Abstract
The physiological relevance of the activation of hepatocyte growth factor (Hgf) by the plasminogen (Plg) system of proteases and its contribution to tissue repair are largely undefined. Here, we investigated whether the defective liver repair in mice lacking Plg is due to impaired activation of Hgf. Loss of Plg in vivo suppressed Hgf activation and signaling through its Met tyrosine kinase receptor. Without Plg, hepatocytes were unresponsive to Hgf-induced proliferation and migration, with a more pronounced impairment in hepatocyte movement within the hepatic environment. Most notably, circumventing the defect in proteolytic activation of Hgf by the downstream expression of an activated Met receptor corrected the functional deficits and improved liver repair in Plg-deficient mice. These findings support a fibrinolysis-unrelated role for Plg in modulating cell proliferation and migration by activation of Hgf.
Tissue repair requires a prompt proliferative response in concert with the timely reorganization of the extracellular matrix. Each one of these processes can be disrupted by the loss of individual growth factors or proteases, but the precise regulatory relationship between these molecules in supporting tissue repair is not fully understood. Multiple in vitro studies have inferred that proteases in the plasminogen (Plg)2 activation system may be important in the proteolytic activation of the hepatocyte growth factor (Hgf) (1–4), the ligand for the Met tyrosine kinase receptor that exerts potent mitogenic and motogenic properties to mesenchymal and epithelial cells. This concept is made even more attractive by the fact that Hgf is structurally related to Plg, with multiple kringle domains and a catalytically inactive serine protease-like domain. However, the physiological relevance of Plg to Hgf activation and Hgf-related reparative processes are controversial and effectively unexplored in vivo.
We previously reported that a genetically imposed loss of circulating Plg severely impairs clearance of necrotic cells and the repopulation of injured zones by newly formed cells but without compromising the general hepatic proliferative response (5). Despite the indisputable role of Plg in fibrin clearance (6), complementary studies in mice with no capacity for fibrin deposition have shown that the loss of fibrinolytic function alone in Plg-deficient mice cannot account for the impediment in tissue repair (5). Multiple nonfibrin targets of plasmin-mediated proteolysis are known (e.g. serine and metalloprotease zymogens, and extracellular matrix glycoproteins, latent growth factors), and it is feasible that they may contribute to the focal clearance of necrotic tissue. However, based on recent findings pointing to a strikingly similar defect in hepatic repair in mice lacking Plg or a conditional loss of Met (7), an attractive hypothesis emerged that the Plg activation system supports physiological liver repair by activation of the Met ligand, Hgf. Testing this hypothesis, we found that the loss of Plg impairs Hgf activation, suppresses Met phosphorylation and signaling, and prevents Hgf-induced migration of hepatocytes. Most notably, consistent with a physiologically relevant contribution of Plg to Hgf-Met signaling, the expression of an autophosphorylated Met largely corrected the defective repair in Plg-deficient livers.
EXPERIMENTAL PROCEDURES
Gene-targeted Mice—Mice with a targeted disruption of genes encoding Plg (Plgo), urokinase-type Plg activator (uPAo), tissue-type Plg activator (tPAo), or both (uPAo/tPAo) were of mixed genetic background and generated by matings of double heterozygous mice, as described previously (6, 8). Experimental challenges were performed in 2–6-month-old littermates; animals were housed in standard facilities with a 12-h light cycle and were fed food and water ad libitum. Animal protocols were approved by the Institutional Animal Care and Use Committee of Cincinnati Children's Hospital Research Foundation.
Model of Liver Injury—Gene-targeted and control mice were subjected to an acute toxic injury by a single intraperitoneal injection of 0.5 ml of carbon tetrachloride (CCl4; Sigma)/kg of body weight as a 25% (v/v) solution in corn oil (5). A group of mice also received bromodeoxyuridine (BrdUrd; 2 mg/kg) intraperitoneally 2 h before sacrifice for cell proliferation studies (9). Livers were either paraffin-embedded, snap-frozen, or used for protein isolation.
Protein and mRNA Expression—Protein homogenates were obtained from livers or cultured hepatocytes and quantified by the Bradford method (9). For Hgf quantification, homogenates were centrifuged at 25,000 × g for 20 min and 105,000 × g for 60 min at 4 °C, and the supernatant was passed through an S-Sepharose column, concentrated using Centricon 30 (Chemicon, Temecula, CA), and then subjected to Western analysis under reducing conditions with goat anti-Hgf antibody (catalogue number sc-1357; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) (9). Western analyses of whole protein homogenates were used to quantify phosphorylated Met (anti-Met antibody from Santa Cruz Biotechnology; anti-phosphotyrosine 4G10 antibody from Upstate Cell Signaling Solutions (Charlottesville, VA)) and phosphorylated Erk1/2 (as a ratio to total Erk1/2; both antibodies from Cell Signaling Technology, Inc., Danvers, MA). Specific signals were quantified using the ImageQuant program (Amersham Biosciences). uPA and tPA expression was assessed by zymography in casein- and plasminogen-containing polyacrylamide gel casts, as described previously (9). Hgf mRNA was detected by real time PCR using forward primer 5-TGGTCTATGGTCCTGAAGGC-3 and reverse primer 5-TGGTGCTGACTGCATTTCTC-3, whereas actin was detected by forward primer 5-GCGCAAGTACTCTGTGTGGA-3 and reverse primer 5-GAAAGGGTGTAAAACGCAGC-3, as described previously (10).
