Abstract
This review attempts to touch on the history and application of amperometry at PC12 cells for fundamental investigation into the exocytosis process. PC12 cells have been widely used as a model for neural differentiation and as such they have been used to examine the effects of differentiation on exocytotic release and specifically release at varicosities. In addition, dexamethasone-differentiated cells have been shown to have an increased number of releasable vesicles with increased quantal size, thereby allowing for an even broader range of applications including neuropharmacological and neurotoxicological studies. PC12 cells exhibiting large numbers of events have two distinct pools of vesicles, one about twice the quantal size of the other and each about half the total releasable vesicles. As will be outlined in this review, these cells have served as an extremely useful model of exocytosis in the study of the latency of stimulation-release coupling, the role of exocytotic proteins in regulation of release, effect of drugs on quantal size, autoreceptors, fusion pore biophysics, environmental factors, health and disease. As PC12 cells have some advantages over other models for neurosecretion, including chromaffin cells, it is more than likely that in the following decade PC12 cells will continue to serve as a model to study exocytosis.
Keywords: amperometry, catecholamines, exocytosis, modulation of vesicle fusion, PC12 cells, release characteristics
The key dynamic event in neuronal communication is exocytosis. This is a process that has been investigated extensively for several decades (Helle & Serck-Hanssen 1975, Livett et al. 1983, Holz 1988). The process of exocytosis can be summarized as the docking of neurotransmitter-containing vesicles (storage compartments) to the cell membrane and subsequent release of the contents by fusion of the vesicle and cell membranes (Südhof 2004, Barclay et al. 2005, Westerink 2006). This process allows the conversion of an electrical signal (action potential) to a chemical signal (receptor recognition), which is necessary for exocytotic communication between cells.
Methods to observe and quantify individual exocytotic events have traditionally revolved around electron microscopy and patch-clamp capacitance measurements (Neher & Marty 1982). In 1990, Wightman and coworkers (Leszczyszyn et al. 1990) showed that they could directly monitor individual exocytotic events involving easily oxidized messengers and occurring on the millisecond timescale by use of amperometric measurements at micro-electrodes (Wightman et al. 1991).
Suitability of PC12 cells as a model for neurosecretion
Most work on single-vesicle exocytosis in culture has been with the adrenal chromaffin cell model. The use of adrenal chromaffin cells for neurobiological studies, including neurosecretory studies, is extensively covered in the other chapters in this issue. An important feature of chromaffin cells is that they are derived from laboratory animals, mainly rats and mice, or from slaughterhouses in the case of bovine chromaffin cells. This not only gives rise to some ethical matters regarding animal use, but also inherently increases the variation in the data. On the other hand, a wide array of knock-out animals is nowadays available and the use of these animals considerably added to the increased insight into the process of exocytosis in the past decade. Nonetheless, the use of rat phaeochromocytoma (PC12) cells developed in parallel as an alternative model to investigate exocytosis with amperometry. The popularity of PC12 cells is mainly because of their extreme versatility for pharmacological manipulation, their ease of culture and the large amount of background knowledge on their proliferation and differentiation. Moreover, they more closely resemble neurons with smaller vesicle and quantal size than chromaffin cells.
The adrenal phaeochromocytoma (PC12) cell line was originally isolated from a tumour in the adrenal medulla of a rat in 1976 (Greene & Tischler 1976). Like adrenal chromaffin cells, PC12 cells synthesize and store DA and sometimes noradrenaline, which are released upon depolarization in a Ca2+-dependent way (Greene & Rein 1977). PC12 cells resemble the phenotype of sympathetic ganglion neurons upon differentiation with nerve growth factor (NGF) and can be subcultured indefinitely. The activity of tyrosine hydroxylase (TH) in PC12 cells can be increased by NGF, insulin, glucocorticoids, cholera toxin and a high plating density (Schubert et al. 1980, Tischler et al. 1983). Through a complex interplay between these agents, NGF enhances the effects of glucocorticoids on these cells. Specifically, dexamethasone in combination with NGF leads to increases in transmitter synthesis and vesicle size (Schubert et al. 1980). Although the majority of catecholamine contained in PC12 cells is dopamine, addition of ascorbic acid can lead to production of limited amounts of noradrenaline in some PC12 subcultures (Schubert & Klier 1977, Tischler et al. 1983). PC12 cells possess large dense-core vesicles (LDCVs) which contain catecholamines, whereas acetylcholine is stored in small clear vesicles (Greene & Tischler 1976, Schubert et al. 1980, Travis & Wightman 1998). LDCVs in PC12 cells are slightly smaller (75–120 nm radius; Greene & Tischler 1976, Schubert et al. 1980, Travis & Wightman 1998) compared with chromaffin cells (170 nm radius; Coupland 1968). Release of catecholamines from PC12 cells gives rise to amperometric spikes similar to those observed for isolated chromaffin cells following stimulation to exocytosis (Clark & Ewing 1997).
Amperometry at PC12 cells to monitor secretion: cellular effects
Initial experiments at undifferentiated PC12 cells
Initial studies of PC12 cells focused on the detection of zeptomole quantities of catecholamines released in a regulated manner from the soma of undifferentiated cells (Chen et al. 1994). Current transients for the oxidation of catecholamines were on the timescale expected for exocytotic release (9.3 ms half-width) and were thus similar to events observed at adrenal chromaffin cells. However, catecholamines are present in much lower levels in vesicles of PC12 cells. This is at least partially because of the smaller size of the PC12 vesicle (Schubert et al. 1980) compared with the adrenal cell vesicle (Coupland 1968). The quantity of dopamine oxidized from a single vesicle after stimulated release was first reported to be 190 zmol (114 300 molecules) for a specific passage of PC12 cells (Chen et al. 1994), although it appears to vary in different PC12 cell subcultures. For the cell line obtained from the American Type Culture Collection; Rockville, MD (original experiments), it was approximately 18 times smaller than the amount of release observed from a single adrenal cell vesicle (Wightman et al. 1991).
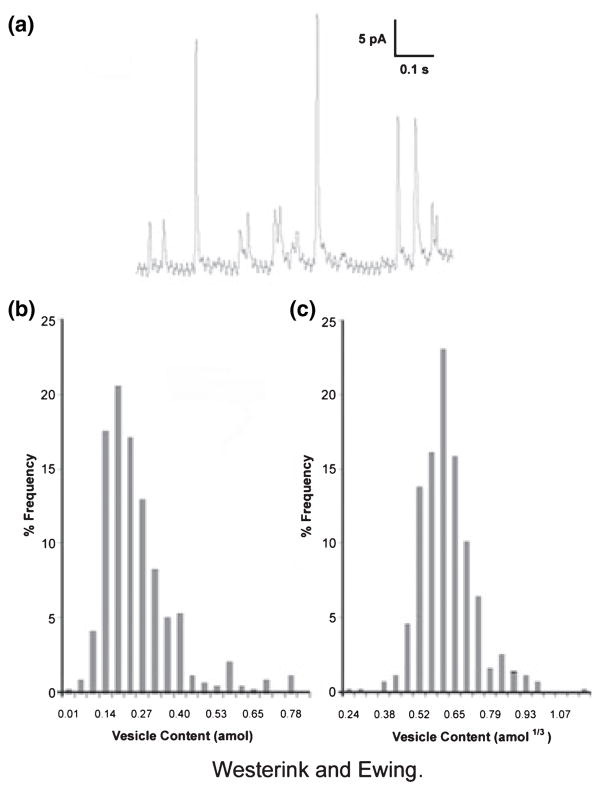
An example of an amperometric recording of depolarization-induced exocytosis from a single PC12 cell is shown in Figure 1a. Quantification is accomplished by integrating the charge (Q) under individual current transients, representing single vesicle release, obtained using amperometry. Using Faraday’s law: Q = nFN, where n is the number of electrons in the electrooxidation (n = 2 for catecholamines), and F is Faraday’s constant (96 485 C/mole), the number of moles (N) of oxidized catecholamines can be determined. The reported quantity of catecholamines from individual vesicles in a cell type is an average value. Histograms generated by plotting the percentage of total release events versus vesicle content in each bin demonstrated the distribution of event sizes. Figure 1b shows a typical histogram generated from exocytotic events measured from PC12 cells. The average vesicle content for this set of PC12 cells was 199 ± 14 zmol (Zerby & Ewing 1996a); however, the distribution ranges from 20 to 800 zmol (Fig. 1b). Histograms plotted in this manner give a skewed (non-Gaussian) distribution and have been modelled mathematically by Wightman et al. (1991) for adrenal cells. The most feasible explanation for this distribution is that vesicles have a range of radii with relatively uniform concentration of catecholamines (Wightman et al. 1991). Thus, if the cube root of the vesicular content (Q1/3 or mole1/3) is utilized for generation of the histograms, then a normal (Gaussian) distribution is obtained (Fig. 1c).
