Abstract
The underlying mechanisms by which tumor cells are resistant to CTL-mediated apoptosis are not clear. Using a human model of B-cell non-Hodgkin's lymphoma (B-cell NHL), we show that intratumoral Treg cells inhibit the proliferation and granule production of activated autologous infiltrating CD8+ T cells. Our results also show that degranulation and subsequent cytotoxic activity of infiltrating CD8+ T cells exposed to lymphoma B cells is completely attenuated by the presence of intratumoral Treg cells. Furthermore, we show that increased numbers of intratumoral Treg cells correlates with the number of CD8+ T cells in biopsy specimens from patients with B-cell NHL, supporting the in vitro findings that intratumoral Treg cells inhibit proliferation of infiltrating CD8+ T cells. Taken together, these data indicate that human lymphoma B cells are sensitive to autologous CTL-mediated cell death but are protected by the inhibitory function of intratumoral Treg cells.
Introduction
B-cell non-Hodgkin's lymphoma (NHL) is characterized by an accumulation of malignant monoclonal B cells arrested at different stages of differentiation. The microenvironment in which NHL is typically found consists of secondary lymphoid organs that are replete with immune cells. Although numerous studies have focused on the biology of the malignant B cell, there is now a growing literature suggesting that tumor infiltrating immune cells may also play a role in determining the outcome of patients with B-cell NHL (1, 2). The cellular component of NHL consists predominantly of malignant B cells, T cells, and monocytes. The specific role of intratumoral T cells and their significance in the pathogenesis of the NHL is not well understood, but there is increasing evidence indicating their relevance (3). In the nonmalignant scenario, T cells play a critical role in regulation of the immune response; yet, in the tumor microenvironment, these same T cells are unresponsive and fail to function appropriately (4, 5). The presence of an immunosuppressive network within the tumor microenvironment may therefore allow tumor cells to grow unchecked by the immune system. In murine models of NHL, it has been shown that induction of antigen-specific T-cell tolerance is one mechanism by which antitumor immunity is disrupted (6-8). Other underlying mechanisms that foster a poor immune response include reduced quality of tumor-associated antigen (TAA) presentation, aberrant cytokine and inflammatory molecule expression, and a shift in costimulatory and coinhibitory molecule expression by TAA-presenting, antigen-presenting cells (9).
An additional mean by which tumors escape immunosurveillance is through an imbalance in the number of regulatory T cells (Treg cells). An accumulation of Treg cells in the tumor microenvironment has been shown to have a significant effect on the functional properties of infiltrating CD4+ T cells and patient outcome (3, 10, 11). In previous work, we have found that Treg cells (CD4+CD25+ T cells with intracellular Foxp3 and CTLA-4 expression) were overrepresented in the tumor sites of NHL. We also found that nearly all CD4+CD25+ T cells isolated from NHL tumors expressed Foxp3, and that intratumoral Treg cells could suppress the functional capabilities, proliferation, and production and secretion of IFN-γ and interleukin-4, of autologous infiltrating CD4+CD25- T cells (3). In accordance with this data, Zhou et al. showed in a murine lymphoma model that tumor induced suppression of CD4+ T cells was regulated in part by expansion of the Treg pool (12). Taken together, these data suggest that the presence of Treg cells within the tumor microenvironment results in a dampening of the CD4+ T-cell response. What remains unanswered is whether or not Treg cells directly affect tumor-associated CD8+ CTL (CD8+ CTL) function, and more importantly, the ability of CTLs to kill autologous tumor cells.
Previous studies have shown that lymphoma B cells are resistant to CTL-mediated cell death (3-5), and this has been attributed to changes in their genetic or protein expression profile (3-5, 13, 14) as well as to abnormal CTL function (15-17). Additionally, there is accumulating data suggesting that Treg cells are capable of inhibiting CD8+ T cells under different pathologic conditions such as infection (18, 19) or allograft transplantation in vivo (20, 21). Murine studies indicate that Treg cells can abrogate CD8+ T cell-mediated tumor rejection by specifically suppressing cytotoxicity of expanded CD8+ cells (22-24). However, little is known about the effect of intratumoral Treg cells on CTL activity in a human NHL model.
To determine the significance of Treg cells on CD8+ T-cell function, we characterized the role of Treg cells in modulating antitumor responses. We show that intratumoral Treg cells isolated from NHL tumors completely inhibit proliferation and granule production of infiltrating CD8+ T cells obtained from the same tumor specimen. Suppression of CD8+ function by Treg cells results in an inability of tumor specific CD8+ T cells to kill autologous tumor cells. We therefore show for the first time that infiltrating CD8+ T cells from NHL tumors are functional, and that their activity is suppressed when Treg cells are present.
Materials and Methods
Patient samples
Patients providing written informed consent were eligible for this study if they had a tissue biopsy that on pathologic review showed B-cell lymphoma and adequate tissue to perform experiments. The biopsy specimens were reviewed and classified using the WHO Lymphoma classification. This study was approved by the Institutional Review Board of the Mayo Clinic/Mayo Foundation. Biopsy specimens from a total of 28 patients were used for the study (see Table 1).
Table 1.
