Abstract
We previously reported that short-term (2 h) plating of cat atrial myocytes on the extracellular matrix protein, laminin (LMN) decreases adenylate cyclase activity and β1-adrenergic receptor (β1-AR) stimulation of L-type Ca2+ current (ICa,L). The present study sought to determine whether LMN-mediated down-regulation of β1 signalling is due to down-regulation of adenylate cyclase and to gain insight into the signalling mechanisms responsible. β1-AR stimulation was achieved by 0.01 μm isoproterenol (isoprenaline) plus 0.1 μm ICI 118551, a selective β2-AR antagonist. Atrial myocytes were plated for at least 2 h on uncoated cover-slips (−LMN) or cover-slips coated with LMN (+LMN). As previously reported, β1-AR stimulation of ICa,L was significantly smaller in +LMN compared to −LMN atrial myocytes. In −LMN myocytes, 10 μm LY294002 (LY), a specific inhibitor of PI-(3)K, had no effect on β1-AR stimulation of ICa,L. In +LMN myocytes, however, LY significantly increased β1-AR stimulation of ICa,L. Western blots revealed that compared with −LMN myocytes, +LMN myocytes showed a significant increase in Akt phosphorylation at Ser-473, which was prevented by LY. In another approach, +LMN myocytes were infected (multiplicity of infection (MOI), 100; 24 h) with replication-defective adenoviruses (Adv) expressing dominant-negative inhibitors of focal adhesion kinase (FAK) (Adv-FRNK or Adv-Y397F-FAK) or Akt (Adv-dnAkt). Compared with control cells infected with Adv-β-galactosidase, cells infected with Adv-FRNK, Adv-Y397F-FAK or Adv-dnAkt each exhibited a significantly greater β1-AR stimulation of ICa,L. In −LMN myocytes LY had no effect on forskolin (FSK)-stimulated ICa,L. However, in +LMN myocytes LY significantly increased FSK-stimulated ICa,L. Similar results were obtained in +LMN atrial myocytes infected with Adv-FRNK. We conclude that LMN binding to β1-integrin receptors acts via FAK/PI-(3)K/Akt to inhibit adenylate cyclase activity and thereby down-regulates β1-AR-mediated stimulation of ICa,L. These findings provide new insight into the cellular mechanisms by which the extracellular matrix can modulate atrial β-AR signalling.
In heart, sympathetic nerve activity acts primarily via β-adrenergic receptor (β-AR) signalling to regulate excitation–contraction coupling. β-AR signalling also plays important roles in cellular apoptosis, hypertrophy, heart failure and cardiac remodelling. Although cardiac muscle exhibits both β1-ARs and β2-ARs, β1-ARs are the predominant subtype. However, remodelling of the failing heart decreases β1-AR signalling both in humans (Bristow et al. 1986) and experimental animals (Kiuchi et al. 1993). The mechanisms underlying this change in β1-AR signalling are not entirely understood.
Atrial disease is commonly associated with atrial fibrosis, i.e. structural remodelling, which is associated with increases in extracellular matrix (ECM) proteins. The cardiac ECM is composed of a variety of proteins including laminin, fibronectin and collagen. Adult cardiomyocytes primarily attach to laminin via low-affinity cell surface receptors known as integrins (Terracio et al. 1991). Integrins comprise a family of heterodimeric transmembrane proteins consisting of a variety of alpha (α) and beta (β) chains. Along with its role in cell attachment, the ECM/β-integrin complex is an important modulator of intracellular signalling, i.e. so-called ‘outside-in’ signalling (Hynes, 1992; Schwartz et al. 1995; Dedhar, 1999). Our previous studies indicate that atrial myocytes exhibit both β1-AR- and β2-AR-mediated stimulation of L-type Ca2+ current (ICa,L) and that β1-ARs are the predominant β-AR signalling mechanism (Wang et al. 2000b). However, plating atrial myocytes on laminin (LMN) acts via β1-integrins to decrease β1-AR stimulation of ICa,L (Wang et al. 2000b) and adenylate cyclase/cAMP activity (Wang et al. 2000a). In neonatal rat ventricular myocytes over-expression of a full-length β1A-integrin also markedly decreased β-AR stimulation of ICa,L (Cheng et al. 2004). These studies establish that β1-integrin signalling can modulate β-AR stimulation of ICa,L, although the underlying cellular mechanisms are not clear.
One possible mechanism may involve integrin-mediated activation of focal adhesion kinase (FAK) (see Samarel, 2005). FAK (and its related proline-rich tyrosine kinase (PYK2)) can bind the p85 subunit of phosphatidylinositol-3′ kinase (PI-(3)K) (Schaller, 2001) and thereby activate downstream Akt in response to integrin engagement and clustering. Moreover, PI-(3)K signalling inhibits cAMP-dependent PKA signalling (Leblais et al. 2004; Kerfant et al. 2004; Alloatti et al. 2005; Dedkova et al. 2007). The present study therefore sought to determine whether LMN acts via FAK/PI-(3)K/Akt to decrease adenylate cyclase-mediated β1-AR signalling in atrial myocytes. These results provide insight into the potential role of the ECM in the remodelling of β-AR signalling. Part of this work has been reported in abstract form (Lipsius et al. 2006).
Methods
Adult cats of either sex were anaesthetized with sodium pentobarbital (50 mg kg−1, i.p.). Once fully anaesthetized, a bilateral thoracotomy was performed, and the heart was rapidly excised and mounted on a Langendorff perfusion apparatus. After enzyme (collagenase; type II, Worthington Biochemical) digestion, atrial myocytes were isolated as previously reported (Wu et al. 1991). The animal protocols and experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of Loyola University of Chicago, Stritch School of Medicine (Maywood, IL, USA). The Institutional Animal Care and Use Committee of Loyola University of Chicago prescribed the rules for the animal care and supervised their enforcement. This study used 26 animals.
