Abstract
The objective of this research was to investigate whether immobilized anti-inflammatory cytokines will signal changes in the inflammatory profile of cultured monocytes. A fusion protein of recombinant human IL-1 receptor antagonist and elastin-like peptide (IL-1ra-ELP) was expressed in E. Coli. THP-1 human monocytes were cultured on either carboxyl-terminated self-assembled monolayers (SAMs), or SAMs with either covalently immobilized or soluble IL-1ra-ELP. LPS-stimulated monocytes exposed to either soluble or immobilized IL-1ra-ELP were prevented from cell differentiation, showed attenuated expression of pro-inflammatory cytokines, and had increased production of anti-inflammatory and pro-wound healing cytokines. These results suggest that immobilized anti-inflammatory cytokines have the potential to be immunomodulatory biomaterials.
Introduction
Ligand decorated surfaces are a common in vitro strategy to promote specific biological interactions on the surface of biomaterials that are otherwise not prone to direct cell adhesion [1, 2]. Most approaches have employed the physical adsorption or chemical coupling of cell binding proteins (e.g. fibronectin or collagen) or adhesion peptides (e.g. integrin binding RGD peptide) to various biomaterials [3] [4]. In this report, we describe an alternative approach for the fabrication of bioactive surfaces by immobilization of an anti-inflammatory cytokine to a self-assembled monolayer (SAM) of hydrophilic alkane thiols. The immobilized cytokine is intended to bind to specific membrane-bound receptors and the hydrophilic SAM is intended to minimize non-specific surface interactions [5].
In brief, cytokines are short-lived, small (10–30 kDa) glycoproteins produced de novo in response to an immune stimulus to mediate and regulate immunity, inflammation, cell growth and differentiation, and hematopoiesis. Cytokines are secreted predominantly by lymphocytes, monocytes and macrophages, but act on a broad array of cells by binding to specific membrane receptors. Binding to membrane-bound receptors triggers second messenger complexes that carry the signal to the nucleus to alter gene expression [6, 7]. Cellular responses to cytokine binding include up- or down-regulation of membrane protein expression, cell growth/proliferation/differentiation, and secretion of effector molecules. IL-1, IL-6, IL-8 and TNF-α are the prototypical pro-inflammatory cytokines because of their early and intense response to the invading agents such as bacterium or toxin; whereas, IL-10, IL-1ra, soluble TNF receptor and TGF-β are commonly identified as anti-inflammatory and/or immune-suppressive cytokines [8].
Here, we utilize the IL-1 family of cytokines that consists of two agonists (IL-1α and IL-1β), a specific receptor antagonist (IL-1ra), and two transmembrane receptors, types I and II [9]. Isoforms IL-1α, IL-1β and IL-1ra are all single chain 17 kDa glycoproteins. The type I receptor is biologically active while the type II receptor is a dummy receptor that sequesters IL-1 at the plasma membrane or as a soluble receptor. When IL-1 binds to the functional type I receptor in the cell membrane it recruits a second protein to join the complex (IL-1R AcP) that activates intracellular signaling cascades leading to transcription of several pro-inflammatory genes in a broad array of cell types. The antagonist IL-1ra binds with comparable avidity to both types of receptors, but blocks the activating step of IL-1R AcP association with type I receptor [9]. The balance between IL-1 and IL-1ra and their binding to the functional and dummy receptors regulates the functions of adaptive immunity in local tissues.
An imbalance of IL-1 and IL-1ra has been implicated in the pathogenesis of rheumatoid arthritis [10], inflammatory bowel disease [11], asthma [12], cancer [13], CNS diseases [14]. The recombinant form of naturally occurring IL-1ra is the only cytokine clinically approved for reducing IL-1 activities; however, clinical trials are underway for IL-1 Traps [15], antibodies to IL-1α, and antibodies to IL-1 receptor type I [16].
Given current interest in cytokines as an anti-inflammatory therapy, and the widespread use of biomolecule immobilization to biomaterials, it is surprising that so little has been done concerning cytokine immobilization. The few examples that do exist to date fall into two categories: biophysical studies of cytokine-receptor binding kinetics at surfaces [17–21], and cell culture studies on surfaces immobilized with cytokines [22–25]. All but one of the cell culture studies [23] employed immobilized TGF-β to modulate smooth muscle cell, epithelial cell and osteoblast growth on PEG, PDMS and titanium alloy surfaces, respectively.
The current study demonstrates that an immobilized human recombinant IL-1ra-ELP fusion protein (IL-1ra-ELP) (Figure 1a) modulates the inflammatory profile of lipopolysaccharide (LPS) stimulated cultured human monocytes. Specifically, LPS-stimulated THP-1 monocytes that were exposed to either soluble or immobilized IL-1ra-ELP did not differentiate, showed attenuated expression of pro-inflammatory cytokines, and had enhanced production of anti-inflammatory and pro-wound healing cytokines. The extent of signaling by immobilized and soluble fusion protein were similar in magnitude, indicating roughly equivalent bioactivity and that cultured monocytes are clearly being signaled by the immobilized IL-1ra-ELP.
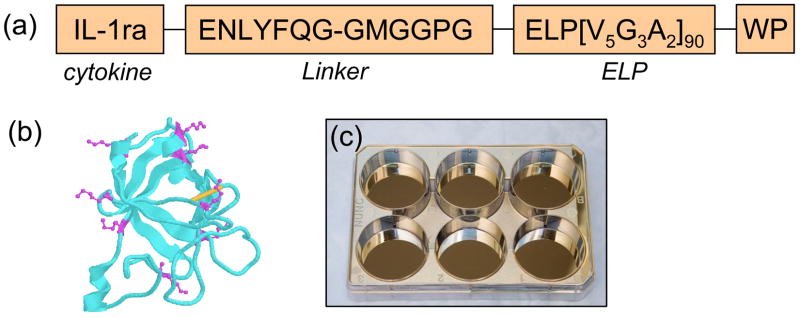
Figure 1.
(a) peptides sequences of IL-1ra-ELP fusion protein (Mw=54,044 Da). The IL-1ra-ELP fusion protein was produced by bacterial expression in E. coli. (b) X-ray crystallography of IL-1ra with the lysines highlighted in magenta. (c) An image of six-well tissue culture polystyrene plates with a 5 nm Cr adhesion layer and a 45 nm Au layer evaporated yielding optically transparent gold-coated slides.
Experimental
IL-1ra-ELP fusion protein expression
IL-1ra-ELP is a 54 kDa protein construct consisting of a 18 kDa IL-1ra domain and an 36 kDa ELP tag. Figure 1a shows the peptide sequence of ELP moiety, and Figure 1b shows the ribbon structure of IL-1ra. Eight of nine lysine side groups are displayed in magenta. A single disulfide bond in the structure is shown in yellow. The expression vectors containing human recombinant IL-1ra-ELP fusion protein genes were transformed into the E. coli strain BLR(DE3) and the protein was expressed utilizing a hyper expression protocol as detailed previously [26, 27].
