Abstract
Introduction
Distraction osteogenesis (DO) is characterized by the induction of highly vascularized new bone formation through an intramembranous process largely devoid of the formation of cartilage.
Materials and Methods
To test the hypothesis that DO is strictly dependent on vascualrization, we inhibited vascular endothelial growth factor (VEGF) activity by antibody blockade of both receptors VEGFR1 (Flt-1) and VEGFR2 (Flk-1) or only VEGFR2 (Flk-1) in a previously developed murine tibia DO model. During normal DO, VEGFR1 (Flt-1), VEGFR2 (Flk-1), VEGFR3 (Flt4) and all four VEGF ligand (A, B, C, and D) mRNAs are induced.
Results
The expression of mRNA for the receptors generally paralleled those of the ligands during the period of active distraction. Bone formation, as assessed by μCT, showed a significant decrease with the double antibody treatment and a smaller decrease with single antibody treatment. Vessel volume, number, and connectivity showed progressive and significant inhibition in all of these of parameters between the single and double antibody blockade. Molecular analysis showed significant inhibition in skeletal cell development with the single and double antibody blockade of both VEGFR1 and 2. Interestingly, the single antibody treatment led to selective early development of chondrogenesis, whereas the double antibody treatment led to a failure of both osteogenesis and chondrogenesis.
Conclusions
Both VEGFR1 and VEGFR2 are functionally essential in blood vessel and bone formation during DO and are needed to promote osteogenic over chondrogenic lineage progression.
Key words: distraction osteogenesis, angiogenesis, vascular endothelial growth factor, vascular endothelial growth factor receptors, bone morphogenetic protein
INTRODUCTION
Distraction osteogenesis (DO) is a bone regenerative response and is used as a surgical therapy in the treatment of skeletal injuries and deformities and in the correction of limb length abnormalities.(1) First described by Codvilla in 1905 for the treatment of limb length discrepancies, it was not until the work of Ilizarov 50 yr later that the technique of DO gained widespread clinical use as a method for enhancing bone regeneration in clinical orthopedics and oral maxillofacial surgery.(1,2) DO generates new bone formation through the application of tensile forces to the skeleton after creation of a controlled osteotomy.(3–5) The process is characterized by three separate phases: (1) a latency phase that immediately follows the osteotomy for some duration during which time no mechanical perturbation is applied; (2) an active or distraction phase during which the two bony segments are mechanically separated; and (3) a consolidation phase in which active distraction has ended and healing and maturation of the newly formed regenerate bone takes place.(3–7)
Like fracture healing, DO enhances the demand on the surrounding tissues to increase blood flow.(5,7) Although less studied than fracture healing, investigations have shown that there is an intense angiogenic response after DO. Precursor cells of new capillaries are abundant within the fibrous interzone regions of the distraction gap.(7) Although small amounts of cartilage are occasionally observed bone formation during DO occurs primarily through an intramembranous process. This characteristic distinguishes it from the large induction of endochondral bone formation as is observed in most settings of fracture healing. An optimal angiogenic response has been shown to be directly related to the rate of distraction, and numerous investigators have speculated that it is this characteristic that drives bone formation through the intramembranous pathway.(8,9) It should be noted that, whereas both fracture repair and bone formation during DO require increased blood flow, a greater overall extent of vascualrization is observed during DO as compared with fracture healing.(10)
Pacicca et al.,(11) using a rat model of DO, showed that several molecules known to regulate angiogenic processes, including vascular endothelial growth factor (VEGF), basic fibroblastic growth factor, and angiopoietin 1 and 2, were expressed during DO, and VEGF was localized within the distraction gap along the leading edge of tissue where nascent osteogenesis was occurring. Subsequently, we developed a murine model of DO. This model showed that VEGFA, both angiopoietin (Ang) 1 and 2 factors, and Tie2 (Ang receptor) were critical for the regeneration of bone in this process. Other angiogenic factors shown to be prominent were hypoxia-induced factor 1α (Hif1α), pleiotropin/OSF1, and multiple MMP(s). All of these factors were shown during the active phase of distraction. The expression of many of these mRNAs was also shown to be temporally related to each activation cycle of the distraction device.(12)
During bone healing, angiogenesis has been shown to be of vital importance and intricately involved in the inflammatory response,(13,14) soft callus tissue formation, and the transition from cartilaginous callus to bone.(14,15) To further elucidate the functional role of VEGF signaling during bone formation in distraction osteogensis, two blocking antibodies MF-1 (anti-VEGFR1) and DC101 (anti-VEGFR2) were used to specifically disrupt the activities of VEGFR 1 and VEGFR2.(16,17) These receptors have differing affinities for the VEGF ligands, which are known mediators of neoangiogenesis, and act as endothelial cell–specific mitogens showing both overlapping and individual roles in these processes.(18,19)
MATERIALS AND METHODS
Materials
Male C57B6 mice (9–12 wk old) were purchased from Jackson Laboratories (Bar Harbor, ME, USA). DO devices were obtained from KLS Martin L.P. (Jacksonville, FL, USA). Both anti-VEGF antibodies, MF-1 (anti-VEGFR1) and DC101 (anti-VEGFR2), were obtained from ImClone through a materials transfer agreement. All reagents for the PCR analysis were from Applied Biosystems(Foster City, CA, USA), and plate assays were read on an ABI 7700 Sequence Detector (Applied Biosystems, Foster City, CA, USA). All external and internal primers with the exception of that used for osteocalcin were from commercial sets available from Applied Biosystems.
Experimental design
All animal research was performed under an approved IACUC protocol. Animals were randomly distributed into 1 of 16 groups (5 control time points, 5 single antibody, and 5 double antibody experimental time points, and 1 reference that was the unoperated mid-diaphyseal tibia bone at day 0). Time points of the study were day 7, which is the end of the latency period; day 10, which is 3 days of active distraction; day 17, which is 10 days of active distraction and is the last day of distraction; day 20, which is 3 days into the period of consolidation; and day 31, which is 14 days into the period of consolidation and is the end of the experiment. In the antibody blockade groups, the antibody administration began at 3 days after surgery, and administration was continued thereafter every 3 days. A total of at least 129 mice were used to complete the study.
μCT evaluation for vessels and bone tissue formation was performed on separate sets of samples. The group size for both μCT experiments was N = 3, and a total of 18 mice were used in aggregate for the μCT experiments. Vessel evaluation was performed at 17 days after surgery, and bone evaluation was performed at 31 days after surgery. The group size for histological assessments was N = 5 (15 mice total). Comparative analysis between the three experimental groups was performed on 31-day postsurgery specimens. An additional group of 21-day postsurgery control (no treatment) specimens was included in the immunohistological analysis. The mRNA assessments were carried out on three separate pools of total RNA, with each pool prepared from N = 2 or 3 mice for each time point. At least 30 controls, 30 single-, and 30 double antibody–treated animals were used for these experiments. Pooled sample sets from the unoperated bones were used as the reference, which was prepared from at least six animals.
Surgical procedure and antibody administration
The surgical method of producing murine DO was performed as previously described.(12) Briefly the surgical protocol included a longitudinal incision, elevation of the tibia, and blunt separation of the underlying muscles with care not to remove all of the periosteum. The distraction device was attached to the upper part of the tibia, just below the knee, in two parts, with ligature wire (3M Unitek, Monrovia, CA, USA) fixation. A transverse tibial osteotomy was created with the use of a serrated scalpel blade in the proximal diaphysis of the tibia, whereas the fibula was left intact. Both proximal and distal segments were approximated, and alignment of the device was confirmed by X-ray. All animals tolerated the procedure well and were mobile on awakening from anesthesia.
