Abstract
Background
Frequent use of nonsteroidal anti-inflammatory drugs (NSAIDs) has been shown to reduce the risk of colorectal adenomas in randomized trials. We examined the persistence of the protective effect after the cessation of randomized aspirin treatment and whether it is affected by the duration and frequency of subsequent NSAID use.
Methods
We used data from the Aspirin/Folate Polyp Prevention Study (AFPPS), in which 1121 subjects were randomly assigned to receive placebo or aspirin (81 or 325 mg/d) for 3 years. After the end of treatment and a follow-up colonoscopy, AFPPS participants were invited to remain under follow-up until their next surveillance colonoscopies, scheduled 3–5 years later. Information regarding use of NSAIDs during posttreatment follow-up was gathered periodically via questionnaires. Average weekly NSAID use was classified as sporadic (<2 days per week), moderate (2 to <4 days per week), or frequent (≥4 days per week). The analysis was stratified according to randomized aspirin groups and posttreatment NSAID use; placebo subjects who later were sporadic NSAID users formed the reference group. The primary outcomes were all adenomas and advanced lesions. Adjusted relative risks and 95% confidence intervals were computed with generalized linear models. All statistical tests were two-sided.
Results
A total of 850 subjects underwent a posttreatment colonoscopy, on average 4 years after the end of study treatment. The protective effect of 81 mg of aspirin for colorectal adenomas persisted with continued posttreatment NSAID use. The risk of any adenoma among frequent NSAID users was 26.8% vs 39.9% among placebo subjects who later used NSAIDs sporadically (adjusted relative risk = 0.62, 95% confidence interval [CI] = 0.39 to 0.98; Ptrend with NSAID use frequency = .03). The unadjusted absolute risk reduction was 13.1 percentage points (95% CI = −0.3 to 26.5 percentage points) (P = .07). Results for 325 mg of aspirin were similar, although not statistically significant. For advanced lesions, small numbers of endpoints limited the analysis, but findings among subjects randomly assigned to 81 mg of aspirin suggested a protective association regardless of posttreatment NSAID use.
Conclusion
Long-term and frequent use of NSAIDs may enhance the chemopreventive effect of aspirin against colorectal neoplasia.
CONTEXT AND CAVEATS
Prior knowledge
Randomized trials have demonstrated that aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) reduce the risk of colorectal adenomas. However, it is unclear whether this protective effect persists after the cessation of randomized NSAID treatment and/or is affected by the duration and frequency of subsequent NSAID use.
Study design
An observational follow-up of the Aspirin/Folate Polyp Prevention Study, in which 1121 subjects who were randomly assigned to receive placebo or aspirin for 3 years were, after the end of treatment and a follow-up colonoscopy, invited to remain under follow-up until their next surveillance colonoscopies. Information regarding the subject’s use of NSAIDs and other medications during follow-up was obtained periodically via questionnaires.
Contribution
The risk of all adenomas was substantially reduced among subjects who continued to use NSAIDs after 3 years of aspirin treatment in the randomized trial. There was an apparent strengthening of the chemopreventive effect of aspirin associated with use of NSAID for at least 4 days per week.
Implications
Long-term and frequent use of NSAIDs may enhance the chemopreventive effect of aspirin against colorectal neoplasia.
Limitations
Due to the observational nature of the study, the findings are susceptible to bias and confounding. The study subjects are not representative of the general population. Small numbers of advanced lesions limited the statistical power of some analyses. The questionnaires did not capture dose information.
From the Editors
The antineoplastic properties of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) have been documented in many observational studies and randomized trials (1–4). However, most observational studies suggest that this protective effect dissipates after discontinuing regular use [reviewed in (3)], and the data regarding the persistence of the effect are not extensive (5). Recently, we reported that the protective effects of calcium supplementation on the risk of colorectal adenoma recurrence persisted up to 5 years after the end of randomized treatment (6), with a statistically significant 37% lower risk for all adenomas for the calcium group compared with the placebo group, suggesting a long-term antineoplastic effect. However, another randomized trial that studied a selective cyclooxygenase (COX)-2 inhibitor, rofecoxib, reported a rebound in the incidence of colorectal adenomas in the rofecoxib group, with a statistically significant 21% increased risk 1 year after treatment cessation (7). These results emphasize the importance of investigating patterns of risk after a chemopreventive agent is stopped.
This analysis was designed to address the following questions: 1) Is the protective effect of aspirin on the risk of colorectal adenomas durable, or does it diminish with sustained use (tachyphylaxis)? 2) Is there a carryover protective effect for aspirin, similar to the effect of calcium, or is there an increased risk after discontinuation, similar to what was seen for rofecoxib? 3) Do these effects hold for both all adenomas and advanced lesions?
Materials and Methods
Study Design
Details of and findings from the Aspirin/Folate Polyp Prevention Study (AFPPS) have been previously published (1,8). Briefly, AFPPS is a double-blind, placebo-controlled randomized trial (clinicaltrials.gov Identifier: NCT00272324) to assess the antineoplastic effects of aspirin and folate in subjects with a recent history of colorectal adenomas. Nine clinical centers took part in the trial. Originally, the study was designed to evaluate aspirin as a chemopreventive agent by comparing three treatment arms: placebo, 81 mg of aspirin per day, and 325 mg of aspirin per day. Shortly after enrollment began (100 subjects had already been randomly assigned to aspirin or placebo), the trial was expanded to a 3 × 2 factorial design to incorporate the additional randomization of each treatment arm to 1 mg of folic acid per day or folate placebo. Human subjects committees at each institution approved the research. All participants provided written informed consent.
