Abstract
The objective of this study was to investigate the kinetics of Hsp60, Hsp70, Hsp90 protein, and messenger RNA (mRNA) expression levels and to correlate these heat shock protein (Hsp) levels with tissue damage resulting from exposure to high temperatures for varying amounts of time. One hundred broilers were heat-stressed for 0, 2, 3, 5, and 10 h, respectively, by rapidly increasing the ambient temperature from 22 ± 1°C to 37 ± 1°C. Obvious elevations of plasma creatine kinase indicate damage to myocardial cells after heat stress. Hsp70 and Hsp90, and their corresponding mRNAs in the heart tissue of heat-stressed broilers, elevated significantly after 2 h of heat exposure and decreased quickly with continued heat stress. However, the levels of hsp60 mRNA in the heart of heat-stressed broilers increased sharply (P < 0.01) at 2 h of heat stress but then decreased quickly after 3 h, while the level of Hsp60 protein in the heart increased (P < 0.01) at 2 h of heat stress and maintained a high level throughout heat exposure. The results indicate that the elevation of the three Hsps, especially Hsp60 in heart, may be important markers at the beginning of heat stress and act as protective proteins in adverse environments. The reduction of Hsp signals in the cytoplasm of myocardial cells implies that myocardial cell lesions may have an adverse impact on the function of Hsps during heat stress. Meanwhile, the localization of Hsp70 in blood vessels of broiler hearts suggests another possible mechanism for protection of the heart after heat exposure.
Keywords: Hsp, hsp mRNA, Heart, Broiler, Heat stress
Introduction
Heat stress is one of the most challenging environmental conditions affecting commercial poultry. Compared to other species of domestic animals, broiler chickens are more sensitive to high ambient temperatures (Geraert et al. 1993). As environmental temperature increases, food consumption, growth rate, feeding efficiency, egg shell quality, and survival ability all decline (van der Hel et al. 1992; Geraert et al. 1996; Mashaly et al. 2004). Additionally, pale, soft exudative-like changes in meat quality have also been observed in broilers exposed to acute or chronic heat stress pre-slaughter (Northcutt et al. 1994; Sandercock et al. 2001; Lu et al. 2007).
As living organisms, chickens have protective measures against environmental challenges. The heat shock proteins (Hsps) are a set of proteins synthesized in response to physical, chemical, or biological stresses, including heat exposure (McCormick et al. 2003a; Ganter et al. 2006; Staib et al. 2007). Hsps are a group of evolutionarily conserved proteins that are, conventionally, classified according to molecular size ranging from 10 to 150 kDa (Benjamin and McMillan 1998). It has been demonstrated that Hsps act as molecular chaperones in protein assembly and disassembly (Bukau et al. 2000; Hartl and Hayer-Hartl 2002; Haslbeck et al. 2005), protein folding and unfolding (Hartl 1996; Mayer and Bukau 2004; Zietkiewicz et al. 2004), protein translocation (Ryan and Pfanner 2001; Zhang et al. 2006), and the refolding of damaged proteins (Hightower 1991; Glover and Lindquist 1998; Marques et al. 2006). Hsps play an important role in the protection and repair of cells and tissues. One relevant feature of Hsps is that overexpression of one or more Hsp genes confers protection against subsequent stress (McCormick et al. 2003a; Zhang et al. 2007; Luh et al. 2007). In the heart, induction of Hsp72 during ischemia and reperfusion could indicate the appearance of damaged proteins as well as the attempt to repair those damaged proteins and overcome the trauma (Morimoto and Santoro 1998). Overexpression of Hsp70 is associated with myocardial protection (Plumier et al. 1995; Jayakumar et al. 2001; Liu et al. 2007). The activation of chaperone expression may constitute a potential protective tool allowing a rapid reestablishment of protein synthesis and myocardial function (Gray et al. 1999). Overexpression of other Hsps including Hsp32 and Hsp60 is also reported to be protective against cardiac injury (Lau et al. 1997; Szalay et al. 2005). However, little is known regarding variation in the levels of Hsp60, Hsp70, and Hsp90 protein and messenger RNA (mRNA) and the correlation with damage in gallinaceous tissue at various time intervals after the stress event.
The purpose of this study was to investigate the kinetics of Hsp60, Hsp70, Hsp90 protein, and mRNA expression, the distribution of Hsps in myocardial cells of heat-stressed broilers, and the correlation of these Hsp levels with tissue damage resulting from exposure to high temperatures of different duration.
Materials and methods
Animals and experimental design
One-day-old male AA broiler chicks (n = 100) were obtained from the Nanjing Changjiaying Commercial Fowl Company. The birds were housed in large coops (20 birds per coop), and the coops were placed in a climate-controlled chamber. The birds were given 4 weeks to acclimate to their new surroundings and to recover from environmental stress. During this period, the broilers were reared under standard conditions. The relative humidity of the chamber was maintained at 60% ± 10%. The room temperature (RT) was gradually decreased from 40°C to 35°C during the first week. As the chickens grew, the RT was gradually decreased to 22 ± 1°C and maintained at this temperature by controlled ventilation and heating until day 30. The total broiler population was vaccinated against Newcastle disease and infectious bursal disease on the seventh and 14th days, respectively. At 30 days of age, RT was abruptly increased from 22 ± 1°C to 37 ± 1°C except for broilers in the control coop. To regulate the heat, the temperature was monitored in the center of each coop. The relative humidity of the chamber during the course of the heat stress was maintained at 50% ± 5%. The birds were given access to a commercial broiler feed and water ad libitum during the heat stress. The mortality of the experimental broilers was recorded during the heat stress period. At the prearranged time of heat stress, all living birds were killed rapidly by decapitation and defined randomly by the duration of heating: 2, 3, 5, and 10 h of heat stress. Blood samples (10 ml) were obtained, transferred into blood collection tubes containing heparin anti-coagulant (50 IU ml−1), and immediately chilled on ice. The plasma samples for subsequent enzyme determination were obtained after centrifugation of whole blood at 1,500 × g for 5 min and were frozen and stored at −20°C. After exsanguination, the birds were manually eviscerated, and the cardiac tissue from right atrium of broilers was quickly dissected and placed into 1.5-ml tubes. The tubes were frozen in liquid nitrogen and then stored at −70°C until use. Further tissues samples (1.5 cm thick) were fixed in freshly prepared 4% paraformaldehyde in phosphate-buffered saline (pH7.0) for subsequent histological analysis.