IL-1ra-ELP bioactivity
The bioactivity of IL-1ra-ELP was benchmarked against commercially available human recombinant IL-1ra (rhIL-1ra) (R&D Systems, Minneapolis, MN) by IL-1ra inhibition of IL-1 induced proliferation of RPMI 1788 cells [28, 29] using ATP production as the cell viability assay. Briefly, cells were seeded at 2000–3000 cells/well in a 96 well plate in RPMI 1640 media supplemented with 10% serum, 50 μM β-Mercaptoethanol, 1mM sodium pyruvate, 10mM Hepes and 5.71 pM (100 pg/mL) IL-1β (Mw: 17.5 kDa, R&D Systems, Minneapolis, MN). IL-1ra-ELP and human recombinant IL-1ra solution in a 1% BSA diluent (R&D Systems, Minneapolis, MN) were added to the wells at increasing molar excesses to determine the critical dosage for inhibiting the IL-1 induced proliferation increase. ATP levels were measured on the fifth day using Cell Titer-Glo® luminescent test kits (Promega, Madison, WI). Results are reported as EC50 indicating the dosage necessary to eliminate 50% of the IL-1β induced proliferation of RPMI 1788 cells.
Self-assembled monolayer preparation
A 5 nm Cr adhesion layer and a 45 nm Au layer were thermally evaporated (CHA Industries, Fremont, CA) on the inverted six-well tissue culture polystyrene plates (Nunc, Rochester, NY) without breaking vacuum to yield optically transparent gold-coated slides for optical microscope observation as shown in Figure 1c. Self-assembled monolayers (SAMs) were generated on the gold by incubation of the gold surface in a 2 mM solution of mercaptohexadecanoic acid (MHA, Sigma, St. Louis, MO) in ethanol overnight at 37.5 °C incubator. The SAMs were rinsed in ethanol followed by PBS. The bare SAM of MHA had a hydrophilic air/water contact angle of 15 ± 2°, measured as previously described [30].
Cytokine immobilization
Carboxyl groups on the SAM were activated for protein immobilization with a solution of 0.4 M 1-ethyl-3-(3dimethylamino-propyl) carbodiimide (EDC, Sigma, St. Louis, MO) and 0.1 M N-hydroxysuccinimide (NHS, Sigma, St. Louis, MO) in Dulbecco’s PBS for 15 min at room temperature. The activated SAMs were then rinsed with deionized water. An IL-1ra-ELP fusion protein was immediately immobilized on the SAMs by incubation with a 10 μg/ml solution of IL-1ra-ELP in PBS for 1 h on an orbital shaker, and rinsed with PBS [31]. Experiments employing SAMs with 0.87 ± 0.1 ng/mm2 immobilized IL-1ra-ELP are referred to as “Imm IL-1ra” in figures and tables, whereas experiments employing 1 μg/ml soluble IL-1ra-ELP are referred to as “Sol IL-1ra”. All samples were sterilized for cell culture by exposure to an ethanol solution for 10 min with subsequent rinsing with sterile PBS. Control surfaces consisted of untreated carboxyl SAMs. Bovine serum albumin (R&D Systems, Minneapolis, MN) was covalently immobilized on the MHA SAM as a secondary control by to the same procedure.
Surface Plasmon Resonance (SPR)
The quantitative SPR determination of protein surface concentration has been described in detail previously [32]. Experiments were carried out using a BIACORE X SPR system (Biacore Inc., Piscataway, NJ). IL-1ra-ELP solutions in PBS were brought into contact with EDC-NHS activated SAMs using a flow rate of 5μL/min for up to 20 min. After washing with PBS to remove loosely bound protein, the absolute coupling angle change, given in response units (RU), was converted to weight of adsorbed protein where 1000 RU (0.1 degree change) corresponds to 1 ng/mm2 of surface bound protein [33]. Incubation of EDC-NHS activated SAMs with 10 μg/mL of IL-1ra-ELP as described above yielded a surface density of 0.87 ± 0.1 ng/mm2. The amount of total protein in the six-well tissue culture plates was calculated to be 1.8 μg/well for immobilized IL-1ra-ELP and 6.0 μg/well for soluble IL-1ra-ELP.
Stability of immobilized IL-1ra-ELP
SAMs immobilized with 0.87± 0.1 ng/mm2 of IL-1ra-ELP were incubated in RPMI 1640 media for 5 days at 37 °C in an incubator in the absence of THP-1 monocytes. 100 μl of supernatant were collected at pre-determined time points and assayed for IL-1ra using LINCOplex detection kits (Linco Research, Inc., St. Charles, MO).
Estimated half-life of soluble IL-1ra-ELP
SAMs with soluble IL-1ra-ELP at a concentration of 1 μg/mL were incubated in the RPMI 1640 media for 5 days at 37 °C in the absence of THP-1 monocytes. 100 μl of supernatant was collected at pre-determined time point and assayed for IL-1ra using LINCOplex detection kits (Linco Research, Inc., St. Charles, MO).
Cell culture and cytokine expression
THP-1 human monocytes, purchased from the Duke University Cell Culture Facility (Durham, NC), were suspended in RPMI 1640 media that was supplemented with 10% FBS, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 10 mM HEPES, 4.5 g/L glucose, 100 U/mL penicillin G and 100 μg/mL streptomycin. 105 cells/mL were cultured on the gold coated surfaces of six-well tissue culture polystyrene plates with pairs of wells representing one of four different conditions: MHA SAM (SAM), MHA SAM with immobilized BSA (Imm BSA), MHA SAM with immobilized IL-1ra-ELP (Imm IL-1ra), and MHA SAM with soluble IL-1ra-ELP (Sol IL-1ra). Cells in half of the wells (one each of SAM, Imm BSA, Imm IL-1ra and Sol IL-1ra) were stimulated with 1 μg/mL LPS (Sigma, St. Louis, MO) at the time of cell seeding, while the cells in the other three wells were left unstimulated (three replicates). 1 μg/mL LPS was used because higher dosages of LPS led to cell death or disability, and lower LPS dosage showed little stimulation of cells (data not shown). 100 μl of cell culture supernatant collected from each condition at 1, 6, 24, 48 and 72 h post seeding was centrifuged at 1,500 rcf for 5 min to remove cell debris and was frozen at −80 °C till further use. The supernatant was thawed and assayed for the cytokines IL-1β, IL-4, IL-6, IL-8, TNF-α, IFN-γ, MCP-1, MIP-1α, IL-1ra, and VEGF using LINCOplex detection kits (Linco Research, Inc., MO) in a Bio-Plex Suspension Array System (Bio-Rad, Hercules, CA). The LINCOplex assay was shown to produce results comparable with ELISA assay [data not shown]. The level of IL-1β in culture supernatant was also tested using ELISA kits (R&D Systems, MN) because the expression level of IL-1β was below the detection limit of the LINCOplex assay.
