during normal nonlearning or nonremembering situations. When acetylcholine is overactivated (as with cholinesterase inhibitors) or when a nAChR agonist is administered, the first response is further activation of the GABA interneuron. However, because α7 nAChRs rapidly desensitize, the acetylcholine input is lost and the glutamate neuron is released from GABA inhibition (disinhibited). This disinhibition leads to glutamate release onto other hippocampal neurons involved in memory and the activation of LTP long-term potentiation, which is an underlying substrate for memory.
Fig. 2.
Model of the study hypothesis. Acetylcholine activates nAChRs on GABA interneurons in the hippocampus (A), which in turn leads to inhibition of primary hippocampal glutamate neurons. This situation would keep activation of glutamate neurons under check during normal nonlearning or nonremembering situations. Because α7 nAChRs rapidly desensitize, the acetylcholine input to GABA interneurons is reduced (B), and the normally robust GABA output (C) is dramatically reduced (D), thereby releasing the glutamate neuron from GABA inhibition. This disinhibition leads to enhanced glutamate release onto other hippocampal neurons involved in memory and the activation of long-term potentiation, which is an underlying substrate for memory.
It is clear that the situation is more complicated. However, the model provides a basis for new drug discovery. Not only do allosteric nAChR compounds exhibit subtype specificity, they could potentially be specific for various combinations and stoichiometries of subunits (Zwart et al., 2008). This is a double-edge sword. High selectivity opens the door to specificity of action with reduced side effects. However, greater knowledge of the true expression and stoichiometry of human nAChR subtypes will be required. Such uncertainty enhances the need for animal testing and particularly for studies of efficacy in nonhuman primates, which have greater translational value in this regard than rodents. Low side-effect profiles also could be characteristic of silent desensitizers, which fail to first activate ligand-gated ion channel receptors. With the amyloid hypothesis in doubt (Holmes et al., 2008), the search for compounds that can both improve cognition and produce neuroprotection, possibly slowing the disease process, becomes paramount.
Acknowledgments
Ki determinations, receptor binding profiles, and agonist and antagonist functional data were generously provided by the National Institute of Mental Health Psychoactive Drug Screening Program (Contract NO-1MH32004) (NIMH PDSP). The NIMH PDSP is directed by Dr. Bryan L. Roth, The University of North Carolina at Chapel Hill, and Jamie Driscol (Project Officer at NIMH, Bethesda MD). For experimental details, please refer to the PDSP website http://pdsp.med.unc.edu/.
This work was supported by the National Institutes of Health National Institute on Aging [Grant 1R01-AG029617]; by a Merit Review Award from the Veterans Administration; and by the Alzheimer's Drug Discovery Foundation.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.145292.
ABBREVIATIONS: nAChR, nicotinic acetylcholine receptor; DMPP, dimethylphenylpiperazinium; DMTS, delayed matching-to-sample; MAP, mean arterial pressure; PNU-120596, 1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)-urea; JWB1-84-1, 2-(4-(pyridin-3-ylmethyl)-piperazin-1-yl)ethanol; JAY2-22-33, 2-(methyl(pyridine-3-ylmethyl)amino)ethanol.