AFPPS recruitment began on July 6, 1994, and ended on March 20, 1998. Eligible subjects had at least one of the following: one or more histologically confirmed colorectal adenomas removed within 3 months before recruitment, one or more histologically confirmed adenomas removed within 16 months before recruitment and a lifetime history of two or more confirmed adenomas, or a histologically confirmed adenoma at least 1 cm in diameter removed within 16 months before recruitment. We also required a complete colonoscopy within 3 months before enrollment, with no known polyps left in the bowel. At enrollment, eligible subjects completed a questionnaire regarding personal characteristics, medical history, and lifestyle habits, and they were asked to avoid the use of aspirin, NSAIDs, and supplements containing folate for the duration of active treatment. Potential participants then entered a 3-month, single-blind run-in period, during which subjects were asked to take the folate placebo and 325 mg of aspirin per day. Subjects with at least 80% compliance and no adverse effects to aspirin were randomly assigned to receive (aspirin) placebo, low-dose aspirin (81 mg/d), or high-dose aspirin (325 mg/d), and to receive (folate) placebo or folate (1 mg/d).
A follow-up colonoscopy was scheduled for 3 years after the qualifying examination. After the 3-year colonoscopy, subjects discontinued randomized aspirin treatment but were invited to continue randomized study treatment with folate or folate placebo. All subjects were also invited to remain under follow-up until the next surveillance colonoscopy, as scheduled by their physicians, usually 3–5 years after the 3-year examination, which occurred at the end of randomized aspirin treatment. Subjects could decline to participate in this second phase and exit the study, continue blinded randomized treatment with folate or placebo (active folate extension), or remain in the study under observational follow-up but discontinue randomized folate treatment (observational extension).
The primary outcome of AFPPS—adenoma occurrence—was determined by colonoscopy and pathology review (Figure 1). Information regarding any intestinal endoscopy or surgery that occurred during the study was obtained from the clinical centers. In addition to routine pathological assessment at each clinical center, histological slides of each lesion removed from the bowel during the study were sent to the AFPPS pathologist (Dale C. Snover, Department of Pathology, Fairview Southdale Hospital, Minneapolis, MN) for uniform review of the pathological diagnoses. Advanced adenomas were defined as neoplastic polyps with a villous component of at least 25% or that contained advanced dysplasia or invasive cancer, or with an estimated diameter of at least 1 cm (as assessed by the endoscopist).
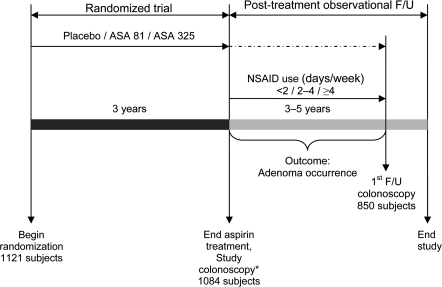
Figure 1.
Analytic design. We focused on two exposures: ASA treatment assignment during the randomized study period (subjects were randomly assigned to placebo, 81 mg of ASA per day [ASA 81], or 325 mg of ASA per day [ASA 325]) and self-reported use of NSAIDs (<2, 2–4, or ≥4 days per week) during the posttreatment F/U period. The primary outcome was defined as the occurrence of adenoma in the first posttreatment F/U colonoscopy. *Results have been published (1). ASA = aspirin; F/U = follow-up; NSAIDs = nonsteroidal anti-inflammatory drugs.
Participants completed a study questionnaire every 4 months (if they were taking study pills) or annually (if they were under observational follow-up). The questionnaires collected information regarding the occurrence of important medical events (eg, intestinal procedures, overnight hospitalizations, cancer, stroke, myocardial infarction, gastrointestinal bleeding), the occurrence of intestinal surgery or endoscopy, their use of aspirin and/or NSAIDs and other over-the-counter and prescribed medications and dietary supplements, and their adherence to study treatment since the previous questionnaire. The study questionnaires also contained a list of the most commonly used NSAIDs at the time (24 drugs, including aspirin-based medications). Participants were asked to report the total number of days that they had taken each of the listed products since the date the previous questionnaire was completed. All follow-up ended on December 31, 2006.
Statistical Analysis
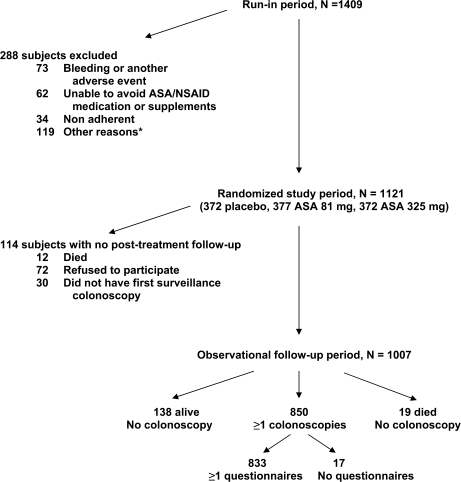
Our aim was to explore the effects of long-term use of NSAIDs on the occurrence of neoplastic colorectal polyps after 3 years of aspirin treatment. Of the 1121 subjects who were randomly assigned to receive aspirin or placebo, 1007 completed the 3-year aspirin treatment period and agreed to participate in posttreatment follow-up (with or without randomized folate treatment). Of these 1007 subjects, 850 underwent at least one colonoscopy after the original year 3 examination but before December 31, 2006, and were included in this analysis (Figure 2). For the purpose of this analysis, we focused solely on randomized aspirin treatment (randomized period) and the self-reported use of NSAIDs after the end of randomized aspirin treatment (observational follow-up) (Figure 1).
Figure 2.
Study flow diagram for the randomized study period and the posttreatment observational follow-up period. *Other reasons for exclusion before randomization: one died, 47 ineligible for reasons related to the folate component (eg, anemia), 28 concurrent illnesses, 26 declined to continue, and 17 other reasons. ASA = aspirin; NSAID = nonsteroidal anti-inflammatory drug.
Fisher exact tests (for categorical variables) and analysis of variance (for continuous variables) were used to compare randomized treatment groups. We also compared the 850 subjects included in this analysis with the 138 subjects who continued in the study after the end of randomized aspirin treatment but did not have a colonoscopy during the posttreatment follow-up phase.
Because 90% of the subjects who had any posttreatment follow-up examinations had only one such colonoscopy (Table 1), we defined our primary outcome as the occurrence of adenomas that were detected in the first colonoscopy performed at least 6 months after the end of randomized aspirin treatment but before December 31, 2006 (Figure 1). Exclusion of colonoscopies performed within 1–2 years after the termination of aspirin treatment did not materially affect the findings, and thus, we do not present these analyses. We also evaluated the occurrence of small tubular adenomas and advanced lesions in separate analyses.
