Abstract
Protein disulfide isomerases (PDIs) aid protein folding and assembly by catalyzing formation and shuffling of cysteine disulfide bonds in the endoplasmic reticulum (ER). Many members of the PDI family are expressed in mammals, but the roles of specific PDIs in vivo are poorly understood. A recent homology-based search for additional PDI family members identified anterior gradient homolog 2 (AGR2), a protein originally presumed to be secreted by intestinal epithelial cells. Here, we show that AGR2 is present within the ER of intestinal secretory epithelial cells and is essential for in vivo production of the intestinal mucin MUC2, a large, cysteine-rich glycoprotein that forms the protective mucus gel lining the intestine. A cysteine residue within the AGR2 thioredoxin-like domain forms mixed disulfide bonds with MUC2, indicating a direct role for AGR2 in mucin processing. Mice lacking AGR2 were viable but were highly susceptible to colitis, indicating a critical role for AGR2 in protection from disease. We conclude that AGR2 is a unique member of the PDI family, with a specialized and nonredundant role in intestinal mucus production.
Keywords: colitis, endoplasmic reticulum, mucin, goblet cell, protein processing
Formation of intrachain and interchain disulfide bonds is essential for the folding and multimerization of proteins that travel through the secretory pathway. Members of the protein disulfide isomerase (PDI) family are critical for efficient formation of correctly arranged disulfide bonds in the endoplasmic reticulum (ER). PDI family members are defined by the presence of 1 or more thioredoxin-like domains and by localization to the ER. At least 19 human PDI family members have been identified (1–3), and each has a known mouse ortholog. Many PDI thioredoxin-like domains have a motif that contains 2 cysteine residues (CXXC). Depending on the redox state of the PDI, these CXXC motifs can mediate formation, shuffling, or reduction of disulfides in client proteins in the ER. Other PDI thioredoxin-like domains contain a single cysteine, often within a CXXS motif, that can form a mixed disulfide with a cysteine residue in a client protein. Such PDI–client interactions may isomerize disulfide bonds and can serve to retain incompletely folded clients in the ER (4, 5). Studies involving expression of mammalian PDIs in yeast demonstrated partial functional overlap between PDIs (6, 7), but the roles of specific PDIs in vivo have been difficult to determine.
Anterior gradient homolog 2 (AGR2, also known as AG2, HAG-2, or GOB-4) was classified recently as a member of the PDI family based on a search for sequences with homology to known family members (3). Murine Agr2 was originally identified in a screen for mRNAs expressed selectively in intestinal goblet cells (8). The major function of goblet cells is the production of mucus, which lines the intestine and provides protection against environmental insults (9). The distinctive viscoelastic properties of mucus depend on the unusual characteristics of glycoproteins called gel-forming mucins. The major intestinal mucin is MUC2, which contains >5000 aa and is processed in the ER and the Golgi apparatus. Processing leads to extensive O-glycosylation of the central mucin repeats and formation of intrachain and interchain disulfide bonds involving >200 cysteine residues concentrated in the cysteine-rich amino-terminal and carboxyl-terminal domains (9–11). After mucins are glycosylated, folded, and multimerized, they enter secretory granules where they can be stored before release into the lumen. AGR2 was originally presumed to be another secreted protein produced by goblet cells (8). However, AGR2 has a carboxyl-terminal KTEL sequence, and a recent analysis indicated that this sequence was recognized by ER retention receptors and served to localize AGR2 predominantly to the ER of transfected cells (12). In addition, AGR2 has a single thioredoxin-like domain with a CXXS motif (3). Because AGR2 has these structural characteristics of a PDI and is expressed selectively in mucus-producing cells, we hypothesized that AGR2 plays a special role in the production of mucus.
Here, we report that AGR2 is a PDI that is essential for the production of intestinal mucus. We show that AGR2 is present within the ER of intestinal secretory epithelial cells, interacts with MUC2 as it is processed by mucus-producing cells, and is absolutely required for in vivo mucus production and protection from colitis. These results establish that AGR2 is a PDI family member that has a unique and specialized role in intestinal mucus production and protection against disease.
Results
AGR2 Is Present Within the ER of Intestinal Secretory Epithelial Cells.
