Abstract
Atrophy is regarded a sensitive marker of neurodegenerative pathology. In addition to confirming the well-known presence of decreased global grey matter and hippocampal volumes in Alzheimer's disease, this study investigated whether deep grey matter structure also suffer degeneration in Alzheimer's disease, and whether such degeneration is associated with cognitive deterioration. In this cross-sectional correlation study, two groups were compared on volumes of seven subcortical regions: 70 memory complainers (MCs) and 69 subjects diagnosed with probable Alzheimer's disease. Using 3T 3D T1 MR images, volumes of nucleus accumbens, amygdala, caudate nucleus, hippocampus, pallidum, putamen and thalamus were automatically calculated by the FMRIB's Integrated Registration and Segmentation Tool (FIRST)—algorithm FMRIB's Software Library (FSL). Subsequently, the volumes of the different regions were correlated with cognitive test results. In addition to finding the expected association between hippocampal atrophy and cognitive decline in Alzheimer's disease, volumes of putamen and thalamus were significantly reduced in patients diagnosed with probable Alzheimer's disease. We also found that the decrease in volume correlated linearly with impaired global cognitive performance. These findings strongly suggest that, beside neo-cortical atrophy, deep grey matter structures in Alzheimer's disease suffer atrophy as well and that degenerative processes in the putamen and thalamus, like the hippocampus, may contribute to cognitive decline in Alzheimer's disease.
Keywords: Alzheimer's disease, subcortical atrophy, thalamus, putamen, FIRST
Introduction
Characteristic degenerative pathology in Alzheimer's disease consists of atrophy and the increased presence of neurofibrillary tangles and amyloid plaques. This degenerative pathology gradually accumulates over a period of years, long before clinical symptoms become manifest (Jack, Jr et al., 1999; Smith et al., 2007). Herein, atrophy is considered a better marker for impaired functioning of brain regions than the deposits of insoluble proteins in plaques and tangles, since only a small portion of neurons contains tangles and the number of neurons that die, exceeds by far the number that contains tangles (Smith, 2002). Description of cerebral atrophy patterns in the process of Alzheimer's disease is considered important, because it may provide information on the pathogenesis of Alzheimer's disease and on the contribution of various brain structures to cognitive decline.
Structural imaging studies have already identified several diagnostic markers of Alzheimer's disease related to atrophy: hippocampal atrophy (de Leon et al., 1995), amygdalar atrophy (Horinek et al., 2007), medial temporal lobe atrophy (Scheltens et al., 1992; de Leon et al., 1993), precuneus atrophy (Karas et al., 2007), and global grey matter atrophy (Karas et al., 2004). Most studies have focused on the neocortex, and especially the hippocampus, regarding their crucial roles in higher cognitive functions and memory processes respectively, whereas the basal nuclei and thalamus have received less attention. There are, however, many reasons to assume that degeneration of deep grey matter structures, besides the hippocampus and the amygdala, may also occur in the process of Alzheimer's disease and may contribute to cognitive deterioration. In 1990, Braak et al. noted the presence of amyloid depositions in the striatum (Braak and Braak, 1990). Klunk et al. added that amyloid depositions are even present in the striatum in very early stages of the disease in PS1 mutation carriers (Klunk et al., 2007). Both studies indicated that the striatum is prone to Alzheimer's disease pathology. Regarding subcortical atrophy and Alzheimer's disease, Ferrarini et al. reported the changes in shape of the ventricle system in areas adjacent to the amygdala, thalamus, and caudate nucleus in patients with Alzheimer's disease, indicating atrophic changes in those regions (Ferrarini et al., 2006). Direct measurement of the caudate nucleus in Alzheimer's disease patients revealed diminished volumes of this structure, although proportionate to whole brain atrophy (Almeida et al., 2003). Direct measurement of subcortical structures in Parkinson's disease, another neurodegenerative disorder accompanied in advanced stages by dementia, also showed decreased volumes of putamen and pallidum (Geng et al., 2006). The impaired functioning of the basal nuclei has previously been related to cognitive dysfunction, since neurodegenerative extrapyramidal syndromes commonly show symptoms of cognitive dysfunction such as visuospatial deficits, depression, anxiety and, in progressed stages, clinical dementia syndrome (Borroni et al., 2007). Atrophy of the thalamus has also been associated with cognitive decline in neurodegenerative disorders other than Alzheimer's disease such as multiple sclerosis, Huntington's disease and Lewy-Body dementia (Barber et al., 2002; Kassubek et al., 2005; Houtchens et al., 2007). Although little is known about the specific role that subcortical and thalamic structures have in cognitive processes, it is well recognized that the thalamus is essential for generating attention (Newman, 1995) and it's anterior and medial nuclei are involved in declarative memory functioning (Van der Werf et al., 2000). In spite of all these data, direct measurement of volumes of all large subcortical structures in Alzheimer's disease patients, compared to those without cognitive impairment has not been assessed before; similarly, the correlation between the decreasing of their volume and cognitive functioning has never been reported in the literature.
