Abstract
This review is an attempt to identify and place in context some of the many questions about voltage-gated proton channels that remain unsolved. As the gene was identified only 2 years ago, the situation is very different than in fields where the gene has been known for decades. For the proton channel, most of the obvious and less obvious structure–function questions are still wide open. Remarkably, the proton channel protein strongly resembles the voltage-sensing domain of many voltage-gated ion channels, and thus offers a novel approach to study gating mechanisms. Another surprise is that the proton channel appears to function as a dimer, with two separate conduction pathways. A number of significant biological questions remain in dispute, unanswered, or in some cases, not yet asked. This latter deficit is ascribable to the intrinsic difficulty in evaluating the importance of one component in a complex system, and in addition, to the lack, until recently, of a means of performing an unambiguous lesion experiment, that is, of selectively eliminating the molecule in question. We still lack a potent, selective pharmacological inhibitor, but the identification of the gene has allowed the development of powerful new tools including proton channel antibodies, siRNA and knockout mice.
A few years ago, a H+-ATPase researcher made the astonishing remark that his field was finished – all the major questions had been answered! It had never even occurred to me that this was possible – I have worked mainly in areas that are rife with problems to solve. The voltage-gated proton channel is a poster child of unsolved problems! Before I begin to list several important unanswered questions, I will briefly introduce this little-known channel.
First, the voltage-gated proton channel is an ion channel. For reasons that escape me, some people seem to want to call it a ‘proton pump.’ It is nothing like a pump. The proton channel cannot move protons against an electrochemical gradient – it is a passive pathway across the membrane. It does not need or use ATP. Proton channels open and close just like other ion channels, generating noise and single-channel currents. They open with depolarization, like the classical Na+, K+ and Ca2+ channels of excitable cells. Although these properties define the proton channel as an ion channel, it does have a number of distinctive, if not unique properties. Its unitary conductance is ∼103 smaller than most other channels, roughly 15 fS at room temperature and physiological pH (Cherny et al. 2003). Many channels are selective, but imperfectly so; the proton channel has apparently perfect selectivity. Proton channels have stronger temperature dependence, both of conductance and gating kinetics, than almost any other ion channel. Reminiscent of inward rectifier K+ channels, the voltage dependence of proton channel gating is not absolute, but varies with the permeant ion concentration. Inward rectifiers conduct mainly inward K+ current; proton channels conduct mainly outward H+ current. Finally, the proton channel may be unique in lacking a water-filled pore that acts as the conduction pathway. Protons can travel in ways that other ions cannot, such as Grotthuss conduction in water (de Grotthuss, 1806; Pomès, 2006) and hydrogen-bonded chain conduction through proteins (Nagle & Morowitz, 1978). It is virtually certain that the conducted species is the proton, H+, not the hydronium ion, H3O+ (DeCoursey & Cherny, 1997, 1998). The lack of a continuous aqueous pore could explain the extreme selectivity of the proton channel.
The pH- and voltage-dependence of its gating makes the proton channel an exquisitely designed proton extrusion mechanism. Eliminating excess acid from cells is its general function. However, proton channel activity has other consequences, to be discussed later, that enable it to perform other specialized functions. The best known and best characterized function occurs in phagocytes. When these cells engulf microbes, the enzyme NADPH oxidase begins to produce superoxide anion, the precursor to a host of bactericidal reactive oxygen species. NADPH oxidase is electrogenic (Henderson et al. 1987; Schrenzel et al. 1998), and proton channels provide the bulk of charge compensation (Henderson et al. 1988a,1988b; Murphy & DeCoursey, 2006), which prevents extreme depolarization that would stop enzyme function (DeCoursey et al. 2003). Recent evidence that proton channels are required for histamine release by human basophils (Musset et al. 2008b) might be explained by proton extrusion, charge compensation or some other mechanism.
Molecular and genetic properties
Has the right gene been identified?
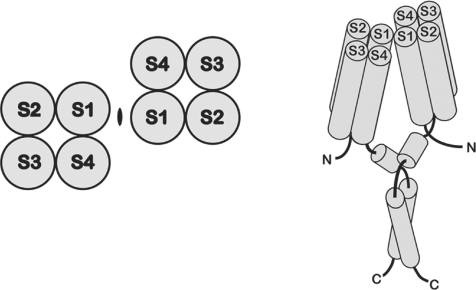
The historical controversies over whether the gp91phox component of NADPH oxidase might function as a voltage-gated proton channel (Henderson et al. 1995; Henderson & Meech, 1999; Bánfi et al. 2000; DeCoursey et al. 2000, 2001b; Maturana et al. 2001) have been thoroughly discussed (Touret & Grinstein, 2002; DeCoursey et al. 2002; Henderson & Meech, 2002; Maturana et al. 2002; DeCoursey, 2003, 2008; Musset et al. 2008a) and little new can be added. What can be stated without any doubt is that bona fide voltage-gated proton channel genes were identified in 2006. The Clapham lab identified and characterized the human proton channel gene, HV1 (Ramsey et al. 2006). The Okamura lab (Sasaki et al. 2006) identified a voltage-gated proton channel, CiVSOP, in Ciona intestinalis, a sea squirt, in a project to classify the genome of this creature, and found a homologue in the mouse, mVSOP. A gene coding for a voltage-sensitive phosphatase had been identified previously (Murata et al. 2005); the proton channel was a fortuitous discovery in an effort to catalogue other homologues. The proton channel gene products have several remarkable and unexpected properties. As shown in Fig. 1, the proton channel has four putative membrane-spanning regions, S1–S4, which are generally similar to the S1–S4 regions of many voltage-gated ion channels (Sasaki et al. 2006; Ramsey et al. 2006) as well as the voltage-sensitive phosphatase in Ciona (Murata et al. 2005). However, the protein lacks the S5–S6 regions, which in ordinary ion channels form the conduction pathway. The proton channel is thus a voltage sensor without a conventional pore.
Figure 1. Topology of the voltage-gated proton channel.
Voltage-gated proton channels resemble the voltage-sensing domain (VSD) of ordinary voltage-gated cation channels. Hydropathy plots of the protein coded for by the proton channel gene indicate four membrane-spanning regions that resemble S1–S4 of voltage-gated K+ channels (Ramsey et al. 2006; Sasaki et al. 2006). The proton channel appears to assemble as a dimer (Koch et al. 2008; Lee et al. 2008; Tombola et al. 2008). The top view of the channel (with the membrane in the plane of the page) is on the left, the side view on the right. In the latter, the S1–S4 cylinders represent alpha helices spanning the membrane and both the N and C termini are intracellular. Proposed interaction sites are just external to S1 and within a predicted coiled-coil region in the C terminus. (From Lee et al. 2008, with permission.)
Genes that are highly homologous to the proton channel are present in at least two dozen species, with selected species given in Table 1. Other species with similar genes, listed in descending order of predicted protein similarity (to the human HVCN1) include: Pan troglodytes (chimpanzee), Sus scrofa (pig), Equus caballus (horse), Monodelphis domestica (grey, short-tailed opossum), Rattus norvegicus (rat), Ornithorhynchus anatinus (platypus), Trichoplax adhaerens (placozoan), Nematostella vectensis (starlet sea anemone), Tetraodon nigroviridis (puffer fish), Macaca fascicularis (crab-eating macaque), Laccaria bicolor (basidiomycete fungus), Aspergillus niger (black mold) and Physcomitrella patens (moss) which is 23% identical to HV1 (NCBI BLAST). Each protein in Table 1 has four predicted transmembrane domains that have substantial homology, fairly long C and N terminal domains, and short linkers with the highest homology in the intracellular S2–S3 linker. In most species, the C terminus contains a long predicted intracellular coiled-coil region, where intersubunit interaction may occur (Koch et al. 2008; Lee et al. 2008; Tombola et al. 2008).
Table 1.
Voltage-gated proton channel (HVCN1) family
| GeneID | Protein identity (%) | Gene aliases | Organism | MW | Length (AA) |
|---|---|---|---|---|---|
| 84329 | 100 | VSOP, MGC15619, UNQ578/PRO1140 | Homo sapiens (Human) | 31 683 | 273 |
| 709745 | 93.4 | LOC709745 | Macaca mulatta (rhesus monkey) | 31 523 | 273 |
| 616570 | 86.2 | – | Bos taurus (cow) | 31 872 | 272 |
| 608547 | 85.3 | – | Canis lupus familiaris (dog) | 31 216 | 268 |
| 74096 | 78.0 | BTS, VSOP, 0610039P13Rik, AI450555 | Mus musculus (mouse) | 31 242 | 269 |
| 416871 | 53.5 | RCJMB04–1c7 | Gallus gallus (chicken) | 27 599 | 235 |
| 496712 | 45.6 | — | Xenopus (Silurana) tropicalis (western clawed frog) | 26 575 | 230 |
| 496219 | 43.6 | — | Xenopus laevis (African clawed frog) | 26 596 | 230 |
| 436618 | 40.9 | zgc:92181 | Danio (Brachydanio) rerio (zebrafish) | 27 110 | 235 |
| 778897 | 26.1 | VSOP, VSX1 | Ciona intestinalis(transparent sea squirt) | 38 501 | 342 |
| 586317 | 22.3 | VSOP | Strongylocentrotus purpuratus(purple sea urchin) | 37 483 | 328 |
Characteristics of proteins coded by selected proton channel genes. Species for which the expressed gene product has been demonstrated by voltage clamp to function as a proton channel are in bold: human (Ramsey et al. 2006), mouse and Ciona (Sasaki et al. 2006), and sea urchin (personal communication, Y. Okamura). VSOP, voltage sensor domain-only protein. Identity with the human protein is from EMBL-EBI (http://www.ebi.ac.uk/Tools/es/cgi-bin/jobresults.cgi/needle). Some aliases are from UniProt (http://www.uniprot.org/). The isotopically averaged molecular weight (MW) of a monomer is given using Protein Calculator v. 3.3 (http://www.scripps.edu).
Is the functional channel really a dimer, and how independent are its pathways?
This year, three groups presented diverse evidence indicating that the voltage-gated proton channel functions as a dimer, and each subunit has its own conduction pathway (Koch et al. 2008; Lee et al. 2008; Tombola et al. 2008). Before describing this evidence, it should be noted that these studies were exclusively based on the expressed proton channel gene product, not on measurements in native channels. In view of speculation that the oligomerization state might determine functional properties (Koch et al. 2008), a clear demonstration of the architecture of the assembled native proton channel, especially in phagocytes, would be most welcome.
Koch et al. (2008) tagged the C terminus of the mouse channel, mVSOP, with either HA or Myc, co-expressed the two constructs and then fished with antibodies to the tags. Each antibody detected the other tag, showing that the proton channel comprised multimers that contain both tags. They then used fluorescence resonance energy transfer (FRET) on the Ciona intestinalis proton channel, CiVSOP, engineering donor and acceptor fluorophores on the extracellular S3–S4 linker (at S242C), and determined a distance of 42.2 Å between them, a proximity consistent with multimerization. To determine the number of subunits, they generated tandem dimers of CiVSOP in both sequences: WT-S242C and S242C-WT. Co-expression should lead to a FRET signal if tetramers formed, but there was no FRET, speaking against tetramerization, although not unequivocally ruling out a trimer, for example. Finally, Western blots of mVSOP revealed mainly monomers but with a faint band at the dimer position. After treatment with the cross-linker DSS (disuccinimidyl suberate), a strong dimer band was observed. There is evidently weak interaction in the cytoplasmic regions. This interaction was demonstrated by truncating N or C termini, or both. The channel still functioned (electrophysiologically) as a monomer with both C and N termini removed, although gating kinetics was altered. Evidently, the channel can function as a monomer, but usually occurs as a dimer. Clearly, each monomer contains an independent conduction pathway. The authors speculated that a transition between monomer and dimer might underlie the ‘activation’ of proton channels in phagocytes.
MacKinnon' group (Lee et al. 2008) found that the human proton channel HV1 migrated as a monomer on Western blots, but with increasing concentrations of the crosslinking agent DSS, predominantly occurred in dimer form. They introduced Cys residues to identify putative interaction sites. All mutants migrated as monomers in reducing conditions but mild to strong oxidation led to dimer formation, with key residues identified as C249 (one of two naturally occurring Cys in HV1) in the C terminus (where coiled-coil interaction was proposed) and I127 near the external end of S1. The proposed interaction regions are shown in Fig. 1.
Tombola et al. (2008) used a completely different approach. They tagged the human proton channel with GFP and observed that photobleaching of the green fluorescent protein (GFP) occurred in two discrete steps, strongly suggesting that HV1 assembles as a dimer. Next they introduced a Cys residue at an intracellular location (N214C, see Table 3) that proved to be accessible to the thiol modifying reagent MTSET; modification introduces a positively charged group that abolished current. Tandem dimers of all possible combinations, WT–WT, N214C–WT, WT–N214C and N214C–N214C, were compared. Currents were 40% inhibited with one mutant subunit and 90% with both. Two other MTS reagents were found to produce twice the inhibition in the double mutant as in a single mutant channel. These results are consistent with two independent pores, but conceivably (albeit improbably) could also occur if the MTS reagents only partially reduced the current of a common pore. Dimerization was found to involve the cytoplasmic domain, using chimeras between HV1 and CiVSP, a related phosphatase that is thought to exist as a monomer (Murata et al. 2005; Kohout et al. 2008). The N and C termini of HV1 were required for dimer formation, assessed by photobleaching. Finally, enforcing monomeric expression by substituting both N and C termini from CiVSP into HV1 resulted in proton currents that, like those reported by Koch et al. (2008), activated substantially more rapidly than the WT dimer.