Statistical Analysis
One-way ANOVA was used to evaluate the differences in the mean values among the treatment groups. All pair-wise multiple comparison procedures were performed by a Tukey’s post hoc test. P-values of less than 0.05 were considered to be significantly different.
Results
IL-1ra-ELP fusion protein bioactivity
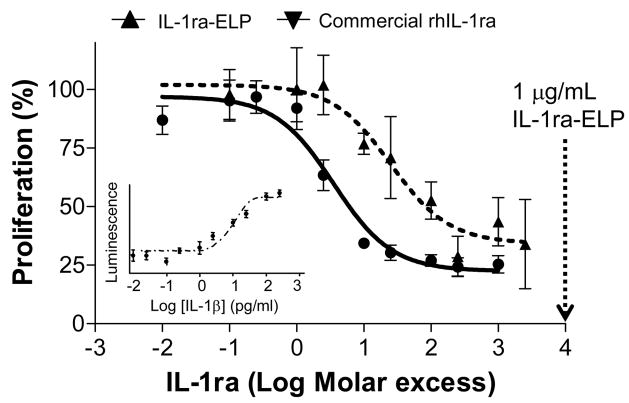
Figure 2 shows the results of the bioactivity comparison between IL-1ra-ELP fusion protein and commercially available rhIL-1ra, both of which showed significant inhibition of the stimulatory effect of IL-1β. Based on the standard curve of IL-1β induction of proliferation shown in the inset of Figure 2, 100 μg/mL IL-1β was necessary to induce proliferation of RPMI 1788 cells. Data for commercial rhIL-1ra and IL-1ra-ELP were fit to a nonlinear 4-parameter logistic equation to determine the EC50, the molar excess of inhibitor needed to inhibit 50% of the stimulatory effect of IL-1β
Figure 2.
(a) Bioactivity of IL-1ra-ELP fusion protein and commercial rhIL-1ra using the inhibition assay of IL-1β stimulated RPMI 1788 cells. The percentage of inhibited proliferation is plotted as a function of excess molar IL-1ra added in 10−2–104. The amount of inhibitor, IL-1ra, for inhibition of stimulatory effect of IL-1β is determined based on the 4-parameter sigmoidal fitting curve. EC50 of IL-1ra-ELP = 211.21 ± 0.31 pM (R2= 0.92) and EC50 Commercial rhIL-1ra = 20.21 ± 0.85 pM (R2= 0.97) Data presented as mean ± SE (n=4). Inset: standard curve of IL-1β induction of RPMI 1788 proliferation.
where Y0 is the minimal curve asymptote, i.e. minimum proliferation in this case, EC50 is the dosage necessary to eliminate 50% of the IL-1β induced proliferation and the Hillslope is the steepness of the dose-response curve.
Commercial rhIL-1ra yielded EC50=20.21±0.85 pM while IL-1ra-ELP yielded EC50= 211.21± 0.31 pM. This means commercial rhIL-1ra is 10-fold more effective than the 1L-1ra-ELP fusion protein in inhibiting IL-1β [28, 29]. The bioactivity data of IL-1ra-ELP fusion protein also suggests that a 10,000-fold excess of soluble IL-1ra-ELP at 1 μg/ml would significantly inhibit the action of IL-1ra in the LPS-stimulated THP-1 monocytes.
Stability of immobilized IL-1ra-ELP on the SAM
Table 1 contains the amount of IL-1ra-ELP and secreted IL-1ra detected in supernatant samples drawn from SAM, Imm IL-1ra, and Sol IL-1ra with and without THP-1 monocytes. For samples without THP-1 monocytes, IL-1ra-ELP was detected only for the trivial case of Sol IL-1ra (c), and only at 1 and 6 hrs. For samples with THP-1 monocytes, IL-1ra was detected for both immobilized (d) and soluble (e) IL-1ra-ELP, but only at 6, 24, 48 and 72 hrs. The level of IL-1ra detected in theses two cases (d and e) are very similar, suggesting the contribution of cleaved IL-1ra from the immobilized IL-1ra on the surface is a negligible contribution to the detected levels of the cytokine. This conclusion is further supported by the lack of IL-1ra-ELP detected from Imm IL-1ra without THP-1 monocytes (b). Therefore the NHS-EDC immobilization chemistry for IL-1ra-ELP molecules provides robust stability of IL-1ra-ELP on the SAM surface up to 5 days.
Table 1.
The amount of IL-1ra-ELP detected from SAM(a), Imm IL-1ra(b) and Sol IL-1ra(c) in the absence of THP-1 monocytes and secreted IL-1ra from the Imm IL-1ra(d) and Sol IL-1ra(e) in the presence of THP-1 monocytes over time course as detected using the LINCOplex assay. No IL-1ra on the Imm IL-1ra in the absence of THP-1 monocytes was detected over the time course tested up to 5 days showing the immobilized IL-1ra-ELP is stable on the SAM surfaces. Severed IL-1ra-ELP is the IL-1ra-ELP detached from the surface possibly due to unstable chemical coupling or enzymatic action from the monoctyes. Secreted IL-1ra is the IL-1ra secreted from THP-1 monocytes responding to the external stimuli. Added IL-1ra-ELP is the soluble IL-1ra-ELP directly added into the media. * The concentration of IL-1ra at 1 hr was close to the maximun detection limit of LINCOplex assay. Sol IL-1ra w/o THP-1 was tested up to 3 days and Sol IL-1ra with THP-1 was tested up to 5 days. N.D. not detected.
| Sampled IL-1ra and/or IL-1ra-ELP (ng/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | Samples | Detected proteins | 1hr | 6hr | 24hr | 48hr | 72hr |
| w/o THP-1 | (a) MHA SAM | N.A. | N.D. | N.D. | N.D. | N.D. | N.D. |
| (b) Imm IL-1ra | Severed IL-1ra-ELP | N.D. | N.D. | N.D. | N.D. | N.D. | |
| (c) Sol IL-1ra | Added IL-1ra-ELP | 50.28±6.36 | 0.30±0.10 | N.D. | N.D. | N.D. | |
| w/THP-1 | (d) Imm IL-1ra | Secreted IL-1ra+ severed IL-1ra-ELP | N.D. | 0.01±0.00 | 0.16±0.13 | 0.38±0.12 | 0.66±0.13 |
| (e) Sol IL-1ra | Secreted IL-1ra + added IL-1ra-ELP | N.D. | N.D. | 0.20±0.06 | 0.30±0.03 | 0.54±0.06 | |
Estimated half-life of soluble IL-1ra-ELP
Table 1 provides a rough estimate of the half-life for soluble IL-1ra-ELP added into the RPMI 1640 media. The concentration of IL-1ra-ELP sampled from SAMs incubated with soluble IL-1ra-ELP without THP-1 monocytes (Table 1c) decreased from ~ 50 ng/mL at 1 h to ~ 0.3 ng/mL at 6 h, indicating that the half life of the soluble IL-1ra-ELP in the RPMI 1640 is a few hours.