Patient characteristics
| No. | Age | Gender | Histology | Treatment | Site of disease |
|---|---|---|---|---|---|
| MF1059 | 66 | male | Mantle cell lymphoma | Untreated | Extranodal |
| MF1065 | 52 | male | Follicular grade 1 | Chemotherapy | Extranodal |
| MF1076 | 69 | male | Large cell lymphoma | Untreated | Lymph node |
| MF1077 | 67 | female | Follicular grade 3 | Chemotherapy | Lymph node |
| MF1093 | 42 | male | Follicular grade 1 | Untreated | Lymph node |
| MF1115 | 72 | male | Small lymphocytic lymphoma | Untreated | Extranodal |
| MF1128 | 60 | male | Follicular grade 2 | Chemotherapy | Extranodal |
| MF1139 | 58 | female | Marginal zone lymphoma | Untreated | Extranodal |
| MF1143 | 44 | male | Small lymphocytic lymphoma | Rituximab | Lymph node |
| MF1160 | 86 | male | Small lymphocytic lymphoma | Chemotherapy | Lymph node |
| MF1171 | 62 | male | Follicular grade 2 | Chemotherapy | Extranodal |
| MF1196 | 64 | male | Small lymphocytic lymphoma | Untreated | Extranodal |
| MF1200 | 62 | male | Small lymphocytic lymphoma | Untreated | Extranodal |
| MF1201 | 54 | male | Mantle cell lymphoma | Chemotherapy | Lymph node |
| MF1240 | 59 | female | Marginal zone lymphoma | Untreated | Extranodal |
| MF1243 | 58 | female | Follicular grade 3 | Chemotherapy | Lymph node |
| MF1252 | 67 | male | Lymphoplasmacytic | Untreated | Lymph node |
| MF1260 | 70 | female | Mantle cell lymphoma | Chemotherapy | Extranodal |
| MF1261 | 64 | male | Follicular grade 1 | Untreated | Extranodal |
| MF1267 | 69 | female | Marginal zone lymphoma | Untreated | Extranodal |
| MF1270 | 54 | female | Small lymphocytic lymphoma | Untreated | Lymph node |
| MF1275 | 61 | female | Marginal zone | Chemotherapy | Extranodal |
| MF1297 | 70 | male | Follicular grade 2 | Untreated | Extranodal |
| MF1314 | 73 | male | Marginal zone lymphoma | Untreated | Extranodal |
| MF1319 | 59 | male | Large cell lymphoma | Chemotherapy | Lymph node |
| MF1327 | 50 | male | Small lymphocytic lymphoma | Untreated | Extranodal |
| MF1332 | 72 | male | Marginal zone lymphoma | Untreated | Extranodal |
| MF1341 | 62 | male | Mantle cell lymphoma | Chemotherapy | Lymph node |
Cell isolation and purification
CD8+ T cells and CD19+ lymphoma B cells were isolated by using CD8 and CD19 microbeads (positive selection). CD4+CD25- or CD4+CD25+ T-cell subsets were purified by using CD4+CD25+ Regulatory T-cell Isolation kit (Miltenyi Biotec, Auburn, CA) as previously described (3). Purity was checked by fluorescence-activated cell sorting (FACS) analysis and was typically >95%.
Immunohistochemistry
Paraffin-embedded tissue was obtained from Mayo Clinic Tissue Registry and cut serially at 5 μm. The tissue sections were deparaffinized in three changes of xylene and cleared through graded ethanol series. Endogenous peroxidase was quenched by incubation in 50% methanol/H2O2. After rinsing with tap water, all sections were pretreated 30 minutes with 50 mmol/L EDTA (pH 8) using a steamer and cooled for an additional 5 minutes. All immunohistochemical staining was done automatically on DAKO (Carpinteria, CA) Autostainerplus using the following antibodies and their corresponding detection systems: Foxp3 (Abcam, Cambridge, MA; 1 μg/mL, DAKO Advance+ DAB+), CD25 (Novocastra, Norwell, MA; 1:100, DAKO Advance+, DAB+), CD8 (DAKO; 1:100, DAKO Advance+, DAB+), CD3 (DAKO polyclone; 1:200, DAKO Advance+, DAB+), CD4 (Novocastra; 1:300, DAKO Advance+, DAB+), CD20cy (DAKO; 1:60, DAKO Advance+, DAB+), or mouse IgG1 control (DAKO; 1 μg/mL, DAKO Envision+, DAB+). All sections were stained with hematoxylin and rinsed well in tap water. All slides were observed with light microscopy (Olympus AX70, ×200/aperture 0.46, ×400/aperture 0.75, ×600/aperture 0.80; Olympus America, Melville, NY) with images captured with a SPOT RT camera and software (Diagnostic Instruments, Burlingame, CA).
Flow cytometry and intracellular staining
Cells (1 × 106) were washed in PBS containing 0.5% bovine serum albumin and incubated with CD3-, CD4-, CD8-, CD19-, and CD25-specific fluorochrome-conjugated antibodies and analyzed on a FACSCalibur flow cytometry (Becton Dickinson, San Diego, CA). For intracellular staining of perforin (PFN) and granzyme B (GzmB), cells were fixed with 2% paraformaldehyde for 15 minutes, washed, permeabilized with 0.5% saponin for 30 minutes, and stained with FITC-CD3, Percp-CD8, and phycoerythrin-conjugated PFN (1:1,000 dilution; BD PharMingen, San Diego, CA), or APC-conjugated Gzm B (1:200 dilution; GB12; Caltag, Burlingame, CA) for 30 minutes at 4°C. After washing, cells were analyzed by flow cytometry. Isotype controls were done for each sample.
Carboxyfluorescein succinimidyl ester labeling and T-cell proliferation assay
Cells were washed, counted, and resuspended at 1 × 107/mL in PBS. A stock solution of carboxyfluorescein succinimidyl ester (CFSE; 5 mmol/L) was diluted 1:100 with PBS and added to the cells for a final concentration of 5 μmol/L. After 10 minutes at 37°C, cells were washed thrice with 10 volumes of PBS containing 10% fetal bovine serum. CFSE-labeled responding cells were cocultured with or without stimulating cells in the presence or absence of phytohemagglutinin (PHA; 2.5 μg/mL) at 37°C and 5% CO2. Cells were harvested at day 3, washed, and stained with fluorochrome-conjugated antibodies for detection of surface markers for 30 minutes at 4°C. Cells were analyzed by flow cytometry. The percentage of CFSEdim was measured and calculated as the percentage of proliferated cells.
Degranulation assay
Degranulation assays were done by determining CD107a surface mobilization on infiltrating CD8+ T cells from B-cell NHL during activation or exposure to lymphoma B cells. The assay was set up based upon a similar approach described by Betts et al. with a few modifications (25). Lymphoma B cells were plated at 2 × 105 per well in a 48-well plate and incubated at 37°C for overnight. On the next day, the culture supernatant was removed from the wells, and the effector cells were added to the wells at various effector/target (E/T) ratios. Effector cells: (a) CD8+ T cells without any treatment (CD8-R), (b) CD8+ T cells activated with PHA (2.5 μg/mL) for 5 days, (c) CD8+ T cells cocultured with CD4+CD25- T cells in the presence of PHA (2.5 μg/mL) for 5 days, (d) CD8+ T cells cocultured with intratumoral Treg cells in the presence of PHA (2.5 μg/mL) for 5 days. Control wells containing either only effector cells or tumor targets were also set up with each assay. CD107a-PE (10 μL) was added to each well at the same time as addition of effector cells. The plate was then centrifuged for 3 minutes at 1,000 rpm to facilitate immediate contact between the effector cells and the tumor targets at the bottom of the wells and incubated at 37°C for overnight. At the end of the incubation period, the cells were stained with anti-human CD8-Percp antibody and analyzed using a FACSCalibur flow cytometry.