Wound Healing Assay—Migration of hepatocytes was assessed by monitoring the filling of a ∼500-μm wound inflicted to monolayers of primary hepatocytes in culture, as described previously (11). In brief, hepatocytes were isolated from either plasminogen-sufficient (Plg+) or Plgo mice following a two-step collagenase perfusion of the liver and cultured in William's E medium containing 5% mouse serum, 1 nm dexamethasone, and 10 nm insulin (9, 10) in the presence or absence of human recombinant single chain Hgf (scHgf), activated human Hgf (aHgf) (both at 40 ng/ml; R&D Systems, Inc., Minneapolis, MN), or purified human plasmin, active uPA, or tPA (American Diagnostica, Inc., Stamford, CT). The contribution of proliferation and migration to wound healing was determined by the addition of mitomycin C (2 ng/ml; Sigma) or cytochalasin D (50 μm; Sigma) to the culture medium. The distance between the wound edges was measured using Qcapture imaging software by utilization of spatial calibration after image capture in light microscopy (Olympus America Inc., San Jose, CA). The distance was measured between the cytoplasm of the cells on either side of wound, with three measurements per field for a total of 10 fields for each culture well using a ×10 objective.
Liver Cell Proliferation and Migration—Hepatocyte proliferation was quantified in primary hepatocytes (cultured in medium containing BrdUrd at a 1:1000 dilution) or in liver sections by incubation with nuclease/anti-BrdUrd antibodies according to the manufacturer's protocol (BD Biosciences). To evaluate for migration, cultured hepatocytes and liver sections were stained with Alexa Fluor-phalloidin according to the manufacturer's instructions (Invitrogen). BrdUrd staining and actin rearrangement were monitored by confocal microscopy (Carl Zeiss MicroImaging, Inc., Thornwood, NY).
Expression of Activated Met—Recombinant adenoviruses expressing cyto-Met and GFP were prepared using the pAdEasy system under the control of human cytomegalovirus promoter. The cyto-Met is a transgene construct that encodes for a truncated, membrane-anchored Met that undergoes spontaneous activation and was obtained from Dr. Marco Tripodi (National Institute for Infectious Disease L. Spallanzani, IRCCS, Rome, Italy). Cyto-Met was cloned into the pShuttle-CMV vector along with an internal ribosome entry site-GFP minigene (Clontech) and expressed in HEK 293 packaging cells to produce the recombinant adenovirus Ad-cyto-MetGFP according to the manufacturer's protocol; a control recombinant adenovirus lacking cyto-Met (referred to as Ad-GFP) was produced, following the same protocol (supplemental Fig. 1). For in vitro wound-healing assays, Plgo hepatocytes were infected with recombinant adenovirus (multiplicity of infection = 10) after an initial culture for 3 h and wounding of the hepatocyte monolayer. One hour later, the culture medium was changed, and the monolayers were monitored for wound healing by daily examination under a fluorescence microscope. To determine whether activation of Met corrects the defective repair induced by Plg deficiency, Ad-cyto-MetGFP or Ad-GFP was administered (1010 plaque-forming units/g of body weight) into the tail vein 2 days after administration of CCl4 to Plgo or Plg+ mice. Mice were examined daily and sacrificed 12 days later.
Statistical Analysis—Values are expressed as mean ± S.D., and statistical significance was determined by unpaired t test, with a significance level of p < 0.05.
RESULTS
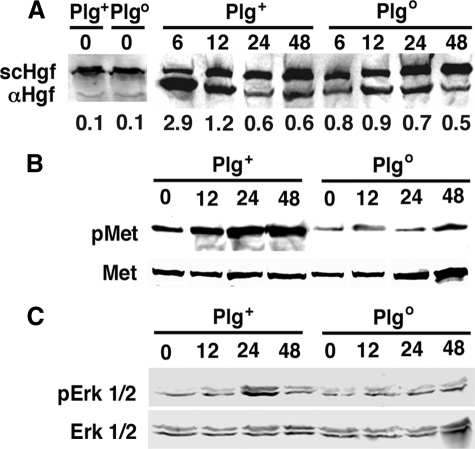
Suppressed Activation of Hgf in Plgo Livers—We first determined whether Plg modulates Hgf activation by quantifying the expression of the α-heavy chain Hgf (αHgf), which is detected after Hgf activation, in livers of Plg+ and Plgo mice at different time points after a single intraperitoneal dose of CCl4; mouse survival was similar in both genotypes after CCl4 administration, as reported previously (5). In livers of Plg+ mice, we found that the expression of αHgf promptly increased at 6 and 12 h, as demonstrated by an increased ratio of αHgf to scHgf (2.9 and 1.2, respectively) and remained detected at lower levels at 24 and 48 h (Fig. 1A). Although this lower level of αHgf detection was present in livers of Plgo mice, the early increases at 6 and 12 h were lower, limited to ratios of 0.8 and 0.9, respectively. Because even low levels of αHgf may be sufficient to activate Met signaling, we next measured the level of phosphorylation of Met (pMet in Fig. 1) and Erk1/2 (pErk1/2). In Plg+ mice, the prominent expression of αHgf at 6–12 h was associated with a rise in phosphorylation of Met that persisted to at least 48 h, whereas the lack of increase of αHgf in Plgo livers kept the levels of phosphorylation of Met near base line (Fig. 1B) and blocked the typical rise of phosphorylated Erk1/2 at 24 h (Fig. 1C). These findings provided the first evidence that the loss of Plg limited Hgf activation in vivo and impeded Met signaling during the reparative response of the liver to an injury. To explore the relationship between these processes and the defective tissue healing, we next determined the functional relationship between Plg and Hgf in the promotion of hepatocyte migration.