Table 3.
S4 regions of voltage-gated K+ and H+ channels and a voltage-sensing phosphatase (VSP)
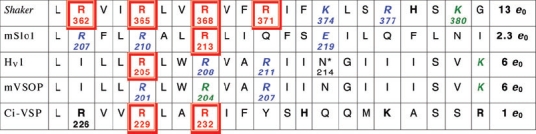 |
A sequence of 21 amino acids in the putative S4 regions of the Shaker K+ channel, the maxi-K calcium-activated K+ channel (mSlo), the human proton channel (HV1), the mouse proton channel (mVSOP), and a voltage-sensing phosphatase in Ciona (Ci-VSP). Potentially charged amino acids are indicated in bold. Single neutralization mutations at the numbered positions in red boxes reduce the gating charge; those in italics (in blue) do not affect gating charge, or were inconclusive (in green) (Aggarwal & MacKinnon, 1996; Seoh et al. 1996; Bezanilla, 2000; Murata et al. 2005; Ramsey et al. 2006; Sasaki et al. 2006; Ma et al. 2006; Musset et al. 2008a; Hossain et al. 2008). Total gating charge (e0) estimates are listed in the final column; for HV1 and mVSOP, these are from the limiting slope method (Musset et al. 2008a). The mouse R204Q mutant did not express well; R207Q was identical to wild type, and for R201Q activation was faster and the gH–V relationship was shifted by −50 mV and had a slightly steeper slope (z = 1.9 versus 1.4 for wt) (Sasaki et al. 2006). In human proton channels, gating was faster for all three Arg mutants, and for R205A the midpoint was shifted positively, and the slope (from gH–V relationships) was less steep by 1/3 (zδ= 0.57 versus 0.90 for wt) (Ramsey et al. 2006). The VSD of the Shaker K+ channel can be transformed into a proton channel by the R362H mutation (Starace & Bezanilla, 2004), and R362X where X = Cys, Ala, Ser or Val produces non-selective cationic ‘omega’ current through the voltage sensor (Tombola et al. 2005). *The N214R mutation greatly attenuates conduction (Tombola et al. 2008). A ClustalW alignment of proteins containing S4 regions homologous to HV1 was manually adjusted to reflect predicted transmembrane regions. In some cases, plausible alternative alignments can be obtained by shifting a sequence by three residues. These previously unpublished alignments were generously provided by S. M. E. Smith (Emory University).
In summary, the diversity of evidence indicating that the expressed proton channel exists as a dimer lends strong support to this conclusion. It also seems clear that each monomer forms a separate conduction pathway. As mentioned above, all of these studies were done on expressed proton channels, which in the next section are shown to have subtle but distinct differences from native channels. Furthermore, there is little or no evidence regarding whether the two protomers function independently or in a concerted manner (as speculated by Lee et al. 2008). Concerted gating might help explain the sigmoidal activation kinetics and certain peculiarities of gating charge measurements (below), and might conceivably be involved in the conversion of the resting channel to its ‘enhanced gating mode’ during phagocyte activation (as speculated by Koch et al. 2008).
Why are there differences between expressed and native proton channels?
In almost every respect, the electrophysiological properties of the proton channel gene products resemble those of native proton channels. Features common to both include: (1) a depolarization-activated conductance; (2) sigmoidal activation kinetics; (3) generally slow activation kinetics; (4) potent inhibition by Zn2+ that, (a) slows current turn-on, (b) shifts the gH–V relationship positively, and (c) exhibits profound pH dependence reflecting competition with H+ for an external binding site; (5) a 40 mV shift of the gH–V relationship when pHo is increased by one unit or pHi is decreased by one unit; (6) extraordinarily strong temperature dependence (Kuno et al. 1997; DeCoursey & Cherny, 1998; Ramsey et al. 2006); and (7) perfect selectivity for protons over all other ions. However, in a detailed study (Musset et al. 2008a), one difference was observed between expressed and native proton currents. In heterologous expression systems, expressed human and murine proton channels, HV1 and mVSOP, both opened at potentials about 30 mV more negative at any given pH gradient, ΔpH, than did native proton channels in over a dozen cell types. As it turns out, this deviant behaviour affects a central characteristic of proton channels – the regulation of their gating by pH that results in only outward current in the steady state (ergo acid extrusion), as discussed below (Sensitivity of gating to ΔpH). This aberrant voltage dependence may reflect a requirement for an additional co-factor or accessory protein, or a difference between the expression systems (COS-7 and HEK-293) and native cells, although the small endogenous proton currents in HEK-293 cells exhibit normal voltage dependence. Rather than speculate further, I will sit back and wait to see how this is resolved.
Permeation
How is perfect selectivity achieved?
A hallmark of the voltage-gated proton channel is its extremely high selectivity. Selectivity determined from measurement of the reversal potential, Vrev, provides two clear results. First, when pH is changed, Vrev changes by an amount that approaches the Nernst potential for protons, EH (DeCoursey, 2003). Second, when the predominant cation or anion in the bath is substituted, there is no detectable change in Vrev (Barish & Baud, 1984; Mahaut-Smith, 1989a; Bernheim et al. 1993; Demaurex et al. 1993; Kapus et al. 1993; DeCoursey & Cherny, 1993, 1994a, 1996a; Qu et al. 1994; Eder et al. 1995; Gordienko et al. 1996; Kuno et al. 1997) provided that liquid junction potentials are corrected (Neher, 1992) and that one avoids creating conditions in which Na+−H+ antiport activity changes pHi (DeCoursey & Cherny, 1994a; Demaurex et al. 1995; Klee et al. 1999). Calculated with the Goldman–Hodgkin–Katz voltage equation (Goldman, 1943; Hodgkin & Katz, 1949; Hille, 2001), the relative permeability of H+ is 106−108 greater than that of any other ion (Demaurex et al. 1993; Kapus et al. 1993; Bernheim et al. 1993; DeCoursey & Cherny, 1994a,1994b, 1996a, 1997; Cherny et al. 1995, 2001b; Gordienko et al. 1996; DeCoursey et al. 2001b; Schilling et al. 2002), even under the improbable worst-case assumption that all deviation from Nernst is due to permeation of the other cation. This high relative permeability results in part from the extremely low proton concentration, typically ∼106 lower than the predominant cation. However, ion substitution experiments reveal that ion species other than H+ have no detectable effect on Vrev and hence, the selectivity of the proton channel is effectively perfect.
Virtually all proton conduction occurs by a Grotthuslike mechanism (de Grotthuss, 1806; Pomès, 2006) in which the proton hops from one molecule to another. In the special case of a ‘water wire’, the proton hops from one water molecule to another, forming a hydronium ion, H3O+, at each stop along the way. A pivotal proposal by John Nagle and colleagues was that protons could cross membranes through proteins by a hydrogen-bonded chain (HBC) mechanism (Nagle & Morowitz, 1978; Nagle & Tristram-Nagle, 1983). The proton hops, as in the Grotthus mechanism, but the pathway may include hydroxyl, amino and carboxyl side groups of amino acids as well as intervening water molecules. An HBC mechanism that included side chains would enable perfect proton selectivity; other ions are excluded (Nagle & Tristram-Nagle, 1983).
In Table 2, proton conduction through the voltage-gated proton channel is compared with proton conduction through the water-filled gramicidin channel, as well as with proton conduction in bulk water. The gramicidin channel has been useful as a model ion channel – it is relatively small, structurally simple and robust – it still functions at 5–6 m HCl (Eisenman et al. 1980; Cukierman et al. 1997)! The gramicidin channel has a narrow cylindrical pore that is filled with a single-file row of a dozen water molecules (Levitt et al. 1978). Gramicidin is a non-selective cation channel, but it conducts protons much better than any other ion, because protons can hop through a water wire without displacing the water molecules. In contrast a Na+ or K+ ion must wait patiently for the water molecules to diffuse through before it can permeate. For roughly the same reason, protons diffuse in bulk water 5 times faster than K+ (Danneel, 1905; Robinson & Stokes, 1959). Gramicidin conducts up to 2 × 109 H+ s−1 (Cukierman, 2000), faster than any other narrow-pore channel conducts any other ion. The impression that any proton that appears at the mouth of gramicidin permeates is supported by a nearly direct proportionality between proton current and proton concentration over 5 orders of magnitude (from pH 4.5 to pH −0.5) (DeCoursey, 2003). Proton conductivity of bulk water is similarly proportional to concentration up to pH ∼0 (Owen & Sweeton, 1941). In contrast, although the single-channel conductance of voltage-gated proton channels increases at lower pHi, the increase is only 4-fold between pHi 6.5 and 5.5 (Cherny et al. 2003). It would be intriguing to obtain single-channel currents at lower pH, but biological membranes seem unwilling to cooperate in this endeavour.
Table 2.
Proton conduction in water and through gramicidin and voltage-gated proton channels
| Property | Bulk water | Gramicidin | Proton channel |
|---|---|---|---|
| Selectivity (PH+/PNa+) | 71 | 38–602 | 106−108(3) |
| Deuterium isotope effect (IH+/ID+) | 1.414 | 1.355 | 1.96 |
| Activation energy (kcal mol−1) | 2.67 | 3–88 | 18–279 |
The activation energies listed are for proton permeation through open channels. The temperature dependence of gramicidin includes measurements of several modified dioxolane-linked channels.
The impression from Table 2 is that proton permeation through gramicidin is not too different from conduction in bulk water, but permeation through voltage-gated proton channels is something completely different. Deuterons permeate both gramicidin and voltage-gated proton channels, but the isotope effect is substantially greater for permeation through voltage-gated proton channels. The temperature dependence of the open-channel conductance is stronger for the voltage-gated proton channel than for almost any other ion channel. Together with these factors, the concentration dependence mentioned above supports the conclusion that traversing voltage-gated proton channels is challenging for protons. The contrast between proton permeation through gramicidin, a water-filled ion channel, and voltage-gated proton channels has led to the proposal that the conduction pathway in the latter is not likely to comprise a simple water wire, but instead may comprise a HBC that includes at least one titratable group (DeCoursey & Cherny, 1994a,1994b, 1997, 1998; Henderson & Meech, 1999; Cherny et al. 2001b; Maturana et al. 2001; DeCoursey, 2003; Demaurex & Petheö, 2005).
Other proton selectivity mechanisms may exist, however. The perfect proton selectivity of the M2 viral proton channel (Chizhmakov et al. 1996; Lin & Schroeder, 2001; Mould et al. 2000) has been explained by two different mechanisms. One is a traditional HBC mechanism in which protonation/deprotonation of a ring of His residues ensures selectivity (Pinto et al. 1997; Schweighofer & Pohorille, 2000; Shuck et al. 2000; Lear, 2003). Alternatively, channel opening might complete a water wire that experiences a constriction that is sufficiently narrow to prevent cation (or water) permeation, but still permits proton transfer between adjacent waters (Sansom et al. 1997; Kukol et al. 1999; Smondyrev & Voth, 2002; Kass & Arkin, 2005; Chen et al. 2007; Stouffer et al. 2008).
There are several examples of proton pathways that almost certainly involve protonation/deprotonation of a titratable group during proton conduction (DeCoursey, 2003, 2008). Strong candidates are produced by particular mutations of the voltage-sensing domain (VSD) of K+ channels. Mutating any one of the first four Arg residues in S4 (the fourth putative membrane-spanning region, Table 3) to His produces proton selective transporters; R365H and R368H are proton carriers whereas R362H and R371H are voltage-gated proton channels with opposite voltage dependence (Starace et al. 1997; Starace & Bezanilla, 2001; Starace & Bezanilla, 2004). The central Arg→His are alternately exposed to internal or external solutions during gating, whereas Arg→His positioned on either end apparently forms a solitary constriction that has simultaneous access to both sides of the membrane, one in the closed (R362H) and the other in the open (R371H) conformation of the central K+ pore. If R362 is mutated into any of several other amino acids, the result is non-selective cation current through the VSD (Tombola et al. 2005, 2007). At two other locations in the VSD, Ile→His mutations (I241H in S1 and I287H in S2) produce proton channels (Campos et al. 2007). Finally, in the VSD of voltage-gated Na+ channels, Arg→His mutation of one of the S4 residues (R663H) produces a proton selective channel (Struyk & Cannon, 2007), whereas Arg→X mutation produces non-selective cation current (Sokolov et al. 2005). It is difficult to imagine that the proton selectivity that is uniquely achieved by positioning a His residue at these locations does not result from protonation/deprotonation of the His during conduction. Unfortunately, it is also difficult to conceive an experiment that would reveal with certainty whether this is the case.
Finally, there are several recent examples of non-selective channels that conduct protons paradoxically well, including TRPV1 (Hellwig et al. 2004), TRP-ML1 (Soyombo et al. 2006), TRPM7 (Jiang et al. 2005; Numata & Okada, 2008) and colicin A (Slatin et al. 2008). The relative proton permeability, PH+/PCs+ > 1000 for TRPV1 and PH+/PCs+= 104−106 for TRP-ML1, is astonishing, and that PH+/PK+= 12 000 for colicin A is truly astounding in view of this channel having a lumen ∼10 Å in diameter (Slatin et al. 2008). Whether the proton permeability of these channels can be explained by conventional paradigms remains to be determined.
Where is the permeation pathway?
