Abstract
Lentiviral vectors are promising tools for gene therapy in the CNS. It is therefore important to characterize their interactions with the immune system in the CNS. This work characterizes transgene expression and brain inflammation in the presence or absence of immune responses generated after systemic immunization with lentiviral vectors. We characterized transduction with SIN-LV vectors in the CNS. A dose—response curve using SIN-LV-GFP demonstrated detectable transgene expression in the striatum at a dose of 102, and maximum expression at 106, transducing units of lentiviral vector, with minimal increase in inflammatory markers between the lowest and highest dose of vector injected. Our studies demonstrate that injection of a lentiviral vector into the CNS did not cause a measurable inflammatory response. Systemic immunization after CNS injection, with the lentiviral vector expressing the same transgene as a vector injected into the CNS, caused a decrease in transgene expression in the CNS, concomitantly with an infiltration of inflammatory cells into the CNS parenchyma at the injection site. However, peripheral immunization with a lentiviral vector carrying a different transgene did not diminish transgene expression, or cause CNS inflammation. Systemic immunization preceding injection of lentiviral vectors into the CNS determined that preexisting antilentiviral immunity, regardless of the transgene, did not affect transgene expression. Furthermore, we showed that the transgene, but not the virion or vector components, is responsible for providing antigenic epitopes to the activated immune system, on systemic immunization with lentivirus. Low immunogenicity and prolonged transgene expression in the presence of preexisting lentiviral immunity are encouraging data for the future use of lentiviral vectors in CNS gene therapy. In summary, the lentiviral vectors tested induced undetectable activation of innate immune responses, and stimulation of adaptive immune responses against lentiviral vectors was effective in causing a decrease in transgene expression only if the immune response was directed against the transgene. A systemic immune response against vector components alone did not cause brain inflammation, possibly because vector-derived epitopes were not being presented in the CNS.
INTRODUCTION
LONG-TERM SUCCESS in gene transfer and gene therapy may be limited by deleterious immune reactions due to activation of immune responses against the viral vectors or even the transgenes themselves (Lowenstein, 2002; Lowenstein and Castro, 2003). In the CNS immune responses concern both innate and adaptive immune responses. Innate immune activation is the local acute stimulation of inflammatory immune responses seen in the CNS shortly after vector injection. The results of the effector arm of the adaptive immune response are seen in the brain after a systemic immune response against a particular vector, wherein the immune system recognizes vector-derived antigen epitopes that continue to be presented within the CNS (Lowenstein, 2004). Of even further concern, vis-à-vis clinical trials, is the existence of systemic immune responses preceding vector administration in patients who undergo gene therapy (Lowenstein, 2004). Any and all of these responses can potentially be detrimental to transgene expression and gene therapy clinical trials. Developing a vector system with limited immunogenicity, stable transgene expression, and promiscuous transducing abilities is thus of central importance in gene therapy. As important as minimizing the immune responses is the understanding of their molecular and cellular bases, in order to predict their appearance and design gene transfer strategies that will not cause stimulation, or be the target of the effector arms of the immune system (Yewdell et al., 1999; Yewdell and Hill, 2002; Bennett, 2003; Jooss and Chirmule, 2003; Follenzi et al., 2004).
Lentiviral vectors are one of the most promising gene therapy vehicles for CNS diseases (Kordower et al., 2000; Consiglio et al., 2001; Kay et al., 2001; Azzouz et al., 2004; Biffi et al., 2004; Wong et al., 2005). These vectors are widely used because of their ability to transduce dividing as well as nondividing cells. Pseudotyping the virus with the vesicular stomatitis virus (VSV) envelope glycoprotein allows the virus to transduce a broad range of tissues and cells and increases its therapeutic applications. Previous studies have demonstrated long-term stable gene transfer and gene therapy in experimental models in the absence of immune responses (Kordower et al., 2000; Consiglio et al., 2001; Kay et al., 2001; Azzouz et al., 2004; Biffi et al., 2004; Wong et al., 2005). However, these studies have generally not monitored the presence of innate or adaptive antilentiviral or antitransgene immune responses, nor have they specifically examined the fate of transduced cells after specific immunization against lentiviral vectors.
Lentiviruses differ from other therapeutic vectors, such as adenoviruses, because they integrate into the host genome instead of remaining episomal (Kay et al., 2001). Host genome integration is thought to allow for longer, more stable, transgene expression. Another lentivirus characteristic that makes them promising therapeutic vectors is their intrinsic low immunogenicity, seen as a reduced capacity to induce inflammation and innate immune responses. High-capacity adenoviruses and adeno-associated viruses (AAVs) are similar to lentiviruses, in that none of the genomes in these vectors encode any viral proteins. This ought to be associated with a lower capacity either to initiate or be a target of immune responses. This trait has proven beneficial for the high-capacity adenovirus vectors because it allows for long-term transgene expression even in the presence of a strong systemic immune response against adenovirus (Thomas et al., 2000a). Nevertheless, the virion proteins that are part of the input vector capsids can be immunogenic, and provide antigenic epitopes to the antigen-presenting cells. Especially in the case of certain serotypes of AAV, such as AAVs that uncoat slowly (Lowenstein, 2004; Thomas et al., 2004), this is thought to occur. This could explain why AAV still develops an immune response that targets AAV-transduced cells, and causes diminished transgene expression (Lowenstein, 2004; Peden et al., 2004). Some of these immune responses are likely to be due to the rate at which these viruses uncoat their capsid proteins. Thus, the rate and the total length of time during which protein sources of potentially antigenic epitopes are made available for antigen presentation will determine the length of time a given vector could potentially be recognized by immune cells. If capsid turnover is rapid, by the time effector T cells become activated no capsid antigenic epitopes may be presented on target cells anymore, thus, making the transduced cells invisible to the immune system. However, if virion uncoating is slow, antigenic epitopes may be presented for a long enough time to be recognized by T cells, even in the absence of de novo synthesis in transduced cells.