In this study we used FMRIB's Integrated Registration and Segmentation Tool (FIRST) (Patenaude, 2007) to automatically measure the volumes of amygdala, hippocampus, nucleus accumbens, caudate nucleus, putamen, pallidum and thalamus in subjects that entered the out-patient memory clinic of our hospital. The aim of this study is to assess whether differences exist between volumes of the basal nuclei and the thalamus of subjects with Alzheimer's disease compared to elderly subjects without cognitive impairment. We hypothesize that smaller volumes of the basal nuclei and thalamus exist in Alzheimer's disease patients due to more severe atrophic changes in those regions, compared with the volume loss accompanying ‘normal ageing’ in elderly subjects by whom no cognitive impairment can be objectified. We also hypothesized that smaller volumes of the deep grey matter structures, when corrected for age, gender, years of education, intra cranial volume (ICV) and neocortical grey matter volume, correlate with poorer cognitive test results.
Methods
Study design
Two hundred and nineteen consecutive patients, who visited the out-patient's clinic for memory deficits of the geriatric department of the Leiden University Medical Center (LUMC) between January 1, 2006 and October 1, 2007 were recruited for this study. All subjects who visited the clinic complained of memory loss and were examined according to a standardized protocol, which included a whole brain MRI, a neuropsychological screening, and a general medical and neurological examination, by a neurologist or geriatrist. Cognitive testing took place prior or after MRI testing with a maximal interval of 14 days. The eventual diagnose was determined in a multidisciplinary consensus meeting which employed the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria for diagnosing probable Alzheimer's disease (McKhann et al., 1984). This cross-sectional case-control study was approved by the local medical-ethical committees. Written informed consent was obtained from all subjects or from a close relative if a patient was demented.
Subjects
Subjects came to the memory clinic for a variety of indications, mostly memory complaints experienced by the patient or notified by people in his or her environment.
In the multidisciplinary meeting, patients were categorized as having possible or probable Alzheimer's disease, mild cognitive impairment, or a neurological or psychiatric disorder. The remaining patients, by whom objectively no memory deficits could be detected, were classified as MCs. Attention was given to factors that might contribute to memory complaints in this heterogeneous group. Part of the MCs suffered mild depressions Geriatric Depression Scale (GDS) <10 (n = 12), while in another part memory was declined due to psycho-social factors such as the loss of a family member or problems at work (n = 17). For over half of the MCs no clear explanation for subjectively experienced memory decline was found. Part of them might have a very early stage of dementia, undetectable by our present means. For our study we included 139 subjects, of whom complete datasets were available. Seventy of them were MCs and 69 were probable Alzheimer's disease patients.
In total 80 subjects were excluded. Among them were subjects who did have cognitive impairment detected during neuropsychological testing but did not meet the criteria for probable Alzheimer's disease (n = 33), or were diagnosed with other forms of dementia (Frontal Temporal Dementia n = 3, Lewy Body Dementia n = 1, Vascular Dementia n = 3, Parkinson's dementia n = 2). Other exclusion criteria for this study included; other neurological disorders (n = 14) e.g. normal pressure hydrocephalus, intracranial tumors, stroke etc.; severe mood-disorders with GDS ≥ 10 (n = 10); alcohol abuse (n = 8); insufficient scan quality (n = 2); and re-examination of the subject neuropsychologically (n = 4).
Neuropsychological assessment
The cognitive functioning of all subjects was assessed using a standardized neuropsychological test battery, including the Cambridge Cognitive Examination-Revised (CAMCOG-R) (Roth et al., 1999) incorporating the Mini Mental State Examination (MMSE) (Folstein et al., 1975). Furthermore all subjects were examined for signs of depression using the GDS (Skeith and Yesavage, 1986) to rule out a severe depression as contributory factor to memory loss or cognitive decline.
MR data acquisition
MRI was performed using a 3.0 T whole body MRI scanner (Philips Medical Systems, Best, The Netherlands). Volume measurements of the basal nuclei and the thalamus were performed on 3D-T1-weighted MR images (acquisition parameters were as follows: TR = 9.8 ms; TE = 4.6 ms; flip angle = 8°; section thickness = 1.2 mm; number of sections = 120; no section gap; whole brain coverage; FOV = 224 mm; matrix = 192, reconstruction matrix = 256). Routine T2-weighted MRI and FLAIR were performed to rule out a mass lesion as contributory factor to memory loss or cognitive decline.
Measurement of volumes of deep grey matter structures
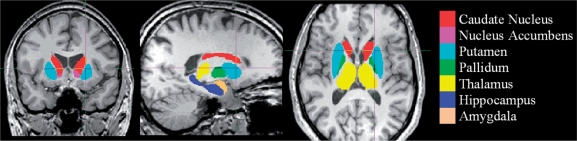
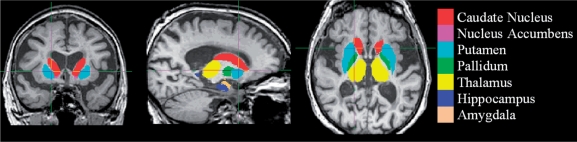
The algorithm FIRST, was applied to separately estimate left and right volumes of seven subcortical regions; amygdala, hippocampus, nucleus accumbens, caudate nucleus, putamen, pallidum and thalamus. FIRST is part of FMRIB's Software Library (FSL) and performs both registration and segmentation of the mentioned subcortical regions (Patenaude, 2007; Patenaude et al., 2007). During registration, the input data (3D T1 images) are transformed to the MNI (Montreal Neurological Institute) 152 standard space, by means of affine transformations based on 12 degrees of freedom (i.e. three translations, three rotations, three scalings and three skews). After subcortical registration, a sub-cortical mask is applied, to locate the different subcortical structures, followed by segmentation based on shape models and voxel intensities. Absolute volumes of subcortical structures are calculated, taking into account the transformations made in the first stage (Patenaude, 2007). Finally a boundary correction is used to determine which boundary voxels belong to the structure or not. In this study a Z-value of 3 was used, corresponding to a 99.998% certainty that the voxel belonged to the subcortical structure. After registration and segmentation of all 139 MR scans, all segmentated subcortical regions were visually checked for errors in registration and segmentation. None were found. Examples of subcortical segmentation, after boundary correction, of a subject classified as MC and of a subject diagnosed with probable Alzheimer's disease, are presented in respectively Figs 1 and 2.