Table 1(c) and (e) also compares IL-1ra sampled from SAMs incubated with soluble IL-1ra-ELP without THP-1 monocytes and with THP-1 monocytes, respectively. The observation that no IL-1ra was detected at 1 and 6 h with THP-1 monocytes (e) suggests that the soluble IL-1ra-ELP added to the culture was scavenged initially by IL-1 receptors (IL-1R) of the THP-1 monocytes, but then reappears at later time points as THP-1 monocytes continue to secret IL-1ra (secreted IL-1ra).
Cell morphology of THP-1 monocytes
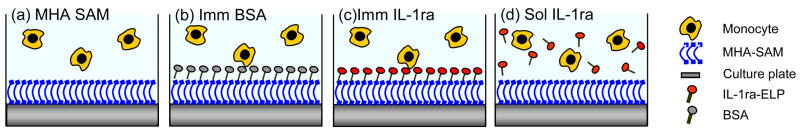
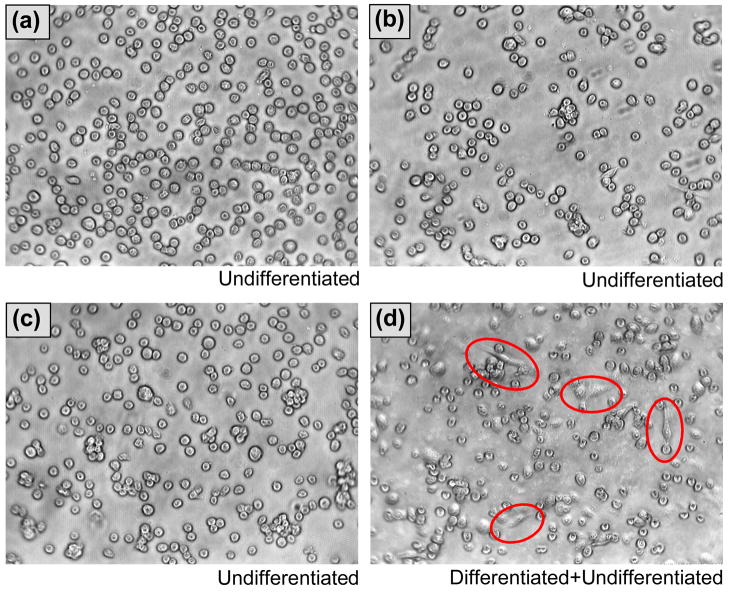
Figure 3 schematically illustrates the four experimental conditions used in the cell culture experiments, i.e. MHA SAM on gold (primary control), MHA SAM with immobilized BSA (secondary control), MHA SAM with immobilized IL-1ra-ELP, and MHA SAM with soluble IL-1ra-ELP added to the cell culture medium. Each surface was seeded with either LPS-stimulated or unstimulated THP-1 monocytes. Monocytes were stimulated by adding 1 μg/mL of LPS to the culture well at the time when the THP-1 monocytes were seeded. Figure 4(a)–(d) are light microscopy images of unstimulated THP-1 monocytes on a MHA SAM (a), and LPS-stimulated THP-1 monocytes on MHA SAMs with immobilized IL-1ra-ELP (b) and soluble IL-1ra-ELP (c), and LPS-stimulated THP-1 monocytes on a MHA SAM (d). Unstimulated THP-1 monocytes on the MHA SAM maintained a rounded, undifferentiated morphology, as expected for a hydrophilic surface. LPS stimulation caused a small number of cells (< 50 cells per well) to adopt a flattened and attached morphology at 72 h on the MHA SAM (Figure 3(d)); however, no flattened or attached LPS-stimulated cells could be found at 72 h in the presence of either immobilized or soluble IL-1ra-ELP (Figure 3(a)–(c)).
Figure 3.
Schematic illustration of human THP-1 monocytes interfering with the four experimental conditions used in the cell culture experiments. i.e. (a) MHA SAMs (SAM), (b) SAMs with immobilized BSA, (c) SAMs with immobilized IL-1ra-ELP (Imm IL1-ra), and (d) MHA SAMs with soluble IL-1ra (Sol IL-1ra), each of which were seeded with either presence or absence of 1 μg/ml of LPS. LPS added to the culture well at the time when the THP-1 monocytes were seeded.
Figure 4.
(a–d) phase contrast images of THP-1 monocytes to show the effect of immobilized and soluble IL-1ra-ELP fusion protein on THP-1 differentiation. Cell images cultured on (a) MHA SAM without LPS stimulation, (b) immobilized IL-1ra-ELP on the MHA SAM surface with LPS stimulation, (c) soluble IL-1ra-ELP (1 μg/ml) was directly added into the media with LPS stimulation and (d) MHA SAM with LPS stimulation, were collected 72 h after seeding. (a–c) all possessed a rounded, undifferentiated morphology. Cells circled in (d) indicate a flattened, differentiated morphology of THP-1 cells. Magnification 200X.
The morphologies of unstimulated THP-1 monocytes on the MHA SAM and the MHA SAM with immobilized BSA were identical as all cells exhibited a rounded, undifferentiated shape. Similar morphologies were also observed for LPS-stimulated THP-1 monocytes on the two controls, MHA SAM and MHA SAM with immobilized BSA (mostly rounded with few differentiated cells).
THP-1 cell viability determined by Trypan Blue dye exclusion for the MHA SAM (a) at 72 h (Figure 3) was 93% while that of LPS-stimulated THP-1 monocytes on the MHA SAM (d) and MHA SAMs with immobilized (b) and soluble IL-1ra-ELP (c) was 86%, as compared to around 95% viability at a time of cell seeding.