In the present study, we have characterized the immune responses to lentivirus injected into the brain, using well-characterized paradigms we have previously developed to understand the immune responses to first-generation and high-capacity adenoviruses. The experiments described here demonstrate that lentivirally transduced cells can be identified by the immune system only if an immune response is raised against the transgene. Otherwise, the immune system remains unable to recognize the presence of lentivirally transduced cells in the brain, indicating that immune responses against the capsid and virion of lentiviral vectors are unable to recognize infected cells in the brain. A rapid turnover of vector components could easily explain the stability of lentivirally transduced cells in the brain, in the presence of robust immune responses to vector components.
MATERIAL AND METHODS
Vectors
The vectors used were pRRLsinPPT.CMV.GFP.WPRE (expressing green fluorescent protein [GFP]; Lenti-GFP) and pRRLsinPPT.CMV.GFP.F-IX.WPRE (expressing soluble human clotting factor IX [FIX]; Lenti-FIX). Construction of these vectors has been described in detail elsewhere (Follenzi et al., 2000). High-titer VSV-pseudotyped lentiviral vectors were produced in 293T cells by transient transfection with the lentiviral transfer vector construct pRRL-SIN-PPT-CMV-GFP-WPRE (expressing GFP) or pRRL-SIN-PPT-CMV-hF.IX-WPRE (expressing human clotting factor IX cDNA), the late-generation packaging constructs pCMVΔR8.74 and pRev, and the VSV envelope-expressing construct pMD2.G and purified by ultra-centrifugation as described (Follenzi et al., 2002). Vector particle content was measured by human immunodeficiency virus type 1 (HIV-1) p24 antigen immunocapture (PerkinElmer, Norwalk, CT). Expression titers for GFP-expressing vectors were determined on HeLa cells by fluorescence-activated cell-sorting (FACS) analysis (FACSCalibur; BD Biosciences Immunocytometry Systems, San Jose, CA) and were between 6 × 109 and 1010 transducing units (TU)/ml. The p24 content was in the range of 50–100 μg/ml.
Western blot analysis
Bald lentiviral vector particles (produced as described above without the VSV-G-expressing construct) and VSV-pseudotyped lentiviral vector particles were matched for p24 content. VSV-pseudotyped lentiviral vector particles and VSV-pseudotyped retroviral vector particles (produced as described above by transient transfection with a murine leukemia virus [MLV]-based transfer and gag-pol packaging construct [Naldini et al., 1996] and pMD2.G) were matched for transducing activity on HeLa cells. All vector particles were lysed in boiling 2% sodium dodecyl sulfate (SDS)—Tris buffer, quantified for protein content, and boiled in Laemmli buffer under reducing conditions. Proteins were separated on 10% SDS—polyacrylamide gels and transferred to Hybond-ECL membrane (Amersham Biosciences/GE Healthcare Discovery Systems) by high-intensity wet blotting (Millipore, Bedford, MA). Filters were probed with the indicated rat serum diluted 1:100 in Tris-buffered saline containing 5% bovine serum albumin and specific binding was detected with an enhanced chemiluminescence system (ECL; Amersham Biosciences/GE Healthcare Discovery Systems). The same filters were treated with a stripping buffer (2% SDS, 62.5 mM Tris-HCl [pH 6.8], and 100 mM 2-mercaptoethanol) at 50°C for 30 min, washed, and reprobed with monoclonal anti-VSV antibody (Sigma, St. Louis, MO).
Animals and surgical procedures
