Abstract
Cytolytic enzymes (CEs) are critical mediators of anti-viral and -tumor immunity; however, as a number of molecules belong to this enzyme family, our understanding of CEs remains limited. Specifically, it remains unclear what combinations of granzymes and perforin (Perf) are expressed by various immune cells and how CE content relates to cellular differentiation. Using polychromatic flow cytometry, we simultaneously measured expression of the most common human CEs [granzyme A (gA), granzyme B (gB), and Perf] alongside markers of αβ and γδ T cell maturation (CD45RO, CCR7, CD27, CD57). Additionally, we measured CE content in NK cell subsets (defined by their expression of CD16 and CD56). We found that among a wide variety of immune cells, CE content was linked to cellular maturity. Moreover, common expression patterns were shared across cell types, such that gB+ cells always contained gA, and Perf+ cells were primarily gA+ gB+. Most importantly, CD57 expression correlated strongly with simultaneous expression of gA, gB, and Perf. Thus, the use of CD57 provides a means to easily isolate viable cells with high cytolytic potential, without the need for lethal fixation/permeabilization techniques.
Keywords: cytotoxicity, differentiation, CTL, NK cells
INTRODUCTION
In human and murine immune systems, cytotoxic cells play a critical role in the elimination of infected, transformed, or foreign cells. A wide variety of cells, arising from different lineages, possesses cytotoxic ability, which is mediated by two possible pathways. One pathway involves the engagement of death receptors (such as the TNF-αR or CD95) [1] and is likely to be important in autoimmune conditions [2, 3], hepatitis [4], and graft-versus-host disease [5]. The other pathway, which is critical for tumor surveillance and the killing of cells infected with intracellular pathogens, involves the release of a variety of cytolytic enzymes (CEs) [5].
Among CEs, a number of granule-associated proteases (or granzymes) have been recognized. The most abundant among these are granzymes A (gA) and B (gB), which induce target cell apoptosis through caspase-dependent and -independent pathways [6,7,8,9]. Target cell death is induced most efficiently when these granzymes are released in the presence of the delivery molecule perforin (Perf) [10,11,12]; however, the function of Perf has been subject to debate. Although early studies proposed that Perf facilitated granzyme delivery by inducing membrane pores on the cell surface [13], recent work has suggested that membrane permeabilization is not required [14] or that the size of Perf pores on the cell surface is too small to allow successful entry of large proteins such as granzymes [15]. Moreover, as the physiologically relevant form of gB may exist as a much larger macromolecular complex linked to proteoglycans and/or Perf [14], it is highly unlikely that CE entry occurs exclusively through cell-surface pores. Regardless of its mechanism of action, the functional importance of Perf is clear: Perf-deficient mice are highly susceptible to viral infection [16, 17], spontaneous tumors [18,19,20], and metastatic cancer [18,19,20]. Thus, efficient cytolytic activity requires the cooperative function of Perf and granzymes.
In contrast, some studies of gA- or gB-deficient mice suggest considerable redundancy in granzyme function, as both strains of mice are equally susceptible to pox virus infection [21,22,23,24,25]. However, there is also evidence that the functions of gA and gB (and others) are distinct. Cytotoxic cells from gB-deficient mice induce apoptosis more slowly than wild-type [26], cells from gA-deficient animals induce apoptosis normally [27], and distinct cell-death pathways appear to be targeted by gA, gB, and gC [5]. Thus, effective immunity to a wide array of pathogens may require the cooperative action of multiple granzymes.
To clarify how the various granzymes and Perf work together and how expression of these molecules is regulated, multiparameter studies are required. Using PCR- and microarray-based technology, the expression of gA and Perf mRNA has been studied in bulk cell populations after in vitro and in vivo cell activation [28]. In these studies, kinetics and relative levels of expression differed by activation system or cell type, suggesting differential regulation of Perf and granzymes and the existence of specialized CTL subsets [29]. A recent single-cell study of CTL mRNA confirmed that individual cells can express various combinations of CEs [30]; however, only activated, naïve cells were studied, and the mRNA-based approach could not measure the substantial levels of preformed gA and Perf proteins found in CTL. Thus, further work is required in single-cell, multiparameter systems. Polychromatic flow cytometry systems are ideally suited for such studies, as demonstrated recently by Takata and Takiguchi [31], who described CE expression patterns within subsets of CD8+ T cells. These authors found that CEs are expressed in an ordered manner, from cells completely lacking expression of CEs to cells expressing all three CEs [31]. Additionally, cells capable of immediate cytolytic activity could be defined by their expression of the three CEs or alternatively, by a particular combination of five cell-surface molecules (i.e., CD45RA+/− CCR7− CD27− CD28− CCR5low/–) [31].