References
- Abdulla FA, Bradbury E, Calaminici MR, Lippiello PM, Wonnacott S, Gray JA, and Sinden JD (1996) Relationship between up-regulation of nicotine binding sites in rat brain and delayed cognitive enhancement observed after chronic or acute nicotinic receptor stimulation. Psychopharmacology 124 323-331. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Santos MD, Alkondon M, Pereira EF, Maelicke A (2001) Modulation of nicotinic receptor activity in the central nervous system: a novel approach to the treatment of Alzheimer disease. Alzheimer Dis Assoc Disord 15 (Suppl 1): S19-S25. [DOI] [PubMed] [Google Scholar]
- Briggs CA, McKenna DG, Monteggia LM, Touma E, Roch JM, Arneric SP, Gopalakrishnan M, and Sullivan JP (1999) Gain of function mutation of the α7 nicotinic receptor: distinct pharmacology of the α7V242T variant. Eur J Pharmacol 366 301-308. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ and Brezenoff HE (1980) Opposing influences on behavior mediated by muscarinic and nicotinic receptors in the rat posterior hypothalamic nucleus. Psychopharmacology 67 249-254. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ and Jackson WJ (1991) Beneficial effects of nicotine administered prior to a delayed matching-to-sample task in young and aged monkeys. Neurobiol Aging 12 233-238. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Jackson WJ, Jonnala RR, and Terry AV Jr (1999) Differential improvement in memory-related task performance to nicotine by aged male and female rhesus monkeys. Behav Pharmacol 10 681-690. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ and Terry AV Jr (2003) The potential role of cotinine in the cognitive and neuroprotective actions of nicotine. Life Sci 72 2931-2942. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ (2004) Neuronal nicotinic receptor subtypes: defining therapeutic targets. Mol Interv 4 285-295. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Beach WB, Terry AV Jr, and Jonnala RR (2005) inventors; Medical College of Georgia, assignee. Novel analogs of choline for neuroprotection and cognitive enhancement in neurodegenerative disorders. U.S. Patent 6,881,738. 2005 Apr 19.
- Buccafusco JJ, Powers JC, Hernandez MA, Prendergast MA, Terry AV Jr, and Jonnala RR (2007a) MHP-133, a drug with multiple CNS targets: potential for neuroprotection and enhanced cognition. Neurochem Res 32 1224-1237 [Erratum in: Neurochem Res 2007 Oct;32]. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Shuster LC, and Terry AV Jr (2007b) Disconnection between activation and desensitization of autonomic nicotinic receptors by nicotine and cotinine. Neurosci Lett 413 68-71. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Geerts H, Sorra K, and Amatniek J (2007) Beyond in vitro data: a review of in vivo evidence regarding the allosteric potentiating effect of galantamine on nicotinic acetylcholine receptors in Alzheimer's neuropathology. J Alzheimer's Dis 11 491-507. [DOI] [PubMed] [Google Scholar]
- Decsi L and Karmos-Várszegi M (1969) Fear and escape reaction evoked by the intrahypothalamic injection of d-tubocurarine in unrestrained cats. Acta Physiol Acad Sci Hung 36 95-104. [PubMed] [Google Scholar]
- Frazier CJ, Strowbridge BW, and Papke RL (2003) Nicotinic receptors on local circuit neurons in dentate gyrus: a potential role in regulation of granule cell excitability. J Neurophysiol 89 3018-3028. [DOI] [PubMed] [Google Scholar]
- Fujii S, Ji Z, and Sumikawa K (2000) Inactivation of alpha7 ACh receptors and activation of non-alpha7 ACh receptors both contribute to long term potentiation induction in the hippocampal CA1 region. Neurosci Lett 286 134-138. [DOI] [PubMed] [Google Scholar]
- Grady SR, Grun EU, Marks MJ, and Collins AC (1997) Pharmacological comparison of transient and persistent [3H]dopamine release from mouse striatal synaptosomes and response to chronic l-nicotine treatment. J Pharmacol Exp Ther 282 32-43. [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, et al. (2008) Long-term effects of Aβ42 immunization in Alzheimer's disease: follow-up of a randomized, placebo-controlled phase I trial. Lancet 372 216-223. [DOI] [PubMed] [Google Scholar]
- Hurst RS, Hajós M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, et al. (2005) A novel positive allosteric modulator of the α7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci 25 4396-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnala RR, Terry AV Jr, and Buccafusco JJ (2002) Nicotine increases the expression of high affinity nerve growth factor receptors both in vitro and in vivo. Life Sci 70 1543-1554. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, File SE, and Neal MJ (2000) Evidence for a complex influence of nicotinic acetylcholine receptors on hippocampal serotonin release. J Neurochem 75 2409-2414. [DOI] [PubMed] [Google Scholar]