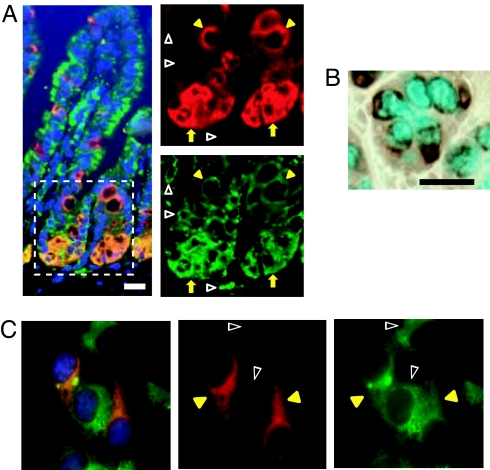
We first examined the localization of AGR2 in the intestine. In the small intestine, AGR2 was found within a subset of cells near the base of the crypt, where Paneth cells and pregoblet cells are found (13), and in cells with goblet cell morphology along the villi (Fig. 1A). AGR2-stained regions also stained with antibodies recognizing the ER proteins GRP78 and GRP94, indicating that AGR2 was present within the ER of these intestinal epithelial cells. The majority of epithelial cells lining the intestinal villi are absorptive enterocytes, and the lack of AGR2 staining in most of the epithelium is consistent with prior studies showing that AGR2 is not present in these nonsecretory cells (8, 14). Immunohistochemical staining of colon goblet cells showed AGR2 concentrated in areas surrounding Alcian blue-stained mucous granules, but little, if any, AGR2 staining was seen within these granules (Fig. 1B). Consistent with a previous report (12), an epitope-tagged AGR2 construct colocalized with the archetypical family member PDI (also known as P4HB) in transfected cells (Fig. 1C), providing further evidence that AGR2, like other PDI family members, is present within the ER.
Fig. 1.
AGR2 is localized to the ER. (A) Mouse intestinal villi were stained with antibodies to AGR2 (red) and the ER proteins GRP78 and GRP94 (green). The outlined area within the composite image at left is magnified in the images on the right to show AGR2 localization in the ER in a subset of cells at the base of the crypt area (yellow arrows) and in goblet cells above the crypts (yellow arrowheads). AGR2 was absent from the ER of other cells (open white arrowheads). (Magnification: 400×.) (B) AGR2 staining (brown) surrounding mucous granules in the colon. Mucus was stained with Alcian blue. (Scale bar: 25 μm.) (C) COS7 cells were transfected with AGR2-FLAG and stained with antibodies to FLAG (red) and the archetypical PDI (P4HB; green). DAPI (blue) was used to visualize nuclei. (Left) A composite image. Yellow arrowheads indicate cells expressing both AGR2-FLAG and P4HB, and open white arrowheads indicate cells expressing P4HB but not AGR2-FLAG. (Magnification: 400×.)
AGR2 Is Essential for Mucus Production in the Intestine.
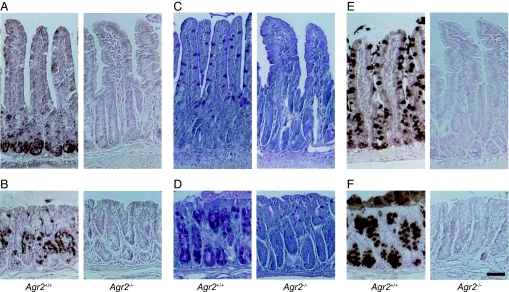
To investigate the role of AGR2 in vivo, we produced mice with a null mutation in the Agr2 gene. Agr2 mRNA and AGR2 protein were expressed in Agr2+/+ control mice but could not be detected in Agr2−/− mice (Fig. 2 A and B and Fig. S1). Periodic acid-Schiff, which stains heavily glycosylated proteins, including mucins, revealed mucus-containing goblet cells within the small intestine and colon of control mice, but staining was markedly reduced in Agr2−/− mice (Fig. 2 C and D). Furthermore, MUC2 core protein was abundant in control mice but undetectable in Agr2−/− mice (Fig. 2 E and F). Pulse–chase analysis revealed synthesis of MUC2 in colon explants from control mice, but there was little or no MUC2 synthesis detected in Agr2−/− explants, even in the presence of a proteasome inhibitor (Fig. S2). Taken together, these results demonstrate that AGR2 has an essential role in mucin and mucus production in the intestine.
Fig. 2.
Agr2−/− mice are devoid of intestinal mucus. (A and B) Immunohistochemical detection of AGR2 protein in small intestine (A) and colon (B) of Agr2+/+ and Agr2−/− mice. (C and D) Periodic acid-Schiff staining of glycoproteins in small intestine (C) and colon (D). (E and F) Immunohistochemical detection of MUC2 protein in small intestine (E) and colon (F). (Scale bar: 50 μm.)
AGR2 Is Not Required for Establishment of Intestinal Secretory Epithelial Cell Lineages.