Using similar technology but different cell-surface markers, we measured the expression of gA, gB, and Perf simultaneously and compared expression patterns across various cell types, differentiation stages, and specificities. We found that all types of T cells (γδ+, αβ+, and CD4+) and NK cells shared the characteristic pattern of CE expression (first described by Takata and Takiguchi [31] for CD8+ T cells). This suggests that the pathways responsible for regulating CE expression are conserved across a wide variety of phenotypically and functionally distinct cells. Moreover, CE expression patterns are related to cellular maturity in all cell types, not only CD8+ T cells. Finally, we found that cells simultaneously expressing gA, gB, and high levels of Perf could be identified simply on the basis of their expression of high levels of CD57.
MATERIALS AND METHODS
Subjects
PBMC samples were obtained from healthy or HIV-infected adult subjects enrolled in various studies at the National Institutes of Health (NIH; Bethesda, MD, USA) or the Fred Hutchinson Cancer Research Center/University of Washington (Seattle, WA, USA). The HIV-infected individuals were randomly selected and had variable CD4 T cell counts, HIV viral loads, and antiretroviral treatment histories. The appropriate Institutional Review Boards approved the studies, and volunteers provided written consent.
Cells and antibodies
After Hypaque-Ficoll (Pharmacia/Amersham, Piscataway, NJ, USA) density gradient centrifugation, cells were analyzed fresh or cryopreserved at −140°C in 90% FCS and 10% DMSO. Cryopreserved cells were thawed and rested for 6 h prior to staining with antibody panels. In all staining panels, the following antibodies to CEs were used (obtained from BD PharMingen, San Jose, CA, USA, unless otherwise noted): anti-gA-FITC or -PE, anti-gB-allophycocyanin (APC; Caltag/Invitrogen, Carlsbad, CA, USA), and anti-Perf Alexa 680 (conjugated in-house). For T cell panels, cells were stained with the following antibodies: CD3 Cy7-APC, CD27-PE, CD45RO-energy coupled dye (ECD) (Beckman Coulter, Miami, FL, USA), CD11a Cy7-PE (conjugated in-house), CD8 quantum dot (Qdot) 585 (conjugated in-house), CD4 Qdot 545 (conjugated in-house), CD57 Qdot 800 (conjugated in-house), and CCR7 Pacific Blue (conjugated in-house). For studies of NK cells, samples were stained with antibodies to: CD32-FITC, CD16 Cy5-PE, CD4 Cy5.5-PE (conjugated in-house), CD8 Cy7-PE (conjugated in-house), CD3 Cy7-APC, CD57 Cascade Blue (conjugated in-house), CD56 biotin (followed by second-step staining with streptavidin-Qdot 705). For studies of γδ T cells, samples were stained with antibodies to: TCR Vδ1-FITC (Endogen/Pierce, Rockford, IL, USA), CD45RO-ECD (Beckman Coulter), TCR Vδ2 (conjugated in-house), CD8 Cy5.5-PE (conjugated in-house), CD4 Cy7-PE (conjugated in-house), CD3 Cy7-APC, CD27 Qdot 605 (conjugated in-house), and CD57 Cascade Blue. Quantum dots were obtained from Quantum Dot Corp. (Hayward, CA, USA) but are now supplied by Invitrogen (Eugene, OR, USA).
CEs in antigen-specific cells
Samples from healthy and HIV+ donors expressing HLA-A2 or -A3 were selected for analysis based on their known IFN-γ response to various peptides in a stimulation assay. Peptide MHC class I (pMHCI) multimers were made from CMV phosphorylated p65 residues 495–503 (NLVPMVATTV), EBV BMLF1 residues 259–267 (GLCTLVAML), and HIV Gag RK9 epitope (RLRPGGKKK) using various Qdot fluorochromes. Cells were stained with the tetramer first (at 37ºC for 10 min) and then with the T cell panel described above for an additional 10 min.
Flow cytometry and analysis
Data were collected on our 20-parameter LSRII flow cytometer (BD Biosciences, San Diego, CA, USA; configuration described previously [32]) and then analyzed using FlowJo (Treestar, Ashland, OR, USA) and the Pestle and Spice software suite (M. Roederer).
Statistical analysis
Graphs were prepared using JMP software (SAS Institute, Cary, NC, USA). The outlier box-plot shown on each graph summarizes the distribution of the data. The ends of the box are the 25th and 75th quantiles. The difference between the quartiles is the interquartile range (IQR). The line across the middle of the box identifies the median. The whiskers extend from the ends of the box to the outermost data point that falls within the distances computed as upper quartile +1.5*(IQR) and lower quartile –1.5*(IQR). For some low-frequency cellular subsets, the number of cells present was too low for accuracy; data are omitted in such cases, and this is noted in the figure legend.