Fig. 1.
FIRST-segmentation: MC.
Fig. 2.
FIRST-segmentation: probable Alzheimer's disease.
Brain tissue volume was estimated with SIENAX (Smith et al., 2002), part of FSL (Smith et al., 2004). SIENAX starts by extracting brain and skull images from the single whole-head input data (Smith et al., 2002). Next, tissue-type segmentation with partial volume estimation is carried out (Zhang et al., 2001) in order to calculate total volume of brain tissue (including separate estimates of volumes of grey matter, white matter). For this study we used the absolute volumes generated by the algorithm. To obtain neocortical grey matter volume (NeoCor GM) independent from the deep grey matter structures of interest, we subtracted the volumes of the hippocampus and amygdala from the peripheral grey matter volume as given by SIENAX. ICV was calculated by adding the volumes of cerebral spinal fluid, total grey matter and total white matter together.
Statistical analysis
SPSS v 14.0 for Windows was used for data analysis. The MCs were compared for age, educational level (in years of education including primary school), and ICV with subjects diagnosed with probable Alzheimer's disease by one-way independent ANOVA, and compared for gender by a chi-quadrate test. The performed analysis was 2-fold. The first part was designed to assess whether the means of cortical and deep grey matter volumes (FIRST and SIENAX-output) in probable Alzheimer's disease-subjects were decreased compared with subjects without cognitive impairment. A general linear model was used in which the volumes of deep grey matter structures were included as dependent factors and diagnosis as independent. In this model age and ICV were included as co-variates, and gender as fixed factor. The Beta differences between the estimated means, adjusted for age, ICV and gender, for the two groups were calculated, as their corresponding P-values. A P-value ≤0.05 was considered significant. Furthermore, Pearson correlation coefficients were calculated between the neo-cortical grey matter and the different deep grey matter structures, total brain volume and total white matter volume.
In the second part, correlations between neuropsychological test results (CAMCOG-R score, executive subtest of CAMCOG-R and MMSE score) and deep grey matter volumes were assessed on linearity for MCs and subjects with probable Alzheimer's disease separately. For each deep grey matter structure a linear regression model was designed, in which the different cognitive test scores were included as the dependent variables and the volume of the deep grey matter structure as independent variable. Age, gender, years of education and neo cortical grey matter volume were also added as independent variables in these models, because of their expected influence on cognitive test scores. ICV was added as independent variable to adjust for differences in deep grey matter volume due to differences in head size between patients. A collinearity test was performed to rule out multicollinearity between the independent variables, in which a R2 above 64% was considered being a strong correlation.
Results
Group demographic features are presented in Table 1. MCs differed significantly from probable Alzheimer's disease subjects in age (F = 38.1, P < 0.001) in years of education (F = 9.97, P = 0.002), and in ICV (one-way ANOVA; F = 5.06, P = 0.026), but not in gender (χ2 = 2.68, P = 0.101). The significance of difference between ICV disappeared if male and female were compared for separately. Although the male–female ratio was not significantly different between the MCs and probable Alzheimer's disease patients, it was incorporated as independent factor in the statistical analyses because of the relatively large number of female probable Alzheimer's disease subjects and the known existence of different brain tissue volumes between the sexes. Age, educational level and ICV were taken in as co-variates in the following analyses.
Table 1.
Demographics and group characteristics
| Memory complainers |
Probable Alzheimer's disease |
|||||
|---|---|---|---|---|---|---|
| M | F | Total | M | F | Total | |
| N | 35 | 35 | 70 | 25 | 44 | 69 |
| Age in Y Mean (SD) | 65 (13) | 67 (12) | 66 (13) | 76 (5.8) | 77 (8.2) | 77 (7.4) |
| Edu in Y Mean (SD) | 12 (3.9) | 12 (3.5) | 12 (3.7) | 11. (4.5) | 8.9 (3.5) | 9.8 (4.0) |
| ICV in cm3 Mean (SD) | 1565 (87.5) | 1390 (118) | 1477 (135) | 1541 (121) | 1361 (92.4) | 1426 (135) |
| CAMCOG-R Mean (SD) | 90 (7.0) | 90 (7.3) | 90 (7.1) | 69 (15) | 61 (14) | 64 (15) |
| CAMCOG-R exe Mean (SD) | 15 (2.6) | 15 (3.1) | 15 (2.9) | 9.6 (3.6) | 9.2 (3.5) | 9.4 (3.5) |
| MMSE Mean (SD) | 28 (1.6) | 27 (2.7) | 27 (2.3) | 20 (5.0) | 17 (4.4) | 18 (4.7) |
The groups MC and probable Alzheimer's disease significantly differ in age (one-way ANOVA: F = 38.1, P < 0.001) years of education (one-way ANOVA; F = 9.97, P = 0.002), and ICV (one-way ANOVA; F = 5.06, P = 0.026) but not in gender (X2-value = 2.68, P = 0.101).
