Abstract
CDX1 is a transcription factor that plays a key role in intestinal development and differentiation. However, the downstream targets of CDX1 are less well defined than those of its close homologue, CDX2. We report here the identification of downstream targets of CDX1 using microarray gene-expression analysis and other approaches. Keratin 20 (KRT20), a member of the intermediate filament and a well-known marker of intestinal differentiation, was initially identified as one of the genes likely to be directly regulated by CDX1. CDX1 and KRT20 mRNA expression were significantly correlated in a panel of 38 colorectal cancer cell lines. Deletion and mutation analysis of the KRT20 promoter showed that the minimum regulatory region for the control of KRT20 expression by CDX1 is within 246 bp upstream of the KRT20 transcription start site. ChIP analysis confirmed that CDX1 binds to the predicted CDX elements in this region of the KRT20 promoter in vivo. In addition, immunohistochemistry showed expression of CDX1 parallels that of KRT20 in the normal crypt, which further supports their close relationship. In summary, our observations strongly imply that KRT20 is directly regulated by CDX1, and therefore suggest a role for CDX1 in maintaining differentiation in intestinal epithelial cells. Because a key feature of the development of a cancer is an unbalanced program of proliferation and differentiation, dysregulation of CDX1 may be an advantage for the development of a colorectal carcinoma. This could, therefore, explain the relatively frequent down regulation of CDX1 in colorectal carcinomas by hypermethylation.
Keywords: colorectal cancer, epithelial, cell lines, methylation, cancer stem cells
Members of the keratin family provide structural support for the cell. They are also involved in apoptosis and protect cells from stress (1). Mutations of some keratins lead to genetic diseases, for example, of the skin and the liver (2–4). Keratin 20 (KRT20), a member of the keratin family, has been cloned and classified as a type-I keratin with a highly conserved amino acid sequence (5). It is mainly expressed in the cytoplasm of epithelial cells in the small and large intestine and in Merkel cells of the skin (5). As KRT20 is expressed in the epithelial cells of the crypt, it has been widely studied as a marker of differentiation in normal epithelium and colorectal cancer (CRC) samples (6–9). Previous studies have suggested that KRT20 is phosphorylated in association with mucin secretion and filament organization (10, 11). Transgenic mice with mutations at a conserved Arg to His site (R80H) show collapse of intermediate filaments. Despite its clearly important functions in the intestine, little is known about the regulation of KRT20.
CDX1 is a homeobox transcription factor that is important for intestinal development and is specifically expressed in the small and large intestine in both mice and humans (12–15). CDX1 plays a role in intestinal epithelial cell differentiation (16) and is down-regulated in a number of CRC-derived cell lines as well as in patient samples (17–19). This suggests that absence of CDX1 expression is an advantage for the development of colorectal carcinomas. However, there have so far been only limited studies of the downstream targets of CDX1 (20–22). To examine further the functional role of CDX1, we have investigated its downstream targets using microarray gene-expression analysis and other approaches. Our results show that CDX1 directly regulates KRT20, which further supports the role of CDX1 in maintaining differentiation in the intestinal crypt and therefore supports an important role for CDX1 in colorectal carcinogenesis.
Results
Identification of CDX1 Downstream Targets by Microarray Analysis.
The CDX1 cDNA transfected cell line, HCT116-CDX1, showed a substantial increase in CDX1 message and protein expression as compared to its CDX1-negative parent, HCT116, while the siRNA transfected line LS174T-siRNA showed a substantial reduction in message and an 85% reduction in CDX1 protein expression relative to its CDX1-positive parent, LS174T. Protein expression was checked by Western blot [supporting information (SI) Fig. S1]. The specificity of the CDX1 knock down was confirmed by the fact that there was no reduction in CDX2 protein expression in the siRNA transfected LS174T cell line as compared to the vector control. RNA from both pairs of stably transfected cell lines was submitted to microarray gene-expression analysis.
The expression of 105 genes was up-regulated at least 1.5-fold in HCT116-CDX1 as compared to its control, but unchanged in LS174T-siRNA in comparison with its control. The expression of 181 genes was down-regulated at least 1.5-fold in the LS174T-siRNA but unchanged in HCT116-CDX1. No genes were found that were down-regulated in HCT116-CDX1 and up-regulated in LS174T-siRNA. However, there were 5 genes that were up-regulated in HCT116-CDX1 and down-regulated in the LS174T-siRNA (Table 1). These genes were therefore considered to be prime candidates for direct regulation by CDX1.
Table 1.