Cytotoxicity assay
A flow-based cytotoxicity assay was used to measure in vitro cellular cytotoxicity of infiltrating CD8+ T cells against lymphoma B cells as previously described (26, 27). Target cells (autologous lymphoma B cells or lymphoma B-cell lines) were labeled with 250 nmol/L of CFSE and added to 48-well plate (2 × 105 per well) along with different amount of effector cells in complete RPMI for 24 hours. Effector cells: (a) CD8+ T cells without any treatment (CD8-R), (b) CD8+ T cells activated with PHA (2.5 μg/mL) for 5 days, (c) CD8+ T cells cocultured with CD4+CD25- T cells at 2:1 ratio in the presence of PHA (2.5 μg/mL) for 5 days, (d) CD8+ T cells cocultured with intratumoral Treg cells at 2:1 ratio in the presence of PHA (2.5 μg/mL) for 5 days. CD8+ T cells cocultured with CD4+CD25+/- T cells were subjected to CD8-positive selection on the day CD8+ T cells were exposed to target cells. In parallel, target cells were incubated alone to measure basal apoptosis. Immediately before analysis, 1 μg/mL ( final concentration) of 7-amino-actinomycin D (7-AAD; Calbiochem, La Jolla, CA) was added to each sample and incubated for 20 minutes at 4°C in the dark. The percentage of apoptotic (7-AADlo+7-AADhi) cells are used to calculate the percentage of specific lysis according to the following formula: % specific lysis = 100 × (% sample lysis - % basal lysis) / (100 - % basal lysis). Sample lysis is the cell lysis in the presence of effectors at a given E/T ratio, and basal lysis is the cell lysis in the absence of effectors.
Statistical analysis
Fisher's exact test was used to compare differences in nominal variables, whereas the rank sum test, paired Student's t test, or the Kruskal-Wallis test was used for continuous variables. P < 0.05 was considered significant. Direct correlation between percentage of CD4+CD25+ T cells and CD8+ T cells was done with the use of regression analysis.
Results
Foxp3 expression in B-cell NHL
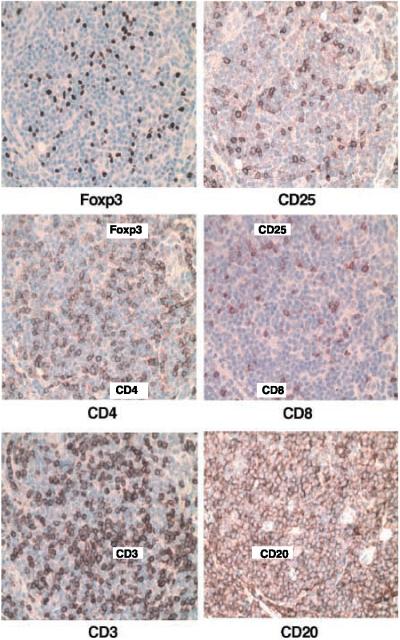
In previous work, we identified a subset of CD4+CD25+ Foxp3+ T cells in the biopsy specimens of B-cell NHL by flow cytometry (3). In the present study, we did immunohistochemistry to confirm the presence of regulatory T cells in NHL tissue. A series of tissue slides (serial sections) from NHL tumor biopsy specimens (n = 11) were stained with antibodies specific for Foxp3, CD25, CD4, CD8, CD3, and CD20. Each specimen also stained with an isotype control and minimal to no staining was detected (data not shown). As shown in a representative case in Fig. 1, CD3+ T cells, including both CD4+ and CD8+ subsets, are present, together with malignant CD19+ B cells, in lymphoma tissue. As expected, cells expressing Foxp3 or CD25 were also present. Although Foxp3-expressing cells could be detected in all specimens tested, the number and pattern, perifolliclar or intrafollicular, varied between specimens (data not shown). The staining pattern of Foxp3+ cells correlated well with the staining of CD25+ cells. This data is in accordance with our flow cytometry results showing that the vast majority of CD4+CD25+ T cells in NHL tumors express Foxp3 and are therefore Treg cells (3).
Figure 1.
Identification of Foxp3-expressing cells in biopsy specimens of patients with B-cell NHL. Immunohistochemical analysis of Foxp3 expression in NHL tumor tissue was done as described in Materials and Methods. Expression of CD25, CD4, CD8, CD3, and CD20 was also performed. From the same region of a serial section of the tumor specimen. Original magnification,×40. Representative specimen (n = 11).
Intratumoral Treg cells suppress proliferation of autologous infiltrating CD8+ T cells in B-cell NHL
We next wanted to determine the influence of Treg cells on proliferation of CD8+ T cells. Infiltrating CD8+ T cells (n = 3; responding cells) were labeled with CFSE and cultured either alone or with intratumoral Treg cells (stimulating cells) in the presence (activated) or absence (resting) of PHA at a ratio of 4:1 and 2:1 (responding cells/stimulating cells). As a control, CD8+ T cells were also cultured in the same conditions with infiltrating CD4+CD25- T cells. Our results show that intratumoral Treg cells completely suppress proliferation of PHA-activated infiltrating CD8+ T cells at both a 4:1 and 2:1 ratio (Fig. 2). Of note, infiltrating CD4+CD25- T cells partially inhibited proliferation of PHA-activated infiltrating CD8+ T cells, reducing it from 28% to 16% at a 4:1 ratio and 20% to 10% at a 2:1 ratio when compared with CD8+ T cells cultured alone, suggesting that some infiltrating CD4+CD25- T cells in biopsy specimens of B-cell NHL also exhibit inhibitory function.
Figure 2.
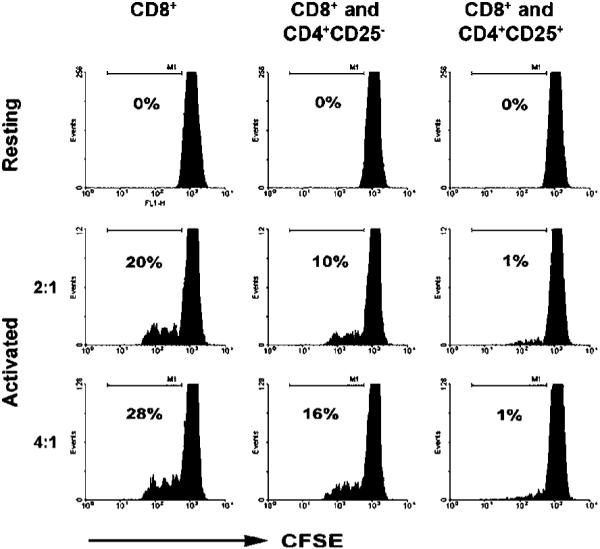
Intratumoral Treg cells inhibit the proliferation of infiltrating CD8+ T cells in B-cell NHL. Proliferation of infiltrating CD8+ T cells was determined by coculturing CFSE labeled CD8+ T cells with autologous CD4+CD25- or CD4+CD25+ T cells in the presence (activated) or absence of PHA (resting) for 3 days. The percentage of cells that lost CFSE expression were considered to have undergone proliferation. Representative of three independent experiments.