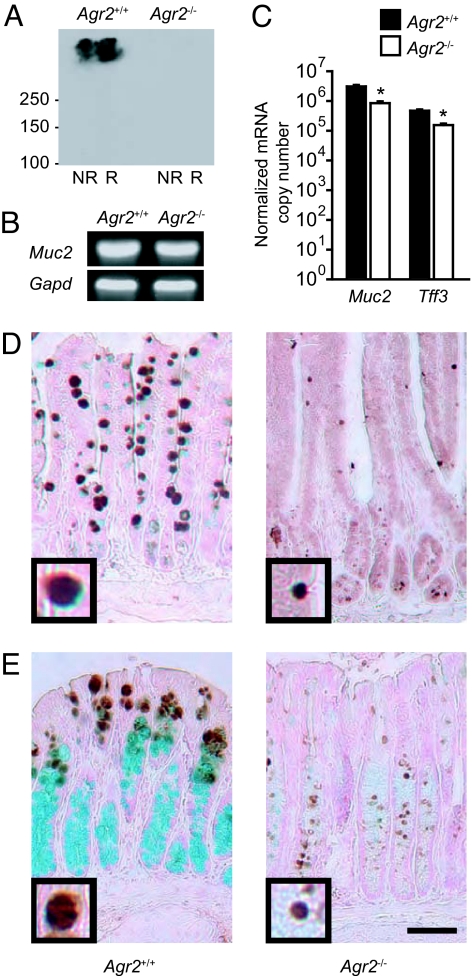
Intestinal epithelial stem cells near the base of the crypt give rise to absorptive enterocytes and to a common precursor for all 3 secretory cell types (goblet cells, Paneth cells, and enteroendocrine cells) (15). Because AGR2 is found in all 3 secretory cell types in the small intestine, it has been suggested that AGR2 might have a general role in establishing secretory cell fate (14). However, analyses of proteins and mRNA transcripts expressed selectively in the Paneth and enteroendocrine cells showed that these lineages were still present in Agr2−/− mice (Fig. S3). To determine whether loss of mucus and MUC2 resulted from loss of the goblet cell lineage, we analyzed expression of goblet cell-specific proteins and mRNAs. MUC2 protein was undetectable by either immunohistochemistry (Fig. 2 E and F) or immunoblotting (Fig. 3A). In contrast, Muc2 mRNA was easily detectable in control and Agr2−/− mice (Fig. 3B), although quantitative RT-PCR demonstrated that levels were 3-fold lower in Agr2−/− mice (Fig. 3C). Tff3, the mRNA encoding the goblet cell secretory product intestinal trefoil factor (16), was also present but reduced 3-fold in Agr2−/− mice. TFF3 protein was detectable in Agr2−/− mice, although the number of TFF3-stained cells and the size of the TFF3-containing granules were substantially reduced (Fig. 3 D and E). A reduction in TFF3-containing granule size has also been described in mice that lack MUC2 because of a null mutation in the Muc2 gene (17). In summary, we found that the goblet cell lineage and Muc2 mRNA were present but MUC2 protein absent in Agr2−/− mice.
Fig. 3.
AGR2 is required for production of MUC2 protein but not for specification of the goblet cell lineage. (A) Immunoblotting for MUC2 protein in nonreduced (NR) and reduced (R) samples from small intestine of Agr2+/+ and Agr2−/− mice. (B) Detection of Muc2 mRNA in small intestine by reverse transcription followed by PCR. Gapd mRNA was used as a control. (C) Expression of Muc2 and Tff3 mRNAs in small intestine from Agr2+/+ and Agr2−/− mice was measured by quantitative RT-PCR. Results are means ± SEM for 4 mice per group. *, P < 0.001 compared to Agr2+/+ mice. (D and E) TFF3 protein (brown) in the small intestine (D) and colon (E) was identified by immunohistochemistry. Mucus was stained with Alcian blue. (Scale bars: 50 μm.) Insets at the lower left corner of each photomicrograph (3× higher magnification) show typical TFF3-stained granules.
A Cysteine Residue in the Thioredoxin-Like Domain of AGR2 Forms Mixed Disulfide Bonds with MUC2.
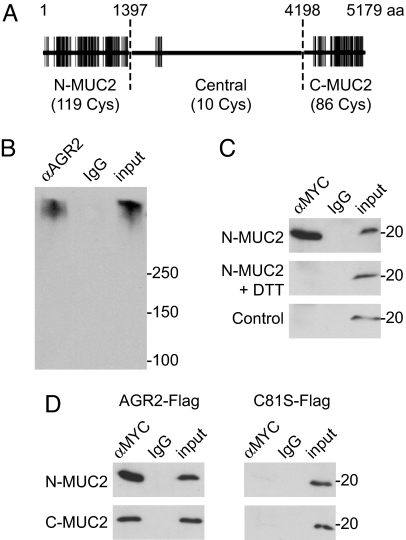
We next considered whether AGR2 might have a direct role in processing of MUC2, which contains 215 cysteine residues (Fig. 4A). PDIs form mixed disulfide bonds with client proteins during processing in the ER. To determine whether AGR2 associates with MUC2, we performed coimmunoprecipitation experiments. We found that MUC2 from a human intestinal cell line coimmunoprecipitated with AGR2 (Fig. 4B). To analyze the AGR2–MUC2 interaction further, we coexpressed wild-type or mutant AGR2 with cysteine-rich regions of MUC2. Wild-type AGR2 coimmunoprecipitated with a 1,397-aa, cysteine-rich region from the amino terminus of MUC2 (Fig. 4C). This association was disrupted by treating cells with reducing agent. AGR2 contains a single thioredoxin-like domain with a CXXS motif, and mutation of the cysteine residue in that motif abolished the association of AGR2 with the amino terminus of MUC2 (Fig. 4C). A 981-aa, cysteine-rich region from the carboxyl terminus of MUC2 also associated with wild-type AGR2 but not with mutant AGR2 lacking the cysteine residue. These results show that the single cysteine residue in the AGR2 thioredoxin-like domain can form mixed disulfide bonds with cysteines in the amino-terminal and carboxyl-terminal portions of MUC2.