Three hundred fifty microliters of VEGF receptor antibodies was administered intraperitoneally (1.5 mg/ml for both DC101 and MF1 in 1× PBS or DC101 alone) starting at day 3 and were given every third day until death. This injection protocol and dosing was as previously used to obtain receptor inhibition that blocked tumor growth.(16,17) Control animals underwent the same surgery and were injected with mouse nonimmune serum. Active distraction (0.15 mm/d) was initiated on day 7 after surgery. During the active distraction period antibody injections and distractions were done concurrently. Antibody delivery was continued through the end of the consolidation period. Animals that were harvested during active distraction were all killed 1 h after the last activation of the distraction device. No systemic effects on the overall health of the mice were visualized in either the control or the experimental groups.
RNA isolation and ribonuclease protection assay
RNA was isolated by Trizol extraction and quantified using its OD260 nm. The integrity of the RNA was visualized by a denaturing RNA gel electrophoresis. To determine the effect of the inhibitors on molecular events of bone formation, the expression of a panel of extracellular matrix protein mRNAs reflective of both chondrogenic and osteogenic cellular activities was initially examined by ribonuclease protection analysis. Because probes for multiple mRNAs are labeled in common and the hybridization of multiple mRNAs is in the same solution, the only potential variable that would effect these measurements would be labeling bias for the individual probe sequences. Thus, the intensity of each of the protected products will be representative of the absolute levels of each of the mRNAs and provides a measure of the absolute amounts of skeletogenic tissue formation. Ribonuclease protection assay (RPA) was carried out as previously described to assess the expression of osteopontin (OPN), bone sialoprotein (BSP), collagen α1 type II (Col 2A1), collagen α1 type X (Col10A1), and osteocalcin (OC). All values were normalized to the expression of the L32 housekeeping gene.(20)
Quantitative real-time RT-PCR
All reagents for the RT-PCR analysis were from Applied Biosystems, and plate assays were read on an ABI 7700 Sequence Detector (Applied Biosystems, Foster City, CA, USA). One microgram of total RNA was used for each preparation of cDNA. All cDNA preparations were generated by random hexamer priming. Reverse transcription reactions were initiated at 25°C for 10 min and subsequently carried out for an additional 60 min at 37°C. The cDNAs were diluted at a ratio of 1:50 in RNase-free water for all PCR reactions. All external and internal primers were from commercial sets available from Applied Biosystemswith the exception of osteocalcin. The amplicon sizes and the 5′ primer of each set are summarized in Table 1. All internal primers were labeled with FAM sequence. Each plate contained two negative controls and a positive control probe. β-actin was used in all experimental samples for normalization of each target sequence. Results of these studies were derived from the average of three replicate experiments. All graphic data for mRNA expression are presented as days after surgery and with numeric values presented as the relative fold expression to the reference (day 0).
Table 1.
RT-PCR Primers
| Unigene ID | Amplicon length | Context sequence | |
| VegfA | Mm. 282184 | 77 | CTCCACCATGCCAAGTGGTCCCAGG |
| VegfB | Mm. 15607 | 93 | CAGACACCTGTAGGTGCCGGAAGCC |
| VegfC | Mm. 1402 | 80 | ATCAAACATGCAGTTGTTACAGAAG |
| VegfD | Mm. 297978 | 92 | CCACTGCCTGGGACAGAAGACCACT |
| VegfR1 | Mm. 389712 | 67 | AATTACCCTGGTAAAGCAACTAAGA |
| VegfR2 | Mm. 285 | 86 | TAGAAGGTGCCCAGGAAAAGACCAA |
| VegfR3 | Mm. 3291 | 92 | GCGCAGCCTCCGGAGGCGGCAGCAG |
| Osterix | Mm. 263284 | 137 | GGCGTCCTCTCTGCTTGAGGAAGAA |
| BMP2 | Mm. 103205 | 58 | CAGGAAGAAGCCGTGGAGGAACTTC |
| OC | Mm. 425160 | 114 | GCAATAAGGTAGTGAACAGACTCC |
| Runx2 | Mm. 391013 | 115 | GCAAAGCTTCTTTTGGGATCCGAGC |
| Col2A1 | Mm. 2423 | 96 | GGCCAGGATGCCCAGGAGGCTGGCA |
| Sox5 | Mm. 1752 | 60 | TCCAGCTGCAGCAGTTCTATGCTGC |
| Sox9 | Mm. 286407 | 101 | CGAGCACTCTGGGCAATCTCAGGGT |
| β actin | Mm. 391967 | 115 | TACTGAGCTGCGTTTTACACCCTTT |
The unigene, reference number and reverse primer sequence of genes. The forward primer and internal primer that are labeled with FAM sequence are proprietary.
The fractional cycle number at which the fluorescence passes the fixed threshold (CT values) was used for quantification by using a comparative CT method. This method is described within the Applied Biosystems instructional manual for the instrumentation. In this method, the threshold (CT values) is determined when fluorescence of probe reaches its exponential phase of accumulation. Sample values are normalized to the threshold value for β-actin (Actb) for each time point: ΔCT = XCT (exp) – XCT (Actb). The CT value for day 0 was used as a reference. ΔΔCT = XCT (exp) – XCT (exp day 0). The fold change in mRNA expression for each time point was plotted in a graph using day 0 as a reference: 2−ΔΔCT(day 0) = 1 as previously described.(21)
Assessments of bone and vascular formation by μCT
Vasculature formation was assessed by μCT using a Scanco μCT 40 system (Scanco Medical, Basserdorf, Switzerland) at a resolution of 12 μm. The following method, as adapted from Duvall et al.,(22) was used to assess vessel formation. After the animals were killed, the thoracic cavity was opened, and the inferior vena cava was severed. The vasculature was flushed with 0.9% normal saline containing heparin sodium (100 U/ml) through a needle inserted into the left ventricle. The specimens were pressure fixed with 10% neutral buffered formalin. Formalin was flushed from the vessels using heparinized saline, and the vasculature was injected with a radiopaque silicone rubber compound containing lead chromate (Microfil MV-122; Flow Tech, Carver, MA, USA). Samples were stored at 4°C overnight for contrast agent polymerization.
Preceding μCT imaging, the fixators were removed from the mouse, and the hind limbs were dissected, leaving most of the muscles in place over the osteotomy site. The tissue specimens were fixed for 4 days in 10% neutral buffered formalin. Tissues were subsequently treated for 48 h in a formic acid–based solution, Cal Ex II (Fisher Scientific, Pittsburgh, PA, USA), to decalcify the bone and facilitate image thresholding of the hind limb vasculature from the surrounding tissues. The data from these experiments were compared by one-way ANOVA followed by Bonferroni test for the multiple comparisons, using the SPSS11.0 software. p = 0.05 or 0.01 was considered as the significant level.
Separate specimens were used to quantify the mineralized tissue formation within the distraction gap. For these studies, hind limbs were disarticulated immediately after death, and the fixator was carefully removed, leaving the soft tissue covering intact. The specimens were fixed for 3 days at 4°C in neutral buffered 4% paraformaldehyde, after which they were stored in neutral phosphate-buffered saline at 4°C. At the time when the μCT measurements where carried out, specimens were embedded in 2% agarose to provide structural support to the regenerate during μCT scanning. Specimens were scanned at a resolution of 16 μm using a Scanco μCT 40 system (Scanco Medical, Basserdorf, Switzerland). For these measurements, a region of interest (ROI) was defined for each of the experimental sample groups. The ROI was the distance along the diaphyseal axis that spans the region at which the cortex of the native bone (as seen in a transverse section) discontinues proximally to the point at which it continues distally. After determining the ROI for each specimen, the contours of the distraction callus were outlined. A global thresholding algorithm was applied with a fixed, constant threshold for all specimens. This threshold was chosen as the intensity (grayvalue) that corresponded to ∼45% of the average intensity of intact cortical bone in these specimens. Voxels with intensities higher than the threshold were considered to contain mineralized tissue. A constrained 3D Gaussian filter (filter width = 0.8, filter support = 1 voxel) was used to partially suppress image noise. Total bone volume and average mineral density were each compared across groups using a Kruskal-Wallis test (ANOVA by ranks).(23)
Demineralized histology and immunohistochemistry
For histological assessment, the distraction sites with surrounding muscle and soft tissues and were fixed, decalcified, sectioned, and stained as previously described.(24) Serial sections were generated, and slides were taken every 100 μM. Slides were stained with H&E. Similar immunohistological procedures to those that we used previously were carried in this study.(11) Antigens were retrieved by incubating sections at 65°C in 10 mg/ml hyaluronidase for 1 h. Endogenous peroxidase activity was extinguished by incubating twice in 3% hydrogen peroxide for 15 min. Three percent weight of reconstituted nonimmune serum from the animal source in which the secondary antibody was produced was resuspended in PBS and used as a blocking reagent for nonspecific background binding. Sections were incubated with the primary antibody, Sox9:goat polyclonal IgG (1:10,000 1% nonimmune serum PBS solution) for overnight at 4°C. Secondary labeling was accomplished by incubation with the appropriate biotin conjugated secondary antibody (10 μg/ml 1% nonimmune serum PBS solution) at room temperature followed by incubation with Vectastain ABC reagent (Vector Laboratories, CA, USA) for 30 min. Color was developed with the diaminobenzidine (DAB) chromogen kit (Vector Laboratories, CA, USA) for 3 min, checking for color development under the microscope. For each staining run, negative control sections were incubated with 1% nonimmune serum PBS solution without the primary antibody.