Electrophysiological recordings from myocytes were performed in the perforated (nystatin) patch whole-cell configuration. CsCl (5 mm) was added to all external solutions to block K+ conductances. L-type Ca2+ current (ICa,L) was activated by depolarizing pulses from a holding potential of −40 mV to 0 mV for 200 ms every 5 s. Peak ICa,L amplitude was measured in relation to steady-state current. β1-AR stimulation was achieved by 0.01 μm isoproterenol (ISO) in the presence of 0.1 μm ICI 118,551, a specific β2-AR blocking agent, i.e. ISO-β1-AR stimulation. The present experiments compared two groups of freshly isolated atrial myocytes obtained from the same hearts: control cells that were plated on uncoated glass cover-slips (−LMN), and cells plated on glass cover-slips coated with LMN (+LMN; 40 μg ml−1) for at least 2 h, as previously described (Wang et al. 2000a). Control experiments have shown that atrial myocytes plated on poly l-lysine, a non-specific substrate for cell attachment, failed to show changes in β-AR signalling similar to cells plated on LMN (Wang et al. 2000b). Cells were exposed to LY294002 (LY) for approximately 15 min before recordings were performed. Western blots were used to analyse Akt phosphorylation (Ser-473), as previously described (Dedkova et al. 2003).
In some experiments atrial myocytes were infected (100 MOI) with adenoviruses in 24 h culture. Adv-FRNK is an adenovirus that expresses the GFP-tagged, C-terminal region of focal adhesion kinase (FAK), known as FAK-related non-kinase or FRNK. The adenovirus was generated in our laboratories (Heidkamp et al. 2002) from a chick cDNA construct kindly provided by Dr Tom Parsons of the University of Virginia. Our previous studies have demonstrated that FRNK overexpression displaces endogenous FAK from focal adhesions and costameres, reduces FAK phosphorylation at multiple sites including the Y397 autophosphorylation site, and thereby blocks FAK-dependent downstream signalling (Heidkamp et al. 2002). Adv-Y397F-FAK expresses an HA-tagged, full-length mouse FAK in which the FAK autophosphorylation site has been mutated to phenylalanine. Adv-Y397F-FAK was kindly provided by Dr Tadashi Kasahara, Kyoritsu College of Pharmacy in Tokyo, Japan (Sakurai et al. 2002). When overexpressed in a human glioma cell line, Adv-Y397F-FAK caused a dose-dependent decrease in the association of the p85 subunit of PI-(3)K with endogenous FAK, and also reduced the downstream phosphorylation of Akt at Ser-473 (Sakurai et al. 2002). Therefore, Y397F-FAK is a dominant-negative (dn) inhibitor of FAK-dependent signalling to PI-(3)K and Akt, presumably by interfering with the binding of PI-(3)K to FAK. Adv-dnAkt is a replication-defective adenovirus expressing a dominant-negative mutant of Akt (Fujio & Walsh, 1999) and was purchased from Vector Biolabs (Philadelphia, PA, USA). The mutant consists of the murine Akt coding sequence fused in frame with the HA epitope, and bearing two mutations (T308A; S473A) rendering the transgene inactive by phosphorylation. Prior studies have demonstrated that this mutant functions in a dominant-negative fashion to inhibit IGF1-PI-(3)K-Akt signalling in neonatal and adult cardiomyocytes (Fujio et al. 2000). A control adenovirus expressing nuclear-encoded β-galactosidase (Adv-β-gal) was used to control for non-specific effects of adenoviral infection (Heidkamp et al. 2001). Adenoviruses were amplified and purified using HEK293 cells, and the multiplicity of infection (MOI) for each virus was determined by dilution assay in HEK293 cells grown in 96-well clusters. Myocytes were plated in DMEM: medium 199 (4: 1) culture medium onto LMN-coated glass cover-slips, and infected (100 MOI, 24 h) with each adenovirus. Preliminary experiments using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) staining of Adv-β-gal-infected cells determined that a concentration of 100 MOI infected 93 ± 3% (N= 3 expts, 400–700 cells per expt) of cultured myocytes.
Drugs used in this study included: isoproterenol, forskolin, ICI 118,551, LY294002 (all from Sigma Chemical Co., St Louis, MO, USA).
Data are presented as mean ± standard error of the mean. Measurements were analysed using either paired or unpaired Student's t tests for significance at P < 0.05. Multiple comparisons were performed with a Student-Newman-Keuls test with significance at P < 0.05.
Results
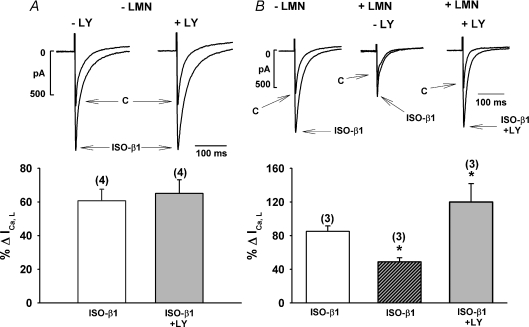
In Fig. 1 we determined the effects of ISO-β1-AR stimulation of ICa,L recorded from both −LMN and +LMN atrial myocytes in the absence and presence of LY294002 (LY), a specific inhibitor of PI-(3)K (Vlahos et al. 1994). The top traces in panels A and B show original recording of ICa,L and the bottom bar graphs summarize the percentage changes in ICa,L induced by ISO-β1-AR stimulation. In a −LMN atrial myocyte (A; upper), compared to control ICa,L (C), ISO-β1-AR stimulation elicited a typical increase in peak ICa,L amplitude. In another −LMN atrial myocyte, prior exposure to 10 μm LY had no discernable effect on ISO stimulation of ICa,L. The corresponding bar graph (below) summarizes the mean data and indicates that in −LMN atrial myocytes, ISO-β1-AR stimulation of ICa,L was unaffected by LY (ISO-β1-AR, 61 ± 7%; N= 4 versus ISO-β1-AR + LY, 65 ± 8%, N= 4). Figure 1B (upper) shows recordings from another −LMN atrial myocyte in which ISO-β1-AR stimulation elicited a typical increase in ICa,L. In a +LMN atrial myocyte from the same heart, ISO-β1-AR stimulation of ICa,L was markedly decreased compared to control (−LMN). This is consistent with our previous report that LMN down-regulates ISO-β1-AR stimulation of ICa,L (Wang et al. 2000b). In another +LMN atrial myocyte prior exposure to LY enhanced ISO-β1-AR stimulation of ICa,L. The bar graph (below) summarizes the mean data and indicates that compared with −LMN atrial myocytes (85 ± 6%; N= 3), ISO-β1-AR stimulation of ICa,L is significantly decreased in +LMN atrial myocytes (48 ± 5%; N= 3, P > 0.05), and that inhibition of PI-(3)K by LY enhances ISO-β1-AR stimulation of ICa,L in +LMN cells (120 ± 21%; N= 3, P < 0.05).