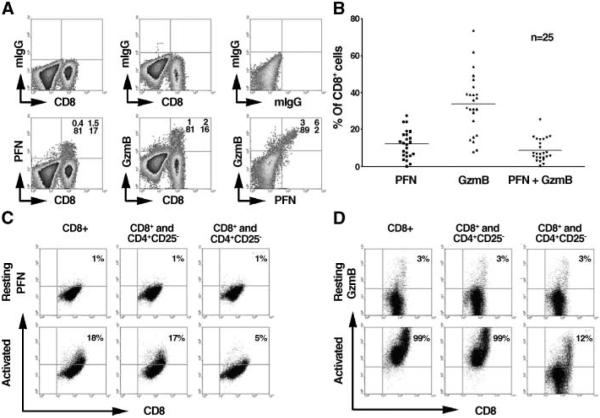
Intratumoral Treg cells inhibit the production of PFN and GzmB by infiltrating CD8+ T cells in B-cell NHL
Synthesis of cytolytic granules (PFN and GzmB) by CD8+ T cells is critical to their ability function as cytotoxic T cells. Therefore, our next goal was to determine the effect of intratumoral Treg cells on cytolytic granule production by infiltrating CD8+ T cells. First, we determined the intracellular expression profile of PFN and GzmB in infiltrating CD8+ T cells from fresh biopsy specimens using multicolor intracellular staining (Fig. 3A). Mononuclear cells isolated from NHL biopsy specimens were costained with anti-bodies specific to CD3, CD8, and PFN or GzmB. Matched isotype controls were used to distinguish PFN+, GzmB+, or PFN+GzmB+ cells. As shown in Fig. 3A, PFN or GzmB were exclusively expressed by infiltrating CD8+ T cells. PFN and GzmB expression could be detected individually and in some cases was found to be coexpressed. Data acquired from 25 fresh NHL samples indicate that the mean percentage of CD8-positive T cells that were PFN+ was 10% (10 ± 6%), GzmB+ 38% (38 ± 15%), and PFN+GzmB+ 8% (8 ± 6%; Fig. 3B). These data suggest that a majority (>50%) of resting infiltrating CD8+ T cells do not express PFN or GzmB.
Figure 3.
Intratumoral Treg cells dose-dependently inhibit the production of PFN and GzmB by infiltrating CD8+ T cells in B-cell NHL. A, intracellular staining of PFN or GzmB expression as well as coexpression of PFN and GzmB in CD8+T cells from fresh biopsy specimens of B-cell NHL. Isotype controls are shown for each sample (top). B, summary of the percentage of CD8+ T cells expressing PFN, GzmB, or both from 25 patient samples. C to D, intracellular expression of PFN (C) and GzmB (D) in infiltrating CD8+ T cells cocultured either alone or with infiltrating CD4+CD25- T cells or intratumoral Treg cells in the presence or absence of PHA for 3 days. Representative of three independent experiments.
Next, we determined the influence of Treg cells on PFN and GzmB expression (Fig. 3C and D). Therefore, we cultured infiltrating CD8+ T cells alone, with intratumoral Treg cells, or with infiltrating CD4+CD25- T cells at a ratio of 4:1 in the presence or absence of PHA (n = 3). The cells were then subjected to intracellular staining for PFN and GzmB and analyzed by flow cytometry. As shown in Fig. 3C and D, 1% of resting purified infiltrating CD8+ T cells expressed intracellular PFN and 3% expressed GzmB. As expected, activation with PHA dramatically increased intracellular expression of PFN from 1% to 18% and GzmB from 3% to 99%. When CD8+ T cells were cocultured with intratumoral Treg cells, we saw attenuation of activation-induced expression of PFN from 18% to 5%. Similarly, GzmB expression was decreased from 99% to 12%. In the presence of infiltrating CD4+CD25- T cells, activation-induced expression of PFN and GzmB by infiltrating CD8+ T cells remained unchanged. These findings indicate that intratumoral Treg cells strongly inhibit the production of PFN and GzmB granules by infiltrating CD8+ T cells in B-cell NHL.
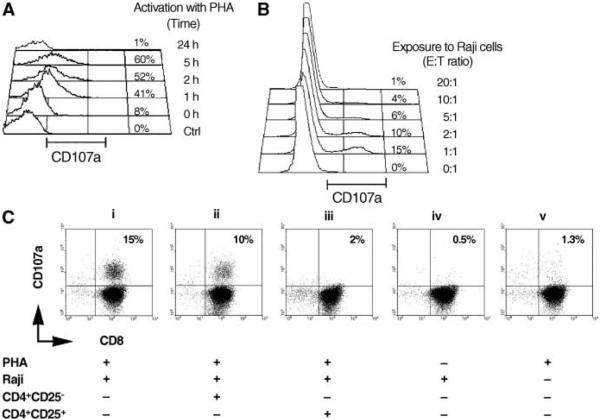
Intratumoral Treg cells inhibit the degranulation of infiltrating CD8+ T cells in B-cell NHL
We next sought to determine the effect of intratumoral Treg cells on granule release by infiltrating CD8+ T cells. Degranulation is an essential mechanism by which CD8+ T cells lyse tumor cells, and it has been shown that granule release is accompanied by the surface mobilization of CD107a (lysosome associated-membrane protein-1) on cytolytic cells. Therefore, CD107a surface expression has been used as a surrogate marker of degranulation by cytolytic cells such as natural killer cells (28, 29) and CTLs (25, 30). When we examined CD107a expression on resting or PHA activated infiltrating CD8+ T cells, we saw that a negligible amount of surface expression was detectable on resting cells. However, activation of infiltrating CD8+ T cells using PHA increased the surface expression of CD107a (Fig. 4A). No effect on CD107b expression was seen (data not shown). CD107a surface expression on infiltrating CD8+ T cells reached its highest level after 5 hours of stimulation and declined to undetectable levels after 24 hours. This is in agreement with the observation that CD107a surface expression is transient (31). Furthermore, when infiltrating CD8+ T cells were exposed to lymphoma B cells (Raji cells), CD107a surface expression was significantly up-regulated, particularly with an E/T ratio of 1:1 (Fig. 4B).
