Abstract
In the American Thoracic Society/European Respiratory Society consensus classification, idiopathic interstitial pneumonias are classified into seven clinicopathologic entities. The classification is largely based on histopathology, but depends on the close interaction of clinician, radiologist, and pathologist. An accurate diagnosis can be very difficult, especially when deciding between idiopathic pulmonary fibrosis and fibrotic nonspecific interstitial pneumonia; better diagnostic markers are needed. The prognosis of idiopathic pulmonary fibrosis is very poor, with median survival of 2–4 yr after the diagnosis, yet the course of individual patients is highly variable. Predicting prognosis in the individual patient is challenging but various clinical and radiologic variables have been identified. According to several recent clinical trials, the natural history of this disease may involve periods of relative stability punctuated by acute exacerbations of disease that result in substantial morbidity or death. Nonspecific interstitial pneumonia is characterized by a distinct histopathologic appearance and a better prognosis than idiopathic pulmonary fibrosis. However, there is still confusion and controversy over the relationship between idiopathic pulmonary fibrosis and fibrotic nonspecific interstitial pneumonia.
Keywords: acute exacerbation; idiopathic pulmonary fibrosis, classification, natural history, prognosis; nonspecific interstitial pneumonia
The idiopathic interstitial pneumonias (IIPs) are a group of diffuse parenchymal lung diseases of unknown etiology with varying degrees of inflammation and fibrosis. In 1969, Liebow and Carrington published a landmark histopathologic classification schema for the IIPs consisting of five patterns: usual interstitial pneumonia (UIP), bronchiolitis obliterans interstitial pneumonia and diffuse alveolar damage, desquamative interstitial pneumonia, lymphocytic interstitial pneumonia (LIP), and giant cell interstitial pneumonia (1). Katzenstein and Myers emphasized the need for consistent histopathologic criteria for the classification of the IIPs and incorporated several key distinguishing features: the temporal heterogeneity of inflammation and fibrosis, the extent of inflammation, the extent of fibroblastic proliferation, the extent of accumulation of intraalveolar macrophages, and the presence of honeycombing or hyaline membranes (2).
The histopathologic classification of the IIPs has evolved over time, most recently codified in the American Thoracic Society/European Respiratory Society (ATS/ERS) 2002 consensus classification statement (3) (Table 1). This classification separates the IIPs into seven clinicopathologic entities (in order of relative frequency): idiopathic pulmonary fibrosis (IPF; frequency, ∼ 47 to 64%), nonspecific interstitial pneumonia (NSIP; frequency, ∼ 14 to 36%), respiratory bronchiolitis–associated interstitial lung disease, respiratory bronchiolitis–associated interstitial lung disease/desquamative interstitial pneumonia (frequency, ∼ 10 to 17%), cryptogenic organizing pneumonia (frequency, ∼ 4 to 12%), acute interstitial pneumonia (frequency < 2%), and LIP (frequency < 2%). Although the histopathologic patterns provide the basis for the ATS/ERS classification, it is no longer the “gold standard” for the classification of the IIPs (3). The ATS/ERS consensus statement emphasizes the importance of dynamic interactions among clinicians, radiologists, and pathologists to arrive at a final clinico-radiologic-pathologic diagnosis. This new multidisciplinary gold standard for the diagnosis of the IIPs is a combined clinico-radiographic-pathologic analysis arrived at by a dynamic, integrated process among clinicians, radiologists, and pathologists (when a surgical lung biopsy is available) that improves diagnostic confidence and interobserver agreement and should be routinely employed in the diagnosis of the IIPs (3, 4).
TABLE 1.
HISTOPATHOLOGIC CLASSIFICATION SCHEMES OF IDIOPATHIC INTERSTITIAL PNEUMONIAS
| ATS/ERS (2002) (3)
|
|||
|---|---|---|---|
| Liebow and Carrington (1969) (1) | Katzenstein and Myers (1998) (2) | Histologic Pattern | Clinico-Radiographic-Pathologic Diagnosis |
| UIP | UIP | UIP | Idiopathic pulmonary fibrosis |
| DIP | DIP | DIP | Desquamative interstitial pneumonia |
| RB-ILD | RB | Respiratory bronchiolitis interstitial lung disease | |
| LIP | LIP | Lymphoid interstitial pneumonia | |
| GIP | |||
| BIP | OP | Cryptogenic organizing pneumonia | |
| AIP | DAD | Acute interstitial pneumonia | |
| NSIP | NSIP | Nonspecific interstitial pneumonia | |
Definition of abbreviations: AIP = acute interstitial pneumonia; BIP = bronchiolitis obliterans interstitial pneumonia and diffuse alveolar damage; DAD = diffuse alveolar damage; DIP = desquamative interstitial pneumonia; GIP = giant cell interstitial pneumonia; LIP = lymphocytic interstitial pneumonia; NSIP = nonspecific interstitial pneumonia; OP = organizing pneumonia; RB = respiratory bronchiolitis; RB-ILD = respiratory bronchiolitis–interstitial lung disease; UIP = usual interstitial pneumonia.
The discussion of all seven forms of IIP is beyond the scope of this short review; several reviews on these entities have been recently published (5–8). Table 2 summarizes some of the key features of the IIPs. The pathologic, physiologic, and radiologic aspects of these diseases and the approach to treatment are discussed in more detail by other authors in this series. This review will focus on IPF and NSIP.
TABLE 2.