Figure 1. Effects of 10 μm LY294002 (LY) on β1-AR stimulation of ICa,L in −LMN and +LMN atrial myocytes.
A, original recordings of ICa,L (upper) showing the effects of ISO-β1-AR stimulation in the absence and presence of LY in two different −LMN atrial myocytes. Summary graph (lower) shows that ISO-β1-AR stimulation of ICa,L was not different in −LMN atrial myocytes in the absence (open bar) or presence (shaded bar) of LY. B, original recordings of ICa,L (upper) showing the effects of ISO-β1-AR stimulation of ICa,L in a −LMN atrial myocyte and +LMN atrial myocyte obtained from the same heart in the absence and presence of LY. Summary data (lower) shows that compared with −LMN atrial myocytes (open bar), ISO-β1-AR stimulation of ICa,L is significantly smaller in +LMN atrial myocytes (hatched bar). Moreover, in +LMN atrial myocytes, LY significantly increased ISO-β1-AR stimulation of ICa,L (shaded bar) compared to control +LMN atrial myocytes (hatched bar). Numbers in parentheses indicate the number of cells tested. *P < 0.05.
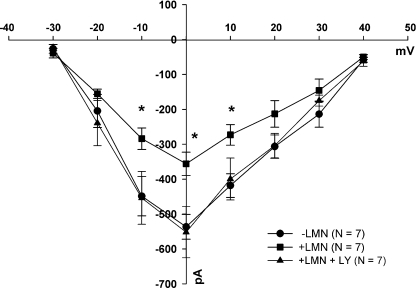
Figure 2 shows current–voltage relationships of ISO-β1-AR-stimulated ICa,L obtained from −LMN (•) and +LMN (▪) atrial myocytes. Compared with −LMN atrial myocytes, ISO-β1-AR stimulation of ICa,L was significantly decreased at −10, 0 and +10 mV in +LMN cells obtained from the same hearts. Prior exposure of +LMN cells to LY (▴) restored ISO-β1-AR stimulation of ICa,L to levels comparable to ISO-β1-AR stimulation of ICa,L in −LMN cells (•). Together, these findings indicate that in freshly isolated atrial myocytes not plated on LMN, PI-(3)K plays no apparent role in β1-AR-mediated stimulation of ICa,L. However, in atrial myocytes plated on LMN, PI-(3)K signalling inhibits β1-AR stimulation of ICa,L.
Figure 2. Current–voltage relationships of ISO-β1-AR-stimulated ICa,L in −LMN (•) and +LMN atrial myocytes in the absence (▪) and presence (▴) of 10 μm LY294002 (LY).
ISO-β1-AR stimulation of peak ICa,L was significantly smaller in +LMN atrial myocytes (▪) compared to −LMN atrial myocytes (•) at −10, 0 and +10 mV (*P < 0.05). In +LMN atrial myocytes previously exposed to LY (▴), ISO-β1-AR stimulation of peak ICa,L was enhanced and similar to that recorded from −LMN atrial myocytes (•). Numbers in parentheses indicate the number of cells tested.
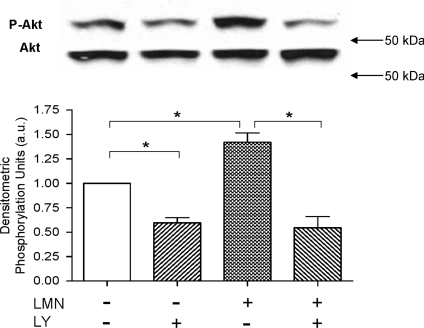
Protein kinase B (Akt) is a primary down-stream target of PI-(3)K (see Toker & Cantley, 1997). As shown in Fig. 3 we used Western blots to determine whether plating atrial myocytes on LMN stimulates Akt phosphorylation (Ser-473) and whether it is dependent on PI-(3)K signalling. Compared to control −LMN atrial myocytes, exposure to LY significantly decreased basal Akt phosphorylation. In +LMN atrial myocytes compared to −LMN cells, Akt phosphorylation was significantly increased and this effect was blocked by LY. The summary graph indicates that compared with freshly isolated (−LMN) atrial myocytes, atrial cells plated on LMN expressed a significant increase in Akt phosphorylation, which was blocked by LY-induced inhibition of PI-(3)K signalling (N= 3). Together, these findings indicate that LMN activates PI-(3)K-dependent Akt activity, and suggests a role for this signalling pathway in LMN-mediated down-regulation of β1-AR signalling (Fig. 1).
Figure 3. Western blots showing the effects of LMN on Akt phosphorylation in the absence and presence of 10 μm LY294002 (LY).
The two upper rows show original blots of phosphorylated Akt (P-Akt; Ser-473) and total Akt (Akt). In the corresponding graph (below), control Akt phosphorylation in −LMN atrial myocytes was normalized to 1 (open bar). In −LMN atrial myocytes, LY significantly decreased basal Akt phosphorylation. In +LMN atrial myocytes Akt phosphorylation was significantly increased compared to −LMN atrial myocytes (open bar). In +LMN atrial myocytes, prior exposure to LY significantly decreased Akt phosphorylation. These results indicate that LMN stimulates PI-(3)K-dependent Akt phosphorylation. *P < 0.05.