Table 1.
Characteristics of the 850 subjects in the AFPPS with at least one colonoscopy after the end of the randomized treatment*
| Characteristic | Placebo group (n = 285) | Low-dose aspirin group (n = 284) | High-dose aspirin group (n = 281) |
| Before randomization in AFPPS | |||
| Mean age, y (SD) | 57.5 (9.3) | 57.6 (9.1) | 57.7 (9.0) |
| Male subjects, No. (%) | 181 (64) | 189 (67) | 177 (63) |
| Race, No. (%) | |||
| White | 238 (84) | 252 (89) | 251 (89) |
| Black | 19 (7) | 15 (5) | 8 (3) |
| Hispanic | 19 (7) | 9 (3) | 13 (5) |
| Other | 9 (2) | 8 (3) | 9 (3) |
| Body mass index in kg/m2, No. (%) | |||
| <25 | 85 (30) | 88 (31) | 86 (31) |
| 25 to <30 | 140 (49) | 133 (47) | 134 (48) |
| ≥30 | 59 (21) | 62 (22) | 61 (22) |
| Alcohol user, No. (%) | 195 (73) | 181 (66) | 195 (71) |
| Cigarette smoking status, No. (%) | |||
| Never | 132 (46) | 128 (45) | 107 (38) |
| Former | 114 (40) | 115 (41) | 135 (48) |
| Current | 38 (13) | 40 (14) | 38 (14) |
| Positive first-degree family history of colorectal cancer, No. (%) | 79 (34) | 89 (37) | 102 (45) |
| Mean lifetime no. of adenomas (SD) | 2.4 (2.2) | 2.3 (1.9) | 2.6 (2.5) |
| During randomized aspirin treatment period | |||
| Randomized folate treatment assignment, No. (%) | 133 (51) | 137 (52) | 136 (53) |
| Mean time on randomized aspirin treatment, mo (SD) | 32.7 (3.7) | 32.3 (2.9) | 32.6 (3.4) |
| Frequency of nonprotocol NSAID use during the randomized period in days per month, No. (%) | |||
| 0 | 208 (73) | 218 (77) | 213 (76) |
| 1–4 | 53 (19) | 42 (15) | 49 (17) |
| >4 | 24 (8) | 24 (8) | 19 (7) |
| Subjects with adenomas at treatment completion, No. (%) | 143 (50) | 118 (42) | 138 (49) |
| During posttreatment observational follow-up period | |||
| Colonoscopies, No. (%) | |||
| 1 | 253 (89) | 260 (92) | 251 (89) |
| 2 | 28 (10) | 20 (7) | 23 (8) |
| ≥3 | 4 (1) | 4 (1) | 7 (3) |
| Subjects whose first posttreatment colonoscopy reached the cecum, No. (%) | 273 (96) | 267 (94) | 271 (96) |
| Mean time from the end of treatment to first colonoscopy, mo (SD) | 44.9 (14.1) | 47.7 (14.7) | 44.8 (14.4) |
| Median no. of questionnaires completed up to the first posttreatment colonoscopy (IQR) | 9 (4–11) | 9 (4–13) | 9 (4–10) |
| Frequency of NSAIDs use before the first colonoscopy in days per week†, No. (%) | |||
| <2 | 203 (73) | 194 (70) | 192 (69) |
| 2–4 | 32 (12) | 28 (10) | 36 (13) |
| ≥4 | 43 (15) | 56 (20) | 49 (18) |
AFPPS = Aspirin/Folate Polyp Prevention Study; low-dose aspirin = 81 mg; high-dose aspirin = 825 mg; NSAID = nonsteriodal anti-inflammatory drug; IQR = interquartile range.
Seventeen subjects did not complete any questionnaire from the end of aspirin treatment to the first posttreatment colonoscopy.
We took into account participants’ responses on all questionnaires that were completed after the end of randomized aspirin treatment and up to the first posttreatment colonoscopy to assess NSAID use (Figure 1). The questionnaires asked participants for the number of days they had used each of the NSAIDs reported (including aspirin-based medications) since they had completed the previous questionnaire. We used this information to calculate the total number of days of NSAID use during the observational follow-up and computed the average weekly NSAID use during this period. On the basis of the distribution of NSAID use in the study cohort, we classified subjects as sporadic NSAID users (average use <2 days per week), moderate NSAID users (average use 2 to <4 days per week), or frequent NSAID users (average use ≥4 days per week). These groups were compared with regard to characteristics at baseline (ie, before randomization) and at follow-up by use of Fisher exact tests (for categorical variables) and analysis of variance (for continuous variables). Subjects were stratified into nine groups by their randomized treatment assignment and their NSAID use during observational follow-up (Figure 1); those who had been randomly assigned to placebo and who later were sporadic NSAID users were used as the reference group in the primary analysis. Subjects who reported taking no NSAIDs at all after the end of aspirin treatment were used as the reference group in a separate analysis. Because of the small numbers of subjects who had advanced lesions detected during the posttreatment follow-up (63 sporadic NSAID users, seven moderate NSAID users, and 15 frequent NSAID users), we combined moderate and frequent NSAID users into one category (“NSAID users”) in the analysis that used advanced lesions as the outcome while maintaining the same reference category.