Figure 1.
(a) Example of a current–time trace for exocytosis at a single undifferentiated PC12 cell following depolarization with KCl. The resulting current transients correspond to the oxidation of catecholamine at the electrode tip upon release from the cell. The area under each current transient is equivalent to the total charge produced by the oxidation of the catecholamine content of one vesicle. (b) Distribution of the amount of catecholamine released following potassium stimulation of undifferentiated PC12 cells. The total number of moles of catecholamine detected for each exocytosis event observed in the first 40 s of KCl-stimulated release is collected into bins and plotted as the per cent of the total number of vesicles undergoing exocytosis. (c) Cubed-root histogram for amperometric charges from PC12 cells shown in (b).
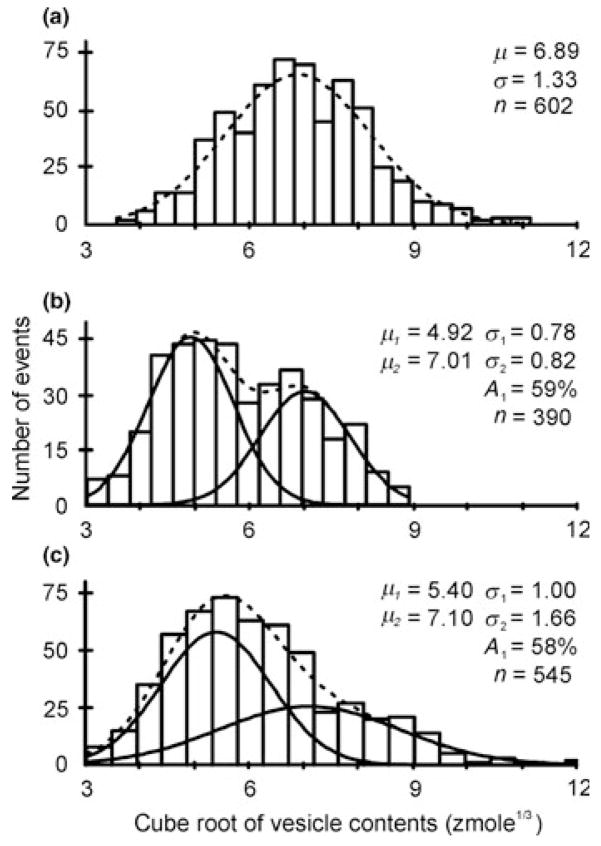
The initial experiments to examine distributions of the cube root of vesicle contents for release from PC12 cells used pooled data from many cells and have been fitted using single Gaussian functions (Finnegan et al. 1996, Zerby & Ewing 1996b, Taylor & Peers 1999). However, it is not always accurate to pool data from individual event at many cells as cell-to-cell variation in the number of events can bias pooled means to only a few cells (Colliver et al. 2000a, Westerink et al. 2000). Thus, it is usually more accurate to examine pooled means than to pool data from many events covering many cells. In addition, it has been shown that in cells that release large numbers of events, the majority of these cells contain two distinct classes of catecholamine-containing vesicles (Fig. 2). The mean vesicle contents between these two classes of vesicles varies by approximately twofold, at 141 and 293 zmol, and each makes up roughly half of the total release events observed (Westerink et al. 2000). It is therefore likely that PC12 cells contain distinct classes of vesicles, although at present it cannot be excluded that the observed results are because of the release of aggregate vesicles or compound fusion.
Figure 2.
Distributions of the cube root of the vesicle contents of three single undifferentiated PC12 cells from which >300 events were recorded during repeated depolarizing with KCl. The distributions were best fitted by double Gaussian functions in 80% of the cells (see, e.g. b and c) and by a single Gaussian function for the cell in (a). The dashed lines represent the sum of the Gaussian distributions (solid lines) with the maximum-likelihood estimates of mean (μ) and variance (σ) indicated in each panel. The estimated number of small events (A1) is indicated as a percentage of the total number of events (n). Modified after Westerink et al. (2000).
Latency of release following chemical stimulation
Amperometry has been used to investigate the effect of different mechanisms of stimulation on the latency of exocytosis from PC12 cells following stimulation (Zerby & Ewing 1996b). PC12 cells possess both nicotinic and muscarinic receptors that can trigger exocytosis through two different mechanisms upon activation. Application of nicotine causes opening of sodium channels, which results in sufficient depolarization of the cell membrane to open voltage-sensitive calcium channels, thereby allowing rapid influx of calcium and subsequent exocytosis (Stallcup 1979). On the other hand, the application of muscarine activates muscarinic receptors that act through intracellular second messengers to release calcium from intracellular stores triggering exocytosis (Berridge & Irvine 1984). To obtain rapid membrane depolarization of the cell, KCl can be applied to promote exocytosis by direct membrane depolarization. The average catecholamine content of the vesicles is unaltered by these different stimuli, but the latencies (time between application of the stimulant and secretion events) vary significantly (Fig. 3; Zerby & Ewing 1996b). The mean latencies for each type of stimulation have been observed to be 6 ± 1 s (105 mM K+); 37 ± 5 s (1 mM nicotine); and 103 ± 11 s (1 mM muscarine). The 6-s delay until release following potassium stimulation apparently represents the diffusion time from the stimulation pipette as the delay is generally <1 s when a continuous, fast superfusion system is used to apply KCl (Westerink et al. 2000). The relatively long latencies until the onset of exocytosis after nicotine and muscarine are surprising as one usually expects latencies comparable with that observed following KCl-induced membrane depolarization. The longer times might reflect a relatively low number of sodium channels on these cells or a slow step in the G-protein coupling by these receptors in PC12 cells, as well as other potential rate-limiting mechanisms.
Figure 3.
Current–time traces for exocytosis at single PC12 cells. A 6-s ejection of stimulant (105 mM K+ (a), 1 mM nicotine (b) or 1 mM muscarine (c) from a microinjector was administered at each arrow. The resulting current transients correspond to the oxidation of dopamine at the electrode tip as it is released from the cell. Reproduced with permission from Zerby & Ewing (1996b).
Experiments at differentiated (NGF-treated) PC12 cell varicosities
PC12 cells can be differentiated with NGF to better resemble a neuronal phenotype. Upon treatment with NGF, PC12 cells extend processes and along these processes or neurites varicosities form (bulbous regions, 1–2 μm in diameter). Varicosities have been previously shown to contain aggregates of small vesicles (20–70 nm in diameter) (Greene & Tischler 1976). Experiments carried out on days 10–14 of NGF-treated PC12 cell cultures showed no release from the cell body, only very infrequent responses from the smooth regions of the neurites, and frequent release when the electrode was located at a varicosity (Zerby & Ewing 1996a). This response was most frequently observed at varicosities located at the intersections of several neurites. The average vesicular catecholamine content observed for exocytosis at varicosities was 178 ± 9 zmol (107 000 molecules) and was not significantly different from that observed at undifferentiated cells; however, a more narrow distribution was observed at the former (Fig. 4). This study demonstrated that functional changes occur during differentiation, specifically the relocation of the sites of exocytosis without significant alteration in the overall mean vesicle catecholamine content, although the narrow distribution of vesicle content might indicate that a tighter distribution of vesicle radii is necessary to pack them into the relatively small varicosities.
Figure 4.