Answering this question would be facilitated by knowing the structure. Although many multimeric channels form their conduction pathway at the interface between subunits, as discussed above, the proton channel appears to be a dimer and each subunit has its own pore (Koch et al. 2008; Tombola et al. 2008; Lee et al. 2008). At present, no published data exist that indicate which amino acids form the conduction pathway, although the N214R mutation greatly attenuates conduction, and thus may be near the inner mouth of the ‘pore’ (Tombola et al. 2008). The general similarity of the proton channel molecule to the voltage-sensing domain of a K+ channel (Sasaki et al. 2006; Ramsey et al. 2006) is highly suggestive. The histidine scanning studies by Bezanilla and colleagues described above (Starace et al. 1997; Starace & Bezanilla, 2001; Starace & Bezanilla, 2004) demonstrate that the VSD has aqueous access channels that lead to a constriction that can provide access of a single (inserted) His residue to external or internal solutions depending on protein movements that occur during gating (channel opening and closing). If the architecture of the proton channel follows this pattern, then the general framework for a proton pathway may reasonably occur in each monomer.
What is the concentration dependence of the proton conductance?
Examination of macroscopic gH data in many studies indicated that gH,max– the limiting gH– increased only 2-fold per unit decrease in pHi (DeCoursey, 1998). The prediction of the Goldman–Hodgkin–Katz equation (Goldman, 1943; Hodgkin & Katz, 1949; Hille, 2001) is a 10-fold increase, which presumes that entry into the channel is rate determining. This discrepancy suggested that permeation, rather than entry, was rate determining. The implicit assumption is that the same number of channels is open during a large depolarization at any pHi. Surprisingly however, when determined at various pHi values, the single-channel conductance increased from 38 fS to 140 fS at pHi 6.5 and 5.5, respectively, nearly a 4-fold increase (Cherny et al. 2003). The open probability was 75% and 95%, respectively, in these conditions. The macroscopic and microscopic conductances can be reconciled if there is an inhibitory process at low pHi that removes functional channels from the fray. Systematic study of this issue would be welcome, but has been hampered because biological membranes do not tolerate extremely low pH. Perhaps incorporation of proton channels into artificial lipid bilayers would enable direct observation over a wider pH range.
At the other extreme, it is curious at how high pHi one could still detect proton currents. At high pH, free protons are exceedingly scarce and buffer (used at its pKa) holds on to its proton tightly. At sufficiently high pH, deprotonation of buffer must become rate determining, at which point proton current should decrease in direct proportion to [H+] (Brönsted & Pedersen, 1923; Eigen & Hammes, 1963), assuming that buffer protonation is diffusion limited. Our attempts to probe this question have been limited by two factors: (1) cells do not survive well at extremely high pH; and (2) it is necessary to monitor Vrev to determine how well one has established the intended pH. Credible outward proton currents with Nernstian (i.e. reasonable) Vrev have been seen in excised patches at pHi 8.5 with pHo 7.5 (V. V. Cherny & T. E. DeCoursey, unpublished data), where only 3 nm permeant ion (H+) was present.
Voltage gating
Is S4 the voltage sensor?
It is well established that the S4 region contributes substantially to the ability of several voltage-gated ion channels to sense membrane potential (Noda et al. 1984; Stühmer et al. 1989). This said, it should be emphasized that charged residues elsewhere in K+ channels also contribute (Seoh et al. 1996), and the mechanism of gating remains controversial. As shown in Table 3, every third amino acid in S4 of the Shaker K+ channel is a potentially charged Arg or Lys and each of the first four Arg residues appears to contribute to voltage sensing (Aggarwal & MacKinnon, 1996; Seoh et al. 1996; Bezanilla, 2000). Three of these Arg residues are conserved in the voltage-gated proton channel (Table 3). However, when these were mutated individually to neutral amino acids, only one appeared to reduce the effective charge moved during gating (channel opening) in the human channel (Ramsey et al. 2006), and none of them contributed in the mouse channel (Sasaki et al. 2006). One limitation of these studies is that they were based on Boltzmann fits to gH–V relationships, which may not capture the entire gating charge moved (Sigworth, 1993). Preferable estimates are from the limiting slope of the g–V relationship (Almers, 1978; Sigworth, 1993). The gating charge movement estimated by this method is 12–14 e0 for Na+ or K+ channels (Hirschberg et al. 1995; Schoppa et al. 1992; Aggarwal & MacKinnon, 1996; Seoh et al. 1996) and 5.4–8 e0 for voltage-gated proton channels (DeCoursey & Cherny, 1996b, 1997; Musset et al. 2008a). Even more direct estimates of gating charge movement come from gating current measurements (Sigworth, 1993). Gating current occurs when channels open or close, but must be isolated from ionic current, for example by blockers. However, this measurement is nearly impossible for voltage-gated proton channels, because: (a) it is impossible to abolish ionic current by eliminating the permeant ion; (b) reducing the permeant ion concentration shifts the gH–V relationship (see Sensitivity of gating to ΔpH) rather than permitting gating in the absence of ionic current; (c) attempts to measure gating currents at EH (the Nernst potential for H+), which would preclude ionic current, still did not reveal detectable gating current, probably because gating is very slow (V. V. Cherny, V. Sokolov & T. E. DeCoursey, unpublished data).
By the best available estimates, half as much gating charge moves in proton channels as in Shaker K+ channels (Table 3). However, K+ channels are tetramers, with four VSD and one pore; hence the charge moved is 3 e0 per VSD. On the other hand, if proton channels are dimers with independently gated pores, the gating charge is 6 e0 for each H+ channel monomer, which is twice that for K+ channels! If both monomers gate cooperatively, the charge would be 3 e0 per proton channel monomer. Incidentally, if gating were cooperative but not precisely simultaneous, unitary H+ currents roughly double the value estimated from noise measurements might be observed, as appears to be the case (Cherny et al. 2003). That only one Arg in S4 seems to contribute to gating charge movement presents a large discrepancy, and suggests that S4 may not be a major participant in voltage sensing in proton channels. A similar conclusion was reached for BK channels, although their total gating charge is relatively petite (Ma et al. 2006). Proton current kinetics changed fairly dramatically even in the Arg mutations that did not affect total charge, however (Sasaki et al. 2006; Ramsey et al. 2006), suggesting that these groups do participate in gating. In summary, the voltage-sensing apparatus and its mechanism in proton channels are largely unknown.
How does gating work?
Voltage-gated proton channels open and close like other ion channels. Single channel currents of ∼10 fA can just barely be resolved under highly favourable conditions: in excised patches, at low pHi, with seal resistances in the teraohm range, and with no other conductances present (Cherny et al. 2003). Proton channel gating generates current fluctuations that exhibit archetypal stochastic behaviour. Distinct fluctuations (noise) appear at voltages at which the gH is activated, and the variance of this noise increases to a maximum near the midpoint of the gH–V relationship, precisely where one would expect the frequency of opening and closing transitions to be maximal. Beyond this, almost nothing is known about the gating mechanism. Given the likelihood of HBC conduction, a proton conduction pathway might appear or disappear in ways that would not be effective for gating conduction of other ions, so we need to keep an open mind. For example, subtle changes in the length or orientation of hydrogen bonds (Pauling, 1939; Scheiner, 1981) or in the pKa of groups in the pathway could enable or disable a HBC.
The similarity between the proton channel and the VSD, combined with the demonstration that the K+ channel VSD can be converted into a proton-selective channel by single mutations of Arg residues in S4 (Starace & Bezanilla, 2001, 2004) raises the obvious possibility that movement analogous to that which is thought to occur during VSD gating also occurs in the proton channel and results in channel ‘opening.’ Changes in accessibility of the four key Arg residues during K+ channel gating led to the conclusion that they ratchet outwards past a solitary constriction, with the first and fourth Arg at the narrow point when the K+ channel is closed or open, respectively (Larsson et al. 1996; Bezanilla, 2000; Horn, 2005; Tombola et al. 2006). Tombola et al. (2008) found that in HV1, the N214R mutation abolished conduction and proposed that the proton channel opens when the polar Asn214 moves into this hypothetical constriction.
Some constraint on the gating process is placed by the extraordinarily strong temperature dependence of gating. Three parameters describing proton channel gating kinetics – the delay and activation time constant τact obtained from fitting proton current turn-on with a delay followed by a rising exponential, and τtail obtained by fitting tail current decay with a single exponential – all had activation energies of 30–38 kcal mol−1 (Q10 values of 6–9) in six types of cells (DeCoursey & Cherny, 1998). Activation energies for the opening and closing of most ion channels, including K+ channels (Bezanilla, 2000), are only about half this large. That all kinetic parameters for proton channel gating had similar large activation energies suggests that both opening and closing involve a single rate-limiting, energetically demanding transition that occurs in multiple independent subunits. Whether the two pores of the proton channel dimer gate independently or cooperatively has not been established.
What is the real kinetics of proton channels?
From the beginning, it has always been evident that proton currents are prone to manifestations of depletion of the permeant ion. Since in many situations the purpose of proton currents is to change pH, this property should not come as a surprise. Nevertheless, someone who is familiar with K+ currents for which the cellular concentration of permeant ion is 10−1m is in for a rude awakening when studying channels for which the cellular concentration of permeant ion is 10−7m. One should first of all be impressed that proton currents are as large as they are – in many cells they are as large or larger than any other conductance present, including K+ currents (Byerly & Suen, 1989; DeCoursey, 1991; Demaurex et al. 1993; DeCoursey & Cherny, 1994b, 1996a; Femling et al. 2006). The conjunction of large currents and minuscule permeant ion concentration leads to depletion of the permeant ion. The most obvious manifestation of depletion is decay or droop of proton currents. Proton channels do not inactivate in any tissue studied to date (p. 532, DeCoursey, 2003). However, proton currents often decay during sustained depolarization. That the cause is proton depletion-induced pH changes has been verified by measuring pHi by pH electrode (Thomas & Meech, 1982; Meech & Thomas, 1987), by fluorescent dye (Demaurex et al. 1993; Kapus et al. 1993; Schwiening & Willoughby, 2002), or by using the proton channel as a pH meter and measuring Vrev of proton currents (Barish & Baud, 1984; DeCoursey, 1991; Kapus et al. 1993; DeCoursey & Cherny, 1994b; Humez et al. 1995; Gordienko et al. 1996; Morihata et al. 2000; Schrenzel et al. 1996). There seems to be a spontaneous tendency for researchers to feel guilty about proton depletion, and to attempt to conceal droopy currents, for example. On the one hand, it makes sense to try to minimize depletion, because it compromises the measurements. On the other hand, it is dangerous to pretend that depletion is not occurring, because this can lead to gross misinterpretation of data.
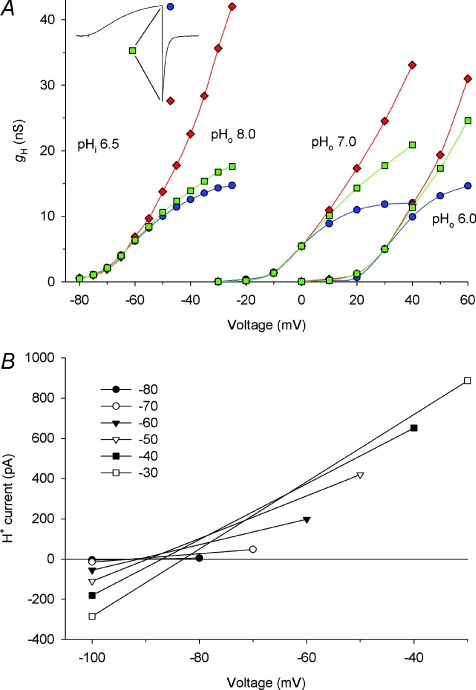
Proton currents are more susceptible to depletion effects than other ion channels for several reasons. (1) The proton concentration is very low. (2) Buffer diffuses more slowly than small monovalent ions. Essentially all of the H+ that carries current was immediately previously bound to buffer. A 10 μm diameter spherical cell dialysed with 100 mm buffer at its pKa contains only 315 000 free H+ at pH 6 (DeCoursey, 2003). These protons can carry a 100 pA H+ current for only 0.5 ms. Although there are 1.6 × 1010 protonated buffer molecules in the cell, 6.25 × 108 (4%) are consumed each second of the 100 pA H+ current. If they are not replenished by diffusion from the pipette solution, pHi will increase. (3) Finally, proton channels in most mammalian cells open quite slowly, with activation time constants, τact, in the range of seconds, in contrast with a few milliseconds in snail neurones (Byerly et al. 1984). By the time what appears to be a steady-state level of activation is achieved during the pulse, depletion has already occurred. Figure 2A illustrates this phenomenon. In order to determine the voltage dependence of ‘steady-state activation’ of the gH, one typically applies a family of pulses. The current at the end of the pulse can be used to calculate the conductance, using Vrev measured previously by the tail-current method (Hodgkin & Huxley, 1952). These values are plotted as blue circles, and they appear to saturate and could be fitted by a Boltzmann function, as we are wont to do. However, the gH ought to be identical at the start of the tail current, and many purists prefer measuring tail current amplitude, because this precludes worries about open-channel rectification. These values are plotted as red diamonds and they show little inclination to saturate. What has happened, of course, is that each pulse removed protons from the cytoplasm, so that pHi was higher at the end of the pulse, and probably also at the start of the next pulse than it was for the previous pulse. The estimates of Vrev in Fig. 2B confirm a 9 mV depolarizing shift of Vrev by the end of the pulse family. Consequently, calculating gH using a single Vrev value does not reflect the true situation. We can attack this problem by realizing that we could use these interpolated Vrev values for each pulse, and obtain the true gH at that instant (green squares). The only problem is that our goal was to determine the gH at one particular pHi, not over a range of continuously changing pHi that spans 0.15 pH units. This distinction is not trivial, because as we will see in the next section, the position of the gH–V relationship depends strongly on pHi.
