Abstract
The chemotactic response of murine peritoneal macrophages to RANTES/CCL5 was inhibited significantly following pretreatment with delta-9-tetrahydrocannabinol (THC), the major psychoactive component in marijuana. Significant inhibition of this chemokine directed migratory response was obtained also when the full cannabinoid agonist CP55940 was used. The CB2 receptor-selective ligand O-2137 exerted a robust inhibition of chemotaxis while the CB1 receptor-selective ligand ACEA had a minimal effect. The THC-mediated inhibition was reversed by the CB2 receptor-specific antagonist SR144528 but not by the CB1 receptor-specific antagonist SR141716A. In addition, THC treatment had a minimal effect on the chemotactic response of peritoneal macrophages from CB2 knockout mice. Collectively, these results suggest that cannabinoids act through the CB2 receptor to trans-deactivate migratory responsiveness to RANTES/CCL5. Furthermore, the results suggest that the CB2 receptor may be a constituent element of a network of G protein-coupled receptor signal transductional systems, inclusive of chemokine receptors, that act coordinately to modulate macrophage migration.
Keywords: cannabinoid, cannabinoid receptor, CB2, CCL5, chemokine, chemokine receptor, chemotaxis, cross-deactivation, delta-9-tetrahydrocannabinol, macrophages, marijuana, RANTES, THC
Introduction
Delta-9-tetrahydrocannabinol (THC) is the major psychoactive component in marijuana. This exogenous cannabinoid exerts a variety of modulatory effects on the immune system, the majority of which have been reported to be immunosuppressive (Klein et al., 1998; Cabral and Dove Pettit, 1998; Cabral and Staab, 2005). In this capacity, THC affects a diverse array of immune cell types, including B lymphocytes (Klein et al., 1985), Natural Killer cells (Specter et al., 1986), T lymphocytes (Zimmerman et al., 1977), macrophages (Raz and Goldman, 1976; Friedman et al., 1986) and macrophage-like cells (Puffenbarger et al., 2000). Cannabinoid effects on cellular systems can be by both receptor-mediated and non-receptor-mediated (Makriyannis et al., 1990; Felder et al., 1992; Berdyshev et al. 2001; Price et al., 2004) modes. In terms of receptor-mediated action, two seven-transmembranal Gi/o protein-coupled receptors have been linked to THC effects on immune function. The first of these, CB1, is found at highest levels in the central nervous system (Matsuda et al., 1990; Galiegue et al., 1995; Herkenham et al., 1998) and testis (Galiegue et al, 1995) but is present also at low levels in various immune cells (Galiegue et al., 1995; Daaka et al., 1996; Waksman et al., 1999). The second receptor, CB2, is found primarily in immune cells (Munro et al., 1993; Galiegue et al., 1995) and appears to play a major role in immune modulation (Klein et al., 1998; Cabral and Dove Pettit, 1998; Cabral and Staab, 2005).
Of the various immune cell populations affected by THC and other cannabinoids, macrophages and macrophage-like cells appear to be a major target (Munro et al., 1993; Cabral et al., 1995; Waksman et al., 1999; Puffenbarger et al., 2000). Ultrastructural abnormalities have been observed in alveolar macrophages of humans who have been heavy users of marijuana (Mann et al., 1971) and in peritoneal macrophages of mice exposed in vitro to various concentrations of THC (Raz and Goldman, 1976). Various functional defects of alveolar and peritoneal macrophages from humans, rats or mice following in vivo or in vitro exposure to marijuana or THC also have been reported. These alterations have included decreases in cell motility, ability to spread in vitro, release of β-glucuronidase, phagocytosis of yeast particles, and inactivation of Staphylococcus aureus and S. albus (Huber et al., 1975; Chari-Briton, 1976; McCarthy et al., 1976; Drath et al., 1979; Huber et al., 1978; Lopez-Cepero et al., 1986; Specter et al., 1991; Tang et al., 1992). In addition, THC has been reported to affect macrophage processing of soluble protein antigens (McCoy et al., 1995; 1999).
A critical activity of macrophages that is exerted early in the inflammatory process is the ability to migrate in response to stimuli. This migratory activity is distinctive from that of stimulus-independent random cellular motion (Lauffenburger and Horwitz, 1996; Mitchison and Cramer, 1996). The two major modes of stimulus-dependent cellular motility are chemokinesis and chemotaxis. Chemokinesis is a process whereby cells exhibit random motion that is dependent on a chemo-stimulant (Becker et al., 1977;Keller et al., 1978). On the other hand, chemotaxis is a process in which cell motility is directed toward a concentration gradient of chemo-stimulant (Harris H, 1953, 1954; Jin and Hereld, 2006; Kehrl, 2006). In this chemotactic process, macrophage interaction with chemoattractants not only initiates a rapid and directed movement, but also is associated with a complex array of cellular events that includes changes in ion fluxes, alterations in integrin avidity, production of superoxide anions, and secretion of lysosomal enzymes (Murdoch and Finn, 2000). “Classical” chemoattractants include bacterial-derivedN-formyl peptides, the complement fragment peptides C5a and C3a, andlipids such as leukotriene B4 and platelet-activatingfactor (Schiffman et al., 1975; Goldman and Goetzl, 1982; Hanahan, 1986; Gerard and Gerard, 1994). Chemokines represent a second group of chemoattractants. These 8- to 17-kD molecular mass range cytokines are selective for leukocytes in vitro and elicit accumulation of inflammatory cells in vivo (Baggiolini et al., 1994, 1997; Kim, 2004; Le et al., 2004). Chemokines have been categorized into four groups on the basis of their cysteine motifs. The CC chemokines (β-chemokines) have two adjacent cysteine residues proximal to the amine terminus while those for the CXC chemokines (α-chemokines) are separated by an amino acid. The CX3X chemokines (δ-chemokines) have three amino acids between the two cysteines. The C chemokines (γ-chemokines) have only one cysteine near the amine terminus. As in the case for cannabinoid receptors, the specific effects of chemokines on target cells are mediated by G-protein-coupled receptors (Murdoch and Finn, 2000; Charo et al., 2006). Ligation of chemokines with their cognate receptors initiates a series of signal transductional events that results in regulation of leukocyte trafficking in inflammation, tissue injury, tumor development and host response to infection (Charo et al., 2006). Correlative to chemokine nomenclature, four families of chemokine receptors have been defined based on the motif of the cysteine residues within the chemokines they bind.
In the present study we demonstrate that THC inhibits the chemotactic response of murine peritoneal macrophages to RANTES/CCL5. The inhibitory effect was shown to be linked functionally to the CB2 receptor, suggesting that cannabinoids can signal through this receptor to trans-deactivate the chemokine receptor-mediated migratory response.
Materials and Methods
Mice
Eight-week old female (B6C3)F1 and C57BL/6 mice were obtained from Taconic Laboratories (Hudson, NY). CB2 (−/−) mice on a C57BL/6 background were obtained from Dr. Nancy E. Buckley (California Polytechnic University, Pomona, CA). CB2 deficiency was confirmed by polymerase chain reaction (PCR) as described (Buckley et al., 2000; Chuchawankul et al., 2004). Animals were quarantined for one week prior to initiation of experiments and were used as a source of peritoneal macrophages. All animal procedures were conducted in accordance with guidelines established by the Virginia Commonwealth University Institutional Animal Care and Use Committee (IACUC).
Cell Culture
Thioglycollate-elicited peritoneal macrophages were obtained by injecting mice intraperitoneally with 1 ml 10% Brewer’s yeast thioglycollate. Five days later, cells were harvested and screened for purity for macrophages by FACScan analysis using monoclonal antibody for the murine macrophage marker F4/80 (Serotec, Kidlington, Oxford, UK). Cells that were greater than 95% positive for F4/80 were used in studies. Macrophages (107 /ml) in RPMI 1640 medium lacking serum and supplemented with 1% L-glutamine, 1% nonessential amino acids, 1% MEM vitamins, 0.01M HEPES and penicillin [100 U/ml]/streptomycin [100 μg/ml]/fungizone [0.25 μg/ml]) were used in chemotaxis assays.