Fig. 4.
AGR2 forms mixed disulfide bonds with MUC2. (A) Schematic representation of MUC2, showing the distribution of cysteine residues (vertical lines) in the amino-terminal and carboxyl-terminal, cysteine-rich regions and the central region, which contains relatively few cysteine residues and is composed mainly of a large number of tandem repeats of a heavily glycosylated, threonine-rich sequence. (B) AGR2 was immunoprecipitated from LS174T cells, and associated MUC2 was detected by immunoblotting. IgG from nonimmune serum was used as a control. A total of 4% of the volume of lysate used for the immunoprecipitation was run on the gel for comparison (input). (C) HEK293T cells were cotransfected with an MYC-tagged amino-terminal fragment of MUC2 (N-MUC2) and AGR2-FLAG. N-MUC2-containing complexes were immunoprecipitated with anti-MYC, and associated AGR2 was detected by immunoblotting with anti-FLAG antibody. AGR2 did not coimmunoprecipitate with N-MUC2 if cells were treated with the reducing agent DTT before lysis. An MYC-tagged construct lacking the N-MUC2 fragment (EGFP-MYC) was used as a control (Bottom). (D) FLAG-tagged AGR2 coimmunoprecipitated with MYC-tagged amino-terminal and carboxyl-terminal fragments of MUC2, but a FLAG-tagged AGR2 mutant lacking the cysteine residue (C81S) did not.
AGR2-Deficient Mice Had an Increased Incidence of Rectal Prolapse and Were More Susceptible to Experimentally Induced Acute Colitis.
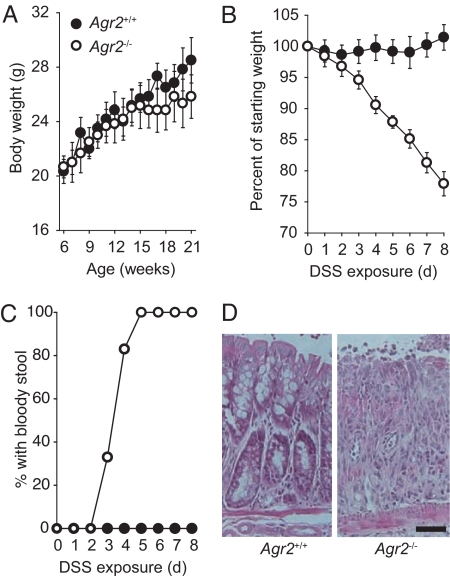
A total of 30 litters produced by breeding an Agr2+/− male with an Agr2+/− female included 240 pups that survived until weaning at 3–4 weeks of age, and 31% of these pups were Agr2−/−, indicating that lack of AGR2 did not adversely affect viability during early life. Mice that lack intestinal mucin because of null mutations in Muc2 may develop spontaneous colonic inflammation and weight loss, depending on genetic background and/or environmental factors (17, 18). Despite a lack of intestinal mucus, young Agr2−/− mice appeared healthy, did not have diarrhea, and had body weights similar to those of wild-type littermates until at least 4 months of age (Fig. 5A). Routine histological examination of the colon again revealed the lack of mucus-filled goblet cells but showed no apparent inflammation (Fig. S4A). DNA microarrays were used to further analyze the effects of AGR2 deficiency (Table S1). The transcript with the largest fold decrease encodes cytochrome P450 1A1, a xenobiotic-metabolizing enzyme that is regulated in response to toxins and certain dietary components (19). There were prominent increases in mast cell proteases in small intestine from Agr2−/− mice. Toluidine blue staining of mast cells confirmed that there were increased numbers of these innate immune cells in the intestine in Agr2−/− mice (Fig. S4B). We also found decreased expression of kallikrein 1 and several related peptidases. Goblet cells are a prominent source of kallikreins, and goblet cell kallikrein expression is substantially reduced in inflammatory bowel disease (20). Despite the lack of overt disease, colon from Agr2−/− mice had increased expression of several proinflammatory cytokines (Table S2). In addition, expressions of Hsp90b1 (an ER chaperone) and Sec61a1 (which encodes an ER pore protein involved in the removal of misfolded proteins) were increased in Agr2−/− colon. Similar changes in these genes were recently identified in mice with Muc2 mutations that cause MUC2 misfolding (21). Although we did not detect overt colitis in young mice, 5 of 8 Agr2−/− mice that we followed for 1 year after birth developed rectal prolapse, a condition that is seen in various mouse models of intestinal inflammation (22, 23). None of the 7 Agr2+/+ littermate controls developed rectal prolapse (P = 0.03 by Fisher's exact test). In summary, Agr2−/− mice had increases in mast cells, increased expression of some genes associated with inflammation and MUC2 misfolding, and an increased incidence of rectal prolapse.