CLINICAL, RADIOLOGIC, AND HISTOLOGIC FEATURES, TREATMENT, AND PROGNOSIS OF THE IDIOPATHIC INTERSTITIAL PNEUMONIAS
| Idiopathic Pulmonary Fibrosis | NSIP | DIP, RB-ILD | Cryptogenic Organizing Pneumonia | Acute Interstital Pneumonia | Lymphocytic Interstitial Pneumonia | |
|---|---|---|---|---|---|---|
| Duration of illness | Chronic (> 12 mo) | Subacute to chronic (mo to yr) | Subacute (wk to mo); | Subacute (< 3 mo) | Abrupt (1 to 2 wk) | Chronic (> 12 mo) |
| HRCT | • Peripheral, subpleural, basal predominance | • Peripheral, subpleural, basal, symmetric | • DIP: diffuse ground-glass opacity in the middle and lower lung zones | • Subpleural or peribronchial | • Diffuse, bilateral | • Diffuse |
| • Ground-glass opacities often with lobular sparing | • Centrilobular nodules, | |||||
| • Patchy consolidation | • Ground-glass attenuation, | |||||
| • Reticular opacities | • Ground-glass attenuation | • Nodules | ||||
| • Architectural distortion | • Septal and bronchovascular thickening, | |||||
| • Consolidation (uncommon) | • RB-ILD: bronchial wall thickening; centrilobular nodules; patchy ground-glass opacity | |||||
| • Traction bronchiectasis/ bronchiolectasis | ||||||
| • Lower lobe volume loss | • Thin-walled cysts | |||||
| • Honeycombing | • Subpleural sparing may be seen | |||||
| Treatment | Poor response to corticosteroid or cytotoxic agents | Corticosteroid responsiveness | Smoking cessation, effectiveness of corticosteroid unknown | Corticosteroid responsiveness | Effectiveness of corticosteroid unknown | Corticosteroid responsiveness |
| Prognosis | 5-yr mortality, 80% (median survival 2–3 yr) | Cellular NSIP: 5-yr mortality < 10% (median survival > 10 yr) Fibrotic NSIP: 5-yr mortality 10% (median survival 6–8 yr) | RB-ILD: no deaths reported DIP: 5-yr mortality < 5% | 5-yr mortality < 5% (deaths rare) | 60% mortality in < 6 mo | Limited data, not well defined |
Definition of abbreviations: NSIP = nonspecific interstitial pneumonia; RB-ILD = respiratory bronchiolitis–interstitial lung disease.
Adapted by permission from Reference 64.
IPF
IPF is the most common interstitial lung disease of unknown etiology. According to the current ATS/ERS definition, IPF is a distinctive type of chronic fibrosing interstitial pneumonia of unknown cause limited to the lungs and associated with a surgical lung biopsy showing a histopathologic pattern of UIP (see Visscher and Myers, pages 322–329).
WHO IS AT RISK OF IPF?
IPF occurs worldwide and the majority of the patients reported are white (10–14). IPF mainly affects people over 50 yr of age; approximately two-thirds are over the age of 60 yr at the time of presentation. The incidence is estimated at 10.7 cases per 100,000 per year for males and 7.4 cases per 100,000 per year for females (15, 16). The prevalence of IPF is estimated at 20/100,000 for males and 13/100,000 for females (15, 16). The majority of patients with IPF are current or former smokers, and cigarette smoking has been identified in some studies as a risk factor for developing IPF (12).
WHAT CAUSES IPF?
The pathogenesis of IPF remains unknown. During the past decade, there has been a shift away from the pathogenesis theory of generalized inflammation progressing to widespread parenchymal fibrosis toward a paradigm of disordered fibroproliferation and alveolar epithelial cell function (17). A complete discussion of the current understanding of the pathogenesis of IPF is found elsewhere in this monograph.
HOW IS IPF DIAGNOSED?
Clinical Features
Most patients present with gradual onset (> 6 mo) of dyspnea and/or a nonproductive cough. Constitutional symptoms are rare. Bibasilar fine inspiratory crackles (so-called Velcro crackles) are the most frequent physical examination finding and digital clubbing is seen in 25 to 50% of patients. Features of right heart failure and peripheral edema develop late in the clinical course.
Pulmonary function tests often reveal restrictive impairment (decreased static lung volumes), reduced diffusing capacity for carbon monoxide, and arterial hypoxemia exaggerated or elicited by exercise (see Martinez and Flaherty, pages 315–321).
Bronchoalveolar lavage (BAL) fluid cellular analysis shows increased neutrophils and eosinophils; lymphocytosis is not a usual feature.
The chest roentgenogram typically reveals diffuse reticular opacities (see Misumi and Lynch, pages 307–314). The chest radiograph lacks diagnostic specificity. In addition, the chest radiograph correlates poorly with the histopathologic pattern, the anatomic distribution of disease, and the severity of disease, with the exception of honeycombing, which is quite specific for IPF. High-resolution computed tomography (HRCT) is significantly more sensitive and specific for the diagnosis of IPF and has replaced conventional chest radiography as the preferred imaging method (Table 2) (19). The presence of extensive ground-glass opacities, nodules, upper lobe or mid-zone predominance of findings, and significant hilar or mediastinal lymphadenopathy should question the radiographic diagnosis of IPF. HRCT can make a confident, highly specific diagnosis of IPF in half to two-thirds of patients with IIP (20, 21).
Surgical Lung Biopsy
The definitive diagnosis of IPF requires a histopathologic pattern of UIP found on surgical lung biopsy (3, 22). The specific features of UIP are described by Visscher and Myers (9). In addition to UIP pattern, the diagnosis of IPF requires the following: (1) exclusion of other known causes of interstitial lung disease, (2) characteristic abnormalities on HRCT scan, and (3) a restrictive ventilatory defect and/or impaired gas exchange (22). In the absence of a surgical lung biopsy, a presumptive diagnosis of IPF can be made based on clinical, radiologic, and physiologic criteria (Table 3). In the immunocompetent adult, the presence of all major diagnostic criteria and at least three of the four minor criteria increases the likelihood of a correct clinical diagnosis of IPF (20).