Age in Y = age in years; Edu in Y = educational level in years including primary school; ICV = Intra Cranial Volume; CAMCOG-R exe = CAMbridge COGnition examination Revised.executive functioning; M = male; F = female.
Decreased volumes of putamen and thalamus in Alzheimer's disease subjects
The mean volumes for all substructures in cubic centimetres, computed from the calculated volumes by the FIRST-algorithm, are shown in Table 2 separately for MCs and probable Alzheimer's disease patients. A distinction is made for male and female mean volumes. Also total brain volume, white matter and neocortical grey matter are shown. All structures, except the right amygdala in females, the left and right caudate nucleus and total white matter in males, show a decrease in size in absolute volume if MCs are compared to probable Alzheimer's disease patients. When age, gender and ICV differences between subjects are corrected for, the left and right hippocampus, putamen and thalamus of probable Alzheimer's disease subjects are significantly smaller than those of MCs (as shown in Table 2). Also total brain volume and neocortical grey matter volume are significantly reduced in probable Alzheimer's disease subjects. Table 2 also shows the Pearson correlation coefficients between neo-cortical grey matter volume and the volumes of the deep grey matter structures, and their corresponding P-values. Both left and right volumes of the nucleus accumbens, hippocampus, putamen and thalamus are significantly correlating to the volume of the neocortical grey matter in male and female subjects. In male subjects the right pallidum also correlates significantly with the neocortical grey matter volume, and in female subjects the right amygdala correlates significantly with neocortical grey matter volume.
Table 2.
Mean volumes of deep grey matter structures in cubic centimeter per diagnosis and gender: General Linear Model applied for estimating group differences (adjusted for age, gender and intra cranial volume)
| Memory complainers Mean volumes (SD) (cm3) |
Probable Alzheimer's disease Mean volumes (SD) (cm3) |
B (cm3) | Pearson-corr NeoCor GM |
||||
|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | ||
| L AMYG | 1.66 (0.28) | 1.44 (0.22) | 1.62 (0.35) | 1.36 (0.27) | −0.030 | 0.13 | 0.06 |
| R AMYG | 1.72 (0.28) | 1.47 (0.26) | 1.57 (0.32) | 1.48 (0.37) | −0.054 | 0.23 | 0.33** |
| L HIPP | 4.20 (0.60) | 3.83 (0.74) | 3.73 (0.67) | 3.43 (0.69) | −0.244* | 0.58** | 0.33** |
| R HIPP | 4.21 (0.71) | 3.97 (0.72) | 3.57 (0.78) | 3.32 (0.65) | −0.295* | 0.61** | 0.39** |
| L NACC | 0.64 (0.16) | 0.51 (0.16) | 0.48 (0.19) | 0.42 (0.14) | −0.049 | 0.42** | 0.45** |
| R NACC | 0.47 (0.17) | 0.36 (0.15) | 0.35 (0.15) | 0.27 (0.10) | −0.035 | 0.32* | 0.40** |
| L CN | 4.41 (1.00) | 4.09 (0.81) | 4.49 (0.90) | 3.77 (0.82) | 0.027 | 0.18 | 0.21 |
| R CN | 4.96 (0.90) | 4.49 (0.60) | 5.01 (0.98) | 4.09 (0.78) | −0.188 | 0.08 | −0.02 |
| L PUT | 5.90 (0.77) | 5.24 (0.61) | 5.25 (0.62) | 4.53 (0.83) | −0.381** | 0.53** | 0.37** |
| R PUT | 6.47 (0.86) | 5.56 (0.75) | 5.80 (0.81) | 5.05 (0.78) | −0.285* | 0.45** | 0.34** |
| L GP | 2.36 (0.99) | 2.26 (0.73) | 2.11 (0.55) | 1.97 (0.64) | −0.029 | 0.22 | −0.12 |
| R GP | 2.39 (0.88) | 2.16 (0.73) | 2.03 (0.51) | 1.83 (0.67) | −0.234 | 0.32* | 0.07 |
| L TH | 7.63 (1.07) | 7.40 (1.03) | 7.20 (0.87) | 6.18 (1.12) | −0.362* | 0.52** | 0.51** |
| R TH | 7.68 (0.95) | 7.21 (0.92) | 6.88 (0.73) | 6.17 (1.08) | −0.409** | 0.61** | 0.46** |
| BRAIN | 1204 (107) | 1085 (123) | 1113 (101) | 974 (76.9) | −41.7** | 0.64** | 0.63** |
| WHITE | 506 (65.7) | 458 (57.5) | 522 (93.9) | 448 (60.6) | 6.40 | −0.17 | 0.09 |
| NeoCor GM | 511 (78.4) | 463 (72.6) | 412 (92.8) | 395 (87.0) | −39.3** | – | – |
*P ≤ 0.05, **P ≤ 0.01.
L: left, R: right; AMYG: amygdala; HIPP: hippocampus; NACC: nucleus accumbens; CN: caudate nucleus; PUT: putamen; GP: globus pallidus; TH: thalamus; BRAIN: total of white and grey matter; WHITE: volume of white matter; NeoCor GM: neocortical grey matter minus volumes of hippocampus and amygdala; M: male; F: female.
Bold figures represent significant B differences between memory complainers and probable Alzheimer's disease patients after adjustment for age, gender and ICV.
