Abstract
The accessory Sec system of Streptococcus gordonii is essential for transport of the glycoprotein GspB to the bacterial cell surface. A key component of this dedicated transport system is SecA2. The SecA2 proteins of streptococci and staphylococci are paralogues of SecA and are presumed to have an analogous role in protein transport, but they may be specifically adapted for the transport of large, serine-rich glycoproteins. We used a combination of genetic and biochemical methods to assess whether the S. gordonii SecA2 functions similarly to SecA. Although mutational analyses demonstrated that conserved amino acids are essential for the function of SecA2, replacing such residues in one of two nucleotide binding folds had only minor effects on SecA2 function. SecA2-mediated transport is highly sensitive to azide, as is SecA-mediated transport. Comparison of the S. gordonii SecA and SecA2 proteins in vitro revealed that SecA2 can hydrolyze ATP at a rate similar to that of SecA and is comparably sensitive to azide but that the biochemical properties of these enzymes are subtly different. That is, SecA2 has a lower solubility in aqueous solutions and requires higher Mg2+ concentrations for maximal activity. In spite of the high degree of similarity between the S. gordonii paralogues, analysis of SecA-SecA2 chimeras indicates that the domains are not readily interchangeable. This suggests that specific, unique contacts between SecA2 and other components of the accessory Sec system may preclude cross-functioning with the canonical Sec system.
GspB is a cell-surface glycoprotein expressed by Streptococcus gordonii that mediates binding of this organism to distinct sialylated carbohydrate structures on human platelets and salivary glycoproteins (2, 48, 49). This unusual adhesin belongs to a family of extremely large serine-rich glycoproteins that are found in most streptococci and staphylococci. Several recent reports have documented that these serine-rich glycoproteins contribute to the virulence of these gram-positive bacterial organisms (33, 42, 43, 45, 47, 55). It is not yet known whether they also enhance colonization, but the expression of these proteins has been linked to biofilm formation (17, 38).
Two serine-rich repeat domains of GspB undergo glycosylation in the cytoplasm of S. gordonii through the combined action of four proteins (1, 50). This modification with carbohydrate appears to occur very rapidly, and it is essential for the stability of GspB. The glycosylated GspB preprotein is then transported to the cell surface by a dedicated transport system (termed the accessory Sec system) comprised of S. gordonii SecA2 (SecA2Sg), SecY2, and the accessory Sec proteins Asp1 to Asp5 (4, 51, 52).
The precise functions of the various accessory Sec system components are unknown. Based on the similarity to SecA of Escherichia coli (SecAEc), it has been suggested that SecA2Sg may have an analogous role in mediating GspB transport. However, the functional properties of SecA2Sg have not been examined directly. It is important to note that SecA2 proteins have also been identified in listeria and mycobacteria, yet the extent to which SecA2 may play a direct role in protein or lipoprotein transport in these organisms is unclear (20, 40, 41). As in streptococci and staphylococci, the SecA2 proteins are not essential for viability but contribute to virulence (7, 8, 28, 29). However, they differ from the streptococcal and staphylococcal SecA2 proteins in several ways. Perhaps most significantly, other components of the accessory Sec system (SecY2, Asp1, Asp2, and Asp3) appear to be absent from listeria and mycobacteria. Furthermore, phylogenetic analysis suggests that these SecA2 proteins are not true orthologues of the SecY2-associated SecA2 proteins (40). Moreover, whereas the SecY2-associated SecA2 of Streptococcus parasanguinis localizes predominantly to the cytoplasmic membrane (11), the Mycobacterium smegmatis SecA2 localizes mainly to the cytosol (41). Thus, the functional properties of SecY2-associated SecA2 proteins cannot necessarily be inferred from those of either the canonical SecA proteins or the SecA2 proteins that interact with the general Sec system.
In this report, we describe a more detailed analysis of SecA2Sg, performed by examining the effects of selected substitutions in various domains of this protein on GspB export. In addition, we assess whether SecA2Sg is an ATPase and whether it functions similarly to SecA. Our results indicate that conserved residues in the putative nucleotide binding domains are essential for SecA2Sg function. In addition, purified SecA2Sg has an ATPase activity comparable to that of SecASg in vitro. However, there appear to be subtle differences in the way these enzymes are regulated. Furthermore, our results indicate that while SecASg and SecA2Sg share key residues, there are sufficient differences between these paralogues to prevent a simple exchange of domains.
MATERIALS AND METHODS
Bacterial strains, plasmids, and reagents.
The bacterial strains and plasmids used in this study are listed in Table 1. S. gordonii strains were grown in Todd-Hewitt broth (THB; Difco Laboratories) at 37°C in a 5% CO2 environment. An M99-derived strain that expresses GspB736flag was constructed as previously described (5) but using chloramphenicol as a selectable marker. This enabled the simultaneous use of the nisin-inducible complementation vector pMSP3535, which encodes resistance to erythromycin. A segment of plasmid pB736flag, which includes codons 604 to 736 of GspB fused in-frame to a 3X FLAG (Sigma) coding sequence, was removed by digestion with HindIII and BamHI and then cloned in the suicide vector pEVP3, which had been digested similarly. The resulting plasmid, p736BflagC, was used to transform the secA2 mutant strain PS516 by natural transformation as described previously (4). Transformants were initially selected on sheep blood agar containing 5 μg/ml chloramphenicol. Subsequent retention of the chromosomally integrated plasmid did not require antibiotic selection.
TABLE 1.
Strains and plasmids used in the present study
| Strain or plasmid | Relevant characteristicsa | Reference/source |
|---|---|---|
| Strains | ||
| M99 | Streptococcus gordonii parental strain | 46 |
| PS516 | M99 ΔsecA2; in-frame deletion | 4 |
| PS1226 | PS516 gspB::pB736flagC Cmr | This study |
| PS1025 | gspA444::gspB145-736flag ΔgtfA | 5 |
| PS1139 | gspB736flagΔ8-68(G75L G79A G80C)ΔsecA2 ΔgtfA | 3 |
| Plasmids | ||
| pEVP3 | cat (gram positive and E. coli) ori (E. coli) | 12 |
| pB736flagC | gspB codons 604 to 736 with a three-FLAG tag in pEVP3 | This study |
| pET28c | lacI kan T7 promoter | Novagen |
| pET28c.secA | 6His-SecA expression plasmid | This study |
| pET28c.secA2 | 6His-SecA2 expression plasmid | This study |
| pET28c.secA2ΔHindIII | 6His-SecA21-707 (SecA2ΔIRA1) expression plasmid | This study |
| pET28c.secA2A | 6His-SecA21-534A535-838 expression plasmid | This study |
| pET28c.secAA2 | 6His-SecA1-534A2535-793 expression plasmid | This study |
| pET28c.secANBDA2 | 6His-SecA1-222A2220-793 expression plasmid | This study |
| pET28c.secANBD3A2 | 6His-SecA1-121A2114-793 expression plasmid | This study |
| pMAL-c2X | lacIq Ampr Ptac promoter | New England Biolabs |
| pMAL.secA | MalE-SecA expression plasmid | This study |
| pMAL.secA2 | MalE-SecA2 expression plasmid | This study |
| pMSP3535 | erm ori (gram positive and E. coli) nisin-inducible promoter | 9 |
| pM99secA2 | secA2 of M99 in pMSP3535 | 2 |
| pM99secA2E205A | secA2(E205A) in pMSP3535 | This study |
| pM99secA2Y303A | secA2(Y303A) in pMSP3535 | This study |
| pM99secA2R490K | secA2(R490K) in pMSP3535 | This study |
| pM99secA2R490A | secA2(R490A) in pMSP3535 | This study |
| pM99secA2D493A | secA2(D493A) in pMSP3535 | This study |
| pM99secA2W724A | secA2(W724A) in pMSP3535 | This study |
| pM99secA2Y124A | secA2(Y124A) in pMSP3535 | This study |
| pM99secA2A488V | secA2(A488V) in pMSP3535 | This study |
| pM99secA2A | secA21-534A535-838 in pMSP3535 | This study |
| pM99secAA2 | secA1-534A2535-793 in pMSP3535 | This study |
| pM99secANBDA2 | secA1-222A2220-793 in pMSP3535 | This study |
| pM99secANBD3A2 | secA21-121A114-793 in pMSP3535 | This study |
Cmr, chloramphenicol resistance; kan, kanamycin resistance; erm, erythromycin resistance; Ampr, ampicillin resistance.