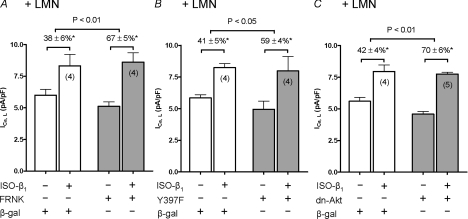
Integrin signalling activates FAK (see Samarel, 2005) and FAK is known to stimulate PI-(3)K/Akt signalling (Chen & Guan, 1994; Schaller, 2001). Therefore, to determine the role of FAK in β1-AR stimulation of ICa,L we infected +LMN atrial myocytes in overnight culture with two different adenoviruses (Adv-FRNK or Adv-Y397F-FAK) which act as dominant-negative inhibitors of FAK. Control cells were infected with Adv-β-galactosidase (β-gal). All cells were plated on laminin (+LMN) for overnight culture. Each cell served as its own control. As summarized in Fig. 4A and B, selective ISO-β1-AR stimulation significantly increased ICa,L in atrial myocytes infected with either Adv-β-gal (open bars), Adv-FRNK (shaded bars) or Adv-Y397F (shaded bars). However, as shown in Fig. 4A, compared with control cells infected with Adv-β-gal (38 ± 6%; N= 4), ISO-β1-AR stimulation of ICa,L was significantly greater in cells infected with Adv-FRNK (67 ± 5%, N= 4; P < 0.01). Similarly, Fig. 4B shows that compared with control cells infected with Adv-β-gal (41 ± 5%; N= 4), ISO-β1-AR stimulation of ICa,L was significantly greater in cells infected with Adv-Y397F (59 ± 4%, N= 4; P < 0.05). These findings suggest that LMN-mediated activation of FAK is a key step in the down-regulation of β1-AR signalling. Because Akt is a downstream target of PI-(3)K, we determined the role of Akt by infecting +LMN atrial myocytes with Adv-dn-Akt, a dominant-negative inhibitor of Akt. As summarized in Fig. 4C, ISO-β1-AR stimulation significantly increased ICa,L in atrial myocytes infected with either Adv-β-gal (open bars) or Adv-dn-Akt (shaded bars). However, compared with control cells infected with Adv-β-gal (42 ± 4%; N= 4), ISO-β1-AR stimulation of ICa,L was significantly greater in cells infected with Adv-dn-Akt (70 ± 6%; N= 5, P < 0.01). In conjunction with the findings presented in Figs 1, 2 and 3, these results suggest that LMN acts via FAK/PI-(3)K/Akt signalling to down-regulate β1-AR stimulation of ICa,L. Although control cells infected with either Adv-FRNK, Adv-Y397F or Adv-dn-Akt tended to exhibit smaller peak ICa,L densities compared with control cells infected with Adv-β-gal, the differences were not statistically significant. A small decrease in peak ICa,L induced by inhibition of PI-(3)K may be related to a report in mouse ventricular myocytes that PI-(3)K/Akt signalling directly stimulates ICa,L (Sun et al. 2006). However, this direct stimulatory effect of basal PI-(3)K signalling may be offset by the inhibitory effect of PI-(3)K to inhibit adenylate cyclase/cAMP-mediated stimulation of ICa,L.
Figure 4. The effects of FRNK, Y397F or dn-Akt adenoviral infection on ISO-β1-AR stimulation of ICa,L.
All experiments were performed on +LMN atrial myocytes incubated overnight. Each cell studied served as its own control. Control cells were infected with Adv-β-galactosidase (β-gal; open bars). Test cells were infected with either Adv-FRNK (A), Adv-Y397F (B) or Adv-dn-Akt (C) (shaded bars). Control and test cells in each panel were obtained from the same hearts. ISO-β1-AR stimulation elicited a significant increase in ICa,L in all cell groups studied (*P < 0.05). A, ISO-β1-AR stimulation of ICa,L was significantly greater in cells infected with Adv-FRNK (67 ± 5%) compared to control cells (38 ± 6%; P < 0.01). B, ISO-β1-AR stimulation of ICa,L was significantly greater in cells infected with Adv-Y397F (59 ± 4%) compared to control cells (41 ± 5%; P < 0.05). C, ISO-β1-AR stimulation of ICa,L was significantly greater in cells infected with Adv-dn-Akt (70 ± 6%) compared to control cells (42 ± 4%; P < 0.01). Numbers in parentheses indicate the number of cells studied.
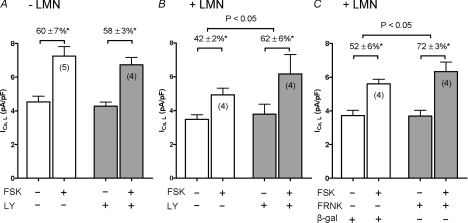
We previously reported that plating atrial myocytes on LMN decreases forskolin (FSK)- and IBMX-mediated stimulation of ICa,L (Wang et al. 2000a) as well as basal, FSK- and IBMX-stimulated cAMP production (Wang et al. 2000a). These findings led to the conclusion that LMN down-regulates adenylate cyclase activity. PI-(3)K signalling also inhibits adenylate cyclase activity (Leblais et al. 2004; Kerfant et al. 2004; Alloatti et al. 2005; Dedkova et al. 2007). Therefore, to determine whether FAK/PI-(3)K signalling is inhibiting adenylate cyclase activity we stimulated ICa,L with 1 μm FSK in −LMN and +LMN atrial myocytes in the absence and presence of LY. As summarized in Fig. 5A, in −LMN atrial myocytes FSK-induced stimulation of ICa,L was not significantly different in the absence (open bars) or presence (shaded bars) of LY (FSK, 60 ± 7%versus FSK + LY, 57 ± 3%). As shown in Fig. 5B, FSK-mediated stimulation of ICa,L was smaller in +LMN atrial myocytes (42 ± 2%; open bars) compared to −LMN atrial myocytes (60 ± 7%; Fig. 5A, open bars). This is similar to our previous report (Wang et al. 2000a). However, in +LMN atrial myocytes (Fig. 5B) FSK-mediated stimulation of ICa,L was significantly enhanced by LY (FSK, 42 ± 2%; (open bars) versus FSK + LY, 62 ± 6%; (shaded bars)) (P < 0.05). Additional experiments (Fig. 5C) showed that FSK-mediated stimulation of ICa,L in +LMN atrial myocytes infected with Adv-FRNK (72 ± 3%; shaded bars) was significantly greater than in control +LMN atrial myocytes infected with Adv-β-gal (52 ± 6%; open bars) (P < 0.05). These results indicate that FSK-mediated stimulation of ICa,L responded similarly to ISO-β1-AR stimulation of ICa,L (Figs 1, 2 and 4) and therefore suggests that LMN acts via FAK/PI-(3)K/Akt to down-regulate β1-AR signalling via inhibition of adenylate cyclase.