Decreased volumes of left putamen and thalamus correlate independently to poorer cognitive test results
No multi-collinearity between the selected variables, age, years of educations, ICV, neocortical grey matter volume, and volumes of the deep grey matter structures, was found (all R2 were found below 0.52). Therefore, all were entered in the linear regression model which was set up for each deep grey matter structure separately. β-Regression coefficients of the correlations between volumes of deep grey matter structures and the main cognitive tests scores (the CAMCOG-R, a sub-test on executive function of the CAMCOG-R and the MMSE) of MCs and subjects with probable Alzheimer's disease, are displayed in Table 3. The corresponding P-values are displayed between brackets. This table shows that volumes of the left hippocampus, left putamen and left thalamus in probable Alzheimer's disease subjects correlate significantly to all three cognitive test scores. Also, the right thalamus shows a significant correlation in probable Alzheimer's disease subjects with the MMSE score. The remaining correlations between right hippocampus, right putamen and right thalamus and cognitive test scores were stronger compared with the correlations of the other deep grey matter structures, but not found significant. In all the regression models between the hippocampus, putamen and thalamus and the cognitive test scores of probable Alzheimer's disease subjects, the volume of the deep grey matter structure constantly forms the strongest predictor of cognitive outcome compared to age, gender, years of education, ICV and neocortical grey matter volume.
Table 3.
Linear Regression analysis: β-coefficients (P-values) of correlations between volumes of deep grey matter regions and main cognitive test results (adjusted for age, gender, years of education, intra cranial volume and neocortical grey matter volume)
| CAMCOG-R | CAMCOG-R exe | MMSE | ||||
|---|---|---|---|---|---|---|
| MC | pAD | MC | pAD | MC | pAD | |
| L AMYG | −0.02 (0.86) | 0.13 (0.40) | −0.02 (0.89) | 0.13 (0.39) | 0.07 (0.58) | 0.20 (0.16) |
| R AMYG | 0.05 (0.67) | 0.20 (0.17) | −0.04 (0.73) | 0.15 (0.30) | 0.09 (0.50) | 0.08 (0.59) |
| L HIPP | 0.29 (0.01) | 0.28 (0.05) | 0.12 (0.33) | 0.33 (0.02) | 0.30 (0.03) | 0.28 (0.05) |
| R HIPP | 0.16 (0.22) | 0.18 (0.19) | 0.11 (0.42) | 0.22 (0.12) | 0.29 (0.06) | 0.20 (0.15) |
| L NACC | 0.34 (0.01) | 0.04 (0.74) | 0.08 (0.58) | 0.13 (0.35) | 0.23 (0.15) | 0.11 (0.41) |
| R NACC | 0.31 (0.02) | 0.17 (0.21) | 0.12 (0.37) | 0.26 (0.08) | 0.19 (0.21) | 0.14 (0.31) |
| L CN | −0.04 (0.74) | 0.24 (0.10) | −0.09 (0.45) | 0.26 (0.09) | −0.09 (0.50) | 0.17 (0.24) |
| R CN | −0.12 (0.30) | 0.07 (0.64) | −0.07 (0.53) | 0.14 (0.39) | 0.03 (0.83) | 0.14 (0.31) |
| L PUT | 0.19 (0.14) | 0.50 (<0.00) | −0.06 (0.70) | 0.52 (<0.00) | 0.18 (0.26) | 0.50 (<0.00) |
| R PUT | 0.20 (0.11) | 0.25 (0.08) | −0.09 (0.48) | 0.14 (0.36) | 0.17 (0.24) | 0.19 (0.21) |
| L GP | 0.07 (0.52) | −0.09 (0.48) | 0.11 (0.30) | −0.16 (0.22) | −0.08 (0.52) | −0.13 (0.33) |
| R GP | 0.02 (0.89) | −0.08 (0.53) | 0.03 (0.82) | 0.02 (0.90) | −0.08 (0.52) | −0.09 (0.47) |
| L TH | 0.07 (0.60) | 0.37 (0.01) | 0.00 (0.96) | 0.43 (<0.00) | −0.16 (0.33) | 0.37 (0.02) |
| R TH | 0.39 (0.01) | 0.21 (0.17) | 0.08 (0.64) | 0.18 (0.27) | 0.27 (0.15) | 0.31 (0.04) |
CAMCOG-R exe = CAMbridge COGnition examination Revised executive functioning; L = left; R = right; AMYG = amygdala; HIPP = hippocampus; NACC = nucleus accumbens; CN = caudate nucleus; PUT = putamen; GP = globus pallidus; TH = thalamus.
Bold figures represent significant correlation-coefficients after adjustment for age, gender, years of education, ICV and neocortical grey matter.
In the linear regression models for MCs the left hippocampus, both the volume of the left and right nucleus accumbens, and the right thalamus correlate significantly to the CAMCOR_R score. The volume of the left hippocampus also correlates significantly to the MMSE score. In all regression models of the MCs, however, the correlation between educational level and cognitive test score exceeds in strength the correlation between volume of the deep grey matter and the cognitive test score, with all P <0.01.
The volumes of the caudate nucleus, amygdala and the pallidum do not show any significant correlations, with any of the cognitive test scores, in any of the groups.