Figure 4.
Intratumoral Treg cells inhibit the degranulation of infiltrating CD8+ T cells in B-cell NHL. A, expression of CD107a on PHA-activated infiltrating CD8+ T cells in freshly isolated biopsy specimens of B-cell NHL at the indicated time points. B, CD107a expression on infiltrating CD8+ T cells exposed to Raji cells at the indicated E/T ratios. C, CD107a surface expression on activated CD8+ T cells exposed to Raji cells alone (i) or in the presence of CD4+CD25-T cells (ii)orTreg cells (iii). Resting CD8+T cells (iv) or activated CD8+ T cells in the absence of Raji (v) serve as negative controls.
Next, we determined the effect of intratumoral Treg cells on CD107a expression on infiltrating CD8+ T cells exposed to tumor cells (Fig. 4C). Before exposure to Raji cells, PHA-activated CD8+ T cells were cocultured either alone (i) or with infiltrating CD4+CD25- T cells (ii) or intratumoral Treg cells (iii). Raji cells were added to the cultures, and CD107a expression on CD8+ T cells was assessed. CD107a expression on resting CD8+ T cells exposed to Raji cells (iv), and activated CD8+ T cells without exposure to Raji cells (v) served as controls. When activated CD8+ T cells were exposed to Raji cells (i), CD107a expression increased (15 ± 2%, n = 5) compared with that found on activated cells alone (v; 1.3 ± 0.2%, n = 5) or on resting cells exposure to Raji cells (iv; 0.5 ± 0.15%, n = 5). When intratumoral Treg cells were added to the culture, CD107a surface expression was significantly reduced (2 ± 0.6%, n = 5). We saw a moderate inhibition of CD107a surface expression on activated CD8+ T cells when the cells were exposed to infiltrating CD4+CD25- T cells (ii; 10 ± 1.6%, n = 5). These results indicate that intratumoral Treg cells attenuate CD107a surface expression on activated infiltrating CD8+ T cells during exposure to tumor cells.
Intratumoral Treg cells inhibit the ability of infiltrating CD8+ T cells to lyse lymphoma B cells
Because intratumoral Treg cells inhibit the proliferation, granule production, and degranulation of infiltrating CD8+ T cells, we next wanted to determine the effect of intratumoral Treg cells on cytotoxicity of infiltrating CD8+ T cells when exposed to lymphoma B cells. We first examined the ability of resting and activated CD8+ T cells to lyse lymphoma B cell lines Raji, DoHH2, and Karpas. As shown in Fig. 5A, resting CD8+ T cells were not capable of killing target cells. However, activation of infiltrating CD8+ T cells with PHA induced a significant cytotoxic response to the lymphoma B cell lines compared with resting CD8+ T cells (n = 3, P = 0.0002), although the cytotoxic activity was different among three lines. Activated CD8+ T cells killed DoHH2 and Raji cells in a dose-dependent fashion with nearly complete destruction of target cells at an E/T ratio 50:1.
Figure 5.
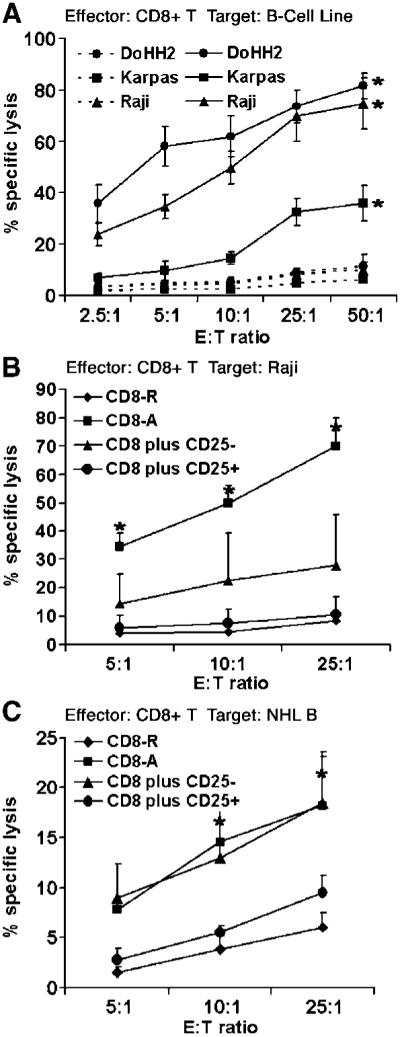
Intratumoral Treg cells inhibit the cytotoxicity of infiltrating CD8+ T cells to lymphoma B cells. A, cytotoxic activity, measured by specific lysis of targets cells, was analyzed in activated (solid line) or resting (dashed line) CD8+ T cells at the indicated E/T ratio. Target cells include DoHH2, Karpas, and Raji cells (n = 3). Cytotoxic activity of resting or activated infiltrating CD8+ T cells cocultured with CD4+CD25- or CD4+CD25+ T cells toward Raji (n =3; B) or autologous lymphoma B-cells (n =6; C) at the indicated E/T ratios. The percentage of 7-AAD+ cells was used to calculate the % lysis of target cells by CD8+ T cells. Points, mean; bars, SD. *, P < 0.05.
We then wanted to determine the effect of intratumoral Treg cells on the cytotoxic activity of activated infiltrating CD8+ T cells (Fig. 5B). Using Raji cells as a target, we cocultured activated infiltrating CD8+ T cells treated with intratumoral Treg cells or infiltrating CD4+CD25- T cells at E/T ratios of 5:1, 10:1, and 25:1. We found that activated CD8+ T cells showed significant dose-dependent cytolytic activity toward Raji cells. Coculture of activated infiltrating CD8+ T cells with intratumoral Treg cells (n = 3) attenuated the cytotoxic activity against Raji cells (11 ± 6.06% at E/T ratio 25:1) compared with activation-induced CD8+ T cells alone (69.8 ± 10.01% at E/T ratio 25:1, P = 0.00183) or with CD4+CD25- cells (27.7 ± 7.9% at E/T ratio 25:1, P = 0.056). In the presence of intratumoral Treg cells, the CD8+ T-cell cytolytic activity was similar to the cytolytic activity seen in unstimulated CD8+ T cells.
