Summary
The small heat shock protein Hsp27 has been shown to be involved in a diverse array of cellular processes, including cellular stress response, protein chaperone activity, regulation of cellular glutathione levels, apoptotic signaling, and regulation of actin polymerization and stability. Furthermore, mutation within Hsp27 has been associated with the human congenital neuropathy Charcot-Marie Tooth (CMT) disease. Hsp27 is known to be expressed in developing embryonic tissues; however, little has been done to determine the endogenous requirement for Hsp27 in developing embryos. In this study, we show that depletion of XHSP27 protein results in a failure of cardiac progenitor fusion resulting in cardia bifida. Furthermore, we demonstrate a concomitant disorganization of actin filament organization and defects in myofibril assembly. Moreover, these defects are not associated with alterations in specification or differentiation. We have thus demonstrated a critical requirement for XHSP27 in developing cardiac and skeletal muscle tissues.
Keywords: HSP27, HSP, actin, muscle, myogenesis, cardiogenesis, cardiac, heart, development, Xenopus
INTRODUCTION
Formation of a functioning heart requires the organization and synchronization of many cellular and tissue-level processes, including proper cell movements and polarity establishment, specification and differentiation of cardiac precursors, and morphogenesis of the heart. During gastrulation, a bilaterally symmetric pair of fields within the anterior lateral plate mesoderm are initially specified as cardiac precursors. Once the cardiac precursor fields are specified, they undergo two separate migration events as neurulation proceeds. The large-scale tissue movements involved in neurulation result in the anterior movement of the cardiac progenitors toward more anterior regions. This process seems to be largely controlled by FGF family members, including FGF8 and FGF4 (Beiman et al., 1996; Ciruna and Rossant, 1999; Gisselbrecht et al., 1996; Sun et al., 1999) as well as G-coupled proteins (Quertermous, 2007; Scott et al., 2007; Zeng et al., 2007). Soon after migration to more anterior positions in the embryo, the cardiac progenitors migrate ventrally as an epithelial sheet towards the anterior ventral midline where they proceed to fuse, proliferate and form a linear heart tube (DeHaan, 1963; Goetz and Conlon, 2007; Kolker et al., 2000; Mohun et al., 2003; Trinh and Stainier, 2004).
Several requirements for the ventral migration and fusion of the cardiac fields have thus far been identified, including proper cardiomyocyte differentiation (Reiter et al., 1999; Yelon et al., 2000), interaction with or signaling from the endoderm (Alexander et al., 1999; Kikuchi et al., 2000; Reiter et al., 1999; Schier et al., 1997), epithelial organization of the cardiac fields, and migration cues from the midline (Trinh and Stainier, 2004). Recent work by Trihn and Stainier (2004) has demonstrated a requirement for fibronectin (Fn) in the migrating cardiac precursor fields in zebrafish; fish mutant for Fn exhibit cardia bifida, which is characterized by unfused cardiac progenitors that independently differentiate into cardiac tissue (Trinh and Stainier, 2004). Their results indicate that Fn at the junction between the endoderm and mesoderm is required for epithelial integrity within the cardiac fields. Furthermore, deposition of Fn at the ventral midline regulates the timing of migration. Other studies have shown that mutant mice lacking Fn display defects in cardiogenesis, despite normal specification of the cardiac precursors (George et al., 1993, 1997). It is also well established that the anterior endoderm is required for cardiac progenitor migration (reviewed in Lough and Sugi, 2000). For example, many genes involved in endoderm differentiation and maturation, including Gata4, Gata5, one-eyed pinhead (oep), casanova, and miles apart result in cardia bifida when mutated in mice or fish (Kuo et al., 1997; Kupperman et al., 2000; Reiter et al., 1999; Stainier, 2001). Furthermore, studies have shown that abrogation of proper myocardial differentiation can also result in cardia bifida (Reiter et al., 1999; Yelon et al., 2000). In the present study, we report a requirement for the small heat shock protein, Hsp27, in proper cardiac fusion.
Hsp27, also called Hsp25 in mice and HspB1 in humans, is one of the most widely distributed and most studied of the small heat shock proteins (sHSPs; reviewed in Ferns et al., 2006). Changes in Hsp27 expression have been observed in cells and tissues exposed to numerous stress conditions, including oxidative damage (Arrigo, 2001; Baek et al., 2000; Dalle-Donne et al., 2001; Escobedo et al., 2004; Huot et al., 1996; Komatsuda et al., 1999; Mehlen et al., 1995), metal toxicity (Bonham et al., 2003; Leal et al., 2002; Somji et al., 1999), and ischemia (Hollander et al., 2004; Reynolds and Allen, 2003; Shelden et al., 2002; Vander Heide, 2002), as well as in disease states such as cardiac hypertrophy (Knowlton et al., 1998; Scheler et al., 1999), and muscle myopathies (Benndorf and Welsh, 2004). In addition, a role for HSP27 function has been implicated in cellular processes, including protein chaperone activity (Jakob et al., 1993), regulation of cellular glutathione levels (Arrigo, 2001; Baek et al., 2000), apoptotic signaling (Bruey et al., 2000; Paul et al., 2002), inhibition of actin polymerization (Benndorf et al., 1994; Miron et al., 1991; Rahman et al., 1995), and stabilization of actin filament arrays (Huot et al., 1996; Lavoie et al., 1993a,b, 1995). Furthermore, mutation within Hsp27 has been associated with the human congenital disorder Charcot-Marie-Tooth disease (CMT; Evgrafov et al., 2004). CMT is a progressive neuropathy of the peripheral nervous system, and is the single most-common inherited neuropathy at an estimated prevalence of 1 in 2,500 (Skre, 1974).
Hsp27 is known to be expressed during both skeletal and cardiac muscle development in several organisms, including human (Shama et al., 1999), mouse (Gernold et al., 1993), pig (David et al., 2000), and zebrafish (Mao et al., 2005; Mao and Shelden, 2006). A role for Hsp27 in heart development has been implied from studies showing that over-expression of Hsp27 can protect mice from induced immunoblotted heart failure (Brundel et al., 2006; Liu et al., 2007). However, the precise requirement for Hsp27 during normal cardiac development is not known. Here we report the sequence and expression of the Xenopus laevis orthologue of heat shock protein 27 (XHsp27). We demonstrate using antisense morpholinos that XHSP27 is required for proper fusion of cardiac precursors and for actin organization in developing cardiac and skeletal muscle. We further demonstrate that cardiac specification and differentiation appear unaltered as assayed by several markers of cardiac precursor and differentiated cardiomyocyte populations.
MATERIALS AND METHODS
Embryo Culture and Injections
Preparation and injection of X. laevis embryos was carried out as previously described (Wilson and Hemmati-Brivanlou, 1995). Embryos were staged according to Nieuwkoop and Faber (1967). An antisense morpholino oligonucleotide was designed against the translation start site of XHsp27. XHSP27 morpholinos were obtained from Gene Tools, LLC with the following sequence: 5′AAT TCT GCG TTC TGA CAT TTT CTC T 3′. The human β-globin standard control morpholino from Gene Tools was used as control. For the in vitro translation studies, TBX20MO was used to show specificity of the HSP27MO (Brown et al., 2005). HSP27MO was injected at 60 ng/embryo.