Discussion
First of all, in addition to the expected finding of reduced hippocampal and neocortical grey matter volumes in Alzheimer's disease, the current study revealed significantly reduced volumes of both left and right putamen and thalamus in patients diagnosed with probable Alzheimer's disease relative to MCs. This volume reduction was found independent of age, gender and ICV. Second, we also found, that reduced volumes of the putamen and the thalamus independently correlated with impaired cognitive functioning in elderly subjects, when controlled for age, gender, educational level, ICV and neocortical grey matter volume. To the best of our knowledge, this is the first study that explicitly associates smaller volumes of putamen and thalamus with Alzheimer's disease, and reveals their relation with cognitive functioning.
The robust finding of smaller volumes of hippocampus, putamen and thalamus in patients diagnosed with probable Alzheimer's disease, strongly suggests that degenerative pathology affects these structures more or earlier in the process of Alzheimer's disease than other deep grey matter structures. Volumes of brain structures, especially grey matter volume, have been said to be of protective value in Alzheimer's disease (Wolf et al., 2004). Our analysis supports this idea as well, since the smaller the volumes of hippocampus, putamen and thalamus, the more impaired the cognitive results are. In fact, in the Alzheimer's disease group, especially the left volumes of the hippocampus, putamen and thalamus formed the strongest predictors for cognitive performance. Hippocampal atrophy and its contribution to memory decline in the process of Alzheimer's disease has been described more than once and is widely accepted (de Leon et al., 1995; Laakso et al., 1995). On the contrary, putaminal and thalamic volume reduction in Alzheimer's disease and their relations to cognitive decline have not been reported before, and these findings seem to open a new perspective on Alzheimer's disease. The basal nuclei and thalamus are known to participate in many different neuronal pathways, with functions that are not just restricted to motor behaviour, but are also related to emotional, motivational, associative and cognitive abilities (Herrero et al., 2002). Strength of our study was the availability of cognitive data for all subjects. Our data show that volume reduction of the left putamen and left thalamus are both significantly associated with global cognitive decline in elderly subjects visiting a memory clinic and exceed in strength the left hippocampal correlation to cognitive performance. To interpret this, data from other studies are needed. The putamen has been correlated to Alzheimer's disease, since amyloid deposits are present early in the disease process (Braak and Braak, 1990). At the moment however, very little is known on the putaminal role in cognition. Recent literature shows that as part of the striatum, the putamen is found active in probabilistic learning tasks (Graybiel, 2005; Bellebaum et al., 2008) and working memory tasks (Dahlin et al., 2008). Furthermore the putamen has been correlated with the emergence of dementia in other neurodegenerative disorders, like Parkinson's disease, due to dopaminergic or other neurochemical deficits (Emre, 2003), indicating once more that its effect on cognitive impairment might be greater than previously assumed. Whether the putamen in the process of Alzheimer's disease influences cognition due to impaired putaminal primary cognitive functions, inadequate neurochemical functioning, or discontinuing the cortico-thalamic projections, could not be answered in this study, and is an interesting topic for further research. In contrast to the putamen, slightly more is known on the thalamus and its relation to cognition. The thalamus consists of multiple nuclei and is classically metaphorized as an active relay centre. The thalamus serves both sensory and motor mechanisms (Herrero et al., 2002). Cognitively it is involved in directing attention and suppressing irrelevant sensory input (Newman, 1995) and its anterior, medial-dorsal, intralaminar and midline nuclei are important for memory functions (Van der Werf et al., 2000). Thalamic atrophy has been related to cognitive performance in other neurodegenerative disorders, like Huntington's disease (Kassube et al., 2005) and multiple sclerosis (Houtchens et al., 2007), and herein affected mostly the executive functioning of patients. As part of the limbic system, atrophy of the anterior part of the thalamus in Alzheimer's disease has been described. Callen et al. reported that the anterior part of the thalamus was significantly smaller in Alzheimer's disease patients than in healthy controls (Callen et al., 2001). However, the size reduction of the thalamus in our study cannot entirely be explained by reduction of the anterior nuclei alone and suggests that other nuclei of the thalamus are also prone to atrophy in Alzheimer's disease, in which the large medial-dorsal nucleus is an important candidate. Supportive to this thought was the finding of Braak et al. in 1991, that in all limbic nuclei of the thalamus extracellular amyloid deposits and neurofibrillary tangles occur, although the most severe involvement was found in the medial-dorsal nucleus (Braak and Braak, 1991). In summary, our study adds to the present knowledge, that is the presence of neurofibrillary tangles and amyloid plaques in the striatum and thalamus early in the disease process of Alzheimer's disease, the finding of a clear reduction in size of the putamen and the thalamus compared to controls without memory deficits. Moreover the reduction in size of the putamen and thalamus, especially the left side, was correlated to global cognitive decline. At the present no clear explanation can be given on difference in correlational strength between left and right side of the putamen and thalamus. Part might be due to the dominant presence of the right-handedness among subjects in this population. Another explanation might be that the tested cognitive functions are more prominent on the left side. Whether shrinkage of the putamen and thalamus is a primary or a secondary phenomenon in the pathology of Alzheimer's disease, also remains speculative.