Generalized linear models for the Poisson distribution with a natural logarithm link function and adjustment for over- or underdispersion of the data (8) were used to compute the relative risks and 95% confidence intervals of having at least one adenoma. Adjusted models included age, time to first colonoscopy after the end of randomized treatment, number of lifetime adenomas before randomization (as continuous variables), body mass index (<25, 25–30, ≥30 kg/m2) at study enrollment (ie, before randomization), sex, clinical center, and the presence of adenomas during the randomized aspirin treatment (yes, no) as indicator variables. In addition, we conducted secondary analyses with further adjustment for randomized folate treatment, first-degree family history of colorectal cancer (yes, no), cigarette smoking status (never, former, current), and alcohol use (yes, no). Inclusion of these covariates in the models did not materially change the results; thus, only the most parsimonious models are presented. To evaluate response trends according to the frequency of NSAID use, we coded sporadic, moderate, and frequent NSAID use as 0, 1, and 2, respectively, and used this coding as a continuous variable. P values were computed with two-sided Wald tests. Because of the small number of advanced lesions in some exposure categories, crude rather than adjusted models might be more statistically appropriate for analyses of this endpoint. However, because unadjusted and adjusted estimates in this analysis were very similar, for consistency of presentation, we present only the adjusted estimates for all adenomas and for advanced lesions. A Pearson chi-square goodness-of-fit test was used to evaluate our models, and no statistically significant lack of fit was found. We used 2 × 2 contingency tables and Pearson chi-square tests to assess unadjusted absolute risk reductions.
The possibility that randomized folate treatment assignment or adenoma occurrence during the randomized aspirin treatment period could modify our findings was evaluated with product interaction terms and Wald tests in separate analyses. Two-sided P values less than .05 were considered to indicate statistical significance. All statistical tests were two-sided.
Results
Characteristics of the subjects in the three aspirin treatment arms are summarized in Table 1. There were no statistically significant differences among the three arms with regard to demographic and lifestyle factors at AFPPS study entry, including age, sex, race, body mass index, first-degree family history of colorectal cancer, alcohol use, cigarette smoking status, and the lifetime number of adenomas before randomization. The three treatment groups were also similar with regard to the percentage of subjects who were randomly assigned to folate treatment, the duration of aspirin treatment, or the use of nonprotocol NSAIDs during the randomized treatment period. However, as suggested by previously published findings (1), subjects who were randomly assigned to receive low-dose aspirin were less likely than subjects in the other two treatment arms to have an adenoma detected during the 3-year treatment period (P = .080). Posttreatment follow-up characteristics were also comparable across the three randomized aspirin groups. Of the 850 subjects included in this analysis, 764 (90%) had one colonoscopy and 86 (10%) had two or more colonoscopies during the observational follow-up after the end of aspirin treatment. The average time from the end of the study treatment to the first posttreatment endoscopy was 45.8 months (SD = 14.4 months). During this time, the median number of study questionnaires completed by subjects was 9 (interquartile range = 4–12; 17 subjects did not complete any questionnaires). The response rate for the study questionnaires (the number completed as a proportion of the number expected) was approximately 85%. The most commonly used NSAIDs were the same in the three treatment groups: aspirin and aspirin-based drugs (used by 59% of subjects), ibuprofen (used by 46%), naproxen (used by 20%), rofecoxib (used by 8%), and celecoxib (used by 8%).
In general, subjects who had a posttreatment colonoscopy were similar to the 138 subjects who did not have a posttreatment colonoscopy with regard to demographic and lifestyle factors. However, subjects with a posttreatment colonoscopy were more likely to have had adenomas during the treatment phase of the study (47% vs 22%, P < .001) and advanced lesions (11% vs 5%, P = .03) detected during the 3-year aspirin treatment period.
Among the 833 subjects who completed posttreatment questionnaires, 589 (71%) took NSAIDs at most intermittently and were classified as sporadic users (average use <2 days per week), 96 (12%) were moderate users (average use 2 to <4 days per week), and 148 (18%) were frequent users (average use ≥4 days per week); 165 subjects (20%) reported no NSAID use at all (Table 1). Frequent NSAID users were similar to subjects with less frequent NSAID use with regard to most demographic, lifestyle, and clinical factors (data not shown). However, frequent NSAID users were statistically significantly older (P = .01) and had a higher body mass index (P = .03) than the sporadic or moderate NSAID users.
The effects of aspirin use on adenoma risk during the treatment phase of the trial are summarized in Table 2. As reported previously (1), aspirin treatment moderately reduced the risk of colorectal adenomas; the adjusted relative risk for all adenomas for the two aspirin groups combined vs the placebo group was 0.90 (95% confidence interval [CI] = 0.77 to 1.05). When we considered each aspirin group independently, a borderline statistically significant risk reduction was observed for the low-dose aspirin group (adjusted relative risk [RR] = 0.84, 95% CI = 0.70 to 1.00).
Table 2.
Risk of adenoma with randomized aspirin treatment and subsequent NSAID use*
| Study phase | No. of subjects with adenoma/Total no. of subjects (%) | Adjusted RR (95% CI) | Ptrend† | |
| Randomized trial‡ | ||||
| Placebo | 143/285 (50.2) | 1.00 (reference) | ||
| Aspirin | 256/565 (45.3) | 0.90 (0.77 to 1.05) | ||
| Low-dose aspirin | 118/284 (41.6) | 0.84 (0.70 to 1.00) | ||
| High-dose aspirin | 138/281 (49.1) | 0.96 (0.81 to 1.14) | NA | |
| Posttreatment observational follow-up§ | ||||
| Aspirin treatment group | Posttreatment NSAID use‖ | |||
| Placebo | <2 days per week | 81/203 (39.9) | 1.00 (reference) | |
| Placebo | 2–4 days per week | 12/32 (37.5) | 1.02 (0.61 to 1.69) | |
| Placebo | ≥4 days per week | 18/43 (41.9) | 0.91 (0.59 to 1.39) | .46 |
| Aspirin | <2 days per week | 153/386 (39.6) | 1.00 (0.80 to 1.24) | |
| Aspirin | 2–4 days per week | 21/64 (32.8) | 0.84 (0.57 to 1.25) | |
| Aspirin | ≥4 days per week | 30/105 (28.6) | 0.67 (0.47 to 0.94) | .02 |
| Low-dose aspirin | <2 days per week | 76/194 (39.2) | 1.01 (0.78 to 1.30) | |
| Low-dose aspirin | 2–4 days per week | 8/28 (28.6) | 0.78 (0.43 to 1.41) | |
| Low-dose aspirin | ≥4 days per week | 15/56 (26.8) | 0.62 (0.39 to 0.98) | .03 |
| High-dose aspirin | <2 days per week | 77/192 (40.1) | 0.98 (0.76 to 1.27) | |
| High-dose aspirin | 2–4 days per week | 13/36 (36.1) | 0.89 (0.55 to 1.43) | |
| High-dose aspirin | ≥4 days per week | 15/49 (30.6) | 0.72 (0.46 to 1.12) | .08 |
Included 833 participants who completed study questionnaires and had at least one colonoscopy during the posttreatment follow-up interval. “Aspirin” refers to both doses combined. RR = relative risk; CI = confidence interval; low-dose aspirin = 81 mg; high-dose aspirin = 325 mg; NA = not applicable; NSAIDs = nonsteroidal anti-inflammatory drug.