All experiments were undertaken in accordance with the guidelines of the regional Animal Ethics Committee and were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University.
Circulating plasma CK
The activities of plasma creatine kinase (CK) were assessed using a commercial kit modified for use with a multi-well plate spectrophotometer as previously described by Sun et al. (2005).
Semi-quantitative detection of Hsps by enzyme-linked immunosorbent assay
After complete washing in ice-cold physiological saline, the heart samples were homogenized on ice in 10 volumes of homogenization buffer [0.15 M NaCl, 20 mM Tris–HCl (pH8.0), 1 mM ethylenediaminetetraacetic acid, 1 mM phenylmethylsulphonyl fluoride, 0.1 μM E-46, 0.08 μM aprotinin, 0.1 μM leupeptin, and 0.1% NP-40] (Shaila et al. 2005) using an Ultra-Turrax homogenizer, and the homogenates were centrifuged at 12,000 × g for 20 min at 4°C to remove cellular debris. The supernatant was collected and stored at −20°C for protein quantification.
The levels of Hsp60, Hsp70, and Hsp90 in the heart of both heat-stressed and control broilers were measured using commercially available enzyme-linked immunosorbent assay kits (QRCT-3223113EIA\UTL, goat anti-chicken Hsp60; QRCT-332031EIA\UTL, goat anti-chicken Hsp70; QRCT-3003012EIA\UTL, goat anti-chicken Hsp90, Adlitteram Diagnostic Laboratories, USA). Quantification of samples was performed using a standard curve. β-actin (QRCT-3230311EIA\UTL, goat anti-chicken β-actin, Adlitteram Diagnostic Laboratories) was used to control the bias caused by the procedure of protein extraction. Immunological detection of the proteins was performed essentially according to the manufacturer’s instructions. The quantity of Hsps in each sample was normalized using the following formula:
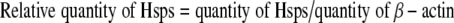 |
Detection of hsps mRNA by real-time reverse transcription–polymerase chain reaction
Isolation of total RNA
After being washed in ice-cold physiological saline, 0.5 g of the tissue stored at −70°C was ground in liquid nitrogen with a pestle and mortar. Total RNA was isolated from the ground tissue using TRIZOL reagent (Invitrogen, USA) following the manufacturer’s instructions.
Design of primers for hsps mRNA and glyceraldehyde-3-phosphate dehydrogenase mRNA detection
Sequences of broiler Hsp60, Hsp70, Hsp90, and GAPDH were obtained from GenBank (accession nos. NM_001012916, AY288298, X07265, K01458, respectively). Using these sequences, primers were designed for each of the Hsps and GAPDH (Table 1) for conventional and real-time reverse transcription–polymerase chain reaction (RT-PCR) amplification.
Table 1.
Primers for real time RT-PCR amplification
| Gene | Amplicon Size (bp) | Sense primer (5′-3′) | Antisense primer (5′-3′) |
|---|---|---|---|
| Hsp60 | 249 | GAAGTTTGACCGAGGCTACATC | ACAGCAACAACCTGAAGACCA |
| Hsp70 | 372 | AGCGTAACACCACCATTCC | TGGCTCCCACCCTATCTC |
| Hsp90 | 327 | AGTCCCAGTTCATTGGCTAC | TCCAGTCATTGGTGAGGCT |
| GAPDH | 230 | TGAAAGTCGGAGTCAACGGA T | ACGCTCCTGGAAGATAGTGAT |
Preparation of hsps mRNA and GAPDH mRNA standards
To quantify the hsp60 mRNA, hsp70 mRNA, hsp90 mRNA, and GAPDH mRNA expression, mRNA standard curves were prepared. One-step RT-PCR was performed using the primers described above. The purified RT-PCR products were inserted into pGEM-T Easy vectors (Promega, USA). Recombinant clones were transformed into Escherichia coli DH5α high-efficiency competent cells. The plasmids were purified from white colonies using a TaKaRa MiniBEST plasmid purification kit Ver. 2.0 (TaKaRa, Japan). The cloned PCR fragments were sequenced by TaKaRa Biotechnology Co., Ltd. to assess the direction of insertion. The sequence-confirmed plasmids were linearized using the vector-specific restriction enzyme ScaI (TaKaRa) and subjected to in vitro transcription using a Riboprobe in vitro Transcription System (Promega). A further 10-fold dilution series of the RNA transcript was used for the construction of standard curves by plotting the cycle threshold (Ct) values obtained against the known concentration of the serially diluted standard RNA.
