Abstract
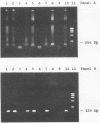
A simple, sensitive, and specific polymerase chain reaction (PCR) protocol for the detection of human immunodeficiency virus type 1 (HIV-1) is described. We have improved all three PCR steps: sample preparation, DNA amplification, and detection of the amplified product. Some of the improvements have been described previously, but they have never been combined into a complete PCR protocol. Peripheral blood mononuclear cells were lysed directly in a buffer containing sodium dodecyl sulfate, Triton X-100, and proteinase K. This crude cell lysate was amplified in a two-step PCR, first with outer primers and then with inner primers nested within the first primers. The PCR product was visualized by agarose gel electrophoresis and ethidium bromide staining. Thus, we avoided conventional DNA extraction as well as hybridization for the detection of the PCR product. The samples were analyzed with four sets of nested primers (JA4 through JA7, JA9 through JA12, JA13 through JA16, and JA17 through JA20) designed to amplify HIV-1 gag, env gp120, env gp41, and pol sequences, respectively. We were able to amplify HIV-1 sequences in all samples from 90 HIV-1-seropositive individuals with mostly mild symptoms. Of these individuals, 24 were negative in HIV-1 isolation and 9 were selected because they were infected by African and Haitian HIV-1 strains. Eighty-five (94%) individuals were positive with at least three of four primer sets. Samples from 26 healthy blood donors, as well as cells infected in vitro with human immunodeficiency virus type 2 and human T-cell leukemia virus type I, were negative in PCR, thus demonstrating the specificity of the amplification.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Abrahamsson B., Nagy K., Aurelius E., Gaines H., Nyström G., Fenyö E. M. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS. 1990 Feb;4(2):107–112. doi: 10.1097/00002030-199002000-00002. [DOI] [PubMed] [Google Scholar]
- Asjö B., Morfeldt-Månson L., Albert J., Biberfeld G., Karlsson A., Lidman K., Fenyö E. M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986 Sep 20;2(8508):660–662. [PubMed] [Google Scholar]
- Eisenstein B. I. The polymerase chain reaction. A new method of using molecular genetics for medical diagnosis. N Engl J Med. 1990 Jan 18;322(3):178–183. doi: 10.1056/NEJM199001183220307. [DOI] [PubMed] [Google Scholar]
- Guatelli J. C., Gingeras T. R., Richman D. D. Nucleic acid amplification in vitro: detection of sequences with low copy numbers and application to diagnosis of human immunodeficiency virus type 1 infection. Clin Microbiol Rev. 1989 Apr;2(2):217–226. doi: 10.1128/cmr.2.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Moudgil T., Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989 Dec 14;321(24):1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Ou C. Y., Jason J., Holmberg S. D., Longini I. M., Jr, Schable C., Mayer K. H., Lifson A. R., Schochetman G., Ward J. W. Duration of human immunodeficiency virus infection before detection of antibody. Lancet. 1989 Sep 16;2(8664):637–640. doi: 10.1016/s0140-6736(89)90892-1. [DOI] [PubMed] [Google Scholar]
- Imagawa D. T., Lee M. H., Wolinsky S. M., Sano K., Morales F., Kwok S., Sninsky J. J., Nishanian P. G., Giorgi J., Fahey J. L. Human immunodeficiency virus type 1 infection in homosexual men who remain seronegative for prolonged periods. N Engl J Med. 1989 Jun 1;320(22):1458–1462. doi: 10.1056/NEJM198906013202205. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Kwok S. Y., Hopsicker J. S., Sannerud K. J., Sninsky J. J., Edson J. R., Balfour H. H., Jr Absence of HIV-1 infection in antibody-negative sexual partners of HIV-1 infected hemophiliacs. Transfusion. 1989 Mar-Apr;29(3):265–267. doi: 10.1046/j.1537-2995.1989.29389162735.x. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Kwok S. Y., Sninsky J. J., Hopsicker J. S., Sannerud K. J., Rhame F. S., Henry K., Simpson M., Balfour H. H., Jr Human immunodeficiency virus type 1 detected in all seropositive symptomatic and asymptomatic individuals. J Clin Microbiol. 1990 Jan;28(1):16–19. doi: 10.1128/jcm.28.1.16-19.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Smith D. B., Foote S. J., Samaras N., Peterson M. G. Colorimetric detection of specific DNA segments amplified by polymerase chain reactions. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2423–2427. doi: 10.1073/pnas.86.7.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Loche M., Mach B. Identification of HIV-infected seronegative individuals by a direct diagnostic test based on hybridisation to amplified viral DNA. Lancet. 1988 Aug 20;2(8608):418–421. doi: 10.1016/s0140-6736(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Martin L. S., McDougal J. S., Loskoski S. L. Disinfection and inactivation of the human T lymphotropic virus type III/Lymphadenopathy-associated virus. J Infect Dis. 1985 Aug;152(2):400–403. doi: 10.1093/infdis/152.2.400. [DOI] [PubMed] [Google Scholar]
- Modrow S., Hahn B. H., Shaw G. M., Gallo R. C., Wong-Staal F., Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1987 Feb;61(2):570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Smit V. T., Boot A. J., Smits A. M., Fleuren G. J., Cornelisse C. J., Bos J. L. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1988 Aug 25;16(16):7773–7782. doi: 10.1093/nar/16.16.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist V. A., Albert J., Ohlsson E., Hinkula J., Fenyö E. M., Wahren B. Human immunodeficiency virus type 1 p24 production and antigenic variation in tissue culture of isolates with various growth characteristics. J Med Virol. 1989 Nov;29(3):170–175. doi: 10.1002/jmv.1890290305. [DOI] [PubMed] [Google Scholar]