FIGURE 1.
Decreased Hgf activation and suppression of Met signaling in Plgo mice. A, Western analysis depicts the liver expression of αHgf and scHgf 0–48 h after CCl4 administration to Plg+ and Plgo mice. Each lane represents pooled Hgf from three livers; the numbers below the panels represent a ratio of αHgf to total Hgf (αHgf + scHgf). The decrease in αHgf is associated with decreased phosphorylation of Met (pMet; B) and Erk1/2 (pErk1/2; C). B and C show representative Western signals from three separate sets of experiments, with each lane representing protein homogenate from one liver. The numbers above the Western blots denote hours after CCl4 administration.
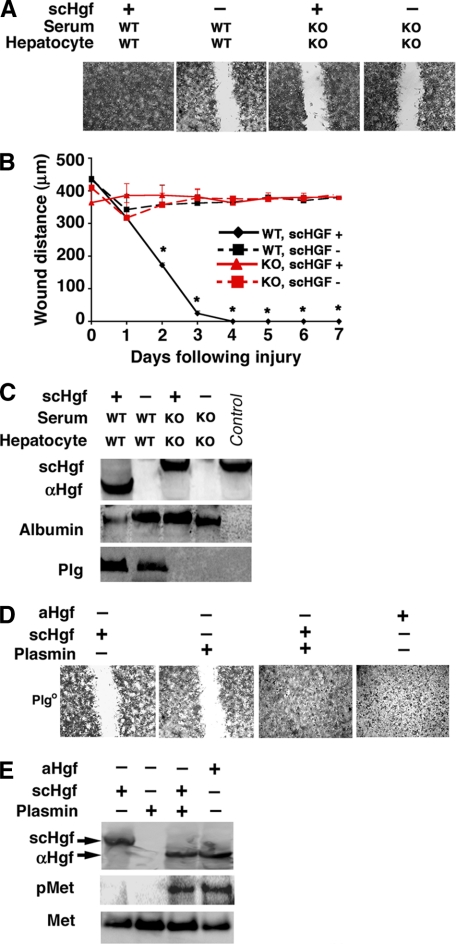
Impaired Hgf-induced Wound Healing in the Absence of Plg— To directly determine the relevance of physiological levels of Plg in Hgf-induced migration, we monitored the healing of a physical wound inflicted on a monolayer of hepatocytes in a primary culture system where the source of Plg is the serum and cultured cells. When Plg is constitutively present, the addition of scHgf induced a prompt wound repair within 2–3 days. In contrast, the removal of Plg by using serum and hepatocytes from Plgo mice completely prevented the wound repair (Fig. 2, A and B). Analysis of the conditioned media from these experiments showed that the conversion of scHgf to αHgf was impaired in the absence of Plg (Fig. 2C), whereas analysis of cell lysates found no evidence of endogenous production of Hgf by Plg+ or Plgo hepatocytes in culture (supplemental Fig. 2). To determine the ability/inability of the active form of Plg to activate Hgf, we performed wound healing assays using serum-free media with or without the active protease plasmin. We found that neither scHgf nor plasmin promoted wound healing, but the addition of plasmin to media containing scHgf enabled the full recovery of the hepatocyte monolayer (Fig. 2D) and induced Met phosphorylation (Fig. 2E). Thus, Hgf activation and Hgf-induced wound healing require the maintenance of physiological levels of Plg.
FIGURE 2.