Fig. 5.
Agr2−/− mice are highly susceptible to DSS-induced colitis. (A) Mean body weights of mice with no DSS exposure (6 mice per group; P > 0.2 for Agr2−/− compared with Agr2+/+ mice at all ages). Error bars represent SEM. (B) Weight change during DSS administration in Agr2−/ − mice (6 mice per group; P < 10−5 at 8 days). (C) Appearance of blood in the stool during DSS administration. Agr2−/ − mice were more likely than control mice to develop bloody stools during low-dose DSS treatment (P = 1 × 10−5 by Fisher's exact test). (D) Hematoxylin and eosin staining of colon from DSS-exposed mice. (Scale bar: 50 μm.)
To determine whether Agr2−/− mice had altered susceptibility to experimentally induced colitis, we used a widely used model involving oral administration of dextran sodium sulfate (DSS) that mimics some features of human inflammatory bowel disease (24, 25). Administration of DSS in the drinking water for 7–8 days produces a dose-dependent acute colitis characterized by diarrhea, weight loss, and colonic mucosal ulceration. We exposed Agr2+/+ controls and Agr2−/− mice to a concentration of DSS (1.5%) that is lower than the dose usually used to induce colitis. All 6 Agr2+/+ control mice maintained a normal body weight, and none had bloody stool (Fig. 5 B and C). In contrast, all 6 DSS-exposed Agr2−/− mice had bloody stool and lost weight. DSS exposure resulted in obvious colonic epithelial damage in Agr2−/− mice but not in the controls (Fig. 5D). These findings indicate that Agr2−/− mice had increased susceptibility to DSS-induced colitis.
Discussion
This study establishes that AGR2 is a PDI with a highly specific and nonredundant role in vivo. In the absence of AGR2, mice were unable to produce intestinal mucin and became highly susceptible to experimentally induced colitis. These findings advance our understanding of functional diversity within the large family of mammalian PDIs and illustrate how 1 PDI can influence disease susceptibility.
AGR2 has the 2 key attributes of a PDI: ER localization and a functional thioredoxin-like domain. AGR2 was initially assumed to be a secreted protein, based on the presence of a predicted signal peptide at the amino terminus (8). However, more recent analysis identified an ER localization signal and thioredoxin-like domain sequences, suggesting that AGR2 is a PDI (3, 12). We found that AGR2 is localized to the ER in intestinal epithelial cells and did not detect AGR2 in secretory granules or in the intestinal lumen. PDI thioredoxin-like domains contain cysteine residues that can form mixed disulfide bonds with client proteins during processing in the ER. We showed that AGR2 associates with MUC2, and that the association between AGR2 and MUC2 depends on a mixed disulfide bond formed by a conserved cysteine residue in the AGR2 thioredoxin-like domain. Mixed disulfide bonds between PDIs and client proteins can catalyze shuffling of disulfide bonds in the client protein or allow for thiol-mediated retention of partially processed clients in the ER (1, 2).