Relative quantification of samples by SYBR Green I-based real-time RT-PCR
SYBR Green I-based one-step real-time quantitative RT-PCR amplification was performed using an iCycler iQ real-time PCR detection system (Bio-Rad, USA). Test samples were assayed in 25-μl reaction mixtures containing 5.4 μl of reaction mix, 1 μl of SYBR Green I, 0.5 μl of each forward and reverse primer, 2 μl of RNA, 0.5 μl of reverse transcriptase, and 15.6 μl of nuclease-free water. A template control was also included in the tests. The thermal profile for SYBR Green I-based one-step real-time RT-PCR consisted of 50 min reverse transcription at 42°C and one 3 min cycle of Taq DNA polymerase activation at 95°C, followed by 40 cycles of PCR at 94°C for 30 s (denaturation), 58°C for 30 s (annealing), and 72°C for 30 s (extension). In each run, a dilution series of the in-vitro-transcribed standard RNA was also included along with the clinical RNA samples. The Ct values of each sample were determined by one-step real-time RT-PCR, and the copy numbers of samples were obtained from the standard curves of hsp70 mRNA and GAPDH mRNA. The hsp70 mRNA of all samples was normalized using the following formula:
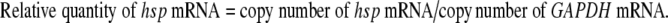 |
Histopathological detection of heart tissues in heat-stressed broilers
Following exsanguination, the birds were manually eviscerated, and the hearts were quickly dissected and fixed in freshly prepared 4% paraformaldehyde within 10 min. After 24 h fixation, samples were dehydrated in a graded series of alcohol and embedded in a paraffin block. Sections (4 μm) were cut, mounted on polylysine-coated slides, and stained by hematoxylin and eosin (HE) staining.
Immunohistochemical detection of Hsps
The sections from heart tissues were examined immunohistochemically (IHC) using the streptavidin–biotin–peroxidase complex (sABC) procedure (85-6643, Zymed Laboratories Inc., South San Francisco, CA, USA). After being dewaxed and rehydrated, the sections were placed in 10 mM citric acid buffer (pH6.0) and then heated in a microwave oven at 800W for 3 min and at 400W for 10 min, respectively. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 20 min. Nonspecific binding sites were blocked by incubating for 20 min in blocking solution (ready-to-use). Sections were overlaid at 4°C overnight with Hsp60 monoclonal antibodies [SPA-806, cross-react with Hsp60 in chickens; StressGen, USA: diluted 1:100 with Tween 20/Tris-buffered saline containing 0.5% bovine serum albumin (BSA)], Hsp70 monoclonal antibodies (SPA-820, cross-react with Hsp70 in chickens; StressGen: diluted 1:800 with Tween 20/Tris-buffered saline containing 0.5% BSA), Hsp90 monoclonal antibodies (SPA-830, cross-react with Hsp90 in chickens; StressGen: diluted 1:25 with Tween 20/Tris-buffered saline containing 0.5% BSA). Samples were then treated with a biotinylated anti-mouse secondary antibody for 20 min at RT, followed by horseradish peroxidase–streptavidin for 20 min at RT. The reaction was developed with 3-3′-diaminobenzidine (diluted ready-to-use, 00-2014, Zymed Laboratories Inc.). The slides were counterstained with Mayer’s hematoxylin for 30 s and then mounted. The corresponding negative control sections were prepared by omitting the antibodies. This control gave a negative result.
Statistical analysis
Statistical analysis of the differences between each group was carried out using a one-way analysis of variance using the Statistical Package for Social Sciences (SPSS version 11.5). Comparison of the mean value of the control group with that of each experimental group was performed using the Duncan test for multiple comparisons. Differences were regarded as significant at P < 0.05.
Results
Mortality and concentrations of plasma CK
At the beginning of heat exposure, all broilers began to pant constantly and lay down with their wings opened. Concurrently, their feed intake decreased and the water consumption increased correspondingly. Some broilers died suddenly in response to the heat stress with the peak time of death between 2 and 3 h after beginning the heat stress. After 3 h of heat stress, the number of dying broilers gradually decreased. There was no broiler death after 5 h of heat stress. The mortality in the five groups exposed to heat stress was 0, 20%, 30%, 35% and 40%, respectively (Table 2). The plasma CK level in broilers is shown in Table 2. Although the plasma CK concentrations show a slight increase (P > 0.05) at 2 h of heat stress, an obvious elevation of plasma CK occurs after 3 h of heat stress (P < 0.01), and the peak level is reached (P < 0.01) after 10 h of heat stress.
Table 2.
Mortality and plasma CK after different times of heat stress
| Period of exposure to heat stress | |||||
|---|---|---|---|---|---|
| 0 h (control) | 2 h | 3 h | 5 h | 10 h | |
| Mortality | 0 | 20% | 30% | 35% | 40% |
| Plasma CK (U/ml) | 53.4 ± 7.89 | 80.28 ± 19.19 | 85.05 ± 17.04** | 78.98 ± 18.4** | 111.23 ± 22.8** |
Values indicated are mean ± SD.
**Significant at 0.01 level when comparing the heat-stressed groups with the control
The levels of three detected Hsps and their corresponding hsp mRNAs in the heart
Hsp60, Hsp70, and Hsp90 protein expression data, normalized to heart tissue β-actin, and the hsp60, hsp70, and hsp90 mRNA transcription ratio data normalized to GAPDH mRNA are shown in Figs. 1, 2 and 3, respectively. Generally, the three hsp mRNAs detected in the heart tissue of heat-stressed broilers are significantly elevated by 2 h of heat exposure and then return to close to baseline levels by 3 h of heat exposure. Meanwhile, all three Hsp proteins detected in the heart tissue of heat-stressed broilers increased significantly by 2 h of heat exposure and continued increasing over the time of the heat shock. However, the levels of the three Hsp proteins and their corresponding hsp mRNA in the heart tissues of heat-stressed broilers differ from each other, especially with respect to Hsp60 protein and hsp60 mRNA.
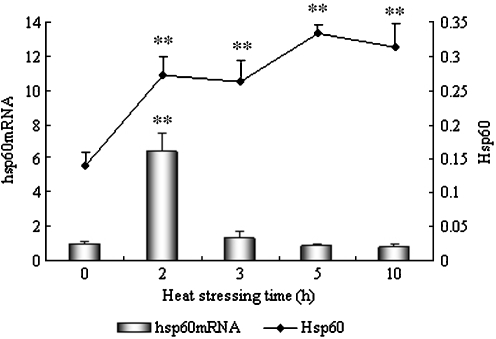
Fig. 1.