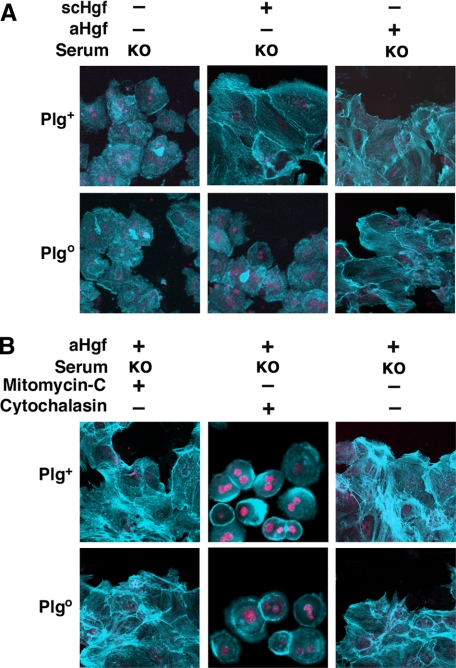
Defective wound healing in monolayer of hepatocytes cultured without Plg. Photomicrographs show the filling of wounds inflicted on monolayers of hepatocytes from Plgo or Plg+ mice cultured for 7 days in media containing serum from either genotype with or without scHgf (A; panels display representative results of three separate experiments). In B, the distance between hepatocytes on either margin of the wound is shown after 1–7 days of culture (mean ± S.D.; n = 3 culture wells per group and per time point; *, p < 0.01). In C, Western analyses show the levels of αHgf and scHgf, albumin, or Plg in conditioned media from culture wells containing Plg+ and Plgo hepatocytes cultured for 24 h (a fifth“control” lane contains purified scHgf). In D, photomicrographs show representative wound healing assays in Plgo hepatocytes in the presence of plasmin, scHgf, or aHgf in serum-free media. In E, the conditioned media from experiments depicted in D were used in Western analysis to detect the expression of αHgf and scHgf, whereas the hepatocyte lysates were used to detect Met phosphorylation (pMet). WT, from Plg+ mice; KO, from Plgo mice; +, scHgf or aHGF added; -, nothing added. Magnification for A and C was ×100. Data from C–E are representative results of three separate experiments.
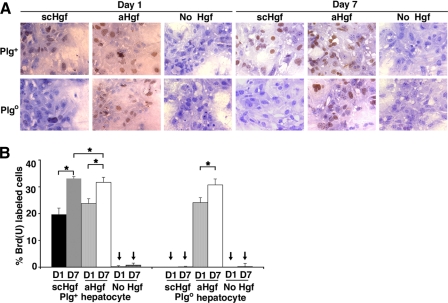
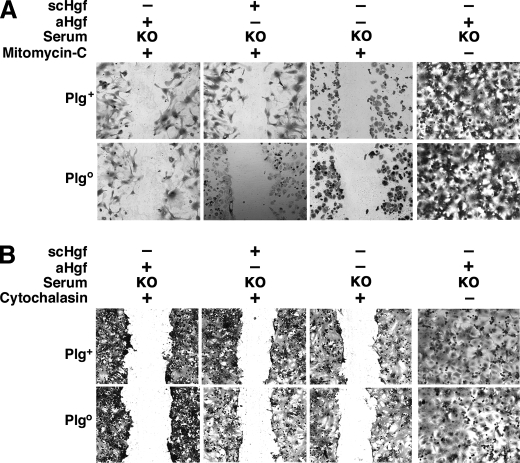
Plg Regulation of Hgf-induced Proliferation and Migration— Because wound healing requires both cellular proliferation and migration, we investigated whether Plg-mediated activation of Hgf selectively modifies its mitogenic or migratory properties. First, measuring BrdUrd uptake by hepatocytes during wound healing, we found that Hgf-induced proliferation was severely impaired in the absence of Plg (Fig. 3, A and B) in a fashion similar to mitomycin C-induced inhibition of mitosis (Fig. 4A). Interestingly, despite the defective proliferation, the cells partially filled the wound by cytoplasmic elongations, suggesting the induction of cell motility (Fig. 4A, left panels). When migration was inhibited by exposure to cytochalasin, there was no cytoplasmic elongations or evidence of healing (Fig. 4B). To more clearly define the contribution of hepatocyte motility to wound healing, we monitored actin staining and found that the loss of Plg prevented Hgf-induced actin reorganization in hepatocytes (Fig. 5A). The contribution of these cellular changes to wound healing was further demonstrated by a pronounced lack of actin rearrangement in the presence of cytochalasin (Fig. 5B). These results show that the well recognized properties of Hgf as a promoter of hepatocyte proliferation and migration are dependent on Plg availability. They also raised the possibility that cell migration may be a greater contributor to wound healing. This possibility is particularly relevant in view of our previous report that hepatocyte proliferation in vivo is generally unaffected in Plgo livers despite a severe defect in repair after CCl4 injury (5).
FIGURE 3.
Defective Hgf-induced proliferation and migration in Plgo hepatocytes. A, BrdUrd uptake in hepatocytes during wound healing of Plg+ and Plgo hepatocytes cultured in media containing serum from mice with respective genotypes and the addition of scHgf, aHgf, or no Hgf after 1 and 7 days of culture (magnification, ×400). B, average percentage ± S.D. of BrdUrd-labeled hepatocytes at both time points. n = 3–4 for each experimental group and time point; *, p < 0.05; arrows point to no detection of labeled hepatocytes.
FIGURE 4.
Contribution of proliferation and migration to wound healing. Photomicrographs show the partial (A) or complete (B) deficit of wound healing in monolayers of hepatocytes from Plgo or Plg+ mice cultured for 7 days with mitomycin C (A) or cytochalasin (B) added to media containing Plgo serum with or without scHgf or aHgf. KO, from Plgo mice; +, Hgf, mitomycin C, or cytochalasin added; -, nothing added; magnification, ×100. The panels display representative results of three separate experiments.
FIGURE 5.
Control of Hgf-induced actin rearrangement by Plg. Immunostaining shows actin rearrangement during wound healing of Plgo or Plg+ hepatocyte cultured for 24 h in the presence of scHgf or aHgf (A), with or without mitomycin C or cytochalasin (B). KO, from Plgo mice; +, Hgf, mitomycin C, or cytochalasin added; -, nothing added; magnification, ×2000. The panels display representative results of three separate experiments.