Drugs
Delta-9-tetrahydrocannabinol (THC; Ki = 40.7nM), a partial agonist for CB1 and CB2, was obtained from the National Institute on Drug Abuse (Rockville, MD). Additional cannabinoid analogs included the CB1 and CB2 full agonist CP55940 (Ki = 0.92 nM) and the highly selective CB2 ligand O-2137-2 (CB1 Ki = 2700nM, CB2 Ki = 11nM). The highly selective CB1 agonist ACEA (Ki = 1.4 nM) that displays > 1400-fold selectivity over CB2 was purchased from Tocris Cookson, Inc. (Ellisville, MO). The CB1 and CB2 antagonists SR141716 (CB1 Ki = 2nM, CB2 Ki > 1000nM) and SR144528 (CB1 Ki = 400nM, CB2 Ki = 0.6nM), respectively, were obtained from Sanofi Recherche (Montpellier, France). Stock solutions of cannabinoids (10−2M) were prepared in 100% ethanol and stored at 20°C. Experimental concentrations were obtained by dilution of cannabinoid stock solutions in RPMI 1640 medium used for macrophage culture to yield a final ethanol concentration of 0.01%. Vehicle controls consisted of 0.01% ethanol in medium.
Chemotaxis Assay
Chemotaxis was measured using transwell inserts pre-loaded in 35 mm standard tissue culture plates (Corning Inc., Corning, NY), in which the upper and lower compartments were separated by a polycarbonate filter with 8 μm pores (Corning Inc., Corning, NY). Peritoneal macrophages (1×107 /ml) were pre-incubated in RPMI 1640 lacking serum and containing vehicle (0.01% ethanol) or cannabinoid (10−6M – 10−12M) for 3 h at 4°C. This time regimen for drug exposure was obtained through initial optimization experiments. Serum was omitted from the culture medium since it contains lipids and other factors that have the capacity to stimulate macrophage migration that could confound interpretation of migratory responses as attributable to RANTES. For experiments using antagonists, cells were exposed to SR141716A (10−6M) or SR144528 (10−6M) for 1h prior to treatment with THC or CP55940 for 3h. Following vehicle or cannabinoid treatment, 100 μl of drug- or vehicle-treated cell suspension (106cells) were placed in the upper chamber of the transwell insert. For assessment of chemotaxis (directed migration against a chemokine concentration gradient) the lower compartment was loaded (600μl) with medium containing murine RANTES (1 ng/ml; R&D Systems, Minneapolis, MN). This concentration of RANTES was selected based on preliminary titration for a chemoattractant response that approximated a mid-point in the linear phase of the dose-response curve. For assessment of chemokinesis (enhanced random migration to chemokine), RANTES (1 ng/ml) was included in both the top and bottom chambers to eliminate the chemoattractant concentration gradient. In addition, for a select number of experiments, RANTES was eliminated from both chambers. The assembled migration plate chamber system was incubated (1h) at 37°C in a 5%CO2 atmosphere. To determine the number of cells that migrated to the bottom chamber, the upper chamber (i.e., polycarbonate filter) was removed and video still images (1mm2) in five random fields of each bottom chamber were captured using an Olympus CK2 inverted microscope (Opelco, Washington, DC) with an attached XV-GP230 digital video camera (Panasonic, Yokohama, Japan) interfaced to a Dell Dimension XPS1450 computer using Videum 100 hardware and Window NT software (Winnov, Sunnyvale, CA). The number of cells migrating into the bottom compartment/transwell plate was calculated as the sum of the five 1 mm2 fields and was represented as cells/mm2/well. Each sample group was run in duplicate and each experiment was performed in triplicate. Migration for each sample group was represented as the mean (±SD) of the total number of migrating cells counted in five fields of duplicate wells. A greater than 2-fold increase in cell migration to the chemoattractant RANTES in the lower compartment as compared to that in the absence of RANTES in the lower compartment was indicative of a positive response.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Real time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) using SYBR Green for CB1 and CB2 and GAPDH primers was used to assess for the presence of CB1 and CB2 mRNA. Total RNA from peritoneal macrophages was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA) according the manufacturer’s instructions. The RNA then was isolated by chloroform:isopropanol extraction and resuspended in 50 μl PCR grade water. The isolated RNA was treated with RNase-free DNase I Amplification grade (Invitrogen, Carlsbad, CA) to remove residual genomic DNA. The reverse transcription (RT) step was performed in a Bio-Rad iCycler (BioRad, Richmond, CA) using the SuperScript III First-Strand Synthesis System (Invitrogen) that included random hexamers as primer to generate complementary DNA (cDNA). SYBR Green real-time PCR was performed using the RT2 PCR Primer Set for mouse CB1 (Cnr 1: PPM04603A) or CB2 (Cnr 2: PPM04826A) as described by the manufacturer (SuperArray Bioscience Corp., Frederick, MD). Briefly, each 25:l PCR mix consisted of 12.5:l 2X RT2 Real-Time SYBR Green PCR Master Mix (SuperArray), 1.0:l first strand cDNA template, and 1.0:l RT2 PCR Primer Set brought to a final volume of 25:l with DEPC-treated water. Tubes containing the PCR mix were placed in a SmartCycler (Cepheid, Sunnyvale, CA) and PCR was performed using the following program: 95°C, 15 min; 40 cycles of (95°C, 30 sec; 55°C, 30 sec; and 72°C, 30 sec). The resulting PCR products were visualized by electrophoresis (100V) using 4% OmniPur Agarose PCr Plus (VWR, West Chester, PA) gel in TBE. A pCD-SKR6 plasmid template (gift from Dr. L. Matsuda, Medical University of South Florida, Charleston, SC; Matsuda et al., 1990) and a pUC18-mCB2 plasmid template (gift from T. Bonner, NIMH, Bethesda, MD) served as positive PCR controls for CB1 and CB2, respectively. Using this approach, amplification products of 167 bp and 207 bp were generated for CB1 and CB2, respectively.
Multiprobe Ribonuclease Protection Assay
Total RNA prepared from peritoneal macrophages using TRIzol reagent (Invitrogen) was redissolved after isopropanol precipitation directly in 1X hybridization buffer (BD Biosciences/PharMingen, San Diego, CA). A Riboquant Multi-probe Ribonuclease Protection Assay (RPA) was used to assess for levels of murine chemokine receptor mRNA (mcr-5 probe template set; BD Biosciences/PharMingen). The ribo-probes were labeled with 32P[UTP] (ICN, Costa Mesa, CA) to a specific activity of greater than 3000Ci/mmol. The isolated RNA samples then were hybridized with the probe overnight at 56°C and the protected fragments were resolved on a 6% polyacrylamide gel containing 6M urea. Imaging of the protected fragments was performed using a 445 SI Phosphorimager (Molecular Dynamics, Sunnyvale, CA). The pixel intensity of each band was quantified using ImageQuant 4.1 software (Molecular Dynamics) and the amount of chemokine receptor mRNA was normalized for loading by dividing the pixel value for the chemokine receptor band by the sum of the pixel values for the mRNAs of the housekeeping genes, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and a ribosomal protein, L32.
SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Immunoblotting
Peritoneal macrophages suspended in sterile PBS were centrifuged (3000 × g, 10 min, 4°C) and the pellet was resuspended in an equal volume of sterile water containing a Protease Inhibitor Cocktail (100:1) (Sigma, St. Louis, MO; 4-(2-aminoethyl) benzenesulfonyl fluoride, pepstatin A, E-64, bestatin, leupeptin, aprotinin). The cells then were subjected to three cycles of freeze-thaw and the lysate homogenized. Protein concentrations were determined by the Bradford assay (Bradford, 1976). Protein samples (15 μg/sample) were separated on a 12% polyacrylamide gel and transferred to a Transblot Transfer nitrocellulose membrane (BioRad, Hercules, CA). The membranes were incubated individually with anti-CCR1, anti-CCR5, and anti-CB2 antibody. The antibody to CCR1 (CKR1 H-52, Santa Cruz Biotechnologies, CA) was directed against the extracellular amino terminus whereas the antibody to CCR5 (CKR5 D-19, Santa Cruz) was directed against the carboxy terminal domain. The antibody to CB2 (CB2-YL) was elicited in rabbits using the peptide [YLQGLGPEGKEEAPRSS] comprising amino acids 320–336 of the murine CB2 linked chemically to keyhole limpet hemocyanin, affinity-purified, and assessed for specificity as described (Dove Pettit et al., 1998).
Statistical Analysis
Analysis of variance (ANOVA) was performed using Dunnett’s test and was followed by a Student’s t-test to allow for comparison of each sample to the vehicle, in addition to comparison between treatment groups.