AGR2-deficient mice lacked intestinal mucus and MUC2. We considered several mechanisms that might contribute to the absence of MUC2 in AGR2-deficient mice. Although it has been suggested that AGR2 might play a critical role in establishing cell fate in the intestinal epithelium (14), we found that Paneth cell, enteroendocrine, and goblet cell lineages were still present in AGR2-deficient mice. The goblet cell mRNA Muc2 was decreased 3-fold in these mice. Decreases in Muc2 mRNA were also reported in mice with mutations in the Muc2 coding sequence that result in MUC2 misfolding (21), suggesting that defects in MUC2 protein synthesis may cause a secondary decrease in Muc2 mRNA. However, the complete absence of MUC2 protein seen in Agr2−/− mice cannot be explained entirely by the reduction in Muc2 mRNA. We examined MUC2 synthesis by pulse–chase experiments and were able to detect newly synthesized MUC2 in control colon explants but not in Agr2−/− explants. MUC2 synthesis was not normalized by proteasome inhibitor treatment, which suggests that the absence of MUC2 is not due to ER-associated protein degradation of improperly processed MUC2. It is possible that impaired translation of Muc2 mRNA is an important contributor to MUC2 protein deficiency in AGR2-deficient mice. ER stress resulting from impaired protein processing may interfere with translation. However, there was only modest evidence for activation of the ER stress response in Agr2−/− mice. Specifically, by using microarrays we found a significant increase in GRP94 (Hsp90b1; Table S2) and trends toward increased expression (by 1.3- to 1.7-fold) of Grp78/Bip (Hspa5), Ero1, Atf4, and Atf6, which did not reach statistical significance after adjustment for multiple comparisons. Another possibility is that some MUC2 is synthesized in Agr2−/− mice, but problems with processing result in a form of MUC2 that is not recognized by the MUC2 antibodies we used, fails to be glycosylated, and does not function normally. Although further studies will be required to precisely determine which steps in mucin synthesis are affected by AGR2 deficiency, it is clear that this PDI family member is essential for intestinal mucin production.
AGR2-deficient mice were more susceptible to intestinal disease. The Agr2−/− mice that we generated and studied were viable, but with aging they often developed rectal prolapse, a common feature of mouse models of colitis (22, 23). Even in the absence of overt spontaneous colitis, microarray analysis did reveal an increase in mast cells, which are important in intestinal innate immunity, and in expression of some proinflammatory cytokines. In the colitis model, Agr2−/− mice rapidly developed severe disease characterized by profound weight loss, intestinal bleeding, and epithelial damage in response to a dose of DSS that did not cause disease in littermate controls. Because Muc2−/− mice are also more susceptible to DSS colitis (17, 21), it is likely that the increased susceptibility of Agr2−/− mice is primarily due to absence of MUC2. Tff3−/− mice also exhibit increased susceptibility to DSS (26), and it is possible that the reduction in TFF3 in Agr2−/− mice helps account for the severe disease these mice developed in response to DSS.
This work provides new information about how specific PDIs play specialized roles in vivo. AGR2 is one of 19 known PDI family members expressed in humans and other mammals. Differences in the number and type of thioredoxin-like domains and in cell type-specific expression patterns of PDIs (1–3) suggest that there is likely to be considerable diversity in PDI function, but the in vivo functions of mammalian PDIs are largely unknown. In a previous study involving gene targeting of a PDI family member, loss of the PDI ERp57, which works together with calnexin and calreticulin during processing of many glycoproteins in the ER (27), resulted in embryonic lethality (28). Our work demonstrates that a different PDI family member, AGR2, has a distinct and selective biological role in mucus production. MUC2 is an unusual protein that contains a central region that undergoes extensive O-linked glycosylation, and it also contains amino-terminal and carboxyl-terminal regions containing more than 200 cysteine residues that form intrachain and interchain disulfide bonds during mucin folding and multimerization (10, 11, 29). AGR2 may be especially important for meeting the demanding processing requirements of MUC2. The presence of AGR2 in non-mucus-producing cells, such as Paneth cells and enteroendocrine cells (14), suggests that AGR2 may interact with proteins other than MUC2, but further work will be required to identify the full range of AGR2 clients. In any case, this work provides a striking and novel demonstration that a single member of the mammalian PDI family can have a selective and nonredundant role in vivo.
Our analysis of AGR2-deficient mice provides an example of how loss of function of a PDI family member can influence disease susceptibility. The susceptibility of Agr2−/− mice to colitis may be relevant to human disease, because polymorphisms in the AGR2 gene were associated with increased risk of ulcerative colitis and Crohn's disease in 2 human populations (30), and abnormalities in MUC2 synthesis can be seen in humans with ulcerative colitis (21). In addition to mucus-producing cells, AGR2 is also expressed in cancers of the breast (31–33), pancreas (34), and prostate (35). In cancer model systems, overexpression or suppression of AGR2 can affect cell differentiation, cell migration, metastasis, and tumor growth (14, 32, 36). The molecular mechanisms of these effects have not been clearly identified, but in light of the work presented here may involve alterations in the processing of cysteine-containing secreted or cell surface proteins expressed by those cells. Other recent studies suggest roles for the archetypical mammalian PDI (P4HB) in neurodegenerative diseases (37) and in thrombus formation (38, 39). Improved understanding of the specific roles of individual mammalian PDIs may therefore provide important new clues about disease pathogenesis and susceptibility.
Materials and Methods
Immunodetection of Proteins in Mouse Intestine.