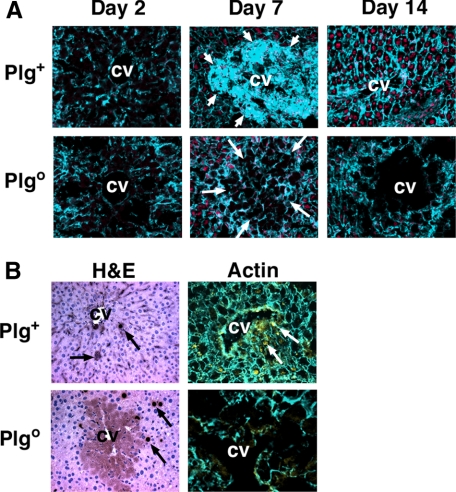
Impaired Movement of Hepatocytes in Plgo Livers—To assess the contribution of hepatocyte migration to the defective liver repair induced by Plg deficiency in vivo, we monitored actin rearrangement in Plgo livers after CCl4 administration. In the acute phase of injury, we found no difference in actin staining between Plg+ and Plgo livers. At the time of resolution of the centrilobular injury (7 days), an intense actin staining was detected in hepatocytes repopulating the injured area in Plg+ livers, with restoration of normal staining by 14 days (Fig. 6A). In contrast, the loss of Plg prevented the development of centrilobular staining at 7 days and throughout the study period. Interestingly, the number of newly formed BrdUrd-stained hepatocytes was similar in Plg+ and Plgo livers, but their pattern of distribution differed in Plgo livers, with proliferating cells failing to populate the injured centrilobular environment (Fig. 6B). Thus, in agreement with the findings from wound healing experiments, a defective movement of newly formed hepatocytes along the liver lobule emerges as a likely physiological process responsible for the abnormal hepatic repair induced by Plg deficiency.
FIGURE 6.
Impaired movement of hepatocytes within the lobule of Plgo livers. A, immunostaining after one dose of CCl4 shows a lack of actin staining in injured centrilobular hepatocytes in Plg+ and Plgo livers 2 days later. Subsequently, intense actin rearrangement occurs during the repopulation of injured areas by hepatocytes (short arrows) in Plg+ livers, with normalization of actin staining in the lobule at 14 days. In contrast, minimal actin staining is noted in Plgo livers at all time points (long arrows point to injured centrilobular areas). In B, BrdUrd-labeled hepatocytes are present within repaired centrilobular areas of Plg+ livers 14 days after CCl4 but remain outside the injured centrilobular areas of Plgo mice. BrdUrd-labeled cells are those with dark brown nuclei on hematoxylin/eosin staining (H&E; arrows in left panels) and yellow when double-stained with actin (arrows in right panels). CV, central vein; magnification, ×400 (A and the left panel of B) and ×1000 (right panel of B). 3–4 mice were examined for each group and each time point.
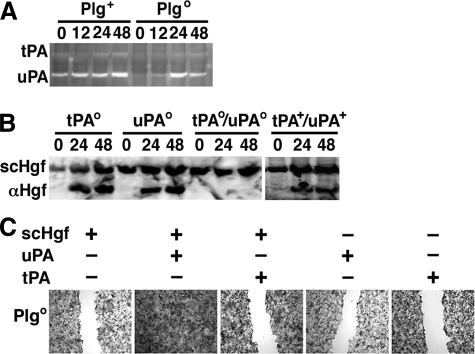
Regulation of Hgf-induced Wound Healing by uPA—Although our experimental data strongly suggested that the availability of Plg is key to Hgf activation and function, previous reports implicated Plg activators in the proteolytic processing of Hgf in vitro (12–16). To examine this possibility, we first determined the residual expression of tPA and uPA by zymographic analyses of the liver after CCl4. Although the expression of both tPA and uPA increased in the first 48 h in Plg+ livers, the loss of Plg resulted in an overall decrease in tPA expression and a delayed increase in uPA expression to 24 and 48 h (Fig. 7A). To examine the contribution of tPA and/or uPA to Hgf activation, we quantified αHgf in livers from tPAo, uPAo, tPAo/uPAo, or tPA+/uPA+ mice after CCl4 administration. The peak levels of αHgf tended to be higher in tPAo than in uPAo livers, but the absence of plasminogen activation by the simultaneous loss of tPA/uPA completely prevented the emergence of αHgf during liver repair (Fig. 7B). To directly examine whether tPA or uPA independently promote Hgf activation, we added them individually to culture media in wound-healing assays. We found that uPA, but not tPA, promoted Hgf-induced wound healing (Fig. 7C). Although these in vitro data suggested that uPA might independently promote Hgf-induced wound healing, previous reports show that the defective liver repair in uPAo mice is mild (17, 18). Further, the ability of plasmin alone to promote Hgf function (Fig. 2D) and the previous reports of a similar defective repair in livers lacking Plg or Met provided a rationale for the hypothesis that plasmin(ogen) is required for the regulatory role of Hgf in promoting liver repair via activation of Met signaling.
FIGURE 7.