Translation Inhibition by Morpholinos
In vitro translations were performed using TNT Coupled Reticulocyte Lysate System (Promega, Madison, WI) following the manufacturer’s protocol. Reactions were carried out in the presence or absence of HSP27MO or TBX20MO. A carboxy-terminal hemagluttin tagged version of HSP27 was generated using the pSP64T-HA vector (generous gift of Masazuma Tada). An HA-tagged TBX20 was also used in the in vitro translation study (Brown et al., 2005). We have recently demonstrated that X. laevis SHP2 is uniformly expressed throughout early development (Brown et al., 2005) and anti-PTP1D/SHP2 primary antibody was used at 1:2,500 (Transduction Laboratories) as a loading control with peroxidase-conjugated AffiniPure donkey anti-mouse (H + L) 2° antibody (1:10,000). HA-tagged proteins were probed with anti-HA primary antibody (Covance, Berkeley, CA) at 1:1,000 dilution, and peroxidase-conjugated AffiniPure Donkey anti-mouse (H+L) secondary antibody (Jackson ImmunoResearch Laboratories) at 1:10,000 dilution. For in vivo translation analyses, embryos were injected with MOs and mRNA at the one-cell stage. At stage 19, 40 embryos per treatment were collected and lysed in 300 μl of lysis buffer: 140 mM NaCl, 50 mM Tris (pH 7.6), 10 mM EDTA, 1% Surfact-Amps Triton X-100 (Pierce, Rockford, IL), Complete EDTA-free Protease Inhibitor (Roche, Nutley, NJ), and 25 mM PMSF (Roche). Lysates were resolved on 12% SDS-PAGE gels, immunoblotted, and visualization was carried out using luminol-based chemiluminescence solutions at 1:1 ratio: solution A: 100 mM Tris pH 8.5, 2.5 mM Luminol (Sigma, St. Louis, MO), 0.4 mM p-Coumaric acid (Sigma); solution B: 100 mM Tris pH 8.5, 0.02% H2O2 (Sigma).
Whole-Mount In Situ Hybridization
Whole-mount in situ hybridization was performed as previously described (Harland, 1991). Probes used included Tbx20 (Brown et al., 2003), Nkx2.5 (Brown et al., 2005), Gata4 and Gata6 (generous gifts of Roger Patient; (Jiang and Evans, 1996)), Mlc1v′ (IMAGE clone 4408657, GenBank Accession No.: BG884964), and Titin Novex 3 (Brown et al., 2006).
Immunodetection
Embryos were prepared for whole-mount immunohistochemistry as previously described (Kolker et al., 2000). Briefly, fixed embryos were incubated overnight at 4°C with an antibody against myosin heavy chain α (MHC) (Abcam, Cambridge, MA), at a dilution of 1:500. Following washes, the embryos were incubated overnight at 4°C with a Cy3-conjugated anti-mouse secondary antibody (Sigma) at a dilution of 1:100. For imaging, embryos were cleared with 2:1 benzyl benzoate: benzyl alcohol and viewed on a Leica MZFLIII fluorescence dissecting microscope. For immunostaining of histological sections, embryos were collected at the indicated stages, fixed for 2 h in 4% paraformaldehyde, and embedded in OCT cryosectioning medium (Tissue Tek, Tokyo, Japan). Cryostat sections (14 μm) were rinsed with wash buffer (PBS with 1% Triton and 1% heat inactivated calf serum) and incubated at 4°C with phalloidin overnight (conjugated to Alexa 488 flourophore) (Molecular Probes, Carlsbad, CA) and either anti-MHC (1:500) or anti-tropomyosin (1:50). Sections were then rinsed with wash buffer and incubated with anti-mouse Cy3-conjugated secondary antibody (1:200; Sigma). Sections were rinsed and incubated for 20 min at room temperature with DAPI, cover slipped and visualized on either a Zeiss LSM410 confocal microscope or a Nikon Eclipse E800 flourescence microscope.
For western blot analysis, stage 29 and 33 embryos (N = 10) per stage were collected, lysed, and sonicated in 100 μl of lysis buffer used in the in vivo morpholino studies previously. Ten micrograms of each lysate was run on a 4%–12% SDS-PAGE gel and transferred. Western blots were probed with antibodies against actin (Santa Cruz, Santa Cruz, CA) and α-tubulin (Abcam) at dilutions of 1:100 and 1:1,000, respectively, in 5% nonfat milk in TBS-T. Actin was detected using peroxidase-conjugated AffiniPure donkey anti-mouse (H + L) secondary antibody (Jackson ImmunoResearch Laboratories). α-tubulin was detected using peroxidase-conjugated AffiniPure donkey anti-rabbit (H + L) secondary antibody (Jackson ImmunoResearch Laboratories). Both secondary antibodies were used at 1:10,000 dilution. Visualization was carried out using Super Signal West Pico Chemiluminescent Substrate (Pierce).
Transmission Electron Microscopy
Briefly, stage 37 embryos were fixed in 2% paraformaldehyde/2.5% gluteraldehyde overnight (Goetz et al., 2006). Embryos were postfixed in ferrocyanide-reduced osmium and embedded in Spurr’s epoxy resin. Transverse ultra-thin (70 nm) sections were mounted on copper grids and post-stained with 4% aqueous uranyl acetate followed by Reynolds’ lead citrate. Sections were imaged with a LEO EM-910 transmission electron microscope.
RESULTS
XHsp27 Expression Is Developmentally Regulated in Differentiating Cardiac and Skeletal Muscle
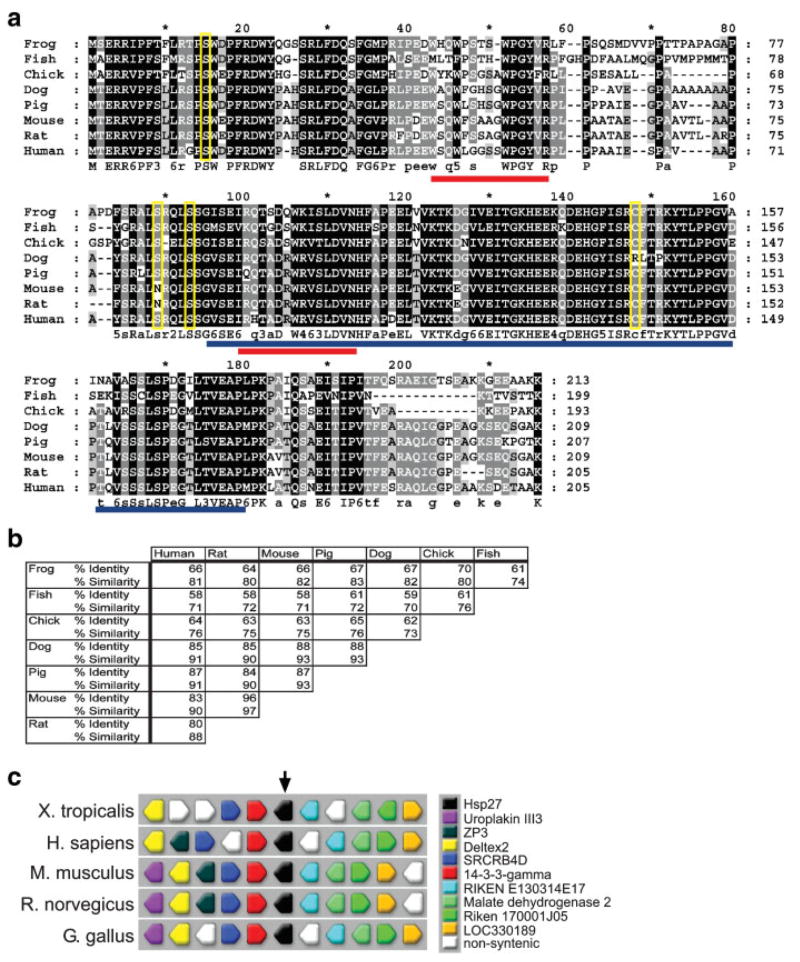