Cytokine expression assays
Table 2 qualitatively compares the relative expression levels of ten cytokines and growth factors assayed at 72 h from THP-1 monocytes with and without LPS stimulation that were exposed to: (1) MHA SAM control, and MHA SAMs with (2) immobilized IL-1ra-ELP, or (3) exposed to soluble IL-1ra-ELP. Very low levels of cytokines were observed for surfaces exposed to unstimulated THP-1 monocytes; at 72 h, the levels of IL-1β, TNF-α, IL-6, IL-8, MCP-1, IFN-γ, IL-1ra and IL-4 detected from the cell culture supernatant were less than 50 pg/mL, and the concentration of MIP-1α and VEGF were less than 100 pg/mL. In contrast, LPS stimulated THP-1 monocytes expressed detectable levels for seven out of ten cytokines under all surface conditions, while IL-1β, IFN-γ and IL-4 were undetectable under all conditions using the LINCOplex assay. IL-1β was therefore tested again with ELISA because of its clear link to the IL-1ra inhibition pathway. Of the seven detectable cytokines, only MCP-1 showed no modulation from the presence of immobilized or soluble IL-1ra-ELP. Conversely, IL-1β, TNF-α, IL-6, IL-8, MIP-1α, IL-1ra and VEGF showed differential expressions with and without LPS stimulation, and with and without the presence of IL-1ra-ELP.
Table 2.
Qualitative comparison of the relative expression levels of the 10 cytokines assayed at 72hrs under the four experimental conditions with and without LPS stimulation. *The level of IL-1β was tested using ELISA assay because of detection limit of Lincoplex. N.D. is non-detectable, i.e. zero. “+”, “++” and “+++” mean relatively low, moderate and high, respectively. “−” means detected less than 50 pg/ml for IL-1β, IL-4, IL-6, IL-8, TNF-α, IFN-γ, MCP-1 and IL-1ra and 100 pg/ml for MIP-1α and VEGF
| Samples | LPS Stimulated | No LPS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Treated | Control | Treated | ||||||
| Cytokines | MHA SAM | Imm BSA | Imm IL-1ra | Sol IL-1ra | MHA SAM | Imm BSA | Imm IL-1ra | Sol IL-1ra | |
| Inflammatory | IL-1β* | ++ | ++ | + | + | − | − | − | − |
| TNF-α | ++ | ++ | + | + | − | − | − | − | |
| IL-6 | ++ | ++ | + | + | − | − | − | − | |
| IL-8 | ++ | ++ | + | + | − | − | − | − | |
| MIP-1α | ++ | ++ | + | + | − | − | − | − | |
| MCP-1 | ++ | ++ | ++ | ++ | − | − | − | − | |
| IFN-γ | N.D | N.D | N.D | N.D | N.D | N.D | N.D | N.D | |
| Wound healing | VEGF | + | + | ++ | ++ | − | − | − | − |
| IL-1ra | + | +++ | ++ | ++ | − | − | − | − | |
| IL-4 | N.D | N.D | N.D | N.D | N.D | N.D | N.D | N.D | |
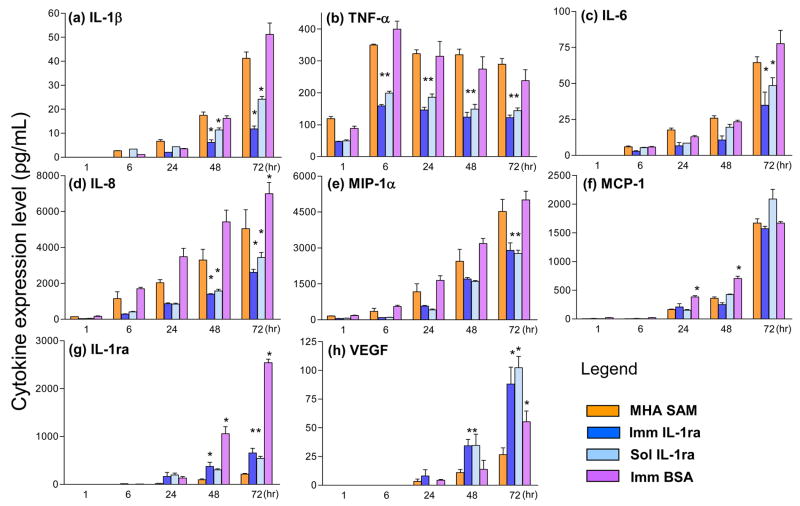
Figure 5 contains bar graphs of expression levels for the cytokines in Table 2 that were sampled above background: IL-1β, TNF-α, IL-6, IL-8, MIP-1α, MCP-1, IL-1ra and VEGF. These were detected under the four experimental conditions in Figure 3 with LPS-stimulated cells. All were detected using the LINCOplex assay, except for IL-1β that was detected by ELISA. ELISA was chosen to quantify the levels of IL-1β because the highest levels observed for IL-1β were well below the detection limit of the LINCOplex assay system. TNF-α expression was highest at 6 h and then decreased gradually over time, while IL-1β, IL-6, IL-8, MCP-1, MIP-1α, IL-1ra and VEGF expression increased over time up to 72 h. IL-1ra was not detected up to 6 h from SAMs with immobilized IL-1ra-ELP, further indicating the stability of the immobilized IL-1ra-ELP.
Figure 5.
Bar graphs of expression levels of IL-1β, TNF-α, IL-6, IL-8, MIP-1α, MCP-1, VEGF and IL-1ra at 1, 6, 24, 48 and 72 hrs from LPS-stimulated monocytes on MHA SAMs, SAMs with immobilized IL-1ra-ELP, and MHA SAMs with soluble IL-1ra-ELP as detected using the LINCOplex assay. IL-1β expression under the same conditions as measured by ELISA due to detection limit of the LINCOplex. * Significant relative to the SAM; p<0.05
Most importantly, the expression levels of pro-inflammatory cytokines IL-1β, TNF-α, IL-6 and IL-8, and the chemokine MIP-1α were significantly attenuated by both soluble and immobilized IL-1ra-ELP relative to the MHA SAM control (p < 0.05); whereas, the expression levels of pro-wound healing VEGF and anti-inflammatory IL-1ra were significantly enhanced by the IL-1ra-ELP fusion protein (p < 0.05). The expression level of the chemokine MCP-1 was not affected by the presence of soluble or immobilized IL-1ra-ELP.
Finally, the expression levels of IL-1β, TNF-α, IL-6, IL-8, MIP-1α and MCP-1 on immobilized BSA were not statistically different from the MHA SAM control at all time points (p < 0.05). Only anti-inflammatory IL-1ra at 48 and 72 hr, and pro-wound healing VEGF at 72 h, had expression levels on immobilized BSA that were statistically different from the MHA SAM control (p < 0.05).
Discussion
Pro-inflammatory IL-1 is produced by lymphocytes and monocytes in response to microbial invasion and tissue damage as a signal to other cells to adopt an inflammatory profile [9]. IL-1 induces its own gene upregulation and expression [34], and also stimulates production of IL-1 [34], TNF-α [35], IL-6 [36], and chemokines [37]. TNF-α also induces synthesis of IL-1 [38]. These positive feedback loops may contribute to the increased expression of inflammatory cytokines by LPS over time as consistently observed in this study.