Hgf activation by uPA and tPA. A, zymographic analysis using liver protein lysates shows a decrease in tPA expression and a delay in uPA expression in Plgo livers when compared with Plg+ livers 0–48 h after CCl4. B, Western analysis shows the expression of αHgf in tPAo or uPAo livers but not in tPAo/uPAo livers at 24 and 48 h after CCl4. C, photomicrographs depict the filling of wounds to monolayers of hepatocytes from Plgo mice cultured for 7 days with the addition of uPA or tPA added to media containing serum from Plgo genotype with or without scHgf. +, Hgf or uPA or tPA added; -, nothing added; magnification for C, ×100. The panels display representative results of three separate experiments.
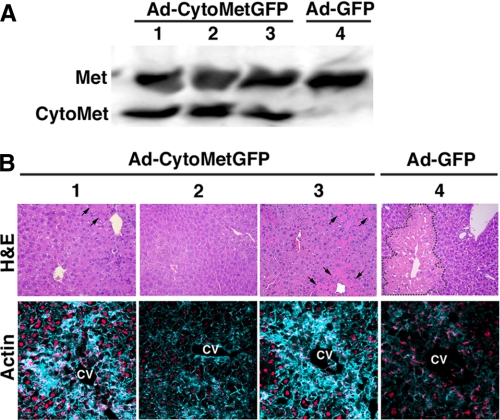
Improved Defective Repair in Plgo Livers by the Activation of Met—To determine whether impaired Hgf activation is the primary mechanism underlying the defective repair in Plgo liver, we bypassed the impaired Hgf activation by expressing an exogenous Met (named cyto-Met) that undergoes spontaneous activation independent of binding to Hgf (19). This was initially done in a wound-healing assay in which monolayers of Plgo hepatocytes were infected with the recombinant adenovirus Ad-cyto-MetGFP (multiplicity of infection = 10) added to Plgo serum-containing media for 1 h after wounding; separate culture wells were infected with the Ad-GFP virus that does not express cyto-Met (to serve as a control). Hepatocytes cultured with Ad-cyto-MetGFP completely filled the wound, whereas hepatocytes infected with Ad-GFP were unable to heal the wound (supplemental Fig. 3). Then we determined whether the Met activation corrects the defective hepatocyte motility caused by Plg deficiency during liver repair in vivo. This was done by administering 1010 plaque-forming units of Ad-cyto-MetGFP or Ad-GFP intravenously to Plgo or Plg+ mice 2 days after CCl4, a time when the extensive centrilobular injury is fully established. We did not administer the virus before CCl4, because the administration of Hgf (and the presumed binding/activation of Met) prior to CCl4 has been shown to decrease the extent of liver injury (20, 21). Livers of Plgo mice treated with Ad-cyto-MetGFP expressed cyto-Met as far out as 12 days (or 14 days after CCl4; Fig. 8A). Notably, these livers had normal visual appearance (not shown) and normalized or greatly decreased the residual centrilobular injury (Fig. 8B). In contrast, Plgo mice receiving the control Ad-GFP adenovirus showed a persistent impairment in clearance of injured hepatocytes (Fig. 8B), whereas Plg+ control mice receiving either virus construct had normal repair (not shown). In addition, the expression of phosphorylated Met induced actin rearrangement and repopulated the centrilobular area by hepatocytes in Plgo livers (lower panel of Fig. 8B). Collectively, these data provide evidence that activation of Met restores molecular circuits that are sufficient to restore cellular motility and to rescue the defective tissue repair imposed by plasminogen deficiency.
FIGURE 8.
Improved repair in Plgo livers by activated Met. A, Western analysis shows the hepatic expression of autophosphorylated Met (cyto-Met) 12 days after administration of Ad-cyto-MetGFP or Ad-GFP adenovirus. B, representative photomicrographs of sections of Plgo livers stained with either hematoxylin/eosin (top) or actin (bottom) 14 days after CCl4 and 12 days after adenovirus administration. Numbers represent individual mice; area of centrilobular injury is shown within dashed lines (in mouse 4) or by arrows (mice 1–3). Actin rearrangement is present in resolving centrilobular injuries of mice 1 and 3 and as a base-line staining in normalized centrilobular area of mouse 2. CV, central vein; magnification, ×200.
DISCUSSION
The demonstration that Plg (or more specifically plasmin) is critical to the activation of Hgf broadens the physiological functions of Plg to include the modulation of cell proliferation and movement during tissue repair by promoting the proteolytic processing of growth and/or scattering factor(s). Although Hgf activation in vivo is probably not exclusively dependent on Plg, our data suggest that the full expression of the biological properties of Hgf in liver repair requires physiological levels of Plg. Although Hgf-induced hepatocyte proliferation and migration in vitro are modulated by Plg, the findings of normal hepatocyte proliferation in response to CCl4 in Plgo mice do not support a critical role for Plg in Hgf-induced mitosis in vivo. In contrast, the ability of activated Met to restore actin rearrangement and improve wound repair in vitro and in vivo suggests that impaired Met signaling induced by defective Hgf activation is a chief mechanism underlying the defective liver repair in Plgo mice.