Relative risks adjusted for age, sex, clinical center, number of lifetime adenomas before randomization, and duration of follow-up until the end of randomized treatment.
P value for the response trend with NSAID frequency of use among each randomized treatment group from a two-sided Wald test.
Relative risks adjusted for aspirin dose, age, sex, center, body mass index, time to year 3 study colonoscopy (overall aspirin treatment effects) or time to first colonoscopy after the end of randomized treatment (posttreatment NSAID effects), number of lifetime adenomas before randomization, and presence of adenomas during the randomized period.
NSAID use defined as the average number of days per week subjects took NSAIDs between the end of randomized aspirin treatment and the subsequent posttreatment colonoscopy.
The protective effect of randomization to one of the aspirin groups persisted during the posttreatment period for subjects who continued to use aspirin or other NSAIDs, and there was a statistically significant trend of decreasing risk with increasing frequency of later NSAID use (Ptrend = .02) (Table 2). However, subjects randomly assigned to either active aspirin arm who used NSAIDs only sporadically during observational follow-up were no longer at a reduced risk of adenomas compared with placebo subjects who also were sporadic NSAID users (adjusted RR = 1.00, 95% CI = 0.80 to 1.24), nor were they at increased risk. There was no association between NSAID use during the posttreatment period and the risk of adenomas among subjects who had been randomly assigned to placebo (Table 2). We obtained similar results when we evaluated the risk of small tubular adenomas (data not shown).
The protective effect of low-dose aspirin against colorectal adenomas during the treatment phase persisted only among subjects who continued to take NSAIDs during the observational follow-up phase (Ptrend with NSAID use = .03) (Table 2). Subjects randomly assigned to 81 mg of aspirin who later used NSAIDs at least 4 days per week had a markedly reduced risk of all adenomas compared with placebo subjects who were at most sporadic NSAID users (39.9% vs 26.8%; adjusted RR = 0.62, 95% CI = 0.39 to 0.98) (Table 2). The reduction in unadjusted absolute risk was 13.1 percentage points (95% CI = −0.3 to 26.5 percentage points) (P = .07). We obtained similar results when we restricted the reference category to placebo subjects who took no NSAIDs after the end of randomized treatment (data not shown).
High-dose aspirin did not confer a statistically significant reduction in adenoma risk during the randomized treatment period of the trial (Table 2). In this analysis, subjects who were randomly assigned to high-dose aspirin and who later were frequent users of NSAIDs had a nonstatistically significantly lower risk for all adenomas than placebo subjects who were at most sporadic NSAID users (30.6% vs 39.9%; adjusted RR = 0.72, 95% CI = 0.46 to 1.12; Ptrend with NSAID use = .08). The corresponding reduction in unadjusted absolute risk was 9.3 percentage points (95% CI = −5.3 to 23.8 percentage points) (P = .23).
The association between randomized aspirin treatment with later NSAID use and the risk of colorectal adenoma in the posttreatment observational period was not statistically significantly modified by randomized folate treatment assignment (P = .89). Stratification by adenoma occurrence during the randomized aspirin treatment period of the trial did not modify the posttreatment results statistically significantly either. Among subjects who were diagnosed with at least one adenoma during study aspirin treatment, randomization to low-dose aspirin with later frequent NSAID use was associated with a 50% reduction in the risk of adenomas (adjusted RR vs placebo subjects with sporadic NSAID use = 0.50, 95% CI = 0.25 to 0.99). Among those without adenomas during the randomized treatment period, the reduction in risk was 22% (adjusted RR vs placebo subjects with sporadic NSAID use = 0.78, 95% CI = 0.41 to 1.47). The multiplicative interaction between the frequency of NSAID use during observational follow-up and the detection of adenomas during the treatment period in the low-dose aspirin group was not statistically significant (P = .60). The results for high-dose aspirin were similar to those for low-dose aspirin (data not shown).
Findings regarding the risk of advanced lesions during the randomized treatment phase are summarized in Table 3. During the randomized treatment period, aspirin (81 or 325 mg) conferred a protective effect against advanced lesions compared with placebo (RR = 0.73, 95% CI = 0.50 to 1.09), and the effect was more pronounced, although not statistically significant, in the low-dose aspirin group (adjusted RR = 0.66, 95% CI = 0.41 to 1.07). A comparable analysis for the posttreatment follow-up period was hampered by the small number of advanced lesions detected. Therefore, we conducted this analysis by combining the moderate and frequent NSAID users into a single category of NSAID users (Table 3). None of the relative risks was statistically significant. However, the lower limit of the confidence limits for each exposure group included reductions in risk as large as 50% in comparison with the reference category, and each point estimate suggested reduced risks, except for subjects randomized to high-dose aspirin who subsequently were NSAID users.
Table 3.