Adult Sprague Dawley rats (body weight, 250 g; Charles River Laboratories UK, Margate, UK) were used. We performed three different experiments. Animals in experimental group 1 (illustrated in Fig. 1) were anesthetized with halothane and received an intrastriatal injection of 102–107 infectious units of Lenti-GFP into the left striatum (0.6 mm forward and 3.4 mm from bregma and 5.0 mm depth from the dura), using a 10-μl Hamilton syringe fitted with a 26-gauge needle. Virus was administered in a volume of 3 μl and each injection was performed over a 3-min period. After the injection, the needle was left in place for a further 5 min before retraction. Animals were perfused—fixed 30 days later and processed for immunocytochemistry.
FIG. 1.
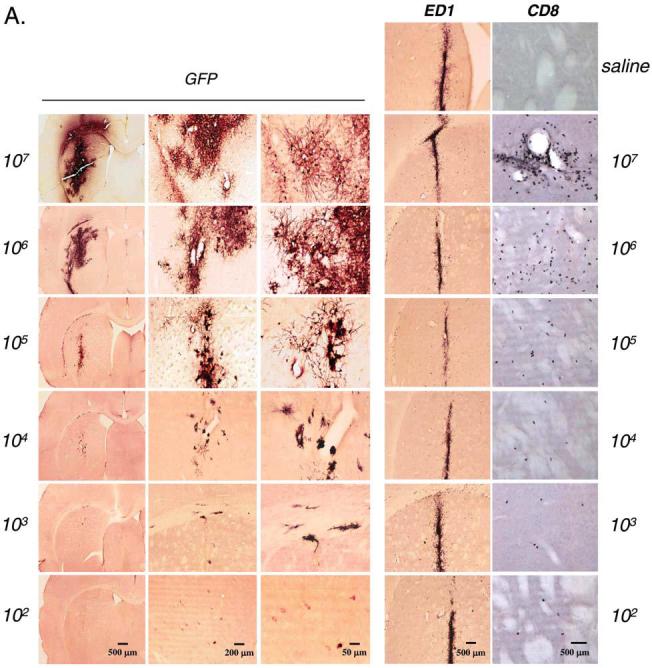
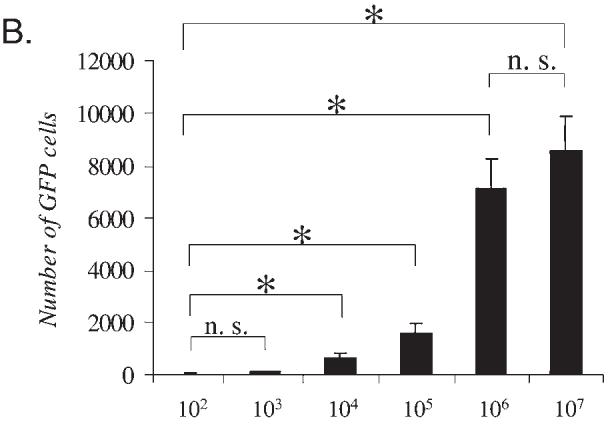
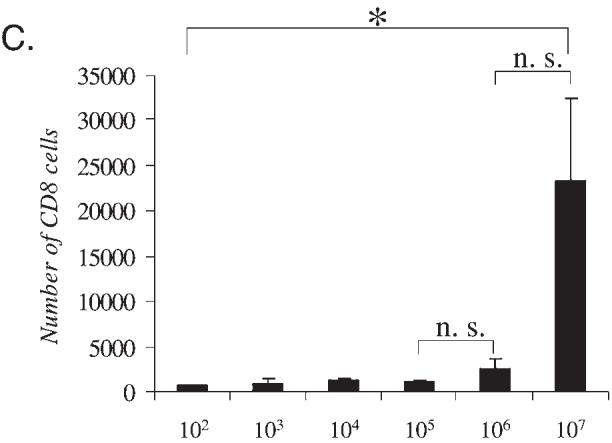
Dose response for Lenti-GFP in the CNS: Transgene expression and markers for innate inflammation. Immunohistochemistry for GFP, ED1, and CD8 was performed in serial brain sections of rats injected with Lenti-GFP via the striatum and perfused—fixed 30 days later. (A) Increase in expression of the transgene GFP from 102 to 107 TU injected directly into the striatum. The innate inflammatory response is illustrated by the influx of macrophage/microglial cells (immunoreactive for ED1) and T cells (immunoreactive for CD8). The number of immunoreactive GFP+ cells increased with injection of 102 to 106 TU; there was no difference at either dose in the amount of cells immunoreactive for ED1. However, the number of CD8+ cells was significantly increased at 107 TU. Quantification of these data is shown in (B) and (C), which illustrates that there is no further increase in transduced cells after the injection of 107 IU of Lenti-GFP into the striatum (B) and that there is a significant increase in CD8+ cells with injection of 107 IU (C). Scale bars are shown in (A). *p < 0.05.
Animals in experimental group 2 (illustrated in Fig. 3) were anesthetized with halothane, held in a stereotaxic frame, and injected intrastriatally as described above. The animals in this group (total, n = 15) were injected via the left striatum with 1 × 105 infectious units of Lenti-GFP. Thirty days later, one-third of the animals (n = 5) were intradermally injected with either sterile saline solution or 2.5 × 107 infectious units of Lenti-GFP or Lenti-FIX. The injection volume was 100 μl.
FIG. 3.
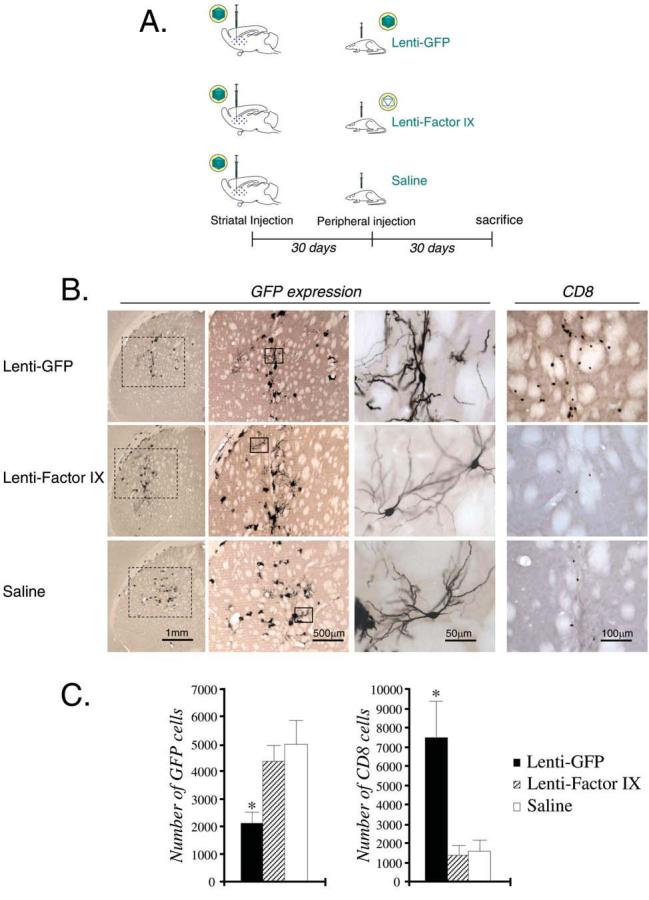
CNS immune responses to lentiviral vector: Systemic immunization after CNS injection of lentiviral vector. (A) Schematic illustration of the experimental procedure, in which rats were injected with 105 TU of Lenti-GFP intrastriatally and immunized systemically 30 days later with either Lenti-GFP, Lenti-FIX, or saline. (B) GFP expression, and T cell (CD8+) infiltration, in the striatum of each group of animals 30 days after systemic immunization. (C) Quantification of the serial sections in (B), and statistical analysis (*p < 0.05; versus Lenti-FIX and saline groups). In (B), of the columns labeled GFP, the leftmost column illustrates a low-power view of the striatum, and the middle and right columns illustrate increasingly higher power views of striatal cells expressing GFP. Scale bars are shown in (B).
Animals in experimental group 3 (illustrated in Fig. 4) were anesthetized with halothane and injected intradermally in the back with either sterile saline solution (n = 5) or 2.5 × 107 infectious units of Lenti-GFP (n = 5) or Lenti-FIX (n = 5), using a 500-μl insulin syringe. The intradermal injections were performed in a volume of 100 μl. Thirty days later, these animals (total, n = 15) were anesthetized, held in a stereotaxic injection frame, and injected via the left striatum (0.6 mm forward and 3.4 mm from bregma and 5.0 mm depth from the dura) with 1 × 105 infectious units of Lenti-GFP, using a 10-μl Hamilton syringe fitted with a 26-gauge needle. Virus was administered in a volume of 3 μl and each injection was performed over a 3-min period. After the injection, the needle was left in place for a further 5 min before retraction.
FIG. 4.