Summary of the genes that are up-regulated in HCT116-CDX1 and down-regulated in LS174T-siRNA cell lines in comparison with the vector controls
| Gene Bank Accession No. | Gene symbol | Function in adult human | Fold change in HCT116-CDX1 | Fold change in LS174T-siRNA |
|---|---|---|---|---|
| NM_019010 | KRT20 | Intermediate filament | +9.3 | −5.1 |
| NM_001562 | IL 18 | T cell activation | +5.3 | −4.0 |
| NM_025113 | C13orf18 | Unknown | +3.9 | −70.3 |
| NM_001935 | DPP4 | T cell activation | +3.4 | −13.2 |
| NM_013404 | MSLN | Unknown | +2.9 | −5.5 |
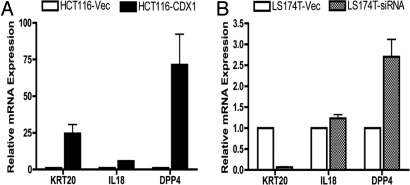
The expression pattern of these 5 candidate genes in HCT116-CDX1, as determined by quantitative PCR (qPCR), gave results for IL 18, dipeptidylpeptidase 4 (DPP4), and KRT20 that were consistent with the microarray data (Fig. 1A). IL 18 is a regulator of IFN γ and Fas ligand-dependent cytotoxicity by T lymphocytes (23). It is expressed in intestinal epithelial cells (24) and has been suggested to play a role in the maintenance of intraepithelial lymphocytes (25) and in antitumor immunity (23). DPP4 (also known as CD26) has a role in activation of T cells (26). Expression of DPP4 has been shown to correlate closely with that of other markers of intestinal epithelial differentiation, such as brush border formation and some related enzyme activities. KRT20 is a well-known marker of intestinal epithelium differentiation.
Fig. 1.
Quantitative RT-PCR analysis of 3 candidate genes identified from the microarray analysis. (A) Relative RNA expression of KRT20, IL18, and DPP4 in HCT116-Vec and HCT116-CDX1 cells. (B) Relative RNA expression of KRT20, IL18, and DPP4 in LS174T-Vec and LS174T-siRNA cells. All qPCR was performed in triplicates in 3 independent experiments. Results are represented as Mean ± SEM.
Among the 5 genes tested on HCT116-CDX1, only the qPCR results for KRT20 were consistent with the microarray data from LS174T-siRNA (Fig. 1B). Given the strong association of KRT20 expression with intestinal epithelial differentiation, we therefore decided to focus on characterizing the relationship between CDX1 and KRT20.
Analysis of CDX1, KRT20, and A33 RNA Expression in CRC Cell Lines.
Our previous work has shown that about 20% of CRC cell lines have low or absent CDX1 mRNA expression because of hypermethylation of the CDX1 promoter region (19). In this study we examined the expression of CDX1 in an additional 32 CRC cell lines, as well as in 6 that had been studied previously, using semiquantitative RT-PCR. In agreement with the earlier work, we found that 10 out of the 38 cell lines had low or absent expression of CDX1 mRNA, and that was regulated by promoter hypermethylation, as demonstrated by methylation-specific PCR (Table S1). Combining these results with our previous data (19), 12 out of 68 of the CRC cell lines lack CDX1 expression because of hypermethylation, and 1 lacking expression is hemimethylated (Table S2).
The mRNA expression of the transmembrane protein A33, which has been shown to be a direct target of CDX1 (21), was also checked in our panel of cell lines. The results (see Tables S1, S3, and S4) show that CDX1 and A33 expression are highly correlated (P < 0.01). The expression of CDX1 and KRT20 was also highly correlated (P < 0.01). The homeobox gene CDX2, a close relative of CDX1 with apparently related functions (27), was expressed at the mRNA level in most of the CRC cell lines and showed no obvious relationship with expression of either KRT20 or A33.
CDX1 Regulates KRT20 by 2 Putative Binding Sites in the KRT20 Promoter.
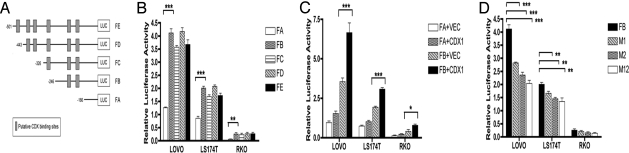
The promoter region sequence of KRT20 was obtained from the University of California, Santa Cruz Genome Bioinformatics site (http://www.genome.ucsc.edu). It contains 6 putative CDX consensus binding sites based on predictions by the TRANSFAC software and published studies (21, 28). CDX1 and CDX2 binding sites cannot be distinguished. There is 68% homology between human and mouse KRT20 in the 500-bp upstream of the 5′ UTR (Fig. 2). This region is therefore presumed to be the minimal promoter, as the homology of the region further upstream drops to below 50%. Deletions from 500-bp to 2-kb upstream of the transcription start site do not show a significant reduction in luciferase activity when tested in a reporter assay (data not shown). Primers were therefore designed to amplify 5 fragments (see Fig. 2, FA–FE) with different combinations of the predicted 6 CDX binding sites found in the 500-bp upstream region of KRT20. These fragments were cloned into the pGL3 luciferase vector for transient transfection assays (Fig. 3A). Three cell lines were chosen for these assays: (i) RKO, which was fully methylated and expressed neither CDX1 nor KRT20; (ii) LOVO, which was partially methylated and expressed both CDX1 and KRT20 at low levels; and (iii) LS174T, which was umethylated and expressed both CDX1 and KRT20 at high levels.
Fig. 2.
Sequence alignment of the human and mouse KRT20 promoters upstream of the initiation of translation. The sequences of the hKRT20 and mKRT20 gene promoters were aligned using the Clustal W algorithm. The translation start site ATG is at the end of each sequence. Conserved nucleotides are shaded in gray. Six potential CDX binding sites were predicted by the Transcription Element Search System (http://www.cbil.upenn.edu/cgi-bin/tess/tess) and are indicated by the black boxes. The 5′ ends of the fragments (FA–FE) of the KRT20 promoter used in the transient transfection deletion experiments are indicated by forward arrows. The common reverse 3′ end primer, R1, is indicated by a reverse arrow. Mutations were made in the 2 CDX binding sites labeled M1 and M2.