Oligonucleotide primers (Table 2) were synthesized by Sigma-Genosys. Peroxidase-conjugated antibodies and anti-FLAG monoclonal antibodies were purchased from Sigma. Antibiotics and other chemicals were obtained from Sigma or Fisher.
TABLE 2.
Primers used for the construction of secA and secA2 variants
| Name | Sequence |
|---|---|
| 5′A2 | 5′-AAGGATCCCAGAGGAGTCAAATGGTTAAAAA-3′ |
| 3′A2 | 5′-AAAAGCATGCTATGGGAAGTACATTACGAC-3′ |
| 5′A | 5′-AAGGATCCTAAGGATAAAAATGGCAAATAT-3′ |
| 3′A | 5′-TTTTCTCGAGTGTCATTCCAACTCCCCCTC-3′ |
| 5′MBPA | 5′-AAGGATCCATGGCAAATATTTTAAGAACA-3′ |
| 5′MBPA2 | 5′-AAGGATCCAGTCAAATGGTTAAAAAT-3′ |
| T7t | 5′-GCTAGTTATTGCTCAGCGGT-3′ |
| E205AFa | 5′-CAGTTATCATCGATGCGATTGACTCT-3′ |
| E205ARa | 5′-AGAGTCAATCGCATCGATGATAACTG-3′ |
| Y303AF | 5′-CAAGACGCTGTGGTACGACAAAGTGAA-3′ |
| Y303AR | 5′-TTCACTTTGTCGTACCACAGCGTCTTG-3′ |
| R490KFb | 5′-ACATCAATGGCTGGTAGAGGAACAGAT-3′ |
| R490KRb | 5′-TCCTCTACCAGCCATTGATGTAGCTAC-3′ |
| R490AFb | 5′-ACATCAATGGCTGGTGCAGGAACAGAT-3′ |
| R490ARb | 5′-TCCTGCACCAGCCATTGATGTAGCTAC-3′ |
| D493AF | 5′-TGGTCGAGGAACAGCTATTAAATTAGGTC-3′ |
| D493AR | 5′-GACCTAATTTAATAGCTGTTCCTCGACCA-3′ |
| W724AF | 5′-AAGGCGATTGATGAGAACGCGGTTGAA-3′ |
| W724AR | 5′-TTCAACCGCGTTCTCATCAATCGCCTT-3′ |
| Y124SF | 5′-CCCAATTCCTCTCTAGCTTTGCGAGATGCG-3′ |
| Y124SR | 5′-TCGCAAAGCTAGAGAGGAATTGGGAGTGAC-3′ |
| A488VFb | 5′-TACATCAATGGTTGGTCGAGGAACAGATAT-3′ |
| A488VRb | 5′-TCTGTTCCTCGACCAACCATTGATGTAGCT-3′ |
| IRA2F | 5′-CGTGGMCGTTCWGGYCGKCARGGWGATCCAGG-3′ |
| IRA2R | 5′-CCTGGATCWCCYTGMCGRCCWGAACGKCCACG-3′ |
| A2SSDF | 5′-GATGAAGCTAGAACTCCTTTGATTATATCTGG-3′ |
| A1NBDR | 5′-AATCAAAGGAGTTCTAGCTTCATCGATCAGAATGGA-3′ |
| NBD3F | 5′-ATGCCTSTWTAYYTGAATGCSTTGKCWGG-3′ |
| NBD3R | 5′-CCWGMCAASGCATTCARRTAWASAGGCAT-3′ |
Includes a silent mutation that adds a ClaI site (underlined).
Includes a silent mutation that removes an NcoI site.
Targeted substitutions within SecA2.
The plasmid pM99secA2 contains the entire secA2 gene, along with an optimized ribosome binding site, in the nisin-inducible expression vector pMSP3535 (2). Mutations in the secA2 sequence were generated by a two-stage PCR procedure. In the first stage, primers 5′A2 and a reverse mutant primer (Table 2) or the complementary forward mutant primer along with primer 3′A2 were used to amplify the 5′- or 3′-end segment of secA2, respectively. The two PCR products were combined for the second stage and then amplified using primers 5′A2 and 3′A2. The 2.5-kb PCR product was then cloned in pMSP3535 or, when possible, restriction fragments thereof were used to replace the corresponding segment of pM99secA2. In some cases, silent mutations that added or removed a restriction site were included in the primer sequence in order to facilitate tracking of the mutation (Table 2). The presence of only the intended alteration in any segment generated by PCR was confirmed by DNA sequence analysis (Sequetech). Plasmids were introduced to PS1226 by natural transformation, and transformants were selected and maintained using 60 μg/ml erythromycin.
Construction of SecA-SecA2 chimeras.
Chimeric secA-secA2 constructs were generated by a two-stage PCR procedure. For the SecA-SecA2 chimera designated SecA21-534A535-838, the ribosome binding site and codons 1 to 534 of secA2 were amplified using primer pairs 5′A2 and IRA2R and codons 535 to 838 of secA were amplified using primer pairs IRA2F and 3′A. The two PCR products were combined and then amplified by overlap extension using primers 5′A2 and 3′A. The SecA1-534A2535-793, SecA1-222A2220-793, and SecA1-121A2114-793 chimeric sequences were generated by a similar process, using the following primer pairs, respectively, for the first-stage reactions: 5′A and IRA2R or IRA2F and 3′A2, 5′A and A1NBDR or A2SSDF and 3′A2, or 5′A and NBD3R or NBD3F and 3′A2. Primers 5′A and 3′A2 were then used for the second-stage PCRs. The second-stage PCR products were cloned in pMSP3535 and used to transform PS1226 as described above.
Analysis of secreted and protoplast proteins.
Proteins from S. gordonii culture supernatants or protoplasted cells were prepared and analyzed by Western blotting as described previously (3), with minor modifications. Strains were grown for 18 h in THB containing erythromycin (60 μg/ml) and then either fractionated immediately or diluted fivefold into fresh THB containing nisin (200 ng/ml) and incubated for 6 h in the absence of erythromycin (the final cell density of all cultures was approximately 8 × 108 CFU per ml). For analysis of secreted products, cells were removed by centrifugation at 16,000 × g for 5 min, and the spent medium was combined directly with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and heated at 70°C for 10 min. For analysis of cytoplasmic components, protoplasts (generated by digestion of the cell wall with mutanolysin) were lysed by suspension in SDS-PAGE sample buffer, followed by boiling for 5 min. When probing for GspB736flag, the proteins present in 25 μl of spent culture medium or in protoplasts recovered from 25 μl of the culture were loaded onto gels and compared by Western blotting for the relative amounts of the secreted and the nonsecreted protein. Anti-FLAG monoclonal antibody was used at 1 μg/ml, along with peroxidase-conjugated anti-mouse antibodies at a 1:10,000 dilution. A 50-kDa cytoplasmic protein that cross-reacts with the anti-FLAG antibody served as a loading control (not shown). For detection of the SecA2 variants and SecA-SecA2 chimeras, a rabbit polyclonal anti-SecA2 serum was used at a 1:2,000 dilution, along with peroxidase-conjugated anti-rabbit antibodies at a 1:25,000 dilution.
Effect of sodium azide on secretion of GspB736flag.
The inhibition of protein transport by sodium azide was performed as described by Jongbloed et al. (24), with modifications. Strains were grown for 18 h in 4 ml of THB containing 60 μg/ml erythromycin. After centrifugation at 3,200 × g for 10 min, cells were suspended in 2 ml fresh medium, and then 500-μl aliquots were combined immediately with 500 μl of THB containing either 0, 3, 9, or 30 mM sodium azide. The cultures were incubated at 37°C for 90 min, and then the medium was prepared and analyzed for GspB736flag content as described above.
Overexpression and purification of SecA and SecA2.