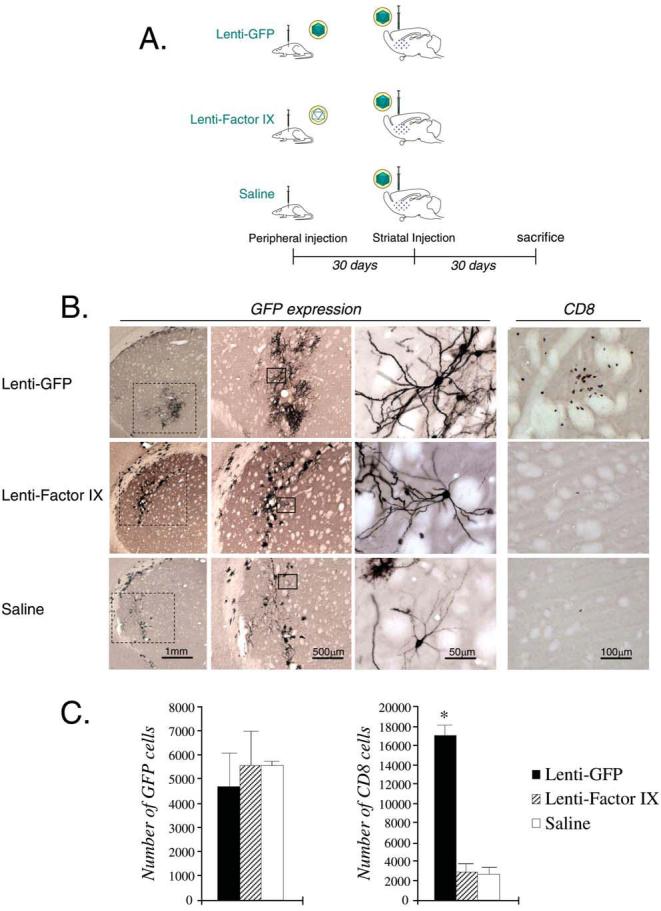
CNS immune responses to lentiviral vector: Systemic immunization preceding CNS injection of lentiviral vector. (A) Schematic illustration of the experimental procedure, in which rats were injected with Lenti-GFP, Lenti-FIX, or saline systemically, and injected intrastriatally 30 days later with 105 TU of Lenti-GFP. (B) GFP expression, and T cell (CD8+) infiltration, in the striatum of each group of animals. (C) Quantification of the serial sections in (B), and statistical analysis (*p < 0.05; versus Lenti-FIX and saline groups). In (B), of the columns labeled GFP, the leftmost column illustrates a low-power view of the striatum, and the middle and right columns illustrate increasingly higher power views of striatal cells expressing GFP. Scale bars are shown in (B).
Sixty days after the start of the experiment, the animals in both groups were injected intraperitoneally with pentobarbitone and transcardially perfused—fixed with oxygenated heparinized Tyrode solution, followed by 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4. The brain tissues were postfixed for a further 6 hr in the same fixative and stored in PBS at 4°C.
Immunohistochemistry
Sections of the striatum (50 μm, serially cut with a Vibratome tissue slicer) were treated according to a free-floating immunohistochemistry method to detect transgene expression and inflammatory or immune cell markers. Serial sections were cut throughout most of the forebrain, including all of the striatum. Endogenous peroxidase activity was quenched with 0.3% H2O2 in PBS, nonspecific Fc-binding sites were blocked with 10% horse serum, and sections were incubated overnight at room temperature with primary antibody diluted in PBS containing 1% horse serum and 0.5% Triton X-100 (antibody solution). The following monoclonal mouse or rabbit anti-rat primary antibodies were used: anti-GFP (diluted 1:1000; Molecular Probes Europe, Leiden, The Netherlands), anti-CD8 (diluted 1:500; Serotec, Kidlington, UK), and ED1 (diluted 1:1000; Serotec). Sections were then incubated with biotin-conjugated anti-mouse or anti-rabbit secondary antibodies (Dako-Cytomation, Cambridge, UK) in antibody solution. Antibody binding was detected with avidin—biotin peroxidase with diaminobenzidine as chromogen. These sections were mounted on gelatinized glass slides and were dehydrated before cover-slipping. All immunocytochemistry technical details have been described elsewhere (Southgate et al., 2000; Thomas et al., 2000a).
Unbiased quantitative stereological analysis
Quantification of cells in the striatum was performed on serial sections. The whole striatum was cut in serial sections, and every sixth section was immunoreacted for either the transgene GFP or either of the immunological markers. Thus, for the quantification, we took the three sections containing >90–95% immunoreactive cells, and quantification was performed by stereological methods (Sterio, 1984) with the use of a computer-assisted image analysis system (Stereo Investigator; Micro-BrightField, Williston, VT) and a Zeiss microscope connected to a digital camera through a Zeiss zoom set, as described previously (Suwelack et al., 2004). Cells were counted within 200 × 200-μm optical disectors in the x–y axis, covering the whole surface area of the analyzed regions, using the principle of the optical fractionator. Positive cells were counted only when they cut the superior and left limits of the square. Results were expressed as the total number of positive cells in the anatomical region analyzed, that is, the striatum, from +0.2 mm bregma to +1.20 mm bregma, where the vast majority of immunoreactive cells were located. To express values as total amount of cells, a correction was made to take into account that every sixth section had been counted. Data are expressed as means ± SEM. Statistical analysis was performed by one-way analysis of variance (ANOVA) after post hoc analysis. The null hypothesis was rejected for an α risk equal to 5%.
RESULTS
Dose-dependent transgene expression
Viral doses ranging from 102 to 107 transducing units of Lenti-GFP were injected into the striata of adult rats. Thirty days later, the brains were sectioned and analyzed for the presence of GFP by immunohistochemistry. Higher viral doses showed increased numbers of transduced cells expressing GFP (Fig. 1A). A quantitative analysis of GFP expression showed increasing numbers of transduced cells with increasing doses of Lenti-GFP from 102 to 106, but no significant difference was observed between 106 and 107 transducing units (Fig. 1B). The sections were also stained for ED1 (macrophages and microglial cells) and CD8 (T cells) to determine the presence of infiltrating immune cells. ED1+ cells were present along the injection site and few CD8+ cells were present, but both were also present in saline-injected brains. ED1- and CD8-immunoreactive cells probably remained from the initial innate, acute immune response that occurred from the injection. Numbers of ED1+ and CD8+ cells were identical in all groups and did not show a dose-dependent progressive increase.