Risk of advanced adenomas with randomized aspirin treatment and subsequent NSAID use*
| Study phase | No. of subjects with adenoma/ Total no. of subjects (%) | Adjusted RR (95% CI) | |
| Randomized trial† | |||
| Placebo | 38/285 (13.3) | 1.00 (reference) | |
| Aspirin | 56/565 (9.9) | 0.73 (0.50 to 1.09) | |
| Low-dose aspirin | 26/284 (9.2) | 0.66 (0.41 to 1.07) | |
| High-dose aspirin | 30/281 (10.7) | 0.81 (0.51 to 1.29) | |
| Posttreatment observational follow-up‡ | |||
| Aspirin treatment group | Posttreatment NSAID Use§ | ||
| Placebo | <2 days per week | 25/203 (12.3) | 1.00 (reference) |
| Placebo | ≥2 days per week | 4/75 (5.3) | 0.44 (0.15 to 1.27) |
| Aspirin | <2 days per week | 28/386 (7.3) | 0.61 (0.35 to 1.04) |
| Aspirin | ≥2 days per week | 14/169 (8.3) | 0.68 (0.35 to 1.32) |
| Low-dose aspirin | <2 days per week | 12/194 (6.2) | 0.54 (0.27 to 1.08) |
| Low-dose aspirin | ≥2 days per week | 3/84 (3.6) | 0.31 (0.09 to 1.04) |
| High-dose aspirin | <2 days per week | 16/192 (8.3) | 0.67 (0.36 to 1.25) |
| High-dose aspirin | ≥2 days per week | 11/85 (12.9) | 0.99 (0.48 to 2.05) |
Included 833 participants who completed study questionnaires and had at least one colonoscopy during the posttreatment follow-up period. “Aspirin” refers to both doses combined. RR = relative risk; CI = confidence interval; low-dose aspirin = 81 mg; high-dose aspirin = 325 mg; NSAIDs = nonsteroidal anti-inflammatory drug.
Relative risks adjusted for age, sex, clinical center, number of lifetime adenomas before randomization, and duration of follow-up until the end of randomized treatment.
Relative risks adjusted for aspirin dose, age, sex, center, body mass index, time to year 3 study colonoscopy (overall aspirin treatment effects) or time to first colonoscopy after the end of randomized treatment (posttreatment NSAID effects), number of lifetime adenomas before randomization, and presence of adenomas during the randomized period.
NSAID use defined as the average number of days per week subjects took NSAIDs between the end of randomized aspirin treatment and the subsequent posttreatment colonoscopy.
All analyses were repeated with aspirin and nonaspirin NSAIDs considered separately. The findings were similar to those shown above (data not shown).
Discussion
In this analysis of adenoma occurrence after the end of randomized aspirin treatment, we found that continued use of aspirin and/or other NSAIDs extended the chemopreventive effects of aspirin that were seen during the treatment period. There was an apparent strengthening of the chemopreventive effect associated with NSAID use of at least 4 days per week on average. There was no evidence of tachyphylaxis or tolerance, nor was there an indication of a rebound in the incidence of adenomas after the end of randomized treatment. Although our numbers for advanced lesions were too small to draw confident conclusions, low-dose aspirin and subsequent NSAID use also seemed to yield a persistent reduction in risk. These findings imply that aspirin does not lose its antineoplastic properties over time and that these effects persist and may even be accentuated with prolonged NSAID use.
During the randomized treatment phase of the study, low-dose aspirin had a chemoprotective effect against adenomas, whereas high-dose aspirin did not (1). This finding was unexpected. In this secondary analysis, both doses of aspirin were associated with a decreased risk for all adenomas among subjects who frequently used NSAIDs after the end of randomized aspirin treatment, although the risk reduction was not statistically significant among subjects who had been randomly assigned to high-dose aspirin. These results seem to support the hypothesis that chance was responsible for the dose–response pattern observed in the analysis of adenoma occurrence during randomized aspirin treatment (1). For advanced lesions, small numbers of endpoints hampered the analysis, and no firm conclusions can be drawn regarding the delayed effects of aspirin treatment.
A recent observational study reported that the use of two standard tablets of aspirin per week (equivalent to a dose of 650 mg of aspirin) was associated with a statistically significant reduction in the risk of colorectal cancer, with a trend of decreasing risk with increasing number of tablets taken per week (9). A more recent analysis of data from two large randomized trials with aspirin concluded that a daily dose of 300 mg or more of aspirin taken for 1–7 years could prevent colorectal cancer after a latency period of about 10 years (10). Our observational follow-up data suggest a trend of reduced risk of colorectal adenomas with increased frequency of NSAID use after 3 years of daily aspirin use. The implications of this secondary prevention of adenomas on the incidence of colorectal cancer are not clear, but our findings are consistent with the two studies mentioned above, given that most cancers in the colon and rectum evolve from the same types of precancerous neoplastic lesions that we studied (11). It is reasonable to argue that by reducing the incidence of colorectal adenomas, long-term use of NSAIDs could reduce the future risk of colorectal cancer.
In this analysis, we did not find an increased risk of adenomas after the end of randomized treatment. Indeed, for advanced adenomas, the chemopreventive potency of aspirin seemed to persist even in the absence of later NSAID use, suggesting a carryover effect of the aspirin treatment, at least for a few years. Such an effect might occur if, for example, aspirin has a longer latent period of effect for advanced adenomas than for small tubular adenomas. However, as mentioned above, the numbers of advanced adenomas are too small and our statistical power is too limited to rule out chance as a possible explanation for this result.
It is unclear why we found no reduction of adenoma risk associated with later NSAID use among subjects who had been randomly assigned to placebo. Previous studies have described inverse associations after relatively short exposures of 1–3 years (3,12). In this analysis, it is possible that longer and/or more intense use of these drugs would be required to see such an effect in placebo subjects because of the 3 years of NSAID abstinence during the randomized treatment period of our study.
There is extensive evidence supporting the antineoplastic properties of aspirin and other NSAIDs (2–4,13–15). Regarding colorectal adenomas and colorectal cancer, experimental studies (16–21) have reported a protective effect of aspirin. More importantly, results of observational studies (5,22–27) and randomized clinical trials (1,10,12,28,29) have consistently supported the inverse association of aspirin with adenoma risk. The proposed mechanisms underlying the anticarcinogenic activity of aspirin and other NSAIDs may be related to their ability to inhibit the activity of COX enzymes. These enzymes are responsible for the synthesis of prostaglandins and other metabolites that are associated with inflammatory processes and carcinogenesis (19,30–35). Some experimental studies (32,36–42) have described anticarcinogenic mechanisms independent of COX enzymes. Indeed, aspirin in the doses we used has relatively little effect on the activity of COX-2 (43), the enzyme isoform that has been most frequently associated with neoplastic processes, a finding that supports the idea that COX-2 inhibition may not be necessary for the chemopreventive effects of NSAIDs.