Levels of Hsp60 and hsp60 mRNA in the heart of heat-stressed broilers. *P < 0.05, **P < 0.01 when comparing the heat-stressed groups with the control. Values indicated are mean ± SD
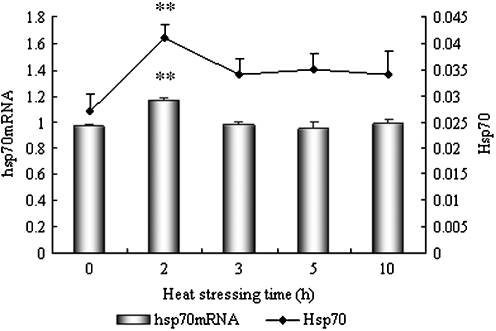
Fig. 2.
Levels of Hsp70 and hsp70 mRNA in the heart of heat-stressed broilers. *P < 0.05, **P < 0.01 when comparing the heat stressed groups with the control. Values indicated are mean ± SD
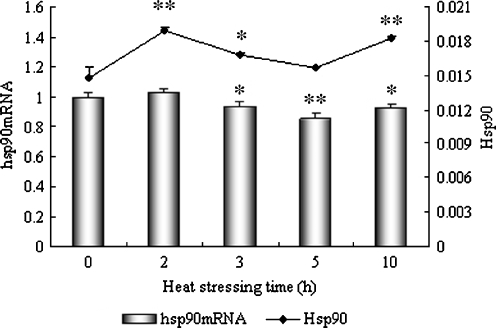
Fig. 3.
Levels of Hsp90 and hsp90 mRNA in the heart of heat-stressed broilers. *P < 0.05, **P < 0.01 when comparing the heat stressed groups with the control. Values indicated are mean ± SD
Figure 1 shows the levels of Hsp60 and its corresponding mRNA in the heart of heat-stressed broilers. The levels of hsp60 mRNA increased sharply in the heart (P < 0.01) and reached a peak level (6.7-fold increase compared with control) at 2 h of heat stress. However, the levels of hsp60 mRNA decreased quickly at 3 h of heat stress, and there was no significant difference between heat-stressed birds and control birds after 3, 5, and 10 h of heat stress. Over the same time period, the levels of Hsp60 protein in the hearts of broilers increased (P < 0.01) dramatically at 2 h of heat stress and maintained this high level during the course of the heat exposure. Over the time of the heat stress, the levels of Hsp60 gradually increased to a peak level after 5 h (P < 0.01) of heat stress.
The level of Hsp70 protein and hsp70 mRNA in the heart of heat-stressed broilers is displayed in Fig. 2. Similar to hsp60 mRNA, the amount of hsp70 mRNA in the heart of heat-stressed birds increased significantly (P < 0.01) at the beginning of heat stress (2 h) when compared to control birds. However, the levels of hsp70 mRNA quickly returned to normal levels after 3, 5, and 10 h of heat exposure as compared to controls. At the same time, the level of Hsp70 increased in the heart tissue and reached a peak level (P < 0.01) at 2 h of heat stress. Although the Hsp70 levels showed a slight induction in the heart tissue of heat-stressed broilers after 3, 5, and 10 h of heat stress, there was no significant difference when compared to control chickens.
Figure 3 shows the level of Hsp90 and its corresponding mRNA in the heart tissue of heat-stressed broilers. The level of hsp90 mRNA in the heart increased slightly at 2 h of heat stress (P > 0.05), but decreased significantly at 3 h (P < 0.05) and 5 h (P < 0.01) of heat stress, respectively. However, compared to the controls, the level of hsp90 mRNA increased again at 10 h (P < 0.05) of heat stress. An immediate and significant induction of the Hsp90 protein occurred after 2 h of heat stress (P < 0.01) where it also reached its peak expression. The level of Hsp90 at 3 h of heat stress was decreased (P < 0.05) compared to the 2-h time point, but was still higher (P < 0.05) than in controls. The level of Hsp90 in the hearts of broilers displayed obvious induction at 10 h (P < 0.01), but not at 5 h (P > 0.05) of heat stress when compared to the control group.
Histopathological and immunohistochemical observation
Histopathologically, granular and vacuolar degeneration were characterized by light pink granulation, slightly enlarged intracellular spaces, and loss of striations in the cytoplasm of the myocardial cells of heat-stressed broilers (Fig. 4a). However, there were no obvious histological changes in the control group.
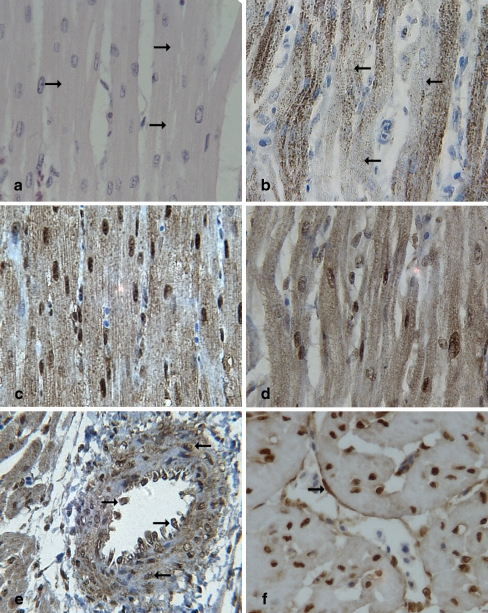
Fig. 4.