For expression of N-terminally His6-tagged SecA2Sg (hereinafter referred to as 6His-SecA2), the secA2 gene was removed from pM99secA2 by digestion of the plasmid with BamHI and SalI and was cloned into pET28c (Novagen) that had been similarly digested, forming pET28c.secA2. This resulted in an in-frame fusion of secA2 with the His6 coding sequence, along with a short spacer sequence. Plasmids used to express the secA-secA2 chimeras were constructed in the same manner. A SecA21-707 expression plasmid was generated by deleting a HindIII fragment spanning the 3′ end of secA2 from pET28c.secA2. For 6His-SecA, the entire secA gene was amplified from strain M99 chromosomal DNA using primers 5′A and 3′A. The 2.5-kb PCR product was digested with BamHI and XhoI and ligated to pET28c that had been digested similarly, forming pET28c.secA. Plasmids were propagated initially in E. coli TOP10 cells (Invitrogen), sequenced to ensure the absence of any mutations in secA or secA2, and then introduced to BL21(DE3) cells (Novagen). For expression of maltose binding protein (MalE) fusion proteins, secA or secA2 was amplified from pET28c.secA or pET28c.secA2 using primers 5′MBPA and T7t or 5′MBPA2 and T7t, respectively. The PCR products were digested with BamHI and XhoI (secA) or BamHI and SalI (secA2) and then ligated to pMAL-c2X (New England Biolabs) that had been digested with BamHI and SalI. The sequences were verified as described above, and the plasmids were then used to transform the E. coli strain BL21 (Novagen).
To express the fusion proteins, strains were grown in Luria broth containing 50 μg/ml kanamycin (pET constructs) or carbenicillin (pMAL constructs) at 25°C to an optical density at 600 nm of 0.7. Expression was then induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 0.1 mM, and incubation was continued for an additional 4 h. Cells were harvested by centrifugation at 3,200 × g, and the pellets were stored frozen at −80°C. For purification of His6-tagged proteins, cell pellets were suspended in 5 mM imidazole, 500 mM NaCl, 20 mM Tris, pH 7.5, and cells were disrupted by sonication. After removal of cellular debris by centrifugation for 15 min at 3,200 × g, the cell lysate was filtered through a 0.45-μm filter and applied to Ni-nitrilotriacetic acid agarose (Qiagen). The resin was washed extensively with 30 mM imidazole, 500 mM NaCl, 20 mM Tris, pH 7.5, and the bound protein was then eluted into 100 mM EDTA, 500 mM NaCl, 20 mM Tris, pH 6.5. Where indicated, 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) was included in all buffers used for lysis and purification.
For purification of MalE fusion proteins, cell pellets were suspended in TBS (150 mM NaCl, 50 mM Tris, pH 7.5). Cells were disrupted by sonication, and the insoluble debris was removed as described above. The lysate was applied to amylose resin (New England Biolabs), which was then washed extensively with TBS. The bound protein was eluted into TBS containing 10 mM maltose.
The eluted 6His-SecA, MalE-SecA, and MalE-SecA2 proteins were concentrated and exchanged into TK buffer (100 mM KCl, 50 mM Tris, pH 7) in a 100-kDa-cutoff centrifugal filter device (Amicon). 6His-SecA, 6His-SecA2, and His6-tagged chimeras that had been purified in the presence of CHAPS were exchanged into TK buffer containing 400 mM NaCl and 0.1% CHAPS. Protein concentrations were determined by using Quick Start Bradford dye reagent (Bio-Rad), with bovine serum albumin as a standard.
Production of anti-SecA2.
A rabbit polyclonal antiserum was generated against polyacrylamide gel slices containing 6His-SecA2 (Covance).
ATPase assays.
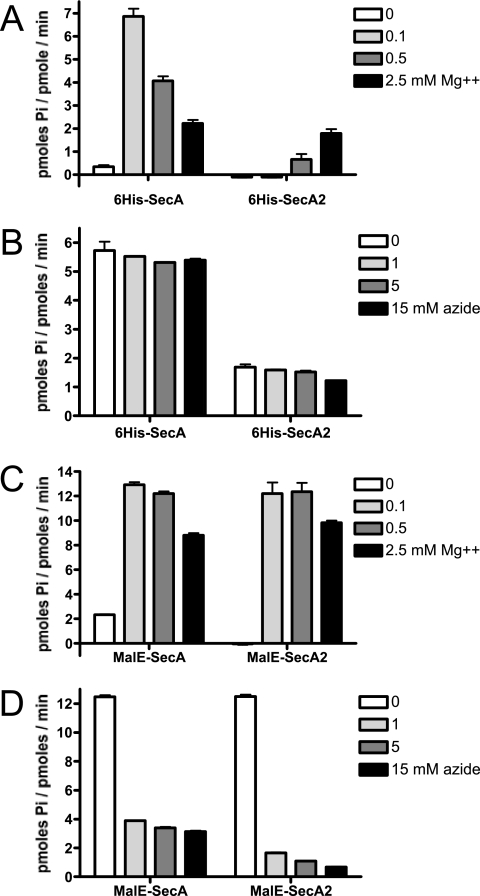
ATP hydrolysis rates were measured by a modified malachite green assay (30), using PiColorLock Gold reagent as described by the manufacturer (Innova Biosciences). In brief, enzymes were diluted to 0.2 μM in TK buffer, and 100-μl (20 pmol) amounts were added to wells of a microtiter plate. Control wells contained buffer only. For measurement of the effect of azide on ATPase activity, the enzymes were diluted into TK buffer containing 3, 9, or 30 mM sodium azide. Reactions were initiated by the addition of 100 μl of a 2× substrate solution (100 mM Tris, pH 7.5, 0.5 mM ATP) that included 0, 0.2, 1, or 5 mM MgCl2, yielding final Mg2+ concentrations of 0, 0.1, 0.5, and 2.5 mM, as indicated in Fig. 4. Plates were incubated at 30°C for 30 min, and the amount of inorganic phosphate (Pi) released was determined by adding the PiColorLock Gold reagent, measuring the absorbance at 635 nm, and extrapolating from a Pi standard curve. Enzymatic rates were calculated after subtraction of amounts of Pi in control wells that had no enzyme.
FIG. 4.
Comparison of basal ATPase rates in vitro. (A) Effect of Mg2+ on the activity of the His6-tagged proteins. (B) Effect of azide on the activity of the His6-tagged proteins. (C) Effect of Mg2+ on the activity of the MalE fusion proteins. (D) Effect of azide on the activity of the MalE fusion proteins. 6His-SecA and 6His-SecA2 were purified in the presence of 0.1% CHAPS. The activity of 6His-SecA purified in the absence of CHAPS was not detectably different from that of 6His-SecA purified with CHAPS (data not shown). The enzymatic rates of ATP hydrolysis were determined after 30 min of incubation at 30°C. For determination of the effects of azide on ATP hydrolysis rates (B and D), the reaction mixtures included Mg2+ at concentrations that promoted maximal activity (0.1 mM Mg2+ for 6His-SecA and MalE-SecA, 0.5 mM for MalE-SecA2, and 2.5 mM for 6His-SecA2). Error bars show standard deviations. Pi, Pi.
RESULTS
SecA2Sg is highly similar to SecAEc.
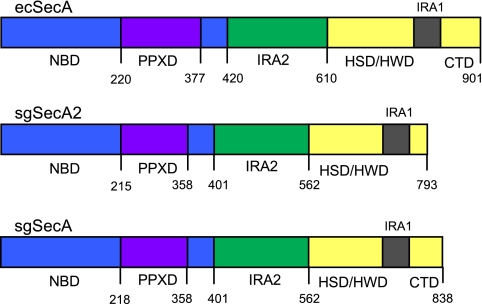
SecAEc is comprised of four major structural domains (Fig. 1) which together facilitate interactions with ATP, preproteins, the SecB chaperone, and the SecYEG translocon (recently reviewed in references 14 and 37). Two discontinuous regions of SecAEc, the nucleotide binding folds NBD and IRA2 (also referred to as NBF1 and NBF2), form two faces of a nucleotide binding cleft and are responsible for the binding and hydrolysis of ATP. A preprotein cross-linking domain (PPXD) extends out of NBD. The C domain includes a helical scaffold domain (HSD), a helical wing domain (HWD), an intramolecular regulator of ATPase (IRA1), and the extreme carboxy-terminal region. The scaffold domain serves as a platform to which the two nucleotide binding folds are anchored. The IRA1 region has been shown to coordinate preprotein binding and ATP hydrolysis (54). The same region has recently been referred to as a “two-helix finger” domain which may push the preprotein through the SecYEG channel (15, 56). The extreme carboxy-terminal region of SecAEc interacts with SecB and may regulate or increase the fidelity of preprotein binding (16, 18). Residues within nearly every domain of SecAEc have been implicated in SecY interactions during the process of translocation (23, 31, 54, 56).