IL-1ra, the primary mediator of cartilage degradation during osteoarthritis [39], recently has been developed as a protein drug that competitively inhibits the binding of IL-1 to IL-1Rs [40]. Human recombinant IL-1ra anti-inflammatory cytokine (Anakinra) has been administrated subcutaneously, and is known to be a safe and well-tolerated therapy [41]; however, the relatively short half life of this compound of four to six hours following administration is problematic. This short life time of injected IL-1ra results in a low efficacy compared to anti-TNF therapies. Therefore, the development of new approaches to elongate the half-life of IL-1ra is desirable.
LPS-stimulated THP-1 human monocytes is a well-established in vitro model of the monocytes/macrophages that are stimulated by endotoxin [42]. This model is commonly used to study response to IL-1 stimulation because they respond in a similar manner to peripheral blood monocytes and produce IL-1 at comparable levels [42]. The rapid induction of IL-1β mRNA is followed by an equally rapid clearance of the mRNA, leading to an equilibrium mRNA level that appears to be relatively independent of LPS dose used to stimulate the cells. This suggests that THP-1 human monocytes have established a precise mechanism for maintaining a constant level of IL-1 message and presumably of the IL-1 protein itself [43]. Furthermore, THP-1 cells may present a more homogenenous population of non-stimulated nomocytic cells than can be obtained from peripheral blood monocytes culture. However, it is well known that primary monocytes are more responsive than are cultured systems [44]. This may therefore provide some benefit in future studies.
The addition of IL-1ra to LPS-stimulated THP-1 monocytes has been shown to inhibit LPS-induced IL-1β synthesis [45]. The addition of IL-1ra before, at the same time, or 4 h after stimulation with LPS showed no significant difference on inhibition of cytokine production; however, the inhibition of LPS-induced IL-1 production was shown to be reduced when IL-1ra was added 8 h after LPS stimulation [45].
The bioactivity of the soluble fusion protein was demonstrated via inhibition of IL-1β-induced proliferation of RPMI 1788 cells. IL-1ra-ELP showed a 50% inhibition of 100 pg/mL IL-1β at 37-fold molar excess compared to a 4-fold molar excess for rhIL-1ra. The inhibition experiments also showed that a 10,000-fold molar excess of soluble IL-1ra-ELP (1μg/mL) would substantially inhibit the stimulatory effect of IL-1β.
The anti-inflammatory properties of IL-1ra-ELP were demonstrated by sampling cytokines from the supernatant of monocytes cultured on MHA SAMS on gold that were exposed to soluble or immobilized fusion protein. Unstimulated THP-1 monocytes maintained a rounded, undifferentiated morphology under all surface conditions. In contrast, LPS stimulation caused a small number of cells to adopt a flattened and attached morphology on bare SAMs; whereas no differentiated cells could be found on SAMs treated with soluble or immobilized IL-1ra-ELP. Images of LPS-stimulated monocytes exposed to SAMs with immobilized BSA also contained differentiated cells. While these morphological observations are somewhat empirical, they are clear indications that the immobilized fusion protein affects monocyte activation. Follow up studies could be made more precise if accompanied by assays for molecular markers of cell differentiation.
These morphological data suggest that no LPS-stimulated monocytes differentiated in the presence of the IL-1ra-ELP fusion protein are because they are binding to the IL-1ra moiety. Although the total fusion protein per well differs only by a factor of three between immobilized and soluble IL-1ra-ELP (1.8 μg/well and 6.0 μg/well, respectively), the tradeoff between IL-1ra density and accessibility may be quite different. For example, the soluble form likely has greater accessibility to IL-1 receptors (IL-1Rs), while the immobilized form has higher effective density at the surface but likely a reduced accessibility. Soluble fusion protein can also interact with both attached and detached cells and is removed from solution when bound, while immobilized fusion protein can only interact with cells that interrogate the surface and is not removed from the surface when bound.
In either case, there appears to be more than enough IL-1ra to affect cell function. A rough calculation starts with the observations that IL-1 in the femtomolar range can induce a response in monocytes by triggering only 2–3% IL-1R receptors [46], and that there are approximately 100 IL-1Rs per monocyte [7]. The number of IL-1Rs available per well in our experiments is estimated to be 6 × 107 molecules/well (105 cells/mL × 6 mL/well × 100 receptors/cell), while the number of IL-1ra-ELP ligands is estimated to be 2.0 × 1013 molecules/well for immobilized fusion protein (1.8 μg/well × (1.1 × 1013 molecules/μg)) and 6.6 × 1013 molecules/well for soluble fusion protein (6 μg/well × (1.1 × 1013 molecules/μg)).
Stability testing of immobilized IL-1ra-ELP on the MHA SAM in the absence of monocytes showed that immobilized IL-1ra-ELP was stable at the SAM surface. The stability of the NHS-EDC chemistry was supported by the lack of IL-1ra detected from in the supernatant of SAMs immobilized with fusion protein without monocytes for up to 5 days, and with THP-1 monocytes up to 6 h. The half-life of soluble IL-1ra-ELP in the media without THP-1 cells also appeared to be on the order of a few hours. It is interesting to note that no IL-1ra was detected at early time points in the supernatant of THP-1 cells with added soluble IL-1ra-ELP, while high levels of IL-1ra were detected in the supernatant without THP-1 cells. This may be due to binding of the soluble IL-1ra-ELP by the IL-1Rs on the membrane of the THP-1 monocytes. In either the immobilized or soluble cases, the IL-1a detected in supernatant with THP-1 monocytes at 24, 48 and 72 h is thus likely to be of cellular origin rather than from fusion protein added at the start of the experiment.
Conclusion
This paper provides in vitro evidence that the IL-1ra-ELP fusion protein is bioactive, and that the immobilized fusion protein is a potent mediator of the inflammatory profile of LPS-stimulated THP-1 human monocytes. The results of this study further indicate that immobilized IL-1ra has the potential to mediate the inflammatory response to the biomaterials surface. While these results are a compelling first demonstration of the potential of this strategy. and the temptation is great to over interpret these results or to suggest specific mechanisms. The current study was limited in that it examined only the gold standard anti-inflammatory cytokine, IL-1ra, against the gold standard in vitro model, THP-1 monocytes, on the gold standard model substrate, alkane thiol self-assembled monolayers. There are a number of unresolved issues that must be addressed before this approach can be translated to an in vivo therapy for implanted biomaterials. These are: (1) developing a better understanding of the signaling mechanism that induces this anti-inflammatory response to immobilized ligand by performing companion gene expression studies; (2) examining other candidate anti-inflammatory/pro-wound healing cytokines such as immobilized IL-10, TGF-β1, and soluble TNF-α receptor; (4) examining whether heterotypic signaling from multiple cytokines is a more potent signaling paradigm than monolithic single cytokine signaling; (5) optimizing cytokine immobilization to real-world biomaterials; (5) testing this effect in vitro on biomaterial substrates; and (6) performing animal studies to confirm the effect in vivo.