Localization of Hsps in the myocardial cells of heat-stressed broilers (all images are at ×400 magnification). All images displayed the heart tissues of 3-h heat-stressed broilers. a Light pink granulation and loss of striations (right arrow) in the cytoplasm of the myocardial cells of heat-stressed broilers (HE staining); b Hsp60-positive signals were mainly detected in the cytoplasm of the myocardial cells (IHC staining), and Hsp60 staining was distinctly lower in the cytoplasm of granular degenerated areas (left arrow); c Strong positive signals of Hsp90 were detected both in the nucleus and the cytoplasm of the myocardial cells, mainly in the nucleus of the myocardial cells (IHC staining); d Positive staining of Hsp70 distributed both in the cytoplasm and the nucleus (IHC staining); e and f In the cross-section of the heart, positive signals of Hsp70 were localized in the endothelial cells (right arrow) and the wall of heart blood vessels (left arrow) of the heat-stressed broilers (IHC staining)
Immunohistochemically, there were no obvious differences in Hsp distribution in the myocardial cells of the heat-stressed groups compared to the control groups. Hsp60-positive signals were mainly detected in the cytoplasm of the myocardial cells (Fig. 4b). Interestingly, after heat stress, Hsp60 expression in the cytoplasm of the heart tissues was more prominent in intact areas than in degenerated areas. In myocardial cells, Hsp60 staining was distinctly lower in the cytoplasm of granular degenerated areas. Strong positive signals of Hsp90 were detected both in the nucleus and cytoplasm of the myocardial cells, and Hsp90 signals in the nucleus were more intense than those in the cytoplasm (Fig. 4c). Positive staining of Hsp70 protein was localized mainly to the cytoplasm and nuclei of the myocardial cells (Fig. 4d). However, slightly positive Hsp70 signals were also observed in the vessel wall of the heart (Fig. 4e, f). No positive signal of Hsp60 was found in the vessel walls of broiler heart. No staining for Hsps was detected in the negative control sections from the heart.
Discussion
Prokaryotes and eukaryotes respond to stressors at the cellular and molecular level by universal expression of Hsps (Craig 1985; Lindquist 1986; Morimoto et al. 1990). These Hsps are believed to exert a protective effect for some tissues against stresses such as exposure to high temperature, ischemia/reperfusion, and cytotoxic substances (Kawana et al. 2000; Eichler et al. 2005; Hagiwara et al. 2007). Hsps help to maintain the metabolic and structural integrity of the cell and act as a protective response to external stresses.
Our results demonstrate that the transcription of hsp60 mRNA, hsp70 mRNA, and hsp90 mRNA increased significantly in the heart and reach their highest levels when chickens are exposed to heat stress for 2 h; simultaneously, the amounts of all three tested proteins (Hsp60, Hsp70, and Hsp90) increased quickly in the heart of broilers at the early stage of 2-h heat stress, which is consistent with their respective mRNA response to high temperature (Figs. 1, 2, and 3). Cells from all organisms respond to increases in temperatures of 3°C to 5°C above normal by rapid gene transcription and subsequent mRNA translation to yield a class of highly conserved proteins known as heat shock proteins (Locke and Noble 1995). It is concluded from our results that the elevations of Hsp60, Hsp70, and Hsp90 (especially Hsp60) occur in the heart at 2 h of heat stress, and they may act as important markers and protective proteins in response to the adverse environmental conditions. Several studies have shown that elevation of Hsps in the heart provide protection against stress-induced myocardial injury. For example, overexpression of Hsp72 reduced infarct size in an in vivo transgenic mouse model of myocardial ischemia and reperfusion (Hutter et al. 1996). Furthermore, myocytes constitutively expressing high levels of hsp90β showed significantly higher survival against heat stress (Richard et al. 1995). Interestingly, our results also show that the levels of Hsp60 mRNA transcription in the heart decrease sharply after 3 h of heat stress, whereas the levels of Hsp60 are still maintained at higher concentrations after 3, 5, and 10 h of heat exposure as compared to the control. There was a fivefold increase in the Hsp60 levels in the hearts of patients with dilated cardiomyopathy in comparison to normal subjects (Latif et al. 1999). The overexpression of Hsp60 may be the reason for the increased requirement for ATP under heat stress in the heart with the increasing rate of heart contraction (Koelkebeck and Odom 1995). In the present study, we observe that the hsp90 mRNA transcription varies with its corresponding Hsp90 protein expression in the same pattern (Fig. 3). These phenomena exist in turkey lymphocytes and in in vitro experiments in broilers (Miller and Qureshi 1992, Wang and Edens 1993). It is also known from studies of chicken embryo cells that the avian stress response has a number of posttranscriptional controls (Takenaka and Hightower 1993). The reasons are still unknown and should be investigated.
The localization of Hsps may be related to the protection function of molecular chaperones (Georgopoulos and Welch 1993; Craig et al. 1994; Hendrick and Hartl 1995). The immunohistochemical results demonstrate the localization of Hsp60, Hsp70, and Hsp90 in the cardiac muscle of broilers. However, the subcellular localization of the three Hsps in the myocardial cells were different. As revealed by IHC, Hsp60 was primarily expressed in the cytoplasm of myocardial cells, consistent with previous findings in muscle fibers in humans (Kervinen et al. 2003). Hsp60 is known to be located in cardiac myocytes which are specifically rich in mitochondria that generate the high amount of ATP needed for normal cardiac contractile function (Kreisel et al. 1994; Soltys and Gupta 1996; Cechetto et al. 2000). When exposed to heat stress, the heart working rate is increased (Knowlton 1994; McCully et al. 1995). ATP depletion is known to have detrimental effects which range from protein aggregation and collapse of the cytoskeleton to loss of ionic balance. Heat stress and subsequent Hsp60 accumulation give rise to an ATP-sparing effect (Kabakov and Gabai 1997). Therefore, mitochondrial HSP60 plays an important role in maintaining mitochondrial integrity, function, and capacity for ATP generation, which are the crucial factors in determining survival of myocardial cells undergoing heat stress injury. Molecular chaperones influence several cellular components including the cytoskeleton, an assortment of filamentous and tubular polymers composed of microtubules, microfilaments, and intermediate filaments (Liang and MacRae 1997). Actin microfilaments and intermediate filaments in rat brain tumor cells damaged by heat stress reverted to normal after 8 h of recovery that correlated with the induction of Hsp70 (Wang et al. 1998). Similar to Hsp70, Hsp90 is a molecular chaperone that may also possess the function of protecting the cytoskeleton. Hsp90 is able to bind to actin and tubulin, major constituents of the cell cytoskeleton (Fostinis et al. 1992; Kellermayer and Csermely 1995). Hsp90 may also significantly contribute to the preservation of the structural integrity of the cell after stress. Environmental stress often leads to depletion of ATP in the stressed cells, which is highly detrimental to these cytoskeletal structures (Kabakov and Gabai 1997). It is believed that Hsp90 possesses the ability to refold denatured proteins into their proper conformation (Wiech et al. 1992; Nathan et al. 1997). Therefore, the expression of Hsp90 and Hsp70 in the nucleus and cytoplasm of the myocardial cells may be related to the refolding of denatured proteins induced by heat exposure.