FIG. 1.
Comparison of the domain organization of SecAEc (ecSecA), SecASg (sgSecA), and SecA2Sg (sgSecA2). The domain boundaries of SecASg and SecA2Sg were approximated as determined by the Superfamily 1.69 structural comparison of proteins (http://supfam.mrc-lmb.cam.ac.uk/SUPERFAMILY/hmm.html) and refined by comparing the primary sequence alignments with that of SecAEc (sequences were aligned with the Multalin algorithm [13]; see Fig. S1 in the supplemental material). CTD, extreme carboxy-terminal domain. HSD, HWD, IRA1, and CTD comprise the C domain.
A comparison of the primary amino acid sequences of SecAEc, SecASg, and SecA2Sg, together with structural predictions, indicates that the overall domain configuration of the two S. gordonii paralogues is nearly identical to that of SecAEc, having two nucleotide binding folds, a preprotein binding domain located within NBD, and HSD and HWD domains (Fig. 1; see also Fig. S1 in the supplemental material). Perhaps the most significant difference between SecA2Sg and the SecA orthologues is the absence of a SecB binding site at the extreme carboxy-terminal end of SecA2Sg. This is not entirely surprising, since homologues of SecB have not been detected in gram-positive bacteria.
A comparison of the primary amino acid sequence alignment also indicates that SecA2Sg has most of the conserved residues known to be essential for SecAEc function (Table 3; see also Fig. S1 in the supplemental material). Of note, SecA2Sg has the nine conserved motifs (Q, I, Ia, Ib, and II through VI) known to line the nucleotide binding cleft of SecAEc and other helicases (35, 44), although the IRA2 motifs IV and VI have nonconserved residues. The alignment also shows that SecA2Sg has a tyrosine residue in the PPXD that is essential for preprotein binding and release by SecAEc (27) and a tryptophan residue in IRA1 that coordinates preprotein binding with ATP hydrolysis (54). This suggests that SecA2Sg is likely to coordinate the activities of preprotein binding and ATP hydrolysis in a manner similar to that of SecAEc.
TABLE 3.
Amino acid residues of SecA2Sg that correspond to key SecAEc residuesa
| SecA2Sg residue | SecAEc residue | Domain, function in SecAEc | Reference |
|---|---|---|---|
| K98 | K108 | Motif I of NBD (Walker A box), ATP binding and hydrolysis | 32 |
| T99 | T109 | Motif I of NBD (Walker A box), ATP binding and hydrolysis | 32 |
| D204 | D209 | Motif II of NBD (Walker B box), ATP binding and hydrolysis | 26, 32 |
| E205 | E210 | Motif II of NBD (Walker B box), catalytic base | 32, 57 |
| Y303 | Y326 | PPXD, preprotein binding and release | 27 |
| T374 | T393 | Motif III of NBD, essential for translocation | 36 |
| R490 | R509 | Motif V of IRA2, essential for translocation | 26, 32, 35, 44 |
| G491 | G510 | Motif V of IRA2, nucleotide binding | 44 |
| D493 | D512 | Motif V of IRA2, nucleotide binding | 53 |
| R518 | R566 | Motif VI of IRA2, interacts with NBD residues | 25, 44 |
| R526 | R574 | Motif VI of IRA2, ATP hydrolysis | 22 |
| R529 | R577 | Motif VI of IRA2, essential for translocation | 44 |
| W724 | W775 | IRA1, coordination of ATP hydrolysis and preprotein binding | 54 |
Conserved residues for which the effect of the mutation has been documented.
Selected conserved residues are essential for the function of SecA2Sg.
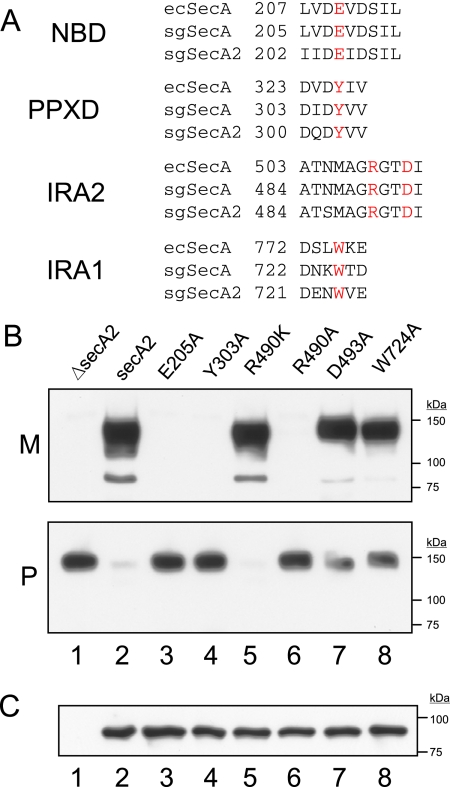
The conservation of key amino acids suggested that SecA2Sg functions analogously to SecAEc and, if so, that these residues would also be essential for the proper functioning of SecA2Sg. To test this directly, we made substitutions for conserved residues in NBD, PPXD, IRA2, or IRA1 (Fig. 2A) whose functions have been characterized in SecAEc and examined the effects on transport of GspB. We have previously used a carboxy-terminal FLAG-tagged truncated version of GspB (GspB736flag) as a model substrate for the accessory Sec system (3, 5). GspB736flag lacks the C-terminal cell wall anchoring motif and is freely secreted into the culture medium. As for native GspB, export of this truncated protein is dependent on a functional accessory Sec system. Mutations were generated in the secA2 sequence of the nisin-inducible expression plasmid pM99secA2, and the variant alleles were used to complement a secA2 mutation in the GspB736flag-expressing strain PS1226.
FIG. 2.
The effects of replacement of select residues of SecA2 on the export of GspB736flag. (A) Alignment of the regions flanking the target residues (indicated in red). SecAEc, ecSecA; SecASg, sgSecA; SecA2Sg, sgSecA2. (B and C) Lanes correspond to the secA2 mutant strain PS1226 carrying the pMSP3535 vector only (lane 1) or the vector with wild-type secA2 (lane 2) or secA2 variants as indicated (lanes 3 to 8). (B) GspB736flag was detected by using an anti-FLAG monoclonal antibody. Lanes contain proteins corresponding to 25 μl of cultures grown for 6 h in the presence of nisin. Nearly identical results were seen even in the absence of nisin induction, indicating that very low levels of SecA2 are required for complementation (data not shown). M, medium fraction; P, protoplast fraction. (C) SecA2 variants were detected by using an anti-SecA2 polyclonal antiserum. Lanes contain protoplast proteins corresponding to 50 μl of cultures grown in the presence of nisin for 6 h.
In a PS1226-derived strain carrying the vector only (Fig. 2B, lane 1), GspB736flag was not exported into the culture medium, and the preprotein was retained within the protoplasted cells. The nonexported, glycosylated preprotein appeared as a single band at 150 kDa. When complemented with wild-type secA2 in trans, GspB736flag was secreted into the culture medium, and very little of the preprotein was retained in the protoplasts (Fig. 2B, lane 2). As described previously (5), secreted GspB736flag appeared as a broad band of glycosylated forms that migrated under SDS-PAGE with an apparent molecular mass of 130 to 150 kDa, along with a minor amount of a nonglycosylated form having an apparent molecular mass equivalent to the predicted mass of 80 kDa. The SecA2E205A (Fig. 2B, lane 3) and SecA2Y303A (lane 4) variants, which have alterations in NBD and PPXD, respectively, failed to restore transport. A conservative arginine-to-lysine substitution for a residue in IRA2 of SecAEc (R509) has been shown to abolish protein transport (32). However, the analogous mutation (R490K) in SecA2Sg had no effect on transport (Fig. 2B, lane 5). In contrast, a nonconservative substitution for the same residue (R490A) abolished the ability of SecA2 to support translocation (Fig. 2B, lane 6). Another residue in IRA2 of SecAEc (D512) has been noted to be important for nucleotide binding (53). A nonconservative substitution for the analogous residue in SecA2Sg (D493A), however, resulted in only a minor decrease in GspB736flag export (Fig. 2B, lane 7). Lastly, a substitution for the conserved tryptophan residue in IRA1 (Fig. 2B, lane 8) resulted in a modest reduction in transport, which parallels the effect of mutating the analogous residue in SecAEc (54). None of the transport defects could be attributed simply to differences in SecA2 expression levels, as equivalent amounts of the wild-type and mutant SecA2 proteins were detected upon Western blotting of the protoplasts (Fig. 2C).