Our study has several strengths. Compliance with the study regimen was excellent: More than 85% of subjects reported taking study pills at least 90% of the time, even during the year before the year 3 colonoscopy that marked the end of the randomized aspirin treatment period (1). We had a large sample size: Of the 1121 subjects originally randomly assigned to placebo or aspirin, 1007 agreed to continue in the follow-up phase and 850 had at least one colonoscopy. Furthermore, all biopsy specimens were examined in a blinded fashion by a single study pathologist in a uniform endpoint review (1,8).
This study also has a number of limitations. Most importantly, because in this analysis, the posttreatment follow-up period was observational, our findings are susceptible to bias and confounding. For example, posttreatment NSAID use was based on self-report and thus, our categorization of subjects according to reported use may not have been entirely accurate. In addition, frequent NSAID users were older than the other subjects and tended to have a higher body mass index. Although our estimates were adjusted for these factors, we could not deal similarly with other potentially confounding factors that we did not identify. Subjects in this analysis were a higher risk subset of an already selected population that met the eligibility requirements for the randomized trial because the participants with neoplastic lesions detected during the treatment period were more likely to have a colonoscopy during the posttreatment phase than those who were free of adenomas. Thus, our study subjects are not representative of the general population. Furthermore, small numbers of advanced lesions limited our statistical power and prevented us from drawing firm conclusions regarding the association of NSAIDs with this endpoint.
To evaluate response trends, we relied on the reported frequency of use because our questionnaires did not capture dose information, and this is another important weakness of our analysis. Furthermore, there was no uniform interval between the end of the randomized study and the first posttreatment colonoscopy. However, our statistical models were adjusted for this varying time at risk, and it is unlikely that it affected our results. It is possible that the particular characteristics of the NSAIDs taken after the end of randomized treatment could have played a role in our findings. For example, specific types of NSAIDs and/or higher doses could have been responsible for the apparent strengthening of the chemopreventive effect of aspirin during the subsequent follow-up interval.
In summary, our study provides further evidence of the potential chemopreventive effect of NSAIDs against colorectal adenomas in patients with a history of these polyps. Our findings suggest that prolonged and frequent use of these agents can have strong beneficial effects. We did not find either loss of effectiveness with continued use or increased risk following discontinuation of use. This study raises the possibility that NSAIDs taken 4–7 days per week might be sufficient to prevent adenomas and, by implication, colorectal cancer. Because our results imply that the strength of the inverse association between NSAID use and adenoma risk may increase with frequency of use and because more frequent use is also likely to cause more adverse events, further investigation is important to understand ways to maximize the possible benefits of these drugs while minimizing their potential harms.
Funding
This work was supported in part by funding from the National Cancer Institute, National Institutes of Health (CA 046927 and CA 098286) to Dartmouth College (J.A.B. was the principal investigator).
Footnotes
The study sponsors had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
J. A. Baron is currently a paid consultant to Bayer AG (a manufacturer of aspirin and NSAIDs) and is a former paid consultant to Merck & Co, Inc (which marketed Vioxx [rofecoxib], another NSAID). Bayer AG provided the aspirin and aspirin placebo for the randomized trial.
References
- 1.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 2.Asano TK, McLeod RS. Nonsteroidal anti-inflammatory drugs and aspirin for the prevention of colorectal adenomas and cancer: a systematic review. Dis Colon Rectum. 2004;47(5):665–673. doi: 10.1007/s10350-003-0111-9. [DOI] [PubMed] [Google Scholar]
- 3.Baron JA. Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog Exp Tumor Res. 2003;37:1–24. doi: 10.1159/000071364. [DOI] [PubMed] [Google Scholar]
- 4.Reddy BS. Strategies for colon cancer prevention: combination of chemopreventive agents. Subcell Biochem. 2007;42:213–225. doi: 10.1007/1-4020-5688-5_10. [DOI] [PubMed] [Google Scholar]
- 5.Flossmann E, Rothwell PM. Commentary: aspirin and colorectal cancer an epidemiological success story. Int J Epidemiol. 2007;36(5):962–965. doi: 10.1093/ije/dym200. [DOI] [PubMed] [Google Scholar]
- 6.Grau MV, Baron JA, Sandler RS, et al. Prolonged effect of calcium supplementation on risk of colorectal adenomas in a randomized trial [see comment] J Natl Cancer Inst. 2007;99(2):129–136. doi: 10.1093/jnci/djk016. [DOI] [PubMed] [Google Scholar]
- 7.Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas [see comment] Gastroenterology. 2006;131(6):1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 8.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial [see comment] JAMA. 2007;297(21):2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 9.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294(8):914–923. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flossmann E, Rothwell PM. British Doctors Aspirin T, the UKTIAAT. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369(9573):1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 11.Leslie A, Carey FA, Pratt NR, Steele RJC. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002;89(7):845–860. doi: 10.1046/j.1365-2168.2002.02120.x. [DOI] [PubMed] [Google Scholar]
- 12.Benamouzig R, Deyra J, Martin A, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125(2):328–336. doi: 10.1016/s0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Perez A, Garcia Rodriguez LA, Lopez-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC Cancer. 2003;3:28. doi: 10.1186/1471-2407-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94(4):252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence [see comment] J Natl Cancer Inst. 2007;99(8):608–615. doi: 10.1093/jnci/djk132. [DOI] [PubMed] [Google Scholar]