Fig. 3.
Dual-luciferase of KRT20 promoter activity in 3 CRC cell lines. (A) Schematic diagram of the KRT20 promoter fragments used in the assays. Each fragment was amplified using the corresponding forward primer and a common reverse primer, R1, and cloned immediately upstream of the luciferase promoter in the pGL3 vector. Putative CDX binding sites are shown as shaded gray boxes. (B) Luciferase reporter assays of the 5 deleted KRT20 fragments (FA–FE) transfected into 3 CRC cell lines: LOVO, LS174T, and RKO. (C) Luciferase reporter assay following transient cotransfection of either fragment A or B of the KRT20 promoter with CDX1 cDNA or vector control. (D) Luciferase reporter assay of the FB fragments and its mutant derivatives: M1, M2, and M12. All of the luciferase activities were normalized with respect to Renilla luciferase activity. Error bars indicate the mean ± SEM of 3 independent experiments.
Deletion of the region between –501 and –246 bp (i.e., FB–FE) did not significantly reduce the KRT20 promoter activity as measured by the relative luciferase activity, suggesting that the CDX putative sites in this region do not contribute significantly to the regulation of KRT20. In contrast, there was a significant reduction in KRT20 luciferase activity between FB and FA in all 3 cell lines, although more markedly in LOVO and LS174T (Fig. 3B). This suggests that the 2 CDX putative binding sites located within the region 246-bp upstream of the transcription start site may be the major contributors to the regulation of KRT20 transcription by CDX1. KRT20 luciferase activity remains low in all cases in the CDX1- and KRT20-negative cell line RKO.
To further confirm the role of the 2 CDX binding sites within the –246-bp region in the regulation of KRT20 transcriptional activity, FA and FB fragments were cotransfected with either pCMV/CDX1 cDNA or pRC/CMV vector control into the same 3 cell lines. As shown in Fig. 3C, there is no significant increase in luciferase activity in any of the 3 cell lines when FA is cotransfected with CDX1, as compared to the vector control. However, cotransfection of CDX1 and FB significantly induces KRT20 luciferase activity even in RKO, consistent with the assumption that activity is determined by the binding of CDX1 to the putative CDX binding sites in fragment FB.
As an additional test of the relevance of the 2 putative CDX binding sites in fragment FB, we studied the effect of mutation of one or both of these sites on the induced luciferase activity. Mutations in either site M1 or M2 reduced the luciferase activity by 30 to 40% in comparison with the intact FB, and this was further reduced when both sites were mutated (Fig. 3D). The decrease in promoter activity when sites M1 and M2 are both mutated adds to the evidence that both these CDX binding sites are required for maximum induction of KRT20 expression by CDX1.
Characterization of an Anti-CDX1 Monoclonal Antibody.
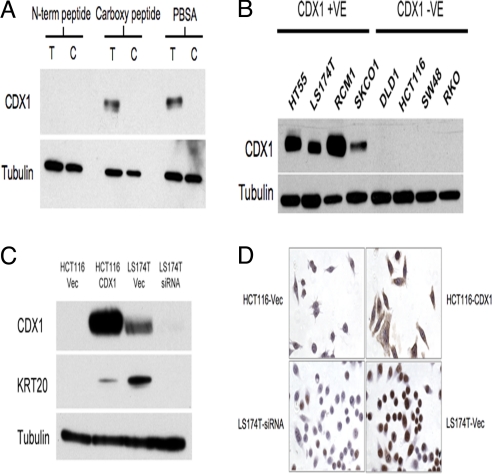
To investigate the expression of the CDX1 protein and to obtain further evidence for KRT20 as a downstream target of CDX1 using a ChIP assay, we developed a monoclonal antibody (mAb) raised against a peptide corresponding to an N-terminal amino acid sequence of CDX1. The specificity of the antibody was confirmed by the fact that only the N-terminal peptide blocked the CDX1 mAb activity on an immunoblot of a lysate from HCT116-CDX1 (Fig. 4A). The anti-CDX1 antibody specificity was further established by the complete concordance between its reactivity on immunoblots and CDX1 mRNA expression from 4 CDX1-positive and 4 negative cell lines (Fig. 4B). In all cases, the positive immunoblots identified a single band of about 32 kDa, which corresponds approximately to the expected size of the CDX1 protein. The antibody also detected the expected band in LS174T-Vec, but not in the LS174T-siRNA cells (Fig. 4C). The KRT20 protein is, furthermore, only expressed when the CDX1 protein is expressed.
Fig. 4.
Characterization of the anti-CDX1 mAb. (A) Peptide-blocking immunoblot of the anti-CDX1 mAb. CDX1 mAb was incubated either with N-term or carboxyl peptides or PBSA before immunoblotting with lysates from either T: HCT116-CDX1 or C: HCT116-Vec. (B) Immunoblot of CDX1-expressing and -nonexpressing CRC cell lines. (C) Immunoblot of the 2 pairs of stably transfected CRC cell lines, HTC116-Vec and HCT116-CDX1, and LS174T-Vec and LS174T-siRNA using anti-CDX1 and anti-KRT20 monoclonal antibodies. Anti β-tubulin was used as a loading control in (A), (B), and (C). (D) Immunohistochemistry using the anti-CDX1 and anti-KRT20 mAbs on the CDX1-expressing cell line LS174T and the non-expressing cell line HCT116.