Next, we examined the influence of intratumoral Treg cell on the cytotoxic activity of infiltrating CD8+ T cells toward autologous lymphoma B cells. As shown in Fig. 5C, coculture of infiltrating activated CD8+ T cells with intratumoral Treg cells attenuated the cytotoxic activity against autologous lymphoma B cells (9.6 ± 1.62% at E/T ratio 25:1, n = 6) compared with activation-induced CD8+ T cells alone (18 ± 5% at E/T ratio 25:1, P = 0.015). Coculture of infiltrating CD8+ T cells with CD4+CD25- T cells had minimal effect of CTL activity compared with activated CD8+ T cells alone (18 ± 4.2% at E/T ratio 25:1, P = 0.497). These results indicate that intratumoral Treg cells strongly suppress the cytotoxic function of infiltrating CD8+ T cells and suggest that infiltrating CD8+ T cells could successfully destroy autologous lymphoma B cells were it not for the protective suppressive effect of intratumoral Treg cells present at the site of B-cell NHL.
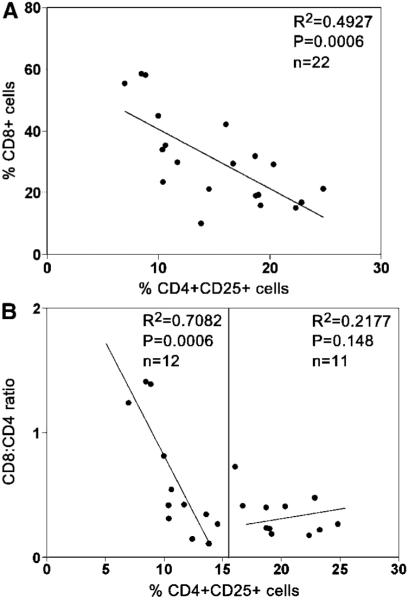
Increased numbers of intratumoral Treg cells are associated with decreased infiltrating CD8+ T-cell numbers in B-cell NHL
As shown above using an in vitro assay, we observed significant attenuation of infiltrating CD8+ T-cell function by intratumoral Treg cells. Dampening of immune function within the tumor microenvironment may have clinical significance, and measurement of Treg numbers may provide us with an indirect measure of Treg activity. Therefore, we measured the percentage of CD8+ T cells, the CD8/CD4 ratio, as well as the frequency of intratumoral Treg cells, CD4+CD25+, in 22 freshly isolated biopsy specimens from patients with B-cell NHL and examined the relationship between intratumoral Treg cells and infiltrating CD8+ T-cell population as well as the value of CD8/CD4 ratio. We found that there was a strong inverse relationship between the frequency of intratumoral Treg cells and the percentage of CD8 cells in biopsy specimens (n = 22, R2 = 0.4927, P = 0.0006; Fig. 6A). The samples that had a high percentage of intratumoral Treg cells had a low percentage of infiltrating CD8+ T cells. Conversely, the samples that had a low percentage of intratumoral Treg cells showed a high percentage of infiltrating CD8+ T cells. Because the CD8/CD4 ratio has been used as a clinical prognostic index for patients with cancer (32), we also compared the frequency of intratumoral Treg cells with the CD8/CD4 ratio in our samples (Fig. 6B). Interestingly, a linear inverse relationship could be seen between intratumoral Treg cells and the CD8/CD4 ratio when the frequency of intratumoral Treg cells was <15% (n =12, R2 = 0.7082, P = 0.0006). However, we found that the CD8/CD4 ratio was uniformly low when the frequency of intratumoral Treg cells was >15% (n =11, R2 = 0.2177, P = 0.148). These findings suggest that increased numbers of Treg cells in the tumor microenvironment suppress the relative number of CD8+ T cells.
Figure 6.
Increased numbers of intratumoral Treg cells are associated with decreased numbers of infiltrating CD8+ T cells in B-cell NHL. A, relationship between the percentage of CD4+CD25+ Treg cells and the percentage of CD8+of CD3+ T cells in 22 fresh biopsy samples of B-cell NHL. Liner line was drawn to calculate R2. B, relationship between the percentage CD4+CD25+ T cells and the ratio of CD8+ to CD4+ T cells in 23 fresh biopsy samples of B-cell NHL. The liner lines were drawn based on percentage of CD4+CD25+ T cells. The value of 15% makes two populations.
Discussion
Orchestration of an effective immune response against tumor cells is a complex process that involves numerous branches of the immune system. Subtle alterations in costimulatory molecule expression, cytokine secretion, and ineffective migration of immune cells to the inflammatory milieu can vastly alter the clearance of antigen. Recent studies by our group, as well of others, have highlighted the significance of Treg cells in this process and suggest that Treg cells contribute to the ineffective immune response observed against tumors(3, 10, 11, 33, 34). Much of this work has focused on CD4+CD4+ T cells or has been accomplished using murine models of solid tumors. However, there is increasing literature suggesting a role for Treg cells in hematologic malignancies as well (12, 35-38).
In previous work, we have shown that intratumoral Treg cells significantly suppress the functional capabilities of autologous infiltrating CD4+CD25- T cells in NHL, and that Treg cells migrate to areas involved by lymphoma in response to factors secreted by the malignant B cells (3). The ability of tumor cells to attract Treg cells, and thereby dampen the immune response, is intriguing and suggests that a dynamic relationship exists between tumor cells and those of the immune system. However, one question that still remained was whether or not Treg cells had a direct effect on CTL activity, and in turn, the viability of human tumor cells.
Classically, Treg cells are phenotypically characterized by expression of CD4, CD25high, and Foxp3 (39-41). Currently, the tools do not exist to isolate T cells for experimental use based on intracellular Foxp3 expression; thus, we are only able to use CD25 as a marker for purification. In other human systems, this may be problematic; however, in our studies on NHL tumor specimens, we have found that irrespective of the CD25 expression level (high versus low), all CD4+CD25+ cells expressed Foxp3 (3). Additionally, the staining pattern of Foxp3+ cells correlates well with the staining of CD4+ and CD25+ cells (Fig. 1). However, in other systems, Foxp3 expression may not necessarily confer regulatory activity. Recent work by Gavin et al. (42) found that small amounts of Foxp3 expression could be detected in in vitro activated CD4+ and CD8+ T cells. Unlike classic regulatory T cells, these cells were unable to suppress Th1cytokine synthesis. However, they were not able to directly determine weather or not these cells had suppressive capabilities. In our experiments, Foxp3 expression was confined to the CD4+ population and was not detected in CD8+ T cells (data not shown). Further characterization of Foxp3 expression and regulatory activity of human T cells in both the normal and malignant scenerio will hopefully shed light recent findings.