Figure 2. Proton depletion complicates measurement of the voltage dependence of the proton conductance, gH.
A, the gH–V relationship estimated by three methods at each of three pHo values in a COS-7 cell transfected with the mouse proton channel gene, mVSOP. The pipette contained pHi 6.5 with 100 mm BisTris buffer. At pHo 8.0, 2 s pulses were applied in 5 mV increments every 20 s from Vhold=−100 mV. At pHo 7.0 or 6.0, Vhold was −60 or −40 mV, respectively, and 6 s pulses were applied every 20 s. As indicated in the inset, gH was estimated from proton current measured at the end of each pulse (Iend, blue circles) or at the start of the tail current (Itail, red diamonds) assuming a single constant measured value for Vrev. Alternatively, gH was calculated as the slope conductance (green squares) between Iend and Itail, which does not require an estimate of Vrev. B, actual Vrev estimated by the X axis intercept of a line connecting Iend and Itail for the indicated pulses in the same cell at pHo 8.0. (From Musset et al. 2008a.)
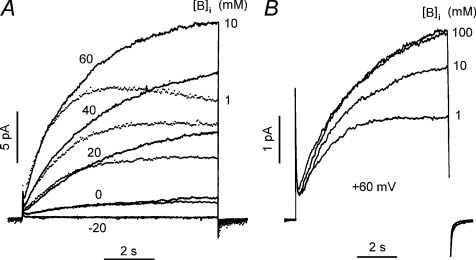
Obviously, increasing the buffer concentration reduces the rate and extent of depletion (DeCoursey, 1991; Kapus et al. 1993; Demaurex et al. 1993; DeCoursey & Cherny, 1994b, 1996b; Kuno et al. 1997). Most labs routinely use ≥ 100 mm buffer, which reduces but does not prevent depletion; in the experiment in Fig. 2 there was 100 mm buffer in all solutions. Figure 3 illustrates the effects of buffer concentration on proton currents in an excised inside-out patch of membrane. Several features are remarkable. First, it is clear that depletion occurs even in excised patches. One can easily imagine that in whole-cell configuration depletion might be a problem, but both H+ currents and unstirred compartment volumes ought to be much smaller in patches. Still, the membrane migrates some distance up from the tip of the pipette (Milton & Caldwell, 1990; Ruknudin et al. 1991), so there may be a substantial unstirred volume from which protons will be removed by depolarizing pulses (in inside-out patches). The impression from Fig. 3A is that the pulses begin well, but increasingly, the current at lower [buffer] sags below the one at higher [buffer]. Although frank droop is seen only during the pulse to +60 mV, it is glaringly obvious that the time course of the current is radically different at low [buffer], even at +20 mV where the current is much smaller. Clearly, lack of droop does not signify that the time course of the current reflects pure gating kinetics. At lower [buffer], τact will be artificially fast. In Fig. 3B, we see that 10 mm buffer was not enough, despite the normal appearance of the family in Fig. 3A, because the currents are larger and ‘activate’ even more slowly at 100 mm buffer. We sometimes use 200 mm buffer, after Gabor Petheö and Nic Demaurex showed us this was possible, but even then, can we be sure that the time course of the current reflects the true time course of gating? The deconvolution of the true gating kinetics of proton channels from diffusional effects on the time course of recorded currents may require serious modelling. In the meanwhile, it is best to remember that much of what you see is not a direct reflection of gating kinetics, and that one must guard against being seduced into over-interpreting data.
Figure 3. High buffer concentration reduces but does not eliminate depletion.
These records were obtained in inside-out patches of membrane from two rat alveolar epithelial cells, at pHo 7.5 and pHi 6.5. As one might expect, diffusion limitation is less severe in patches than in whole-cell configuration. However, depletion still occurs in patches. A, the currents in were recorded at the indicated voltages during pulses from a holding potential of −40 mV with [buffer] in the bath (corresponding to intracellular) either 10 mm (continuous lines) or 1 mm (dotted lines). B, proton currents in another patch during pulses to + 60 mV at the indicated BisTris concentrations. Note the radically different kinetics at different buffer concentration. Pulses were applied at 100 mm buffer before and after the other concentrations were applied. (From DeCoursey & Cherny, 1996b.)
Depletion is more pronounced in whole-cell configuration. Proton accumulation outside the cell (Zifarelli et al. 2008b) and proton depletion inside the cell (Swietach et al. 2003) during constant proton efflux have been modelled. Certainly depletion during proton efflux is more severe inside the cell, and replenishment depends on diffusion of protonated buffer from the pipette solution, whose rate of equilibration has also been evaluated (Pusch & Neher, 1988). The effective diffusion coefficient for protons in cells is slowed by the effects of fixed and mobile intrinsic buffers (Junge & McLaughlin, 1987) by at least two (Vaughan-Jones et al. 2002; Swietach et al. 2003) or even three orders of magnitude (Stewart et al. 1999), particularly at low pH (Swietach & Vaughan-Jones, 2005; Swietach et al. 2007) where the intrinsic buffering capacity is increased (p. 483, DeCoursey, 2003). In practical terms, establishing control of pHi by 100 mm buffer requires 8–10 min in large cells of diameter 120 μm (Byerly & Moody, 1986), and 1–3 min in small cells of diameter 10–20 μm (Demaurex et al. 1993; Kapus et al. 1993), depending on pipette geometry. Adequate control is never achieved at low (<20 mm) buffer concentration (Byerly & Moody, 1986; DeCoursey, 1991; Demaurex et al. 1993).
The inevitability of depletion has several consequences. As mentioned, one must be alert to this phenomenon and not over-interpret data. With depletion in mind, we often use the threshold for channel activation, Vthreshold, as a rough indicator of the voltage dependence of gating. This parameter is arbitrary, because if gH decreases exponentially with hyperpolarization, no true threshold exists; the Vthreshold detected will depend on gH,max and noise levels. However, Vthreshold is a useful and practical compromise for several reasons. It is easy to determine. It precludes the need for large depolarizations that cause extensive depletion. By definition, currents near Vthreshold are very small and produce little depletion. It does not assume any specific gating model, nor does it require force-fitting data to a Boltzmann function. In practice, Vthreshold gave results similar to more traditional methods of analysis (Musset et al. 2008a). We suspect that Vthreshold is a more reliable indication of the voltage dependence of proton channel gating than parameters obtained from Boltzmann fits, which are strongly influenced by depletion and the specific conditions of the measurements.
Sensitivity of gating to ΔpH
Which groups sense pHo and pHi?
A quintessential feature of voltage-gated proton channels is that their voltage dependence is strongly regulated by the pH gradient, ΔpH (defined as pHo– pHi) (Cherny et al. 1995). The gH–V relationship shifts 40 mV per unit change in ΔpH over a wide range encompassing all physiologically attainable values. The result of this extraordinary regulation by pH is that proton channels open only when the electrochemical proton gradient is outward, when opening will result in acid extrusion from the cell. Consequently, it is universally accepted that acid extrusion, especially in situations of excessive metabolic activity, is a major function of voltage-gated proton channels. All native voltage-gated proton channels share the property of ΔpH-dependent gating, and the voltage at which the gH first turns on, Vthreshold, can be predicted by: Vthreshold= 0.79 Vrev+ 18 mV (DeCoursey, 2003). This behaviour can be modelled quite simply by assuming that protonation of sites on the channel accessible to the external solution stabilizes the closed channel and protonation from the inside stabilizes the open channel. The model works only if the regulatory protonation sites are accessible to just one side of the membrane at a time, and accessibility is transferred by an unspecified conformational change in the protein (Cherny et al. 1995). At this moment, we have no idea what or where these protonation sites are, or whether these hypothetical sites exist at all.
An informal survey of amino acid mutations that significantly alter pH sensing in 35 membrane proteins revealed the following: 20 involved His residues, singly or in clusters of up to six, 15 involved Glu, 7 Asp, 6 Arg, 6 Lys, and 3 Gly. The apparent preference for Glu over Asp is surprising, but may be fortuitous. In some cases, a single residue seems to provide the entire pH sensitivity. In many others, the pH sensitivity seems to involve a complex interplay between groups of nearby amino acids; sometimes mutation of only one residue of the group has no effect. The ClC-0 chloride channel is sensitive to pHi, but when mutation of 22 candidate amino acids failed to reveal a sensor, the idea of a proton sensor was abandoned in favour of a different mechanism involving OH− (Zifarelli et al. 2008a).
How do the pH sensors communicate with the voltage sensor?
The mechanism of this coupling has not even been imagined. One can hardly speculate on this mechanism, despite its obvious importance, because we do not know what or where the sensors are or how gating works.
Pharmacology
Zinc' secrets revealed
One structure–function question has been solved, namely the location of the external Zn2+ binding site on the human proton channel. Zn2+ is among the most potent inhibitors of voltage-gated proton channels (Mahaut-Smith, 1989b). The effects of Zn2+ on proton currents closely resemble the effects of polyvalent cations on nearly all voltage-gated ion channels; namely, the gH–V relationship is shifted positively and the turn-on of current during depolarizing pulses is slowed (larger τact). Qualitatively, these effects correspond to the electrostatic effects of positioning two positive charges at the outer end of the channel – with the result that the voltage sensor is tricked into thinking the membrane potential is more negative than it really is (Frankenhaeuser & Hodgkin, 1957). The efficacy of Zn2+ in inhibiting the proton channel is greatly reduced at low pHo, and the details of the competition between H+ and Zn2+ could be explained only by assuming that the external Zn2+ binding site comprised 2 or 3 (but not 1) titratable groups with pKa 6.2–7.0, suggesting His residues (Cherny & DeCoursey, 1999). The human proton channel turns out to have two His residues that are accessible to the external solution, and their mutation individually to Ala lowers Zn2+ sensitivity by an order of magnitude; the double His→Ala mutant has only weak Zn2+ sensitivity (Ramsey et al. 2006).
To a rough approximation, mouse and human proton channels have similar Zn2+ sensitivity (Ramsey et al. 2006; Sasaki et al. 2006), but the Ciona proton channel is less sensitive (Sasaki et al. 2006). There are several His residues at suggestive locations in the mouse proton channel, and fewer in the Ciona channel (Sasaki et al. 2006; DeCoursey & Cherny, 2007), but whether this explains the weaker Zn2+ binding remains to be determined.
The internal Zn2+ binding site has not been identified. Applied intracellularly, Zn2+ has less dramatic effects that are qualitatively consistent with surface charge effects, namely shifting the gH–V relationship negatively, slowing tail current decay (larger τtail) and decreasing the limiting gH, gH,max (Cherny & DeCoursey, 1999; Petheöet al. 2003).
What about a potent, selective inhibitor?
Unfortunately, Zn2+ is not a very selective inhibitor, which greatly hampers attempts to evaluate possible functions of proton channels. A potent and selective toxin or venom would be very handy, but so far, none has been discovered.
How are H+ channels regulated?
Where is(are) the phosphorylation site(s)?
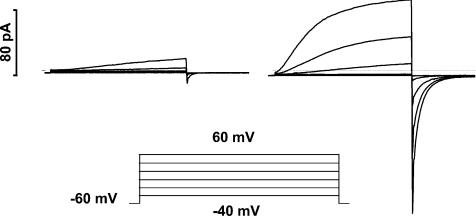
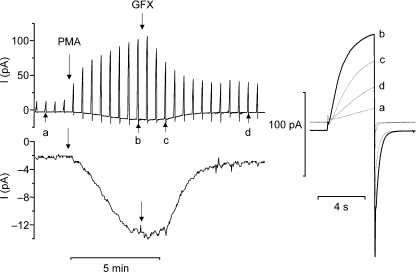
In phagocytes, voltage-gated proton channels can be transformed into an ‘enhanced gating mode’ that seems to coincide with NADPH oxidase activity (Bánfi et al. 1999). As illustrated in Fig. 4, agents that activate NADPH oxidase, such as PMA or arachidonic acid, profoundly alter the gating of proton channels. PMA shifts the gH–V relationship by −40 mV, speeds activation, slows deactivation and increases gH,max (DeCoursey et al. 2000, 2001a,2001b; Bankers-Fulbright et al. 2001; Cherny et al. 2001a). These effects are prevented or largely reversed by the PKC inhibitors GF109203X (GFX) or staurosporine (Bankers-Fulbright et al. 2001; Mori et al. 2003; Morgan et al. 2007), which suggests that phosphorylation by PKC of either the channel or a regulatory molecule creates the enhanced gating mode. On a more phenomenological level, PKC stimulates both NADPH oxidase activity and proton efflux in intact human neutrophils (Henderson et al. 1987; Nanda & Grinstein, 1991; Kapus et al. 1992). Accepting that phosphorylation is a major mechanism of proton channel regulation, where is(are) the phosphorylation site(s)? The next question is, by what mechanism do these sites exert such a strong influence on proton channel gating? Does the level of ‘activation’ of proton channels reflect a balance between phosphorylation and dephosphorylation? If so, which kinases and phosphatases are involved?