The presented data suggest that in Alzheimer's disease some structures are affected by atrophy (i.e. the neocortical grey matter and hippocampus, putamen and thalamus) whereas others are relatively preserved (i.e. the amygdala, nucleus accumbens, caudate nucleus, pallidum and white matter). In previous studies it has been shown, however, that atrophy of the amygdala and caudate nucleus are also associated with Alzheimer's disease; our data do not confirm this, though. The volumetric data show that the amygdala is smaller in the probable Alzheimer's disease group compared to the MCs, but this reduction in size was not significant when controlled for age, gender and ICV. We applied a rather strict analysis to assess significance between MCs and probable Alzheimer's disease subjects, therefore possible shrinkage of the amygdala could have lost its significance. Another explanation might be that amygdala is affected later in the disease process of Alzheimer's disease than the hippocampus, the putamen and thalamus, and therefore not prominent in MR imaging of patients that attended the memory clinic for the first time. The caudate nucleus was also not significantly smaller in MCs than in probable Alzheimer's disease patients, as were the nucleus accumbens, pallidum and white matter volumes. None of these volumes showed a significant correlation with any of the cognitive test scores except for the nucleus accumbens which was significantly correlated to the CAMCOG-R score in MCs, but not in probable Alzheimer's disease subjects. Our study did not show a significant reduction in size of the nucleus accumbens in probable Alzheimer's disease subjects compared to MCs, but found it strongly correlated to the neocortical grey matter volume. A possible explanation for this relation to neocortical grey matter volume and cognitive decline in MCs, is that the nucleus accumbens is affected in old age, but not specifically in Alzheimer's disease. Another explanation is that the structure is just too small, relative to the other deep grey matter structures, to obtain sensitive data with our method.
One limitation of our study was that the MCs could not be regarded as a healthy control group. Several studies have already revealed the presence of smaller left hippocampal volumes and decreased grey matter volumes among MCs, suggesting that this condition for some subjects forms a pre-cursor condition to Alzheimer's disease (van der Flier et al., 2004; Saykin et al., 2006). However the MCs form a relevant control group, since if Alzheimer's disease pathology is present; it is in a very early stage and very likely to be less pronounced than in clinical stages of Alzheimer's disease.
Another limitation of our study could be the relative novelty of the software adopted from FSL. However, we could not detect any significant mismatches in segmentation of the subcortical structures performed by the algorithm (Figs 1 and 2 are a 2D-represenations of segmented subcortical structures by FIRST). Furthermore, the method of semi-automatic volumetry has clear advantages compared to voxel based morphometry and manual segmentation. Voxel based morphometry has shown its value in comparing groups of subjects in patterns of atrophy, but is prone to registration artifacts in the deep grey matter and not suitable for analysis of the pattern of atrophy in an individual subject (Bookstein, 2001; Frisoni and Whitwell, 2008). The method of semi-automatic volumetry can be used to measure volumes of different regions in individual subjects and gives an indication of the actual amount of atrophy that occurs. Therefore, it is more patient specific and can be used as marker for disease progression on an individual level. Compared to manual segmentation, the method used has the advantage that its segmentation is based on voxel intensities, while in manual segmentation the contrast differences can be difficult to detect visually. Furthermore, since the method used is automatic researcher bias in segmentation is absent.
A third limitation of our study is that because of the cross-sectional nature of the study the question whether shrinkage of the thalamus, nucleus accumbens and putamen is a primary or secondary phenomenon to hippocampal or neo cortical loss, could not be answered.
In conclusion, the present study demonstrates that besides global atrophy of the neocortex and atrophy of the medial temporal lobe, parts of the basal nuclei and thalamus are also affected in Alzheimer's disease. Furthermore, our study suggests that putaminal and thalamic atrophy play pivotal roles in cognitive decline in patients with Alzheimer's disease.
Glossary
Abbreviations:
- CAMCOG-R
Cambridge Cognitive Examination-Revised
- FSL
FMRIB's Software Library
- FIRST
FMRIB's Integrated Registration and Segmentation Tool
- ICV
intra cranial volume
- MC
memory complainer
- MMSE
Mini Mental State Examination
References
- Almeida OP, Burton EJ, McKeith I, Gholkar A, Burn D, O’Brien JT. MRI study of caudate nucleus volume in Parkinson's disease with and without dementia with Lewy bodies and Alzheimer's disease. Dement Geriatr Cogn Disord. 2003;16:57–63. doi: 10.1159/000070676. [DOI] [PubMed] [Google Scholar]
- Barber R, McKeith I, Ballard C, O’Brien J. Volumetric MRI study of the caudate nucleus in patients with dementia with Lewy bodies, Alzheimer's disease, and vascular dementia. J Neurol Neurosurg Psychiatry. 2002;72:406–7. doi: 10.1136/jnnp.72.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellebaum C, Koch B, Schwarz M, Daum I. Focal basal ganglia lesions are associated with impairments in reward-based reversal learning. Brain. 2008;131:829–41. doi: 10.1093/brain/awn011. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. ‘Voxel-based morphometry’ should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–62. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Borroni B, Turla M, Bertasi V, Agosti C, Gilberti N, Padovani A. Cognitive and behavioral assessment in the early stages of neurodegenerative extrapyramidal syndromes. Arch Gerontol Geriatr. 2007;47:53–61. doi: 10.1016/j.archger.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Alzheimer's disease: striatal amyloid deposits and neurofibrillary changes. J. Neuropathol Exp Neurol. 1990;49:215–24. [PubMed] [Google Scholar]