TABLE 3.
AMERICAN THORACIC SOCIETY/EUROPEAN RESPIRATORY SOCIETY CRITERIA FOR DIAGNOSIS OF IDIOPATHIC PULMONARY FIBROSIS IN ABSENCE OF SURGICAL LUNG BIOPSY*
| Major Criteria (Must Have All Four) |
|---|
| 1. Exclusion of other known causes of ILD, such as certain drug toxicities, environmental exposures, and connective tissue diseases |
| 2. Abnormal pulmonary function studies that include evidence of restriction (reduced VC, often with an increased FEV1/FVC ratio) and impaired gas exchange (increased P[A-a]O2, decreased PaO2 with rest or exercise or decreased DlCO) |
| 3. Bibasilar reticular abnormalities with minimal ground-glass opacities on HRCT scans |
| 4. Transbronchial lung biopsy or BAL showing no features to support an alternative diagnosis |
| Minor Criteria (Must Have Three of Four) |
| 1. Age > 50 yr |
| 2. Insidious onset of otherwise unexplained dyspnea on exertion |
| 3. Duration of illness > 3 mo |
| 4. Bibasilar, inspiratory crackles |
Definition of abbreviations: BAL = bronchoalveolar lavage; DlCO = diffusing capacity of the lung for carbon monoxide; HRCT = high-resolution computed tomography; ILD = interstitial lung disease; P(A-a)O2 = alveolar-arterial pressure difference for O2.
Reprinted by permission from Reference 22.
See text for details.
Increasingly, HRCT has been shown to provide a high degree of specificity in the diagnosis of IPF. Several studies have demonstrated that, when present, these clinical criteria are highly specific (> 90%) for the presence of UIP on surgical lung biopsy (20, 21). Their sensitivity, however, is only around 50%. Therefore, in a subset of patients with IPF, the diagnosis can be confirmed with a high degree of confidence even in the absence of surgical lung biopsy (3, 22).
In rare cases of IPF, the HRCT pattern may be more informative than the surgical lung biopsy pattern. Biopsies that show a fibrosing NSIP pattern in patients with clinical and radiographic features consistent with IPF (especially honeycomb lung) tend to behave like, and should therefore be regarded as, IPF. This further emphasizes the importance of a combined clinico-radiographic-histopathologic diagnostic approach to IPF.
WHAT IS THE NATURAL HISTORY AND PROGNOSIS OF IPF?
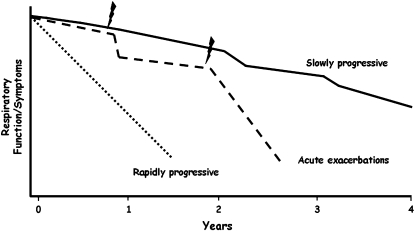
The natural history of IPF is not well defined. While the course of IPF is typically described as one of relentless decline in respiratory function, the course of individual patients is highly variable and some patients remain stable for extended periods of time without treatment (Figure 1).
Figure 1.
Schematic drawing of the potential clinical course of patients with idiopathic pulmonary fibrosis (IPF). The majority of patients with IPF show a relatively slow decline in functional status after diagnosis. Others appear to have episodes of acute clinical deterioration (acute exacerbations) that precede and possibly initiate the terminal phase of their illness. A minority of patients appear to have a short duration of illness with a more rapidly progressive clinical course. Jagged mark = acute exacerbations.
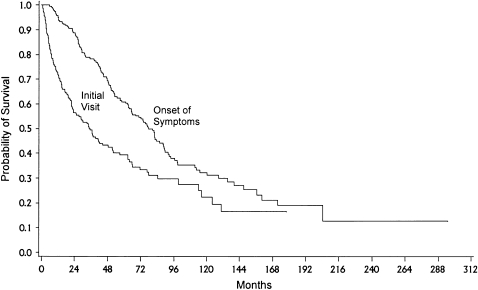
Estimates of survival in IPF are dependent on the time point from which they are calculated: time of first radiographic abnormality, time of onset of symptoms, or time of diagnosis. Basilar reticular opacities are often visible on chest imaging studies several years before the development of symptoms. Among asymptomatic patients with IPF (diagnosed by radiographic abnormalities found on routine chest X-ray screening and lung biopsy showing UIP), symptoms developed about 1,000 d after the recognition of the radiologic abnormality (23). Many studies have reported that the median duration of symptoms before the diagnosis of IPF is 1 to 2 yr (23–31). Thus, the diagnosis of IPF may be delayed 5 or more yr from the time of onset. Compared with survival from the time of diagnosis, median survival from the development of symptoms is approximately 48 mo shorter, illustrating the impact the time point chosen can have on survival estimates (Figure 2). The median survival in most studies performed after the development of new IIP classification is between 2 and 4 yr (average, 3 yr) after the diagnosis is made, and the 5-yr survival is between 20 and 40% (24–31). Survival of the prevalent cases is longer than that of incident cases, likely because prevalence studies exclude those who died quickly of aggressive disease (i.e., survival bias) (32).
Figure 2.
Kaplan-Meier plot of survival probability from the time of the onset of symptoms (median survival was 80.8 mo; 95% confidence interval, 65.5–89.3) compared with the time from initial visit (median survival was 35.2 mo; 95% confidence interval, 23.2–48.5; n = 238). Reprinted by permission from Reference 13.