XHsp27, the X. laevis orthologue of Hsp27 (HSP25, HspB1; GenBank Accession No.: EF066483) was initially identified in a screen for differentially expressed cardiac genes as an expressed sequence tag (GenBank Accession No.: AW766262) identified in the developing heart (D. Brown and F.L.C., unpublished data). This EST was subsequently sequenced and a BLASTn search revealed that the EST is highly similar to several members of the small heat shock protein 27 subfamily. The X. laevis Hsp27 transcript consists of at least 1,123 nucleotides and encodes 213 putative amino acids (Fig. 1a). The XHsp27 transcript appears to align with a single locus within the Xenopus tropicalis genome (JGI, Version 4.1, scaffold 72), consisting of three exons separated by two introns. This organization appears to mirror that of mouse Hsp27 (Ferns et al., 2006), which also consists of three exons. In addition, a synteny search using Metazome (www.metazome.net) reveals that the genomic locus within X. tropicalis is highly syntenic with the Hsp27 orthologue locus on human chromosome 7, mouse chromosome 5, rat chromosome 12, and chick chromosome 19 (Fig. 1c). Protein alignments performed against Hsp27 orthologues in human (GenBank Accession No.: BC073768), rat (GenBank Accession No.: NM_031970), mouse (GenBank Accession No.: AK003119), pig (Gen-Bank Accession No.: NM_001007518), dog (GenBank Accession No.: NM_001003295), chick (GenBank Accession No.: NM_205290), and zebrafish (GenBank Accession No.: NM_001008615) reveal conservation of identities in the range of 64–70% (Fig. 1a,b). An α-crystallin-hsps domain within the transcript is conserved between the Hsp27 orthologues, as identified by the NCBI conserved domain search (cd No.: cd00298; Fig. 1a). The crystallin domain is critical in homo- and heterodimerization between various sHSPs (Feil et al., 2001). Furthermore, one of two putative actin interacting domains appears to be highly conserved between XHsp27 and the various orthologues, with a second domain showing a smaller degree of conservation (Fig. 1a; Mao et al., 2005). Thus, this transcript is likely the X. laevis orthologue of Hsp27 and we refer to this transcript as XHsp27.
FIG. 1.
XHsp27 is a conserved member of the Hsp27 subfamily of proteins. (a) Protein sequence alignments of X. laevis Hsp27 with various Hsp27 orthologues. Alignment was performed using the GeneDoc program. Blue underline indicates conserved crystallin domain. Red underline indicates putative actin interacting domains. (b) Percent identity and similarity between Hsp27 orthologues. (c) Synteny between X. tropicalis, human, mouse, rat, and chick Hsp27 loci as revealed by Metazome. Hsp27 is indicated in black. Upstream and downstream genes are colored as indicated.
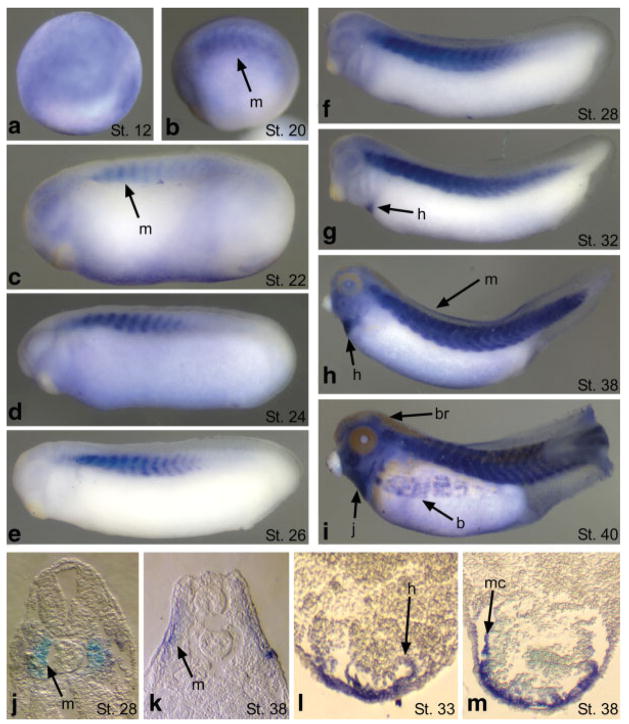
By whole-mount in situ hybridization, XHsp27 appears to be expressed diffusely throughout the developing embryo during gastrulation, consistent with recent findings in zebrafish (Fig. 2; (Mao and Shelden, 2006)). Shortly after gastrulation XHsp27 becomes restricted to thin dorsal-ventral stripes within a subdomain of each developing myotome within the somitic mesoderm (Fig. 2c). This expression initially begins in the anterior most somites and proceeds in a wave towards the posterior end, mirroring the wave of somite formation and development (Fig. 2b–l). As myogenesis progresses, the thickness of each vertical stripe expands to encompass the entire myotome and this expression remains in the developing muscle until at least stage 40 (Fig. 2b–i). During cardiac precursor fusion and linear heart tube formation, XHsp27 expression commences throughout the developing myocardium and remains expressed throughout the developing heart at all stages examined (Fig. 2g–i, l, m). Furthermore, as muscle development continues during early tadpole stages, XHsp27 expression becomes evident in other muscle domains such as those in the developing jaw and the body wall (Fig. 2i). Expression is also detected in the brain of tadpole stage embryos (Fig. 2i). These results indicate that XHsp27 is a developmentally regulated gene and may be involved in gastrulation, cardiac and skeletal myogenesis, and neural development.
FIG. 2.
XHsp27 is expressed in the gastrula, and developing skeletal and cardiac muscle. Whole mount in situ hybridization of X. laevis embryos using an antisense probe specific for XHsp27 at the indicated stages. (a) Dorsal is to the top. (b–i) Anterior is to the left. (j–m) Transverse sections through somitic (j, k) and cardiac (l, m) regions. Dorsal is to the top. b, body muscle; br, brain; h, heart; j, jaw muscle, m, myotome; mc, myocardium.
XHSP27 Morpholinos Specifically Inhibit HSP27 Translation
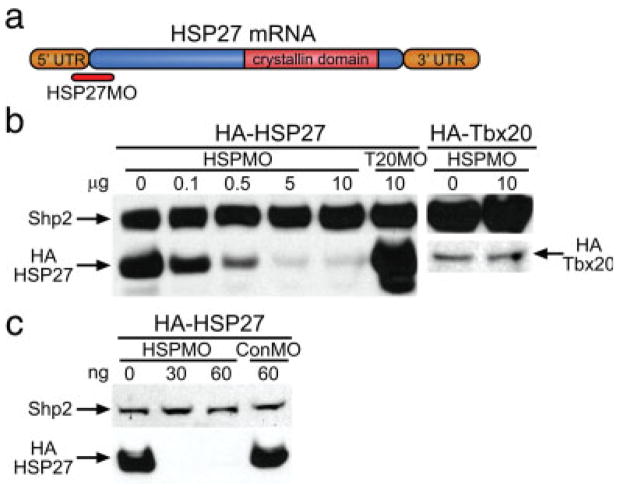
To test whether XHsp27 protein is required during embryogenesis, we sought to knock down endogenous XHSP27 protein levels using antisense morpholino oligonucleotides. To this end, we designed morpholinos targeted against the start site of XHsp27, which we refer to as HSP27MO (Fig. 3a). Unfortunately, attempts to detect endogenous or in vitro translated X. laevis HSP27 were unsuccessful using several of the available commercial HSP27 antibodies. Thus we sought to test the efficiency and specificity of morpholino translation inhibition using a hemagglutinin (HA) epitope-tagged version of XHSP27 both in vitro and in vivo. For the in vitro inhibition study, transcription/translation reactions were incubated with HA-HSP27 construct alone and together with increasing concentrations of HSP27MO (Fig. 3b) and as a negative control TBX20MO (Brown et al., 2005). Furthermore, HSP27MO was incubated with HA-Tbx20 to show specificity of the HSP27MO. Results from these assays show that HSP27MO efficiently blocks translation of HA-XHsp27 in vitro while TBX20MO and ControlMO do not. In contrast, HSP27MO does not block translation of HA-TBX20 (Fig. 3b).