Results
Murine Thioglycollate-Elicited Peritoneal Macrophages Express the Chemokine Receptors CCR1 and CCR5 and the Cannabinoid Receptor CB2
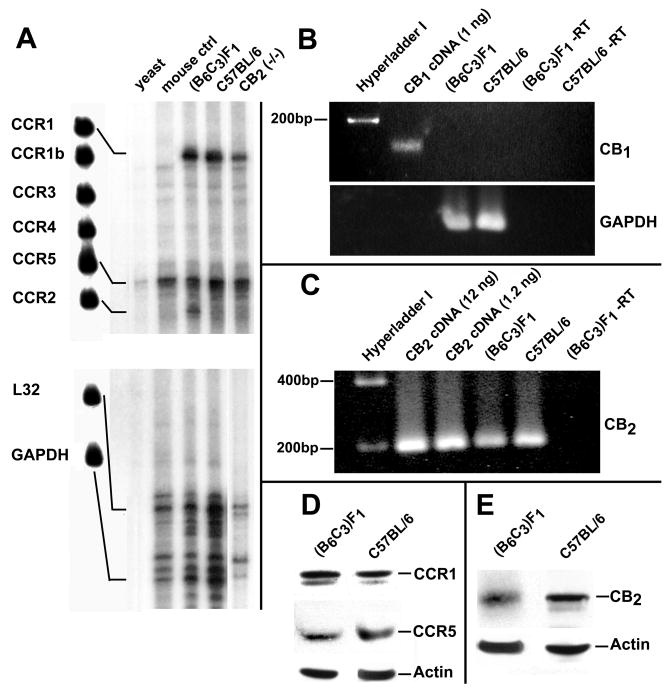
RANTES binds to the chemokine receptors CCR1, CCR3, and CCR5 (Murphy, 2002; Bajetto et al., 2002; Charo et al., 2006). Thus, in order to determine the CC chemokine receptor gene expression profile of (B6C3)F1 and C57BL/6 murine thioglycollate-elicited peritoneal macrophages, a multiprobe RNase Protection assay was employed. Using a template set for CC chemokine receptors, it was demonstrated that the predominant chemokine receptor mRNAs detected for (B6C3)F1 micewere those for CCR1, CCR2 and CCR5 (Fig. 1). C57BL/6 mice contained approximately equal levels of mRNA for CCR1 and CCR5 but, in contrast to (B6C3)F1 mice, contained low levels of mRNA for CCR2. Because RANTES is a major agonist for CCR1 and CCR5, but not CCR2, the presence of protein for the former two receptors also was determined (Fig. 1). Consistent with the mRNA data, approximately equivalent levels of protein for CCR1 and CCR5 were detected in thioglycollate-elicited macrophages of (B6C3)F1 and C57BL/6 mice.
Figure 1.
Thioglycollate-Elicited Murine Peritoneal Macrophages Express CCR1, CCR5 and CB2. (Left Panel) Multi-probe ribonuclease protection assay depicting mRNAs for CC chemokine receptors in murine peritoneal macrophages. The left-most lane (Lane 1) depicts the undigested probes for the CC chemokines CCR1, CCR1b, CCR2, CCR3, CCR4, CCR5 and the constitutively expressed internal controls L32 and GAPDH. Lanes 2 and 3 represent yeast and mouse controls, respectively. (Right Two Uppermost Panels) Product from real-time SYBR Green RT-PCR illustrating the presence of message for CB1 and CB2 receptors in peritoneal macrophages from (B6C3)F1 and C57BL/6 mice. Amplification products of 167 bp and 207 bp were generated for CB1 and CB2, respectively. (Bottom Middle Left Panel) Western immunoblot depicting immunoreactive product for CCR1, CCR5, or the actin control from homogenates of peritoneal macrophages from (B6C3)F1 and C57BL/6 mice. (Bottom Right Panel) Western Immunoblot depicting immunoreactive product for the CB2 in homogenates of peritoneal macrophages from (B6C3)F1 or C57BL/6 mice.
SYBR Green RT-PCR was employed to assess for the presence of CB2 mRNA in peritoneal macrophages from (B6C3)F1 and C57BL/6 mice. A 207 bp amplicon, consistent with the fragment size predicted for the CB2, was detected from total RNA of peritoneal macrophages (Fig. 1). Western immunoblot analysis using a murine CB2 domain-specific antibody confirmed the presence of the CB2 in murine peritoneal macrophages at the protein level (Fig. 1). Evidence for the presence of CB1 mRNA or protein in thioglycollate-elicited peritoneal macrophages from (B6C3)F1 and C57BL/6 mice using SYBR Green RT-PCR or Western immunoblot analysis, respectively, was not obtained (Data not shown).
Treatment with THC in vivo Results in Inhibition of the Chemotactic Response of Murine Peritoneal Macrophages to RANTES in vitro
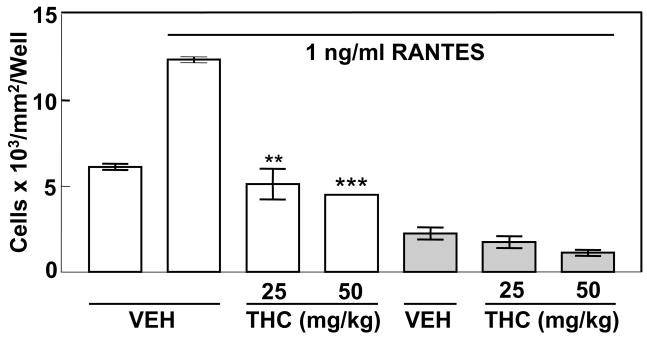
(B6C3)F1 mice were inoculated with thioglycollate and 5 days later were administered a single intraperitoneal injection of vehicle (ethanol:emulphor:saline, 1:1:18) or THC (25 mg/kg or 50 mg/kg). Peritoneal macrophages were harvested 24h later and were subjected to migration assay. In vivo administration of 25 mg/kg or 50 mg/kg THC resulted in a significant and greater than 50% inhibition of cell migration in response to RANTES as compared to that observed for cells of mice receiving vehicle (Fig. 2). No significant differences in migration were obtained between vehicle and drug treated cells when RANTES was placed in both the top and bottom compartments to eliminate the RANTES concentration gradient. These results are consistent with THC as exerting an inhibitory effect on the macrophage chemotactic response to RANTES.
Figure 2.
Treatment in vivo with THC Results in Inhibition of the Chemotactic Response to RANTES. (B6C3)F1 mice were injected intraperitoneally with 10% thioglycollate to elicit macrophages. Five days later, the mice were injected intraperitoneally with Vehicle (1:1:18, ethanol:emulphor:saline) or THC (25 mg/kg or 50 mg/kg). Migration of macrophages to 1ng/ml RANTES was assessed in vitro using transwell tissue culture inserts. Results are presented as the mean ± SD. For RANTES placed only in the bottom chamber, SD was compared with that of vehicle-treated macrophages exposed to RANTES in the bottom chamber. For RANTES placed in both chambers (shaded bars), SD was compared with that of vehicle-treated macrophages exposed to RANTES in both chambers. When RANTES was added only to the bottom compartment, THC as compared to the vehicle control exerted a major inhibitory effect on cell migration to the bottom compartment. When RANTES was added to both the upper and lower compartments to eliminate the chemoattractant concentration gradient, THC as compared to the vehicle control did not result in significant inhibition of cell migration to the bottom well. These results indicate that THC inhibits directed migration (i.e., chemotaxis) to a RANTES concentration gradient rather enhancement of random movement (i.e., chemokinesis) to RANTES. **p<0.01, ***p<0.001.