Antisera against AGR2 (ab22208; Abcam) and MUC2 (sc-15334 from Santa Cruz Biotechnology and HPA006197 from Sigma) and antibodies against other proteins of interest were used for immunofluorescence, immunohistochemistry, immunoblotting, and immunoprecipitation (including pulse–chase analysis) as detailed in SI Methods.
Heterologous Expression of AGR2 and MUC2 Constructs.
Plasmids encoding EGFP-tagged and Myc-tagged amino-terminal (10) and carboxyl-terminal (11) portions of human MUC2 were gifts from G. C. Hansson (Göteburg University, Gothenburg, Sweden). The production of a control EGFP-myc construct and FLAG-tagged AGR2 constructs is described in SI Methods. Cos-7 and HEK-293T cells were grown in DMEM with 10% FBS and transfected by using Lipofectamine 2000 (Invitrogen) or calcium phosphate and were analyzed 36–48 h after transfection.
Generation of Agr2−/− Mice.
The gene-targeting strategy is described in SI Methods and summarized in Fig. S1. Mice were housed in a specific pathogen-free facility. The University of California, San Francisco Committee on Animal Research approved the use of mice for these experiments.
Measurements of Intestinal mRNA Expression.
RNA isolates from small intestine and colon of Agr2−/− and control Agr2+/+ littermates were analyzed by using quantitative RT-PCR and Agilent oligonucleotide microarrays as described in detail in SI Methods.
Colitis Model.
Agr2−/− mice and control Agr2+/+ littermates were given drinking water containing 1.5% DSS (40 kDa; MP Biomedicals). Body weight was measured and stools were examined for the presence of gross blood each day. After 8 days, mice were killed for histological analysis.
Statistical Analysis.
Statistical analyses of microarray data are described in SI Methods. For other data, P values for comparisons between Agr2+/+ and Agr2−/− mice were obtained by using Student's t test unless otherwise indicated.
Supplementary Material
Acknowledgments.
We thank R. Barbeau for performing the microarray analyses, Y.S. Choi for technical advice, G. C. Hansson for providing the MUC2 expression constructs, and W. E. Finkbeiner, R. M. Locksley, A. Ma, D. Sheppard, and E. van Anken for comments. This work was supported by funding from the National Heart, Lung, and Blood Institute, National Institutes of Health/National Center for Research Resources University of California, San Francisco-Clinical and Translational Science Institute Grant Number UL1 RR024131, the Sandler Asthma Basic Research Center, and Korea Research Foundation Grant KRF-2005-013-E00017. G.Z. was supported in part by funding from the National Natural Science Foundation of China Grant 30770941, and C.V. was supported by a Fellowship from the Belgian-American Educational Foundation and by the Leon Fredericq Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray data have been deposited in the Gene Expression Omnibus (GEO) Database, www.ncbi.nlm.nih.gov/geo (accession no. GSE12503).
This article contains supporting information online at www.pnas.org/cgi/content/full/0808722106/DCSupplemental.
References
- 1.van Anken E, Braakman I. Versatility of the endoplasmic reticulum protein folding factory. Crit Rev Biochem Mol Biol. 2005;40:191–228. doi: 10.1080/10409230591008161. [DOI] [PubMed] [Google Scholar]
- 2.Ellgaard L, Ruddock LW. The human protein disulphide isomerase family: Substrate interactions and functional properties. EMBO Rep. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Persson S, et al. Diversity of the protein disulfide isomerase family: Identification of breast tumor induced Hag2 and Hag3 as novel members of the protein family. Mol Phylogenet Evol. 2005;36:734–740. doi: 10.1016/j.ympev.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Anelli T, et al. ERp44, a novel endoplasmic reticulum folding assistant of the thioredoxin family. EMBO J. 2002;21:835–844. doi: 10.1093/emboj/21.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anelli T, et al. Thiol-mediated protein retention in the endoplasmic reticulum: The role of ERp44. EMBO J. 2003;22:5015–5022. doi: 10.1093/emboj/cdg491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunther R, et al. Functional replacement of the Saccharomyces cerevisiae Trg1/Pdi1 protein by members of the mammalian protein disulfide isomerase family. J Biol Chem. 1993;268:7728–7732. [PubMed] [Google Scholar]
- 7.Laboissiere MC, Sturley SL, Raines RT. The essential function of protein-disulfide isomerase is to unscramble non-native disulfide bonds. J Biol Chem. 1995;270:28006–28009. doi: 10.1074/jbc.270.47.28006. [DOI] [PubMed] [Google Scholar]
- 8.Komiya T, Tanigawa Y, Hirohashi S. Cloning of the gene gob-4, which is expressed in intestinal goblet cells in mice. Biochim Biophys Acta. 1999;1444:434–438. doi: 10.1016/s0167-4781(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Vilar J, Hill RL. The structure and assembly of secreted mucins. J Biol Chem. 1999;274:31751–31754. doi: 10.1074/jbc.274.45.31751. [DOI] [PubMed] [Google Scholar]