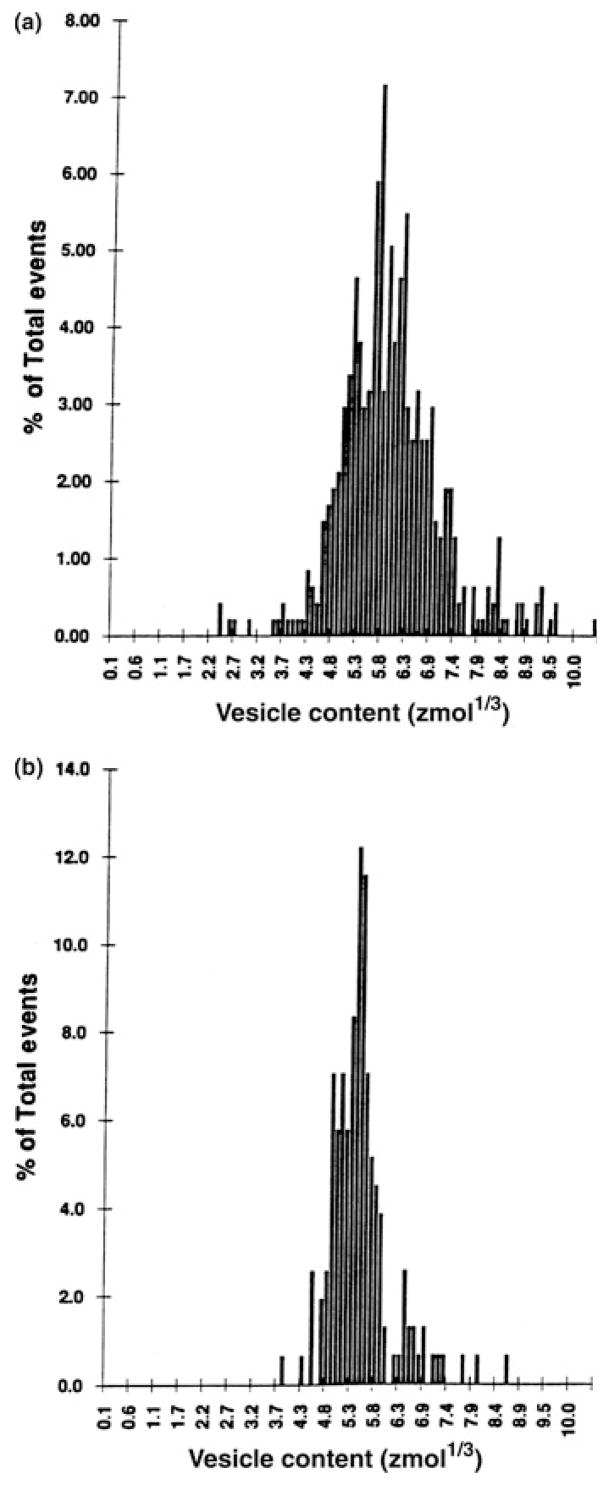
Distribution of the cube root of vesicle catecholamine content for KCl-stimulated release plotted as the percent of the total number of vesicles undergoing exocytosis from (a) the soma of undifferentiated cells (n = 17 cells, 475 events) and from (b) varicosities of NGF-differentiated PC12 cells (n = 16 cells, 156 events). Reproduced with permission from Zerby & Ewing (1996a).
Dexamethasone increases calcium channel function and exocytosis
Dexamethasone, a potent synthetic glucocorticoid, is used to treat a wide variety of conditions as an anti-inflammatory agent or immunosuppressant. Dexamethasone has also been used to differentiate PC12 cells into neuroendocrine chromaffin-like cells. These dexamethasone-differentiated cells have been monitored with patch clamp and amperometry and it was found that treatment for 5–7 days dramatically increases quantal size, excitability and coupling between calcium channels and vesicle release sites, leading to rapid exocytosis and endocytosis (Elhamdani et al. 2000).
These findings can be extended by comparison of amperometric measurements of exocytosis in undifferentiated and dexamethasone-differentiated PC12 cells from the same research group (Westerink et al. 2000, Westerink & Vijverberg 2002a). Approximately 90% of dexamethasone-differentiated PC12 cells responded with vesicular neurotransmitter release when depolarized with KCl, compared with ~50% in undifferentiated cells. During stimulation the release frequency in dexamethasone-differentiated PC12 cells amounts to ~5 Hz, whereas it averages only ~2 Hz in undifferentiated cells. The amount of releasable vesicles in dexamethasone-differentiated PC12 is also much larger (~500 vs. ~50). Finally, vesicle content increased from ~200 to ~650 zmol following differentiation with dexamethasone. Surprisingly, the cube root of vesicle content is distributed normally in single dexamethasone-differentiated PC12 indicating a more homogenous vesicle population. These characteristics allowed dexamethasone-differentiated PC12 to become a preferred model for studying modulation of neurotransmitter release at the single-cell level in neuropharmacological and neurotoxicological studies.
Amperometry at PC12 cells to monitor secretion: manipulation of exocytotic proteins
The use of amperometry at PC12 cells has led to considerable insight into the function of proteins underlying vesicle fusion. The basic molecular machinery underlying vesicle fusion is well summarized in a large number of reviews (Burgoyne & Morgan 2003, Südhof 2004, Westerink 2006). Therefore, in this section only an overview of recent achievements using amperometry at PC12 cells is given.
SNARE proteins
Secretory vesicles become docked at the cell membrane through formation of the SNARE-complex consisting of vesicle-associated synaptobrevin and plasma membrane-associated synaptosomal-associated protein of 25 kDa (SNAP-25) and syntaxin. Obviously, disruption of the SNARE complex or cleavage of SNARE proteins prevents vesicle docking, priming and release. This was elegantly demonstrated in PC12 cells transfected with Botulinum neurotoxin C1 light chain (BoNT/C1), which cleaves syntaxin and SNAP-25. In PC12 cells expressing BoNT/C1, ATP-evoked exocytosis was inhibited almost completely as revealed using amperometry (Fisher & Burgoyne 1999). More recently, syntaxin was shown to be involved in fusion pore formation as well. The fusion pore is a channel-like structure connecting the vesicle and plasma membranes, which is formed during the initial phase of exocytosis. Leakage of catecholamines through the fusion pore results in the detection of foot signals that precede the actual exocytotic event (Chow et al. 1992, Alvarez de Toledo et al. 1993). An amperometric study showed the influence of specific proteins on the process of release via the pore. Point mutations in syntaxin, a protein thought to form transmembrane segments in the pore, reduced the flux of catecholamines through the fusion pore as well as its conductance (Han et al. 2004), underlining the importance of syntaxin in regulated exocytosis.
Synaptotagmin
The proposed Ca2+-sensor synaptotagmin (Syt), which exists in several isoforms, prevents the SNARE complex from further interactions until an increase in the intracellular Ca2+ concentration displaces Syt and catalyses membrane fusion. Using amperometry, it was shown that silencing Syt-I in PC12 cells significantly reduced evoked exocytosis. The reduction in exocytosis was reversed following rescue with human Syt-I (Moore et al. 2006), indicating that Syt-I plays a major role in regulated secretion. It is not clear if Syt-I is regulatory by enhancing or inhibiting full expansion of the pore. As it appears that membrane mechanics provide enough energy for expansion of the pore (Cans et al. 2003), it is highly possible that protein regulation is inhibitory. However, as exocytosis is not completely abolished, these findings also indicate that one or more proteins can replace Syt-I. PC12 cells over-expressing Syt-I display a prolonged open time of the fusion pore, whereas Syt-IV decreases the fusion pore open time (Wang et al. 2001). Transfection with Syt-IV, or increasing endogenous Syt-IV by forskolin treatment, increases the frequency and duration of kiss-and-run events. Full fusion is inhibited by mutation of a Ca2+ ligand in the C2A domain of Syt-I, whereas kiss-and-run exocytosis is inhibited by mutation of a homologous Ca2+ ligand in the C2B domain of Syt-IV (Wang et al. 2003).
In permeabilized PC12 cells, elevating the intracellular Ca2+ concentration reduced the fusion pore lifetime, indicating that Ca2+ acts during the actual fusion process. Both, opening of the fusion pore and dilatation of the pore were accelerated by Ca2+, suggesting separate Ca2+ control over each of these steps. This was further confirmed by the fact that Ca2+ ligand mutations in either the C2A or C2B domains of Syt-I reduced fusion pore opening, but had opposite actions on the rate of fusion pore closure (Wang et al. 2006). Exactly how the Syt proteins regulate pore is still a matter of debate and ongoing interest.
SCAMP2
Another protein critically involved in fusion pore formation is secretory carrier membrane protein 2 (SCAMP2). Expression of a point mutant of SCAMP2 in PC12 cells resulted in the inhibition of exocytosis. Interestingly, dilatation of the opened fusion pores was also hampered, resulting in a relative abundance of stand-alone foot signals, thus linking SCAMP2 to the process of fusion pore formation (Liu et al. 2005). As such, amperometry at (transfected) PC12 cells is a powerful tool to further complete the detailed picture of the molecular regulatory mechanisms underlying exocytosis.