RESULTS
VEGF receptor and ligand expression during DO
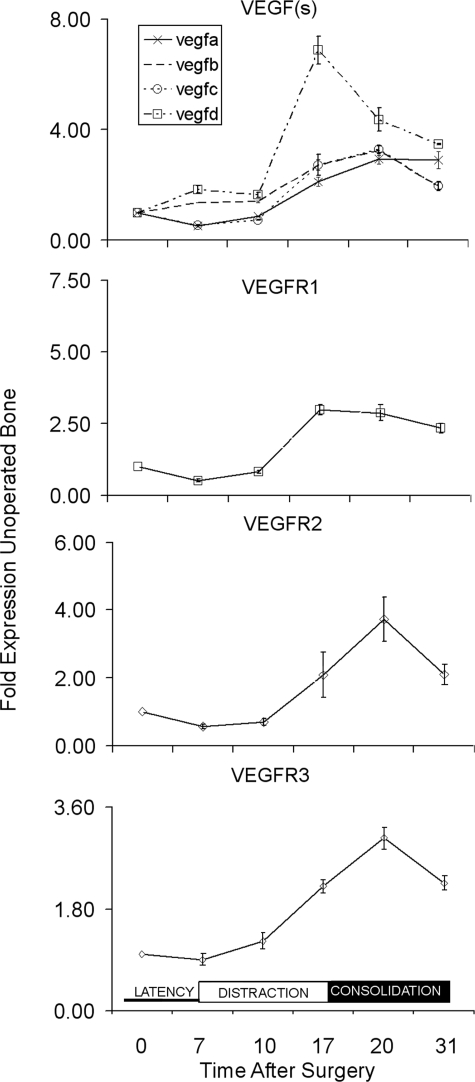
The expression of all four VEGF mRNAs was induced throughout the period of distraction and consolidation (Fig. 1). The induction of VEGFD preceded that of all the other ligands showing a definitive ∼6-fold peak in expression at the end of the distraction period (day 17), after which its expression decreased. In contrast, the expression of the other ligands showed only ∼2-fold induction that peaked after the consolidation period had been initiated at day 20. Whereas VEGFA showed the same pattern of induction as VEGFB and VEGFC, its elevated expression did not decrease within the 31-day experimental period.
FIG. 1.
Temporal expression of VEGF ligands and VEGF receptor mRNAs across the time course of bone regeneration during distraction osteogenesis. The mRNA expression as normalized to β-actin is expressed as a fold change relative to day 0 (control unoperated) as a reference: 2−ΔΔCT(day 0) = 1. The nature of the individual mRNAs is denoted in the figure and key in the figure. Total time after surgery is denoted in the figure. The three phases of the time course of distraction that correspond to the time after surgery are schematically denoted in the bottom panel of the figure with latency phase encompassing times from 0 to 7 days, active distraction from days 8 to 17, and consolidation days from 18 to 31. All values are representative of three to four replicates from two pools of mRNAs. Error bars are the SD of each set of measurements.
All three VEGF receptors also showed an induction of their expression over the time course of DO. The expression of both VEGFR2 and VEGFR3 generally paralleled that of seen for the ligands, showing induction beginning at ∼10 days after surgery (3 days after the initiation of active distraction) that continued to rise until they peaked during the consolidation period (day 20), after which time they began to decrease. It is also interesting to note that the VEGR1 receptor reached its peak expression by the end of the distraction period (day 17) and showed a more constant level of induction throughout the period of consolidation in a similar manner to the VEGFA ligand.
Radiographic assessments of skeletal and vasculature tissue formation
To elucidate the functional roles of the VEGF receptors in promoting angiogenesis and osteogenesis, antibody blockade against VEGFR1 and VEGFR2 was used to inhibit both receptors together or VEGFR2 alone. Faxitron radiographic analyses were used as an initial qualitative assessment of skeletal tissue formation. Control specimens showed robust bone formation by the end of consolidation (day 31). In the experimental animals, however, blockade of VEGFR2 alone or both VEGFR1 and VEGFR2, respectively, showed a progression in the inhibition in of bone formation within the osteotomy gap (Fig. 2A).
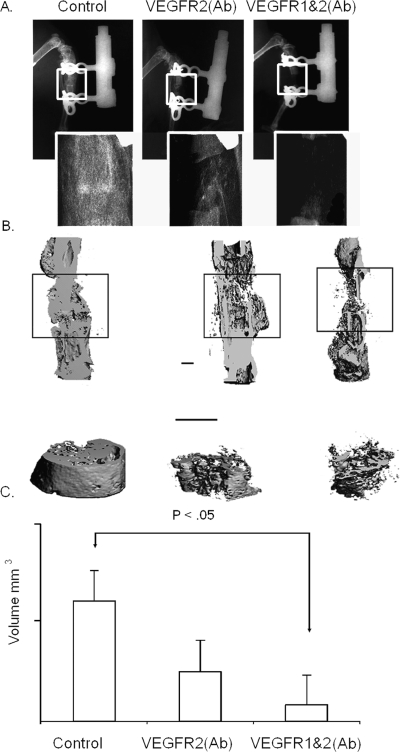
FIG. 2.
Radiographic assessment of bone formation during distraction osteogenesis in response to VEGFR blockade. All images are orientated with the proximal end of the bone at the top. (A) Faxitron analysis of distraction the bone regenerate at 31 days after the initiation of experiment. The gross ×1 appearance and ×5 magnification of the distraction site are seen in each X-ray image. (B) Representative μCT images of bone formation in the tissue regenerate from control mice and in the single antibody blockade– and double antibody blockade–treated mice harvested at 31 days after the time of surgery. Top images present a longitudinal cut away of the 3D reconstructions of the tibia. The boxed areas circumscribe the regenerate formed between the two ends of bone that were distracted. Bottom image contains a higher magnification the 3D reconstructions of the total volume of the region of the regenerate contained within the boxed region. Bar = 1 mm. (C) Graphical measurements of the volume of total bone tissue that was formed in the regenerate as determined from the μCT analysis.
μCT analysis was next carried out to provide both a structural and quantitative assessment of mineralized skeletal tissue formation during DO. Antibody blockade of both VEGFR2 and both VEGFR1and 2 led to the selective loss of external periosteal bone formation. In contrast, there was a more progressive loss of trabecular bone formation in the intramedullary space that was seen between the single and double antibody treatments (Fig. 2B). Quantitative measurements made from the μCT assessments showed ∼2- and ∼6-fold less bone formation in comparison with controls with the single and double antibody treatments, respectively (Fig. 2C). The relatively small sample size used in this analysis, however, did not show significant quantitative differences in the amount of mineralized tissue accumulated in the gap between the single and double antibody treatment, although both were statistically less than the control.