Acknowledgments
This research was supported by the NIH grants # DK054932 to W.M.R and GM61232 to A.C., the Whitaker Foundation, and Becton Dickinson and Company. The authors also thank to Dong-Woo Lim, Dr. Kimberly Carlson, and Professor David Pickup of Duke University for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirano Y, Mooney DJ. Peptide and protein presenting materials for tissue engineering. Adv Mater. 2004;16(1):17–25. [Google Scholar]
- 2.Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 3.Alsberg E, Anderson KW, Albeiruti A, Rowley JA, Mooney DJ. Engineering growing tissues. P Natl Acad Sci USA. 2002;99(19):12025–12030. doi: 10.1073/pnas.192291499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hubbell JA. Bioactive biomaterials. Curr Opin Biotech. 1999;10(2):123–129. doi: 10.1016/s0958-1669(99)80021-4. [DOI] [PubMed] [Google Scholar]
- 5.Prime KL, Whitesides GM. Self-assembled organic monolayers-model systems for studying adsorption of proteins at surfaces. Science. 1991;252(5009):1164–1167. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JM. Biological responses to materials. Ann Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 7.Thomson AW, Lotze MT. The cytokine handbook. San Diego: Academicpress; 2003. [Google Scholar]
- 8.Brodbeck WG, Voskerician G, Ziats NP, Nakayama Y, Matsuda T, Anderson JM. In vivo leukocyte cytokine mRNA responses to biomaterials are dependent on surface chemistry. J Biomed Mate Res A. 2003;64A(2):320–329. doi: 10.1002/jbm.a.10425. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. The Interleukin-1 family-10 years of discovery. Faseb J. 1994;8(15):1314–1325. [PubMed] [Google Scholar]
- 10.Bresnihan B, Alvaro-Gracia JM, Cobby M, Doherty M, Domljan Z, Emery P, Nuki G, Pavelka K, Rau R, Rozman B, Watt I, Williams B, Aitchison R, McCabe D, Musikic P. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41(12):2196–2204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Cominelli F, Pizarro TT. Interleukin-1 and interleukin-1 receptor antagonist in inflammatory bowel disease. Aliment Pharmacol Ther. 1996;10(Suppl 2):49–53. doi: 10.1046/j.1365-2036.1996.22164020.x. discussion 54. [DOI] [PubMed] [Google Scholar]
- 12.Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992;89(5):958–67. doi: 10.1016/0091-6749(92)90218-q. [DOI] [PubMed] [Google Scholar]
- 13.Shirakawa F, Tanaka Y, Oda S, Eto S, Yamashita U. Autocrine stimulation of interleukin 1 alpha in the growth of adult human T-cell leukemia cells. Cancer Res. 1989;49(5):1143–7. [PubMed] [Google Scholar]
- 14.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5(8):629–40. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 15.Economides AN, Carpenter LR, Rudge JS, Wong V, Koehler-Stec EM, Hartnett C, Pyles EA, Xu XB, Daly TJ, Young MR, Fandl JP, Lee F, Carver S, McNnay J, Bailey K, Ramakanth S, Hutabarat R, Huang TT, Radziejewski C, Yancopoulos GD, Stahl N. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat Med. 2003;9(1):47–52. doi: 10.1038/nm811. [DOI] [PubMed] [Google Scholar]
- 16.Preas HL, Reda D, Tropea M, Vandivier RW, Banks SM, Agosti JM, Suffredini AF. Effects of recombinant soluble type I interleukin-1 receptor on human inflammatory responses to endotoxin. Blood. 1996;88(7):2465–2472. [PubMed] [Google Scholar]
- 17.De Crescenzo G, Pham PL, Durocher Y, O’Connor-McCourt MD. Transforming growth factor-beta (TGF-beta) binding to the extracellular domain of the type II TGF-beta receptor: Receptor capture on a biosensor surface using a new coiled-coil capture system demonstrates that avidity contributes significantly to high affinity binding. J Mol Biol. 2003;328(5):1173–1183. doi: 10.1016/s0022-2836(03)00360-7. [DOI] [PubMed] [Google Scholar]
- 18.Piehler J, Schreiber G. Fast transient cytokine-receptor interactions monitored in real time by reflectometric interference spectroscopy. Anal Biochem. 2001;289(2):173–186. doi: 10.1006/abio.2000.4920. [DOI] [PubMed] [Google Scholar]
- 19.Seipelt I, Hoffmann SH, Schmidt J, Engels JW, Beckers T. Overexpression, purification, and use of a soluble human interleukin-4 receptor alpha-chain/Ig gamma 1 fusion protein for ligand binding studies - Characterization of ligand binding to soluble IL-4 receptor alpha-chain by surface plasmon resonance measurements and by microtiter-plate-based ELISA with biotinylated IL-4. Biochem Biophys Res Commun. 1997;239(2):534–542. doi: 10.1006/bbrc.1997.7509. [DOI] [PubMed] [Google Scholar]
- 20.Wu ZN, Johnson KW, Choi Y, Ciardelli TL. Ligand-binding analysis of soluble interleukin-2 receptor complexes by surface-plasmon resonance. J Biol Chem. 1995;270(27):16045–16051. doi: 10.1074/jbc.270.27.16045. [DOI] [PubMed] [Google Scholar]
- 21.Webb DJ, Crookston KP, Hall SW, Gonias SL. Binding of transforming growth factor-beta-1 to immobilized human alpha-2-macroglobulin. Arch Bio Chem Biophys. 1992;292(2):487–492. doi: 10.1016/0003-9861(92)90020-w. [DOI] [PubMed] [Google Scholar]
- 22.Fischer U, Hempel U, Becker D, Bierbaum S, Scharnweber D, Worch H, Wenzel KW. Transforming growth factor beta1 immobilized adsorptively on Ti6Al4V and collagen type I coated Ti6Al4V maintains its biological activity. Biomaterials. 2003;24(15):2631–41. doi: 10.1016/s0142-9612(03)00068-1. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y, Hasuda H, Yamauchi T, Komatsu N, Ikebuchi K. Immobilization of erythropoietin to culture erythropoietin-dependent human leukemia cell line. Biomaterials. 2004;25(12):2293–2298. doi: 10.1016/j.biomaterials.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Mann BK, Schmedlen RH, West JL. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22(5):439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 25.Merrett K, Griffith CM, Deslandes Y, Pleizier G, Dube MA, Sheardown H. Interactions of corneal cells with transforming growth factor beta 2-modified poly dimethyl siloxane surfaces. J Biomed Mater Res A. 2003;67(3):981–93. doi: 10.1002/jbm.a.10165. [DOI] [PubMed] [Google Scholar]