The control of Hgf activation by Plg does not exclude the participation of other molecules (e.g. uPA, Hgf activator (Hgfa), hepsin, and others) in the activation of Hgf (3, 12–16, 22, 23). These proteins have been shown to activate Hgf in several in vitro systems. However, evidence for their relevance to living organisms is limited and in some cases not directly supported. For example, hepsin has been proposed to be an important activator of Hgf (directly or through the activation of pro-uPA), but mice lacking hepsin have normal liver development and regeneration (22, 24, 25), suggesting the existence of accessory pathways that are able to promote Hgf activation in vivo. Our findings demonstrate that Plg and uPA are likely members of this pathway. One previous study examining the role of the plasminogen activator inhibitor-1 in lung fibrosis used tranexamic acid to inhibit plasmin activity and found decreased Hgf activation (26). Three additional lines of evidence support a central role for Plg in Hgf activation in the liver and a secondary role for uPA: 1) the milder defective repair in livers of mice lacking uPA (9), 2) no obvious requirement for uPA in wound healing assays, and 3) the complete lack of αHgf chain in livers of mice lacking Plg activation due to the combined loss of uPA plus tPA. Our studies do not negate the possibility that uPA, Hgfa, hepsin, and other molecules might activate Hgf directly, but the data reported herein support a key participation of Plg (plasmin) in the Hgf activation circuit during liver repair.
The identification of cell motility as the primary Hgf function regulated by Plg points to the relevance of Hgf-Met signaling in promoting hepatocyte movement along the lobular framework during liver repair. This concept is also supported by an earlier report showing that Met-deficient mice have impaired hepatocyte motility but differs from previous suggestions of a primary mitogenic effect of the Hgf-Met signaling on hepatocytes (27–30). Our data suggest that the hepatocyte movement within the adjacent microenvironment, even if limited to a short distance, enables existing and newly formed hepatocytes to (re)position themselves within the lobule to replace injured hepatocytes and restore anatomical integrity to the liver. Interfering with this (re)positioning either by loss of Plg-dependent Hgf activation or by Met deficiency has the potential to impair liver regeneration and repair. It is likely that cellular movement may be slowed or prevented by the accumulation of fibrin-rich matrix substrates normally cleared by Plg. However, the previous report that the defective liver repair is not corrected by the genetically superimposed loss of fibrin in Plgo mice was the first indication that Plg influences liver cell movement by fibrin-independent mechanisms (5). Here, the findings that the loss of Plg significantly suppressed activation of Met suggested that the delayed, mild increase was not enough to correct the defective repair in Plg-deficient mice. Consistent with this possibility, we found that Met activation restored cell movement and improved the centrilobular injury of Plgo mice. Collectively, these findings uncover the activation of Hgf as one of the key mechanisms by which proteolytic deficits in the extracellular environment critically affect intracellular signals and important cellular functions.
It is intriguing that the activation of Met enabled the clearance of necrotic cells and provisional matrix despite the absence of Plg. Although we have not yet identified the mechanisms by which Hgf-Met directly or indirectly aids matrix clearance, it is possible that cellular signals triggered by Met may promote matrix reorganization via up-regulation of other proteases. In addition, Plg may also modulate intercellular cross-talk and decrease the availability of Hgf produced by fibrogenic stellate cells (31). Although we have not yet formally investigated these mechanisms, the in vivo relationship between Plg and Hgf reported in this study supports the hypothesis that Plg exerts fibrinolysis-unrelated functions as a promoter of cell proliferation and migration by direct or indirect interaction with Hgf and the induction of Met-dependent intracellular signals to restore anatomical integrity of injured livers.
Supplementary Material
Acknowledgments
We are grateful to Dr. Marco Tripodi for providing the cyto-Met transgene.
This work was supported, in whole or in part, by National Institutes of Health Grants DK55710 (to J. A. B.), AR049822 and HL085357 (to J. L. D.), and DK032512 (Integrative Morphology Core of the Digestive Health Center).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
Footnotes
The abbreviations used are: Plg, plasminogen; tPA, tissue-type plasminogen activator; uPA, urokinase-type plasminogen activator; Hgf, hepatocyte growth factor; scHgf, single chain Hgf; aHgf, activated Hgf; αHgf, α-heavy chain Hgf; Ad, adenovirus.