New insight into the natural history of IPF comes from several recent well-designed clinical trials (33–35). Although these trials confirmed the high mortality associated with this disease, the mean change in lung function was surprisingly small. In a randomized, placebo-controlled trial of IFN-γ1b, subjects who survived to Week 72 had a decrease in mean FVC % predicted from 64.5 to 61.0%, and a decrease in mean DlCO% predicted from 37.8 to 37.0% (33). Similarly, a randomized, placebo-controlled trial of acetylcysteine demonstrated a mean reduction of VC during 12 mo of 190 ml (7.5% of baseline) and mean decline in DlCO of 0.70 mmol/min/kPa. (13.3% of baseline) in the placebo group (34). Finally, in a randomized, placebo-controlled trial of pirfenidone, a decline in VC of only 130 ml was observed in the placebo group at 9 mo (35).
The discordance between the rate of decline in pulmonary function and mortality suggests that the clinical course of IPF may be less a gradual decline and more a steplike process, with periods of relative stability punctuated by periods of acute decompensation that may be associated with high mortality (Figure 1). These acute decompensations have received increasing attention over the last several years and have been termed acute exacerbations of IPF.
Acute Exacerbation of IPF
Acute deterioration in IPF—that is, an abrupt and unexpected worsening of the underlying lung disease—may occur secondary to infections, pulmonary embolism, pneumothorax, or heart failure (36). Often, however, there is no identifiable cause for the acute decline, and these episodes are called “acute exacerbations” or “accelerated phase” of IPF (37–40).
Acute exacerbations of IPF are increasingly recognized as common and highly morbid clinical events (35, 37–44) (Table 4). Martinez and colleagues retrospectively analyzed the course of 168 patients with IPF (45). Over a median period of 76 wk, 21% of these patients died, and 47% of these deaths followed an acute deterioration in the patient's clinical status. Azuma and colleagues prospectively reported 35 patients with untreated IPF and found a 14% incidence (five cases) of acute exacerbation (35). The impact of acute exacerbation on mortality was unclear as the number of cases was small and only one of the patients died. Rice and colleagues in a review of autopsy findings conclude that acute exacerbations associated with a histopathologic pattern of diffuse alveolar damage may be a common terminal event (46). Recent studies have suggested mortality rates from 20 to 86% (35, 41–43).
TABLE 4.
ACUTE EXACERBATIONS IN IDIOPATHIC PULMONARY FIBROSIS
| Publication | Study Population | Study Design | Frequency of Acute Exacerbations | Mortality Rate after Acute Exacerbations |
|---|---|---|---|---|
| Kim, 2006 (41) | 147 | Retrospective cohort | 9.6% (2-yr incidence) | 78% (overall, 82% died within 3 mo) |
| Parambil, 2005 (42) | 7 | Case series | Not available | 86% |
| Azuma, 2005 (35) | 107 | Prospective RCT | 7% (6-mo incidence) | 20% |
| Kubo, 2005 (43) | 56 | Prospective RCT | 57% (3-yr incidence) | 30% |
| Kondoh, 2005 (44) | 27 | Prospective cohort | 22% (mean follow-up 49.5 mo) | 100% |
| Ambrosini, 2003 (39) | 5 | Case series | Not available | 80% |
| Akira, 1997 (40) | 17 | Case series | Not available | 53% |
| Kondoh, 1993 (38) | 3 | Case series | Not available | 0% |
| Kondo, 1989 (37) | 155 | Survey | 57.6% (no time period defined) | 95.5% |
| Kondo, 1989 (37) | 22 | Retrospective cohort | 18% (no time period defined) | 100% |
Acute exacerbation of IPF has not been well defined clinically. Most diagnostic criteria are modeled after those of Kondoh and include acute subjective worsening, new radiographic abnormalities, and evidence of impaired gas exchange (38). Because of the importance of acute exacerbations of IPF to our understanding of the natural history the disease, a consensus definition should be developed and the etiology, risk factors, pathogenesis, treatment, prognosis, and predictors need to be studied. We propose that the definition of acute exacerbation of IPF require subjective and objective evidence of deterioration and exclusion of common alternative etiologies, such as infection, pulmonary embolism, heart failure, and pneumothorax (Table 5).
TABLE 5.
PROPOSED DIAGNOSTIC CRITERIA FOR ACUTE EXACERBATIONS IN PATIENTS WITH IDIOPATHIC PULMONARY FIBROSIS
| 1. Subjective worsening of dyspnea or cough within the last 30 d |
| 2. New ground-glass opacities or consolidation on chest imaging studies (chest radiograph or HRCT) |
| 3. One of the following: |
| a. Decline of ⩾ 10% in absolute FVC |
| b. Decline of ⩾ 10 mm Hg in PaO2 |
| c. Decline of ⩾ 5% in SpO2 |
| 4. Negative respiratory culture for respiratory pathogens (positive culture defined as moderate or heavy growth of sputum or endotracheal aspirate or quantitative culture by mini-BAL ⩾ 103 cfu/ml, protected brush specimen ⩾ 103 cfu/ml or BAL ⩾ 104 cfu/ml) |
| 5. No evidence of pulmonary embolism, congestive heart failure, pneumothorax as cause of acute worsening |
Definition of abbreviations: BAL = bronchoalveolar lavage; HRCT = high-resolution computed tomography.
Predictors of Prognosis of IPF
Predicting survival based on baseline clinical variables has proven challenging. A number of parameters at the time of diagnosis have been proposed as predictors of worse survival in IPF: increasing age, male sex, degree of dyspnea, smoking history, severity of lung function and radiographic abnormality, neutrophilia or eosinophilia on BAL, honeycombing on HRCT, and the extent of fibroblastic foci on surgical lung biopsy specimens (24, 47, 48). Among the demographic and physiologic variables, patient age at the time of diagnosis and reduced lung function appear to be relatively consistent prognostic markers, but even these are inconsistent.