In summary, substitutions for residues in NBD, PPXD, and IRA1 generally had effects on transport comparable to those that have been reported for SecAEc. However, replacement of residues in IRA2 had a less dramatic effect on export than did replacement of the analogous residues of SecAEc. These data indicate that SecA2Sg functions in a manner similar to SecAEc but also suggest that the IRA2 domain might interact with NBD or nucleotides somewhat differently than SecAEc.
SecA2-mediated transport is sensitive to azide.
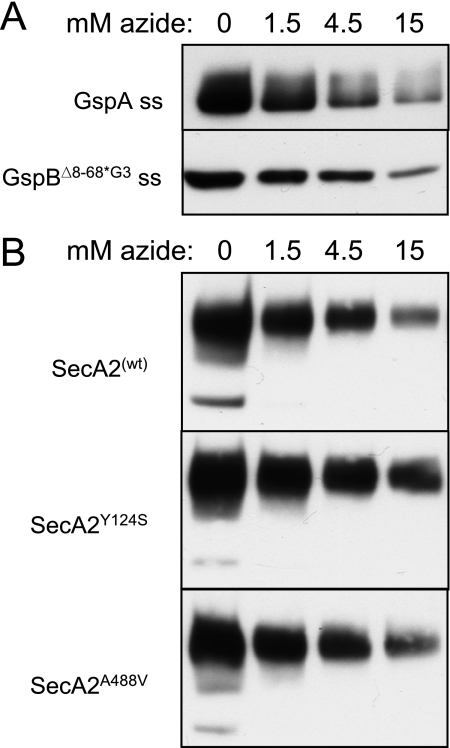
Azide is a potent inhibitor of the ATPase activity of SecA. This small molecule mimics the γ phosphate of ATP and stabilizes the ADP-bound state of the enzyme (6). Whereas azide levels of 2 mM are sufficient to suppress the general Sec system of E. coli, streptococci can readily grow in medium containing this concentration of azide, and 15 mM azide or more has been used to inhibit the general Sec system of Bacillus subtilis and streptococci (5, 10, 24). It is unknown, however, whether these differences in the amount of azide required to inhibit protein transport are due to an intrinsic difference in the sensitivity of the respective SecA proteins to azide. To better assess the impact of azide on SecA2Sg function, we first examined the effect of azide on SecA-dependent export in S. gordonii. We chose to examine the secretion of two GspB736flag variants with modified N-terminal signal sequences that we have previously characterized. The first variant consists of GspB736flag fused to a canonical signal peptide (5). The second GspB736flag variant has undergone replacement of the three glycine residues in the hydrophobic core of the signal sequence, along with a deletion of the extended N region. Alteration of the hydrophobic core redirects the preprotein to the general Sec pathway (3), and deletion of the extended N region renders the signal peptide similar to a canonical signal sequence (5). This second variant was expressed in a strain containing a deletion of secA2, to preclude export via the accessory Sec pathway, as well as a deletion of gtfA to avoid possible hindrance of export by carbohydrate linkage to the serine-rich GspB domains (50). When examined in S. gordonii cells, the presence of 1.5 mM azide led to a slight reduction in the secretion of both GspB736flag variants. Higher concentrations of azide (4.5 and 15 mM) resulted in progressively greater levels of suppression (Fig. 3A).
FIG. 3.
The effect of sodium azide on SecASg- and SecA2Sg-dependent export. Lanes contain protein from 25 μl of spent medium of cultures grown at high density for 90 min. The secreted proteins were detected with an anti-FLAG monoclonal antibody. (A) SecASg-dependent export of GspB736flag variants. The upper panel shows export of a GspA-GspB736flag fusion protein from strain PS1025 (GspA is another cell wall protein of S. gordonii). The lower panel shows export of GspB736flag carrying a modified signal peptide (Δ8-68 G75L G79A G80C) from strain PS1139 (ΔsecA2 ΔgtfA). ss, signal sequence. (B) Export of GspB736flag from strains expressing variants of SecA2. wt, wild type.
To assess whether azide has a comparable effect on SecA2Sg activity, we examined the export of GspB736flag (with its native signal sequence) in the presence of azide. As was seen with SecASg, the transport activity of SecA2Sg was highly sensitive to azide. As little as 1.5 mM azide resulted in a substantial decrease in the amount of GspB736flag exported, and transport was strongly suppressed by 15 mM azide (Fig. 3B, upper panel).
Substitutions for certain residues in NBD or IRA2 of SecAEc have been shown to confer altered sensitivity to azide. For example, the replacement of Y134 in SecAEc with cysteine, serine, or asparagine results in a decreased sensitivity to azide, whereas the replacement of A507 with valine produces a hypersensitive phenotype (21, 31). We introduced substitutions in the analogous residues of SecA2Sg (Y124 in motif Ia of NBD and A488 in motif V of IRA2, respectively) and then compared the effects of azide on export of GspB736flag. Replacement of Y124 in SecA2Sg led to a slightly increased resistance to azide. That is, the SecA2Y124S variant strain exported more GspB736flag than did the wild-type SecA2-expressing strain at high azide concentrations (Fig. 3B, middle panel). In contrast, replacement of A488 had no discernible effect on the sensitivity to azide (Fig. 3B, lower panel). These results demonstrate that, like SecAEc, SecA2Sg is highly sensitive to azide and that replacement of a residue in motif Ia of NBD can reduce the sensitivity to azide. However, alteration of a residue in motif V of IRA2 had no apparent effect on azide sensitivity. This is consistent with the possibility that the action of IRA2 of SecA2Sg during the process of translocation may be subtly different from that of SecAEc.
The biochemical properties of SecA2Sg differ from those of SecASg.
Although SecA2Sg is predicted to be an ATPase, the ability of this putative enzyme to hydrolyze ATP has not been demonstrated directly. For SecAEc, three rates of ATP hydrolysis have typically been assessed in vitro. The rate of ATP hydrolysis by the isolated enzyme in an unactivated state (the intrinsic or basal rate) is relatively low and is highly sensitive to the level of Mg2+ (19). The ATPase activity is increased approximately twofold by the addition of anionic phospholipids or lipid vesicles containing SecYEG (the membrane-associated rate), and it is then maximally stimulated upon the addition of a preprotein substrate (the translocation rate).
As a first step toward characterizing the differences between SecASg and SecA2Sg in vitro, we compared the basal rates of ATP hydrolysis by these proteins. Both proteins were first expressed with N-terminal His6 tags in E. coli. Although 6His-SecA was easily purified to homogeneity by using nickel affinity chromatography, purification of 6His-SecA2 was hampered by the apparent insolubility of this protein. The expression of 6His-SecA2 under a variety of conditions typically used to enhance solubility upon overexpression in E. coli (expression at low temperature, addition of ethanol to enhance the production of chaperones, addition of low levels of the inducing agent, etc.) yielded only minimal improvements in solubility which, at best, was found to be less than 0.1 mg per ml in aqueous solutions. Purification of 6His-SecA2 in the presence of the nondenaturing zwitterionic detergent CHAPS improved the yields of soluble protein, although solubility was still fairly low (approximately 0.5 mg per ml).
The basal rate of ATP hydrolysis by 6His-SecA2 was measured and compared with that of 6His-SecA purified in the presence or absence of CHAPS. Since the basal ATPase activity of SecAEc is highly sensitive to Mg2+ levels, due in part to the effect of this cation on the conformation of SecAEc (19), we assessed the activity of these enzymes over a range of Mg2+ concentrations. As has been reported for SecAEc, 6His-SecA showed maximal activity in the presence of very low levels of Mg2+ (0.1 mM) and was inhibited by higher levels (Fig. 4A). In contrast, 6His-SecA2 activity was highest in 2.5 mM Mg2+, with a maximal rate of hydrolysis that was approximately 30% of that of 6His-SecA. The low rate of ATP hydrolysis by 6His-SecA2 is due in part to the IRA1 domain, as removal of this region resulted in a twofold-higher ATPase activity (see Fig. S2 in the supplemental material). Somewhat surprisingly, the basal hydrolysis rates of 6His-SecA and 6His-SecA2 were unaffected by even high levels of azide (Fig. 4B).