Amperometry at PC12 cells to monitor secretion: vesicular changes
L-DOPA increases quantal size
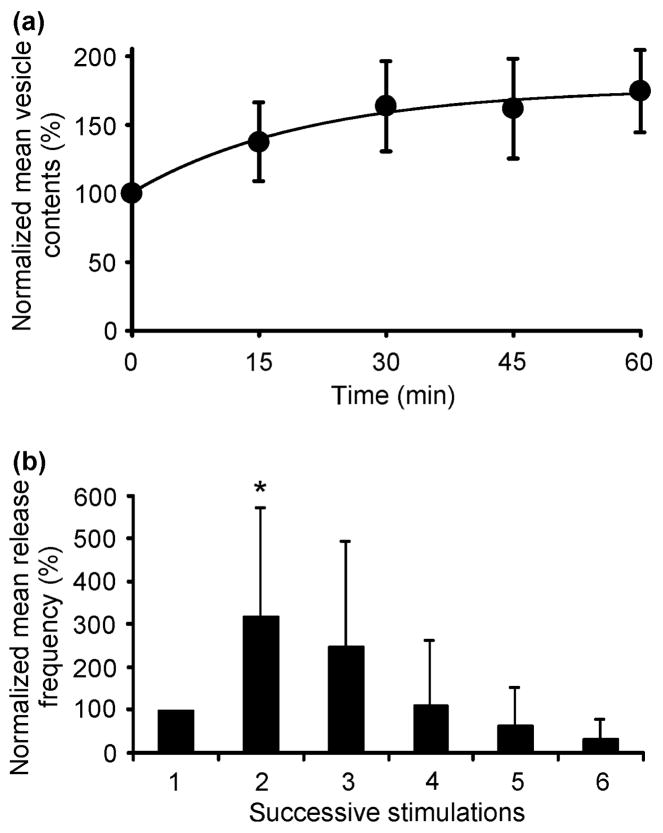
Several early studies used PC12 cells to investigate the effects of different pharmacological agents on the average catecholamine content of PC12 cell vesicles. For example, the quantal size of release events can be increased by treatment of the cells with the dopamine precursor L-3, 4-dihydroxyphenylalanine (L-DOPA) (Kozminski et al. 1998). In one set of experiments, treatment of cells with 50 μM L-DOPA for 40–90 min increased the average quantal size for release by 251% (Pothos et al. 1996). When release events are examined at different times following exposure to L-DOPA, this treatment steadily increases vesicle content, already within 15 min of exposure, and after 60 min of exposure apparently saturates at ~175% of the contents before incubation with L-DOPA (Fig. 5A). Interestingly, L-DOPA initially increases the mean number of exocytosis events in undifferentiated PC12 cells (Fig. 5b). This increase, however, is transient and the release frequency rapidly declines following repeated stimulations indicating depletion of the pool of releasable vesicles (Westerink et al. 2000).
Figure 5.
The mean vesicle content of undifferentiated PC12 cells, determined following KCl-induced exocytosis, increased with increasing duration of superfusion with saline containing 100 μM L-DOPA (a). Each point represents mean ± SD (n = 8 cells) and at all time points the mean vesicle contents was increased significantly when compared with control (t-test, P < 0.01). The drawn line is an exponential curve fitted to the data with a maximum increase amounting to 76% and an exponential time constant with an increase of 21 min. The graph in (b) depicts the relation between the KCl-induced release frequency and the number of successive stimulations at 10–15 min intervals, during which 100 μM L-DOPA-containing saline was superfused. The exocytotic frequency is expressed as a percentage of the value obtained during the first response (set at 100%), which was evoked before exposure to L-DOPA. Each bar represents mean ± SD (n = 8 cells). During the first stimulus after L-DOPA superfusion, the frequency of events was significantly higher than the control value (t-test, P < 0.05). Reproduced with permission from Westerink et al. (2000).
Autoreceptors on PC12 cells
Besides upregulation of vesicle contents by L-DOPA, vesicle content can also be reduced by exposure to amphetamine or reserpine (Sulzer et al. 1995, Kozminski et al. 1998). Importantly, Pothos et al. (1998) used amperometry to demonstrate that quantal size can also be modulated physiologically, e.g. by activation of D2 autoreceptors. In these experiments, PC12 cells were treated with the D2 agonist quinpirole resulting in a ~50% decrease in the quantal size of release events. In contrast to the results for amphetamine and reserpine, which appear to deplete vesicular stores by operating on the vesicle monoamine transporter (VMAT), the experiments with quinpirole provide evidence for a receptor-mediated mechanism that can deplete or modulate quantal release, probably through feedback on the TH-mediated dopamine synthesis pathway.
Variability of vesicle size and the fusion pore
PC12 cells can be loaded with L-DOPA to increase quantal size (Pothos et al. 1996, Kozminski et al. 1998, Colliver et al. 2000b, Westerink et al. 2000). However, it was unclear by what means this enhancement was taking place. Colliver et al. (2000b) used amperometry and transmission electron microscopy to demonstrate that the vesicle size in PC12 increased in the presence of excess L-DOPA and decreased in the presence of reserpine. This was later corroborated for adrenal chromaffin cells by Lindau and coworkers using patch-amperometry (Gong et al. 2003).
More recently, Sombers et al. (2004) have used the ability to vary vesicle size to examine the effect of vesicle size on release via the fusion pore. Although L-DOPA and reserpine increase and decrease vesicle size, respectively, the dense core of the vesicle is mostly unchanged (Colliver et al. 2000b) and it appears that the increased amount of dopamine in the vesicle following L-DOPA exposure is mainly in the clear halo of the vesicle (Sombers et al. 2005). The dense core consists primarily of the catecholamine-storage protein chromogranin A and is thought to expand during exocytosis, facilitating fusion pore expansion (Amatore et al. 2005). Although this is a likely outcome with adrenal chromaffin cell vesicles, it appears that the fusion pore in PC12 cell vesicles can pass through a transition state involving a lipid nanotube. Under conditions where the dense core expands, placing tension on the vesicular membrane, the nanotube is stabilized and constricts. This mechanism has been proposed to explain a higher occurrence of events with a foot for the smaller vesicles with larger ratio of dense core to vesicle volume, following treatment with reserpine (Sombers et al. 2004) and might provide a mechanism for forced release of dopamine from the halo of the vesicle through the fusion pore.
In some cases the fusion pore opens and closes without a full exocytosis event occurring. This phenomenon, termed ‘kiss and run’ has been observed in dopaminergic midbrain neurons using amperometry (Staal et al. 2004). If these events represent a significant communication pathway, then the cell’s ability to modify vesicle size could be a mechanism of neuronal plasticity (learning and memory).
Amperometry at PC12 cells to monitor secretion; environmental factors
Heavy metals
A number of recent studies indicate that environmental and chemical factors can strongly modulate exocytosis in PC12 cells. Of these environmental factors, heavy metals are probably best studied.
Heavy metals have been known for many years to disturb neurotransmission and ion channel function (reviewed in, e.g. Cooper & Manalis 1983, Vijverberg et al. 1994). In PC12 cells, the heavy metal Cd2+ has been shown to block voltage-gated Ca2+ channels (VGCCs; Shafer 1998) and, consequently, inhibit neurotransmitter release (Taylor & Peers 1998, Westerink & Vijverberg 2002b). Additionally, using a combination of amperometry and membrane capacitance measurements, it has been shown that Cd2+, as well as Sr2+ and Ba2+, can induce vesicular catecholamine release in PC12 cells, probably by acting as a partial agonist of the proposed Ca2+-sensor synaptotagmin (Kishimoto et al. 2001).