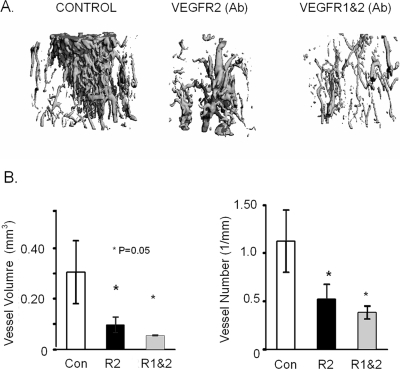
μCT imaging after vascular Microfil perfusion was used to assess the effect of VEGF receptor blockade on the formation of new blood vessels in the distraction gap (Fig. 3). A similar graded effect between the single and double antibody blockade treatments was observed for vascular formation. Decreasing values for vessel volume, number, volume/total volume, connectivity, density, and surface area, with an increase in vessel separation, was seen in the VEGFR 1 and 2 antibody–treated mice compared with the VEGFR2 and nontreatment controls. In this analysis, even with the small sample size, all these indices showed significance both between them and with the controls (Fig. 3B; Table 2).
FIG. 3.
μCT analysis of vessel formation within the DO gap in response of VEGFR blockade. (A) Representative 3D reconstructions of the μCT images of vessel formation in the tissue regenerate from control mice and in the single antibody blockade– and double antibody blockade–treated mice at 17 days after surgery. (B) Graphical measurements of total vessel number and total vessel volume as determined from the μCT.
Table 2.
MCT Values for New Vessel Formation*
| N | Total volume | Vessel volume† | VV/TV† | Conn. D.† | |
| Control | 3 | 6.2957 ± 0.9126 | 0.3057 ± 0.1248 | 0.0475 ± 0.0155 | 20.2568 ± 4.3700 |
| Anti-VEGFR2 | 3 | 4.7322 ± 0.2232‡ | 0.0970 ± 0.0304‡ | 0.0204 ± 0.0055‡ | 3.0194 ± 1.7968‡ |
| Anti-VEGFR 1 and 2 | 3 | 5.4710 ± 0.5889§ | 0.0547 ± 0.0015§ | 0.0101 ± 0.0009§ | 1.4415 ± 0.8488§ |
|
Vessel surface† |
VS/VV† |
Vessel number† |
Vessel thickness |
||
| Control | 3 | 14.2421 ± 4.9915 | 48.3912 ± 8.940 | 1.1216 ± 0.3231 | 0.0423 ± 0.0078 |
| Anti-VEGFR2 | 3 | 4.9554 ± 1.6220‡ | 50.9450 ± 5.7740 | 0.5199 ± 0.1551‡ | 0.0396 ± 0.0042 |
| Anti-VEGFR 1 and 2 | 3 | 4.2074 ± 0.9828§ | 76.5930 ± 16.2774 | 0.3822 ± 0.0663§ | 0.0271 ± 0.0065 |
|
Vessel Separation† |
Degree of anisotropy |
SMI |
|||
| Control | 3 | 0.8939 ± 0.2332 | 1.3967 ± 0.1517 | 4.0451 ± 0.3397 | |
| Anti-VEGFR2 | 3 | 2.0326 ± 0.7441 | 1.4012 ± 0.0543 | 4.1497 ± 0.3588 | |
| Anti-VEGFR 1 and 2 | 3 | 2.6461 ± 0.4830§ | 1.4377 ± 0.0837 | 4.3392 ± 0.5424 |
Values are mean ± SD.
* Parameters that are assayed are as defined in Duvall et al.(22)
† p < 0.05 between all groups.
‡ p < 0.05 between group and control.
§ p < 0.05 between group and control.
Molecular assessments of skeletal cell development
The molecular mechanisms that were regulating the cellular development within the regenerate tissues were assessed by two different approaches. RPA was used to measure both the temporal progression and quantitative amounts of cartilage and bone formation. These results, which are seen in Fig. 4A, confirmed the radiological analysis that the lack of skeletal tissue formation in the experimental groups was reflected in the diminished cellular activities of skeletogenic cells. These results further showed that, whereas the expression of the osteogenic mRNAs was diminished, the chondrogenic mRNAs showed an earlier and more sustained expression in the single antibody–treated animals, although at a much lower level than osteogenic expression.
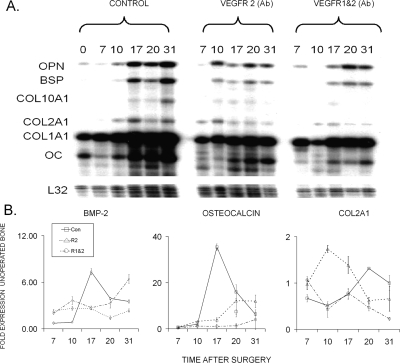
FIG. 4.
Temporal expression of selected extracellular matrix protein mRNAs that are representative of bone and cartilage tissue development across the time course of bone regeneration during distraction osteogenesis. Top panel depicts the autoradiographic gel profile from a representative ribonuclease protection assay for a panel of mRNAs expressed by chondrocytes and osteoblasts. Identification of the various bands for each of the specific mRNAS, days after surgery, and the nature of tissue specimen from which the RNA was extracted is noted. Bottom panels present the quantitative RT-PCR for BMP-2, osteocalcin, and Col2A1 mRNAs. The mRNA expression as normalized to β-actin is expressed as a fold change relative to day 0 (control no surgery) as a reference: 2−ΔΔCT(day 0) = 1. The nature of the individual mRNAs and animal group is denoted in the figure and key in the figure, respectively. Total time after surgery is denoted in the figure. All values are representative of three to four replicates from two pools of mRNAs. Error bars are the SD of each set of measurements.
To quantify how the inhibition of VEGFR signaling and the lack of angiogenesis affected the temporal progression of skeletogenic cell differentiation, real-time qRT-PCR was used to measure the relative levels of BMP-2, Col2A1, and osteocalcin mRNA expression as representative markers for differentiation of chondrogenic and osteogenic cells (Fig. 4B). BMP-2 expression was analyzed as a means of determining the degree to which inhibition of angiogenic development affected the morphogenetic signals that drive skeletal tissue formation. This analysis showed that BMP-2 levels peaked at ∼6-fold the levels of unorperated bones during the period of active distraction. The levels slightly decreased but remained elevated ∼4-fold throughout the remainder of the experimental period. Whereas both antibody treatments led to decreased levels in the expression of BMP-2, its level of expression remained ∼3-fold above that seen in the unoperated tissues. Interestingly, the double antibody treatment did not show lesser amounts than the single treatment, and a sharp increase in the levels of BMP-2 expression was seen in the double antibody–treated tissues at the end of the experimental period.
The patterns of chondrogenic and osteogenic mRNA expression seen for the qRT-PCR findings showed that the relative levels of expression of Col2A1 for the single receptor blockade was greatly increased during the active distraction period, after which it showed a rapid decrease during consolidation. In contrast, the double blockade treatment resulted in a small transient elevation at the very beginning of distraction but thereafter showed a steady decline over the remainder of the time course. Interestingly, the control samples showed an induction of Col2A1 expression throughout the consolidation period consistent with both our previous results(12) and the RPA analysis shown in Fig. 4A.
Osteocalcin expression, on the other hand, had a very sharp and almost 40-fold increase in its expression in the period of active distraction. Thereafter, the levels rapidly declined, although it continued to show a ∼5-fold elevation throughout the consolidation period. In an inverse manner to the expression of Col2A1, the single blockade samples showed elevated osteocalcin expression during the consolidation period, whereas the double antibody blockade–treated samples had no substantial increase at any time throughout DO (Fig. 4B).