The present study finds a slight histological lesion in the hearts of heat-stressed broilers which includes enlarged intracellular spaces and slightly granular degeneration. Myocardial cell damage induced by heat exposure was also reflected in the gradual elevation of plasma CK. In the present study, exposure to acute heat stress causes marked induction (P < 0.01) in plasma CK activity in the broilers. Such elevations are indicative of myocardial damage and are the consequence of disruptions in muscle cell membrane (sarcolemma) function and permeability. Our IHC results reveal that Hsp60 positive staining was distinctly lower in the cytoplasm of granular degenerated areas.
The present finding that Hsp70 is located in the blood vessel walls and vascular endothelial cells of the hearts of these broilers reveals the importance of the blood vessels and endothelial cells in myocardial cell damage and suggests an association between Hsp70 concentration in the blood vessels and the constriction functions that occurs after heat shock treatment. White (1980) discovered a protein of 71,000 molecular weight that accumulated in the microvascular fraction when telencephalon slices were incubated in vinblastine. This inducible protein was verified in future research to be a HSP. Following this discovery, other researchers studied the protection of HSPs in the blood capillary. Wang et al. (1995) reported that induction of Hsp72 in human endothelial cells (ECs) by a thermal or non-thermal mechanism could prevent activated human polymorphonuclear neutrophil leukocyte-mediated ECs necrosis, which may favor increased vascular permeability during the systemic inflammatory response syndrome. House et al. (2001) detected Hsp70 in aortic tissues from heat- and stannous-chloride-treated animals, indicating that these tissues were in a cytoprotected state. McCormick et al. (2003b) reported that clinically applicable thermal preconditioning attenuated leukocyte–endothelial interactions associated with an increased expression of Hsp72. The detailed protective mechanism of the strong expression of heat shock-induced Hsp70 in the blood vessels of the heart is still unclear and needs further study.
Acknowledgments
This study was supported by grants (30170682, 30571400) from the National Natural Science Foundation of China and grants (20050307008) from the Specialized Research Fund for the Doctoral Program of Higher Education of China.
References
- Benjamin IJ, McMillan DR (1998) Stress heat shock proteins molecular chaperones in cardiovascular biology and disease. Circ Res 83:117–132 [DOI] [PubMed]
- Bukau B, Deuerling E, Pfund C, Craig EA (2000) Getting newly synthesized proteins into shape. Cell 101(2):119–122 [DOI] [PubMed]
- Cechetto JD, Soltys BJ, Gupta RS (2000) Localization of mitochondrial 60-kD heat shock chaperonin protein (Hsp60) in pituitary growth hormone secretory granules and pancreatic zymogen granules. J Histochem Cytochem 48:45–56 [DOI] [PubMed]
- Craig EA (1985) The heat shock response. CRC Crit Rev Biochem 18:239–280 [DOI] [PubMed]
- Craig EA, Weissman JS, Horwich AL (1994) Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell 78:365–372 [DOI] [PubMed]
- Eichler TE, Ransom RF, Smoyer WE (2005) Differential induction of podocyte heat shock proteins by prolonged single and combination toxic metal exposure. Toxicol Sci 84(1):120–128 [DOI] [PubMed]
- Fostinis Y, Theodoropoulos PA, Gravanis A, Stournaras C (1992) Heat shock protein HSP90 and its association with the cytoskeleton: a morphological study. Biochem Cell Biol 70:779–786 [DOI] [PubMed]
- Ganter MT, Ware LB, Howard M, Roux J, Gartland B, Matthay MA, Fleshner M, Pittet J (2006) Extracellular heat shock protein 72 is a marker of the stress protein response in acute lung injury. Am J Physiol Lung Cell Mol Physiol 291:L354–L361 [DOI] [PMC free article] [PubMed]
- Georgopoulos C, Welch WJ (1993) Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol 9:601–634 [DOI] [PubMed]
- Geraert PA, Guillaumin S, Leclercq B (1993) Are genetically lean broilers more resistant to hot climate? Br Poult Sci 34(4):643–653 [DOI] [PubMed]
- Geraert PA, Padilha JC, Guillaumin S (1996) Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br J Nutr 75(2):195–204 [DOI] [PubMed]
- Glover JR, Lindquist S (1998) Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94(1):73–82 [DOI] [PubMed]
- Gray CC, Amrani M, Yacoub MH (1999) Heat stress proteins and myocardial protection: experimental model or potential clinical tool? Int J Biochem Cell Biol 31:559–573 [DOI] [PubMed]
- Hagiwara S, Iwasaka H, Matsumoto S, Noguchi T, Yoshioka H (2007) Association between heat stress protein 70 induction and decreased pulmonary fibrosis in an animal model of acute lung injury. Lung 185(5):287–293 [DOI] [PubMed]
- Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–580 [DOI] [PubMed]
- Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Sci 295(5561):1852–1858 [DOI] [PubMed]