Peripheral immunization triggers a systemic immune response
Previous experiments have shown that systemic injection of Lenti-GFP will induce GFP-specific CD8+ cytotoxic T cell responses, and injection of Lenti-FIX will induce circulating anti-FIX antibodies (Follenzi et al., 2004). To confirm that lentiviral vectors were able to mount an immune response on systemic injection, rats were injected via the CNS with Lenti-GFP, and 30 days later they were immunized subcutaneously with either Lenti-GFP or Lenti-FIX, or with saline. Thirty days after peripheral immunization animals were perfused—fixed, after blood had been removed for the analysis of immune responses. The sera were tested for the presence of antilentiviral immune responses (Fig. 2). The animals injected with Lenti-GFP via the CNS and systemically with saline as controls did not show an immune response to any of the lentiviral proteins (Fig. 2A, animals 12 and 15). Sera from the rats immunized subcutaneously with Lenti-GFP and Lenti-FIX targeted several lentiviral proteins (Fig. 2). The strong reactivity with some of the larger proteins in lanes 2 and 3, which are not present in lane 1, indicates a significant immune response to virion (p24 and p17) and envelope (VSV-G) proteins.
FIG. 2.
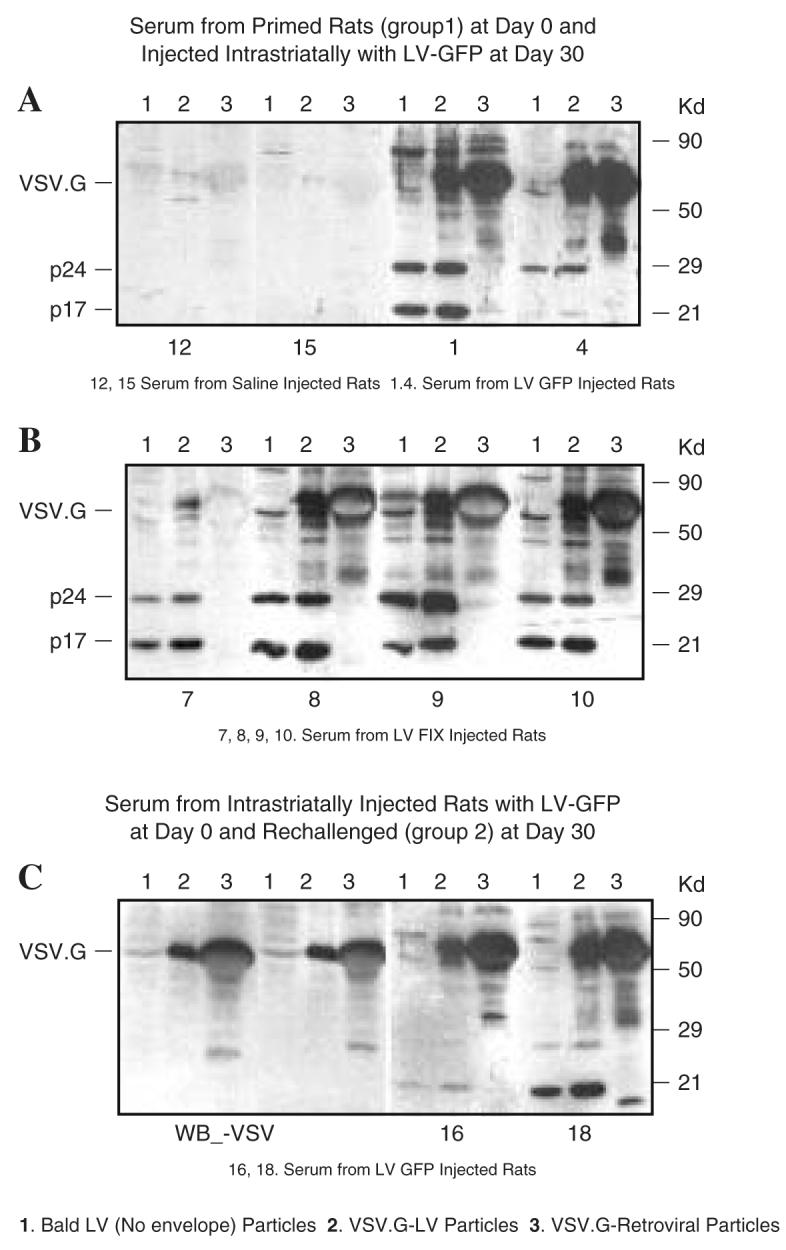
Immune response to VSV-pseudotyped lentiviral vector particles. (A and B) Sera from rats injected subcutaneously with saline (rats 12 and 15), Lenti-GFP (rats 1 and 4), or Lenti-FIX (rats 7–10), and 30 days later injected via the brain with Lenti-GFP, were tested after another 30 days for the presence of antibodies against vector particles by Western blot. The following types of particles were loaded on the gel: lane 1, bald lentiviral particles (no VSV); lane 2, VSV-G-pseudotyped lentiviral particles; lane 3, VSV-G-pseudotyped retroviral particles. The expected migration of HIV-1 p24 capsid, p17 matrix protein, and VSV-G envelope protein is indicated. (C) Left: Anti-VSV antibodies were also used to identify VSV-G protein in the blot. Right: Two representative blots of sera from rats (rats 16 and 18) first injected via the brain with Lenti-GFP, challenged subcutaneously after 30 days with Lenti-GFP and Lenti-FIX, and analyzed after another 30 days.
Peripheral immunization after CNS injection leads to decreased transgene expression and increased inflammation in the CNS
To determine whether peripheral immunization against lentivirus after transduction of the CNS with lentivirus would alter the levels of transgene expression in the CNS, a series of experiments was performed. Figure 3A shows a diagram of the experimental design utilized. Groups of five animals were injected with a nonsaturating dose of 1 × 105 infectious units of Lenti-GFP into the striatum; of these, one group was immunized subcutaneously with Lenti-GFP, and another was immunized subcutaneously with Lenti-FIX, whereas the last group was injected with saline. The brains were sectioned 30 days after the systemic immunization and analyzed by immunohistochemistry for GFP and inflammatory marker expression in the brain (Fig. 3B). The rats that had been immunized with Lenti-GFP displayed significantly lower GFP expression in the striatum compared with those that were immunized with Lenti-FIX or saline (Fig. 3B and C).