FIG. 3.
HSP27 morpholinos specifically block translation in vitro. (a) Diagram depicting XHsp27 mRNA structure and morpholino-targeted region. (b) Western blot demonstrating translation inhibition in vitro using rabbit reticulocyte lysate. Reactions were incubated with the indicated total amounts of HA-Hsp27 mRNA with/without HSP27MO. TBX20MO was included as a negative control. HSP27MO was incubated with HA-Tbx20 mRNA to show specificity of the HSP27MO. HA-HSP27 and HA-TBX20 were visualized using anti-HA antibody, and SHP2 antibody was used as loading control. (c) Western blot demonstrating translation inhibition in vivo by coinjection of HA-Hsp27 mRNA with HSP27MO. Embryos were injected with the indicated amount of HSP27MO, ControlMO, and/or HA-Hsp27 mRNA. Embryos were collected at Stage 20. Western blotting was performed on lysate using anti-HA antibody. SHP2 antibody was used as loading control.
To test whether HSP27MO can knock down XHSP27 translation in vivo, HA-Hsp27 capped mRNA was injected into one-cell stage embryos along with HSP27MO. To insure that the MOs did not bind the mRNA before injection, embryos were first injected with 30 or 60 ng HSPMO or 60 ng ControlMO. Embryos were then reinjected with 100 pg HA-HSP27 capped mRNA prior to first cleavage. Embryos were then collected at stage 20, lysed, submitted to Western blotting with an anti-HA antibody, and with an antibody against the protein phosphatase SHP2 as loading control (Brown et al., 2005; Langdon et al., Development (In Press)). As shown in Figure 3c, embryos injected with HSPMO completely lack HA-HSP27 protein, in contrast to ControlMO injected embryos, which display no inhibition of HA-HSP27 translation.
Knockdown of XHSP27 Protein Translation Results in Partial Cardia Bifida
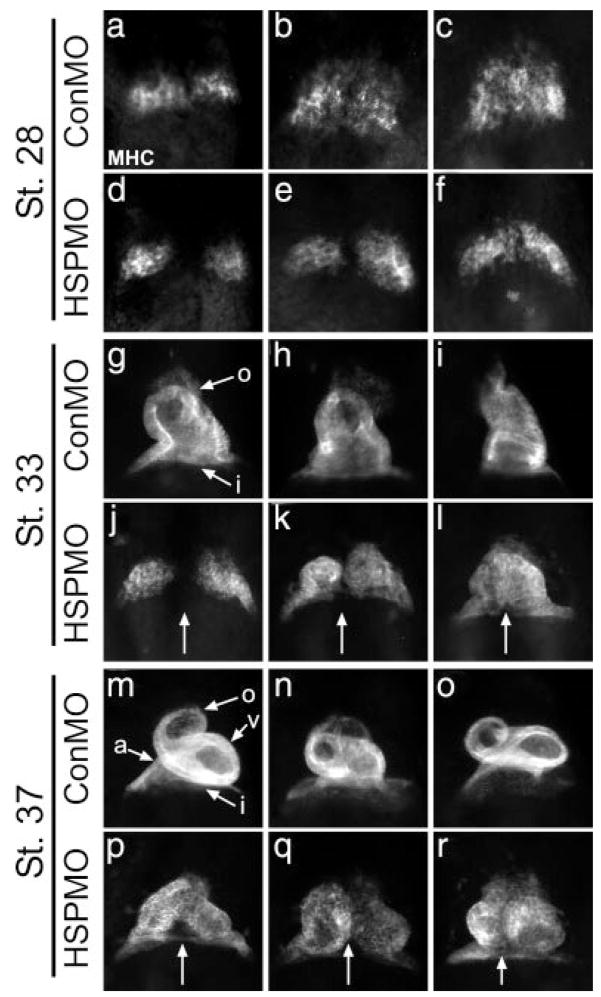
To determine the requirement for XHSP27 during development, we injected HSP27MO into one-cell stage embryos. Despite XHsp27 expression in gastrula embryos, no defects in gastrulation were observed, suggesting that XHSP27 is not required for this process. However, by linear heart tube formation stage (stage 33), defects in heart tube fusion became apparent as assayed by MHC whole-mount antibody staining (Fig. 4j–l). As shown in Figure 4, XHSP27 morphants display a bifurcation in the posterior inflow region of the linear heart tube. The degree of bifurcation varies between embryos, and in the most severe cases the hearts appear to be almost entirely divided, resulting in complete cardia bifida (Fig. 4p–r). Additional Hsp27 morphoinos designed against either the 5-prime UTR or splice donor/acceptor sites led to partial reduction of HSP27 and a similar albeit weaker phenotype while mismatch splice morpholinos gave no phenotype (data not shown). Attempts to rescue the phenotype by misexpression of Hsp27 led to developmental abnormalities at gastrulation and early embryonic lethality precluding analysis of heart development.
FIG. 4.
Depletion of XHSP27 results in unfused or partially fused hearts. Whole-mount antibody staining using anti-myosin heavy chain α (MHC). Embryos were injected at the one-cell stage with either HSPMO or ConMO, fixed, and stained for MHC at the indicated stages. All views are ventral with anterior upwards. Arrows indicate separation between the two cardiac fields or developing hearts. a, atrium; i, inflow tract; o, outflow tract; v, ventricle.
Despite aberrant morphogenesis of the heart, the extant cardiac tissue becomes rhythmically contractile. However, XHSP27 morphant embryos arrest development shortly thereafter (Stage 40). These results suggest that XHSP27 is critical for proper cardiac morphogenesis.
The Cardiac Transcriptional Program Appears to be Unaltered in XHSP27 Morphants
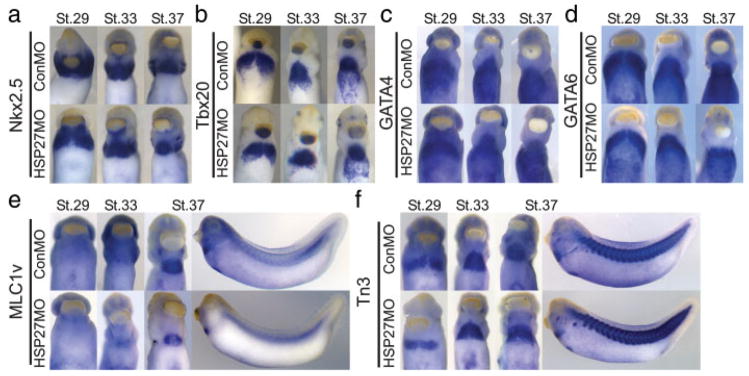
To assess whether the cardiac and skeletal muscle precursors are properly specified and differentiate, whole-mount in situ hybridizations were performed on XHSP27 morphant embryos using a panel of cardiac and skeletal muscle markers. Tbx20, Nkx2.5, Gata4, and Gata6 probes were used to mark both early cardiac precursors and terminally differentiated cardiomyocytes, while titin novex-3 (XTn3) and myosin light chain 1v′ (Mlc1v′) were used to mark differentiated skeletal and cardiac muscle. As shown in Figure 5, the cardiac and skeletal muscle domains appeared normal in all cases. This data, combined with the observation that the hearts are contractile, suggests that the cardiac and skeletal muscle precursors are properly specified, migrate to the correct location within the embryo and can initiate terminal differentiation.