We next examined whether fusion of MalE to SecA2 could enhance protein solubility and recovery. The MalE-SecA2 fusion protein was indeed more amenable to purification and was soluble to at least 1 mg per ml in aqueous solutions. ATP hydrolysis by the MalE-tagged protein was then assessed and compared with that of MalE-SecA. The N-terminal MalE fusion to SecASg resulted in approximately twofold higher rates of hydrolysis in the presence of Mg2+ than were found for 6His-SecA. Activity was still highest, however, when the Mg2+ concentration was low (Fig. 4C). The MalE-SecA2 fusion protein also exhibited elevated rates of ATP hydrolysis, but it differed from MalE-SecA in two aspects. As seen with 6His-SecA2, ATP hydrolysis was undetectable in the absence of Mg2+. Second, the rate was maximal at 0.5 mM Mg2+ and was less strongly suppressed by higher levels.
The effects of sodium azide on the rate of ATP hydrolysis by the MalE fusion proteins were also examined. Both enzymes were strongly inhibited by azide levels as low as 1 mM (Fig. 4D). The ATPase activity of MalE-SecA2 was even more sensitive to azide than that of MalE-SecA (86% reduction compared with 69% reduction, respectively, at 1 mM azide) and was nearly completely suppressed at 15 mM. The combined results indicate that SecA2Sg is an ATPase that can provide the energy for protein transport by the accessory Sec system but that there may be subtle differences in the way that the activity of this motor protein is regulated.
The SecASg and SecA2Sg domains are not readily interchangeable.
Since SecASg and SecA2Sg appeared to be highly similar both structurally and functionally, we hypothesized that these proteins might have a modular organization, such that domains of SecASg and SecA2Sg with common functions could be interchanged with little impact on export activity. In contrast, the exchange of any uniquely adapted domains could result in a gain of function, such as the ability to interact with the paralogous transport system. Indeed, a similar type of exchange between domains of the SecA proteins of organisms as disparate as rickettsiae and E. coli (39) or even mycobacteria and E. coli (34) has produced functional chimeras. Accordingly, construction of SecA-SecA2 chimeras that transport GspB through either the SecYEG or the SecY2 translocon could help to address whether SecA2 or SecY2 is uniquely adapted for a glycosylated preprotein.
To determine whether such uniquely adapted domains could be identified, we generated chimeric forms of SecA and SecA2 by exchanging various segments of the two S. gordonii paralogues. In all, eight chimeras were generated. For brevity, results for four chimeras will be presented here. One type of chimera involved an exchange within a very highly conserved motif near the end of IRA2, just ahead of the junction with the C domain (see Fig. S3 in the supplemental material). In effect, this involved replacement of the conserved PPXD and nucleotide binding folds (62% similarity and 52% identity) to adjoin the less similar C domain (43% similarity and 26% identity). Although both of the chimeric proteins (SecA1-534A2535-793 and SecA21-534A535-838) were expressed at detectable levels (Fig. 5, lanes 3 and 4, respectively), neither was able to support transport of GspB736flag (data not shown).
FIG. 5.
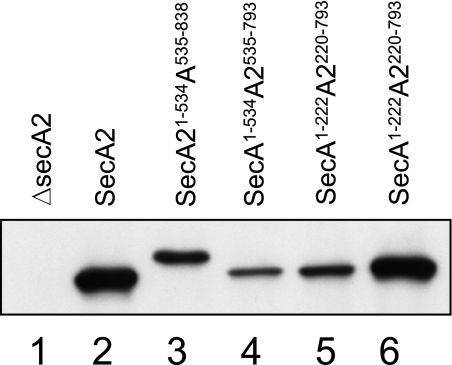
Expression of the SecA-SecA2 chimeras in S. gordonii. Lanes contain protoplast proteins corresponding to 50 μl of cultures grown in the presence of nisin for 6 h. Chimeras were detected by using a polyclonal anti-SecA2 serum. The secA2 mutant strain PS1226 carried pMSP3535 with no insert (lane 1), wild-type secA2 (lane 2), or secA-secA2 constructs as indicated (lanes 3 to 6).
Another chimera involved simply replacing the highly conserved NBD of SecA2Sg, by an exchange at the conserved β strand, or “hinge,” that connects the N-terminal portion of NBD to PPXD. Again, this chimera (SecA1-222A2220-793) was expressed at detectable levels (Fig. 5, lane 5) but failed to transport GspB736flag (data not shown). A fourth chimera, which has a replacement of just the N-terminal end of SecA2 up to the conserved motif I (Walker A box) of NBD (SecA1-121A2114-793), was also examined. Surprisingly, although the segments exchanged are very highly similar (72% similarity and 61% identity) and the chimera was expressed at levels comparable to that of wild-type SecA2 (Fig. 5, lane 6), the SecA1-121A2114-793 chimera also failed to complement the SecA2 mutation (data not shown).
The inability of these chimeric proteins to support transport is evidently not due to a complete loss of the ability to hydrolyze ATP, as all retained low levels of basal ATPase activity (see Fig. S2 in the supplemental material). The lack of function may therefore be due either to disruption of more-complex intramolecular interactions (for example, improper coupling of ATP hydrolysis with the motion of protein transport) or to faulty interaction with the transport channel. Regardless, these data indicate that, despite the high degree of similarity, the SecASg and SecA2Sg domains are not interchangeable.
DISCUSSION
The accessory Sec system of gram-positive bacteria appears to be a uniquely adapted variation of the general Sec system that has evolved specifically for the transport of a family of very large, serine-rich glycoproteins. Our previous studies indicated that SecA2Sg is a key component of this system and, based on its similarity to SecA, that it is likely to be a motor protein, albeit one specifically adapted for the transport of GspB. We therefore compared some of the properties of SecA and SecA2 with a view toward identifying similarities and differences, especially those that might explain the selectivity of this system. Our results demonstrate that SecA2Sg is an ATPase that functions similarly to the extensively characterized but still incompletely understood SecA proteins. However, SecA2Sg has two biochemical properties that differ notably from those of SecA.
The first difference is that SecA2Sg is considerably less soluble than SecA in aqueous solutions. A preliminary assessment of the behavior of the SecA-SecA2 chimeras indicates that the insolubility is not due to any particular region of SecA2Sg but rather is distributed throughout the protein (unpublished results). These findings are consistent with what has been reported for SecA2 of S. parasanguinis, where the protein localized strictly to the cytoplasmic membrane (11).
A second difference between SecA2Sg and SecA is the level of Mg2+ required to stimulate ATPase activity. Whereas the activity of 6His-SecA is highest at a very low Mg2+ concentration (0.1 mM), 6His-SecA2 is completely inactive at Mg2+ concentrations of less than 0.5 mM and requires 2.5 mM Mg2+ for maximal activity. The allosteric regulation of SecAEc by Mg2+ is thought to help prevent a wasteful expenditure of ATP when the protein is not engaged in protein transport (19). If MalE-SecA and MalE-SecA2 do indeed mimic activated forms of the ATPases (see below), the combined data suggest that SecA2 has an intrinsically strong suppression of ATP hydrolysis in the resting state. Regulation of the basal rate of ATP hydrolysis by SecAEc is also due in part to residues in the IRA1 and IRA2 domains (26, 44). The IRA1 domain of SecA2Sg likewise appears to suppress the basal ATPase activity, since deletion of this region led to a higher rate of ATP hydrolysis (see Fig. S2 in the supplemental material). However, the effect of mutations in the IRA2 domain of SecA2Sg did not parallel the effect of the analogous mutations in SecAEc. The findings suggest that the IRA2 of SecA2Sg may interact with NBD or nucleotides in a different manner than the IRA2 of SecAEc.
The high degree of similarity between SecA2Sg and SecASg suggested that these proteins were modular and that at least some of the domains could be interchanged. By exchanging domains of SecA2Sg and SecASg, we sought to identify functionally conserved regions versus those that had become specifically adapted for GspB export. Although we were able to generate numerous chimeras with ATPase activity, none was able to support GspB736flag export. The precise reason for the inability of the SecA-SecA2 chimeras to mediate the translocation of GspB736flag is unclear. These proteins may be defective in preprotein binding, preprotein-stimulated ATP hydrolysis, or interaction with the translocon. It was especially surprising that replacement of the extreme N-terminal end of SecA2Sg with that of SecASg resulted in an inactive chimera, since the exchanged regions show 61% identity (72% similarity). However, two recent studies indicate that residues in this region of SecAEc contact SecY during the process of protein transport (23, 56). Thus, one explanation for the lack of export function of this chimera is a faulty interaction with the SecY2 translocon, and it is possible that each of the SecA2 domains is specifically adapted for interaction with SecY2 or other components of this unique system.