Further characterization of the immune response was done by staining brain sections for the presence of ED1+ and CD8+ cells. Immunization with lentiviral vector did not increase the number of ED1-immunoreactive cells in the striatum. However, the number of CD8+ cells in the brains from rats immunized with Lenti-GFP was significantly higher than in rats immunized with either Lenti-FIX or saline (Fig. 3C). These cells were possibly recognizing an epitope related to GFP because immunization with Lenti-FIX did not induce an increase in the density of CD8+ cells in the striatum.
Peripheral immunization preceding CNS injection
To study the effects of preexisting systemic immunity on transgene expression we initiated an immune response against lentiviral vector in rats before the striatal injections. Figure 4A shows a diagram of the experimental paradigm employed to investigate the response in the brain to a preexisting immune response against lentiviral vectors. There was comparable GFP staining in the brains of rats preimmunized with Lenti-FIX or saline compared with rats preimmunized with Lenti-GFP (Fig. 4B). Quantification of GFP expression showed no significant difference between any of the treatments (Fig. 4C).
Levels of activated microglia/macrophages were also comparable among the different groups (shown by ED1 staining; not illustrated). But on quantification of the CD8+ cells, a significant increase was seen in the Lenti-GFP-immunized rats compared with the Lenti-FIX- or saline-injected rats (Fig. 4C). Again, this suggests that the CD8+ cells are specific to the transgene GFP.
DISCUSSION
Lentiviral vectors have been shown to be efficient in transferring genes into the brain in the context of experimental models of inherited diseases, and neurodegeneration, and they have shown their efficiency both in rodents and in nonhuman primate models of disease. Further, they have also been utilized to model neurodegeneration, taking advantage of their capacity to provide long-term expression in the CNS (Blomer et al., 1997; Kordower et al., 2000; Consiglio et al., 2001; Biffi et al., 2004; Guillot et al., 2004; Lo Bianco et al., 2004; Regulier et al., 2004; Zala et al., 2004). Although inflammatory responses have not been detected, experiments examining this question in detail have not yet been reported.
We have previously characterized in detail the innate and adaptive immune responses to adenoviral vectors (both first generation and high capacity) injected into the brain (Thomas et al., 2000b, 2001a,b, 2002; Lowenstein, 2002; Lowenstein and Castro, 2003). Innate inflammatory immune responses to adenoviral vectors are transient, and do not affect transgene expression. Systemic adaptive immune responses to first-generation adenoviral vectors completely abolish expression from vectors injected into the brain, because the brain presents antigenic epitopes derived from the first-generation adenoviral vectors. On the other hand, novel high-capacity adenoviral vectors are “resistant” to the activated systemic adaptive immune response, because they do not express any potentially antigenic epitopes (Thomas et al., 2000a, 2001b). Thus, we utilized identical paradigms to examine in detail the inflammatory and immune responses to lentiviral vectors. Our prediction was that lentiviruses ought to behave immunologically as high-capacity, helper-dependent adenoviral vectors.
Indeed, the manner in which the immune system responds to lentiviral vectors is similar to how the immune system interacts with high-capacity adenoviral vectors (Thomas et al., 2000a, 2001a,b), and not first-generation recombinant adenoviral vectors or AAV vectors (Bennett, 2003; Peden et al., 2003; Lowenstein, 2004; Thomas et al., 2004). Both lentiviral vectors and high-capacity adenoviral vectors allow sustained transgene expression even when a preexisting immune response against the vector is present. The innate immune response to both vectors in the CNS is minimal and therefore allows the use of both for CNS gene therapies.
A systemic immune response against viral vectors is induced only after injection of the vectors into a site outside of the brain, where vector-derived antigens can prime the systemic immune response. The reason for this is that the adaptive immune system cannot be primed by the careful and selective injection of infectious particulate antigens into the brain parenchyma, as done in our work. Further, because the injection of vectors into the CNS is essentially ignored by the immune system, systemic immunization (either preceding or after injection of vectors into the CNS) induces the same qualitative type of immune responses.
An advantage of lentiviruses is that they transduce nondividing cells, which makes them better for certain therapies, especially where long-term expression may be required. The trait of integrating into the host genome is a powerful aspect of these vectors. Insertion of the transgene into the host genome could prolong expression better than the episomal transgenes found in adenovirus-infected cells, and this is currently being tested by various research groups. Replacing constitutively active promoters with cell type-specific promoters may further improve the use of lentiviral vectors in the CNS. Further, this may also impact immune responses against the vectors and/or the transgenes. Specifically, it has been described that expression of lentiviral vectors directly in the liver decreased systemic immune responses detected against the vectors and the transgenes (Follenzi et al., 2004). Expression in identified cell types in the brain may also potentially reduce immune responses to the transgenes, as was detected in our studies. This might offer stronger and more enhanced transgene expression and could possibly lead to the need for lower doses of the vector during therapy.
On injection of lentiviral vectors into the brain, inflammation was detected only at the highest dose utilized, and transgene expression was stable. However, after systemic priming of an antilentiviral vector immune response, we demonstrated that the immune system recognized the lentivirus-encoded transgene, but not a component associated with the viral vector capsid or virion. After injection of Lenti-GFP into the CNS, followed by systemic immunization, rats displayed brain inflammation and reduced transgene expression only if they were immunized systemically with Lenti-GFP, but not if they were immunized systemically with Lenti-FIX. This indicates that the immune response recognizes the transgene GFP as immunogenic, rather than an antigenic epitope derived from the lentiviral virion. If the immune system were recognizing a component of the lentiviral vector, then brain inflammation and transgene reduction would have been seen in animals immunized systemically with either Lenti-FIX or Lenti-GFP. However, it may well be that a systemic immune response against the lentiviral capsid and/or virion exists. Nevertheless, it is unable to affect brain transgene expression, because the relevant antigens are not being presented at the time the T cells enter the brain. This could be due to lack of presentation of lentiviral virion-derived epitopes in the CNS, or to rapid turnover of these antigens from CNS antigen-presenting cells.