A study by Flaherty and colleagues suggests that the HRCT pattern adds important prognostic information. Among patients with IPF, a HRCT consistent with definite IPF was associated with worse survival than a HRCT that was indeterminate (median survival, 2.08 [95% confidence interval (CI), 1.30–3.98] vs. 5.76 [95% CI, 4.03 to not available], respectively) (49).
Recently, oxygen desaturation during 6 min of walking has been demonstrated to predict survival in IPF (50). End of exercise SpO2, change in SpO2 with exercise, walk distance, and walk velocity were also correlated with survival in a 5-yr follow-up study in patients with IPF (51). Considering its simplicity and practicality, the prognostic value of this test should be investigated further.
Composite indices containing multiple parameters may be more accurate than single parameters. King and colleagues generated a clinico-radiologic-physiologic score from a large cohort of patients with biopsy-proven IPF using clinical, radiographic, and physiologic (including exercise test) features to predict survival (13). Wells and colleagues derived a composite physiologic index (CPI) from simple spirometry and DlCO and demonstrated that CPI was linked to mortality more closely than the individual pulmonary function test values (52). The CPI score is easier to generate than the clinico-radiologic-physiologic score because no radiographic scoring or exercise data are required. However, the validity of these composite indices requires more study.
Finally, several studies have shown that serial changes in dyspnea and lung function are predictive of survival (27–29, 53). Change in FVC during the initial 6 mo seems to be better than change in DlCO as a predictor of mortality in patients with moderately severe disease (27–29).
WHAT IS THE TREATMENT FOR IPF?
The treatment of IPF is discussed by elsewhere in this issue by Walter and coworkers (pages 330–338).
NSIP
For many years, it was recognized that lung biopsy samples from some patients with IIP do not fit into any well-defined histologic pattern. In 1994, Katzenstein and Fiorelli assigned the term “nonspecific interstitial pneumonia” to this histopathologic group (55). Importantly, the NSIP pattern is found on surgical lung biopsies from nonidiopathic forms of interstitial lung disease, such as connective tissue disease, hypersensitivity pneumonitis, poorly resolved diffuse alveolar damage, LIP, and a variety of exposures. It has been suggested that NSIP may represent an injury pattern common to many settings, rather than a specific disease, and that idiopathic NSIP (i.e., the clinical condition) may represent subclinical forms of these alternative diagnoses.
The concept of NSIP as a separate disease entity is attractive, however, because it can explain some of the historical heterogeneity in the clinical behavior of IPF. Many patients previously labeled as having IPF had cellular biopsies (prominent lymphoplasmacytic inflammation), BAL lymphocytosis, a dramatic response to steroids, and better long-term prognosis. On re-review, many of these steroid-responsive, cellular, lymphocyte-predominant cases represented NSIP (i.e., their surgical lung biopsy showed NSIP pattern, not UIP pattern). Based on this observation, the ATS/ERS classification schema for the IIPs included idiopathic NSIP as a provisional clinical diagnosis and recommended further study and characterization of this condition.
Nicholson has proposed that fibrotic NSIP pattern may be a relatively “inactive” UIP pattern (56). However, in a study comparing lung biopsy and subsequent explant tissue in patients with IIP, Katzenstein and colleagues found no explants showing UIP pattern to be preceded by biopsy findings of the NSIP pattern, which was interpreted as evidence against the evolution from NSIP to IPF (57). Furthermore, surgical lung biopsy specimens of early stages from patients with IPF who were detected by CT scan showed the same temporal heterogeneity (i.e., UIP pattern) as seen in advanced cases of IPF; NSIP pattern was not observed (26). Based on these data, current evidence suggest that, in general, idiopathic fibrosing NSIP pattern represents a distinct clinical condition, and not an early (or inactive) stage of IPF (58). The finding of both UIP and NSIP pattern in multiple biopsies from the same patients (59, 60) raises the possibility that NSIP pattern may, on occasion, be a nonspecific manifestation of IPF. However, more data are required to clarify this issue.
WHO IS AT RISK OF NSIP?
NSIP occurs worldwide and the majority of the patients reported are white. The incidence and prevalence are unknown. The mean age of patients with NSIP is a decade younger than the patients with IPF (median age of onset is 40 and 50 yr respectively) (3, 23, 25, 29, 53). NSIP appears to be more common in women, although this needs further clarification. There is not an association with cigarette smoking.
WHAT CAUSES NSIP?
The pathogenesis of NSIP is unknown. Many investigators suspect it to be an autoimmune disease because of the common appearance of this pattern in patients with connective tissue disease, especially systemic sclerosis and polymyositis-dermatomyositis. As mentioned previously, it is also possible that idiopathic NSIP may represent subclinical forms of other nonidiopathic interstitial lung diseases (e.g., hypersensitivity pneumonitis). Clearly, this issue needs additional study.
WHAT ARE THE CLINICAL FEATURES OF NSIP?
The clinical presentation of NSIP generally consists of the subacute onset of dyspnea or nonproductive cough. Constitutional symptoms are rare, although a low-grade fever is sometimes reported. The median duration of symptoms before diagnosis varies from 6 to 18 mo. Clubbing is found in about 10% of patients. In contrast to IPF, serologic abnormalities (antinuclear antibodies and rheumatoid factor) may be positive in low titer. More than half of the patients have an increased percentage of lymphocytes in the BAL fluid. Pulmonary function tests often reveal restrictive impairment, reduced diffusing capacity for carbon monoxide, and arterial hypoxemia exaggerated or elicited by exercise (18). The radiologic and histopathologic findings will be discussed by other authors in this issue (9, 19).
HOW IS NSIP DIAGNOSED?