- Levin ED, Christopher NC, and Briggs SJ (1997) Chronic nicotinic agonist and antagonist effects on T-maze alternation. Physiol Behav 61 863-866. [DOI] [PubMed] [Google Scholar]
- Lukas RJ, Lucero L, Buisson B, Galzi JL, Puchacz E, Fryer JD, Changeux JP, and Bertrand D (2001) Neurotoxicity of channel mutations in heterologously expressed alpha7-nicotinic acetylcholine receptors. Eur J Neurosci 13 1849-1860. [DOI] [PubMed] [Google Scholar]
- Mok MHS and Kew JN (2006) Excitation of rat hippocampal interneurons via modulation of endogenous agonist activity at the alpha7 nicotinic ACh receptor. J Physiol (Lond) 574 699-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran PM (1993) Differential effects of scopolamine and mecamylamine on working and reference memory in the rat. Pharmacol Biochem Behav 45 533-538. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ and Christensen BN (1988) Mecamylamine is a selective non-competitive antagonist of N-methyl-d-aspartate and aspartate-induced currents in horizontal cells dissociated from catfish retina. Neurosci Lett 94 93-98. [DOI] [PubMed] [Google Scholar]
- O'Leary K, Parameswaran N, McIntosh JM, and Quik M (2008) Cotinine selectively activates a subpopulation of α3/α6β2 nicotinic receptors in monkey striatum. J Pharmacol Exp Ther 325 646-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, and Brunzell DH (2008) It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84 329-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME and Cragg SJ (2004) Nicotine amplifies reward-related dopamine signals in striatum. Nature Neurosci 7 583-584. [DOI] [PubMed] [Google Scholar]
- Ropert N and Krnjević K (1982) Pharmacological characteristics of facilitation of hippocampal population spikes by cholinomimetics. Neuroscience 7 1963-1977. [DOI] [PubMed] [Google Scholar]
- Rowell PP and Duggan DS (1998) Long-lasting inactivation of nicotinic receptor function in vitro by treatment with high concentrations of nicotine. Neuropharmacology 37 103-111. [DOI] [PubMed] [Google Scholar]
- Sood A, Warren Beach J, Webster SJ, Terry AV, and Buccafusco JJ (2007) The effects of JWB1-84-1 on memory-related task performance by amyloid Aβ transgenic mice and by young and aged monkeys. Neuropharmacology 53 588-600. [DOI] [PubMed] [Google Scholar]
- Terry AV Jr, and Clarke MSF (1994) Nicotine stimulation of nerve growth factor receptor expression. Life Sci 55PL 91-98. [DOI] [PubMed] [Google Scholar]
- Terry AV Jr, Buccafusco JJ, and Prendergast MA (1999) Dose-specific improvements in memory-related task performance by rats and aged monkeys administered the nicotinic-cholinergic antagonist mecamylamine. Drug Dev Res 47 127-136. [Google Scholar]
- Terry AV Jr, Hernandez CM, Hohnadel EJ, Bouchard KP, and Buccafusco JJ (2005) Cotinine: A neuroactive metabolite of nicotine: potential for treating disorders of impaired cognition. CNS Drug Rev 11 229-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio PJ and Tuominen RK (2001) Cotinine binding to nicotinic acetylcholine receptors in bovine chromaffin cell and rat brain membranes. Nicotine Tob Res 3 177-182. [DOI] [PubMed] [Google Scholar]
- Wanaverbecq N, Semyanov A, Pavlov I, Walker MC, and Kullmann DM (2007) Cholinergic axons modulate GABAergic signaling among hippocampal interneurons via postsynaptic α7 nicotinic receptors. J Neurosci 27 5683-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsby P, Rowan M, and Anwyl R (2006) Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. Eur J Neurosci 24 3109-3118. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, and Kellar KJ (2006) Sazetidine-A, a novel ligand that desensitizes α4β2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol 70 1454-1460. [DOI] [PubMed] [Google Scholar]
- Yen CL, Mar MH, Craciunescu CN, Edwards LJ, and Zeisel SH (2002) Deficiency in methionine, tryptophan, isoleucine, or choline induces apoptosis in cultured cells. J Nutr 132 1840-1847. [DOI] [PubMed] [Google Scholar]
- Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, Broad LM, Williams AC, Zhang D, Ding C, et al. (2008) Sazetidine-A is a potent and selective agonist at native and recombinant α4β2 nicotinic acetylcholine receptors. Mol Pharmacol 73 1838-1843. [DOI] [PubMed] [Google Scholar]