All rats immunized systemically (either Lenti-GFP or Lenti-FIX) after a CNS injection were able to mount a systemic immune response, confirming that both immunization schedules were efficient and able to result in the presence of activated immune cells. However, we did not encounter any evidence of a systemic immune response to lentiviruses in animals that had been injected with lentiviral vector only in the brain. This suggests the existence of systemic immune ignorance to vectors injected exclusively into the brain, a situation identical to that encountered on injection of adenoviral vectors into the CNS (Thomas et al., 2000a, 2001a). The reason for this is as follows. For a vector to elicit immune responses after gene transfer to the brain, either the vector needs to escape to the periphery during administration or antigen-presenting cells need to take up the vector and present the epitopes in the periphery (because surveillance of the brain parenchyma by naive T cells is minimal, if it occurs at all, and therefore naive T cells need to be primed in the periphery).
In the absence of any antigenic lentiviral epitopes being presented, the transgene remains an important source of potentially antigenic epitopes. Our experiments demonstrate that indeed the transgene can act as a target of the immune system. This could prove of potential clinical significance because it would be possible for patients to become immunized against their own gene therapy vectors. In the case of lentiviral vectors a potential immune response against the vector itself may not be of concern, but an immune response to the transgene will need careful monitoring. In particular, because lentiviral vectors are efficient in transducing and activating dendritic cells, they will therefore elicit a potent antitransgene immune response if the transgene product is foreign to the host, and/or potentially immunogenic.
Another potential barrier that needs consideration is the possibility that some individuals may have been previously exposed to infection with a lentivirus, such as HIV. Therefore, a preexisting immune response that recognizes antigens present in lentiviral vectors may be able to inhibit transduction with lentiviral vectors used during gene therapy.
The data we have provided show that preexisting immune cells lead to an increase in CD8+ cells in the brain 30 days after striatal injection, but only if the preexisting systemic immunization was triggered by a lentivirus expressing the identical transgene being expressed in the CNS. Preimmunization of rats with Lenti-FIX, followed by intrastriatal injection of Lenti-GFP, does not lead to increased immune cells at the site of brain injection, indicating that any CD8+ T cells that may have been generated do not recognize any antigenic epitopes in the CNS, possibly because of the rapid turnover of lentiviral virion epitopes.
In summary, we have shown that there is a dose-dependent response of transgene expression to increasing lentiviral doses, which reaches a threshold at 106 transducing units in rats. Peripheral immunization before or after CNS injection with the lentivirus vector shows a decrease in transgene expression, and an increase in infiltrating immune cells in the brain, only if the immune response is targeted against the transgene also expressed in the CNS. Thus, although a systemic immune response against lentiviral vectors was detected, it did not cause brain inflammation; this indicates that lentivirus-derived virion antigens are not being presented in the CNS. Lentiviral vectors thus display a high safety standard, and optimal stability vis-à-vis both pre- and postimmunization paradigms for gene transduction and gene therapy in the CNS and for the treatment of neurological diseases.
ACKNOWLEDGMENTS
Work in the Gene Therapeutics Research Institute (GTRI) is funded by NIH grants 1 RO1 NS44556 (M.G.C.), 1 RO1 NS42893 (P.R.L.), U54 4 NS04-5309 (P.R.L.), and R21 NS47298 (P.R.L.), and by a Linda Tallen and David Paul Kane Annual Fellowship in Gene Therapy for Cancer Research. Work at the Hospital San Rafael (HSR) was funded by grants from Telethon and the Italian Ministry for Scientific Research (L.N.). P.R.L. is holder of the Bram and Elaine Goldsmith Chair in Gene Therapeutics. We are grateful to the Board of Governors at Cedars-Sinai Medical Center for their vision and generous creation and support of the GTRI. We also thank Dr. Shlomo Melmed for support and academic leadership, and Mr. Richard Katzman for excellent administrative support.
REFERENCES
- AZZOUZ M, RALPH GS, STORKEBAUM E, WALMSLEY LE, MITROPHANOUS KA, KINGSMAN SM, CARMELIET P, MAZARAKIS ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- BENNETT J. Immune response following intraocular delivery of recombinant viral vectors. Gene Ther. 2003;10:977–982. doi: 10.1038/sj.gt.3302030. [DOI] [PubMed] [Google Scholar]
- BIFFI A, DE PALMA M, QUATTRINI A, DEL CARRO U, AMADIO S, VISIGALLI I, SESSA M, FASANO S, BRAMBILLA R, MARCHESINI S, BORDIGNON C, NALDINI L. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J. Clin. Invest. 2004;113:1118–1129. doi: 10.1172/JCI19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOMER U, NALDINI L, KAFRI T, TRONO D, VERMA IM, GAGE FH. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J. Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSIGLIO A, QUATTRINI A, MARTINO S, BENSADOUN JC, DOLCETTA D, TROJANI A, BENAGLIA G, MARCHESINI S, CESTARI V, OLIVERIO A, BORDIGNON C, NALDINI L. In vivo gene therapy of metachromatic leukodystrophy by lentiviral vectors: Correction of neuropathology and protection against learning impairments in affected mice. Nat. Med. 2001;7:310–316. doi: 10.1038/85454. [DOI] [PubMed] [Google Scholar]
- FOLLENZI A, AILLES LE, BAKOVIC S, GEUNA M, NALDINI L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat. Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- FOLLENZI A, SABATINO G, LOMBARDO A, BOCCACCIO C, NALDINI L. Efficient gene delivery and targeted expression to hepatocytes in vivo by improved lentiviral vectors. Hum. Gene Ther. 2002;13:243–260. doi: 10.1089/10430340252769770. [DOI] [PubMed] [Google Scholar]