As with all IIPs, the diagnosis of NSIP depends on a combination of clinical, radiologic, and histopathologic findings. NSIP is characterized pathologically by varying degrees of inflammation and fibrosis and by temporal homogeneity (i.e., uniformity of fibrosis), which is distinct from UIP pattern. Nonetheless, fibrotic NSIP pattern can be difficult to distinguish from UIP pattern, and significant interobserver variability exists even among expert histopathologists (30, 61). Therefore, even though the current ATS/ERS classification is based on histopathologic pattern, additional diagnostic methods, such as microarray analysis, are needed to better distinguish the two diseases. (62).
WHAT IS THE TREATMENT FOR NSIP?
Patients with NSIP have a better outcome than patients with IPF, with many showing improvement after treatment with corticosteroids. Importantly, there are no controlled data on which to judge the effectiveness of therapy. Retrospective reviews of patients with NSIP show around a third will improve pulmonary function with therapy and the majority of the rest will stabilize (30, 63). Patients with NSIP may require the addition of immunosuppressive agents as the initial response to corticosteroids alone may not be adequate. Patients with moderate to severe impairment are at particular risk for progressive or poorly responsive disease (53). It has been suggested that patients with fibrosing NSIP be treated with the combination of corticosteroid and immunosuppressant therapy to prevent the development of an irreversible fibrosis (44). Further studies are needed to compare the effect of corticosteroid therapy with corticosteroid and immunosuppressant combination therapy for idiopathic fibrosing NSIP (44).
WHAT IS THE NATURAL HISTORY AND PROGNOSIS OF NSIP?
The clinical course of idiopathic NSIP has not been well studied. The prognosis of cellular NSIP is excellent with few reported deaths (29, 53). The course of fibrotic NSIP is worse, with reported median survival ranging from 6 to 13.5 yr (25, 29, 49, 53, 60). Travis and colleagues reported the 10-yr survival of fibrotic NSIP to be 35% (31). Nicholson and colleagues reported a 5-yr survival of only 43% (30). Nagai and colleagues reported deterioration of lung function despite treatment in 5 of 17 patients, and 2 of them died (48). Kondoh and colleagues showed that 25% worsened or died after a mean follow-up of 92 mo (44). Further studies with long-term follow-up in larger number of patients are required to clarify the course and prognostic marker in this disease.
CONCLUSIONS
Following the development of the ATS/ERS consensus classification, we have been able to classify the IIPs more clearly. HRCT has become central to the diagnosis of the IIPs, and a multidisciplinary approach to the diagnosis of these conditions is paramount. IPF has the worst prognosis among the chronic IIPs. However, the clinical course of IPF may not be as indolent and steadily progressive as classically described. Periods of relative stability may instead be punctuated with acute exacerbations of disease that cause new lung injury, acute decline, and, frequently, death. Predicting prognosis in the individual patient with IPF is challenging but various clinical and radiologic variables have been identified. NSIP has been proposed as a separate entity among the IIPs. There is little evidence to support the idea that NSIP progresses to IPF; rather, NSIP may be a unique form of IIP, although further clarification of the relationship of NSIP to collagen vascular disease, hypersensitivity pneumonitis, and other nonidiopathic forms of interstitial lung disease is needed. NSIP appears more responsive to corticosteroid and immunomodulatory therapy, and has better survival than IPF.
Supported, in part, by National Institutes of Health grant U10 HL080685.
Conflict of Interest Statement: D.S.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.R.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.E.K. has served on advisory boards for Actelion (compensation: 2003 = $11,725; 2004 = $9,940; 2005 = $15,000), for InterMune (2003 = $21,000; 2004 = $15,000; 2005 = $20,000), and for GlaxoSmithKline (2004 = $12,625; 2005 = $10,000), and has served as a consultant for Nektar, Alexza, AstraZeneca, Biogen, Centocor, Fibrogen, Genzyme, Human Genome Sciences, Merck, and CoTherix, and none of these exceeded $10,000 per company per year in any of the preceding 3 yr.
References
- 1.Liebow AA, Carrington DB. The interstitial pneumonias. In: Simon M, Potchen EJ, LeMay M, editors. Frontiers of pulmonary radiology. New York: Grune & Stratteon; 1969. pp. 102–141.