- 16.Barnes CJ, Cameron IL, Hardman WE, Lee M. Non-steroidal anti-inflammatory drug effect on crypt cell proliferation and apoptosis during initiation of rat colon carcinogenesis. Br J Cancer. 1998;77(4):573–580. doi: 10.1038/bjc.1998.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes CJ, Hamby-Mason RL, Hardman WE, Cameron IL, Speeg KV, Lee M. Effect of aspirin on prostaglandin E2 formation and transforming growth factor alpha expression in human rectal mucosa from individuals with a history of adenomatous polyps of the colon. Cancer Epidemiol Biomarkers Prev. 1999;8(4 pt 1):311–315. [PubMed] [Google Scholar]
- 18.Kokoska ER, Smith GS, Miller TA. Nonsteroidal anti-inflammatory drugs attenuate proliferation of colonic carcinoma cells by blocking epidermal growth factor-induced Ca++ mobilization. J Gastrointest Surg. 2000;4(2):150–161. doi: 10.1016/s1091-255x(00)80051-7. [DOI] [PubMed] [Google Scholar]
- 19.Meric J-B, Rottey S, Olaussen K, et al. Cyclooxygenase-2 as a target for anticancer drug development. Crit Rev Oncol Hematol. 2006;59(1):51–64. doi: 10.1016/j.critrevonc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Oshima M, Taketo MM. COX selectivity and animal models for colon cancer. Curr Pharm Des. 2002;8(12):1021–1034. doi: 10.2174/1381612023394953. [DOI] [PubMed] [Google Scholar]
- 21.Yin H, Xu H, Zhao Y, Yang W, Cheng J, Zhou Y. Cyclooxygenase-independent effects of aspirin on HT-29 human colon cancer cells, revealed by oligonucleotide microarrays. Biotechnol Lett. 2006;28(16):1263–1270. doi: 10.1007/s10529-006-9084-9. [DOI] [PubMed] [Google Scholar]
- 22.Harris RE, Beebe-Donk J, Doss H, Burr Doss D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade [review] Oncol Rep. 2005;13(4):559–583. [PubMed] [Google Scholar]
- 23.Giovannucci E, Egan KM, Hunter DJ, et al. Aspirin and the risk of colorectal cancer in women [see comment] N Engl J Med. 1995;333(10):609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 24.Larsson SC, Giovannucci E, Wolk A. Long-term aspirin use and colorectal cancer risk: a cohort study in Sweden. Br J Cancer. 2006;95(9):1277–1279. doi: 10.1038/sj.bjc.6603442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin C, Connelly A, Keku TO, et al. Nonsteroidal anti-inflammatory drugs, apoptosis, and colorectal adenomas. Gastroenterology. 2002;123(6):1770–1777. doi: 10.1053/gast.2002.37053. [DOI] [PubMed] [Google Scholar]
- 26.Tangrea JA, Albert PS, Lanza E, et al. Non-steroidal anti-inflammatory drug use is associated with reduction in recurrence of advanced and non-advanced colorectal adenomas (United States) Cancer Causes Control. 2003;14(5):403–411. doi: 10.1023/a:1024990617158. [DOI] [PubMed] [Google Scholar]
- 27.Cruz-Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002;122(3):641–645. doi: 10.1053/gast.2002.31890. [DOI] [PubMed] [Google Scholar]
- 28.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328(18):1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 29.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer [see comment] [erratum appears in N Engl J Med. 2003;348(19):1939] N Engl J Med. 2003;348(10):883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 30.Shiff SJ, Rigas B. The role of cyclooxygenase inhibition in the antineoplastic effects of nonsteroidal antiinflammatory drugs (NSAIDs) J Exp Med. 1999;190(4):445–450. doi: 10.1084/jem.190.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiff SJ, Shivaprasad P, Santini DL. Cyclooxygenase inhibitors: drugs for cancer prevention. Curr Opin Pharmacol. 2003;3(4):352–361. doi: 10.1016/s1471-4892(03)00087-0. [DOI] [PubMed] [Google Scholar]
- 32.IARC Handbooks of Cancer Prevention. Non-steroidal Anti-inflammatory Drugs. Lyon, France: International Agency for Research on Cancer; 1997. IARC Working Group on the Evaluation of Cancer Preventive Agents; pp. 1pp. 1–202. [Google Scholar]
- 33.Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med. 2007;58:239–252. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- 34.Chan TA. Prostaglandins and the colon cancer connection. Trends Mol Med. 2006;12(6):240–244. doi: 10.1016/j.molmed.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Liao Z, Mason KA, Milas L. Cyclo-oxygenase-2 and its inhibition in cancer: is there a role? Drugs. 2007;67(6):821–845. doi: 10.2165/00003495-200767060-00001. [DOI] [PubMed] [Google Scholar]
- 36.Deasy BM, O’Sullivan-Coyne G, O’Donovan TR, McKenna SL, O’Sullivan GC. Cyclooxygenase-2 inhibitors demonstrate anti-proliferative effects in oesophageal cancer cells by prostaglandin E(2)-independent mechanisms. Cancer Lett. 2007;256(2):246–258. doi: 10.1016/j.canlet.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006;98(11):736–747. doi: 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- 38.Ou Y-C, Yang C-R, Cheng C-L, Raung S-L, Hung Y-Y, Chen C-J. Indomethacin induces apoptosis in 786-O renal cell carcinoma cells by activating mitogen-activated protein kinases and AKT. Eur J Pharmacol. 2007;563(1–3):49–60. doi: 10.1016/j.ejphar.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 39.Boon EMJ, Keller JJ, Wormhoudt TAM, et al. Sulindac targets nuclear beta-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br J Cancer. 2004;90(1):224–229. doi: 10.1038/sj.bjc.6601505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong SP, Ha SH, Park IS, Kim WH. Induction of apoptosis in colon cancer cells by nonsteroidal anti-inflammatory drugs. Yonsei Med J. 1998;39(4):287–295. doi: 10.3349/ymj.1998.39.4.287. [DOI] [PubMed] [Google Scholar]
- 41.Qiao L, Hanif R, Sphicas E, Shiff SJ, Rigas B. Effect of aspirin on induction of apoptosis in HT-29 human colon adenocarcinoma cells. Biochem Pharmacol. 1998;55(1):53–64. doi: 10.1016/s0006-2952(97)00400-0. [DOI] [PubMed] [Google Scholar]
- 42.Smith ML, Hawcroft G, Hull MA. The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur J Cancer. 2000;36(5):664–674. doi: 10.1016/s0959-8049(99)00333-0. [DOI] [PubMed] [Google Scholar]
- 43.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I) J Natl Cancer Inst. 1998;90(20):1529–1536. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]