- FOLLENZI A, BATTAGLIA M, LOMBARDO A, ANNONI A, RONCAROLO MG, NALDINI L. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood. 2004;103:3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- GUILLOT S, AZZOUZ M, DEGLON N, ZURN A, AEBISCHER P. Local GDNF expression mediated by lentiviral vector protects facial nerve motoneurons but not spinal motoneurons in SOD1(G93A) transgenic mice. Neurobiol. Dis. 2004;16:139–149. doi: 10.1016/j.nbd.2004.01.017. [DOI] [PubMed] [Google Scholar]
- JOOSS K, CHIRMULE N. Immunity to adenovirus and adeno-associated viral vectors: Implications for gene therapy. Gene Ther. 2003;10:955–963. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- KAY MA, GLORIOSO JC, NALDINI L. Viral vectors for gene therapy: The art of turning infectious agents into vehicles of therapeutics. Nat. Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- KORDOWER JH, EMBORG ME, BLOCH J, MA SY, CHU Y, LEVENTHAL L, McBRIDE J, CHEN EY, PALFI S, ROITBERG BZ, BROWN WD, HOLDEN JE, PYZALSKI R, TAYLOR MD, CARVEY P, LING Z, TRONO D, HANTRAYE P, DEGLON N, AEBISCHER P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- LO BIANCO C, DEGLON N, PRALONG W, AEBISCHER P. Lentiviral nigral delivery of GDNF does not prevent neurodegeneration in a genetic rat model of Parkinson’s disease. Neurobiol Dis. 2004;17:283–289. doi: 10.1016/j.nbd.2004.06.008. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN PR. Immunology of viral-vector-mediated gene transfer into the brain: An evolutionary and developmental perspective. Trends Immunol. 2002;23:23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN PR. Input virion proteins: Cryptic targets of antivector immune responses in preimmunized subjects. Mol. Ther. 2004;9:771–774. doi: 10.1016/j.ymthe.2004.05.019. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN PR, CASTRO MG. Inflammation and adaptive immune responses to adenoviral vectors injected into the brain: peculiarities, mechanisms, and consequences. Gene Ther. 2003;10:946–954. doi: 10.1038/sj.gt.3302048. [DOI] [PubMed] [Google Scholar]
- NALDINI L, BLOMER U, GALLAY P, ORY D, MULLIGAN R, GAGE FH, VERMA IM, TRONO D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- PEDEN C, BURGER C, MUZYCZKA N, MANDEL RJ. The effects of pre-immunization to wtAAV on single and multiple administrations of rAAV in the brain. Mol. Ther. 2003;7:S312. [Google Scholar]
- PEDEN CS, BURGER C, MUZYCZKA N, MANDEL RJ. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J. Virol. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REGULIER E, ZALA D, AEBISCHER P, DEGLON N. Lentiviral-mediated gene transfer to model triplet repeat disorders. Methods Mol. Biol. 2004;277:199–213. doi: 10.1385/1-59259-804-8:199. [DOI] [PubMed] [Google Scholar]
- SOUTHGATE T, KINGSTON P, CASTRO MG. Gene transfer into neural cells in vivo using adenoviral vectors. In: Gerfen CR, McKay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current Protocols in Neuroscience. John Wiley & Sons; New York: 2000. pp. 4.23.21–24.23.40. [Google Scholar]
- STERIO DC. The unbiased estimation of number and size of arbitrary particles using the disector. J. Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- SUWELACK D, HURTADO-LORENZO A, MILLAN E, NICOLINI VGONZALEZ, WAWROWSKY K, LOWENSTEIN P, CASTRO MG. Neuronal expression of the transcription factor Gli1 using the Tα1 α-tubulin promoter is neuroprotective in an experimental model of Parkinson’s disease. Gene Ther. 2004;11:1742–1752. doi: 10.1038/sj.gt.3302377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS CE, ABORDO-ADESIDA E, MALENIAK TC, STONE D, GERDES G, LOWENSTEIN PR. Gene transfer into rat brain using adenoviral vectors. In: Gerfen JN, McKay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current Protocols in Neuroscience. John Wiley & Sons; New York: 2000a. pp. 4.23.21–24.23.40. [DOI] [PubMed] [Google Scholar]
- THOMAS CE, SCHIEDNER G, KOCHANEK S, CASTRO MG, LOWENSTEIN PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: Toward realistic long-term neurological gene therapy for chronic diseases. Proc. Natl. Acad. Sci. U.S.A. 2000b;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS CE, BIRKETT D, ANOZIE I, CASTRO MG, LOWENSTEIN PR. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol. Ther. 2001a;3:36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- THOMAS CE, SCHIEDNER G, KOCHANEK S, CASTRO MG, LOWENSTEIN PR. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum. Gene Ther. 2001b;12:839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- THOMAS CE, EDWARDS P, WICKHAM TJ, CASTRO MG, LOWENSTEIN PR. Adenovirus binding to the cox-sackievirus and adenovirus receptor or integrins is not required to elicit brain inflammation but is necessary to transduce specific neural cell types. J. Virol. 2002;76:3452–3460. doi: 10.1128/JVI.76.7.3452-3460.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS CE, STORM TA, HUANG Z, KAY MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG LF, RALPH GSCOTT, WALMSLEY LE, BIENEMANN AS, PARHAM S, KINGSMAN SM, UNEY JB, MAZARAKIS ND. Lentiviral-mediated delivery of Bcl-2 or GDNF protects against excitotoxicity in the rat hippocampus. Mol. Ther. 2005;11:89–95. doi: 10.1016/j.ymthe.2004.08.026. [DOI] [PubMed] [Google Scholar]
- YEWDELL JW, HILL AB. Viral interference with antigen presentation. Nat. Immunol. 2002;3:1019–1025. doi: 10.1038/ni1102-1019. [DOI] [PubMed] [Google Scholar]
- YEWDELL JW, NORBURY CC, BENNINK JR. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: Implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv. Immunol. 1999;73:1–77. doi: 10.1016/s0065-2776(08)60785-3. [DOI] [PubMed] [Google Scholar]
- ZALA D, BENSADOUN JC, DE ALMEIDA LPEREIRA, LEAVITT BR, GUTEKUNST CA, AEBISCHER P, HAYDEN MR, DEGLON N. Long-term lentiviral-mediated expression of ciliary neurotrophic factor in the striatum of Huntington’s disease transgenic mice. Exp. Neurol. 2004;185:26–35. doi: 10.1016/j.expneurol.2003.09.002. [DOI] [PubMed] [Google Scholar]