One similarity between SecA2Sg and SecASg is their sensitivity to azide. This sensitivity was observed both in vivo, as measured by the effect of azide on the secretion of GspB736flag, and in vitro, as shown by the effect of azide on the ATPase activity of MalE-SecA2. This is in contrast to what has been reported for the SecA2 of S. parasanguinis, which apparently can function in the presence of 30 mM azide (10). We cannot explain why the basal ATPase activity of the His6-tagged proteins was not inhibited by azide. However, this lack of an effect on basal ATPase activity, in spite of a strong suppression of translocation by azide, also occurs with SecAEc (T. A. Rapoport and D. A. Kendall, personal communications).
A somewhat surprising finding was the behavior of the MalE fusion proteins in comparison to that of the His6-tagged proteins. The MalE fusion proteins displayed characteristics that are typically associated with activated SecA: higher rates of ATP hydrolysis, lower sensitivity to Mg2+, and pronounced sensitivity to azide. To confirm whether MalE-SecA and MalE-SecA2 mimic activated forms of the ATPases, it will be necessary to compare the activities of the MalE fusion proteins with the activity of 6His-SecA or 6His-SecA2 in the presence of their respective translocons or an optimal combination of anionic phospholipids. If MalE-SecA and MalE-SecA2 do correspond to activated or “open” forms, these proteins will be useful for preprotein binding studies, since the activation of SecA upon interaction with lipids or SecYEG is paralleled by a conformational change that favors preprotein binding.
Several of the mutant forms of SecA2Sg generated for this study will also be useful for further in vivo and in vitro analyses. For example, the SecA2W724A variant is anticipated to be active for preprotein binding in the absence of lipids and the translocon (54). In addition, the SecA2Y124S variant may be useful for studying the specificity of the interactions with SecY2 and the GspB preprotein, since secA mutations that affect sensitivity to azide (so-called azi mutants) can simultaneously affect binding with mutant signal peptides and SecY (21, 31). Thus, these experiments not only confirm that SecA2Sg is a motor protein but also establish the groundwork for addressing how the accessory Sec system is uniquely adapted to transport large, serine-rich glycoproteins.
Supplementary Material
Acknowledgments
This work was supported by the Department of Veterans Affairs and by grants RO1AI41513 and RO1AI057433 from the National Institutes of Health.
We thank R. Seepersaud for critical reading of the manuscript.
Footnotes
Published ahead of print on 10 April 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Bensing, B. A., B. W. Gibson, and P. M. Sullam. 2004. The Streptococcus gordonii platelet binding protein GspB undergoes glycosylation independently of export. J. Bacteriol. 186638-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bensing, B. A., J. A. Lopez, and P. M. Sullam. 2004. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibα. Infect. Immun. 726528-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bensing, B. A., I. R. Siboo, and P. M. Sullam. 2007. Glycine residues in the hydrophobic core of the GspB signal sequence route export toward the accessory Sec pathway. J. Bacteriol. 1893846-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bensing, B. A., and P. M. Sullam. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 441081-1094. [DOI] [PubMed] [Google Scholar]
- 5.Bensing, B. A., D. Takamatsu, and P. M. Sullam. 2005. Determinants of the streptococcal surface glycoprotein GspB that facilitate export by the accessory Sec system. Mol. Microbiol. 581468-1481. [DOI] [PubMed] [Google Scholar]
- 6.Bowler, M. W., M. G. Montgomery, A. G. Leslie, and J. E. Walker. 2006. How azide inhibits ATP hydrolysis by the F-ATPases. Proc. Natl. Acad. Sci. USA 1038646-8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braunstein, M., A. M. Brown, S. Kurtz, and W. R. Jacobs, Jr. 2001. Two nonredundant SecA homologues function in mycobacteria. J. Bacteriol. 1836979-6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braunstein, M., B. J. Espinosa, J. Chan, J. T. Belisle, and W. R. Jacobs, Jr. 2003. SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 48453-464. [DOI] [PubMed] [Google Scholar]
- 9.Bryan, E. M., T. Bae, M. Kleerebezem, and G. M. Dunny. 2000. Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid 44183-190. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Q., B. Sun, H. Wu, Z. Peng, and P. M. Fives-Taylor. 2007. Differential roles of individual domains in selection of secretion route of a Streptococcus parasanguinis serine-rich adhesin, Fap1. J. Bacteriol. 1897610-7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, Q., H. Wu, R. Kumar, Z. Peng, and P. M. Fives-Taylor. 2006. SecA2 is distinct from SecA in immunogenic specificity, subcellular distribution and requirement for membrane anchoring in Streptococcus parasanguis. FEMS Microbiol. Lett. 264174-181. [DOI] [PubMed] [Google Scholar]
- 12.Claverys, J. P., A. Dintilhac, E. V. Pestova, B. Martin, and D. A. Morrison. 1995. Construction and evaluation of new drug-resistance cassettes for gene disruption mutagenesis in Streptococcus pneumoniae, using an ami test platform. Gene 164123-128. [DOI] [PubMed] [Google Scholar]
- 13.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1610881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driessen, A. J., and N. Nouwen. 2008. Protein translocation across the bacterial cytoplasmic membrane. Annu. Rev. Biochem. 77643-667. [DOI] [PubMed] [Google Scholar]
- 15.Erlandson, K. J., S. B. Miller, Y. Nam, A. R. Osborne, J. Zimmer, and T. A. Rapoport. 2008. A role for the two-helix finger of the SecA ATPase in protein translocation. Nature 455984-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fekkes, P., C. van der Does, and A. J. Driessen. 1997. The molecular chaperone SecB is released from the carboxy-terminus of SecA during initiation of precursor protein translocation. EMBO J. 166105-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Froeliger, E. H., and P. Fives-Taylor. 2001. Streptococcus parasanguis fimbria-associated adhesin fap1 is required for biofilm formation. Infect. Immun. 692512-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelis, I., A. M. Bonvin, D. Keramisanou, M. Koukaki, G. Gouridis, S. Karamanou, A. Economou, and C. G. Kalodimos. 2007. Structural basis for signal-sequence recognition by the translocase motor SecA as determined by NMR. Cell 131756-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold, V. A., A. Robson, A. R. Clarke, and I. Collinson. 2007. Allosteric regulation of SecA: magnesium-mediated control of conformation and activity. J. Biol. Chem. 28217424-17432. [DOI] [PubMed] [Google Scholar]
- 20.Hou, J. M., N. G. D'Lima, N. W. Rigel, H. S. Gibbons, J. R. McCann, M. Braunstein, and C. M. Teschke. 2008. ATPase activity of Mycobacterium tuberculosis SecA1 and SecA2 proteins and its importance for SecA2 function in macrophages. J. Bacteriol. 1904880-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huie, J. L., and T. J. Silhavy. 1995. Suppression of signal sequence defects and azide resistance in Escherichia coli commonly result from the same mutations in secA. J. Bacteriol. 1773518-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt, J. F., S. Weinkauf, L. Henry, J. J. Fak, P. McNicholas, D. B. Oliver, and J. Deisenhofer. 2002. Nucleotide control of interdomain interactions in the conformational reaction cycle of SecA. Science 2972018-2026. [DOI] [PubMed] [Google Scholar]
- 23.Jilaveanu, L. B., and D. B. Oliver. 2007. In vivo membrane topology of Escherichia coli SecA ATPase reveals extensive periplasmic exposure of multiple functionally important domains clustering on one face of SecA. J. Biol. Chem. 2824661-4668. [DOI] [PubMed] [Google Scholar]