Figure 4. Agents that activate NADPH oxidase in phagocytes also greatly enhance the opening of proton channels.
Identical families of pulses (inset) were applied to a human eosinophil before (left) and after (right) addition of PMA to the bathing solution. The enhanced gating response can be observed in perforated-patch configuration, but not whole-cell. This cell was exposed to a pH 7.0 bath solution with 50 mm NH4+ on both sides of the membrane to clamp pHi near pHo. (From Morgan et al. 2007.)
Does phosphorylation account entirely for proton channel ‘activation’ in phagocytes?
When GFX, a fairly selective PKC inhibitor, is applied to an activated eosinophil, the electron current turns off completely (Fig. 5), indicating that sustained NADPH oxidase activity requires ongoing PKC activity. At the same time, the enhanced features of proton currents are largely, but not completely reversed (Morgan et al. 2007). Two explanations for the less complete reversal of proton channel modulation are possible. First, it might be that deactivation reflects the activity of phosphatases, and those that dephosphorylate proton channels are less active then those that turn off NADPH oxidase. The phosphatase inhibitor, okadaic acid, does slow the reversal of proton current enhancement by GFX (Morgan et al. 2007). Alternatively, the activation of proton channels may involve something more than PKC phosphorylation. If there are additional or alternative pathways of activating proton channels, what are they?
Figure 5. The enhanced gating mode is partially reversed by the PKC inhibitor, GFX.
From a holding potential of −60 mV, test pulses were applied every 30 s to +60 mV, resulting in the upward deflections. Where indicated, 60 nm PMA was added, stimulating increased proton current as well as inward current at −60 mV. This inward current (shown at amplified gain below) is electron current that is generated by the activity of NADPH oxidase, and can be inhibited by the NADPH oxidase inhibitor DPI (diphenylene iodonium). Examples of proton currents during test pulses at right are labelled with lower case letters that show the time each was recorded. At the second arrow, 3 μm GFX was added. (From Morgan et al. 2007.)
What is the relationship between proton channels and NADPH oxidase?
This is one of the most intriguing mysteries regarding the activity of proton channels. As mentioned in ‘Has the right gene been identified?’ the preponderance of evidence speaks against gp91phox functioning as a proton channel. However, there remain several inexplicable interactions between these two molecules. The existence of these interactions contributed substantially to the attraction of the idea that gp91phox might be a proton channel.
1. The first indication of a liaison between proton channels and NADPH oxidase came from studies of neutrophils from patients with chronic granulomatous disease (CGD). In this hereditary disease, any of several hundred known mutations to one of the several critical components of the NADPH oxidase complex that results in enzyme dysfunction produces a severe susceptibility to infections that leads to early morbidity, unless the patient is actively managed (Babior, 1991). PMA-induced pH changes that were interpreted as reflecting conductive proton efflux were substantially diminished in CGD cells, compared to normal cells (Nanda et al. 1993). Examination of patients with a variety of specific mutations led to the conclusions that: (1) gp91phox is not a proton channel, because proton currents persist in its absence; (2) nevertheless, normal assembly of NADPH oxidase is required for activation of the proton conductance (Nanda et al. 1994a,1994b).
2. Proton currents are converted into the enhanced gating mode by numerous activators of NADPH oxidase (Figs 4 and 5). NADPH oxidase activity was first associated with proton channel ‘activation’ in a whole-cell study in which ingredients supportive of NADPH oxidase activity were included in the pipette solution (Bánfi et al. 1999). In perforated-patch studies, agonists that activate both NADPH oxidase and proton channels include: PMA, AA (arachidonic acid), oleic acid, LTB4 (leukotriene B4), IL-5 (interleukin-5), fMLF (formyl-methionyl-leucyl-phenylalanine, a chemotactic peptide) and spontaneous activation presumably due to adherence (DeCoursey et al. 2000, 2001a,2001b; Bankers-Fulbright et al. 2001; Cherny et al. 2001a; Mori et al. 2003; Morgan et al. 2007).
3. Proton channels can sense whether NADPH oxidase is working or not. One of the characteristics of proton channels in the enhanced gating mode is profound slowing of tail current decay, with τtail increasing by 4- to 6-fold (Bánfi et al. 1999; DeCoursey et al. 2000, 2001a, 2001b; Morgan et al. 2007). When the NADPH oxidase inhibitor DPI is introduced, the electron current turns off and at the same time, τtail returns toward its original rapid kinetics (DeCoursey et al. 2000, 2001a). DPI only affects ‘activated’ proton channels, not resting channels in unstimulated cells (DeCoursey et al. 2000). However, there is no immediate effect of DPI on the other enhanced gating properties of proton channels. The association of slowing of τtail with electron currents is uncanny. In experiments on differentiated HL-60 cells, a fraction of cells responded to PMA stimulation with characteristic changes in proton currents seen in other phagocytes; τtail slowed appreciably only in cells in which electron current was detected (V. V. Cherny, D. Morgan and T. E. DeCoursey, unpublished data).
4. On the other hand, all of the features of enhanced gating except for τtail (including the −40 mV shift of the gH–V relationship that produces inward currents) remain enhanced when NADPH oxidase is inhibited by DPI or by removal of substrate O2 (Bánfi et al. 1999). Thus, most enhanced gating features do not require NADPH oxidase activity (electron current).
5. An extension of this phenomenon is observed in granulocytes from patients with CGD. Stimulation of neutrophils or eosinophils from CGD patients by PMA produces most of the enhanced gating features of proton channels that occur in normal cells, except the negative shift of the gH–V relationship is smaller and τtail does not slow appreciably (DeCoursey et al. 2001b; DeCoursey, 2003). By definition, there is no electron current in these cells. Presumably the only difference between these and normal cells is the dysfunction of NADPH oxidase, yet the proton channel response is different.
6. A similar phenomenon occurs in human basophils. These leucocytes are developmentally related to eosinophils and, more distantly, to neutrophils, but they do not express detectable levels of NADPH oxidase (de Boer & Roos, 1986). In a recent study, we found that basophils respond to PMA almost identically to CGD cells: the shift of Vthreshold is only −20 mV instead of −40 mV, and τtail is slowed negligibly (Musset et al. 2008b).
7. In non-leukocytic cells that do not express high levels of NADPH oxidase, there is little evidence of the enhanced gating mode. So far, this has been tested only in rat alveolar epithelial cells (DeCoursey et al. 2000), and in HEK-293 and COS-7 cell lines (Musset et al. 2008a). Nevertheless, the coincidence of proton channel enhancement with NADPH oxidase expression is striking.
In the case of proton channels sensing NADPH oxidase activity (no. 3 above), one proposed mechanism is via local pH (DeCoursey et al. 2000). NADPH oxidase activity in phagocytes generates large quantities of cytoplasmic protons (van Zwieten et al. 1981; Gabig et al. 1984; Borregaard et al. 1984), and according to the model of proton channel gating, the effect of intracellular protons is to stabilize the open proton channel (Cherny et al. 1995). If sufficient protons were generated in the vicinity of proton channels, this could account for τtail slowing, at least qualitatively. Rough calculations do not support the likelihood of sufficiently large pH changes if random distribution of both molecules in the plasma membrane is assumed (p. 553–554, DeCoursey, 2003), but any number of ad hoc explanations could be offered. In summary, no mechanistic explanation for the cross-talk between proton channels and NADPH oxidase has been established.
How about PI(3,4)P2?
The stability of the enhanced gating mode of proton channels in inside-out patches appears to be supported by a combination of ATP, GTPγS and PI(3,4)P2 (phosphoinositide 3,4-bisphosphate). The mechanism was hypothesized to include interaction with the p47phox component of NADPH oxidase (Petheöet al. 2006), adding to the intriguing question of the nature of the relationship between proton channels and NADPH oxidase. The details of the interactions of these regulatory molecules with proton channels need further clarification.
What are the functions of proton channels?
What does the KO look like? What are the consequences of eliminating the voltage-gated proton channel?
One could ask the function of proton channels in each of the cells where they occur. Specific functions that have been proposed in various cells are reviewed elsewhere (DeCoursey, 2003, 2008) and will not be reiterated here. With a few exceptions, the proposed functions are based mainly on pharmacological lesion experiments using Zn2+ in combination with varying amounts of pure speculation. The identification of the proton channel gene now enables several new approaches to this question. Both groups who identified the gene (Ramsey et al. 2006; Sasaki et al. 2006) have generated knockout mice. We eagerly await reports of the manifestations of this genetic loss. Of course, in many knockouts, the functions performed by the missing protein are compensated by other molecules that may not normally act in this capacity. But this puts the apologetics before the horse. Antibodies to external epitopes on the proton channel that alter function in intact cells would be very useful, but to my knowledge do not yet exist. It is now also possible to use siRNA (small interfering RNA, or silencing RNA) to knock down the activity of the channel. The cautionary note for this approach is that the knockdown must be nearly complete. Ion channels are activated (i.e. they open) in a probabilistic manner. Even if 63% (e−1) of all functional proton channels were eliminated, the cell would need only 4.2 mV additional depolarization – the limiting slope of the gH–V relationship (Musset et al. 2008a) – to activate the same proton conductance. Whether this small extra depolarization would significantly affect anything else in the cell is not clear.
The converse of eliminating proton channel function is increased proton conductance. In brown fat cells, a proton leak across mitochondrial inner membrane generates heat. The inappropriate appearance of proton current occurs with certain mutations that insert His into the VSD of a Na+ channel, and may be responsible for some of the consequences of hypokalaemic periodic paralysis (Struyk & Cannon, 2007).
In general, any of the consequences of proton channel activity may serve a useful function in a cell. These consequences include: (1) electrical effects, such as hyperpolarization of the membrane or charge compensation; (2) increasing pHi; (3) decreasing pHo; (4) osmotic effects; and (5) other specialized consequences. Specific situations have been identified in which each consequence of proton channel activity plays an important role.
Charge compensation
Opening proton channels will drive the membrane potential toward the Nernst potential for H+. Since proton channels open mainly when the electrochemical gradient for protons is outward, their activity tends to hyperpolarize the membrane. The importance of electrical effects has been shown abundantly in phagocytes, where proton efflux balances the electrical consequences of the electrogenic electron flux through NADPH oxidase. NADPH oxidase works by translocating electrons from cytoplasmic NADPH across the membrane to reduce O2 to superoxide anion, O2·− (Henderson et al. 1987). As soon as NADPH oxidase is activated, there is rapid and profound depolarization (Seligmann & Gallin, 1980; Whitin et al. 1980; Lazzari et al. 1986; Di Virgilio et al. 1987; Henderson et al. 1987; Pugin et al. 1997; Geiszt et al. 1997; Jankowski & Grinstein, 1999; Bankers-Fulbright et al. 2003; Rada et al. 2004; Demaurex & Petheö, 2005), which is exacerbated by Zn2+ (Henderson et al. 1987; Bánfi et al. 1999; Bankers-Fulbright et al. 2003; Rada et al. 2004; Demaurex & Petheö, 2005). Zn2+ has no direct effect on NADPH oxidase (Yatsuyanagi & Ogiso, 1988; Schrenzel et al. 1998; DeCoursey et al. 2003), but potently inhibits proton channels (Thomas & Meech, 1982; Mahaut-Smith, 1989b; Cherny & DeCoursey, 1999; DeCoursey & Cherny, 2007). When enough proton channels are open, H+ efflux balances electron extrusion, and there is no further change in membrane potential. If the electronic charge were not compensated, depolarization would reach levels that directly inhibit NADPH oxidase by opposing electron translocation (DeCoursey et al. 2003).
A recent speculative explanation for the need for proton channels in basophils is to compensate electrically for Ca2+ influx, which is required for anti-IgE-mediated histamine release (Musset et al. 2008b).
Acid extrusion (increasing pHi)
The classical way to demonstrate involvement of proton channels in acid extrusion is to load a cell with acid, either by the NH4+ pre-pulse method (Roos & Boron, 1981) or by direct injection of HCl (Thomas & Meech, 1982), and then observe the recovery. Under conditions in which other acid extrusion mechanisms are precluded, Zn2+ inhibits pHi recovery in many cells (Nanda et al. 1992; Nordström et al. 1994, 1997; Demaurex et al. 1996; Kuno et al. 1997; Sheldon & Church, 2002; Murphy et al. 2005; Cheng et al. 2008). A complementary result is that transfecting mVSOP into HEK-293 cells greatly accelerated their recovery from an acid load (Sasaki et al. 2006).
Acid secretion (lowering pHo)
The distinction from acid extrusion is mainly teleological – the former has a ‘goal’ of maintaining pHi in an optimal range for cellular functions, whereas the ‘intent’ of acid secretion is to regulate the acidity of the extracellular environment. In the case of respiratory epithelium, proton channel activity may serve to maintain the airway surface liquid at a moderately low pH (Fischer et al. 2002; Schwarzer et al. 2004), although additional functions have been proposed (Schwarzer et al. 2004; Fischer, 2007).
Osmotic effects
This function has been suggested to occur in the phagosome, where it might more properly be termed ‘preventing osmotic effects.’ Since NADPH oxidase activity extrudes enormous quantities of electrons that require charge compensation, as well as pH compensation, precisely which ionic species does the compensation becomes an important consideration. If all of the charge were compensated by K+ flux into the phagosome, for example, the osmotic effect would be at least a 20-fold swelling (Murphy & DeCoursey, 2006). As the observed swelling is much less (Reeves et al. 2002), it is clear that most of the compensation occurs by H+ flux. The osmotic consequences of H+ flux into the phagosome are mild because most of the products of reactions involving H+ that occur in the phagosome (e.g. H2O, H2O2, HOCl) are membrane permeable.