Although it has been reported that Treg cells inhibit CD8+ T-cell proliferation under several pathologic conditions, such as infection (18, 19) and transplantation (20, 21), it was less clear how Treg cells influence CD8+ T cells in a human tumor model. We found that intratumoral Treg cells isolated from tissue biopsy specimens from patients could completely inhibit proliferation of patient-matched autologous CD8+ T cells (Fig. 2). The inability of CD8+ T cells to proliferate in response to antigen within the tumor microenvironment may be one mechanism by which Treg cells hinder the initial steps of a normal immune response. This is in contrast to what has been seen in mouse tumor models where experiments found that Treg cells did not interfere with accumulation or division of tumor-specific CD8+ T cells (23). The exact mechanisms that Treg cells use to inhibit proliferation of CD8+ T in a human model may therefore be unique.
Intratumoral Treg cells suppress not only the proliferation of infiltrating CD8+ T cells but also production and release of PFN and GzmB. Our in vitro experiments indicate that intratumoral Treg cells can dramatically inhibit activation-induced intracellular expression of PFN and GzmB by CD8+ T cells (Fig. 3). We also found that a majority of intratumoral CD8+ T cells express minimal amounts of PFN and GzmB, suggesting that they are relatively inactive. Production of PFN and GzmB is essential for CD8+ T cells to function as effector cells, and it has been shown that the perforin/granzyme pathway is critical for CTL-mediated lymphoma B-cell lysis in B-cell NHL (43). In parallel experiments, we found that expression of CD107a, a measure of degranulation, on infiltrating CD8+ T cells was also inhibited by intratumoral Treg cells (Fig. 4). Degranulation is one of the key steps in the cytolytic process and is required for lysis of tumor cells. Our studies show that expression of CD107a on infiltrating CD8+ T cells was up-regulated upon activation by PHA or exposure to tumor cells. We also found that expression of CD107a on CD8+ T cells, upon exposure to lymphoma B cells, was suppressed by intratumoral Treg cells. Inhibition of perforin and GzmB expression by infiltrating CD8+ T, as well as suppression of granule release, may therefore be an additional mechanism by which by Treg cells suppress normal CTL responses. These results are in accordance with murine studies, which have also shown that Treg cells abolish tumor-specific CD8+ T-cell cytotoxic activity (23).
The ability of Treg cells the shutdown various aspect of CTL mediated immunity is clear; however, it was still unknown weather or not intratumoral CD8+ T cells could kill autologous tumor cells and what effect Treg cells would have on this activity. As shown in Fig. 5, our results show that CD8+ T cells from NHL tumors are functional, and that they can kill both tumor cell lines as well as autologous lymphoma B cells. When we further characterized the CD8+ T cell population, we found that the vast majority (>90%, n = 3) of infiltrating CD8+ T cells lack expression of CD45RA and CCR7, suggesting that they are effector memory cells (data not shown) and should therefore be capable of effective immune response (44). Of note, when we phenotyped the CD8+ T-cell population in a nonmalignant hyperplastic lymph node, we found that a vast majority (>90%, n = 1) of infiltrating CD8+ T cells expressed CD45RA and CCR7, suggesting a naive phenotype (data not shown). The significance of these data and what effect it may have on the immune response to NHL B cells is currently under investigation, but it may indicate that the malignant scenario or the increased number of Treg cells in the tumor microenvironment may influence the development or attraction of CD8+ effector memory cells.
Throughout the experiments we noted that infiltrating CD4+CD25- had the ability to reduce CTL function, albeit to a lesser extent than CD4+CD25+ T cells. In light of these data, we have done preliminary FACS experiments that suggest that a small subpopulation of CD4+CD25- T cells that express Foxp3 can be found in NHL tumors, and they may therefore be responsible for the suppressive activity seen in our studies (data not shown). In support of these initial findings, Nishioka et al. have identified a population of CD4+CD25-Foxp3+ suppressive T cells in aged mice (45). Throughout our experiments, we have found no significant difference in the function and frequency of the various T-cell subpopulations, as a percentage of total T cells, among the different NHL histologic subtypes studied thus far. However, a formal analysis has not been done due to the small number of samples used in this study.
The ability of Treg cells to shutdown antitumor activity suggests that their presence in the tumor microenvironment may have significant effect on CD8+ T-cell function as well as the progression of NHL. Therefore, we next wanted to determine if the occurrence of Treg cells in NHL was related to the number of CD8+ T cells present. When we measured the percentage of CD8+ T cells and intratumoral Treg cells in biopsy specimens from patients with B-cell NHL, we found that there was a strong inverse relationship between the frequency of intratumoral Treg cells and the percentage of CD8+ cells in biopsy specimens. Taken together, these data suggest that Treg cells influence the function of CD8+, and that increased numbers of Treg cells in the tumor microenvironment may suppress the relative number of CD8+ T cells.
In summary, our data provide evidence indicating that Treg cells contribute in part to the ineffective antitumor response seen in B-cell NHL by suppressing numerous facets of the CTL response. Our findings are in accordance with recent finding is mice where regulatory T cells have been shown to suppress CTL activity (22-24). Taken together, these data highlight the importance of the Treg cell within the tumor microenvironment and suggest that CTLs could potentially eradicate tumor cells in the absence of Treg cells. Therefore, we contend that use of novel therapies that would inhibit the function of intratumoral Treg cells will result in significant clinical benefit for patients with B-cell NHL.