- 24.Jongbloed, J. D., H. Antelmann, M. Hecker, R. Nijland, S. Bron, U. Airaksinen, F. Pries, W. J. Quax, J. M. van Dijl, and P. G. Braun. 2002. Selective contribution of the twin-arginine translocation pathway to protein secretion in Bacillus subtilis. J. Biol. Chem. 27744068-44078. [DOI] [PubMed] [Google Scholar]
- 25.Karamanou, S., G. Gouridis, E. Papanikou, G. Sianidis, I. Gelis, D. Keramisanou, E. Vrontou, C. G. Kalodimos, and A. Economou. 2007. Preprotein-controlled catalysis in the helicase motor of SecA. EMBO J. 262904-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karamanou, S., E. Vrontou, G. Sianidis, C. Baud, T. Roos, A. Kuhn, A. S. Politou, and A. Economou. 1999. A molecular switch in SecA protein couples ATP hydrolysis to protein translocation. Mol. Microbiol. 341133-1145. [DOI] [PubMed] [Google Scholar]
- 27.Kourtz, L., and D. Oliver. 2000. Tyr-326 plays a critical role in controlling SecA-preprotein interaction. Mol. Microbiol. 371342-1356. [DOI] [PubMed] [Google Scholar]
- 28.Lenz, L. L., S. Mohammadi, A. Geissler, and D. A. Portnoy. 2003. SecA2-dependent secretion of autolytic enzymes promotes Listeria monocytogenes pathogenesis. Proc. Natl. Acad. Sci. USA 10012432-12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenz, L. L., and D. A. Portnoy. 2002. Identification of a second Listeria secA gene associated with protein secretion and the rough phenotype. Mol. Microbiol. 451043-1056. [DOI] [PubMed] [Google Scholar]
- 30.Lill, R., W. Dowhan, and W. Wickner. 1990. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell 60271-280. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto, G., H. Nakatogawa, H. Mori, and K. Ito. 2000. Genetic dissection of SecA: suppressor mutations against the secY205 translocase defect. Genes Cells 5991-999. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell, C., and D. Oliver. 1993. Two distinct ATP-binding domains are needed to promote protein export by Escherichia coli SecA ATPase. Mol. Microbiol. 10483-497. [DOI] [PubMed] [Google Scholar]
- 33.Obert, C., J. Sublett, D. Kaushal, E. Hinojosa, T. Barton, E. I. Tuomanen, and C. J. Orihuela. 2006. Identification of a candidate Streptococcus pneumoniae core genome and regions of diversity correlated with invasive pneumococcal disease. Infect. Immun. 744766-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owens, M. U., W. E. Swords, M. G. Schmidt, C. H. King, and F. D. Quinn. 2002. Cloning, expression, and functional characterization of the Mycobacterium tuberculosis secA gene. FEMS Microbiol. Lett. 211133-141. [DOI] [PubMed] [Google Scholar]
- 35.Papanikolau, Y., M. Papadovasilaki, R. B. Ravelli, A. A. McCarthy, S. Cusack, A. Economou, and K. Petratos. 2007. Structure of dimeric SecA, the Escherichia coli preprotein translocase motor. J. Mol. Biol. 3661545-1557. [DOI] [PubMed] [Google Scholar]
- 36.Papanikou, E., S. Karamanou, C. Baud, G. Sianidis, M. Frank, and A. Economou. 2004. Helicase motif III in SecA is essential for coupling preprotein binding to translocation ATPase. EMBO Rep. 5807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papanikou, E., S. Karamanou, and A. Economou. 2007. Bacterial protein secretion through the translocase nanomachine. Nat. Rev. Microbiol. 5839-851. [DOI] [PubMed] [Google Scholar]
- 38.Plummer, C., H. Wu, S. W. Kerrigan, G. Meade, D. Cox, and C. W. Ian Douglas. 2005. A serine-rich glycoprotein of Streptococcus sanguis mediates adhesion to platelets via GPIb. Br. J. Haematol. 129101-109. [DOI] [PubMed] [Google Scholar]
- 39.Rahman, M. S., J. A. Simser, K. R. Macaluso, and A. F. Azad. 2005. Functional analysis of secA homologues from rickettsiae. Microbiology 151589-596. [DOI] [PubMed] [Google Scholar]
- 40.Rigel, N. W., and M. Braunstein. 2008. A new twist on an old pathway: accessory Sec systems. Mol. Microbiol. 69291-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rigel, N. W., H. S. Gibbons, J. R. McCann, J. A. McDonough, S. Kurtz, and M. Braunstein. 2009. The accessory SecA2 system of mycobacteria requires ATP binding and the canonical SecA1. J. Biol. Chem. 2849927-9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose, L., P. Shivshankar, E. Hinojosa, A. Rodriguez, C. J. Sanchez, and C. J. Orihuela. 2008. Antibodies against PsrP, a novel Streptococcus pneumoniae adhesin, block adhesion and protect mice against pneumococcal challenge. J. Infect. Dis. 198375-383. [DOI] [PubMed] [Google Scholar]
- 43.Seifert, K. N., E. E. Adderson, A. A. Whiting, J. F. Bohnsack, P. J. Crowley, and L. J. Brady. 2006. A unique serine-rich repeat protein (Srr-2) and novel surface antigen (ɛ) associated with a virulent lineage of serotype III Streptococcus agalactiae. Microbiology 1521029-1040. [DOI] [PubMed] [Google Scholar]
- 44.Sianidis, G., S. Karamanou, E. Vrontou, K. Boulias, K. Repanas, N. Kyrpides, A. S. Politou, and A. Economou. 2001. Cross-talk between catalytic and regulatory elements in a DEAD motor domain is essential for SecA function. EMBO J. 20961-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siboo, I. R., H. F. Chambers, and P. M. Sullam. 2005. Role of SraP, a Serine-rich surface protein of Staphylococcus aureus, in binding to human platelets. Infect. Immun. 732273-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullam, P. M., F. H. Valone, and J. Mills. 1987. Mechanisms of platelet aggregation by viridans group streptococci. Infect. Immun. 551743-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi, Y., E. Takashima, K. Shimazu, H. Yagishita, T. Aoba, and K. Konishi. 2006. Contribution of sialic acid-binding adhesin to pathogenesis of experimental endocarditis caused by Streptococcus gordonii DL1. Infect. Immun. 74740-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takamatsu, D., B. A. Bensing, H. Cheng, G. A. Jarvis, I. R. Siboo, J. A. Lopez, J. M. Griffiss, and P. M. Sullam. 2005. Binding of the Streptococcus gordonii surface glycoproteins GspB and Hsa to specific carbohydrate structures on platelet membrane glycoprotein Ibα. Mol. Microbiol. 58380-392. [DOI] [PubMed] [Google Scholar]
- 49.Takamatsu, D., B. A. Bensing, A. Prakobphol, S. J. Fisher, and P. M. Sullam. 2006. Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins. Infect. Immun. 741933-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takamatsu, D., B. A. Bensing, and P. M. Sullam. 2004. Four proteins encoded in the gspB-secY2A2 operon of Streptococcus gordonii mediate the intracellular glycosylation of the platelet-binding protein GspB. J. Bacteriol. 1867100-7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takamatsu, D., B. A. Bensing, and P. M. Sullam. 2004. Genes in the accessory sec locus of Streptococcus gordonii have three functionally distinct effects on the expression of the platelet-binding protein GspB. Mol. Microbiol. 52189-203. [DOI] [PubMed] [Google Scholar]
- 52.Takamatsu, D., B. A. Bensing, and P. M. Sullam. 2005. Two additional components of the accessory Sec system mediating export of the Streptococcus gordonii platelet-binding protein GspB. J. Bacteriol. 1873878-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vrontou, E., and A. Economou. 2004. Structure and function of SecA, the preprotein translocase nanomotor. Biochim. Biophys. Acta 169467-80. [DOI] [PubMed] [Google Scholar]
- 54.Vrontou, E., S. Karamanou, C. Baud, G. Sianidis, and A. Economou. 2004. Global co-ordination of protein translocation by the SecA IRA1 switch. J. Biol. Chem. 27922490-22497. [DOI] [PubMed] [Google Scholar]
- 55.Xiong, Y. Q., B. A. Bensing, A. S. Bayer, H. F. Chambers, and P. M. Sullam. 2008. Role of the serine-rich surface glycoprotein GspB of Streptococcus gordonii in the pathogenesis of infective endocarditis. Microb. Pathog. 45297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zimmer, J., Y. Nam, and T. A. Rapoport. 2008. Structure of a complex of the ATPase SecA and the protein-translocation channel. Nature 455936-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zito, C. R., E. Antony, J. F. Hunt, D. B. Oliver, and M. M. Hingorani. 2005. Role of a conserved glutamate residue in the Escherichia coli SecA ATPase mechanism. J. Biol. Chem. 28014611-14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.