- Haslbeck M, Miess A, Stromer T, Walter S, Buchner J (2005) Disassembling protein aggregates in the yeast cytosol: the cooperation of Hsp26 with SSA1 and Hsp104. J Biol Chem 25:23861–23868 [DOI] [PubMed]
- Hendrick JP, Hartl FU (1995) The role of molecular chaperones in protein folding. FASEB J 9:1559–1569 [DOI] [PubMed]
- Hightower LE (1991) Heat shock, stress proteins, chaperones and proteotoxicity. Cell 66:191–197 [DOI] [PubMed]
- House SD, Guidon PT Jr, Perdrizet GA, Rewinski M, Kyriakos R, Bockman RS, Mistry T, Gallagher PA, Hightower LE (2001) Effects of heat shock, stannous chloride, and gallium nitrate on the rat inflammatory response. Cell Stress Chaperones 6:164–171 [DOI] [PMC free article] [PubMed]
- Hutter JJ, Mestril R, Tam EKW, Sievers RE, Dillmann WH, Wolfe CL (1996) Overexpression of heat shock protein 72 in transgenic mice decreases infarct size in vivo. Circulation 94:1408–1411 [DOI] [PubMed]
- Jayakumar J, Suzuki K, Sammut IA, Smolenski RT, Khan M, Latif N, Abunasra H, Murtuza B, Amrani M, Yacoub MH (2001) Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury. Circulation 104:I303–307 [DOI] [PubMed]
- Kabakov AE, Gabai VL (1997) Heat shock proteins and cytoprotection: ATP-deprived mammalian cells. R.G. Landes Co., Austin
- Kawana K, Miyamoto Y, Tanonaka K, Han-no Y, Yoshida H, Takahashi M, Takeo S (2000) Cytoprotective mechanism of heat shock protein 70 against hypoxia/reoxygenation injury. J Mol Cell Cardiol 32:2229–2237 [DOI] [PubMed]
- Kellermayer MS, Csermely P (1995) ATP induces dissociation of the 90 kDa heat shock protein (hsp90) from F-actin: interference with the binding of heavy meromyosin. Biochem Biophys Res Commun 211:166–174 [DOI] [PubMed]
- Kervinen H, Huittinen T, Vaarala O, Leinonen M, Saikku P, Manninen V, Mänttäri M (2003) Antibodies to human heat shock protein 60, hypertension and dyslipidemia. A study of joint effects on coronary risk. Atherosclerosis 169:339–344 [DOI] [PubMed]
- Knowlton AA (1994) Heat-shock proteins, stress and the heart. Ann NY Acad Sci 723:128–137 [DOI] [PubMed]
- Koelkebeck KW, Odom TW (1995) Laying hen response to acute heat stress and carbon dioxide supplementation: II changes in plasma enzymes, metabolites and electrolytes. Comp Biochem Physiol Part A: Physiol 122:119–122 [DOI] [PubMed]
- Kreisel W, Hildebrandt H, Schiltz E, Kohler G, Spamer C, Dietz C, Mossner W, Heilmann C (1994) Immuno-gold electron microscopical detection of heat shock protein 60 (hsp60) in mitochondria of rat hepatocytes and myocardiocytes. Acta Histochem 96:51–62 [DOI] [PubMed]
- Latif N, Taylor PM, Khan MA, Yacoub MH, Dunn MJ (1999) The expression of heat shock protein 60 in patients with dilated cardiomyopathy. Basic Res Cardiol l94:112–119 [DOI] [PubMed]
- Lau S, Patnaik N, Sayen R, Mestril R (1997) Simultaneous overexpression of two stress proteins in rat cardiomyocytes and myogenic cells confers protection against ischemia-induced injury. Circulation 96:2287–2294 [DOI] [PubMed]
- Liang P, MacRae TH (1997) Molecular chaperones and the cytoskeleton. J Cell Sci 110:1431–1440 [DOI] [PubMed]
- Lindquist S (1986) The heat-shock responses. Annu Rev Biochem 55:1151–1191 [DOI] [PubMed]
- Liu JC, He M, Wan L, Cheng XS (2007) Heat shock protein 70 gene transfection protects rat myocardium cell against anoxia-reoxygeneration injury. Chin Med J (Engl) 120(7):578–583 [PubMed]
- Locke M, Noble EG (1995) Stress protein: the exercise response. Can J Appl Physiol 20:155–167 [DOI] [PubMed]
- Lu Q, Wen J, Zhang H (2007) Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult Sci 86(6):1059–1064 [DOI] [PubMed]
- Luh SP, Kuo PH, Kuo TF, Tsai TP, Tsao TC, Chen JY, Tsai CH, Yang PC (2007) Effects of thermal preconditioning on the ischemia-reperfusion-induced acute lung injury in minipigs. Shock 28(5):615–622 [DOI] [PubMed]
- Marques C, Guo W, Pereira P, Taylor A, Patterson C, Evans PC, Shang F (2006) The triage of damaged proteins: degradation by the ubiquitin-proteasome pathway or repair by molecular chaperones. FASEB J 20:741–743 [DOI] [PMC free article] [PubMed]
- Mashaly MM, Hendricks GL, Kalama MA, Gehad AE, Abbas AO, Patterson PH (2004) Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult Sci 83(6):889–894 [DOI] [PubMed]
- Mayer MP, Bukau B (2004) Hsp70 chaperones: Cellular functions and molecular mechanism. Cell Mol Life Sci 62:670–684 [DOI] [PMC free article] [PubMed]
- McCormick PH, Chen G, Tlerney S, Kelly CJ, Bouchier-Hayes DJ (2003a) Clinically relevant thermal preconditioning attenuates ischemia-reperfusion injury. J Surg Res 109:24–30 [DOI] [PubMed]
- McCormick PH, Chen G, Tierney S, Kelly CJ, Bouchier-Hayes DJ (2003b) Clinically applicable thermal preconditioning attenuates leukocyte–endothelial interactions. J Am Coll Surg 197:71–78 [DOI] [PubMed]