- Braak H, Braak E. Alzheimer's disease affects limbic nuclei of the thalamus. Acta Neuropathol. 1991;81:261–8. doi: 10.1007/BF00305867. [DOI] [PubMed] [Google Scholar]
- Callen DJ, Black SE, Gao F, Caldwell CB, Szalai JP. Beyond the hippocampus: MRI volumetry confirms widespread limbic atrophy in AD. Neurology. 2001;57:1669–74. doi: 10.1212/wnl.57.9.1669. [DOI] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Backman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–12. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, DeSanti S, Golomb J, Tarshish C, Rusinek H, et al. The hippocampus in aging and Alzheimer's disease. Neuroimaging Clin N Am. 1995;5:1–17. [PubMed] [Google Scholar]
- de Leon MJ, Golomb J, Convit A, DeSanti S, McRae TD, George AE. Measurement of medial temporal lobe atrophy in diagnosis of Alzheimer's disease. Lancet. 1993;341:125–6. doi: 10.1016/0140-6736(93)92610-6. [DOI] [PubMed] [Google Scholar]
- Emre M. What causes mental dysfunction in Parkinson's disease? Mov Disord. 2003;18(Suppl 6):S63–71. doi: 10.1002/mds.10565. [DOI] [PubMed] [Google Scholar]
- Ferrarini L, Palm WM, Olofsen H, van Buchem MA, Reiber JH, Admiraal-Behloul F. Shape differences of the brain ventricles in Alzheimer's disease. NeuroImage. 2006;32:1060–9. doi: 10.1016/j.neuroimage.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Whitwell JL. How fast will it go, doc? New tools for an old question from patients with Alzheimer disease. Neurology. 2008;70:2194–5. doi: 10.1212/01.wnl.0000313844.18381.a9. [DOI] [PubMed] [Google Scholar]
- Geng DY, Li YX, Zee CS. Magnetic resonance imaging-based volumetric analysis of basal ganglia nuclei and substantia nigra in patients with Parkinson's disease. Neurosurgery. 2006;58:256–62. doi: 10.1227/01.NEU.0000194845.19462.7B. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–44. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Horinek D, Varjassyova A, Hort J. Magnetic resonance analysis of amygdalar volume in Alzheimer's disease. Curr Opin Psychiatry. 2007;20:273–7. doi: 10.1097/YCO.0b013e3280ebb613. [DOI] [PubMed] [Google Scholar]
- Houtchens MK, Benedict RH, Killiany R, Sharma J, Jaisani Z, Singh B, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007;69:1213–23. doi: 10.1212/01.wnl.0000276992.17011.b5. [DOI] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas G, Scheltens P, Rombouts S, van Schijndel R, Klein M, Jones B, et al. Precuneus atrophy in early-onset Alzheimer's disease: a morphometric structural MRI study. Neuroradiology. 2007;49:967–76. doi: 10.1007/s00234-007-0269-2. [DOI] [PubMed] [Google Scholar]
- Karas GB, Scheltens P, Rombouts SA, Visser PJ, van Schijndel RA, Fox NC, et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2004;23:708–16. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kassubek J, Juengling FD, Ecker D, Landwehrmeyer GB. Thalamic atrophy in Huntington's disease co-varies with cognitive performance: a morphometric MRI analysis. Cereb Cortex. 2005;15:846–53. doi: 10.1093/cercor/bhh185. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Price JC, Mathis CA, Tsopelas ND, Lopresti BJ, Ziolko SK, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–84. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso MP, Soininen H, Partanen K, Hallikainen M, Lehtovirta M, Hänninen T, et al. Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer's disease: correlation with memory functions. J Neural Transm Park Dis Dement Sect. 1995;9:73–86. doi: 10.1007/BF02252964. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Newman J. Thalamic contributions to attention and consciousness. Conscious Cogn. 1995;4:172–93. doi: 10.1006/ccog.1995.1024. [DOI] [PubMed] [Google Scholar]
- Patenaude B. FMRIB Technical Report TR07BP1. Oxford: 2007. Bayesian statistical models of shape and appearance for subcortical brain segmentation. pp. 1–23. [Google Scholar]
- Patenaude B, Smith S, Kennedy D, Jenkinson M. FMRIB Technical Report TR07BP1. Oxford: 2007. Bayesian Shape and Appearance Models. pp. 1–23. [Google Scholar]
- Roth M, Huppert FA, Mountjoy CQ, Tym E. Cambridge: Cambridge University Press; 1999. The Cambridge Examination for Mental Disorders of the Elderly—Revised. [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–42. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, et al. Atrophy of medial temporal lobes on MRI in ‘probable’ Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–72. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeith JI, Yesavage JA. Clinical Gerontology: a Guide to Assessment and Intervention edition. New York: The Haworth Press; 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version; p. 165. [Google Scholar]
- Smith AD. Imaging the progression of Alzheimer pathology through the brain. Proc Natl Acad Sci USA. 2002;99:4135–7. doi: 10.1073/pnas.082107399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Jicha GA, Cooper G, et al. Brain structural alterations before mild cognitive impairment. Neurology. 2007;68:1268–73. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust and automated longitudinal and cross-sectional brain change analysis. NeuroImage. 2002;17:479–89. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- van der Flier WM, van Buchem MA, Weverling-Rijnsburger AW, Mutsaers ER, Bollen EL, Admiraal-Behloul F, et al. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol. 2004;251:671–5. doi: 10.1007/s00415-004-0390-7. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Uylings HB, Jolles J. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia. 2000;38:613–27. doi: 10.1016/s0028-3932(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Wolf H, Julin P, Gertz HJ, Winblad B, Wahlund LO. Intracranial volume in mild cognitive impairment, Alzheimer's disease and vascular dementia: evidence for brain reserve? Int J Geriatr Psychiatry. 2004;19:995–1007. doi: 10.1002/gps.1205. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans Med Imag. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]