We further confirmed the results by immunohistochemistry. As shown in Fig. 4D, the anti-CDX1 mAb readily shows strong CDX1 staining in LS174T-Vec and HCT116-CDX1, but essentially gave no staining in the CDX1-negative LS174T-siRNA and HCT116-Vec.
CDX1 Regulates KRT20 Directly in Vivo.
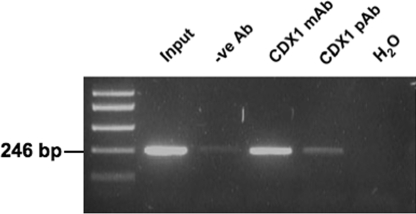
In vivo binding of CDX1 to the KRT20 promoter was studied using a ChIP assay both with our anti-CDX1 mAb and with a commercially available CDX1 polyclonal antibody (Abcam). Normal mouse IgG antibody was used as a negative control. DNA isolated from immunoprecipitated chromatin from the HT55 cell line (another unmethylated CDX1-expressing line) was amplified, as described in Materials and Methods, by primers flanking the 2 putative CDX binding sites in fragment B. Fig. 5 shows that the resulting DNA pulled down by both the anti-CDX1 antibodies contained the sequences flanking the 2 putative CDX binding sites. This demonstrates that CDX1 binds the 2 sites within the first 219 bp of the KRT20 promoter in fragment B, and supports strongly the conclusion that KRT20 mRNA expression is directly regulated by CDX1.
Fig. 5.
Identification of CDX binding sites using ChIP assay. CRC line HT55 lysate was immunoprecipitated with anti-CDX1 mAb, anti-CDX1 polyclonal antibody (pAb), or negative-control anti-mouse IgG antibody as described in Materials and Methods. Eluted DNA was amplified by PCR using primers flanking the 2 CDX binding sites in fragment FB. Nonimmunoprecipitated sheared chromatin DNA is denoted as “Input” and H2O as water control.
Parallel Expression of CDX1 and KRT20 in Normal Crypt.
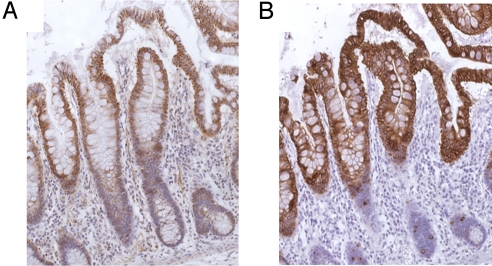
The expression of the CDX1 and KRT20 proteins in the colonic normal crypt was investigated by immunohistochemistry using both the anti-CDX1 polyclonal antibody and an anti-KRT20 monoclonal antibody. CDX1 expression appears to increase from near the bottom to the top of the crypt (Fig. 6A) and largely parallels the expression of KRT20 (Fig. 6B). The CDX1 mAb also shows increased staining from the bottom to the top of the crypt. However, in this case, there is also staining in the cytoplasm of the muscularis mucosae because of cross-reaction with a different protein that is not expressed in the epithelial cells (data not shown). As has already been shown in a study by Moll et al. (5), KRT20 is absent from the bottom of the crypt and gradually increases to its highest level of expression in epithelial cells at the top of the crypt. The CDX1 staining is nuclear, while the KRT20 expression is cytoplasmic. The approximate colocalisation of the CDX1 and KRT20 expression is consistent with regulation of KRT20 by CDX1.
Fig. 6.
Immunohistochemistry of anti-CDX1 pAb and anti-KRT20 mAb in normal intestinal tissues. (A) Using CDX1 pAb (Abcam), CDX1 staining increases from bottom to the top of the crypt in normal colonic mucosa. (B) KRT20 staining shows a similar pattern to CDX1 in normal colonic mucosa.
Discussion
Using microarray gene-expression analysis on paired cell lines that do and do not express the homeobox gene CDX1, we identified KRT20, a well-known marker of differentiated intestinal epithelium, as a candidate for direct regulation by CDX1. This is supported by the strong relationship between CDX1 and KRT20 expression in a panel of CRC-derived cell lines. We also identified 2 key CDX binding sites within 246-bp upstream of the start site in the KRT20 promoter as the main contributors to the regulation of KRT20 expression by CDX1. Using a recently derived anti-CDX1 monoclonal antibody, we have confirmed that CDX1 binds to this region of the CDX1 promoter in vivo. Finally, immunohistochemical analysis of normal colorectal epithelium shows that both CDX1 and KRT20 tend to be colocalized in the upper-differentiated part of the crypt, with CDX1 in the nucleus and KRT20 in the cytoplasm.