References
- 1.Gak, E., Taylor, W. G., Chan, A. M., and Rubin, J. S. (1992) FEBS Lett. 311 17-21 [DOI] [PubMed] [Google Scholar]
- 2.Mars, W. M., Zarnegar, R., and Michalopoulos, G. K. (1993) Am. J. Pathol. 143 949-958 [PMC free article] [PubMed] [Google Scholar]
- 3.Michalopoulos, G. K., and DeFrances, M. C. (1997) Science 276 60-66 [DOI] [PubMed] [Google Scholar]
- 4.Webb, C. P., Hose, C. D., Koochekpour, S., Jeffers, M., Oskarsson, M., Sausville, E., Monks, A., and Vande Woude, G. F. (2000) Cancer Res. 60 342-349 [PubMed] [Google Scholar]
- 5.Bezerra, J. A., Bugge, T. H., Melin-Aldana, H., Sabla, G., Kombrinck, K. W., Witte, D. P., and Degen, J. L. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 15143-15148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bugge, T. H., Kombrinck, K. W., Flick, M. J., Daugherty, C. C., Danton, M. J., and Degen, J. L. (1996) Cell 87 709-719 [DOI] [PubMed] [Google Scholar]
- 7.Huh, C. G., Factor, V. M., Sanchez, A., Uchida, K., Conner, E. A., and Thorgeirsson, S. S. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 4477-4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bugge, T. H., Flick, M. J., Danton, M. J., Daugherty, C. C., Romer, J., Dano, K., Carmeliet, P., Collen, D., and Degen, J. L. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 5899-5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanmukhappa, K., Sabla, G. E., Degen, J. L., and Bezerra, J. A. (2006) BMC Gastroenterol. 6 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shanmukhappa, K., Mourya, R., Sabla, G. E., Degen, J. L., and Bezerra, J. A. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 10182-10187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stolz, D. B., and Michalopoulos, G. K. (1997) J. Cell Physiol. 170 57-68 [DOI] [PubMed] [Google Scholar]
- 12.Jeffers, M., Rong, S., and Vande Woude, G. F. (1996) Mol. Cell Biol. 16 1115-1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naldini, L., Tamagnone, L., Vigna, E., Sachs, M., Hartmann, G., Birchmeier, W., Daikuhara, Y., Tsubouchi, H., Blasi, F., and Comoglio, P. M. (1992) EMBO J. 11 4825-4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naldini, L., Vigna, E., Bardelli, A., Follenzi, A., Galimi, F., and Comoglio, P. M. (1995) J. Biol. Chem. 270 603-611 [DOI] [PubMed] [Google Scholar]
- 15.Pepper, M. S., Matsumoto, K., Nakamura, T., Orci, L., and Montesano, R. (1992) J. Biol. Chem. 267 20493-20496 [PubMed] [Google Scholar]
- 16.Ried, S., Jager, C., Jeffers, M., Vande Woude, G. F., Graeff, H., Schmitt, M., and Lengyel, E. (1999) J. Biol. Chem. 274 16377-16386 [DOI] [PubMed] [Google Scholar]
- 17.Bezerra, J. A., Currier, A. R., Melin-Aldana, H., Sabla, G., Bugge, T. H., Kombrinck, K. W., and Degen, J. L. (2001) Am. J. Pathol. 158 921-929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Currier, A. R., Sabla, G., Locaputo, S., Melin-Aldana, H., Degen, J. L., and Bezerra, J. A. (2003) Am. J. Physiol. 284 G508-G515 [DOI] [PubMed] [Google Scholar]
- 19.Amicone, L., Galimi, M. A., Spagnoli, F. M., Tommasini, C., De Luca, V., and Tripodi, M. (1995) Gene (Amst.) 162 323-328 [DOI] [PubMed] [Google Scholar]
- 20.Kaido, T., Yamaoka, S., Tanaka, J., Funaki, N., Kasamatsu, T., Seto, S., Nakamura, T., and Imamura, M. (1996) Biochem. Biophys. Res. Commun. 218 1-5 [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto, K., and Nakamura, T. (1997) Ciba Found Symp. 212 198-211 [DOI] [PubMed] [Google Scholar]
- 22.Herter, S., Piper, D. E., Aaron, W., Gabriele, T., Cutler, G., Cao, P., Bhatt, A. S., Choe, Y., Craik, C. S., Walker, N., Meininger, D., Hoey, T., and Austin, R. J. (2005) Biochem. J. 390 125-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazawa, K., Shimomura, T., and Kitamura, N. (1996) J. Biol. Chem. 271 3615-3618 [DOI] [PubMed] [Google Scholar]
- 24.Moran, P., Li, W., Fan, B., Vij, R., Eigenbrot, C., and Kirchhofer, D. (2006) J. Biol. Chem. 281 30439-30446 [DOI] [PubMed] [Google Scholar]
- 25.Yu, I. S., Chen, H. J., Lee, Y. S., Huang, P. H., Lin, S. R., Tsai, T. W., and Lin, S. W. (2000) Thromb. Haemost. 84 865-870 [PubMed] [Google Scholar]
- 26.Hattori, N., Mizuno, S., Yoshida, Y., Chin, K., Mishima, M., Sisson, T. H., Simon, R. H., Nakamura, T., and Miyake, M. (2004) Am. J. Pathol. 164 1091-1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burr, A. W., Toole, K., Chapman, C., Hines, J. E., and Burt, A. D. (1998) J. Pathol. 185 298-302 [DOI] [PubMed] [Google Scholar]
- 28.Ishiki, Y., Ohnishi, H., Muto, Y., Matsumoto, K., and Nakamura, T. (1992) Hepatology 16 1227-1235 [PubMed] [Google Scholar]
- 29.Li, Z., Mizuno, S., and Nakamura, T. (2007) Am. J. Physiol. 292 G639-G646 [DOI] [PubMed] [Google Scholar]
- 30.Matsuda, Y., Matsumoto, K., Ichida, T., and Nakamura, T. (1995) J. Biochem. (Tokyo) 118 643-649 [DOI] [PubMed] [Google Scholar]
- 31.Passino, M. A., Adams, R. A., Sikorski, S. L., and Akassoglou, K. (2007) Science 315 1853-1856 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.