FIG. 5.
Specification and differentiation of cardiac and skeletal muscle appear unaltered in HSP27 morphants. Whole-mount in situ hybridizations using antisense probes against (a) Nkx2.5, (b) Tbx20, (c) Gata4, (d) Gata6, (e) Mlc1v′, and (f) Titin novex 3 (Tn3). Embryos were injected with either HSP27MO or ControlMO and fixed at the indicated stages. (a–f) Ventral views with anterior upward. (e, f) Stage 37 embryos shown laterally with anterior to the left. All markers analyzed appear normal between control and HSP27 morphant embryos.
XHSP27 Morphants Display Actin Filament Disorganization in the Developing Heart and Somites
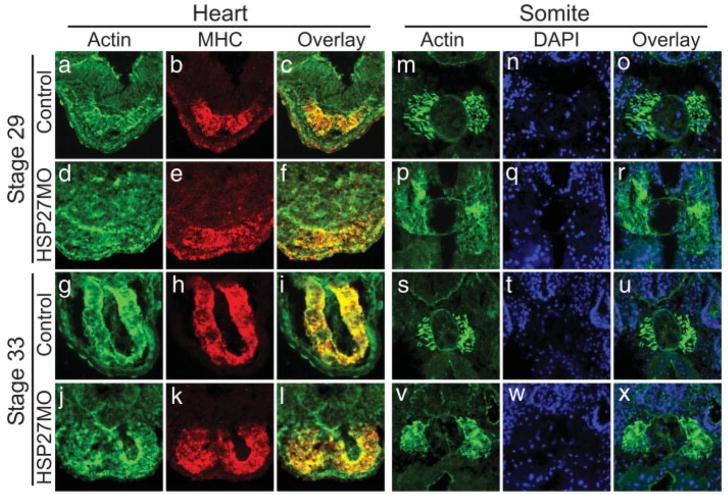
Recent studies have shown that in addition to a requirement for cardiac differentiation and endoderm maturation in heart tube formation, proper epithelial organization, adhesion, and migration are absolutely critical for heart tube formation. Furthermore, HSP27 has been shown to stabilize the actin cytoskeleton in response to stress (Huot et al., 1996; Lavoie et al., 1993a,b, 1995). Thus considering that the cardiomyocytes are apparently specified and differentiate properly, that XHsp27 is not detected in endoderm tissue, and that XHsp27 is known to be involved in cytoskeletal dynamics, we hypothesized that actin organization may be disrupted in the developing myotomes and heart of XHSP27 morphants. To address this possibility, we injected HSP27MO into one-cell stage embryos and collected the embryos at stages during cardiac fusion (Stage 28), linear heart tube formation (Stage 33), and cardiac looping (Stage 37). Embryos were then transversely cryosectioned and cardiac sections were immunostained for MHC using a MHC-specific antibody and for F-actin using phalloidin. Somitic sections were stained for F-actin and with DAPI to mark nuclei. In control hearts, actin staining is most apparent at the basal and apical surfaces of the cells in the forming heart tube, which consists of a single layer of cardiac cells, as well as in fibers perpendicular to the lumen, which appear to correspond with the lateral membranes of cardiac cells (Fig. 6a–c, g–i). However, in XHSP27 morphant hearts, actin staining appears diffuse and disorganized (Fig. 6d–f, j–l). Few distinct fibers are apparent within the morphant hearts, except where lumen formation occurs (Fig. 6l). A more dramatic defect in actin organization is evident within the developing somites. In control morpholino-injected embryos, the somites display thick, highly organized actin bundles oriented along the anterior–posterior axis (Fig. 6m–o, s–u). However, in XHSP27 morphants, the actin appears disorganized and scattered throughout the somites (Fig. 6p–r, v–x). Furthermore, the somitic domain itself appears to be larger and less well-defined (Fig. 6p–r, v–x). This appearance is similar to the appearance of the somites at earlier stages, after which the somites normally become smaller and more compact, possibly because of the formation of the thick actin bundles. These alterations in actin organization are not due to the levels of actin as western blots with an actin specific antibody show equal levels of actin in control and HSP27MO embryos (see Fig. 7) therefore, suggesting that XHsp27 is required for proper regulation of cytoskeletal dynamics during myogenesis.
FIG. 6.
Depletion of XHSP27 results in actin disorganization in developing skeletal and cardiac muscle. Transverse 12 μm sections through the heart (a–l) and somite (m–x). All sections are shown with dorsal upward. Heart sections were immunostained for F-actin using phalloidin (a, d, g, j) and MHC using anti-MHC antibody (b, e, h, k). Overlays are shown in (c, f, i, l). Somite sections were immunostained for F-actin using phalloidin (m, p, s, v) and stained with DAPI to visualize the nuclei (n, q, t, w). Overlays are shown in (o, r, u, x).
FIG. 7.
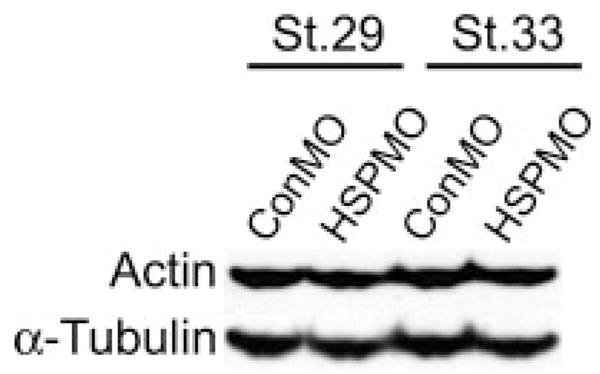
Hsp-depletion does not alter levels of actin. Western blot demonstrating actin levels in control and Hsp27-depleted embryos (Hsp27MO) at the indicated stages. α-tubulin antibody staining was used as a loading control.