Treatment with THC and CP55940 in vitro Results in Inhibition of the Chemotactic Response of Murine Peritoneal Macrophages to RANTES
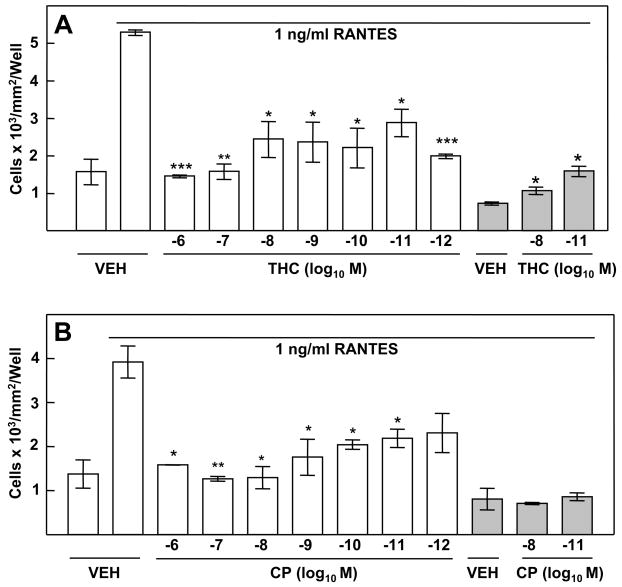
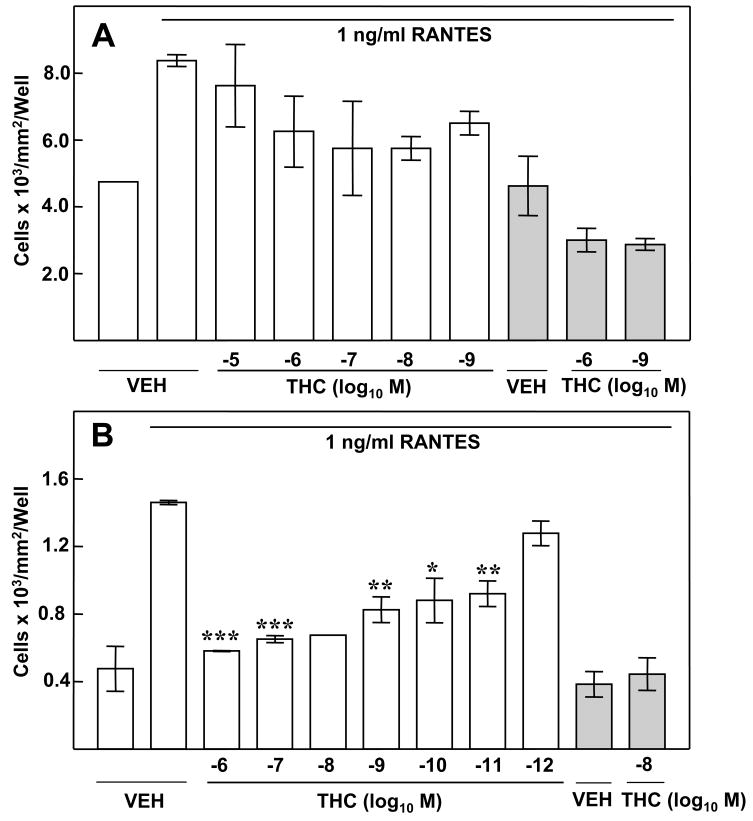
In order to determine whether THC exerted a direct effect on macrophages, in vitro exposure experiments were performed. THC treatment of (B6C3)F1 murine peritoneal macrophages in vitro resulted in a significant inhibition of the chemotactic response to RANTES (Fig. 3A). Cells treated with vehicle exhibited a minimal level of migration (i.e., approximately 1600 cells/mm2/well) to the bottom compartment in the absence of RANTES (Fig 3A). In contrast, when RANTES was added to the bottom compartment to establish a chemokine concentration gradient, a nearly five-fold increase (i.e., in excess of 5000 cells/mm2/well) was obtained for macrophages treated with vehicle. Treatment of macrophages with THC (10−6M 10−12M) resulted in a significant inhibition of migration in response to RANTES. THC, at a concentration as low as 10−12M, exerted a major inhibitory effect on cell migration, with numbers of cells in the bottom compartment approximating those for cells treated with vehicle and not exposed to RANTES. Again, the inhibitory effect of THC on macrophage migration was at the level of chemotaxis rather than chemokinesis. When RANTES was added to both the upper and lower compartments to eliminate the chemoattractant concentration gradient to allow for assessment of random migration to chemokine, approximately 1000 cells/mm2/well were obtained for peritoneal macrophages treated with vehicle. Treatment of these cells with 10−8M or 10−11M THC did not result in significant inhibition of this random movement. Rather, a slight augmentation in random migration to the bottom compartment was recorded.
Figure 3.
Treatment in vitro with THC and CP55940 Results in Inhibition of the Chemotactic Response to RANTES. Migration of peritoneal macrophages to 1ng/ml RANTES was assessed following in vitro treatment (3h) with cannabinoid (10−6 to 10−12 M) or vehicle (0.01% ethanol). (A) Treatment with the partial agonist THC resulted in inhibition of chemotaxis. (B) Treatment with the full agonist CP55940 resulted in a robust dose-related inhibition of chemotaxis to RANTES. Results are presented as the mean ± SD. For RANTES placed only in the bottom chamber, SD was compared with that of vehicle-treated macrophages exposed to RANTES in the bottom chamber. For RANTES placed in both chambers (shaded bars), SD was compared with that of vehicle-treated macrophages exposed to RANTES in both chambers. *p<0.05, **p<0.01, ***p<0.001.
Experiments performed with THC were replicated using CP55940, a full agonist at CB1 and CB2 (Fig. 3B). Again, a minimal level in cell migration was observed for control wells. Approximately 1500 cells/mm2/well were recorded when vehicle-treated cells were placed in the top compartment in the absence of RANTES in the bottom compartment. An approximate fourfold increase in the number of peritoneal macrophages treated with vehicle was obtained when RANTES was placed in the bottom compartment to establish a chemoattractant gradient. Treatment of cells with CP55940 (10−6M – 10−12M) resulted in a significant concentration-related decrease in migration in response to RANTES. A greater than 50% inhibition in migration was obtained for cells treated with CP55940 at 10−6M – 10−9M as compared to vehicle control. CP55940 as compared to vehicle did not affect macrophage migration when RANTES was placed both in the top and bottom compartments to eliminate the chemoattractant gradient, indicating that the effect of CP55940 on migration was at the level of chemotaxis rather than chemokinesis.
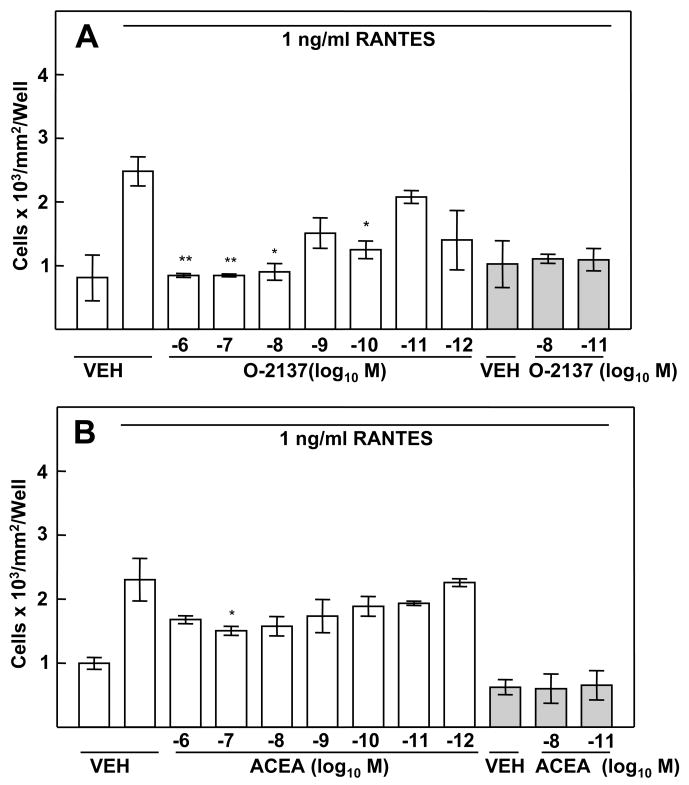
The CB2-selective Ligand O-2137 Exerts a Robust Inhibitory Effect on the Murine Peritoneal Macrophage Chemotactic Response to RANTES
The concentration-related inhibitory effect of THC and CP55940 on the chemotactic response of murine peritoneal macrophages to RANTES implicated a role for a cannabinoid receptor in this process. In order to obtain insight as to the cannabinoid receptor linked to the inhibitory effect, macrophages from (B6C3)F1 mice were treated with compounds exhibiting selective high affinity binding to the CB1 or the CB2 antecedent to assessment of the chemotactic response. Treatment of macrophages with the highly selective CB2 ligand O-2137 resulted in a profound and significant concentration-related inhibition in the chemotactic response to RANTES (Fig. 4A). For drug concentrations of 10−6M–10−8M, a greater than 50% inhibition, as compared to vehicle control, was observed. In contrast, the CB1 specific ligand ACEA (10−6M–10−12M) exerted a minimal inhibitory effect on the peritoneal macrophage chemotactic response to RANTES (Fig. 4B).
Figure 4.
Effect of CB1- and CB2-selective Ligands on the Chemotactic Response to RANTES. (A) Treatment (3h) with the CB2-selective ligand O-2137 resulted in a robust and significant inhibition of chemotaxis. (B) Treatment with the CB1-selective ligand ACEA had a minimal effect on RANTES-induced migration of peritoneal macrophages. Results are presented as the mean ± SD. For RANTES placed only in the bottom chamber, SD was compared with that of vehicle-treated macrophages exposed to RANTES in the bottom chamber. For RANTES placed in both chambers (shaded bars), SD was compared with that of vehicle-treated macrophages exposed to RANTES in both chambers. *p<0.05, **p<0.01.