- 2.Katzenstein ALA, Myers JL. Idiopathic pulmonary fibrosis. Clinical relevance of pathologic classification. Am J Respir Crit Care Med 1998;157:1301–1315. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society/European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty KR, King TE Jr, Raghu G, Lynch JP III, Colby TV, Travis WD, Gross BH, Kazerooni EA, Toews GB, Long Q, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med 2004;170:904–910. [DOI] [PubMed] [Google Scholar]
- 5.Cordier JF. Cryptogenic organizing pneumonia. Clin Chest Med 2004;25:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vourlekis JS, Brown KK, Cool CD, Young DA, Cherniack RM, King TE Jr, Schwarz MI. Acute interstitial pneumonitis: case series and review of the literature. Medicine 2000;79:369–378. [DOI] [PubMed] [Google Scholar]
- 7.Craig PJ, Wells AU, Doffman S, Rassl D, Colby TV, Hansell DM, Du Bois RM, Nicholson AG. Desquamative interstitial pneumonia, respiratory bronchiolitis and their relationship to smoking. Histopathology 2004;45:275–282. [DOI] [PubMed] [Google Scholar]
- 8.Swigris JJ, Berry GJ, Raffin TA, Kuschner WG. Lymphoid interstitial pneumonia: a narrative review. Chest 2002;122:2150–2164. [DOI] [PubMed] [Google Scholar]
- 9.Visscher DW, Myers JL. Histologic spectrum of idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006;3:322–329. [DOI] [PubMed] [Google Scholar]
- 10.Smith C, Feldman C, Levy H, Kaemback JM, Zwi S. Cryptogenic fibrosing alveolitis: a study of an indigenous African population. Respiration (Herrlisheim) 1990;57:364–371. [DOI] [PubMed] [Google Scholar]
- 11.Iwai K, Mori T, Yamada N, Yamaguchi M, Hosoda Y. Idiopathic pulmonary fibrosis: epidemiologic approaches to occupational exposure. Am J Respir Crit Care Med 1994;150:670–675. [DOI] [PubMed] [Google Scholar]
- 12.Baumgartner KB, Samet J, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1997;155:242–248. [DOI] [PubMed] [Google Scholar]
- 13.King TE Jr, Tooze JA, Schwarz MI, Brown K, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 2001;164:1171–1181. [DOI] [PubMed] [Google Scholar]
- 14.Johnston IDA, Prescott RJ, Chalmers JC, Rudd RM. British Thoracic Society study of cryptogenic fibrosing alveolitis: current presentation and initial management. Fibrosing Alveolitis Subcommittee of the Research Committee of the British Thoracic Society. Thorax 1997;52:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coultas DB. Epidemiology of idiopathic pulmonary fibrosis. Semin Respir Med 1993;14:181–196. [Google Scholar]
- 16.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung disease. Am J Respir Crit Care Med 1994;150:967–972. [DOI] [PubMed] [Google Scholar]
- 17.Selman M, King TE Jr, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151. [DOI] [PubMed] [Google Scholar]
- 18.Martinez FJ, Flaherty K. Pulmonary function testing in idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006;3:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misumi S, Lynch DA. Idiopathic pulmonary fibrosis/usual interstitial pneumonia: imaging diagnosis, spectrum of abnormalities, and temporal progression. Proc Am Thorac Soc 2006;3:307–314. [DOI] [PubMed] [Google Scholar]
- 20.Raghu G, Mageto YN, Lockhart D, Schmidt RA, Wood DE, Godwin JD. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: a prospective study. Chest 1999;116:1168–1174. [DOI] [PubMed] [Google Scholar]
- 21.Hunninghake G, Zimmerman MB, Schwartz DA, King TE Jr, Lynch J, Hegele R, Waldron J, Colby T, Muller N, Lynch D, et al. Utility of lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;164:193–196. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS) and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 23.Nagai S, Nagao T, Kitaichi M, et al. Clinical courses of asymptomatic cases with idiopathic pulmonary fibrosis and a histology of usual interstitial pneumonia [abstract]. Eur Respir J 1998;11:131s. [Google Scholar]
- 24.King TE Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA Jr, Flint A, Thurlbeck WM, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med 2001;164:1025–1032. [DOI] [PubMed] [Google Scholar]
- 25.Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, Offord KP. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;157:199–203. [DOI] [PubMed] [Google Scholar]
- 26.Nagai S, Kitaichi M, Hamada K, Nagao T, Hoshino Y, Miki H, Izumi T. Hospital-based historical cohort study of 234 histologically proven Japanese patients with IPF. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:209–214. [PubMed] [Google Scholar]
- 27.Collard HR, King TE Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003;168:538–542. [DOI] [PubMed] [Google Scholar]
- 28.Flaherty KR, Mumford JA, Murray S, Kazerooni EA, Gross BH, Colby TV, Travis WD, Flint A, Toews GB, Lynch JP III, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003;168:543–548. [DOI] [PubMed] [Google Scholar]
- 29.Jegal Y, Kim DS, Shim TS, Lim CM, Do Lee S, Koh Y, Kim WS, Kim WD, Lee JS, Travis WD, et al. Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 2005;171:639–644. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson AG, Colby TV, Dubois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 2000;162:2213–2217. [DOI] [PubMed] [Google Scholar]
- 31.Travis WD, Matsui K, Moss J, Ferrans VJ. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol 2000;24:19–33. [DOI] [PubMed] [Google Scholar]
- 32.Hubbard R, Johnston I, Britton J. Survival in patients with cryptogenic fibrosing alveolitis: a population-based cohort study. Chest 1998;113:396–400. [DOI] [PubMed] [Google Scholar]
- 33.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE Jr; Idiopathic Pulmonary Fibrosis Study Group. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med 2004;350:125–133. [DOI] [PubMed] [Google Scholar]
- 34.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2005;353:2229–2242. [DOI] [PubMed] [Google Scholar]
- 35.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi H, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:1040–1047. [DOI] [PubMed] [Google Scholar]
- 36.Panos RJ, Mortenson R, Niccoli SA, King TE Jr. Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med 1990;88:396–404. [DOI] [PubMed] [Google Scholar]
- 37.Kondo A, Saiki S. Acute exacerbation in idiopathic interstitial pneumonia (IIP). In: Harasawa M, Fukuchi Y, Morinari H, editors. Interstitial pneumonia of unknown etiology. Japan Intractable Diseases Research Foundation Publication No. 27. Tokyo: University of Tokyo Press; 1989. pp. 33–42.