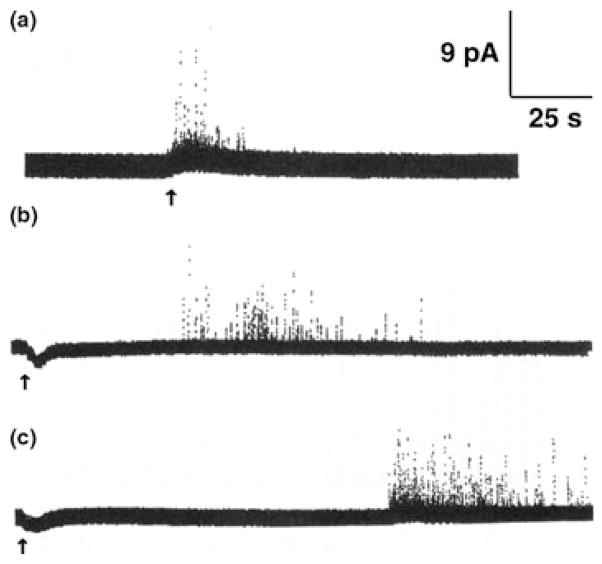
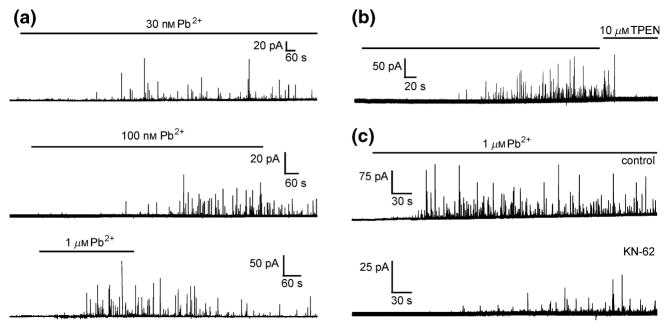
The neurotoxic heavy metal Pb2+ was shown to not only block VGCCs (Shafer 1998), but also to enhance spontaneous neurotransmitter release in populations of PC12 cells (Bressler et al. 1996). This effect was delayed and, as it occurred irrespective of the presence of extracellular Ca2+, was attributed to intracellular effects of Pb2+ (reviewed in Suszkiw 2004, Toscano & Guilarte 2005). Recent amperometric recordings, in combination with the imaging of the intracellular Ca2+ and Pb2+ concentrations, demonstrated the vesicular origin of the enhancement of spontaneous neurotransmitter release in dexamethasone-differentiated PC12 cells (Fig. 6). Pb2+ concentration-dependently induced exocytosis from intact as well as from ionomycin-permeabilized PC12 cells through direct effects on intracellular mechanisms following a concentration-dependent delay (Fig. 6a). Pb2+-induced exocytosis occurred only after partial saturation of intracellular high-affinity buffer components (Fig. 6b; Westerink & Vijverberg 2002a). Pb2+-induced exocytosis could be blocked almost completely by inhibition of Ca2+/calmodulin-dependent protein kinase II (CaM kinase II), indicating that CaM kinase II plays a major role in Pb2+-induced catecholamine exocytosis from PC12 cells (Fig. 6c) (Westerink et al. 2002), although synaptotagmin I is also likely to be involved (Bouton et al. 2001). Thus, PC12 cells have successfully been used to demonstrate that heavy metals can exert inhibitory (block of VGCCs) as well as stimulatory (mimicking Ca2+ as a trigger for exocytosis) effects on neurotransmission.
Figure 6.
Effects of the heavy metal Pb2+ on vesicular catecholamine release from ionomycin-permeabilized dexamethasone-differentiated PC12 cells. (a) Amperometric recordings from permeabilized PC12 cells superfused with Ca2+-free saline containing 5 μM ionomycin and 0.03, 0.1 and 1 μM Pb2+, demonstrating the concentration dependence of Pb2+-induced exocytosis. (b) Amperometric recording from a permeabilized PC12 cells showing vesicular catecholamine release during superfusion with nominal Ca2+-free saline containing 1 μM Pb2+. The membrane-permeable heavy metal chelator TPEN rapidly reduces the intracellular Pb2+ concentration below the threshold for release, whereas the membrane-impermeable chelator EGTA is ineffective (not shown), demonstrating the Ca2+-independence of Pb2+-evoked exocytosis. (c) Amperometric recordings from ionomycin-permeabilized PC12 cells reveal that saline containing 1 μM Pb2+ induced vesicular release (control), which is strongly inhibited by co-application of an inhibitor of CaM kinase II (10 μM KN-62), whereas inhibition of Protein Kinase C does not appear to affect Pb2+-induced exocytosis (not shown). Modified after Westerink & Vijverberg (2002a) and Westerink et al. (2002).
Organic solvents
Organic solvents, particularly toluene, are occasionally used as a drug of abuse and are known to cause a variety of neurotoxic and neuropathological effects. Exposure to toluene causes post-synaptic effects on a variety of receptors (reviewed in Bowen et al. 2006). Using amperometry at PC12 cells, it was shown that toluene induced catecholamine exocytosis in dexamethasone-differentiated PC12 cells, which depended on the influx of extracellular Ca2+ through VGCCs (Westerink & Vijverberg 2002b). However, in another study, it was shown that toluene dose-dependently inhibits the depolarization-induced rise in intracellular Ca2+ in NGF-differentiated PC12 cells (Tillar et al. 2002). These rather contradictory results might be explained by the different effects of NGF and dexamethasone on the density and expression patterns of VGCCs. Nonetheless, the increase in catecholamine release as observed in the amperometric study is in agreement with elevated levels of extracellular dopamine in rat brain (Riegel & French 1999).
Persistent organic pollutants
Several persistent environmental compounds, like polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs), are known to accumulate in biological tissues and cause behavioural symptoms as well as alteration in neurotransmission. Potential mechanisms underlying the effects of PCBs on the catecholaminergic system include changes in dopamine homeostasis, inhibition of vesicular catecholamine uptake and alteration of the intracellular Ca2+ concentration (reviewed in Fonnum et al. 2006, Mariussen & Fonnum 2006). Surprisingly, to date there is only one study in which amperometry was used to demonstrate that acute exposure of PC12 cells to the non-planar congener PCB 4 and to the coplanar congener PCB 126, but not exposure to the non-planar congener PCB 128, increases spontaneous catecholamine exocytosis. Unexpectedly, acute and subchronic exposure to these PCBs failed to cause changes in the contents of catecholamine-containing vesicles in this study (Westerink & Vijverberg 2002c), suggesting that the toxicological focus may need to be redirected from the turnover of catecholamines to other effects including the mechanisms underlying the subtle changes in basal neurotransmitter release and its functional consequences.
Brominated flame retardants, among them PBDEs, have been shown to inhibit vesicular neurotransmitter uptake and induce neuronal cell death (reviewed in Fonnum et al. 2006, Mariussen & Fonnum 2006). Only recently, it was shown that a single exposure of young mice to BDE-47 reduced long-term potentiation recorded in hippocampal slices. This reduction in long-term potentiation was probably caused by a post-synaptic mechanism and coincided with a decrease in post-synaptic glutamate receptor subunits NR2B and GluR1 and autophosphorylated-active αCaM kinase II. The involvement of a post-synaptic mechanism was further underlined by in vitro research in dexamethasone-differentiated PC12 cells revealing that acute exposure to high concentrations of BDE-47 (20 μM) induced only a modest increase in intracellular Ca2+ and basal catecholamine exocytosis (Dingemans et al. 2007).
These combined findings illustrate the usefulness of neuroendocrine PC12 cells as models for past and future neurotoxicological studies.
Amperometry at PC12 cells to monitor secretion: relation to health and disease
The successful combination of amperometry and PC12 cells is now in use for over a decade to study the mechanisms of exocytosis, although the field is still expanding. In recent years, PC12 cells were used to shed some light on the mechanisms underlying the diseased brain.
Hypoxia is a serious condition that can result in ischaemia. Hypoxia results in the inhibition of O2-sensitive K+ channels and, consequently, depolarization. As PC12 cells contain these O2-sensitive channels, they can be used as a chemosensory model to study the cellular effects of hypoxia. In a series of studies, Peers et al. showed that acute hypoxia stimulated basal and depolarization-induced exocytosis in PC12 cells. The enhancement of exocytosis resulted from hypoxia-induced inhibition of the Kv1.2 K+ channel (Taylor & Peers 1998). Additionally, it was shown that chronic hypoxia increased exocytosis in response to acute hypoxia, at least partly by increasing O2-sensitive K+ channel-mediated depolarization. Importantly, the amount of catecholamines released per vesicle also increased (Taylor & Peers 1999), which is probably because of increased expression of the rate-limiting enzyme in dopamine synthesis TH. On the other hand, prolonged hypoxia resulted in the formation of amyloid β-peptides (AβPs). These AβPs cause not only selective augmentation of calcium influx through L-type Ca2+ channels but also induce the formation of a Cd2+-resistant Ca2+ influx pathway (Green & Peers 2001). The hypoxic enhancement of catecholamine exocytosis was markedly reduced by inhibitors of Alzheimer’s AβPs and mimicked by direct application of AβPs under normoxic conditions (Taylor et al. 1999). The formation of AβP and generation of reactive oxygen species from AβP were prerequisites for the hypoxic effect. Consequently, the hypoxia-induced enhancement of exocytosis could be inhibited by antioxidants like ascorbic acid and melatonin (Green et al. 2002). Thus, using PC12 cells as a model it was shown that acute hypoxia induces exocytosis, whereas prolonged hypoxia can induce formation of Ca2+-permeable AβP channels resulting in excessive exocytosis. This excessive secretion in turn probably contributes to AβP pathophysiology following cerebral ischaemia.