Figure 5. The effects of 10 μm LY294002 (LY) or FRNK adenovirus on forskolin (FSK)-mediated stimulation of ICa,L.
Each cell studied served as its own control. Control (open bars) and test (shaded bars) cells in each panel were obtained from the same hearts. FSK (1 μm) elicited a significant increase in ICa,L in all cell groups studied (*P < 0.05). A, in −LMN atrial myocytes, FSK-induced stimulation of ICa,L was not different in the absence (60 ± 7%) or presence (58 ± 3%) of LY. B, in +LMN atrial myocytes, FSK-induced stimulation of ICa,L was significantly greater in the presence (62 ± 6%) than in the absence (42 ± 2%) of LY (P < 0.05). C, control cells were infected with Adv-β-galactosidase (β-gal; open bars) and test cells were infected with Adv-FRNK (FRNK; shaded bars). FSK-induced stimulation of ICa,L was significantly greater in cells infected with Adv-FRNK (72 ± 3%) compared to control (52 ± 6%; P < 0.05). Numbers in parentheses indicate the number of cells studied.
Discussion
In two separate studies, we previously reported that in atrial myocytes LMN binding to β1-integrin receptors decreases adenylate cyclase activity (Wang et al. 2000a) and down-regulates β1-AR stimulation of ICa,L (Wang et al. 2000b). The present study sought to determine whether there is a cause and effect relationship between these two findings and to gain insight into the signalling mechanisms. The new findings of this study are that LMN acts via FAK/PI-(3)K/Akt signalling to inhibit adenylate cyclase and thereby down-regulate β1-AR stimulation of ICa,L.
The present work confirms our previous findings that compared with −LMN atrial myocytes, +LMN atrial myocytes exhibit depressed β1-AR (Wang et al. 2000b) and FSK-mediated stimulation of ICa,L (Wang et al. 2000a). The fact that these changes were absent in cells plated on poly l-lysine, a non-specific substrate for cell attachment, indicates a mechanism of action specific to LMN. Moreover, the effects of LMN are mediated via stimulation of β1-integrin receptors (Wang et al. 2000a,b). Intracellularly, β1-integrins are associated with a variety of catalytic and structural proteins that comprise the costamere or focal adhesion complex. FAK, a non-receptor protein tyrosine kinase (PTK), is a primary downstream target of β1-integrin receptor stimulation (see Samarel, 2005). The present results show that in +LMN atrial myocytes inhibition of FAK by over-expression of non-phosphorylatable, dominant-negative mutants of FAK (Adv-FRNK or Adv-Y397F) enhanced β1-AR stimulation of ICa,L compared with control +LMN atrial myocytes infected with Adv-β-gal. In other words, inhibition of FAK signalling prevented the down-regulation of β1-AR signalling mediated by LMN/β1-integrin receptor stimulation. Therefore, FAK-mediated signalling is a key component involved in LMN-mediated down-regulation of β1-AR signalling.
Different PI-(3)K isoforms are activated via different signalling pathways. The PI-(3)K α, β and δ isoforms are activated by protein tyrosine kinases (PTKs) (see Crackower et al. 2002), such as FAK, and the PI-(3)Kγ isoform is activated via G-protein-coupled receptor stimulation. FAK (and its related kinase PYK2) can bind the p85 subunit of PI-(3)K (Chen & Guan, 1994; Schaller, 2001) and thereby activate downstream signalling to Akt. Indeed, the present experiments show that +LMN atrial myocytes exhibit an increase in PI-(3)K-dependent Akt phosphorylation. In addition, inhibition of PI-(3)K by LY294002 or over-expression of a dominant-negative mutant Akt each prevented LMN-mediated down-regulation of β1-AR signalling. On the other hand, LY294002 had no effect on selective β1-AR stimulation of ICa,L in −LMN cells, suggesting that PI-(3)K/Akt signalling plays no apparent role in β1-AR signalling in freshly isolated atrial myocytes. We therefore conclude that PI-(3)K/Akt signalling is an essential pathway underlying LMN-mediated down-regulation of β1-AR signalling.
Our previous work indicates that LMN inhibits adenylate cyclase activity (Wang et al. 2000a). The present work extends these findings by demonstrating that LMN acts via FAK/PI-(3)K/Akt signalling to inhibit adenylate cyclase-mediated stimulation of ICa,L. Indeed, in +LMN atrial myocytes FSK-mediated stimulation of ICa,L was enhanced by inhibition of PI-(3)K (LY294002) or inhibition of FAK (Adv-FRNK) signalling. These findings are similar to those obtained with β1-AR-mediated stimulation of ICa,L and therefore support the idea that the effects of LMN to down-regulate β1-AR signalling are mediated via down-regulation of adenylate cyclase. Figure 6 is a simplified schematic diagram of the present findings showing that LMN acts via FAK/PI-(3)K/Akt signalling to inhibit adenylate cyclase activity, resulting in the down-regulation of β1-AR-mediated stimulation of ICa,L. Our previous work also showed that direct stimulation of ICa,L by intracellular cAMP or a cAMP analogue is unaffected by LMN (Wang et al. 2000a), indicating that LMN does not alter the calcium channel response to cAMP or modulate phosphatases that regulate phosphorylation of ICa,L. Results obtained from PI-(3)Kγ knockout mice indicate that PI-(3)Kγ is an essential component of a complex controlling PDE3-mediated degradation of cAMP (Patrucco et al. 2004). Although a potential role for PDE-mediated regulation cannot be excluded by the present experiments, it seems unlikely based on our previous findings that stimulation of ICa,L through inhibition of all PDE activities by high doses of IBMX or inhibition of PDE type 3 by milrinone were significantly attenuated by LMN (Wang et al. 2000a). Rather, these findings are consistent with LMN-mediated inhibition of endogenous adenylate cyclase activity.