- 10.Godl K, et al. The N terminus of the MUC2 mucin forms trimers that are held together within a trypsin-resistant core fragment. J Biol Chem. 2002;277:47248–47256. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- 11.Lidell ME, et al. The recombinant C-terminus of the human MUC2 mucin forms dimers in Chinese-hamster ovary cells and heterodimers with full-length MUC2 in LS 174T cells. Biochem J. 2003;372:335–345. doi: 10.1042/BJ20030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raykhel I, et al. A molecular specificity code for the three mammalian KDEL receptors. J Cell Biol. 2007;179:1193–1204. doi: 10.1083/jcb.200705180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 1999;4:D286–D298. doi: 10.2741/karam. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Hao Y, Lowe AW. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res. 2008;68:492–497. doi: 10.1158/0008-5472.CAN-07-2930. [DOI] [PubMed] [Google Scholar]
- 15.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 16.Podolsky DK, et al. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993;268:12230. [PubMed] [Google Scholar]
- 17.Van der Sluis M, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Velcich A, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 19.Ito S, Chen C, Satoh J, Yim S, Gonzalez FJ. Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J Clin Invest. 2007;117:1940–1950. doi: 10.1172/JCI31647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devani M, et al. Kallikrein-kinin system in inflammatory bowel diseases: Intestinal involvement and correlation with the degree of tissue inflammation. Dig Liver Dis. 2005;37:665–673. doi: 10.1016/j.dld.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Heazlewood CK, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laubitz D, et al. Colonic gene expression profile in NHE3-deficient mice: Evidence for spontaneous distal colitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G63–G77. doi: 10.1152/ajpgi.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okayasu I, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 25.Egger B, et al. Characterisation of acute murine dextran sodium sulphate colitis: Cytokine profile and dose dependency. Digestion. 2000;62:240–248. doi: 10.1159/000007822. [DOI] [PubMed] [Google Scholar]
- 26.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 27.Jessop CE, et al. ERp57 is essential for efficient folding of glycoproteins sharing common structural domains. EMBO J. 2007;26:28–40. doi: 10.1038/sj.emboj.7601505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garbi N, Tanaka S, Momburg F, Hammerling GJ. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. Nat Immunol. 2006;7:93–102. doi: 10.1038/ni1288. [DOI] [PubMed] [Google Scholar]
- 29.Asker N, Axelsson MA, Olofsson SO, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J Biol Chem. 1998;273:18857–18863. doi: 10.1074/jbc.273.30.18857. [DOI] [PubMed] [Google Scholar]
- 30.Zheng W, et al. Evaluation of AGR2 and AGR3 as candidate genes for inflammatory bowel disease. Genes Immun. 2006;7:11–18. doi: 10.1038/sj.gene.6364263. [DOI] [PubMed] [Google Scholar]
- 31.Thompson DA, Weigel RJ. hAG-2, the human homologue of the Xenopus laevis cement gland gene XAG-2, is coexpressed with estrogen receptor in breast cancer cell lines. Biochem Biophys Res Commun. 1998;251:111–116. doi: 10.1006/bbrc.1998.9440. [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Rudland PS, Sibson DR, Platt-Higgins A, Barraclough R. Human homologue of cement gland protein, a novel metastasis inducer associated with breast carcinomas. Cancer Res. 2005;65:3796–3805. doi: 10.1158/0008-5472.CAN-04-3823. [DOI] [PubMed] [Google Scholar]
- 33.Innes HE, et al. Significance of the metastasis-inducing protein AGR2 for outcome in hormonally treated breast cancer patients. Br J Cancer. 2006;94:1057–1065. doi: 10.1038/sj.bjc.6603065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Missiaglia E, et al. Analysis of gene expression in cancer cell lines identifies candidate markers for pancreatic tumorigenesis and metastasis. Int J Cancer. 2004;112:100–112. doi: 10.1002/ijc.20376. [DOI] [PubMed] [Google Scholar]
- 35.Zhang JS, Gong A, Cheville JC, Smith DI, Young CY. AGR2, an androgen-inducible secretory protein overexpressed in prostate cancer. Genes Chromosomes Cancer. 2005;43:249–259. doi: 10.1002/gcc.20188. [DOI] [PubMed] [Google Scholar]
- 36.Pohler E, et al. The Barrett's antigen anterior gradient-2 silences the p53 transcriptional response to DNA damage. Mol Cell Proteomics. 2004;3:534–547. doi: 10.1074/mcp.M300089-MCP200. [DOI] [PubMed] [Google Scholar]
- 37.Uehara T, et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 38.Reinhardt C, et al. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–1131. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.