CDX1 also seems to play a key role in the control of the expression of the transmembrane protein, A33, which is almost uniquely associated with intestinal epithelium (21, 29, 30). Other published evidence suggests that ectopic expression of CDX1 in an undifferentiated rat cell line results in differentiated phenotypes with columnar epithelial features, together with induction of differentiation markers, such as villin and aminopeptidase N (16). It seems likely that the expression of other markers of intestinal epithelial differentiation, such as villin, MUC2, and sucrase isomaltase, may also be regulated by CDX1. The overall data are consistent with the idea that CDX1 expression is a key switch for intestinal epithelial differentiation, as suggested by Wong et al. (29) with respect to the development of intestinal metaplasia in Barrett's oesophagus, and presumably also in gastric mucosa (31), where intestinal metaplasia is assumed often to be a precursor to the development of gastric carcinomas. The differential expression of CDX1 appears to be controlled by promoter methylation (19, 29).
A number of intestine-specific genes, including lactase-phlorizin hydrolase, calbindin-D9K, carbonic anhydrase, gut-enriched Kruppel-like factor, MUC2, sucrase isomaltase, and liver intestine (L1)-cadherin have been characterized as being regulated by CDX2 (32–41). The relationship of these downstream targets regulated by CDX1 and CDX2 is not clear. Our data suggest that CDX1 is the proximal determinant of intestinal differentiation, but it is probable that its activity depends on cooperation with CDX2. Recent work by Bonhomme et al. (42) indicates that intestinal differentiation in a mouse knock out for CDX1 proceeds more or less normally. This suggests that CDX2 may substitute for CDX1 in its absence, but not vice versa, as CDX2 knock outs are lethal. Our results, showing CDX2 mRNA expression in most of the CRC cell lines that we studied, are not in agreement with claimed evidence for consistent down-regulation of CDX2 (39–41) in CRC.
The initial microarray analysis suggested that IL 18 and DPP4 may also be directly regulated by CDX1. These 2 genes are related to T cell activation, suggesting CDX1 may also have a role in inflammatory and immune responses in the gut.
We have previously shown that the mRNA expression of CDX1 in partially methylated cell lines could be increased by 5-aza-2′ deoxycytidine treatment and that “postconfluent” growth of these partially methylated cell lines, such as LOVO, led to an increase in CDX1 expression that was correlated with a decrease in methylation of a key region in the CDX1 promoter (19). It is now widely accepted that cancer stem cells are the driving cells of a cancer, which will also contain the differentiated cells derived from them (43). A cancer therefore, to some extent but in a disordered and dysregulated manner, recapitulates the process that occurs in the normal tissue and the same is true in cell lines derived from cancers and grown in vitro. The data on the CDX1 partially methylated cell lines therefore suggests that postconfluent growth increases the proportion of differentiated cells as compared to cancer stem cells in these cultures. This is accompanied by a reduction in overall promoter methylation of CDX1 associated with the triggering of differentiation in the differentiated cell component in the cell culture.
As suggested by Wong et al. (19), selection for reduced expression of CDX1 by methylation in colorectal cancers (17–19) is likely to be associated with a reduced capacity of the cancer stem cells to differentiate and so to contribute to an increased rate of growth. Studying the patterns of expression of CDX1 and its downstream-regulated differentiation markers, such as KRT20, should therefore enable better characterization of cancer stem cells both in CRC cell lines and in primary CRCs.
Materials and Methods
Detailed materials and methods can be found in the SI Text.
Cell Lines and Cell Culture.
CaCO2, CCK-81, COLO320DM, DLD1, HCT116, HT29, LOVO, LS123, LS174T, NCI H548, RCM1, SKCO1, SW48, SW837, and SW1116 were cultured in E4 medium [Cancer Research U.K. (CRUK) Cell Services]. COLO678, LS513, NCI-H716, RKO, SW1222, and SW1417 were cultured in RPMI1640 medium (CRUK). The rest of the cell lines were grown in Iscoves medium (Invitrogen). All of the above media were supplemented with 10% FCS (Autogen Bioclear), 1% glutamine, and 1% Penicillin-streptomycin (Invitrogen). Cell lines were kept in a 10% CO2 incubator except those grown in RPMI1640 medium, which were kept in a 5% CO2 incubator. Cells were grown to 50 to 80% confluence before next passage or further experiments (19).
Stable Transfection and Microarray.
The CDX1 nonexpressing CRC cell line HCT116 was stably transfected with either CDX1 cDNA in the pRC/CMV expression vector (HCT116-CDX1) or with vector control (HCT116-Vec), followed by Geneticin (G418) selection to generate stable clones. The strongest CDX1 expressing clone was then chosen for further study. The CDX1-expressing CRC cell line LS174T was similarly transfected with either a pSilencer vector (Ambion) containing a short sequence of CDX1 siRNA (LS174T-siRNA), or a pSilencer vector containing a scrambled siRNA sequence as a control (LS174T-vec), followed by G418 selection to generate stable clones. The clone that had the greatest reduction in CDX1 expression was, again, selected for further study. The 63-bp length oligonucleotide (Top strand: 5′-GATCCGACTCGGACCAAGGACAAGTTCAAGAGACTTGTCCTTGGTCCGAGTCTTTTTTGGAAA-3′ and bottom strand: 5′-AGCTTTTCCAAAAAAGACTCGGACCAAGGACAAGTCTCTT GAACTTGTCCTTGGTCCGAGTCG-3′) of CDX1 siRNA was designed using Ambion online guidelines. The transfected clones were selected in 1-mg/ml G418 (Invitrogen). Total RNA was extracted by using the RNeasy mini kit (Qiagen) according to the manufacturer's instructions. Ten micrograms of RNA of each sample were sent to the Molecular Biology Core Facility of the Paterson Institute for Cancer Research for gene-expression microarray analysis using the Human genome U133 plus 2.0 chips following the manufacturer's instructions (Affymetrix). Gene expression data were normalized using the RMA (Robust Multichip Analysis) algorithm. A fold change of 1.5 with 95% significance level was selected as the threshold for the comparison between the paired cell lines.