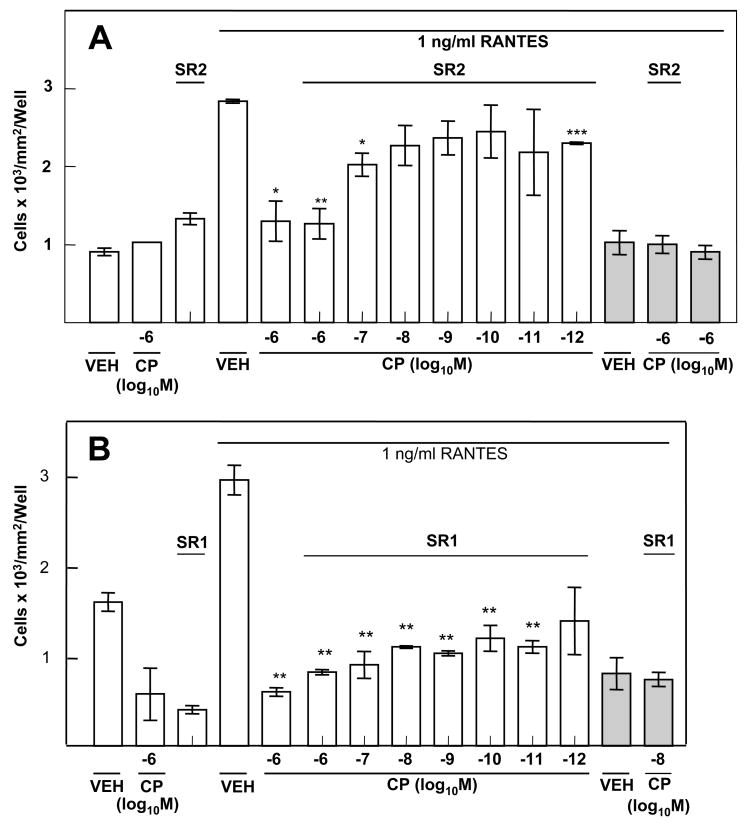
The CB2-specific Antagonist SR144528 Reverses the Inhibitory Effect of CP55940 on the Murine Peritoneal Macrophage Chemotactic Response to RANTES
In order to confirm the data indicating that activation of the CB2 with a cannabinoid receptor selective ligand exerted a major inhibitory effect on the chemotactic response to RANTES, cannabinoid receptor agonist-antagonist experiments were performed. For these experiments, the CB1 or CB2 antagonist was used at a concentration of 10−6M. Treatment of (B6C3)F1 murine peritoneal macrophages with the CB2-specific antagonist SR141716A alone had no major effect on the chemotactic response to RANTES. At equimolar concentrations (i.e., 10−6M) of antagonist and agonist, CP55940 inhibited macrophage chemotaxis to RANTES. However, at lower concentrations (10−7M 10−11M) of CP55940, the inhibitory effect of the agonist was reversed by the CB2 antagonist SR144528 (Fig. 5A). These results were in contrast to those obtained when the CB1 antagonist SR141716A was used (Fig. 5B). SR141716A (10−6M 10−12M) did not block the inhibitory effect of CP55940.
Figure 5.
Effect of Cannabinoid Receptor Antagonists on Chemotaxis to RANTES. (A) The CP55940-mediated inhibition of chemotaxis was reversed by the CB2 antagonist SR144528. (B) Treatment with the CB1 antagonist SR141716A (10−6M) did not block CP55940-mediated inhibition of chemotaxis. Macrophages were treated (1h) with antagonist prior to treatment (3h) with cannabinoid. Results are presented as the mean ± SD. For RANTES placed only in the bottom chamber, SD was compared with that of vehicle-treated macrophages exposed to RANTES in the bottom chamber. For RANTES placed in both chambers (shaded bars), SD was compared with that of vehicle-treated macrophages exposed to RANTES in both chambers. *p<0.05, **p<0.01, ***p<0.001.
THC Does Not Inhibit the Chemotactic Response to RANTES of Peritoneal Macrophages from CB2 Knockout Mice
To confirm the pharmacological data implicative of a functional linkage of the CB2 to cannabinoid-mediated inhibition of macrophage chemotaxis to RANTES, experiments were performed using thioglycollate-elicited peritoneal macrophages from C57BL/6 CB2 knockout mice. THC (10−5M–10−9M) had no significant effect on either the chemotactic or chemokinetic response of macrophages from the knockout mice (Fig. 6A). Since these CB2 null animals were generated on a C57BL/6 genetic background, replicate migration experiments were performed using thioglycollate-elicited peritoneal macrophages from their C57BL/6 CB2 (+/+) wild-type counterparts. Consistent with the data obtained using (B6C3)F1 mice, THC exerted a concentration-related inhibition of the chemotactic response of peritoneal macrophages to RANTES (Fig. 6B).
Figure 6.
Effect of THC on the Chemotactic Response of Peritoneal Macrophages from CB2 Knockout Mice to RANTES. (A) In vitro THC treatment (3h) did not have a significant effect on RANTES-induced migration by peritoneal macrophages from CB2 receptor knockout mice. (B) THC treatment (3h) resulted in a concentration-related inhibition of the chemotactic response of peritoneal macrophages to RANTES from the C57BL/6 CB2 (+/+) wild-type counterpart. Results are presented as the mean ± SD. For RANTES placed only in the bottom chamber, SD was compared with that of vehicle-treated macrophages exposed to RANTES in the bottom chamber. For RANTES placed in both chambers (shaded bars), SD was compared with that of vehicle-treated macrophages exposed to RANTES in both chambers. *p<0.05, **p<0.01, ***p<0.001.
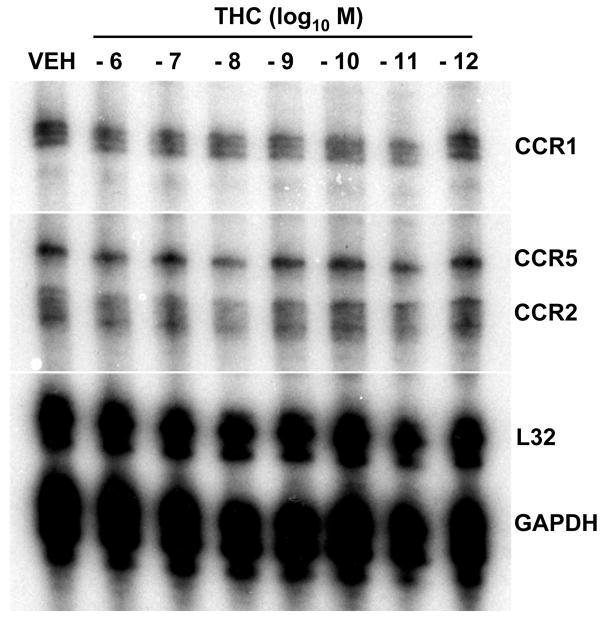
THC Does Not Alter mRNA Levels of CC Chemokine Receptors in Thioglycollate-Elicited Murine Peritoneal Macrophages
Chemotaxis to RANTES results from a complex series of signal transductional activities following ligation of the chemokine to its cognate G protein-coupled receptor. THC treatment of macrophages could affect activation of chemokine receptors and alter their expression and/or compartmentalization. Thus, in order to obtain initial insight as to the mode of action through which THC treatment results in inhibition of chemotaxis, experiments were performed to assess for levels CC chemokine mRNA in peritoneal macrophages. THC (10−6M–10−12M) treatment (3h) had no major effect on total mRNA levels of CCR1, CCR2 or CCR5 (Fig. 7). Similarly, at this concentration range THC had no major effect on total mRNA levels of CB2 (Data not shown).
Figure 7.
Effect of THC on Levels of CC Chemokine Receptor mRNA. Multiprobe ribonuclease protection assay demonstrated that THC (10−6M–10−12M) treatment (3h) had no major effect on levels of CCR1, CCR2, and CCR5 in thioglycollate-elicited (B6C3)F1 murine peritoneal macrophages.