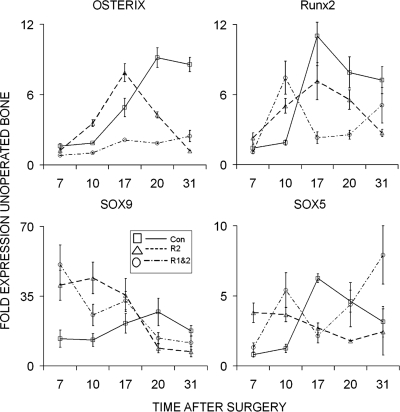
The effect of the antibody blockade on the recruitment and differentiation of skeletogenic stem/progenitor cells was next assessed by examining a set of transcription factors that are known to be determinants for the chondrogenic and osteogenic lineages (Fig. 5). The four transcription factors that were compared included the following: SOX 9, which commits skeletal stem/progenitor cells to the skeletal lineage and is common to both chondrogenic and osteogenic cells; SOX 5, which subsequently contributes to the commitment of cells to the chondrogenic lineage; osterix, which helps commit the lineage to osteogenesis; and RUNX2, which is involved in both terminal hypertrophic chondrocyte differentiation and is necessary with osterix to promote osteogenesis.(25) The results for the expression of osterix and Runx2 seen in the top two panels of this figure are parallel to those seen in the terminal osteocalcin marker for the differentiation of osteogenic cells. There is almost a complete lack of induction of osterix expression in the double antibody–treated samples. These results would indicate that the more complete blockade of angiogenesis produced by the double antibody treatment blocks the progression of any of the recruited cells to an osteoblastic lineage. The expression of SOX9 and SOX5 expression are depicted in the bottom panels. The very high levels of expression of both SOX9 and SOX5 mRNAs in both the single- and double antibody–treated samples at the end of the latency period suggests that skeletal stem/progenitor cells can still be recruited but they remain as common progenitors or selectively progress toward a chondrogenic phenotype when vascularization is compromised.
FIG. 5.
Temporal expression of selected transcription factor mRNAs that are the determinants of chondrocyte and osteoblast differentiation across the time course of bone regeneration during distraction osteogenesis. Graphical analysis for each of RT-PCR analysis is denoted in the figure. The mRNA expression as normalized to β-actin is expressed as a fold change relative to day 0 (control no surgery) as a reference: 2−ΔΔCT(day 0) = 1. The nature of the individual mRNAs and animal group is denoted in the figure and key in the figure, respectively. Total time after surgery is denoted in the figure. All values are representative of three to four replicates from two pools of mRNAs. Error bars are the SD of each set of measurements.
Histological assessments of skeletal cell development
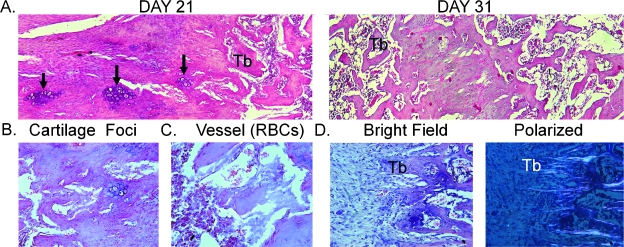
The last set of studies presents a histological and immunohistological analysis of the specimens as a means of validating the cellular activities that were extrapolated from the various mRNA assessments. Micrographs of control regenerate samples taken from the intramedullary space of both 21- and 31-day postsurgery specimens are first presented to highlight the various biological processes and the morphogenesis of the regenerate tissues that are formed (Fig. 6). The regenerate tissue in the control specimens is characterized by robust trabecular bone formation within the intramedullary space. As the tissue is progressively lengthened, new bone formation proceeds from the proximal side (right side of the micrograph) inward to the distal end of the gap (left side of the micrograph). This can be seen by comparing the micrographs from the two different postsurgical times. As observed in this comparison, the formation of mature trabeculae arises from condensation at the proximal side, and as the tissue develops distally, dense layers of cells became aligned in columns, some of which have central cores of cartilage cells. The foci of chondrocyte within the cores of the condensing tissues are seen in the higher-magnification micrographs (Fig. 6B). In between these columns of new bone tissue, new blood vessels are formed that are lined with endothelia-appearing cells with well-defined concave-shaped red blood cells (RBCs) contained with the luminal spaces of these large vessels (Fig. 6C). The aligned nature of the newly formed trabeculae to the long axis of the bone can be further shown by assessing the underlying collagen fiber orientations with polarized light (Fig. 6D).
FIG. 6.
Histological analysis of the tissue formation in control distraction osteogenesis specimens at 21 and 31 days after surgery. (A) Representative ×100 micrographs of sections depicting the intramedullary regions localized between the proximal and distal ends of the osteotomy are presented. The representative images of the tissue formation at both 21 and 31 days after surgery are denoted in the figure. All sections are presented in a left (distal) to right (proximal) orientation in the microphotograph. Sections were stained with hematoxylin and eosin. Regions encompassing new bone formation within the distraction gap begin at the proximal margin where new trabeculae of bone are forming and extend distally into the gap. Nascent trabeculae are denoted (Tb) with the foci of chondrogenic cells within the central regions of condensing cells that will form the new trabeculae indicated with arrows. (B) Micrographs (×200) of selected regions from 21 day postsurgery distractions gaps from control animals depicting chondrocytes embedded in the condensing tissue structures. (C) Micrographs (×400) of newly formed blood vessels are denoted with concave RBCs seen in the lumen of the vessels. (D) Left and right ×100 micrographs show matched bright filed and polarized images of newly formed trabeculae. Underlying collagen fiber alignment is seen in the polarized images.
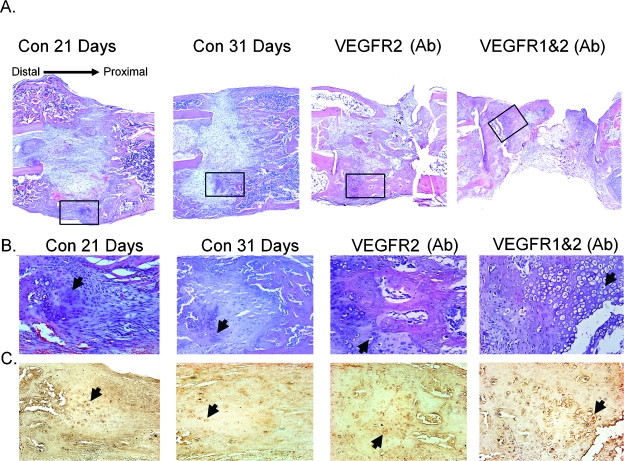
The global effect of VEGFR blockade on tissue formation is presented in Fig. 7A. As can be seen in these micrographs, considerable amounts of tissue formation develop external to intramedullary space of the control specimens. The tissues that formed externally to the distraction gap form a shell of bone that bridges the gap with small areas of cartilage in the central regions within the external callus regions. By 31 days after surgery, no cartilage tissue was seen in the medullary space of the control specimens in contrast to the control specimens at 21 days. In comparison, the single- and double antibody–treated samples developed much lesser amounts of intramedullary trabeculae and external callus tissues. The most severe diminishment in tissue regeneration was seen in the midregions of the double antibody–treated tissues. Both the single and double antibody treatment specimens also showed only a thin edge of developing bone along the surface of the external callus proximal and distal to the gap but not bridging the distraction gap at all. Both single and double antibody blockade treatments developed areas of cartilage tissue both within the intramedullary space, with the single treatment showing lesser amounts than the double treatments. The double antibody blockade treatment, however, showed extensive areas of hypertrophic-looking chondrocyte, whereas the proximal regions of the gap was mainly fibrous and fat tissues (Fig. 7B).
FIG. 7.
Histological analysis of the tissue formation in response to VEGFR blockade. (A) Representative ×40 micrographs of sections depicting global tissue appearance of controls at 21 and 31 days after surgery and VEGFR blockade specimens at 31 days after surgery. Regions encompassing the entire distraction gap and surrounding calluses are presented. Each experimental group is labeled in the figure. Boxed areas are presented in higher ×100 magnification micrographs in B and C. All Sections were cut in longitudinal orientations, and sections were selected from the anatomical center of regenerate such that all four cortices are seen. Sections were stained with H&E. (B and C) Representative micrographs of immunohistological localization of Sox9 protein are depicted. Matched H&E and immunohistological micrographs from 5-μm serials sections correspondending to the boxed regions indicated in A. Arrows are provided as a reference to denote single cell staining for Sox 9 in each section.