RT-PCR.
RT-PCR was done using standard procedures, as described in the SI Text. Normalization was carried out as previously described (44).
Plasmid Constructs and Site-Directed Mutagenesis.
DNA fragments of the human KRT20 promoter were cloned from cell line HDC8 using the primers given in Tables S5 and S6 (see also Fig. 2). Detailed cloning procedures are described in the SI Text. Full-length CDX1 cDNA was cloned as a HindIII-NotI fragment in a pRC/CMV expression vector and is referred to as pRC/CMV-CDX1 (a kind gift from Dr. Jaleh Malakooti). All constructs were prepared using the Endo-free Plasmid Maxi Kit (Qiagen) and verified by DNA sequencing before further study.
Transient Transfection and Luciferase-Reporter Assay.
Cells were trypsinised, counted, and plated at a density of 1 × 106 cells per well in 6-well plates (Corning) 1 day before transfection. Luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega). Transfection efficiencies were normalised to Renilla luciferase activity as described in the SI Text.
Production of Anti-CDX1 Monoclonal Antibody.
The CDX1 anti-human mAb was produced against the human CDX1 peptide ANYGPPAPPPAPPQYPDFSS (referred to as N-term peptide) synthesized by the CRUK Peptide Synthesis Laboratory and used to immunise BALB/c mice. Monoclonal antibodies were raised in the CRUK Tumor Pathology Group laboratory, Oxford, using conventional hybridoma production methods. The mAb was purified from the supernatant and concentrated using the Montage antibody purification kit (Millipore) following the manufacturer's instructions.
Immunoblotting and Peptide Blocking.
Total protein lysate was analyzed using a standard immunoblot protocol. The antibodies and dilutions used were as follows: anti-CDX1 mAb (0.1 μg/ml), anti-KRT20 mAb (Dako) (1:4,000), anti-beta tubulin mAb (Sigma) (1:5,000), and rabbit anti-mouse secondary antibodies conjugated with HRP (1:10,000).
Chromatin Immunoprecipitation.
ChIP was performed using the EZ ChIP Chromatin Immunoprecipitation Kit (Millipore) according to the manufacturer's instructions.
Immunohistochemistry.
Immunohistochemistry was performed following standard procedures. The concentrations of antibody used for immunohistochemistry were: anti-CDX1 pAb (Abcam 1: 500) and anti-KRT20 mAb (Dako 1:200).
Statistical Analysis.
All data are shown as mean ± SEM. For pairwise analysis, Student's t test was used. For all statistical comparisons, P < 0.001 was denoted as ***, P < 0.01 as **, and P < 0.05 as *.
Supplementary Material
Acknowledgments.
We thank Sylvia Bartlett for helpful discussions. The CDX1 expression vector pRC/CMV-CDX1 was a kind gift from Dr. J Malakooti (University of Illinois). We thank Stuart Pepper and Yvonne Hey from the Paterson Institute for Cancer Research for providing the GeneChip Microarray Service and Cancer Research U.K. Peptide Synthesis Laboratory for the CDX1 peptide synthesis service. We also thank Dr. Kenichi Mukaisho from Shiga University of Medical Science for helpful advice on immunohistochemistry. This work was mainly funded by a Cancer Research U.K. program grant, and by support from the Jacqueline Seroussi Memorial Foundation for Cancer Research.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE14380).
This article contains supporting information online at www.pnas.org/cgi/content/full/0812904106/DCSupplemental.