Notably, AβPs have a marked resemblance to some prion proteins. Prion diseases, like Creutzfledt Jakob disease, are characterized by neuro-degeneration and arise from infection with the protease-resistant scrapie form of prion. PC12 cells were used to investigate the cellular mechanisms by which prion protein fragments cause neuronal dysfunction. In PC12 cells prion protein fragment 106–126 also generated a Cd2+-resistant Ca2+ influx pathway, thereby augmenting catecholamine exocytosis in a concentration-dependent manner. This mechanism of action was remarkably comparable with the enhancement of exocytosis following formation of AβPs, although prion protein fragment 106–126 did not increase the amount of catecholamines released per vesicle (Taylor et al. 2001).
Some forms of familial Parkinson’s disease, a neurodegenerative disorder characterized by a loss of dopaminergic neurons in the substantia nigra and the presence of Lewy bodies are caused by mutations in α-synuclein (α-syn) or by the over-expression of wild-type α-syn. Recently, PC12 cells were used to investigate how these mutations or the enhanced expression of α-syn affects catecholamine homeostasis and neurotransmission. Using intracellular patch amperometry (see below), it was demonstrated that PC12 cells over-expressing wild-type or mutated α-syn display elevated levels of cytosolic catecholamines. This effect appeared to result from leakage of catecholamines from the vesicles (Mosharov et al. 2006). Importantly, these PC12 cells displayed impaired depolarization-evoked dopamine release. This is rather surprising as the number of morphologically docked vesicles was increased (Larsen et al. 2006). These findings suggest that exocytosis under these conditions is inhibited by interference with a step following vesicle docking, but preceding vesicle fusion.
The cellular mechanisms underlying the antimanic properties of lithium have also been investigated using PC12 cells. Exposure of NGF-differentiated PC12 cells to lithium results in an increase in mRNA encoding for secretogranin II and VMAT1, proteins associated with filling of LDCVs with catecholamines. Surprisingly, this upregulation is absent in undifferentiated PC12 cells (Cordeiro et al. 2000). The selective increase in mRNA in NGF-differentiated PC12 cells correlated with an increase in regulated secretion of 3H-labelled dopamine from PC12 cell populations (Cordeiro et al. 2004). More recently, using amperometry, it was found that lithium exposure increased K+-evoked exocytosis. The increase in exocytosis was without effects on vesicle contents and spike characteristics, although vesicle diameter was increased by ~15% (Umbach et al. 2005). This is rather surprising as an increase in vesicle diameter and an increase in VMAT are usually correlated and accompanied by an increase in vesicle contents (Colliver et al. 2000b). Either way, the mechanisms underlying the antimanic action of lithium are probably more complex than initially thought, although the future use of PC12 cells will most probably contribute to resolving the underlying mechanisms.
The future of amperometric measurements at PC12 cells
As science and technology progress, new applications become available to further elucidate the mechanism of exocytosis. Additionally, techniques already in use for other cell types may be used to resolve the modulation of regulated secretion in PC12 cells. One of the older applications is voltammetry at carbon paste (Wightman et al. 1976), and later carbon fibre (Gonon et al. 1980, Dayton et al. 1981, Ewing et al. 1983) electrodes. These were originally used to measure levels of neurotransmitters and metabolites in cerebrospinal fluid and in defined brain areas in vivo. Using voltammetry, the concentration profiles, instead of the amount, and identity of neurotransmitters and metabolites can be resolved with high temporal resolution. In the 1990s, cyclic voltammetry was applied by Wightman et al. (1991) to identify the neurotransmitters secreted by chromaffin cells as catecholamines, whereas amperometry was used to determine the amount of secreted catecholamines per vesicle with enhanced temporal resolution. In 1998, it was proven in PC12 cells that the secreted neurotransmitters, as detected with amperometry, were indeed catecholamines, most probably dopamine (Kozminski et al. 1998). In the same study, it was shown that following exposure to L-DOPA, the amount of secreted catecholamines increased, although the measured catecholamine concentration was rather similar in all vesicles clearly suggesting that the concentration of catecholamines within a vesicle is tightly regulated.
Further evidence for the tight regulation of the intravesicular catecholamine concentration was obtained using patch-amperometry. Patch-amperometry is an elegant combination of cell-attached membrane capacitance measurement, thus providing single-vesicle resolution, with amperometry (Dernick et al. 2005). Patch-amperometry was first used in 1997 to simultaneous determine the opening of individual fusion pores and of the kinetics of catecholamine release from the same vesicle in chromaffin cells. This study demonstrated that fusion pore diameter stays at <3 nm for a variable period (up to seconds) before expanding and, importantly, the existence of kiss-and-run exocytosis (Albillos et al. 1997). Several years later, patch amperometry was used with chromaffin cells (Gong et al. 2003) to substantiate previous work combining amperometry and TEM of PC12 cells (Colliver et al. 2000b) in both cases showing that a decrease and increase in quantal size, induced by incubation with reserpine or L-DOPA, are associated with a respective decrease and increase in vesicle size. Thus, vesicles can change size in response to changes in transmitter content to maintain a stable intravesicular neurotransmitter concentration.
Using patch-amperometry in a slightly different way, i.e. intracellular patch electrochemistry, it was possible to measure directly the concentration of oxidizable molecules in the cytosol of chromaffin cells. This study demonstrated that the strict regulation of the catecholamine concentration is not restricted to the vesicle, but also holds for the cytosol (Mosharov et al. 2003). Up to now, because of their smaller vesicle size and cytosolic catecholamine content, it has not been possible to use these techniques at PC12 cells. Realizing the advances in sensitivity needed to carry out patch amperometry at PC12 cells is probably only a matter of time.
Acknowledgments
We thank the USA National Institutes of Health and National Science Foundation for funding work reviewed here and we thank all of our present and past colleagues for work cited herein.
Footnotes
Conflict of interest
There is no conflict of interest.
References
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- Alvarez de Toledo G, Fernandez-Chacon R, Fernandez JM. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- Amatore C, Arbault S, Bonifas I, Bouret Y, Erard M, Ewing AG, Sombers LA. Correlation between vesicle quantal size and fusion pore release in chromaffin cell exocytosis. Biophys J. 2005;88:4411–4420. doi: 10.1529/biophysj.104.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay JW, Morgan A, Burgoyne RD. Calcium-dependent regulation of exocytosis. Cell Calcium. 2005;38:343–353. doi: 10.1016/j.ceca.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bouton CM, Frelin LP, Forde CE, Arnold Godwin H, Pevsner J. Synaptotagmin I is a molecular target for lead. J Neurochem. 2001;76:1724–1735. doi: 10.1046/j.1471-4159.2001.00168.x. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Batis JC, Paez-Martinez N, Cruz SL. The last decade of solvent research in animal models of abuse: mechanistic and behavioral studies. Neurotoxicol Teratol. 2006;28:636–647. doi: 10.1016/j.ntt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Bressler JP, Belloni-Olivi L, Forman S, Goldstein GW. Distinct mechanisms of neurotransmitter release from PC 12 cells exposed to lead. J Neurosci Res. 1996;46:678–685. doi: 10.1002/(SICI)1097-4547(19961215)46:6<678::AID-JNR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- Cans AS, Wittenberg N, Karlsson R, Sombers LA, Karisson M, Orwar O, Ewing AG. Artificial cells: unique insights into exocytosis using liposomes and lipid nanotubes. Proc Natl Acad Sci USA. 2003;100:400–404. doi: 10.1073/pnas.232702599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TK, Luo G, Ewing AG. Amperometric monitoring of stimulated catecholamine release from rat phaeochromocytoma (PC12) cells at the zeptomole level. Anal Chem. 1994;66:3031–3035. doi: 10.1021/ac00091a007. [DOI] [PubMed] [Google Scholar]