It is interesting to note that, whereas tissue from both the single- and double antibody–treated animals showed bands of aligned fibrous tissues surrounding external areas of the gap, no well-developed woven bone formation was histologically seen in the regenerate that formed in osteotomy gaps. Although lesser amounts of mineralization of these tissues must have taken place based on the comparison with the μCT data seen in Fig. 3, no tissues with a woven bone or trabecular appearance were seen in the histological analysis of these samples. The histological results suggest that the mineral deposition is most likely associated with areas of hypertrophic cartilage tissue development.
Immunohistological analysis was next carried out for SOX9. Micrographs depicting the H&E staining and matched serial sections developed with anti-SOX 9 antibody are seen in B and C. These analyses were carried out to assess the spatial distribution and histological nature of cells that commit to the skeletal cell lineages in control DO tissues and in the regenerate tissues in which VEGFR activity has been inhibited. In the regenerates tissues in the distraction gaps from the control animals, antibody staining was predominantly localized within the nuclei of large round chondrocyte-looking cells that were spatially either aligned in long columns in the intramedullary gap regions or in the external callus regions of the in the middle regions of the gap. The most intense staining was detected in the cells from the single- and double antibody–treated animals. It is interesting to note that their were many more labeled cells in the control specimens from 21 days and much fewer and much less intensely reacting cells were observed in the tissues from the controls at 31 days.
DISCUSSION
Previous studies of both fracture healing and distraction osteogenesis have shown the presence of all of the major VEGF ligands (A–D), other ligands that interact with the VEGF receptors including neuroplin (Nrp 1 and 2), and placental growth factor (PIGF). All three receptors VEGFR1–3 have also been shown to be present during skeletal healing.(12–14,26,27) Studies examining either osteoblast-like cell lines or primary cultures of osteogenic cells have also suggested that these cells express multiple VEGF ligands and seem to express all three receptors.(28–34) The current studies corroborate these findings while providing new data that show that both VEGFR1 and VEGFR2 contribute functionally to both angiogenic and osteogenic processes during new bone formation.
Specific studies of fracture healing in the absence of PlGF, which mediates its activities only through VEFGR1, and studies of postnatal cortical bone healing and bone development have shown that VEGFR1 is involved in skeletal stem cell development, cartilage turnover, and bone remodeling.(14,34–36) There is a considerable variability, however, between a number of different studies concerning the functionality of the VEGFR2 and VEGFR3 receptors in osteogenic, chondrogenic, and skeletal stem/progenitor cells.(26,27,37–39) Whereas specific signal transduction and phenotypic responses have been observed in response to VEGFR1 during bone development,(30,34,35,37,40) other studies have shown that both VEGFR 2 and VEGFR3 may also have activity in these cells.(29,32) The general consensus that has emerged from these results suggests that VEGFR1 stimulates both a migratory response in osteoblasts and skeletal stem/progenitor cells and stimulates further osteogenic differentiation.(14,30,31,35,40,41) Other studies have suggested that osteoblast-like cells do not show similar responses to VEGFR2.(30,37) Together these data are generally supportive of a central role for VEGFR1-mediated signaling in bone repair; however, the direct role of VEGFR2 and VEGFR3 within skeletal stem/progenitors has remained unresolved.
Previous studies, in which VEGFR activity was inhibited with sFlt-1(15,42) or in which only VEGFR1 signaling was diminished,(14) have shown only partial reductions in bone formation and repair. The use of sFlt-1 to block VEGF activity, however, will only antagonize the ligands that interact with VEGFR1, whereas incompletely antagonizing the complement of ligands that interact with VEGFR2 and VEGFR3. In this context, this study showed that the in vivo blockade of both VEGFR1 and VEGFR2 or only VEGFR2 activity by itself also leads to a partial but increasing levels of inhibition of bone formation when both receptors were inhibited. The additive and concurrent inhibitory effects on both bone and new blood vessel formation, in the double antibody blockade experiments, showed that multiple VEGF receptors contribute to both bone healing and neoangiogenesis. Thus, the single and double VEGFR antibody blockade produced parallel effects for angiogenesis to those observed for bone formation, showing partial and more complete effects with the single and double antibody treatments, respectively, even though the blockade of VEGFR2 activity will primarily affect endothelia cell growth.(16) The additive inhibition on vasculogenesis by the blockade of both receptors is also consistent with the emerging picture that VEGF-induced vascular endothelial cell migration in vitro is mediated by the multiple VEGF receptors and that multiple VEGF receptors are necessary for the formation of functional vessels during tumorigenesis.(43) In other studies, the molecular mechanisms of cross-talk between VEFGR1 and VEGFR2 were examined for the endothelial cell responses to wound healing, and these studies showed that Flt-1 blockade impeded VEGFR2 internalization and delayed vascular endothelial cell monolayer recovery after wounding.(44)
Whereas the double antibody blockade showed a 4- and 6-fold decrease in both mineralized and blood vessel formation, respectively, complete inhibition was not observed for either process. In this context, the histological and μCT analysis showed a selective inhibition of the periosteal response and external callus formation with both antibody treatment groups while having much lesser affects on the formation of bone in the central region of the gap. In this regard, it is interesting to note that the antibody blockade inhibited BMP-2 its expression was only reduced by one half, and the effects of both antibodies were not additive. The lack of complete inhibition would be consistent, however, with previous studies that has showed that hypoxia will induce BMP-2 expression in vascular endothelia tissues.(45) These differences raise specific questions about both the origin of the skeletal stem/progenitor and vascular stem cells that participate in the processes of bone and vascular tissue formation and which cells express and respond to specific morphogenetic proteins that drive both these processes. It is intriguing to speculate that the small amount of mineralized tissue that does form in the intramedullary space is supported by VEGFR3-mediated activity that is primarily associated with lymphogenesis and not angiogenesis. It is therefore interesting to note that VEGFR3 has been shown to be present in osteogenic populations of cells that differentiate in marrow stromal cultures.(32) In this regard, whereas this receptor was not inhibited in this study, our analysis did show that this receptor had a similar temporal co-expression with VEGFR2.
The presence of lymphatic vessels within the medullary space has recently been identified in studies of local and distal inflammatory responses to prosthetic wear particles.(46) It may be that different stem cell populations predominate in the intramedullary space from those in the periosteal and muscular tissues. Thus, the development of a skeletal stem/progenitor cell population in the marrow cavity can be supported in the absence of VEGFR1 and VEGFR2 signaling but those in the muscular and periosteal tissues cannot. This interpretation is supported by the more selective inhibition of the external callus formation that is seen in the antibody blockade–treated animals relative to their effects on bone formation in the intramedullary space. This concept is also supported by recent studies using a heterotopic bone formation model and green fluorescent protein (GFP)-marked marrow stromal donor cells, which showed that the marrow contained cell populations that differentiated into both endothelial and osteogenic cells when implanted in an ectopic site. Interestingly, in this same study, a unique population of endosteal lining cells was identified showing osteogenic characteristics but apparently did not become encapsulated in mineralized extracellular matrix but remained as lining surface cells.(47) These data in aggregate provide evidence to support the conclusion that all three receptors (VEGFR1, VEGFR2, and VEGFR3) are needed to promote both osteogenesis and angiogenesis during DO and that the co-development of each of these tissues synergizes each other's growth.