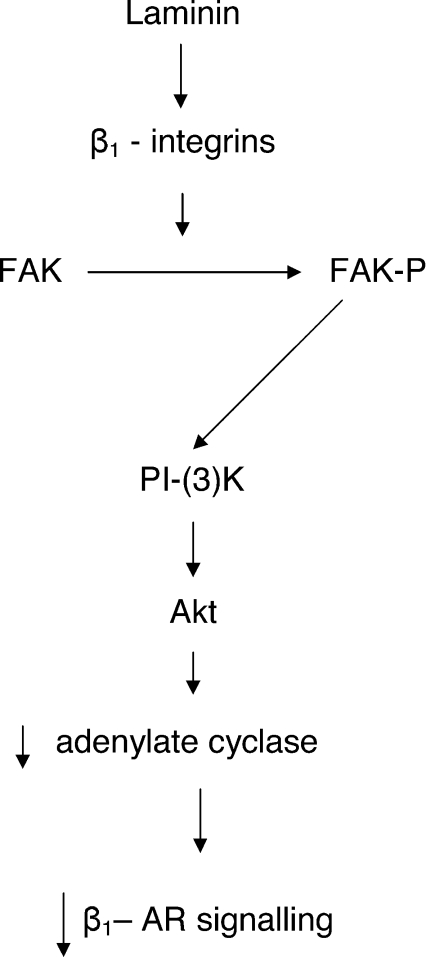
Figure 6. Schematic diagram of proposed signalling mechanisms underlying LMN-mediated down-regulation of β1-AR signalling in atrial myocytes.
Attachment of atrial myocytes to LMN stimulates β1-integrin receptors which in turn leads to focal adhesion kinase (FAK) autophosphorylation. Binding of FAK to the regulatory subunit of PI-(3)K leads to PI-(3)K activation and subsequent downstream activation of Akt. PI-(3)K/Akt activation down-regulates adenylate cyclase and subsequent β1-AR signalling.
PI-(3)K signalling is also thought to be involved in agonist-induced β-AR internalization where β-AR kinase 1 (βARK1) mediates the translocation of PI-(3)K to β-ARs (Naga Prasad et al. 2001, 2002; Nienaber et al. 2003). Naga Prasad et al. (2001) reported that PI-(3)Kγ signalling mediates β2-AR internalization. On the other hand, Oudit et al. (2003) reported that β1- and β2-AR receptor densities were decreased by similar amounts in PI-(3)Kγ-deficient and control mice, suggesting that the PI-(3)Kγ isoform plays no role in the down-regulation of β-ARs in response to chronic β-AR stimulation. In the present study, β-AR internalization was not the focus of the experiments and therefore cannot be excluded as a potential contributing mechanism. However, several experimental findings make it unlikely that β-AR internalization plays a significant role in the present results. First, our previous work indicates that LMN actually increases β2-AR signalling in atrial myocytes (Wang et al. 2000b). In addition, our present and previous findings (Wang et al. 2000a) indicate that LMN down-regulates the effects of FSK, IBMX or milrinone to stimulate ICa,L, and decreases FSK- or IBMX-mediated stimulation of cAMP (Wang et al. 2000a), each of which bypasses β1-AR activation.
We also reported (Wang et al. 2000a,b) that LMN-mediated down-regulation of β1-AR signalling and adenylate cyclase are prevented by agents that inhibit actin polymerization. Studies in several cell systems indicate that PI-(3)K/Akt signalling can alter actin cytoskeletal architecture (Toker & Cantley, 1997; Cenni et al. 2003; Yin & Janmey, 2003). In fact, Akt can interact with actin directly both in vitro and in vivo, and Akt phosphorylation is required for Akt to associate with actin (Cenni et al. 2003). Findings in a variety of cell systems also indicate a close association between cytoskeletal components and adenylate cyclase activity (Zor, 1983; Rasenick & Wang, 1988; Jasper et al. 1995). Inhibition of actin polymerization can also interfere with FAK phosphorylation (Heidkamp et al. 2002) which according to the present findings and our previous results (Wang et al. 2000b) would be expected to prevent LMN-mediated down-regulation of β1-AR signalling. It therefore appears that the architecture of the actin cytoskeleton may be potentially involved at multiple levels of the signalling cascade.
In the present study inhibition of PI-(3)K signalling by LY only affected β1-AR- or FSK-mediated stimulation of ICa,L in +LMN but not in −LMN atrial myocytes. In other words, PI-(3)K signalling does not appear to be involved in β1-AR/adenylate cyclase signalling in freshly isolated atrial myocytes but only in those cells in which the PI-(3)K/Akt signalling pathway has been previously activated, in this case by LMN-mediated stimulation of β1-integrin receptors. These findings are also consistent with those obtained in ventricular myocytes isolated from murine hearts deficient in PI-(3)Kγ (Kerfant et al. 2004), where there were no changes in ICa,L or ISO-induced stimulation of ICa,L or Ca2+ transients (Kerfant et al. 2004). On the other hand, in adult rat ventricular myocytes inhibition of PI-(3)K by LY enhanced β1-AR/Gs-mediated stimulation of ICa,L, Ca2+ transients and contraction (Leblais et al. 2004). PI-(3)Kγ is thought to be the isoform that locally controls cAMP levels (see Kerfant et al. 2006) whereas PI-(3)Kα is the isoform activated via FAK binding to the p85 subunit of PI-(3)K. Given the present findings that LMN acts via FAK signalling to inhibit adenylate cyclase, it may be that LMN activates both PI-3K isoforms, i.e. PI-(3)Kα through FAK signalling and PI-(3)Kγ through an as yet undetermined signalling pathway. For example, FAK could act as a scaffolding protein for the p101 regulatory subunit of PI-(3)Kγ in an analogous fashion to the p85 subunit of PI-(3)Kα. Further experiments will be required to clarify this issue.