- Chow RH, von Rüden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992;356:60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Clark RA, Ewing AG. Quantitative measurements of released amines from individual exocytosis events. Mol Neurobiol. 1997;15:1–16. doi: 10.1007/BF02740612. [DOI] [PubMed] [Google Scholar]
- Colliver TL, Hess EJ, Pothos EN, Sulzer D, Ewing AG. Quantitative and statistical analysis of the shape of amperometric spikes recorded from two populations of cells. J Neurochem. 2000a;74:1086–1097. doi: 10.1046/j.1471-4159.2000.741086.x. [DOI] [PubMed] [Google Scholar]
- Colliver TL, Pyott SJ, Achalabun M, Ewing AG. VMAT-mediated changes in quantal size and vesicular volume. J Neurosci. 2000b;20:5276–5282. doi: 10.1523/JNEUROSCI.20-14-05276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GP, Manalis RS. Influence of heavy metals on synaptic transmission: a review. Neurotoxicology. 1983;4:69–83. [PubMed] [Google Scholar]
- Cordeiro ML, Umbach JA, Gundersen CB. Lithium ions up-regulate mRNAs encoding dense-core vesicle proteins in nerve growth factor-differentiated PC12 cells. J Neurochem. 2000;75:2622–2625. doi: 10.1046/j.1471-4159.2000.0752622.x. [DOI] [PubMed] [Google Scholar]
- Cordeiro ML, Gundersen CB, Umbach JA. Convergent effects of lithium and valproate on the expression of proteins associated with large dense core vesicles in NGF-differentiated PC12 cells. Neuropsychopharmacology. 2004;29:39–44. doi: 10.1038/sj.npp.1300288. [DOI] [PubMed] [Google Scholar]
- Coupland RE. Determining sizes and distribution of sizes of spherical bodies such as chromaffin granules in tissue sections. Nature. 1968;217:384–388. doi: 10.1038/217384a0. [DOI] [PubMed] [Google Scholar]
- Dayton MA, Ewing AG, Wightman RM. Evaluation of amphetamine-induced in vivo electrochemical response. Eur J Pharmacol. 1981;75:141–144. doi: 10.1016/0014-2999(81)90074-1. [DOI] [PubMed] [Google Scholar]
- Dernick G, Gong LW, Tabares L, Alvarez de Toledo G, Lindau M. Patch amperometry: high-resolution measurements of single-vesicle fusion and release. Nat Methods. 2005;2:699–708. doi: 10.1038/nmeth0905-699. [DOI] [PubMed] [Google Scholar]
- Dingemans MML, Ramakers GMJ, Gardoni F, van Kleef RGDM, Bergman A, Di Luca M, van den Berg M, Westerink RHS, Vijverberg HPM. Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environ Health Perspect. 2007;115:865–870. doi: 10.1289/ehp.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhamdani A, Brown ME, Artalejo CR, Palfrey HC. Enhancement of the dense-core vesicle secretory cycle by glucocorticoid differentiation of PC12 cells: characteristics of rapid exocytosis and endocytosis. J Neurosci. 2000;20:2495–2503. doi: 10.1523/JNEUROSCI.20-07-02495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing AG, Bigelow JC, Wightman RM. Direct in vivo monitoring of dopamine released from two striatal compartments in the rat. Science. 1983;221:169–171. doi: 10.1126/science.6857277. [DOI] [PubMed] [Google Scholar]
- Finnegan JM, Pihel K, Cahill PS, Huang L, Zerby SE, Ewing AG, Kennedy RT, Wightman RM. Vesicular quantal size measured by amperometry at chromaffin, mast, phaeochromocytoma, and pancreatic beta-cells. J Neurochem. 1996;66:1914–1923. doi: 10.1046/j.1471-4159.1996.66051914.x. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Burgoyne RD. The effect of transfection with Botulinum neurotoxin C1 light chain on exocytosis measured in cell populations and by single-cell amperometry in PC12 cells. Pflugers Arch. 1999;437:754–762. doi: 10.1007/s004240050842. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Mariussen E, Reistad T. Molecular mechanisms involved in the toxic effects of polychlorinated biphenyls (PCBs) and brominated flame retardants (BFRs) J Toxicol Environ Health A. 2006;69:21–35. doi: 10.1080/15287390500259020. [DOI] [PubMed] [Google Scholar]
- Gong LW, Hafez I, Alvarez de Toledo G, Lindau M. Secretory vesicles membrane area is regulated in tandem with quantal size in chromaffin cells. J Neurosci. 2003;23:7917–7921. doi: 10.1523/JNEUROSCI.23-21-07917.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon F, Buda M, Cespuglio R, Jouvet M, Pujol JF. In vivo electrochemical detection of catechols in the neostriatum of anaesthetized rats: dopamine or DOPAC? Nature. 1980;286:902–904. doi: 10.1038/286902a0. [DOI] [PubMed] [Google Scholar]
- Green KN, Peers C. Amyloid beta peptides mediate hypoxic augmentation of Ca2+ channels. J Neurochem. 2001;77:953–956. doi: 10.1046/j.1471-4159.2001.00338.x. [DOI] [PubMed] [Google Scholar]
- Green KN, Boyle JP, Peers C. Hypoxia potentiates exocytosis and Ca2+ channels in PC12 cells via increased amyloid beta peptide formation and reactive oxygen species generation. J Physiol. 2002;541:1013–1023. doi: 10.1113/jphysiol.2002.017582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Rein G. Release, storage and uptake of catecholamines by a clonal cell line of nerve growth factor (NGF) responsive phaeochromocytoma cells. Brain Res. 1977;129:247–263. doi: 10.1016/0006-8993(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a nor-adrenergic clonal line of rat adrenal phaeochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Wang CT, Bai J, Chapman ER, Jackson MB. Transmembrane segments of syntaxin line the fusion pore of Ca2+-triggered exocytosis. Science. 2004;304:289–292. doi: 10.1126/science.1095801. [DOI] [PubMed] [Google Scholar]
- Helle KB, Serck-Hanssen G. The adrenal medulla: a model for studies of hormonal and neuronal storage and release mechanisms. Mol Cell Biochem. 1975;6:127–146. doi: 10.1007/BF01732006. [DOI] [PubMed] [Google Scholar]
- Holz RW. Control of exocytosis from adrenal chromaffin cells. Cell Mol Neurobiol. 1988;8:259–268. doi: 10.1007/BF00711168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Liu TT, Ninomiya Y, Takagi H, Yoshioka T, Ellis-Davies GC, Miyashita Y, Kasai H. Ion selectivities of the Ca2+ sensors for exocytosis in rat phaeochromocytoma cells. J Physiol. 2001;533:627–637. doi: 10.1111/j.1469-7793.2001.t01-1-00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KD, Gutman DA, Davila V, Sulzer D, Ewing AG. Voltammetric and pharmacological characterization of dopamine release from single exocytotic events at rat phaeochromocytoma (PC12) cells. Anal Chem. 1998;70:3123–3130. doi: 10.1021/ac980129f. [DOI] [PubMed] [Google Scholar]
- Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczyszyn DJ, Jankowski JA, Viveros OH, Diliberto EJ, Near JA, Wightman RM. Nicotinic receptor-mediated catecholamine secretion from individual chromaffin cells. Chemical evidence for exocytosis. J Biol Chem. 1990;265:14736–14737. [PubMed] [Google Scholar]
- Liu L, Liao H, Castle A, Zhang J, Casanova J, Szabo G, Castle D. SCAMP2 interacts with Arf6 and phospholipase D1 and links their function to exocytotic fusion pore formation in PC12 cells. Mol Biol Cell. 2005;16:4463–4472. doi: 10.1091/mbc.E05-03-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livett BG, Boksa P, Dean DM, Mizobe F, Lindenbaum MH. Use of isolated chromaffin cells to study basic release mechanisms. J Auton Nerv Syst. 1983;7:59–86. doi: 10.1016/0165-1838(83)90069-3. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. Neurochemical targets and behavioral effects of organohalogen compounds: an update. Crit Rev Toxicol. 2006;36:253–289. doi: 10.1080/10408440500534164. [DOI] [PubMed] [Google Scholar]
- Moore JM, Papke JB, Cahill AL, Harkins AB. Stable gene silencing of synaptotagmin I in rat PC12 cells inhibits Ca2+-evoked release of catecholamine. Am J Physiol Cell Physiol. 2006;291:C270–C281. doi: 10.1152/ajpcell.00539.2005. [DOI] [PubMed] [Google Scholar]