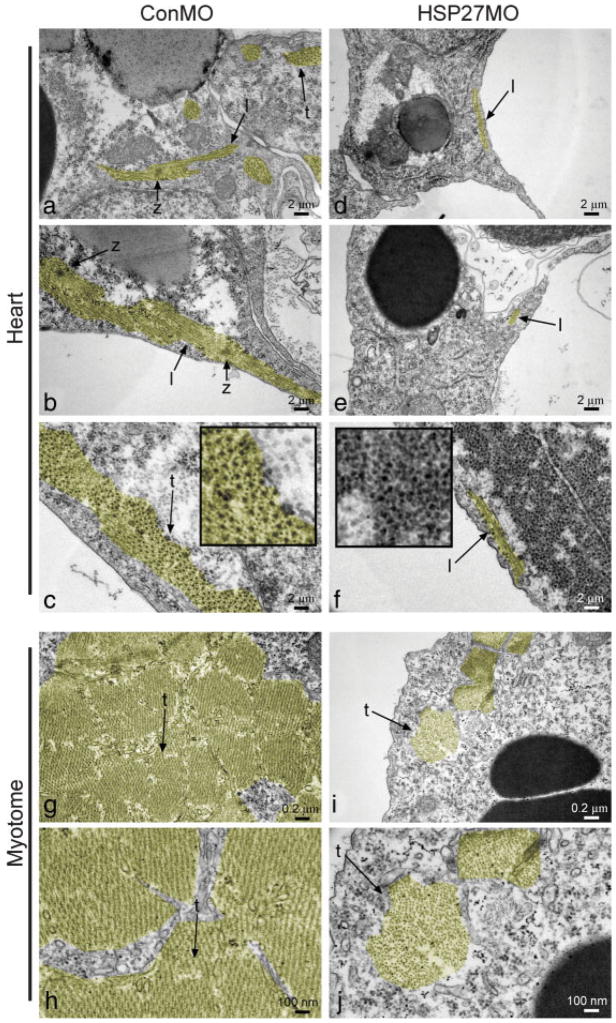
To gain further insight into the nature of microfilament disorganization in XHSP27 morphants, embryos were injected with control or XHSP27 morpholinos, collected at stage 38 and visualized using transmission electron microscopy (TEM). As shown in Figure 8, ControlMO hearts display many myofibril bundles, the majority of which were found to be oriented along the anterior–posterior axis, as demonstrated by transversely sectioned myofibers (see Fig. 8). In regions where longitudinal sections of myofibers are present, clear z-lines are evident showing the fusion between individual sarcomeres (Fig. 8a,b). Furthermore, in transverse myofiber sections, the highly organized myosin structure can be seen (Fig. 8c inset). In contrast, XHSP27 morphant hearts show very few myofiber structures (Fig. 8d,e). In addition, the occasional myofibers are very short and no z-lines or connections between multiple fibers were apparent, indicating a lack of sarcomeric assembly (Fig. 8d,e). In some sections, large aggregates of bodies are apparent that appear very similar to cross-sectional views of myosin in control myofibrils (Fig. 8f inset). However, these aggregates lack any of the obvious structure in spacing or organization characteristic of myofibrils (Fig. 8f inset). Similar results are observed within the developing myotomes in the somitic region of XHSP27 morphants. In the forming skeletal muscle, all myofibers analyzed appeared to be sectioned transversely, indicating that these fibers are arranged along the anterior–posterior axis. ControlMO injected myotomes display thick myofiber structures that generally extend throughout the entire cell (Fig. 8g,h). In higher magnifications, the myofibril structure is visible as highly ordered arrays of microfilaments (Fig. 8h). In contrast, XHSP27 morphants display much fewer apparent myofibers and most of these appear abnormal in morphology (Fig. 8i,j). Magnification of these myofibers reveals a less-ordered structure and an apparent lack of thick myosin filaments in these structures (Fig. 8j). These observations suggest that the lack of actin organization is accompanied by a failure of myofibril assembly and sarcomere formation. Collectively, these results indicate that XHSP27 is required for normal cardiac precursor fusion and muscle formation, and that these defects are accompanied by a failure in actin organization and proper myofibril assembly.
FIG. 8.
XHSP27 morphant ultrastructure analysis reveals a lack in myofibril assembly. Transmission electron microscopy of ventral myocardium in Stage 38 control and XHSP27 morphant hearts. (a–c) Ventral myocardium of ConMO injected embryo. (d–f) Ventral myocardium of XHSP27MO injected embryo. Cardiac muscle fibrils are shown pseudocolored in yellow. Inset in (c) shows magnification of myofibril structure and inset in (f) shows magnification of apparent myosin aggregates. (g, h) Muscle fibers within the myotome in Stage 38 ConMO injected embryos. (i, j) Muscle fibers within the myotome in stage 38 XHsp27 morpholino injected embryos. l, longitudinal myofibers section; t, transverse myofibers section; z, z-line.
DISCUSSION
Developmental Regulation of Hsp27 Expression
HSP27 has been shown to be involved in a diverse array of cellular processes. In general, the majority of functional data on HSP27 comes from experiments performed both in vitro and in cell culture, and much of this research has focused on the function of HSP27 in response to various cellular stressors. However, surprisingly little has been done to address the potential role of HSP27 in developing embryos. In this study, we report the identification of X. laevis HSP27 and demonstrate that this orthologue is expressed in a developmentally regulated manner throughout developing gastrula, skeletal muscle, and cardiac tissues. The temporal and spatial expression appears to be highly conserved between X. laevis and zebrafish (Mao and Shelden, 2006; Tuttle et al., 2007). In frogs and fish, HSP27 is initially detected diffusely throughout the gastrulating embryo, suggesting that HSP27 may be involved in gastrulation. However, embryos depleted of HSP27 protein by antisense morpholinos did not display any defects in gastrulation, suggesting either that XHSP27 does not function in gastrulation or that a functionally redundant heat shock protein is present in X. laevis. During neurulation, HSP27 expression commences in the anteriormost developing somites, followed by a posterior wave of expression in newly forming somites. As development proceeds, expression within the somitic myotomes expands to encompass the entire myotome, suggesting that XHSP27 may be involved in morphogenesis or differentiation of muscle tissue. However, our data from XHSP27-depleted embryos suggest that XHSP27 functions in cytoskeletal dynamics but is not involved in differentiation. In addition to its role in skeletal muscle, XHsp27 is expressed in developing cardiac tissue, beginning at heart tube formation, with this expression continuing through stage 40. During tadpole stages, additional domains of expression within developing jaw and body wall muscles also become evident, suggesting that XHSP27 may be involved in general mechanisms of muscle formation and development.
Hsp27 in Cardiogenesis and Myogenesis
Formation of a linear heart tube requires the coordination of cardiac specification, differentiation, and cell behavior within defined spatial and temporal domains. In the present study, we identify the small heat shock protein, Hsp27, as being integral to this process. Several studies have shown that Hsp27 is expressed in developing muscle tissues. While it is clear that Hsp27 is critical in mediating the cellular response to a wide variety of stressors, it is unclear what role Hsp27 may be playing during cardiogenesis and myogenesis under unstressed physiological conditions. Evidence from studies of embryonic stem cell differentiation has suggested that HSP27 can function as a molecular switch between differentiation and apoptosis (Mehlen et al., 1997). Furthermore, it is known that proper cardiomyocyte differentiation is necessary for cardiac fusion and heart tube formation (Reiter et al., 1999; Yelon et al., 2000). However, while our data does not rule this out as a possible function for HSP27, it at least suggests that a primary function of HSP27 may be to regulate actin dynamics in the context of myogenesis. Our results show that the cardiac and skeletal muscle appears to be specified and to differentiate normally, at least as assayed by several markers of specification and terminal myocyte differentiation. Furthermore, the total amount of cardiac and skeletal tissue appears grossly normal in HSP27-depleted embryos. With the exception of actin, all markers examined appear to be normally expressed and the defects in heart development appear to be primarily morphogenetic in nature. These data suggest that the cause of the cardiac defect is not a failure of cardiomyocyte differentiation. It remains formally possible that the cardiac fusion defects may result from a loss of differentiation or an increase in apoptosis in a subset of cardiac precursors at the mid-line, essentially creating a barrier between the two cardiac fields. However, further studies must be conducted to precisely define whether HSP27 can influence differentiation or apoptosis in the developing embryo.