Other consequences
Another important function of proton flux in phagocytes is to provide the H+ needed in large quantities as a substrate for the production of several reactive oxygen species (H2O2, HOCl, etc.) that kill microbes (Klebanoff, 2005; Nauseef, 2007; Rada et al. 2008). Proton flux into the phagosome delivers this substrate to the site of its utilization.
Conclusion
During the interval between the discovery of voltage-gated proton channels and the identification of their gene, many of the fundamental properties of these channels were determined. Now that the gene has been identified, a host of questions can be addressed, including the whole gamut of structure–function questions. Genetic approaches may facilitate further understanding of the functions of proton channels in the many cells that express them. The surprising similarity of the proton channel architecture to the VSD of other voltage-gated ion channels has the serendipitous effect that progress in understanding one type of channel has already and will continue to contribute in unpredictable ways to the understanding of other channels.
Acknowledgments
I appreciate comments on the manuscript by V. V. Cherny, C. Eder, B. Musset, S. M. E. Smith and R. D. Vaughan-Jones. This work was supported by the Heart, Lung and Blood Institute of the NIH (HL-61437) and by Philip Morris.
References
- Aggarwal SK, MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 1996;16:1169–1177. doi: 10.1016/s0896-6273(00)80143-9. [DOI] [PubMed] [Google Scholar]
- Akeson M, Deamer DW. Proton conductance by the gramicidin water wire: model for proton conductance in the F1F0 ATPases? Biophys J. 1991;60:101–109. doi: 10.1016/S0006-3495(91)82034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. doi: 10.1007/BFb0030498. [DOI] [PubMed] [Google Scholar]
- Babior BM. The respiratory burst oxidase and the molecular basis of chronic granulomatous disease. Am J Hematol. 1991;37:263–266. doi: 10.1002/ajh.2830370410. [DOI] [PubMed] [Google Scholar]
- Bánfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, Krause K-H. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 2000;287:138–142. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]
- Bánfi B, Schrenzel J, Nüsse O, Lew DP, Ligeti E, Krause K-H, Demaurex N. A novel H+ conductance in eosinophils: unique characteristics and absence in chronic granulomatous disease. J Exp Med. 1999;190:183–194. doi: 10.1084/jem.190.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankers-Fulbright JL, Gleich GJ, Kephart GM, Kita H, O’Grady SM. Regulation of eosinophil membrane depolarization during NADPH oxidase activation. J Cell Sci. 2003;116:3221–3226. doi: 10.1242/jcs.00627. [DOI] [PubMed] [Google Scholar]
- Bankers-Fulbright JL, Kita H, Gleich GJ, O’Grady SM. Regulation of human eosinophil NADPH oxidase activity: a central role for PKCδ. J Cell Physiol. 2001;189:306–315. doi: 10.1002/jcp.10022. [DOI] [PubMed] [Google Scholar]
- Barish ME, Baud C. A voltage-gated hydrogen ion current in the oocyte membrane of the axolotl Ambystoma. J Physiol. 1984;352:243–263. doi: 10.1113/jphysiol.1984.sp015289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim L, Krause RM, Baroffio A, Hamann M, Kaelin A, Bader C-R. A voltage-dependent proton current in cultured human skeletal muscle myotubes. J Physiol. 1993;470:313–333. doi: 10.1113/jphysiol.1993.sp019860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- Borregaard N, Schwartz JH, Tauber AI. Proton secretion by stimulated neutrophils: significance of hexose monophosphate shunt activity as source of electrons and protons for the respiratory burst. J Clin Invest. 1984;74:455–459. doi: 10.1172/JCI111442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brönsted JN, Pedersen K. Die katalytische Zersetzung des Nitramids und ihre physikalisch-chemische Bedeutung. Z Phys Chem. 1923;108:185–235. [Google Scholar]
- Byerly L, Meech R, Moody W., Jr Rapidly activating hydrogen ion currents in perfused neurones of the snail, Lymnaea stagnalis. J Physiol. 1984;351:199–216. doi: 10.1113/jphysiol.1984.sp015241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L, Moody WJ. Membrane currents of internally perfused neurones of the snail, Lymnaea stagnalis, at low intracellular pH. J Physiol. 1986;376:477–491. doi: 10.1113/jphysiol.1986.sp016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L, Suen Y. Characterization of proton currents in neurones of the snail, Lymnaea stagnalis. J Physiol. 1989;413:75–89. doi: 10.1113/jphysiol.1989.sp017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos FV, Chanda B, Roux B, Bezanilla F. Two atomic constraints unambiguously position the S4 segment relative to S1 and S2 segments in the closed state of Shaker K channel. Proc Natl Acad Sci U S A. 2007;104:7904–7909. doi: 10.1073/pnas.0702638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wu Y, Voth GA. Proton transport behavior through the influenza A M2 channel: insights from molecular simulation. Biophys J. 2007;93:3470–3479. doi: 10.1529/biophysj.107.105742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YM, Kelly T, Church J. Potential contribution of a voltage-activated proton conductance to acid extrusion from rat hippocampal neurons. Neurosci. 2008;151:1084–1098. doi: 10.1016/j.neuroscience.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Cherny VV, DeCoursey TE. pH-dependent inhibition of voltage-gated H+ currents in rat alveolar epithelial cells by Zn2+ and other divalent cations. J Gen Physiol. 1999;114:819–838. doi: 10.1085/jgp.114.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny VV, Henderson LM, Xu W, Thomas LL, DeCoursey TE. Activation of NADPH oxidase-related proton and electron currents in human eosinophils by arachidonic acid. J Physiol. 2001a;535:783–794. doi: 10.1111/j.1469-7793.2001.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny VV, Markin VS, DeCoursey TE. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J Gen Physiol. 1995;105:861–896. doi: 10.1085/jgp.105.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny VV, Murphy R, Sokolov V, Levis RA, DeCoursey TE. Properties of single voltage-gated proton channels in human eosinophils estimated by noise analysis and direct measurement. J Gen Physiol. 2003;121:615–628. doi: 10.1085/jgp.200308813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny VV, Thomas LL, DeCoursey TE. Voltage-gated proton currents in human basophils. Biologicheskie Membrany. 2001b;18:458–465. [Google Scholar]
- Chernyshev A, Cukierman S. Thermodynamic view of activation energies of proton transfer in various gramicidin A channels. Biophys J. 2002;82:182–192. doi: 10.1016/S0006-3495(02)75385-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyshev A, Pomès R, Cukierman S. Kinetic isotope effects of proton transfer in gramicidin channels in aqueous and methanol containing solutions. Biophys Chem. 2003;103:179–190. doi: 10.1016/s0301-4622(02)00255-7. [DOI] [PubMed] [Google Scholar]
- Chizhmakov IV, Geraghty FM, Ogden DC, Hayhurst A, Antoniou M, Hay AJ. Selective proton permeability and pH regulation of the influenza virus M2 channel expressed in mouse erythroleukemia cells. J Physiol. 1996;494:329–336. doi: 10.1113/jphysiol.1996.sp021495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman S. Proton mobilities in water and in different stereoisomers of covalently linked gramicidin A channels. Biophys J. 2000;78:1825–1834. doi: 10.1016/S0006-3495(00)76732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman S, Quigley EP, Crumrine DS. Proton conduction in gramicidin A and in its dioxolane-linked dimer in different lipid bilayers. Biophys J. 1997;73:2489–2502. doi: 10.1016/S0006-3495(97)78277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danneel H. Notiz über Ionengeschwindigkeiten. Z Elektrochemie Angewandte Physikalische Chemie. 1905;11:249–252. [Google Scholar]
- de Boer M, Roos D. Metabolic comparison between basophils and other leukocytes from human blood. J Immunol. 1986;136:3447–3454. [PubMed] [Google Scholar]
- DeCoursey TE. Hydrogen ion currents in rat alveolar epithelial cells. Biophys J. 1991;60:1243–1253. doi: 10.1016/S0006-3495(91)82158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE. Four varieties of voltage-gated proton channels. Front Biosci. 1998;3:d477–d482. doi: 10.2741/a294. http://www.bioscience.org/1998/v3/d/decourse/d477-482.htm. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE. Voltage-gated proton channels. Cell Mol Life Sci. 2008;65:2554–2573. doi: 10.1007/s00018-008-8056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV. Potential, pH, and arachidonate gate hydrogen ion currents in human neutrophils. Biophys J. 1993;65:1590–1598. doi: 10.1016/S0006-3495(93)81198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV. Na+-H+ antiport detected through hydrogen ion currents in rat alveolar epithelial cells and human neutrophils. J Gen Physiol. 1994a;103:755–785. doi: 10.1085/jgp.103.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV. Voltage-activated hydrogen ion currents. J Membr Biol. 1994b;141:203–223. doi: 10.1007/BF00235130. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV. II. Voltage-activated proton currents in human THP-1 monocytes. J Membr Biol. 1996a;152:131–140. doi: 10.1007/s002329900092. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV. Effects of buffer concentration on voltage-gated H+ currents: does diffusion limit the conductance? Biophys J. 1996b;71:182–193. doi: 10.1016/S0006-3495(96)79215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV. Deuterium isotope effects on permeation and gating of proton channels in rat alveolar epithelium. J Gen Physiol. 1997;109:415–434. doi: 10.1085/jgp.109.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV. Temperature dependence of voltage-gated H+ currents in human neutrophils, rat alveolar epithelial cells, and mammalian phagocytes. J Gen Physiol. 1998;112:503–522. doi: 10.1085/jgp.112.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV. Pharmacology of voltage-gated proton channels. Curr Pharm Des. 2007;13:2400–2420. doi: 10.2174/138161207781368675. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV, DeCoursey AG, Xu W, Thomas LL. Interactions between NADPH oxidase-related proton and electron currents in human eosinophils. J Physiol. 2001a;535:767–781. doi: 10.1111/j.1469-7793.2001.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV, Morgan D, Katz BZ, Dinauer MC. The gp91phox component of NADPH oxidase is not the voltage-gated proton channel in phagocytes, but it helps. J Biol Chem. 2001b;276:36063–36066. doi: 10.1074/jbc.C100352200. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV, Zhou W, Thomas LL. Simultaneous activation of NADPH oxidase-related proton and electron currents in human neutrophils. Proc Natl Acad Sci U S A. 2000;97:6885–6889. doi: 10.1073/pnas.100047297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Morgan D, Cherny VV. The gp91phox component of NADPH oxidase is not a voltage-gated proton channel. J Gen Physiol. 2002;120:773–779. doi: 10.1085/jgp.20028704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- de Grotthuss CJT. Sur la décomposition de l’eau et des corps qu’elle tient en dissolution à l’aide de l’électricité galvanique. Annales de Chimie. 1806;LVIII:54–74. [Google Scholar]
- Demaurex N, Downey GP, Waddell TK, Grinstein S. Intracellular pH regulation during spreading of human neutrophils. J Cell Biol. 1996;133:1391–1402. doi: 10.1083/jcb.133.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N, Grinstein S, Jaconi M, Schlegel W, Lew DP, Krause K-H. Proton currents in human granulocytes: regulation by membrane potential and intracellular pH. J Physiol. 1993;466:329–344. [PMC free article] [PubMed] [Google Scholar]
- Demaurex N, Orlowski J, Brisseau G, Woodside M, Grinstein S. The mammalian Na+/H+ antiporters NHE-1, NHE-2, and NHE-3 are electroneutral and voltage independent, but can couple to an H+ conductance. J Gen Physiol. 1995;106:85–111. doi: 10.1085/jgp.106.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N, Petheö GL. Electron and proton transport by NADPH oxidases. Philos Trans R Soc Lond B Biol Sci. 2005;360:2315–2325. doi: 10.1098/rstb.2005.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Lew PD, Andersson T, Pozzan T. Plasma membrane potential modulates chemotactic peptide-stimulated cytosolic free Ca2+ changes in human neutrophils. J Biol Chem. 1987;262:4574–4579. [PubMed] [Google Scholar]
- Eder C, Fischer H-G, Hadding U, Heinemann U. Properties of voltage-gated currents of microglia developed using macrophage colony-stimulating factor. Pflügers Arch. 1995;430:526–533. doi: 10.1007/BF00373889. [DOI] [PubMed] [Google Scholar]
- Eigen M, Hammes GG. Elementary steps in enzyme reactions (as studied by relaxation spectrometry) Adv Enzymol Relat Areas Mol Biol. 1963;25:1–38. doi: 10.1002/9780470122709.ch1. [DOI] [PubMed] [Google Scholar]
- Eisenman G, Enos B, Hägglund J, Sandblom J. Gramicidin as an example of a single-filing ionic channel. Ann NY Acad Sci. 1980;329:8–20. doi: 10.1111/j.1749-6632.1980.tb15964.x. [DOI] [PubMed] [Google Scholar]