- Mosharov EV, Gong LW, Khanna B, Sulzer D, Lindau M. Intracellular patch electrochemistry: regulation of cytosolic catecholamines in chromaffin cells. J Neurosci. 2003;23:5835–5845. doi: 10.1523/JNEUROSCI.23-13-05835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov EV, Staal RGW, Bove J, Prou D, Hananiya A, Markov D, Poulsen N, Larsen KE, Moore CM, Troyer MD, Edwards RH, Przedborski S, Sulzer D. Alpha-synuclein overexpression increases cytosolic catecholamine concentration. J Neurosci. 2006;26:9304–9311. doi: 10.1523/JNEUROSCI.0519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN, Desmond M, Sulzer D. L-3, 4-dihydroxyphenylalanine increases the quantal size of exocytotic dopamine release in vitro. J Neurochem. 1996;66:629–636. doi: 10.1046/j.1471-4159.1996.66020629.x. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Przedborski S, Davila V, Schmitz Y, Sulzer D. D2-Like dopamine autoreceptor activation reduces quantal size in PC12 cells. J Neurosci. 1998;18:5575–5585. doi: 10.1523/JNEUROSCI.18-15-05575.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel AC, French ED. Acute toluene induces biphasic changes in rat spontaneous locomotor activity which are blocked by remoxipride. Pharmacol Biochem Behav. 1999;62:399–402. doi: 10.1016/s0091-3057(98)00062-8. [DOI] [PubMed] [Google Scholar]
- Schubert D, Klier FG. Storage and release of acetylcholine by a clonal cell line. Proc Natl Acad Sci USA. 1977;74:5184–5188. doi: 10.1073/pnas.74.11.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, LaCorbiere M, Klier FG, Steinbach JH. The modulation of neurotransmitter synthesis by steroid hormones and insulin. Brain Res. 1980;190:67–79. doi: 10.1016/0006-8993(80)91160-9. [DOI] [PubMed] [Google Scholar]
- Shafer TJ. Effects of Cd2+, Pb2+ and CH3Hg+ on high voltage-activated calcium currents in phaeochromocytoma (PC12) cells: potency, reversibility, interactions with extra-cellular Ca2+ and mechanisms of block. Toxicol Lett. 1998;99:207–221. doi: 10.1016/s0378-4274(98)00225-2. [DOI] [PubMed] [Google Scholar]
- Sombers LA, Hanchar HJ, Colliver TL, Wittenberg N, Cans A, Arbault S, Amatore C, Ewing AG. The effects of vesicular volume on secretion through the fusion pore in exocytotic release from PC12 cells. J Neurosci. 2004;24:303–309. doi: 10.1523/JNEUROSCI.1119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombers LA, Maxson MM, Ewing AG. Loaded dopamine is preferentially stored in the halo portion of PC12 cell dense core vesicles. J Neurochem. 2005;93:1122–1131. doi: 10.1111/j.1471-4159.2005.03087.x. [DOI] [PubMed] [Google Scholar]
- Staal RGW, Mosharov EV, Sulzer D. Dopamine neurons release transmitter via a flickering fusion pore. Nature Neurosci. 2004;7:341–346. doi: 10.1038/nn1205. [DOI] [PubMed] [Google Scholar]
- Stallcup WB. Sodium and calcium fluxes in a clonal nerve cell line. J Physiol (Lond) 1979;286:525–540. doi: 10.1113/jphysiol.1979.sp012635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing AG. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suszkiw JB. Presynaptic disruption of transmitter release by lead. Neurotoxicology. 2004;25:599–604. doi: 10.1016/j.neuro.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Taylor SC, Peers C. Hypoxia evokes catecholamine secretion from rat phaeochromocytoma PC-12 cells. Biochem Biophys Res Commun. 1998;248:13–17. doi: 10.1006/bbrc.1998.8905. [DOI] [PubMed] [Google Scholar]
- Taylor SC, Peers C. Chronic hypoxia enhances the secretory response of rat phaeochromocytoma cells to acute hypoxia. J Physiol. 1999;514:483–491. doi: 10.1111/j.1469-7793.1999.483ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SC, Batten TF, Peers C. Hypoxic enhancement of quantal catecholamine secretion. Evidence for the involvement of amyloid beta-peptides. J Biol Chem. 1999;274:31217–31222. doi: 10.1074/jbc.274.44.31217. [DOI] [PubMed] [Google Scholar]
- Taylor SC, Green KN, Smith IF, Peers C. Prion protein fragment 106–126 potentiates catecholamine secretion from PC-12 cells. Am J Physiol Cell Physiol. 2001;281:C1850–C1857. doi: 10.1152/ajpcell.2001.281.6.C1850. [DOI] [PubMed] [Google Scholar]
- Tillar R, Shafer TJ, Woodward JJ. Toluene inhibits voltage-sensitive calcium channels expressed in phaeochromocytoma cells. Neurochem Int. 2002;41:391–397. doi: 10.1016/s0197-0186(02)00048-7. [DOI] [PubMed] [Google Scholar]
- Tischler AS, Perlman RL, Morse GM, Sheard BE. Glucocorticoids increase catecholamine synthesis and storage in PC12 phaeochromocytoma cell cultures. J Neurochem. 1983;40:364–370. doi: 10.1111/j.1471-4159.1983.tb11291.x. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Guilarte TR. Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev. 2005;49:529–554. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Travis ER, Wightman RM. Spatio-temporal resolution of exocytosis from individual cells. Annu Rev Biophys Biomol Struct. 1998;27:77–103. doi: 10.1146/annurev.biophys.27.1.77. [DOI] [PubMed] [Google Scholar]
- Umbach JA, Zhao Y, Gundersen CB. Lithium enhances secretion from large dense-core vesicles in nerve growth factor-differentiated PC12 cells. J Neurochem. 2005;94:1306–1314. doi: 10.1111/j.1471-4159.2005.03277.x. [DOI] [PubMed] [Google Scholar]
- Vijverberg HPM, Oortgiesen M, Leinders T, van Kleef RGDM. Metal interactions with voltage- and receptor-activated ion channels. Environ Health Perspect. 1994;102(Suppl 3):153–158. doi: 10.1289/ehp.94102s3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CT, Grishanin R, Earles CA, Chang PY, Martin TF, Chapman ER, Jackson MB. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 2001;294:1111–1115. doi: 10.1126/science.1064002. [DOI] [PubMed] [Google Scholar]
- Wang CT, Lu JC, Bai J, et al. Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature. 2003;424:943–947. doi: 10.1038/nature01857. [DOI] [PubMed] [Google Scholar]
- Wang CT, Bai J, Chang PY, Chapman ER, Jackson MB. Synaptotagmin-Ca2+ triggers two sequential steps in regulated exocytosis in rat PC12 cells: fusion pore opening and fusion pore dilation. J Physiol. 2006;570:295–307. doi: 10.1113/jphysiol.2005.097378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink RHS. Targeting exocytosis: ins and outs of the modulation of quantal dopamine release. CNS Neurol Disord Drug Targets. 2006;5:57–77. doi: 10.2174/187152706784111597. [DOI] [PubMed] [Google Scholar]
- Westerink RHS, Vijverberg HPM. Ca2+-independent vesicular catecholamine release in PC12 cells by nanomolar concentrations of Pb2+ J Neurochem. 2002a;80:861–873. doi: 10.1046/j.0022-3042.2001.00751.x. [DOI] [PubMed] [Google Scholar]
- Westerink RHS, Vijverberg HPM. Toluene-induced, Ca2+-dependent vesicular catecholamine release in rat PC12 cells. Neurosci Lett. 2002b;326:81–84. doi: 10.1016/s0304-3940(02)00315-4. [DOI] [PubMed] [Google Scholar]
- Westerink RHS, Vijverberg HPM. Vesicular catecholamine release from rat PC12 cells on acute and subchronic exposure to polychlorinated biphenyls. Toxicol Appl Pharmacol. 2002c;183:153–159. doi: 10.1006/taap.2002.9482. [DOI] [PubMed] [Google Scholar]
- Westerink RHS, de Groot A, Vijverberg HPM. Heterogeneity of catecholamine-containing vesicles in PC12 cells. Biochem Biophys Res Commun. 2000;270:625–630. doi: 10.1006/bbrc.2000.2470. [DOI] [PubMed] [Google Scholar]
- Westerink RHS, Klompmakers AA, Westenberg HGM, Vijverberg HPM. Signaling pathways involved in Ca2+- and Pb2+-induced vesicular catecholamine release from rat PC12 cells. Brain Res. 2002;957:25–36. doi: 10.1016/s0006-8993(02)03580-1. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Strope E, Plotsky PM, Adams RN. Monitoring of transmitter metabolites by voltammetry in cerebrospinal fluid following neural pathway stimulation. Nature. 1976;262:145–146. doi: 10.1038/262145a0. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Jr, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerby SE, Ewing AG. Electrochemical monitoring of individual exocytotic events from the varicosities of differentiated PC12 cells. Brain Res. 1996a;712:1–10. doi: 10.1016/0006-8993(95)01383-0. [DOI] [PubMed] [Google Scholar]
- Zerby SE, Ewing AG. The latency of exocytosis varies with the mechanism of stimulated release in PC12 cells. J Neurochem. 1996b;66:651–657. doi: 10.1046/j.1471-4159.1996.66020651.x. [DOI] [PubMed] [Google Scholar]