Discussion
THC, the major psychoactive component in marijuana, has been shown to alter the activities of macrophages and macrophage-like cells, including phagocytosis (Friedman et al., 1986; Lopez-Cepero et al., 1986; Tang et al., 1992; Ehrhart et al., 2005), antigen processing (McCoy et al., 1995; McCoy et al., 1999), and production of chemokines and cytokines (Watzl et al., 1991; Zheng et al., 1992; Puffenbarger et al., 2000). Recent studies indicate that this exogenous cannabinoid, as well as other cannabinoids, also affects the migratory activities of macrophages. Stefano et al. (1998) reported that acute exposure to the endogenous cannabinoid (endocannabinoid) anandamide resulted in transformation of macrophages from an amoeboid and motile state to that of a rounded and non-motile conformation. These investigators proposed that the transforming events were linked to the CB1 since the CB1-specific antagonist SR141716A blocked the transformation. Sacerdote et al. (2000) demonstrated that in vivo and in vitro treatment of rat peritoneal macrophages with CP55940, a full agonist at both CB1 and CB2 receptors, resulted in decreased migration in vitro to the peptide formal-methionyl-leucine-phenylalanine (fMLP). It was indicated that, while both the CB1 and CB2 receptors appeared to be involved in this process, the cannabinoid-mediated effect was linked primarily to the CB2. The chemotactic response of murine macrophages to fMLP also has been shown to be decreased by cannabidiol (Sacerdote et al., 2005), a cannabinoid that binds weakly to CB2. The CB2 antagonist SR144528 prevented this decrease, suggesting a functional linkage to this receptor. On the other hand, Walter et al. (2003) found that the endocannabinoid 2-arachidonylglycerol (2-AG) triggered migration of microglia, macrophages that are resident in the brain, and that the CB2 was involved in this effect. Collectively, these studies suggest that exogenous cannabinoids exert inhibitory effects on macrophage migration while endocannabinoids elicit an opposite effect.
Consistent with these observations, in the present study we demonstrate that THC inhibits the chemotactic or directed migratory response of murine peritoneal macrophages to RANTES, a chemokine that can signal through the chemokine receptors CCR1 and CCR5. This effect was exerted on peritoneal macrophages from mice administered THC or on peritoneal macrophages that were exposed directly to THC in vitro. In the latter context, the inhibition occurred over a wide concentration range (i.e., 10−6 M–10−12 M). These results are consistent with THC as having a direct effect on macrophages which results in inhibition of chemotaxis. The results obtained with THC were replicated using the full CB1/CB2 agonist CP55940. Treatment of murine macrophages in vitro with CP55940 resulted in inhibition of chemotaxis to RANTES over the same concentration range (i.e., 10−6 M–10−12 M) of THC. In order to establish whether the cannabinoid-mediated inhibition was linked to a cannabinoid receptor, a series of experiments was performed in which cannabinoid receptor-selective ligands as well as cannabinoid receptor-specific antagonists were used. Treatment of macrophages in vitro with O-2137, a compound that exhibits high selectivity for the CB2, resulted in a robust inhibition of macrophage chemotaxis. In contrast, the CB1 selective compound ACEA had a minimal effect. In addition, the CB2 antagonist SR144528 blocked CP55940-mediated inhibition of macrophage chemotaxis while the CB1 antagonist SR141716A had a minimal effect. Finally, THC was not able to inhibit the chemotactic response to RANTES of peritoneal macrophages obtained from CB2 knockout mice. Collectively, the results of experiments in which a pharmacological approach was complemented with that using macrophages from CB2 null (i.e., CB2 −/ −) mice support the proposition that the CB2 is linked functionally to the THC-mediated inhibition of chemotaxis to RANTES.
RANTES, for which the current International Union of Pharmacology nomenclature is CCL5 (Murphy, 2002), is one of many chemotactic cytokines that direct the migration of leukocytes to sites of infection and inflammation. In this capacity, these small molecular weight proteins constitute a critical component of innate immune defenses. Four subfamilies of chemokines have been identified based on the relative position of their N terminal cysteine residues. All chemokines bind specific receptors that are seven transmembranal and are coupled to heterotrimeric Gi proteins, a feature that is shared with cannabinoid agonists. However, binding within a chemokine subfamily is somewhat promiscuous. RANTES, for example, can bind CCR1, CCR3, and CCR5, receptors that have specialized roles in leukocyte trafficking (Murdoch and Finn, 2000; Murphy, 2002; Charo et al., 2006). In addition, multiple chemokine receptor types have been identified on individual immune cells and their expression may vary in relation to cell differentiation and activation. For example, monocytes have been reported to express a variety of chemokine receptors, particularly CCR1, CCR2, and CCR5 (Mantovani et al., 2004). It has been demonstrated also that differentiation of monocytes into tissue macrophages is associated with the upregulation of CCR1 and CCR5 and loss of CCR2 expression (Mantovani et al., 2004). In the present study, we examined thioglycollate-elicited peritoneal macrophages from (B6C3)F1 and C57BL/6 mice for their CC chemokine receptor expression profile. These cells were shown to express CCR1 and CCR5, receptors that can bind RANTES. Thus, in the context of our experimental paradigm it is possible that RANTES acted through one or both receptors to induce chemotactic activity. In turn, THC may have affected the functionality of one or both chemokine receptors. Regardless of which of the chemokine receptors found on macrophages is functionally relevant in RANTES-mediated signaling, the results of this study suggest that cannabinoid activation of the CB2 can result in deactivation of other members of the G protein-coupled family such as chemokine receptors. Studies utilizing chemokine receptor-specific antagonists should serve to identify the CC receptor type that is linked to RANTES-mediated chemotactic activity that is targeted by cannabinoids.
The mode by which THC and other analogs that signal through cannabinoid receptors to deactivate CCR1 and/or CCR5 chemokine receptor migratory responsiveness to RANTES remains to be defined. THC and other cannabinoids, as highly lipophilic molecules, can perturb cellular membranes (Martin, 1986; Makriyannis et al., 1990; Cabral and Staab, 2005). Such perturbation could alter conformational strictures requisite for ligand-receptor interaction, disrupt receptor-G protein complexes, and disturb intracellular membranous compartments that are linked to biochemical events in the cascade of signal transduction. However, as suggested by the present study, cannabinoids also may trans-deactivate chemokine receptors and affect their ability to elicit a signal transductional cascade that culminates in the chemotactic migratory response. Indeed, it has been reported that members of the G protein coupled receptor superfamily can associate with each other, forming homodimers and heterodimers that results in alteration in the functionality of one of the involved receptors (Rios et al, 2001). Opioid receptors, for example, have been reported to interact with chemokine receptors to alter their function. Grimm et al (1998) indicated that this interaction resulted in trans-deactivation of chemokine receptors and that it occurred through a process of receptor-mediated heterologous desensitization. In their studies, Met-enkephalin and morphine were shown to inhibit interleukin (IL)-8-induced chemotaxis of human neutrophils and macrophage inflammatory protein (MIP)-1α, RANTES, and monocyte chemoattractant protein (MCP)-1-mediated chemotaxis of human monocytes. This inhibition was indicated as mediated by δ and μG opioid receptors, the activation of which led to phosphorylation of the chemokine receptors CXCR1 and CXCR2 resulting in heterologous desensitization. Rogers et al (2000) reported that activation of opioid and chemokine receptors could lead to reciprocal down-regulation of leukocyte migratory activities. These observations have extended using a number of experimental paradigms (Szabo and Rogers, 2001; Szabo et al., 2001; Szabo et al., 2002; Suzuki et al., 2003; Zhang et al., 2003). Indeed, it has been proposed that cross-desensitization of chemokine receptors by opioids represents a significant element in opioid-mediated immunosuppression (Zhang et al., 2003). The process of heterologous desensitization may also apply to cannabinoid receptors. Ghosh et al. (2006) reported that the CB1/CB2 agonist CP55940, as well as the CB2-selective agonist JW-015, caused significant inhibition of chemokine CXCL12-induced chemotaxis of CD4+ and CD8+ T lymphocytes. These investigators also found that these cannabinoids inhibited CXCL12 induced chemotaxis and transendothelial migration of Jurkat T cells. Rios et al (2006) reported recently that the μ opioid receptor also interacts with the CB1 to effect a reciprocal inhibition of receptor signaling and neuritogenesis.
In the present study, heterologous desensitization may articulate a mode of action by which cannabinoids mediate inhibition of the murine peritoneal macrophage chemotactic response to RANTES. In order to obtain initial insight as to the process by which THC and other cannabinoids cross-deactivate this macrophage activity, a multiprobe RNase protection assay was performed to assess for levels of CC chemokine receptor mRNAs. THC over a concentration range of 10−6M to 10−12M had no effect on macrophage mRNA levels of CCR1 and CCR5. These results are consistent with THC-mediated inhibition of the chemotactic process as occurring at a site upstream to gene expression of the cognate receptors at the mRNA level. Studies are in progress to assess for effects of THC on protein expression and phosphorylation of CCR1, CCR5 and CB2 in the context of the chemotactic response.