Abrogation of HSP27 function in X. laevis embryos results in improper fusion of the cardiac progenitors, resulting in two unfused or partially fused contractile hearts. Our data suggest that the primary role of HSP27 in cardiogenesis is to regulate actin dynamics, and thus cell motility or adhesion. In support of this hypothesis, previous research has shown that cardiac epithelial integrity and cell motility and adhesion are critical for proper fusion of the cardiac primordia (Trinh and Stainier, 2004). In addition, the precardiac field was shown to consist of a single polarized epithelial layer. Our results demonstrate that actin fibers are visible primarily at the cell membrane in the single-cell layered cardiac precursors. However, in HSP27-depleted embryos, few discrete fibers are visible. These results suggest that epithelial organization or polarization is defective in the cardiac fields and that HSP27 is required for this organization. Actin fiber organization appears to be morphologically different in developing skeletal muscle. Within the developing myotomes, thick actin fibers are arranged in an anterior–posterior orientation and do not appear to delineate the membranes in an epithelial manner. However, similar to what is seen in cardiac primordia, actin protein appears to be completely disorganized in HSP27 morphant embryos, again suggesting that cell polarity or tissue integrity is affected by HSP27 loss.
Acknowledgments
This work is supported by an award from the UNC Medical Alumni Association. D.D.B is supported by a UNC Graduate School Dissertation Completion Fellowship. The authors thank Roger Patient for providing the Gata4 and Gata6 probe constructs.
Contract grant sponsor: NIH/NHLBI; Contract grant numbers: RO1 HL075256, R21 HL083965; Contract grant sponsor: NIH (Integrative Vascular Biology program); Contract grant number: T32HL69768.
LITERATURE CITED
- Alexander J, Rothenberg M, Henry GL, Stainier DY. Casanova plays an early and essential role in endoderm formation in zebrafish. Dev Biol. 1999;215:343–357. doi: 10.1006/dbio.1999.9441. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. Hsp27: Novel regulator of intracellular redox state. IUBMB Life. 2001;52:303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- Baek SH, Min JN, Park EM, Han MY, Lee YS, Lee YJ, Park YM. Role of small heat shock protein HSP25 in radioresistance and glutathione-redox cycle. J Cell Physiol. 2000;183:100–107. doi: 10.1002/(SICI)1097-4652(200004)183:1<100::AID-JCP12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Beiman M, Shilo B, Volk T. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev. 1996;10:2993–3002. doi: 10.1101/gad.10.23.2993. [DOI] [PubMed] [Google Scholar]
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- Benndorf R, Welsh MJ. Shocking degeneration. Nat Genet. 2004;36:547–548. doi: 10.1038/ng0604-547. [DOI] [PubMed] [Google Scholar]
- Bonham RT, Fine MR, Pollock FM, Shelden EA. Hsp27, Hsp70, and metallothionein in MDCK and LLC-PK1 renal epithelial cells: Effects of prolonged exposure to cadmium. Toxicol Appl Pharmacol. 2003;191:63–73. doi: 10.1016/s0041-008x(03)00226-6. [DOI] [PubMed] [Google Scholar]
- Brown DD, Binder O, Pagratis M, Parr BA, Conlon FL. Developmental expression of the Xenopus laevis Tbx20 orthologue. Dev Genes Evol. 2003;212:604–607. doi: 10.1007/s00427-002-0276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DD, Davis AC, Conlon FL. Xtn3 is a developmentally expressed cardiac and skeletal muscle-specific novex-3 titin isoform. Gene Expr Patterns. 2006;6:913–918. doi: 10.1016/j.modgep.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DD, Martz SN, Binder O, Goetz SC, Price BMJ, Smith JC, Conlon FL. Tbx5 and Tbx20 act synergistically to control vertebrate heart morphogenesis. Development. 2005;132:553–563. doi: 10.1242/dev.01596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Brundel BJ, Shiroshita-Takeshita A, Qi X, Yeh YH, Chartier D, van Gelder IC, Henning RH, Kampinga HH, Nattel S. Induction of heat shock response protects the heart against atrial fibrillation. Circ Res. 2006;99:1394–1402. doi: 10.1161/01.RES.0000252323.83137.fe. [DOI] [PubMed] [Google Scholar]
- Ciruna BG, Rossant J. Expression of the T-box gene Eomesodermin during early mouse development. Mech Dev. 1999;81:199–203. doi: 10.1016/s0925-4773(98)00243-3. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Milzani A, Di Simplicio P, Colombo R. The actin cytoskeleton response to oxidants: From small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic Biol Med. 2001;31:1624–1632. doi: 10.1016/s0891-5849(01)00749-3. [DOI] [PubMed] [Google Scholar]
- David JC, Landry J, Grongnet JF. Perinatal expression of heat-shock protein 27 in brain regions and nonneural tissues of the piglet. J Mol Neurosci. 2000;15:109–120. doi: 10.1385/jmn:15:2:109. [DOI] [PubMed] [Google Scholar]
- DeHaan RL. Migrating patterns of precardiac mesoderm in the early chick embryo. Exp Cell Res. 1963;29:544–560. doi: 10.1016/s0014-4827(63)80016-6. [DOI] [PubMed] [Google Scholar]
- Escobedo J, Pucci AM, Koh TJ. HSP25 protects skeletal muscle cells against oxidative stress. Free Radic Biol Med. 2004;37:1455–1462. doi: 10.1016/j.freeradbiomed.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, Schagina O, Verpoorten N, Van Impe K, Fedotov V, Dadali E, Auer-Grumbach M, Windpassinger C, Wagner K, Mitrovic Z, Hilton-Jones D, Talbot K, Martin JJ, Vasserman N, Tverskaya S, Polyakov A, Liem RK, Gettemans J, Robberecht W, De Jonghe P, Timmerman V. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004;36:602–606. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- Feil IK, Malfois M, Hendle J, van Der Zandt H, Svergun DI. A novel quaternary structure of the dimeric alpha-crystallin domain with chaperone-like activity. J Biol Chem. 2001;276:12024–12029. doi: 10.1074/jbc.M010856200. [DOI] [PubMed] [Google Scholar]
- Ferns G, Shams S, Shafi S. Heat shock protein 27: Its potential role in vascular disease. Int J Exp Pathol. 2006;87:253–274. doi: 10.1111/j.1365-2613.2006.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–3081. [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Gernold M, Knauf U, Gaestel M, Stahl J, Kloetzel PM. Development and tissue-specific distribution of mouse small heat shock protein hsp25. Dev Genet. 1993;14:103–111. doi: 10.1002/dvg.1020140204. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht S, Skeath J, Doe C, Michelson A. Heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev. 1996;1:3003–3017. doi: 10.1101/gad.10.23.3003. [DOI] [PubMed] [Google Scholar]
- Goetz S, Conlon FL. Cardiac progenitors and the embryonic cell cycle. Cell Cycle. 2007;6:1974–1981. doi: 10.4161/cc.6.16.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Brown DD, Conlon FL. TBX5 is required for embryonic cardiac cell cycle progression. Development. 2006;133:2575–2584. doi: 10.1242/dev.02420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: An improved whole mount method for Xenopus embryos. Methods Cell Biol. 1991;36:675–685. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation. 2004;110:3544–3552. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Jiang Y, Evans T. The Xenopus GATA-4/5/6 genes are associated with cardiac specification and can regulate cardiac-specific transcription during embryogenesis. Dev Biol. 1996;174:258–270. doi: 10.1006/dbio.1996.0071. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Trinh LA, Reiter JF, Alexander J, Yelon D, Stainier DY. The zebrafish bonnie and clyde gene encodes a Mix family homeo-domain protein that regulates the generation of endodermal precursors. Genes Dev. 2000;14:1279–1289. [PMC free article] [PubMed] [Google Scholar]