The LMN-mediated down-regulation of β1-AR signalling in atrial myocytes shows similarities to the remodelling of β-AR function typically seen in the failing heart. In general, the human failing heart exhibits a selective reduction in β1-AR signalling (Bristow et al. 1986; Ungerer et al. 1987) and depression in adenylate cyclase activity (Bristow et al. 1988). Similar results are obtained experimentally in the canine rapid pacing-induced model of heart failure (Marzo et al. 1991; Ishikawa et al. 1994). The present results suggest that the changes in β1-AR signalling and adenylate cyclase seen in heart failure may be mediated, at least in part, via stimulation of integrin/FAK/PI-(3)K/Akt signalling due to enhanced formation of extracellular matrix proteins. However, in the canine pacing-induced model of heart failure, structural remodelling is less evident in left ventricular muscle (Hanna et al. 2004). This suggests that additional factors may operate to remodel ventricular β1-AR signalling. For example, mechanical forces such as stretch (Vila Petroff et al. 2001) or contraction (Dedkova et al. 2007) also may act via integrin-mediated stimulation of FAK/PI-(3)K/Akt signalling.
Acknowledgments
We thank Ms Rekha Iyengar for her technical assistance with the adenoviral experiments. This work was supported by NIH grants HL079038 (S.L.L.) and HL34328 (A.M.S.).
References
- Alloatti G, Marcantoni A, Levi R, Gallo MP, Del Sorbo L, Patrucco E, Barberis L, Malan D, Assolino O, Wymann M, Hirsch E, Montrucchio G. Phosphoinositide 3-kinase γ controls autonomic regulation of the mouse heart through Gi-independent downregulation of cAMP level. FEBS Lett. 2005;579:133–140. doi: 10.1016/j.febslet.2004.11.059. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Ginsberg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S, Stinson EB. β1- and β2-adrenergic-receptor subpopulation in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective β1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Hershberg RE, Port JD, Minobe W, Rasmussen R. β1- and β2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Mol Pharmacol. 1988;35:295–303. [PubMed] [Google Scholar]
- Cenni V, Sirri A, Riccio M, Lattanzi G, Santi ADP, Maraldi NM, Marimiroli S. Targeting of the Akt/PKB kinase to the actin skeleton. Cell Mol Life Sci. 2003;60:2710–2720. doi: 10.1007/s00018-003-3349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-C, Guan J-L. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 1994;91:10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Ross RS, Walsh KB. Overexpression of the integrin β1A subunit and the β1A cytoplasmic domain modifies the β-adrenergic regulation of the cardiac L-type Ca2+ current. J Mol Cell Cardiol. 2004;36:795–798. doi: 10.1016/j.yjmcc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Crackower MA, Oudit GY, Kozieradzki RB, Sarao R, Sun H, Sasaki T, et al. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110:737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Dedhar S. Integrins and signal transduction. Curr Opin Hematol. 1999;6:37–43. doi: 10.1097/00062752-199901000-00007. [DOI] [PubMed] [Google Scholar]
- Dedkova EN, Ji X, Wang YG, Blatter LA, Lipsius SL. Signaling mechanisms that mediate nitric oxide production induced by acetylcholine exposure and withdrawal in cat atrial myocytes. Circ Res. 2003;93:1233–1240. doi: 10.1161/01.RES.0000106133.92737.27. [DOI] [PubMed] [Google Scholar]
- Dedkova EN, Wang YG, Ji X, Blatter LA, Samarel AM, Lipsius SL. Signalling mechanisms in contraction-mediated stimulation of intracellular NO production in cat ventricular myocytes. J Physiol. 2007;580:327–345. doi: 10.1113/jphysiol.2006.126805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna N, Cardin S, Leung T, Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res. 2004;63:236–244. doi: 10.1016/j.cardiores.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Heidkamp MC, Bayer AL, Kalina JA, Eble DM, Samarel AM. GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes. Circ Res. 2002;90:1282–1289. doi: 10.1161/01.res.0000023201.41774.ea. [DOI] [PubMed] [Google Scholar]
- Heidkamp MC, Bayer AL, Martin JL, Samarel AM. Differential activation of mitogen-activated protein kinase cascades and apoptosis by protein kinase C ε and δ in neonatal rat ventricular myocytes. Circ Res. 2001;89:882–890. doi: 10.1161/hh2201.099434. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Sorota S, Kiuchi K, Shannon RP, Komamura K, Katsushika S, Vatner DE, Vatner SF, Homcy CJ. Downregulation of adenylyl-cyclase types V and VI mRNA levels in pacing-induced heart failure in dogs. J Clin Invest. 1994;93:2224–2229. doi: 10.1172/JCI117219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper JR, Post SR, Desai KH, Insel PA, Bernstein D. Colchicine and cytochalasin B enhance cyclic AMP accumulation via postreceptor actions. J Pharmacol Exp Ther. 1995;274:937–942. [PubMed] [Google Scholar]
- Kerfant B-G, Gidrewicz D, Sun H, Oudit GY, Penninger JM, Backx PH. Cardiac sarcoplasmic reticulum calcium release and load are enhanced by subcellular cAMP elevations in PI3Kγ-deficient mice. Circ Res. 2004;96:1079–1086. doi: 10.1161/01.RES.0000168066.06333.df. [DOI] [PubMed] [Google Scholar]