In summary, we have demonstrated that THC and cannabinoids that activate the CB2 inhibit murine peritoneal macrophage chemotaxis to RANTES/CCL5. This inhibitory effect was linked functionally to the CB2. Furthermore, since this chemokine serves as a ligand for CCR1 and CCR5, these results suggest that activation of the CB2 leads to trans-deactivation of these G protein-coupled receptors of the CC chemokine subfamily that have specialized roles in leukocyte trafficking. Thus, as has been suggested for opioid receptors, CB2 “cross-talk” with chemokine receptors, may constitute an integrative component of a network of intercommunicating G protein-coupled receptors that regulate immune responses.
Acknowledgments
The Authors thank Ms. Christina L. Hartman for excellent technical assistance.
Source of Financial Support: These studies were supported through NIH Awards DA015608, DA05832, DA05274 and T32 DA07027.
Abbreviations
- ACEA
N-(2-Chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide
- CCL5
chemokine (C-C motif) ligand 5
- CP55940
(−)-cis-3-[2-Hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- O-2137
1R, 3R)-1-[4-(1,1-Dimethylheptyl)-2,6-dimethoxyphenyl]-3-methylcyclohexanol
- RANTES
Regulated upon Activation Normal T cell Expressed and Secreted
- SR141716A
5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(1-piperidyl)pyrazole-3-carboxamide hydrochloride
- SR144528
(1S-endo)-5-(4-Chloro-3-methylphenyl)-1-((4-methylphenyl)methyl)-N-(1,3,3-trimethylbicyclo(2.2.1)hept-2-yl)-1H-pyrazole-3-carboxamide
- THC
Delta-9-tetrahydrocannabinol
References
- Bajetto A, Bonavia R, Barbero S, Schettini G. Characterization of chemokines and their receptors in the central nervous system: physiopathological implications. J Neurochem. 2002;82:1311–1329. doi: 10.1046/j.1471-4159.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- Berdyshev EV, Schmid PC, Krebsbach RJ, Hillard CJ, Huang C, Chen N, Dong Z, Schmid HO. Cannabinoid-receptor-independent cell signaling by N-acylethanolamines. Biochem J. 2001;360:67–75. doi: 10.1042/0264-6021:3600067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M, Zimmer A. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Toney DM, Fischer-Stenger K, Harrison MP, Marciano-Cabral FM. Anandamide inhibits macrophage-mediated killing of tumor necrosis-sensitive cells. Life Sci. 1995;56:23–24. doi: 10.1016/0024-3205(95)00190-h. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Dove Pettit DA. Drugs and immunity: cannabinoids and their role in decreased resistance to infectious disease. J Neuroimmunol. 1998;83:116–123. doi: 10.1016/s0165-5728(97)00227-0. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Staab A. Effects on the immune system. In: Pertwee R, editor. Handbook on Experimental Pharmacology: Cannabinoids. Springer-Verlag; Berlin: 2005. pp. 85–423. [DOI] [PubMed] [Google Scholar]
- Chari-Bitron A. Effect of δ1-tetrahydrocannabinol on red blood cell membranes and alveolar macrophages. In: Nahas GG, editor. Marihuana: Chemistry, Biochemistry, and Cellular Effects. Springer-Verlag; New York: 1976. pp. 273–281. [Google Scholar]
- Charo IF, Ransohoof RM. Mechanisms of disease: The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Chuchawankul S, Shima M, Buckley NE, Hartmann CB, McCoy KL. Role of cannabinoid receptors in inhibiting macrophage costimulatory activity. Int Immunopharmacol. 2004;4:265–278. doi: 10.1016/j.intimp.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Friedman H, Klein TW. Cannabinoid receptor proteins are increased in Jurkat, human T-cell line after mitogen activation. J Pharmacol Exp Ther. 1996;276:776–783. [PubMed] [Google Scholar]
- Dove Pettit D, Harrison MP, Spencer RF, Cabral GA. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res. 1998;51:391–402. doi: 10.1002/(SICI)1097-4547(19980201)51:3<391::AID-JNR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Drath DB, Shorey JM, Price L, Huber GL. Metabolic and functional characteristics of alveolar macrophages recovered from rats exposed to marijuana smoke. Infect Immun. 1979;25:268–272. doi: 10.1128/iai.25.1.268-272.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, Klein T, Fernandez F, Tan J, Shytle RD. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation. 2005;2:29–41. doi: 10.1186/1742-2094-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Veluz JS, Williams HL, Briley EM, Matsuda LA. Cannabinoid agonists stimulate both receptor- and non-receptor-mediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. Mol Pharmacol. 1992;42:838–845. [PubMed] [Google Scholar]
- Friedman M, Cepero ML, Klein T, Friedman H. Suppressive effect of delta 9-tetrahydrocannabinol in vitro on phagocytosis by murine macrophages. Proc Soc Exp Biol Med. 1986;182:225–228. doi: 10.3181/00379727-182-42332. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gerard C, Gerard NP. C5A anaphylatoxin and its seven transmembrane-segment receptor. Rev Immunol. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Preet A, Groopman JE, Ganju KG. Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Mol Immunol. 2006;43:2169–2179. doi: 10.1016/j.molimm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Goldman DW, Goetzl EJ. Specific binding of leukotriene B4 to receptors on human polymorphonuclear leukocytes. J Immunol. 1982;129:1600–1604. [PubMed] [Google Scholar]
- Grimm MC, Ben-Baruch A, Taub DD, Howard OM, Resau JH, Wang JM, Ali H, Richardson R, Snyderman R, Oppenheim JJ. Opiates transactivate chemokine receptors: delta and mu opioid receptor-mediated heterologous desensitization. J Exp Med. 1998;188:317–325. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan DJ. Platelet activating factor: A biologically active phosphoglyceride. Annu Rev Biochem. 1986;55:483–509. doi: 10.1146/annurev.bi.55.070186.002411. [DOI] [PubMed] [Google Scholar]
- Harris H. Chemotaxis of monocytes. Br J Exp Pathol. 1953;34:276–279. [PMC free article] [PubMed] [Google Scholar]
- Harris H. Role of chemotaxis in inflammation. Physiol Rev. 1954;34:529–562. doi: 10.1152/physrev.1954.34.3.529. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber GL, Simmons GA, McCarthy CR, Cutting MB, Laguarda R, Pereira W. Depressant effect of marihuana smoke on antibactericidal activity of pulmonary alveolar macrophages. Chest. 1975;68:769–773. doi: 10.1378/chest.68.6.769. [DOI] [PubMed] [Google Scholar]
- Huber GL, Shea JW, Hinds WC, Pochay VE, Weker RT, First MW, Sornberger GC. An experimental animal model for quantifying the biological effects of marijuana on the defense system of the lung. Adv Biosci. 1978;22–23:301–328. doi: 10.1016/b978-0-08-023759-6.50027-5. [DOI] [PubMed] [Google Scholar]
- Jin T, Hereld D. Moving toward understanding eukaryotic chemotaxis. Eur J Cell Biol. 2006 doi: 10.1016/j.ejcb.2006.04.008. In press. [DOI] [PubMed] [Google Scholar]
- Kehrl JH. Chemoattractant receptor signaling and the control of lymphocyte migration. Immunol Res. 2006;34:211–228. doi: 10.1385/IR:34:3:211. [DOI] [PubMed] [Google Scholar]
- Kim CH. Chemokine chemokine-receptor network in immune cell trafficking. Curr Drug Target Immune Endoc Metabol Disord. 2004;4:343–361. doi: 10.2174/1568008043339712. [DOI] [PubMed] [Google Scholar]
- Klein TW, Friedman H, Specter S. Marijuana, immunity and infection. J Neuroimmunol. 1998;83:102–115. doi: 10.1016/s0165-5728(97)00226-9. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton CA, Widen R, Friedman H. The effect of delta-9-tetrahydrocannabinol and 11-hydroxy-delta-9-tetrahydrocannabinol on T-lymphocyte and B-lymphocyte mitogen responses. J Immunopharmacol. 1985;7:451–466. doi: 10.3109/08923978509026487. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. 2004;1:95–104. [PubMed] [Google Scholar]