The final aspect of these studies to discuss is the assessment of skeletal stem cell development and lineage progression in the absence of VEGFR signaling. In two recent studies in which VEGFR activity was blocked, similar results were observed. The studies of fracture healing in which VEGFR1 signaling was partially diminished by the absence of placental growth factor (PlGF) showed a massive expansion of chondrogenesis in the callus tissues in the absence of robust periosteal response and a delayed removal of the cartilage in the callus.(14) In a different study in which muscle-derived skeletal stem/progenitor cells were implanted and induced to differentiate with BMP-2 in the absence or presence of sFlt-1, cartilage formed in both experimental groups but in the presence of the VEGFR antagonist, bone did not form, and there was a failure of the cartilage to undergo hypertrophic differentiation.(42) The investigators of the former study suggested that the failure to develop bone resulted from a failure in the recruitment of skeletal stem/progenitor cells and development of an adequate pool of progenitors, whereas the latter group of investigators suggested that the antagonism of the VEGFR signaling did not significantly affect cell recruitment but did block the progression of cartilage formation and subsequent bone formation. It is interesting to note that, in our study, the increased expression of Runx2 that is seen in the double antibody treated toward the end of the consolidation period is consistent with the known expression pattern of Runx2 that is observed when chondrocytes become hypertrophic. This suggests that some the chondrocytes that do develop in the absence of angiogenesis do continue to differentiate and mature in the absence of angiogenic stimuli.
Our analysis of the various transcriptional determinants and extracellular matrix protein markers of the chondrogenic and osteogenic lineages tends to support the conclusions of this later study that mesenchymal cell recruitment does take place but fails to progress in the absence of appropriate VEGFR signaling. This conclusion is based on the extremely high level of SOX9 expression that is expressed in the common osteogeo/chondro progenitor cell(25) and the presence of SOX9+ cells throughout the regenerates of both the control and the antibody blockade–treated tissues and the development of chondrogenic cells within the intramedullary space. It is particularly interesting that in the single blockade of VEGFR2 osterix and Runx2 both appear early during the healing processes but fail to show sustained expression as the time course of healing progresses. This suggests that the skeletal stem/progenitor cells that are recruited will commit to chondrogenic lineage but will not progress to the osteogenic lineage or undergo premature cell death in the absence of vascular development. Whereas the expression of type X collagen was not rigorously defined by RT-PCR, RPA showed that collagen X fails to be expressed almost completely in the double antibody blockade–treated specimens consistent with the conclusion of numerous studies that VEGFR signaling is needed for the progression of chondrocyte hypertrophy.(14,37–39,42) It is also important to note that, whereas there has been a greater emphasis on the consideration of the direct effects of the receptors themselves in driving the patterns of differentiation of the skeletal lineages, the oxygen tension within the tissues may have a direct role itself in effecting the patterns of both the progression of skeletal cell differentiation(48–50) and in skeletal tissue survival.(51)
In conclusion, our data showed that both VEGFR1 and VEGFR2 are functionally essential in the formation of both bone and blood vessels during DO and that vascularization is more important for osteogenesis than chondrogenesis.
ACKNOWLEDGMENTS
This work was partially supported by the Department of Orthopaedic Surgery at Boston University School of Medicine and grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (PO1AR049920) (TAE and LCG) and AR052746 and AR49410 and a VA Merit Review Grant (TLC).
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Codivilla A. On the means of lengthening in the lower limbs. Am J Orthop Surg. 1905;2:353–369. [Google Scholar]
- 2.Ilizarov G. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop. 1990;250:8–26. [PubMed] [Google Scholar]
- 3.Isefuko S, Joyner CJ, Simpson HRW. A murine model of distraction osteogenesis. Bone. 2000;27:661–665. doi: 10.1016/s8756-3282(00)00385-9. [DOI] [PubMed] [Google Scholar]
- 4.Tay BK, Le AX, Gould SE, Helms JA. Histochemical and molecular analysis of distraction osteogenesis in a mouse model. J Orthop Res. 1998;16:636–642. doi: 10.1002/jor.1100160518. [DOI] [PubMed] [Google Scholar]
- 5.Aronson J, Hogue WR, Flahiff CM, Gao GG, Shen XC, Skinner RA, Badger TM, Lumpkin CK., Jr Development of tensile strength during distraction osteogenesis in a rat model. J Orthop Res. 2001;19:64–69. doi: 10.1016/S0736-0266(00)00002-4. [DOI] [PubMed] [Google Scholar]
- 6.Sato M, Ochi T, Nakase T, Hirota S, Kitamura Y, Nomura S, Yasui N. Mechanical tension-stress induces expression of bone morphogenetic protein (BMP)-2 and BMP-4, but not BMP-6, BMP-7, and GDF-5 mRNA, during distraction osteogenesis. J Bone Miner Res. 1999;14:1084–1095. doi: 10.1359/jbmr.1999.14.7.1084. [DOI] [PubMed] [Google Scholar]
- 7.Li G, Simpson AH, Kenwright J, Triffitt JT. Effect of lengthening rate on angiogenesis during distraction osteogenesis. J Orthop Res. 1999;17:362–367. doi: 10.1002/jor.1100170310. [DOI] [PubMed] [Google Scholar]
- 8.Lewinson D, Maor G, Rozen N, Rabinovich I, Stahl S, Rachmiel A. Expression of vascular antigens by bone cells during bone regeneration in a membranous bone distraction system. Histochem Cell Biol. 2001;116:381–388. doi: 10.1007/s004180100331. [DOI] [PubMed] [Google Scholar]
- 9.Meyer U, Meyer T, Wiesman HP, Kruse-Losler B, Vollmer D, Stratmann U, Joos U. Mechanical tension in distraction osteogenesis regulates chondrocytic differentiation. Int J Oral Maxillo Surg. 2001;30:522–530. doi: 10.1054/ijom.2001.0159. [DOI] [PubMed] [Google Scholar]
- 10.Aronson J, Harp JH, Walker CW, Dalrymple GV. Blood flow, bone formation and mineralization during distraction osteogenesis. Trans Orthop Res Soc. 1990;15:589–594. [Google Scholar]
- 11.Pacicca DM, Patel N, Lee C, Salisbury K, Lehmann W, Carvalho R, Gerstenfeld LC, Einhorn TA. Expression of angiogenic factors during distraction osteogenesis. Bone. 2003;33:889–898. doi: 10.1016/j.bone.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho RS, Einhorn TA, Lehmann W, Edgar C, Al-Yamani A, Apazidis A, Pacicca D, Clemens TL, Gerstenfeld LC. The role of angiogenesis in a murine tibial model of distraction osteogenesis. Bone. 2004;34:849–861. doi: 10.1016/j.bone.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann W, Edgar CM, Wang K, Cho TJ, Barnes GL, Kakar S, Graves DT, Rueger JM, Gerstenfeld LC, Einhorn TA. Tumor necrosis factor alpha (TNF-alpha) coordinately regulates the expression of specific matrix metalloproteinases (MMPS) and angiogenic factors during fracture healing. Bone. 2005;36:300–310. doi: 10.1016/j.bone.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Maes C, Coenegrachts L, Stockmans I, Daci E, Luttun A, Petryk A, Gopalakrishnan R, Moermans K, Smets N, Verfaillie CM, Carmeliet P, Bouillon R, Carmeliet G. Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J Clin Invest. 2006;116:1230–1242. doi: 10.1172/JCI26772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prewett M, Huber J, Li Y, Santiago A, O'Connor W, King K, Overholser J, Hooper A, Pytowski B, Witte L, Bohlen P, Hicklin DJ. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- 17.Witte L, Hicklin DJ, Zhu Z, Pytowski B, Kotanides H, Rockwell P, Bohlen P. Monoclonal antibodies targeting the VEGF receptor-2 (Flk-1/KDR) as an anti-angiogenic therapeutic strategy. Cancer Metastasis Rev. 1998;17:155–161. doi: 10.1023/a:1006094117427. [DOI] [PubMed] [Google Scholar]