References
- 1.Omary MB, Ku NO, Toivola DM. Keratins: guardians of the liver. Hepatology. 2002;35:251–257. doi: 10.1053/jhep.2002.31165. [DOI] [PubMed] [Google Scholar]
- 2.Ku NO, Gish R, Wright TL, Omary MB. Keratin 8 mutations in patients with cryptogenic liver disease. N Engl J Med. 2001;344:1580–1587. doi: 10.1056/NEJM200105243442103. [DOI] [PubMed] [Google Scholar]
- 3.Ku NO, et al. Mutation of a major keratin phosphorylation site predisposes to hepatotoxic injury in transgenic mice. J Cell Biol. 1998;143:2023–2032. doi: 10.1083/jcb.143.7.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos M, Paramio JM, Bravo A, Ramirez A, Jorcano JL. The expression of keratin k10 in the basal layer of the epidermis inhibits cell proliferation and prevents skin tumorigenesis. J Biol Chem. 2002;277:19122–19130. doi: 10.1074/jbc.M201001200. [DOI] [PubMed] [Google Scholar]
- 5.Moll R, Schiller DL, Franke WW. Identification of protein IT of the intestinal cytoskeleton as a novel type I cytokeratin with unusual properties and expression patterns. J Cell Biol. 1990;111:567–580. doi: 10.1083/jcb.111.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen ZM, Wang HL. Alteration of cytokeratin 7 and cytokeratin 20 expression profile is uniquely associated with tumorigenesis of primary adenocarcinoma of the small intestine. Am J Surg Pathol. 2004;28:1352–1359. doi: 10.1097/01.pas.0000135520.72965.50. [DOI] [PubMed] [Google Scholar]
- 7.Cross HS, et al. Vitamin D receptor and cytokeratin expression may be progression indicators in human colon cancer. Anticancer Res. 1996;16:2333–2337. [PubMed] [Google Scholar]
- 8.Wildi S, et al. Characterization of cytokeratin 20 expression in pancreatic and colorectal cancer. Clin Cancer Res. 1999;5:2840–2847. [PubMed] [Google Scholar]
- 9.Meyer BI, Gruss P. Mouse Cdx-1 expression during gastrulation. Development. 1993;117:191–203. doi: 10.1242/dev.117.1.191. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Q, et al. Keratin 20 serine 13 phosphorylation is a stress and intestinal goblet cell marker. J Biol Chem. 2006;281:16453–16461. doi: 10.1074/jbc.M512284200. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q, et al. Keratin 20 helps maintain intermediate filament organization in intestinal epithelia. Mol Biol Cell. 2003;14:2959–2971. doi: 10.1091/mbc.E03-02-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonner CA, Loftus SK, Wasmuth JJ. Isolation, characterization, and precise physical localization of human CDX1, a caudal-type homeobox gene. Genomics. 1995;28:206–211. doi: 10.1006/geno.1995.1132. [DOI] [PubMed] [Google Scholar]
- 13.Duprey P, et al. A mouse gene homologous to the Drosophila gene caudal is expressed in epithelial cells from the embryonic intestine. Genes Dev. 1988;2:1647–1654. doi: 10.1101/gad.2.12a.1647. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, Kazenwadel J, James R. Isolation and characterization of the murine homeobox gene Cdx-1. Regulation of expression in intestinal epithelial cells. J Biol Chem. 1993;268:27214–27225. [PubMed] [Google Scholar]
- 15.Mallo GV, et al. Molecular cloning, sequencing and expression of the mRNA encoding human Cdx1 and Cdx2 homeobox. Down-regulation of Cdx1 and Cdx2 mRNA expression during colorectal carcinogenesis. Int J Cancer. 1997;74:35–44. doi: 10.1002/(sici)1097-0215(19970220)74:1<35::aid-ijc7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Soubeyran P, et al. Cdx1 promotes differentiation in a rat intestinal epithelial cell line. Gastroenterology. 1999;117:1326–1338. doi: 10.1016/s0016-5085(99)70283-0. [DOI] [PubMed] [Google Scholar]
- 17.Pilozzi E, Onelli MR, Ziparo V, Mercantini P, Ruco L. CDX1 expression is reduced in colorectal carcinoma and is associated with promoter hypermethylation. J Pathol. 2004;204:289–295. doi: 10.1002/path.1641. [DOI] [PubMed] [Google Scholar]
- 18.Suh ER, Ha CS, Rankin EB, Toyota M, Traber PG. DNA methylation down-regulates CDX1 gene expression in colorectal cancer cell lines. J Biol Chem. 2002;277:35795–35800. doi: 10.1074/jbc.M205567200. [DOI] [PubMed] [Google Scholar]
- 19.Wong NA, et al. Loss of CDX1 expression in colorectal carcinoma: promoter methylation, mutation, and loss of heterozygosity analyses of 37 cell lines. Proc Natl Acad Sci USA. 2004;101:574–579. doi: 10.1073/pnas.0307190101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alkhoury F, Malo MS, Mozumder M, Mostafa G, Hodin RA. Differential regulation of intestinal alkaline phosphatase gene expression by Cdx1 and Cdx2. Am J Physiol Gastrointest Liver Physiol. 2005;289:G285–G290. doi: 10.1152/ajpgi.00037.2005. [DOI] [PubMed] [Google Scholar]
- 21.Johnstone CN, et al. Analysis of the regulation of the A33 antigen gene reveals intestine-specific mechanisms of gene expression. J Biol Chem. 2002;277:34531–34539. doi: 10.1074/jbc.M204865200. [DOI] [PubMed] [Google Scholar]
- 22.Patterson AP, et al. Developmental regulation of apolipoprotein B mRNA editing is an autonomous function of small intestine involving homeobox gene Cdx1. J Biol Chem. 2003;278:7600–7606. doi: 10.1074/jbc.M201601200. [DOI] [PubMed] [Google Scholar]