- Lehner T. The role of CCR5 chemokine ligands and antibodies to CCR5 coreceptors in preventing HIV infection. TRENDS in Immunol. 2002;23:347–351. doi: 10.1016/s1471-4906(02)02252-4. [DOI] [PubMed] [Google Scholar]
- Lopez-Cepero M, Friedman M, Klein T, Friedman H. Tetrahydrocannabinol-induced suppression of macrophage spreading and phagocytic activity in vitro. J Leukoc Biol. 1986;39:679–686. doi: 10.1002/jlb.39.6.679. [DOI] [PubMed] [Google Scholar]
- Makriyannis A, Yang DP, Griffin RG, Das Gupta SK. The perturbation of model membranes by (−)-delta 9-tetrahydrocannabinol. Studies using solid-state 2H- and 13C-NMR. Biochim Biophys Acta. 1990;1028:31–42. doi: 10.1016/0005-2736(90)90262-m. [DOI] [PubMed] [Google Scholar]
- Mann PEG, Cohen AB, Finley TN, Ladman AJ. Alveolar macrophages. Structural and functional differences between non-smokers and smokers of marijuana in vitro. Lab Invest. 1971;25:111–120. [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. TRENDS in Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Martin BR. Cellular effects of cannabinoids. Pharmacol Rev. 1986;1:45–74. [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned CDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McCarthy CR, Cutting MB, Simmons GA, Pereira W, Laguarda R, Huber GL. The effect of marihuana on the in vitro function of pulmonary alveolar macrophages. In: Braude MC, Szara S, editors. Pharmacology of Marihuana. Vol. 1. Raven Press; New York: 1976. pp. 211–216. [Google Scholar]
- McCoy K, Gainey D, Cabral G. Delta-9-tetrahydrocannabinol modulates antigen processing by macrophages. J Pharmacol Exp Ther. 1995;273:1216–1223. [PubMed] [Google Scholar]
- McCoy KL, Matveyeva M, Carlisle SJ, Cabral GA. Cannabinoid inhibition of the processing of intact lysozyme by macrophages: evidence for CB2 receptor participation. J Pharmacol Exp Ther. 1999;289:1620–1625. [PubMed] [Google Scholar]
- Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious disease. Blood. 2000;95:3032–3043. [PubMed] [Google Scholar]
- Murphy PM. International Union of Pharmacology. XXX. Update on Chemokine Receptor Nomenclature. Pharmacol Rev. 2002;54:227–229. doi: 10.1124/pr.54.2.227. [DOI] [PubMed] [Google Scholar]
- Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Cannabinoid receptor-independent actions of the aminoalkylindole WIN 55,212-2 on trigeminal sensory neurons. Br J Pharmacol. 2004;142:257–266. doi: 10.1038/sj.bjp.0705778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puffenbarger R, Boothe C, Cabral G. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29:58–69. [PubMed] [Google Scholar]
- Raz A, Goldman R. Effect of hashish compounds on mouse peritoneal macrophages. Lab Invest. 1976;34:69–76. [PubMed] [Google Scholar]
- Rios CD, Jordan BA, Gomes I, Devi LA. G-protein-coupled receptor dimerization: modulation of receptor function. Pharmacol Ther. 2001;92:71–87. doi: 10.1016/s0163-7258(01)00160-7. [DOI] [PubMed] [Google Scholar]
- Rios CD, Gomes I, Devi LA. Mu and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TJ, Steele AD, Howard OMZ, Oppenheim JJ. Bidirectional heterologous desensitization of opioid and chemokine receptors. Ann NY Acad Sci. 2000;917:19–28. doi: 10.1111/j.1749-6632.2000.tb05369.x. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Martucci C, Vaccani A, Bariselli F, Panerai AE, Colombo A, Parolaro D, Massi P. The nonpsychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J Neuroimmunol. 2005;159:97–105. doi: 10.1016/j.jneuroim.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Massi P, Panerai AE, Parolaro D. In vivo and in vitro treatment with the synthetic cannabinoid CP55, 940 decreases the in vitro migration of macrophages in the rat: involvement of both CB1 and CB2 receptors. J Neuroimmunol. 2000;109:155–163. doi: 10.1016/s0165-5728(00)00307-6. [DOI] [PubMed] [Google Scholar]
- Samson M, Libert F, Doranz B, Rucker J, Leisnard C, Farber C, Saragosti S, Lapoumeromlie C, Cognaux J, Forcielle C, Muydermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R, Collman R, Doms R, Vassart G, Parmentier M. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- Schiffmann E, Corcoran BA, Wahl SM. N-formyl-methionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci USA. 1975;72:1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, Reeves J, Hibbits S, Stine J, Gray P, Proudfoot A, Clapham P. Co-receptor use by HIV and inhibition of HIV infection by chemokine receptor ligands. Immunological Rev. 2000;177:112–126. doi: 10.1034/j.1600-065x.2000.17719.x. [DOI] [PubMed] [Google Scholar]
- Specter SC, Klein TW, Newton C, Mondragon M, Widen R, Friedman H. Marijuana effects on immunity: suppression of human natural killer cell activity of delta-9-tetrahydrocannabinol. Int J Immunopharmacol. 1986;8:741–745. doi: 10.1016/0192-0561(86)90010-x. [DOI] [PubMed] [Google Scholar]
- Specter S, Lancz G, Goodfellow D. Suppression of human macrophage function in by delta-9-tetrahydrocannabinol. J Leukocyte Biol. 1991;50:423–426. doi: 10.1002/jlb.50.5.423. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Salzet M, Rialas CM, Mattocks D, Fimiani C, Bilfinger TV. Macrophage behavior associated with acute and chronic exposure to HIV GP120, morphine and anandamide: endothelial implications. Int J Cardiol 64 Suppl. 1998;1:S3–13. doi: 10.1016/s0167-5273(98)00030-8. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang I, Yau P, Doi R, Chuang R. Interactions of opioid and chemokine receptors: oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp Cell Res. 2002;280:192–200. doi: 10.1006/excr.2002.5638. [DOI] [PubMed] [Google Scholar]
- Szabo J, Chen XH, Xin L, Adler MW, Howard OMZ, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci USA. 2002;99:10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo J, Rogers TJ. Crosstalk between chemokine and opioid receptors results in downmodulation of cell migration. Adv Exp Med Biol. 2001;493:75–79. doi: 10.1007/0-306-47611-8_9. [DOI] [PubMed] [Google Scholar]
- Szabo J, Wetzel M, McCarthy L, Steele A, Henderson E, Howard OMZ, Oppenheim Z, Rogers TJ. Interactions of opioid receptors, chemokines and chemokine receptors. Adv Exp Med Biol. 2001;493:69–74. doi: 10.1007/0-306-47611-8_8. [DOI] [PubMed] [Google Scholar]
- Tang JL, Lancz G, Specter S, Bullock H. Marijuana and immunity: tetrahydrocannabinol-mediated inhibition of growth and phagocyte activity of the murine macrophage cell line, P388D1. Int J Immunopharmacol. 1992;14:253–262. doi: 10.1016/0192-0561(92)90037-l. [DOI] [PubMed] [Google Scholar]
- Waksman Y, Olson JM, Carlisle SJ, Cabral GA. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J Pharmacol Exp Ther. 1999;288:1357–1366. [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzl B, Scuderi P, Watson RR. Marijuana components stimulate human peripheral blood mononuclear cell secretion of interferon-gamma and suppresses interleukin-1 alpha in vitro. Int J Immunopharmacol. 1991;13:1091–1097. doi: 10.1016/0192-0561(91)90160-9. [DOI] [PubMed] [Google Scholar]
- Zhang N, Hodge D, Rogers TJ, Oppenheim JJ. Ca2+-independent protein kinase Cs mediate heterologous desensitization of leukocyte chemokine receptors by opioid receptors. J Biol Chem. 2003;278:12729–12736. doi: 10.1074/jbc.M300430200. [DOI] [PubMed] [Google Scholar]
- Zheng ZM, Specter S, Friedman H. Inhibition by delta-9-tetrahydrocannabinol of tumor necrosis factor alpha production by mouse and human macrophages. Int J Immunopharmacol. 1992;14:1445–1452. doi: 10.1016/0192-0561(92)90017-f. [DOI] [PubMed] [Google Scholar]
- Zimmerman S, Zimmerman AM, Cameron IL, Laurence HL. Delta1-tetrahydrocannabinol, cannabidiol and cannabinol effects on the immune response of mice. Pharmacology. 1977;15:10–23. doi: 10.1159/000136658. [DOI] [PubMed] [Google Scholar]