- 18.Carano RA, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003;8:980–989. doi: 10.1016/s1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- 19.Wang ES, Teruya-Feldstein J, Wu Y, Zhu Z, Hicklin DJ, Moore MA. Targeting autocrine and paracrine VEGF receptor pathways inhibits human lymphoma xenografts in vivo. Blood. 2004;104:2893–2902. doi: 10.1182/blood-2004-01-0226. [DOI] [PubMed] [Google Scholar]
- 20.Gerstenfeld LC, Cho T-J, Kon T, Aizawa T, Tsay A, Barnes GL, Graves DT, Einhorn TA. Impaired fracture healing in the absence of TNF-α signaling: The role of TNF-α in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 21.Wang K, Vishwanath P, Eichler GS, Al-Sebaei MO, Edgar CM, Einhorn TA, Smith TF, Gerstenfeld LC. Analysis of fracture healing by large scale transcriptional profile identified temporal relationships between metalloproteinase and ADAMTS mRNA expression. Matrix Biol. 2005;25:271–281. doi: 10.1016/j.matbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Duvall CL, Robert Taylor W, Weiss D, Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am J Physiol Heart Circ Physiol. 2004;287:H302–H310. doi: 10.1152/ajpheart.00928.2003. [DOI] [PubMed] [Google Scholar]
- 23.Shefelbine SJ, Simon U, Claes L, Gold A, Gabet Y, Bab I, Muller R, Augat P. Prediction of fracture callus mechanical properties using microCT images and voxel-based finite element analysis. Bone. 2005;36:480–488. doi: 10.1016/j.bone.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Gerstenfeld LC, Alkhiary YM, Krall EA, Nicholls FH, Stapleton SN, Fitch JL, Graves DT, Jepsen KJ, Einhorn TA. Three dimensional reconstruction of fracture callus morphogenesis demonstrates asymmetry in callus development. J Histochem Cytochem. 2006;54:1215–1228. doi: 10.1369/jhc.6A6959.2006. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003;19:458–466. doi: 10.1016/S0168-9525(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 26.Byun JH, Park BW, Kim JR, Lee JH. Expression of vascular endothelial growth factor and its receptors after mandibular distraction osteogenesis. Int J Oral Maxillofac Surg. 2007;36:338–344. doi: 10.1016/j.ijom.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Mori S, Akagi M, Kikuyama A, Yasuda Y, Hamanishi C. Axial shortening during distraction osteogenesis leads to enhanced bone formation in a rabbit model through the HIF-1alpha/vascular endothelial growth factor system. J Orthop Res. 2006;24:653–663. doi: 10.1002/jor.20076. [DOI] [PubMed] [Google Scholar]
- 28.Harper J, Gerstenfeld LC, Klagsbrun M. Neuropilin-1 expression in osteogenic cells: Down-regulation during differentiation of osteoblasts into osteocytes. J Cell Biochem. 2001;81:82–92. doi: 10.1002/1097-4644(20010401)81:1<82::aid-jcb1025>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 29.Deckers MM, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Lowik CW. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667–1674. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- 30.Mayer H, Bertram H, Lindenmaier W, Korff T, Weber H, Weich H. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: Autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem. 2005;95:827–839. doi: 10.1002/jcb.20462. [DOI] [PubMed] [Google Scholar]
- 31.Mayr-Wohlfart U, Waltenberger J, Hausser H, Kessler S, Gunther KP, Dehio C, Puhl W, Brenner RE. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30:472–477. doi: 10.1016/s8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- 32.Orlandini M, Spreafico A, Bardelli M, Rocchigiani M, Salameh A, Nucciotti S, Capperucci C, Frediani B, Oliviero S. Vascular endothelial growth factor-D activates VEGFR-3 expressed in osteoblasts inducing their differentiation. J Biol Chem. 2006;281:17961–17967. doi: 10.1074/jbc.M600413200. [DOI] [PubMed] [Google Scholar]
- 33.Tombran-Tink J, Barnstable CJ. Osteoblasts and osteoclasts express PEDF, VEGF-A isoforms, and VEGF receptors: Possible mediators of angiogenesis and matrix remodeling in the bone. Biochem Biophys Res Commun. 2004;316:573–579. doi: 10.1016/j.bbrc.2004.02.076. [DOI] [PubMed] [Google Scholar]
- 34.Uchida S, Sakai A, Kudo H, Otomo H, Watanuki M, Tanaka M, Nagashima M, Nakamura T. Vascular endothelial growth factor is expressed along with its receptors during the healing process of bone and bone marrow after drill-hole injury in rats. Bone. 2003;32:491–501. doi: 10.1016/s8756-3282(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 35.Otomo H, Sakai A, Uchida S, Tanaka S, Watanuki M, Moriwaki S, Niida S, Nakamura T. 2007 Flt-1 tyrosine kinase-deficient homozygous mice result in decreased trabecular bone volume with reduced osteogenic potential. Bone. 2007;40:1494–1501. doi: 10.1016/j.bone.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt-1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 37.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 38.Bluteau G, Julien M, Magne D, Mallein-Gerin F, Weiss P, Daculsi G, Guicheux J. VEGF and VEGF receptors are differentially expressed in chondrocytes. Bone. 2007;40:568–576. doi: 10.1016/j.bone.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 39.Carlevaro MF, Cermelli S, Cancedda R, Descalzi Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: Auto-paracrine role during endochondral bone formation. J Cell Sci. 2000;113:59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- 40.Waltenberger J, Hausser H, Kessler S, Gunther KP, Dehio C, Puhl W, Brenner RE. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30:472–477. doi: 10.1016/s8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- 41.Fiedler J, Leucht F, Waltenberger J, Dehio C, Brenner RE. VEGF-A and PlGF-1 stimulate chemotactic migration of human mesenchymal progenitor cells. Biochem Biophys Res Commun. 2005;334:561–568. doi: 10.1016/j.bbrc.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 42.Peng H, Usas A, Olshanski A, Ho AM, Gearhart B, Cooper GM, Huard J. VEGF improves, whereas sFlt-1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20:2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 43.Li B, Sharpe EE, Maupin AB, Teleron AA, Pyle AL, Carmeliet P, Young PP. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J. 2006;20:1495–1497. doi: 10.1096/fj.05-5137fje. [DOI] [PubMed] [Google Scholar]
- 44.Santos SC, Miguel C, Domingues I, Calado A, Zhu Z, Wu Y, Dias S. VEGF and VEGFR-2 (KDR) internalization is required for endothelial recovery during wound healing. Exp Cell Res. 2007;313:1561–1574. doi: 10.1016/j.yexcr.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 45.Bouletreau PJ, Warren SM, Spector JA, Peled ZM, Gerrets RP, Greenwald JA, Longaker MT. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: Implications for fracture healing. Plast Reconstr Surg. 2002;109:2384–2397. doi: 10.1097/00006534-200206000-00033. [DOI] [PubMed] [Google Scholar]
- 46.Jell G, Kerjaschki D, Revell P, Al-Saffar N. Lymphangiogenesis in the bone-implant interface of orthopedic implants: Importance and consequence. J Biomed Mater Res A. 2006;77:119–127. doi: 10.1002/jbm.a.30548. [DOI] [PubMed] [Google Scholar]
- 47.Bilic-Curcic I, Kalajzic Z, Wang L, Rowe DW. Origins of endothelial and osteogenic cells in the subcutaneous collagen gel implant. Bone. 2005;37:678–687. doi: 10.1016/j.bone.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Hirao M, Tamai N, Tsumaki N, Yoshikawa H, Myoui A. Oxygen tension regulates chondrocyte differentiation and function during endochondral ossification. J Biol Chem. 2006;281:31079–31092. doi: 10.1074/jbc.M602296200. [DOI] [PubMed] [Google Scholar]
- 49.Robins JC, Akeno N, Mukherjee A, Dalal RR, Aronow BJ, Koopman P, Clemens TL. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005;37:313–322. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 50.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Hypoxia inducible factor-1alpha deficiency affects chondrogenesis of adipose-derived adult stromal cells. Tissue Eng. 2007;13:1159–1171. doi: 10.1089/ten.2006.0265. [DOI] [PubMed] [Google Scholar]
- 51.Pfander D, Cramer T, Schipani E, Johnson RS. HIF-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J Cell Sci. 2003;116:1819–1826. doi: 10.1242/jcs.00385. [DOI] [PubMed] [Google Scholar]