- 23.Pages F, et al. Modulation of interleukin-18 expression in human colon carcinoma: consequences for tumor immune surveillance. Int J Cancer. 1999;84:326–330. doi: 10.1002/(sici)1097-0215(19990621)84:3<326::aid-ijc22>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi M, et al. Immunohistochemical and immuno-electron-microscopic detection of interferon-gamma-inducing factor (“interleukin-18”) in mouse intestinal epithelial cells. Cell Tissue Res. 1997;289:499–503. doi: 10.1007/s004410050895. [DOI] [PubMed] [Google Scholar]
- 25.Okazawa A, et al. Human intestinal epithelial cell-derived interleukin (IL)-18, along with IL-2, IL-7 and IL-15, is a potent synergistic factor for the proliferation of intraepithelial lymphocytes. Clin Exp Immunol. 2004;136:269–276. doi: 10.1111/j.1365-2249.2004.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darmoul D, et al. Dipeptidyl peptidase IV (CD 26) gene expression in enterocyte-like colon cancer cell lines HT-29 and Caco-2. Cloning of the complete human coding sequence and changes of dipeptidyl peptidase IV mRNA levels during cell differentiation. J Biol Chem. 1992;267:4824–4833. [PubMed] [Google Scholar]
- 27.Guo RJ, Suh ER, Lynch JP. The role of Cdx proteins in intestinal development and cancer. Cancer Biol Ther. 2004;3:593–601. doi: 10.4161/cbt.3.7.913. [DOI] [PubMed] [Google Scholar]
- 28.Freund JN, Domon-Dell C, Kedinger M, Duluc I. The Cdx-1 and Cdx-2 homeobox genes in the intestine. Biochem Cell Biol. 1998;76:957–969. doi: 10.1139/o99-001. [DOI] [PubMed] [Google Scholar]
- 29.Wong NA, et al. CDX1 is an important molecular mediator of Barrett's metaplasia. Proc Natl Acad Sci USA. 2005;102:7565–7570. doi: 10.1073/pnas.0502031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong NA, et al. EpCAM and gpA33 are markers of Barrett's metaplasia. J Clin Pathol. 2006;59:260–263. doi: 10.1136/jcp.2005.027474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutierrez-Gonzalez L, Wright NA. Biology of intestinal metaplasia in 2008: more than a simple phenotypic alteration. Dig Liver Dis. 2008;40:510–522. doi: 10.1016/j.dld.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 32.Colnot S, et al. Intestinal expression of the calbindin-D9K gene in transgenic mice. Requirement for a Cdx2-binding site in a distal activator region. J Biol Chem. 1998;273:31939–31946. doi: 10.1074/jbc.273.48.31939. [DOI] [PubMed] [Google Scholar]
- 33.Dang DT, Mahatan CS, Dang LH, Agboola IA, Yang VW. Expression of the gut-enriched Kruppel-like factor (Kruppel-like factor 4) gene in the human colon cancer cell line RKO is dependent on CDX2. Oncogene. 2001;20:4884–4890. doi: 10.1038/sj.onc.1204645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drummond FJ, Sowden J, Morrison K, Edwards YH. Colon carbonic anhydrase 1: transactivation of gene expression by the homeodomain protein Cdx2. FEBS Lett. 1998;423:218–222. doi: 10.1016/s0014-5793(98)00103-3. [DOI] [PubMed] [Google Scholar]
- 35.Hinoi T, et al. CDX2 regulates liver intestine-cadherin expression in normal and malignant colon epithelium and intestinal metaplasia. Gastroenterology. 2002;123:1565–1577. doi: 10.1053/gast.2002.36598. [DOI] [PubMed] [Google Scholar]
- 36.Lambert M, et al. cis-Acting elements and transcription factors involved in the intestinal specific expression of the rat calbindin-D9K gene: binding of the intestine-specific transcription factor Cdx-2 to the TATA box. Eur J Biochem. 1996;236:778–788. doi: 10.1111/j.1432-1033.1996.00778.x. [DOI] [PubMed] [Google Scholar]
- 37.Mesquita P, et al. Human MUC2 mucin gene is transcriptionally regulated by Cdx homeodomain proteins in gastrointestinal carcinoma cell lines. J Biol Chem. 2003;278:51549–51556. doi: 10.1074/jbc.M309019200. [DOI] [PubMed] [Google Scholar]
- 38.Suh E, Chen L, Taylor J, Traber PG. A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol Cell Biol. 1994;14:7340–7351. doi: 10.1128/mcb.14.11.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor JK, Levy T, Suh ER, Traber PG. Activation of enhancer elements by the homeobox gene Cdx2 is cell line specific. Nucleic Acids Res. 1997;25:2293–2300. doi: 10.1093/nar/25.12.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Troelsen JT, et al. Regulation of lactase-phlorizin hydrolase gene expression by the caudal-related homoeodomain protein Cdx-2. Biochem J. 1997;322:833–838. doi: 10.1042/bj3220833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto H, Bai YQ, Yuasa Y. Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. Biochem Biophys Res Commun. 2003;300:813–818. doi: 10.1016/s0006-291x(02)02935-2. [DOI] [PubMed] [Google Scholar]
- 42.Bonhomme C, et al. Cdx1, a dispensable homeobox gene for gut development with limited effect in intestinal cancer. Oncogene. 2008;27:4497–4502. doi: 10.1038/onc.2008.78. [DOI] [PubMed] [Google Scholar]
- 43.Ricci-Vitiani L, Pagliuca A, Palio E, Zeuner A, De Maria R. Colon cancer stem cells. Gut. 2008;57:538–548. doi: 10.1136/gut.2007.127837. [DOI] [PubMed] [Google Scholar]
- 44.Pfaffl M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.