- Femling JK, Cherny VV, Morgan D, Rada B, Davis AP, Czirják G, Enyedi P, England SK, Moreland JG, Ligeti E, Nauseef WM, DeCoursey TE. The antibacterial activity of human neutrophils and eosinophils requires proton channels but not BK channels. J Gen Physiol. 2006;127:659–672. doi: 10.1085/jgp.200609504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. Airway surface liquid pH and innate defense by the NADPH oxidase. Ped Pulmonol Suppl. 2007;30:175–177. [Google Scholar]
- Fischer H, Widdicombe JH, Illek B. Acid secretion and proton conductance in human airway epithelium. Am J Physiol Cell Physiol. 2002;282:736–743. doi: 10.1152/ajpcell.00369.2001. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser B, Hodgkin AL. The action of calcium on the electrical properties of squid axons. J Physiol. 1957;137:218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabig TG, Lefker BA, Ossanna PJ, Weiss SJ. Proton stoichiometry associated with human neutrophil respiratory-burst reactions. J Biol Chem. 1984;259:13166–13171. [PubMed] [Google Scholar]
- Geiszt M, Kapus A, Nemet K, Farkas L, Ligeti E. Regulation of capacitative Ca2+ influx in human neutrophil granulocytes. Alterations in chronic granulomatous disease. J Biol Chem. 1997;272:26471–26478. doi: 10.1074/jbc.272.42.26471. [DOI] [PubMed] [Google Scholar]
- Goldman DE. Potential, impedance, and rectification in membranes. J Gen Physiol. 1943;27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordienko DV, Tare M, Parveen S, Fenech CJ, Robinson C, Bolton TB. Voltage-activated proton current in eosinophils from human blood. J Physiol. 1996;496:299–316. doi: 10.1113/jphysiol.1996.sp021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig N, Plant TD, Janson W, Schäfer M, Schultz G, Schaefer M. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J Biol Chem. 2004;279:34553–34561. doi: 10.1074/jbc.M402966200. [DOI] [PubMed] [Google Scholar]
- Henderson LM, Banting G, Chappell JB. The arachidonate-activable, NADPH oxidase-associated H+ channel. Evidence that gp91-phox functions as an essential part of the channel. J Biol Chem. 1995;270:5909–5916. [PubMed] [Google Scholar]
- Henderson LM, Chappell JB, Jones OTG. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem J. 1987;246:325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LM, Chappell JB, Jones OTG. Internal pH changes associated with the activity of NADPH oxidase of human neutrophils: further evidence for the presence of an H+ conducting channel. Biochem J. 1988a;251:563–567. doi: 10.1042/bj2510563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LM, Chappell JB, Jones OTG. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem J. 1988b;255:285–290. [PMC free article] [PubMed] [Google Scholar]
- Henderson LM, Meech RW. Evidence that the product of the human X-linked CGD gene, gp91-phox, is a voltage-gated H+ pathway. J Gen Physiol. 1999;114:771–785. doi: 10.1085/jgp.114.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LM, Meech RW. Proton conduction through gp91phox. J Gen Physiol. 2002;120:759–765. doi: 10.1085/jgp.20028708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3rd edn. Sunderland, MA: Sinauer Associates, Inc.; 2001. [Google Scholar]
- Hirschberg B, Rovner A, Lieberman M, Patlak J. Transfer of twelve charges is needed to open skeletal muscle Na+ channels. J Gen Physiol. 1995;106:1053–1068. doi: 10.1085/jgp.106.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952;116:473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Katz B. The effects of sodium ions on the electrical activity of the giant axon of the squid. J Physiol. 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R. How ion channels sense membrane potential. Proc Natl Acad Sci U S A. 2005;102:4929–4930. doi: 10.1073/pnas.0501640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MI, Iwasaki H, Okochi Y, Chahine M, Higashijima S, Nagayama K, Okamura Y. Enzyme domain affects the movement of the voltage sensor in ascidian and zebrafish voltage-sensing phosphatases. J Biol Chem. 2008;283:18248–18259. doi: 10.1074/jbc.M706184200. [DOI] [PubMed] [Google Scholar]
- Humez S, Fournier F, Guilbault P. A voltage-dependent and pH-sensitive proton current in Rana esculenta oocytes. J Membr Biol. 1995;147:207–215. doi: 10.1007/BF00233548. [DOI] [PubMed] [Google Scholar]
- Jankowski A, Grinstein S. A noninvasive fluorimetric procedure for measurement of membrane potential. J Biol Chem. 1999;274:26098–26104. doi: 10.1074/jbc.274.37.26098. [DOI] [PubMed] [Google Scholar]
- Jiang J, Li M, Yue L. Potentiation of TRPM7 inward currents by protons. J Gen Physiol. 2005;126:137–150. doi: 10.1085/jgp.200409185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge W, McLaughlin S. The role of fixed and mobile buffers in the kinetics of proton movement. Biochim Biophys Acta. 1987;890:1–5. doi: 10.1016/0005-2728(87)90061-2. [DOI] [PubMed] [Google Scholar]
- Kapus A, Romanek R, Qu AY, Rotstein OD, Grinstein S. A pH-sensitive and voltage-dependent proton conductance in the plasma membrane of macrophages. J Gen Physiol. 1993;102:729–760. doi: 10.1085/jgp.102.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapus A, Szászi K, Ligeti E. Phorbol 12-myristate 13-acetate activates an electrogenic H+-conducting pathway in the membrane of neutrophils. Biochem J. 1992;281:697–701. doi: 10.1042/bj2810697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass I, Arkin IT. How pH opens a H+ channel: the gating mechanism of influenza A M2. Structure. 2005;13:1789–1798. doi: 10.1016/j.str.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- Klee R, Heinemann U, Eder C. Voltage-gated proton currents in microglia of distinct morphology and functional state. Neuroscience. 1999;91:1415–1424. doi: 10.1016/s0306-4522(98)00710-6. [DOI] [PubMed] [Google Scholar]
- Koch HP, Kurokawa T, Okochi Y, Sasaki M, Okamura Y, Larsson HP. Multimeric nature of voltage-gated proton channels. Proc Natl Acad Sci U S A. 2008;105:9111–9116. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout SC, Ulbrich MH, Bell SC, Isacoff EY. Subunit organization and functional transitions in Ci-VSP. Nat Struct Mol Biol. 2008;15:106–108. doi: 10.1038/nsmb1320. [DOI] [PubMed] [Google Scholar]
- Kukol A, Adams PD, Rice LM, Brunger AT, Arkin TI. Experimentally based orientational refinement of membrane protein models: a structure for the Influenza A M2 H+ channel. J Mol Biol. 1999;286:951–962. doi: 10.1006/jmbi.1998.2512. [DOI] [PubMed] [Google Scholar]
- Kuno M, Kawawaki J, Nakamura F. A highly temperature-sensitive proton conductance in mouse bone marrow-derived mast cells. J Gen Physiol. 1997;109:731–740. doi: 10.1085/jgp.109.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson HP, Baker OS, Dhillon DS, Isacoff EY. Transmembrane movement of the Shaker K+ channel S4. Neuron. 1996;16:387–397. doi: 10.1016/s0896-6273(00)80056-2. [DOI] [PubMed] [Google Scholar]
- Lazzari KG, Proto PJ, Simons ER. Simultaneous measurement of stimulus-induced changes in cytoplasmic Ca2+ and in membrane potential of human neutrophils. J Biol Chem. 1986;261:9710–9713. [PubMed] [Google Scholar]
- Lear JD. Proton conduction through the M2 protein of the influenza A virus; a quantitative, mechanistic analysis of experimental data. FEBS Lett. 2003;552:17–22. doi: 10.1016/s0014-5793(03)00778-6. [DOI] [PubMed] [Google Scholar]
- Lee S-Y, Letts JA, MacKinnon R. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc Natl Acad Sci U S A. 2008;105:7692–7695. doi: 10.1073/pnas.0803277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt DG, Elias SR, Hautman JM. Number of water molecules coupled to the transport of sodium, potassium and hydrogen ions via gramicidin, nonactin or valinomycin. Biochim Biophys Acta. 1978;512:436–451. doi: 10.1016/0005-2736(78)90266-3. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Doody TC. The mobility of ions in H2H2O. J Am Chem Soc. 1933;55:3504–3506. [Google Scholar]
- Lin TI, Schroeder C. Definitive assignment of proton selectivity and attoampere unitary current to the M2 ion channel protein of influenza A virus. J Virol. 2001;75:3647–3656. doi: 10.1128/JVI.75.8.3647-3656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Lou XJ, Horrigan FT. Role of charged residues in the S1–S4 voltage sensor of BK channels. J Gen Physiol. 2006;127:309–328. doi: 10.1085/jgp.200509421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaut-Smith M. Separation of hydrogen ion currents in intact molluscan neurones. J Exp Biol. 1989a;145:439–454. doi: 10.1242/jeb.145.1.439. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith M. The effect of zinc on calcium and hydrogen ion currents in intact snail neurones. J Exp Biol. 1989b;145:455–464. doi: 10.1242/jeb.145.1.455. [DOI] [PubMed] [Google Scholar]
- Maturana A, Arnaudeau S, Ryser S, Bánfi B, Hossle JP, Schlegel W, Krause K-H, Demaurex N. Heme histidine ligands within gp91phox modulate proton conduction by the phagocyte NADPH oxidase. J Biol Chem. 2001;276:30277–30284. doi: 10.1074/jbc.M010438200. [DOI] [PubMed] [Google Scholar]
- Maturana A, Krause KH, Demaurex N. NOX family NADPH oxidases: do they have built-in proton channels? J Gen Physiol. 2002;120:781–786. doi: 10.1085/jgp.20028713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech RW, Thomas RC. Voltage-dependent intracellular pH in Helix aspersa neurones. J Physiol. 1987;390:433–452. doi: 10.1113/jphysiol.1987.sp016710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton RL, Caldwell JH. How do patch clamp seals form? A lipid bleb model. Pflugers Arch. 1990;416:758–762. doi: 10.1007/BF00370626. [DOI] [PubMed] [Google Scholar]
- Morgan D, Cherny VV, Finnegan A, Bollinger J, Gelb MH, DeCoursey TE. Sustained activation of proton channels and NADPH oxidase in human eosinophils and murine granulocytes requires PKC but not cPLA2α activity. J Physiol. 2007;579:327–344. doi: 10.1113/jphysiol.2006.124248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Sakai H, Morihata H, Kawawaki J, Amano H, Yamano T, Kuno M. Regulatory mechanisms and physiological relevance of a voltage-gated H+ channel in murine osteoclasts: phorbol myristate acetate induces cell acidosis and the channel activation. J Bone Miner Res. 2003;18:2069–2076. doi: 10.1359/jbmr.2003.18.11.2069. [DOI] [PubMed] [Google Scholar]
- Morihata H, Kawawaki J, Sakai H, Sawada M, Tsutada T, Kuno M. Temporal fluctuations of voltage-gated proton currents in rat spinal microglia via pH-dependent and -independent mechanisms. Neurosci Res. 2000;38:265–271. doi: 10.1016/s0168-0102(00)00170-x. [DOI] [PubMed] [Google Scholar]
- Mould JA, Drury JE, Frings SM, Kaupp UB, Pekosz A, Lamb RA, Pinto LH. Permeation and activation of the M2 ion channel of influenza A virus. J Biol Chem. 2000;275:31038–31050. doi: 10.1074/jbc.M003663200. [DOI] [PubMed] [Google Scholar]
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- Murphy R, Cherny VV, Morgan D, DeCoursey TE. Voltage-gated proton channels help regulate pHi in rat alveolar epithelium. Am J Physiol Lung Cell Mol Physiol. 2005;288:L398–L408. doi: 10.1152/ajplung.00299.2004. [DOI] [PubMed] [Google Scholar]
- Murphy R, DeCoursey TE. Charge compensation in phagocytes. Biochim Biophys Acta. 2006;1757:996–1011. doi: 10.1016/j.bbabio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Musset B, Cherny VV, Morgan D, Okamura Y, Ramsey IS, Clapham DE, DeCoursey TE. Detailed comparison of expressed and native voltage-gated proton channel currents. J Physiol. 2008a;586:2477–2486. doi: 10.1113/jphysiol.2007.149427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musset B, Morgan D, Cherny VV, MacGlashan DW, Jr, Thomas LL, Ríos E, DeCoursey TE. A pH-stabilizing role of voltage gated proton channels in IgE-mediated activation of human basophils. Proc Natl Acad Sci U S A. 2008b;105:11020–11025. doi: 10.1073/pnas.0800886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers VB, Haydon DA. Ion transfer across lipid membranes in the presence of gramicidin A: the ion selectivity. Biochim Biophys Acta. 1972;274:313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- Nagle JF, Morowitz HJ. Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci U S A. 1978;75:298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle JF, Tristram-Nagle S. Hydrogen bonded chain mechanisms for proton conduction and proton pumping. J Membr Biol. 1983;74:1–14. doi: 10.1007/BF01870590. [DOI] [PubMed] [Google Scholar]
- Nanda A, Curnutte JT, Grinstein S. Activation of H+ conductance in neutrophils requires assembly of components of the respiratory burst oxidase but not its redox function. J Clin Invest. 1994a;93:1770–1775. doi: 10.1172/JCI117162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A, Grinstein S. Protein kinase C activates an H+ (equivalent) conductance in the plasma membrane of human neutrophils. Proc Natl Acad Sci U S A. 1991;88:10816–10820. doi: 10.1073/pnas.88.23.10816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A, Grinstein S, Curnutte JT. Abnormal activation of H+ conductance in NADPH oxidase-defective neutrophils. Proc Natl Acad Sci U S A. 1993;908:760–764. doi: 10.1073/pnas.90.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A, Gukovskaya A, Tseng J, Grinstein S. Activation of vacuolar-type proton pumps by protein kinase C: role in neutrophil pH regulation. J Biol Chem. 1992;267:22740–22746. [PubMed] [Google Scholar]