- McCully JD, Myrmel T, Lotz M, Krukenkamp IB, Levitsky S (1995) The rapid expression of myocardial hsp70 mRNA and the heat shock 70 kDa protein can be achieved after only a brief period of retrograde hyperthermic perfusion. J Mol Cell Cardiol 27:873–882 [DOI] [PubMed]
- Miller L, Qureshi MA (1992) Molecular changes associated with heat shock treatment in avian mononuclear and lymphoid lineage cells. Poult Sci 71:473–481 [DOI] [PubMed]
- Morimoto RI, Santoro GM (1998) Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol 16:833–838 [DOI] [PubMed]
- Morimoto RI, Tisieres A, Georgopoulos C (1990) The stress response function of the proteins and perspective. In: Morimoto RI, Tisieres A, Georgopoulos C (eds) Stress proteins in biology and medicine. Cold Spring Harbor Laboratory Press, Cold Springs Harbor, NY, pp 1–36
- Nathan DF, Vos MH, Lindquist S (1997) In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc Natl Acad Sci U S A 94:12949–12956 [DOI] [PMC free article] [PubMed]
- Northcutt JK, Foegeding EA, Edens FW (1994) Waterholding properties of thermally preconditioned chicken breast and leg meat. Poult Sci 73:308–316 [DOI] [PubMed]
- Plumier JCL, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G (1995) Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest 95:1854–1860 [DOI] [PMC free article] [PubMed]
- Richard J, Derek M, David SL (1995) Differential cytoprotection against heat stress or hypoxia following expression of specific stress protein genes in myogenic cells. J Mol Cell Cardiol 27(8):1669–1678 [DOI] [PubMed]
- Ryan MT, Pfanner N (2001) Hsp70 proteins in protein translocation. Adv Protein Chem 59:223–242 [DOI] [PubMed]
- Sandercock DA, Hunter RR, Nute GR, Mitchell MA, Hocking PM (2001) Acute heat stress-induced alterations in blood acid–base status and skeletal muscle membrane integrity in broiler chickens at two ages: implications for meat quality. Poult Sci 80:418–425 [DOI] [PubMed]
- Shaila S, Angshuman S, Abhijeet K, Samindranath M, Pal JK (2005) Flufenoxuron, an acylurea insect growth regulator, alters development of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) by modulating levels of chitin, soluble protein content, and HSP70 and p34cdc2 in the larval tissues. Pestic Biochem Physiol 85:84–90
- Soltys BJ, Gupta RS (1996) Immunoelectron microscopic localization of the 60-kDa heat shock chaperonin protein (Hsp60) in mammalian cells. Exp Cell Res 222:16–27 [DOI] [PubMed]
- Staib JL, Quindry JC, French JP, Criswell DS, Powers SK (2007) Increased temperature, not cardiac load, activates heat shock transcription factor 1 and heat shock protein 72 expression in the heart. Am J Physiol Regul Integr Comp Physiol 292:R432–R439 [DOI] [PubMed]
- Sun PM, Liu YT, Zhao YG, Bao ED, Wang ZL (2005) Relationship between heat damages and HSPs mRNA in persistent heat stressed broilers. Agric Sci China 6:227–233
- Szalay L, Shimizu T, Schwacha MG, Choudhry MA, Rue LW III, Bland KI, Chaudry IH (2005) Mechanism of the salutary effects of estradiol on organ function following trauma-hemorrhage: upregulation of hemeoxygenase. Am J Physiol 289:H92–H98 [DOI] [PubMed]
- Takenaka IM, Hightower LE (1993) Regulation of chicken Hsp70 and Hsp90 family gene expression by transforming growth factor-b1. J Cell Physiol 155:54–62 [DOI] [PubMed]
- van der Hel W, Versteqen MW, Pijls L, van Kampen M (1992) Effect of two-day temperature exposure of neonatal broiler chicks on growth performance and body composition during two weeks at normal conditions. Poult Sci 71(12):2014–2021 [DOI] [PubMed]
- Wang SY, Edens FW (1993) Stress induced heat shock protein synthesis in peripheral leukocytes of turkeys, Meleagris gallopavo. Comp Biochem Physiol 106B(3):621–628
- Wang JH, Redmond HP, Watson RW, Condron C, Bouchier-Hayes D (1995) Induction of heat shock protein 72 prevents neutrophil-mediated human endothelial cell necrosis. Arch Surg 130(12):1260–1265 [DOI] [PubMed]
- Wang TT, Chiang AS, Chu JJ, Cheng TJ, Chen TM, Lai YK (1998) Concomitant alterations in distribution of 70 kDa heat shock proteins, cytoskeleton and organelles in heat shocked 9L cells. Int J Biochem Cell Biol 30:745–759 [DOI] [PubMed]
- White FP (1980) The synthesis and possible transport of specific proteins by cells associated with brain capillaries. J Neurochem 35(1):88–94 [DOI] [PubMed]
- Wiech H, Buchner J, Zimmermann R, Jakob U (1992) Hsp90 chaperones protein folding in vitro. Nature 358:169–170 [DOI] [PubMed]
- Zhang XY, Clark AF, Yorio T (2006) Heat shock protein 90 is an essential molecular chaperone for nuclear transport of glucocorticoid receptor b. Invest Ophthalmol Vis Sci 47:700–708 [DOI] [PubMed]
- Zhang HY, Lv NH, Xie Y, Guo GH, Zhan JH, Chen J (2007) Protection of heat shock preconditioning on acute gastric mucosal lesion in scalded rats and its mechanism. Zhonghua Shao Shang Za Zhi 23(1):58–61 [PubMed]
- Zietkiewicz S, Krzewska J, Liberek K (2004) Successive and synergistic action of the Hsp70 and Hsp100 chaperones in protein disaggregation. J Biol Chem 279:44376–44383 [DOI] [PubMed]