- Kerfant B-G, Rose RA, Sun H, Backx PH. Phosphoinositide 3-kinase γ regulates cardiac contractility by locally controlling cyclic adenosine monophosphate levels. Trends Cardiovasc Med. 2006;16:250–256. doi: 10.1016/j.tcm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Kiuchi K, Shannon RP, Komamura K, Cohen DJ, Bianchi C, Homcy CJ, Vatner SF, Vatner DE. Myocardial β-adrenergic receptor function during the development of pacing-induced heart failure. J Clin Invest. 1993;91:907–914. doi: 10.1172/JCI116312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblais V, Jo S-H, Chakir K, Maltsev V, Zheng M, Crow MT, Wang W, Lakatta EG, Xiao R-P. Phosphatidylinositol 3-kinase offsets cAMP-mediated positive inotropic effect via inhibiting Ca2+ influx in cardiomyocytes. Circ Res. 2004;95:1183–1190. doi: 10.1161/01.RES.0000150049.74539.8a. [DOI] [PubMed] [Google Scholar]
- Lipsius S, Wang YG, Ji X, Szotek E, Samarel AM. Laminin downregulates β1-adrenergic receptor stimulation of L-type Ca2+ current via PI-(3)K/Akt signaling in cat atrial myocytes. FASEB J. 2006;20:A1323–A1324. [Google Scholar]
- Marzo KP, Frey MJ, Wilson JR, Liang BT, Manning DR, Lanoce V, Molinoff PB. β-Adrenergic receptor–G protein-adenylate cyclase complex in experimental canine congestive heart failure produced by rapid ventricular pacing. Circ Res. 1991;69:1546–1556. doi: 10.1161/01.res.69.6.1546. [DOI] [PubMed] [Google Scholar]
- Naga Prasad SV, Barak LS, Rapacciuolo A, Caron MG, Rockman HA. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by β-adrenergic receptor kinase 1. A role in receptor sequestration. J Biol Chem. 2001;276:18953–18959. doi: 10.1074/jbc.M102376200. [DOI] [PubMed] [Google Scholar]
- Naga Prasad SV, Laporte SA, Chamberlain D, Caron MG, Barak L, Rockman HA. Phosphoinositide 3-kinase regulates β2-adrenergic receptor endocytosis by AP-2 recruitment to the receptor/β-arrestin complex. J Cell Biol. 2002;158:563–575. doi: 10.1083/jcb.200202113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienaber JJ, Tachibana H, Naga Prasad SV, Esposito G, Wu D, Mao L, Rockman HA. Inhibition of receptor-localized PI3K preserves cardiac β-adrenergic receptor function and ameliorates pressure overload heart failure. J Clin Invest. 2003;112:1067–1079. doi: 10.1172/JCI18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit GY, Crackower MA, Eriksson U, Sarao R, Kozieradzki I, Sasaki T, Irie-Sasaki J, Gidrewicz D, Rybin VO, Wada T, Steinberg SF, Backx PH, Penninger JM. Phosphoinositide K-kinase γ-deficient mice are protected from isoproterenol-induced heart failure. Circulation. 2003;108:2147–2152. doi: 10.1161/01.CIR.0000091403.62293.2B. [DOI] [PubMed] [Google Scholar]
- Patrucco E, Notte A, Barberis L, Selvetella G, Maffel A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, Silengo L, Altruda F, Wetzker R, Wymann MP, Lembo G, Hirsch E. PI3Kγ modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 2004;118:375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Rasenick MM, Wang N. Exchange of guanine nucleotides between tubulin and GTP-binding proteins that regulate adenylate cyclase: cytoskeletal modification of neuronal signal transduction. J Neurochem. 1988;51:300–311. doi: 10.1111/j.1471-4159.1988.tb04870.x. [DOI] [PubMed] [Google Scholar]
- Sakurai S, Sonoda Y, Koguchi E, Shinoura N, Hamada H, Kasahara T. Mutated focal adhesion kinase induces apoptosis in a human glioma cell line, T98G. Biochem Biophys Res Commun. 2002;293:174–181. doi: 10.1016/S0006-291X(02)00192-4. [DOI] [PubMed] [Google Scholar]
- Samarel AM. Costameres, focal adhesions and cardiomyocyte mechanotransduction. Am J Physiol Heart Circ Physiol. 2005;289:H2291–H2301. doi: 10.1152/ajpheart.00749.2005. [DOI] [PubMed] [Google Scholar]
- Schaller MD. Biochemical signaling and biological responses elicited by the focal adhesion kinase. Biochem Biophys Res Commun. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Sun H, Kerfant B-G, Zhao D, Trivieri MG, Oudit GY, Penninger JM, Backx PH. Insulin-like growth factor-1 and PTEN deletion enhances cardiac L-type Ca2+ currents via increased PI3Kα/PKB signaling. Circ Res. 2006;98:1390–1397. doi: 10.1161/01.RES.0000223321.34482.8c. [DOI] [PubMed] [Google Scholar]
- Terracio L, Rubin K, Gullberg D, Balog E, Carver W, Jyring R, Borg TK. Expression of collagen binding integrins during cardiac development and hypertrophy. Circ Res. 1991;68:734–744. doi: 10.1161/01.res.68.3.734. [DOI] [PubMed] [Google Scholar]
- Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- Ungerer M, Böhm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of β-adrenergic receptor kinase and β1-adrenergic receptors in the failing human heart. Circulation. 1987;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- Vila Petroff MG, Kim SH, Pepe S, Dessy C, Marban E, Balligand J-L, Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Wang YG, Samarel AM, Lipsius SL. Laminin acts via β1 integrin signalling to alter cholinergic regulation of L-type Ca2+ current in cat atrial myocytes. J Physiol. 2000a;526:57–68. doi: 10.1111/j.1469-7793.2000.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YG, Samarel AM, Lipsius SL. Laminin binding to β1-integrins selectively alters β1- and β2-adrenoceptor signalling in cat atrial myocytes. J Physiol. 2000b;527:3–9. doi: 10.1111/j.1469-7793.2000.t01-2-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Vereecke J, Carmeliet E, Lipsius SL. Ionic currents activated during hyperpolarization of single right atrial myocytes from cat heart. Circ Res. 1991;68:1059–1069. doi: 10.1161/01.res.68.4.1059. [DOI] [PubMed] [Google Scholar]
- Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- Zor U. Role of cytoskeletal organization in the regulation of adenylate cyclase-cyclic adenosine monophosphate by hormones. Endocr Rev. 1983;4:1–21. doi: 10.1210/edrv-4-1-1. [DOI] [PubMed] [Google Scholar]