- Nanda A, Romanek R, Curnutte JT, Grinstein S. Assessment of the contribution of the cytochrome b moiety of the NADPH oxidase to the transmembrane H+ conductance of leukocytes. J Biol Chem. 1994b;269:27280–27285. [PubMed] [Google Scholar]
- Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Noda M, Shimizu S, Tanabe T, Takai T, Kayano T, Ikeda T, Takahashi H, Nakayama H, Kanaoka Y, Minamino N, Kangawa K, Matsuo H, Raftery MA, Hirose T, Inayama S, Hayashida H, Miyata T, Numa S. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984;312:121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Nordström T, Grinstein S, Brisseau GF, Manolson MF, Rotstein OD. Protein kinase C activation accelerates proton extrusion by vacuolar-type H+-ATPases in murine peritoneal macrophages. FEBS Lett. 1994;350:82–86. doi: 10.1016/0014-5793(94)00738-1. [DOI] [PubMed] [Google Scholar]
- Nordström T, Shrode LD, Rotstein OD, Romanek R, Goto T, Heersche JNM, Manolson MF, Brisseau GF, Grinstein S. Chronic extracellular acidosis induces plasmalemmal vacuolar type H+ ATPase activity in osteoclasts. J Biol Chem. 1997;272:6354–6360. doi: 10.1074/jbc.272.10.6354. [DOI] [PubMed] [Google Scholar]
- Numata T, Okada Y. Proton conductivity through the human TRPM7 channel and its molecular determinants. J Biol Chem. 2008;283:15097–15103. doi: 10.1074/jbc.M709261200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BB, Sweeton FH. The conductance of hydrochloric acid in aqueous solutions from 5 to 65°. J Am Chem Soc. 1941;63:2811–2817. [Google Scholar]
- Pauling L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals; an Introduction to Modern Structural Chemistry. Ithaca, NY: Cornell University Press; 1939. [Google Scholar]
- Petheö GL, Girardin NC, Goossens N, Molnár GZ, Demaurex N. Role of nucleotides and phosphoinositides in the stability of electron and proton currents associated with the phagocytic NADPH oxidase. Biochem J. 2006;400:431–438. doi: 10.1042/BJ20060578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petheö GL, Maturana A, Spät A, Demaurex N. Interactions between electron and proton currents in excised patches from human eosinophils. J Gen Physiol. 2003;122:713–726. doi: 10.1085/jgp.200308891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto LH, Dieckmann GR, Gandhi CS, Papworth CG, Braman J, Shaughnessy MA, Lear JD, Lamb RA, DeGrado WF. A functionally defined model for the M2 proton channel of influenza A virus suggests a mechanism for its ion selectivity. Proc Natl Acad Sci U S A. 1997;94:11301–11306. doi: 10.1073/pnas.94.21.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomès R. Translation of: de Grotthuss, CJT 1806. Memoir on the decomposition of water and of the bodies that it holds in solution by means of galvanic electricity. Biochim Biophys Acta. 2006;1757:871–875. doi: 10.1016/j.bbabio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Pugin A, Frachisse J-M, Tavernier E, Bligny R, Gout E, Douce R, Guern J. Early events induced by the elicitor cryptogein in tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell. 1997;9:2077–2091. doi: 10.1105/tpc.9.11.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Qu AY, Nanda A, Curnutte JT, Grinstein S. Development of a H+-selective conductance during granulocytic differentiation of HL-60 cells. Am J Physiol Cell Physiol. 1994;266:C1263–C1270. doi: 10.1152/ajpcell.1994.266.5.C1263. [DOI] [PubMed] [Google Scholar]
- Rada B, Hably C, Meczner A, Timár C, Lakatos G, Enyedi P, Ligeti E. Role of Nox2 in elimination of microorganisms. Semin Immunopathol. 2008;30:237–253. doi: 10.1007/s00281-008-0126-3. [DOI] [PubMed] [Google Scholar]
- Rada BK, Geiszt M, Káldi K, Tímár C, Ligeti E. Dual role of phagocytic NADPH oxidase in bacterial killing. Blood. 2004;104:2947–2953. doi: 10.1182/blood-2004-03-1005. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves EP, Lu H, Jacobs HL, Messina CGM, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- Robinson RA, Stokes RH. Electrolyte Solutions. London: Butterworths; 1959. [Google Scholar]
- Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Ruknudin A, Song MJ, Sachs F. The ultrastructure of patch-clamped membranes: a study using high voltage electron microscopy. J Cell Biol. 1991;112:125–134. doi: 10.1083/jcb.112.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom MSP, Kerr ID, Smith GR, Son HS. The influenza A virus M2 channel: a molecular modeling and simulation study. Virology. 1997;233:163–173. doi: 10.1006/viro.1997.8578. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- Scheiner S. Proton transfers in hydrogen-bonded systems: cationic oligomers of water. J Am Chem Soc. 1981;103:315–320. [Google Scholar]
- Schilling T, Gratopp A, DeCoursey TE, Eder C. Voltage-activated proton currents in human lymphocytes. J Physiol. 2002;545:93–105. doi: 10.1113/jphysiol.2002.028878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, McCormack K, Tanouye MA, Sigworth FJ. The size of gating charge in wild-type and mutant Shaker potassium channels. Science. 1992;255:1712–1715. doi: 10.1126/science.1553560. [DOI] [PubMed] [Google Scholar]
- Schrenzel J, Lew DP, Krause K-H. Proton currents in human eosinophils. Am J Physiol Cell Physiol. 1996;271:C1861–C1871. doi: 10.1152/ajpcell.1996.271.6.C1861. [DOI] [PubMed] [Google Scholar]
- Schrenzel J, Serrander L, Bánfi B, Nüsse O, Fouyouzi R, Lew DP, Demaurex N, Krause K-H. Electron currents generated by the human phagocyte NADPH oxidase. Nature. 1998;392:734–737. doi: 10.1038/33725. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem. 2004;279:36454–36461. doi: 10.1074/jbc.M404983200. [DOI] [PubMed] [Google Scholar]
- Schweighofer KJ, Pohorille A. Computer simulation of ion channel gating: the M2 channel of influenza A virus in a lipid bilayer. Biophys J. 2000;78:150–163. doi: 10.1016/S0006-3495(00)76581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiening CJ, Willoughby D. Depolarization- induced pH microdomains and their relationship to calcium transients in isolated snail neurones. J Physiol. 2002;538:371–382. doi: 10.1113/jphysiol.2001.013055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligmann BE, Gallin JI. Use of lipophilic probes of membrane potential to assess human neutrophil activation: abnormality in chronic granulomatous disease. J Clin Invest. 1980;66:493–503. doi: 10.1172/JCI109880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoh SA, Sigg D, Papazian DM, Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 1996;16:1159–1167. doi: 10.1016/s0896-6273(00)80142-7. [DOI] [PubMed] [Google Scholar]
- Sheldon C, Church J. Intracellular pH response to anoxia in acutely dissociated adult rat hippocampal CA1 neurons. J Neurophysiol. 2002;87:2209–2224. doi: 10.1152/jn.2002.87.5.2209. [DOI] [PubMed] [Google Scholar]
- Shuck K, Lamb RA, Pinto LH. Analysis of the pore structure of the influenza A virus M2 ion channel by the substituted-cysteine accessibility method. J Virol. 2000;74:7755–7761. doi: 10.1128/jvi.74.17.7755-7761.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth FJ. Voltage gating of ion channels. Q Rev Biophys. 1993;27:1–40. doi: 10.1017/s0033583500002894. [DOI] [PubMed] [Google Scholar]
- Slatin SL, Finkelstein A, Kienker PK. Anomalous proton selectivity in a large channel: colicin A. Biochemistry. 2008;47:1778–1788. doi: 10.1021/bi701900x. [DOI] [PubMed] [Google Scholar]
- Smondyrev AM, Voth GA. Molecular dynamics simulation of proton transport near the surface of a phospholipid membrane. Biophys J. 2002;82:1460–1468. doi: 10.1016/S0006-3495(02)75500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov S, Scheuer T, Catterall WA. Ion permeation through a voltage-sensitive gating pore in brain sodium channels having voltage sensor mutations. Neuron. 2005;47:183–189. doi: 10.1016/j.neuron.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, Kiselyov K. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J Biol Chem. 2006;281:7294–7301. doi: 10.1074/jbc.M508211200. [DOI] [PubMed] [Google Scholar]
- Starace DM, Bezanilla F. Histidine scanning mutagenesis of basic residues of the S4 segment of the Shaker K+ channel. J Gen Physiol. 2001;117:469–490. doi: 10.1085/jgp.117.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starace DM, Bezanilla F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. 2004;427:548–553. doi: 10.1038/nature02270. [DOI] [PubMed] [Google Scholar]
- Starace DM, Stefani E, Bezanilla F. Voltage-dependent proton transport by the voltage sensor of the Shaker K+ channel. Neuron. 1997;19:1319–1327. doi: 10.1016/s0896-6273(00)80422-5. [DOI] [PubMed] [Google Scholar]
- Stewart AK, Boyd CAR, Vaughan-Jones RD. A novel role for carbonic anhydrase: cytoplasmic pH gradient dissipation in mouse small intestinal enterocytes. J Physiol. 1999;516:209–217. doi: 10.1111/j.1469-7793.1999.209aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouffer AL, Acharya R, Salom D, Levine AS, Di Costanzo L, Soto CS, Tereshko V, Nanda V, Stayrook S, DeGrado WF. Structural basis for the function and inhibition of an influenza virus proton channel. Nature. 2008;451:596–599. doi: 10.1038/nature06528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyk AF, Cannon SC. A Na+ channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore. J Gen Physiol. 2007;130:11–20. doi: 10.1085/jgp.200709755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W, Conti F, Suzuki H, Wang XD, Noda M, Yahagi N, Kubo H, Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989;339:597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- Swietach P, Spitzer KW, Vaughan-Jones RD. pH-dependence of extrinsic and intrinsic H+-ion mobility in the rat ventricular myocyte, investigated using flash photolysis of a caged-H+ compound. Biophys J. 2007;92:641–653. doi: 10.1529/biophysj.106.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swietach P, Vaughan-Jones RD. Spatial regulation of intracellular pH in the ventricular myocyte. Ann N Y Acad Sci. 2005;1047:271–282. doi: 10.1196/annals.1341.024. [DOI] [PubMed] [Google Scholar]
- Swietach P, Zaniboni M, Stewart AK, Rossini A, Spitzer KW, Vaughan-Jones RD. Modelling intracellular H+ ion diffusion. Prog Biophys Mol Biol. 2003;83:69–100. doi: 10.1016/s0079-6107(03)00027-0. [DOI] [PubMed] [Google Scholar]
- Thomas RC, Meech RW. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 1982;299:826–828. doi: 10.1038/299826a0. [DOI] [PubMed] [Google Scholar]
- Tombola F, Pathak MM, Gorostiza P, Isacoff EY. The twisted ion-permeation pathway of a resting voltage-sensing domain. Nature. 2007;445:546–549. doi: 10.1038/nature05396. [DOI] [PubMed] [Google Scholar]
- Tombola F, Pathak MM, Isacoff EY. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron. 2005;45:379–388. doi: 10.1016/j.neuron.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Tombola F, Pathak MM, Isacoff EY. How does voltage open an ion channel? Annu Rev Cell Dev Biol. 2006;22:23–52. doi: 10.1146/annurev.cellbio.21.020404.145837. [DOI] [PubMed] [Google Scholar]
- Tombola F, Ulbrich MH, Isacoff EY. The voltage-gated proton channel HV1 has two pores, each controlled by one voltage sensor. Neuron. 2008;58:546–556. doi: 10.1016/j.neuron.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touret N, Grinstein S. Voltage-gated proton ‘channels’: a spectator' viewpoint. J Gen Physiol. 2002;120:767–771. doi: 10.1085/jgp.20028706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zwieten R, Wever R, Hamers MN, Weening RS, Roos D. Extracellular proton release by stimulated neutrophils. J Clin Invest. 1981;68:310–313. doi: 10.1172/JCI110250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones RD, Peercy BE, Keener JP, Spitzer KW. Intrinsic H+ ion mobility in the rabbit ventricular myocyte. J Physiol. 2002;541:139–158. doi: 10.1113/jphysiol.2001.013267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitin JC, Chapman CE, Simons ER, Chovaniec ME, Cohen HJ. Correlation between membrane potential changes and superoxide production in human granulocytes stimulated by phorbol myristate acetate. J Biol Chem. 1980;255:1874–1878. [PubMed] [Google Scholar]
- Yatsuyanagi J, Ogiso T. Zinc inhibition of respiratory burst in zymosan-stimulated neutrophils: a possible membrane action of zinc. Chem Pharm Bull (Tokyo) 1988;36:1035–1040. doi: 10.1248/cpb.36.1035. [DOI] [PubMed] [Google Scholar]
- Zifarelli G, Murgia AR, Soliani P, Pusch M. Intracellular proton regulation of ClC-0. J Gen Physiol. 2008a;132:185–198. doi: 10.1085/jgp.200809999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zifarelli G, Soliani P, Pusch M. Buffered diffusion around a spherical proton pumping cell: a theoretical analysis. Biophys J. 2008b;94:53–62. doi: 10.1529/biophysj.107.111310. [DOI] [PMC free article] [PubMed] [Google Scholar]