Acknowledgments
Grant support: NIH grants CA92104 and CA97274 and Leukemia & Lymphoma Society translational research grant.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Ansell SM, Stenson M, Habermann TM, Jelinek DF, Witzig TE. Cd4+ T-cell immune response to large B-cell non-Hodgkin's lymphoma predicts patient outcome. J Clin Oncol. 2001;19:720–6. doi: 10.1200/JCO.2001.19.3.720. [DOI] [PubMed] [Google Scholar]
- 2.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–69. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 3.Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–46. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irisarri M, Plumas J, Bonnefoix T, et al. Resistance to CD95-mediated apoptosis through constitutive c-FLIP expression in a non-Hodgkin's lymphoma B cell line. Leukemia. 2000;14:2149–58. doi: 10.1038/sj.leu.2401954. [DOI] [PubMed] [Google Scholar]
- 5.Plumas J, Jacob MC, Chaperot L, Molens JP, Sotto JJ, Bensa JC. Tumor B cells from non-Hodgkin's lymphoma are resistant to CD95 (Fas/Apo-1)-mediated apoptosis. Blood. 1998;91:2875–85. [PubMed] [Google Scholar]
- 6.Horna P, Cuenca A, Cheng F, et al. In vivo disruption of tolerogenic cross-presentation mechanisms uncovers an effective T-cell activation by B-cell lymphomas leading to antitumor immunity. Blood. 2006;107:2871–8. doi: 10.1182/blood-2005-07-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sotomayor EM, Borrello I, Rattis FM, et al. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood. 2001;98:1070–7. doi: 10.1182/blood.v98.4.1070. [DOI] [PubMed] [Google Scholar]
- 8.Staveley-O'Carroll K, Sotomayor E, Montgomery J, et al. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc Natl Acad Sci U S A. 1998;95:1178–83. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 10.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 11.Unitt E, Rushbrook SM, Marshall A, et al. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41:722–30. doi: 10.1002/hep.20644. [DOI] [PubMed] [Google Scholar]
- 12.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–36. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bladergroen BA, Meijer CJ, ten Berge RL, et al. Expression of the granzyme B inhibitor, protease inhibitor 9, by tumor cells in patients with non-Hodgkin and Hodgkin lymphoma: a novel protective mechanism for tumor cells to circumvent the immune system? Blood. 2002;99:232–7. doi: 10.1182/blood.v99.1.232. [DOI] [PubMed] [Google Scholar]
- 14.Rimsza LM, Roberts RA, Miller TP, et al. Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: a follow-up study from the Leukemia and Lymphoma Molecular Profiling Project. Blood. 2004;103:4251–8. doi: 10.1182/blood-2003-07-2365. [DOI] [PubMed] [Google Scholar]
- 15.Kudoh S, Wang Q, Hidalgo OF, et al. Responses to T cell receptor/CD3 and interleukin-2 receptor stimulation are altered in T cells from B cell non-Hodgkin's lymphomas. Cancer Immunol Immunother. 1995;41:175–84. doi: 10.1007/BF01521344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stopeck AT, Gessner A, Miller TP, et al. Loss of B7.2 (CD86) and intracellular adhesion molecule 1(CD54) expression is associated with decreased tumor-infiltrating T lymphocytes in diffuse B-cell large-cell lymphoma. Clin Cancer Res. 2000;6:3904–9. [PubMed] [Google Scholar]
- 17.Wang Q, Stanley J, Kudoh S, et al. T cells infiltrating non-Hodgkin's B cell lymphomas show altered tyrosine phosphorylation pattern even though T cell receptor/CD3-associated kinases are present. J Immunol. 1995;155:1382–92. [PubMed] [Google Scholar]
- 18.Boettler T, Spangenberg HC, Neumann-Haefelin C, et al. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860–7. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rushbrook SM, Ward SM, Unitt E, et al. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852–9. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai Z, Li Q, Wang Y, et al. CD4+CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J Clin Invest. 2004;113:310–7. doi: 10.1172/JCI19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin CY, Graca L, Cobbold SP, Waldmann H. Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat Immunol. 2002;3:1208–13. doi: 10.1038/ni853. [DOI] [PubMed] [Google Scholar]
- 22.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-β signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–24. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu P, Lee Y, Liu W, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–91. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 26.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Lecoeur H, Fevrier M, Garcia S, Riviere Y, Gougeon ML. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J Immunol Methods. 2001;253:177–87. doi: 10.1016/s0022-1759(01)00359-3. [DOI] [PubMed] [Google Scholar]
- 28.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Penack O, Gentilini C, Fischer L, et al. CD56dim CD16neg cells are responsible for natural cytotoxicity against tumor targets. Leukemia. 2005;19:835–40. doi: 10.1038/sj.leu.2403704. see comment. [DOI] [PubMed] [Google Scholar]
- 30.Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Evaluation of the CD107 cytotoxicity assay for the detection of cytolytic CD8+ cells recognizing HER2/neu vaccine peptides. Breast Cancer Res Treat. 2005;92:85–93. doi: 10.1007/s10549-005-0988-1. [DOI] [PubMed] [Google Scholar]
- 31.Kannan K, Stewart RM, Bounds W, et al. Lysosome-associated membrane proteins h-LAMP1(CD107a) and h-LAMP2 (CD107b) are activation-dependent cell surface glycoproteins in human peripheral blood mononuclear cells which mediate cell adhesion to vascular endothelium. Cell Immunol. 1996;171:10–9. doi: 10.1006/cimm.1996.0167. [DOI] [PubMed] [Google Scholar]
- 32.Diederichsen AC, Hjelmborg JB, Christensen PB, Zeuthen J, Fenger C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother. 2003;52:423–8. doi: 10.1007/s00262-003-0388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor a) monoclonal antibody. Cancer Res. 1999;59:3128–33. [PubMed] [Google Scholar]
- 34.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–8. [PubMed] [Google Scholar]
- 35.Beyer M, Kochanek M, Darabi K, et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–25. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 36.Beyer M, Kochanek M, Giese T, et al. In vivo peripheral expansion of naive CD4+CD25high Foxp3+regulatory T cells in patients with multiple myeloma. Blood. 2006;107:3940–9. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 37.Marshall NA, Christie LE, Munro LR, et al. Immuno-suppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103:1755–62. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 38.Prabhala RH, Neri P, Bae JE, et al. Dysfunctional T regulatory cells in multiple myeloma. Blood. 2006;107:301–4. doi: 10.1182/blood-2005-08-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 40.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 41.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 42.Gavin MA, Torgerson TR, Houston E, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–64. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaperot L, Manches O, Mi JQ, et al. Differentiation of anti-tumour cytotoxic T lymphocytes from autologous peripheral blood lymphocytes in non-Hodgkin's lymphomas. Br J Haematol. 2002;119:425–31. doi: 10.1046/j.1365-2141.2002.03885.x. [DOI] [PubMed] [Google Scholar]
- 44.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 45.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25[C0]3+ T cells in aged mice. J Immunol. 2006;176:6586–93. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]