- Knowlton AA, Kapadia S, Torre-Amione G, Durand JB, Bies R, Young J, Mann DL. Differential expression of heat shock proteins in normal and failing human hearts. J Mol Cell Cardiol. 1998;30:811–818. doi: 10.1006/jmcc.1998.0646. [DOI] [PubMed] [Google Scholar]
- Kolker S, Tajchman U, Weeks DL. Confocal imaging of early heat development in Xenopus laevis. Dev Biol. 2000;218:64–73. doi: 10.1006/dbio.1999.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsuda A, Wakui H, Oyama Y, Imai H, Miura AB, Itoh H, Tashima Y. Overexpression of the human 72 kDa heat shock protein in renal tubular cells confers resistance against oxidative injury and cisplatin toxicity. Nephrol Dial Transplant. 1999;14:1385–1390. doi: 10.1093/ndt/14.6.1385. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- Kupperman E, An S, Osborne N, Waldron S, Stainier DY. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature. 2000;406:192–195. doi: 10.1038/35018092. [DOI] [PubMed] [Google Scholar]
- Langdon YG, Goetz SC, Berg AE, Swanik JT, Conlon FL. SHP-2 is required for the Maintenance of Cardiac Progenitors. Development. 2007 doi: 10.1242/dev.009290. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993a;268:3420–3409. [PubMed] [Google Scholar]
- Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993b;268:24210–24214. [PubMed] [Google Scholar]
- Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal RB, Cordova FM, Herd L, Bobrovskaya L, Dunkley PR. Lead-stimulated p38MAPK-dependent Hsp27 phosphorylation. Toxicol Appl Pharmacol. 2002;178:44–51. doi: 10.1006/taap.2001.9320. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang X, Qian B, Min X, Gao X, Li C, Cheng Y, Huang J. Over-expression of heat shock protein 27 attenuates doxorubicin-induced cardiac dysfunction in mice. Eur J Heart Fail. 2007;9:729–762. doi: 10.1016/j.ejheart.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Lough J, Sugi Y. Endoderm and heart development. Dev Dyn. 2000;217:327–342. doi: 10.1002/(SICI)1097-0177(200004)217:4<327::AID-DVDY1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Mao L, Bryantsev AL, Chechenova MB, Shelden EA. Cloning, characterization, and heat stress-induced redistribution of a protein homologous to human hsp27 in the zebrafish Danio rerio. Exp Cell Res. 2005;306:230–241. doi: 10.1016/j.yexcr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Mao L, Shelden EA. Developmentally regulated gene expression of the small heat shock protein Hsp27 in zebrafish embryos. Gene Expr Patterns. 2006;6:127–133. doi: 10.1016/j.modgep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Kretz-Remy C, Briolay J, Fostan P, Mirault ME, Arrigo AP. Intracellular reactive oxygen species as apparent modulators of heat-shock protein 27 (hsp27) structural organization and phosphorylation in basal and tumour necrosis factor alpha-treated T47D human carcinoma cells. Biochem J. 1995;312 (Pt 2):367–375. doi: 10.1042/bj3120367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Mehlen A, Godet J, Arrigo AP. hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem. 1997;272:31657–31665. doi: 10.1074/jbc.272.50.31657. [DOI] [PubMed] [Google Scholar]
- Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T, Orford R, Shang C. The origins of cardiac tissue in the amphibian, Xenopus laevis. Trends Cardiovasc Med. 2003;13:244–248. doi: 10.1016/s1050-1738(03)00102-6. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Amsterdam: North Holland; 1967. [Google Scholar]
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quertermous T. Apelin and its g protein-coupled receptor regulate cardiac development as well as cardiac function. Dev Cell. 2007;12:319–320. doi: 10.1016/j.devcel.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Rahman DR, Bentley NJ, Tuite MF. The Saccharomyces cerevisiae small heat shock protein Hsp26 inhibits actin polymerisation. Biochem Soc Trans. 1995;23:77S. doi: 10.1042/bst023077s. [DOI] [PubMed] [Google Scholar]
- Reiter JF, Alexander J, Rodaway A, Yelon D, Patient R, Holder N, Stainier DY. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999;13:2983–2995. doi: 10.1101/gad.13.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LP, Allen GV. A review of heat shock protein induction following cerebellar injury. Cerebellum. 2003;2:171–177. doi: 10.1080/14734220310016114. [DOI] [PubMed] [Google Scholar]
- Scheler C, Li XP, Salnikow J, Dunn MJ, Jungblut PR. Comparison of two-dimensional electrophoresis patterns of heat shock protein Hsp27 species in normal and cardiomyopathic hearts. Electrophoresis. 1999;20:3623–3628. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3623::AID-ELPS3623>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Helde KA, Talbot WS, Driever W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- Scott IC, Masri B, D’Amico LA, Jin SW, Jungblut B, Wehman AM, Baier H, Audigier Y, Stainier DY. The g protein-coupled receptor agtrl1b regulates early development of myocardial progenitors. Dev Cell. 2007;12:403–413. doi: 10.1016/j.devcel.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Shama KM, Suzuki A, Harada K, Fujitani N, Kimura H, Ohno S, Yoshida K. Transient up-regulation of myotonic dystrophy protein kinase-binding protein, MKBP, and HSP27 in the neonatal myocardium. Cell Struct Funct. 1999;24:1–4. doi: 10.1247/csf.24.1. [DOI] [PubMed] [Google Scholar]
- Shelden EA, Borrelli MJ, Pollock FM, Bonham R. Heat shock protein 27 associates with basolateral cell boundaries in heat-shocked and ATP-depleted epithelial cells. J Am Soc Nephrol. 2002;13:332–341. doi: 10.1681/ASN.V132332. [DOI] [PubMed] [Google Scholar]
- Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet. 1974;6:98–118. doi: 10.1111/j.1399-0004.1974.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Somji S, Sens DA, Garrett SH, Sens MA, Todd JH. Heat shock protein 27 expression in human proximal tubule cells exposed to lethal and sublethal concentrations of CdCl2. Environ Health Perspect. 1999;107:545–552. doi: 10.1289/ehp.99107545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier DY. Zebrafish genetics and vertebrate heart formation. Nat Rev Genet. 2001;2:39–48. doi: 10.1038/35047564. [DOI] [PubMed] [Google Scholar]
- Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–382. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- Tuttle AM, Gauley J, Chan N, Heikkila JJ. Analysis of the expression and function of the small heat shock protein gene, hsp27, in Xenopus laevis embryos. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:112–121. doi: 10.1016/j.cbpa.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Vander Heide RS. Increased expression of HSP27 protects canine myocytes from simulated ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2002;282:H935–H941. doi: 10.1152/ajpheart.00660.2001. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Yelon D, Ticho B, Halpern ME, Ruvinsky I, Ho RK, Silver LM, Stainier DY. The bHLH transcription factor hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development. 2000;127:2573–2582. doi: 10.1242/dev.127.12.2573. [DOI] [PubMed] [Google Scholar]
- Zeng XX, Wilm TP, Sepich DS, Solnica-Krezel L. Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev Cell. 2007;12:391–402. doi: 10.1016/j.devcel.2007.01.011. [DOI] [PubMed] [Google Scholar]