- 26.Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat Biotech. 1999;17(11):1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- 27.Chow DC, Dreher MR, Trabbic-Carlson K, Chilkoti A. Ultra-high expression of a thermally responsive recombinant fusion protein in E-coli. Biotech Prog. 2006;22(3):638–646. doi: 10.1021/bp0503742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandenabeele P, Declercq W, Libert C, Fiers W. Development of a Simple, Sensitive and Specific Bioassay for Interleukin-1 Based on the Proliferation of Rpmi 1788 Cells -Comparison with Other Bioassays for Il-1. J Immun Method. 1990;135(1–2):25–32. doi: 10.1016/0022-1759(90)90252-q. [DOI] [PubMed] [Google Scholar]
- 29.Vandenabeele P, Jayaram B, Devos R, Shaw A, Fiers W. Interleukin-1-Alpha Acts as an Autocrine Growth-Factor for Rpmi-1788, an Epstein-Barr Virus-Transformed Human B-Cell Line. Eur J Immun. 1988;18(7):1027–1031. doi: 10.1002/eji.1830180709. [DOI] [PubMed] [Google Scholar]
- 30.Smith JT, Viglianti BL, Reichert WM. Spreading diagrams for the optimization of quill pin printed microarray density. Langmuir. 2002;18(16):6289–6293. [Google Scholar]
- 31.Hyun J, Lee WK, Nath N, Chilkoti A, Zauscher S. Capture and release of proteins on the nanoscale by stimuli-responsive elastin-like polypeptide “switches”. J Am Chem Soc. 2004;126(23):7330–7335. doi: 10.1021/ja049721e. [DOI] [PubMed] [Google Scholar]
- 32.Stenberg E, Persson B, Roos H, Urbaniczky C. Quantitative-Determination of Surface Concentration of Protein with Surface-Plasmon Resonance Using Radiolabeled Proteins. J Colloid Interf Sci. 1991;143(2):513–526. [Google Scholar]
- 33.Smith JT, Tomfohr JK, Wells MC, Beebe TP, Kepler TB, Reichert WM. Measurement of cell migration on surface-bound fibronectin gradients. Langmuir. 2004;20(19):8279–8286. doi: 10.1021/la0489763. [DOI] [PubMed] [Google Scholar]
- 34.Dinarello CA, Ikejima T, Warner SJC, Orencole SF, Lonnemann G, Cannon JG, Libby P. Interleukin-1 Induces Interleukin-1.1. Induction of Circulating Interleukin-1 in Rabbits Invivo and in Human Mononuclear-Cells Invitro. J Immun. 1987;139(6):1902–1910. [PubMed] [Google Scholar]
- 35.Ikejima T, Okusawa S, Ghezzi P, Vandermeer JWM, Dinarello CA. Interleukin-1 Induces Tumor-Necrosis-Factor (Tnf) in Human Peripheral-Blood Mononuclear-Cells Invitro and a Circulating Tnf-Like Activity in Rabbits. J Infect Dis. 1990;162(1):215–223. doi: 10.1093/infdis/162.1.215. [DOI] [PubMed] [Google Scholar]
- 36.Content J, Dewit L, Poupart P, Opdenakker G, Vandamme J, Billiau A. Induction of a 26-Kda-Protein Messenger-Rna in Human-Cells Treated with an Interleukin-1-Related, Leukocyte-Derived Factor. Eur J Biochem. 1985;152(2):253–257. doi: 10.1111/j.1432-1033.1985.tb09191.x. [DOI] [PubMed] [Google Scholar]
- 37.Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, Appella E, Kung HF, Leonard EJ, Oppenheim JJ. Molecular-Cloning of a Human Monocyte-Derived Neutrophil Chemotactic Factor (Mdncf) and the Induction of Mdncf Messenger-Rna by Interleukin-1 and Tumor Necrosis Factor. J Exp Med. 1988;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinarello CA, Cannon JG, Wolff SM, Bernheim HA, Beutler B, Cerami A, Figari IS, Palladino MA, Oconnor JV. Tumor-Necrosis-Factor (Cachectin) Is an Endogenous Pyrogen and Induces Production of Interleukin-1. J Exp Med. 1986;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bresnihan B, Cunnane G. Interleukin-1 receptor antagonist. Rheum Dis Clin N Am. 1998;24(3):615. doi: 10.1016/s0889-857x(05)70029-6. [DOI] [PubMed] [Google Scholar]
- 40.Furst DE, Breedveld FC, Kalden JR, Smolen JS, Burmester GR, Bijlsma JW, Dougados M, Emery P, Keystone EC, Klareskog L, Mease PJ. Updated consensus statement on biological agents, specifically tumour necrosis factor alpha (TNFalpha) blocking agents and interleukin-1 receptor antagonist (IL-1ra), for the treatment of rheumatic diseases, 2004. Ann Rheum Dis. 2004;63(Suppl 2):ii2–ii12. doi: 10.1136/ard.2004.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat Rev Drug Discov. 2004;3(4):330–339. doi: 10.1038/nrd1342. [DOI] [PubMed] [Google Scholar]
- 42.Krakauer T, Oppenheim JJ. Interleukin-1 production by a human acute monocytic leukemia-cell line. Cell Immunol. 1983;80(2):223–229. doi: 10.1016/0008-8749(83)90111-9. [DOI] [PubMed] [Google Scholar]
- 43.Fenton MJ, Clark BD, Collins KL, Webb AC, Rich A, Auron PE. Transcriptional regulation of the human prointerleukin-1-beta gene. J Immunol. 1987;138(11):3972–3979. [PubMed] [Google Scholar]
- 44.Marsh CB, Moore SA, Pope HA, Wewers MD. IL-1ra suppresses endotoxin-induced IL-1-beta and TNF-alpha release from mononuclear phagocytes. Am J Physiol. 1994;267(1):L39–L45. doi: 10.1152/ajplung.1994.267.1.L39. [DOI] [PubMed] [Google Scholar]
- 45.Granowitz EV, Vannier E, Poutsiaka DD, Dinarello CA. Effect of interleukin-1 (Il-1) blockade on cytokine synthesis. 2. Il-1 receptor antagonist inhibits lipopolysaccharide-induced cytokine synthesis by human monocytes. Blood. 1992;79(9):2364–2369. [PubMed] [Google Scholar]
- 46.Arend WP, Welgus HG, Thompson RC, Eisenberg SP. Biological properties of recombinant human monocyte-derived interleukin-1 receptor antagonist. J Clin Invest. 1990;85(5):1694–1697. doi: 10.1172/JCI114622. [DOI] [PMC free article] [PubMed] [Google Scholar]