- 38.Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K. Acute exacerbation in idiopathic pulmonary fibrosis: analysis of clinical and pathologic findings in three cases. Chest 1993;103:1808–1812. [DOI] [PubMed] [Google Scholar]
- 39.Ambrosini V, Cancellieri A, Chilosi M, Zompatori M, Trisolini R, Saragoni L, Poletti V. Acute exacerbation of idiopathic pulmonary fibrosis: report of a series. Eur Respir J 2003;22:821–826. [DOI] [PubMed] [Google Scholar]
- 40.Akira M, Hamada H, Sakatani M, Kobayashi C, Nishioka M, Yamamoto S. CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. AJR Am J Roentgenol 1997;168:79–83. [DOI] [PubMed] [Google Scholar]
- 41.Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006;27:143–150. [DOI] [PubMed] [Google Scholar]
- 42.Parambil JG, Myers JL, Ryu JH. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest 2005;128:3310–3315. [DOI] [PubMed] [Google Scholar]
- 43.Kubo H, Nakayama K, Yanai M, Suzuki T, Yamaya M, Watanabe M, Sasaki H. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest 2005;128:1475–1482. [DOI] [PubMed] [Google Scholar]
- 44.Kondoh Y, Taniguchi H, Yokoi T, Nishiyama O, Ohishi T, Kato T, Suzuki K, Suzuki R. Cyclophosphamide and low-dose prednisolone in idiopathic pulmonary fibrosis and fibrosing nonspecific interstitial pneumonia. Eur Respir J 2005;25:528–533. [DOI] [PubMed] [Google Scholar]
- 45.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE Jr, Flaherty KR, Schwartz DA, Noble PW, Raghu G, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med 2005;142:963–967. [DOI] [PubMed] [Google Scholar]
- 46.Rice AJ, Wells AU, Bouros D, du Bois RM, Hansell DM, Polychronopoulos V, Vassilakis D, Kerr JR, Evans TW, Nicholson AG. Terminal diffuse alveolar damage in relation to interstitial pneumonias: an autopsy study. Am J Clin Pathol 2003;119:709–714. [DOI] [PubMed] [Google Scholar]
- 47.Flaherty KR, Colby TV, Travis WD, Toews GB, Mumford J, Murray S, Thannickal VJ, Kazerooni EA, Gross BH, Lynch JP III, et al. Fibroblastic foci in usual interstitial pneumonia: idiopathic versus collagen vascular disease. Am J Respir Crit Care Med 2003;167:1410–1415. [DOI] [PubMed] [Google Scholar]
- 48.Nicholson AG, Fulford LG, Colby TV, Dubois RM, Hansell DM, Wells AU. The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002;166:173–177. [DOI] [PubMed] [Google Scholar]
- 49.Flaherty KR, Thwaite EL, Kazerooni EA, Gross BH, Toews GB, Colby TV, Travis WD, Mumford JA, Murray S, Flint A, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax 2003;58:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lama VN, Flaherty KR, Toews GB, Colby TV, Travis WD, Long Q, Murray S, Kazerooni EA, Gross BH, Lynch JP III, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003;168:1084–1090. [DOI] [PubMed] [Google Scholar]
- 51.Hallstrand TS, Boitano LJ, Johnson WC, Spada CA, Hayes JG, Raghu G. The timed walk test as a measure of severity and survival in idiopathic pulmonary fibrosis. Eur Respir J 2005;25:96–103. [DOI] [PubMed] [Google Scholar]
- 52.Wells AU, Desai SR, Rubens MB, Goh NS, Cramer D, Nicholson AG, Colby TV, du Bois RM, Hansell DM. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003;167:962–969. [DOI] [PubMed] [Google Scholar]
- 53.Latsi PI, du Bois RM, Nicholson AG, Colby TV, Bisirtzoglou D, Nikolakopoulou A, Veeraraghavan S, Hansell DM, Wells AU. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med 2003;168:531–537. [DOI] [PubMed] [Google Scholar]
- 54.Walter N, Collard HR, King TE Jr. Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc Am Thorac Soc 2006;3:330–338. [DOI] [PubMed] [Google Scholar]
- 55.Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis: histologic features and clinical significance. Am J Surg Pathol 1994;18:136–147. [PubMed] [Google Scholar]
- 56.Nicholson AG, Wells AU. Nonspecific interstitial pneumonia: nobody said it's perfect. Am J Respir Crit Care Med 2001;164:1553–1554. [DOI] [PubMed] [Google Scholar]
- 57.Katzenstein AL, Zisman DA, Litzky LA, Nguyen BT, Kotloff RM. Usual interstitial pneumonia: histologic study of biopsy and explant specimens. Am J Surg Pathol 2002;26:1567–1577. [DOI] [PubMed] [Google Scholar]
- 58.Nagai S, Handa T, Tabuena R, Kitaichi M, Izumi T. Nonspecific interstitial pneumonia: a real clinical entity? Clin Chest Med 2004;25:705–715. [DOI] [PubMed] [Google Scholar]
- 59.Flaherty KR, Travis WD, Colby TV, Toews GB, Kazerooni EA, Gross BH, Jain A, Strawderman RL, Flint A, Lynch JP, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med 2001;164:1722–1727. [DOI] [PubMed] [Google Scholar]
- 60.Monaghan H, Wells AU, Colby TV, du Bois RM, Hansell DM, Nicholson AG. Prognostic implications of histologic patterns in multiple surgical lung biopsies from patients with idiopathic interstitial pneumonias. Chest 2004;125:522–526. [DOI] [PubMed] [Google Scholar]
- 61.Nicholson AG, Addis BJ, Bharucha H, Clelland CA, Corrin B, Gibbs AR, Hasleton PS, Kerr KM, Ibrahim NB, Stewart S, et al. Inter-observer variation between pathologists in diffuse parenchymal lung disease. Thorax 2004;59:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selman M, Pardo A, Barrera L, Estrada A, Watson SR, Wilson K, Aziz N, Kaminski N, Zlotnik A. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med 2006;173:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daniil ZD, Gilchrist FC, Nicholson AG, Hansell DM, Harris J, Colby TV, du Bois RM. A histologic pattern of nonspecific interstitial pneumonia is associated with a better prognosis than usual interstitial pneumonia in patients with cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 1999;160:899–905. [DOI] [PubMed] [Google Scholar]
- 64.King TE Jr. Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med 2005;172:268–279. [DOI] [PubMed] [Google Scholar]