RESULTS
Common characteristic pattern of CE expression
Using freshly isolated PBMC, we examined the CE content of various cell types ex vivo. As expected, most expression of CEs was observed within the CD8+ T cell compartment. Within CD8+ T cells, we could clearly distinguish cells expressing gA or gB, as well as those expressing high versus low levels of Perf (Fig. 1A). The content of Perf correlated with expression of CD57 (a marker of terminally differentiated cells); cells expressing high levels of Perf were uniformly bright for CD57 (Fig. 1A), suggesting a relationship between cell differentiation and CE content.
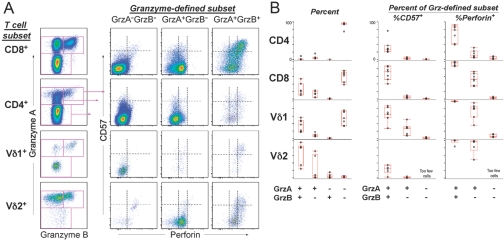
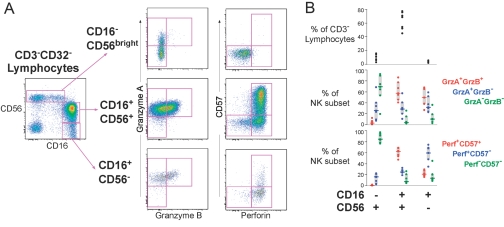
Fig. 1.
Patterns of CE and CD57 expression within various cell populations from healthy donors. (A) gA, gB, Perf, and CD57 expression was compared across CD4+ and CD8+ αβ T cells and γδ T cells expressing vδ1 or vδ2 TCR genes. The left plots show expression of gA and gB, and the three columns of plots on the right show the expression of Perf and CD57 for the granzyme-defined subset. The dotted lines separate no expression, low expression, and high expression of Perf or CD57. CE expression patterns were remarkably consistent across these diverse cell populations. (B) The frequency of cells expressing various combinations of CEs is shown for seven healthy donors. The left plots show the percent of the indicated T cell subset expressing one or both granzymes. The plots on the right show the percent of the granzyme-defined subset expressing CD57 or Perf (low or high expression).
We next compared the CE content of CD8+ (αβ) T cells with CD4+ (αβ) T cells and the two major γδ T cell subsets (which express the vδ1 or vδ2 genes). Across these various T cell types, there was a characteristic pattern for CE expression. Cells that did not express either granzyme (gA– or gB–, Fig. 1A) rarely expressed Perf and generally expressed little or no CD57. Cells frequently expressed gA alone; however, gB was never expressed without gA (Fig. 1A). Many gA+ gB– cells expressed low levels of Perf (Perfdim) and did not express CD57; however, a small population of CD4+ gA+ gB– CD57dim cells was unique for their complete absence of Perf expression. In all T cell subsets, gA+ gB+ populations also contained Perfdim CD57– cells, along with a high proportion of Perfbright CD57+ cells (Fig. 1A). Thus, although the frequency of each CE phenotype differed by T cell type, some common “rules of expression” emerged: First, gA could be expressed alone, but gB expression was always associated with gA. Second, gA+ gB– cells could only express low levels of Perf, as high Perf content required expression of gA and gB. Third, the level of Perf was closely related to the expression of CD57. These patterns of expression were remarkably consistent among various donors and across different cell types (Fig. 1B), except in rare instances. For example, in one of the donors we studied, the small population of Vδ2+ gA– gB– cells detected contained some Perf+ cells. Given the paucity of these cells in this individual and the absence of these cells in most of the donors we studied (Fig. 1B), the significance of this population is likely minimal.
CE content relates to cellular differentiation
To investigate the relationship between T cell differentiation and CE content, we used 10-color flow cytometry to measure CEs simultaneously alongside a number of markers of CD8+ T cell maturity. Figure 2 shows the pattern of CE expression within naïve, central memory, memory, and effector cell populations, as defined by expression of CD45RO and CCR7. In CD8+ T cell populations identified as naïve by their CD45RO– CCR7+ phenotype, the vast majority of cells did not express CEs (Fig. 2). Those CD45RO– CCR7+ cells that expressed CEs did not express CD27 (Fig. 2), suggesting that these cells are likely to be memory cells contaminating the naïve CD45RO– CCR7+ gate. When naïve cells were identified more stringently, according to their CD45RO– CCR7+ CD27+ phenotype, data from four healthy individuals showed that most naïve cells lacked CEs (Fig. 3A).
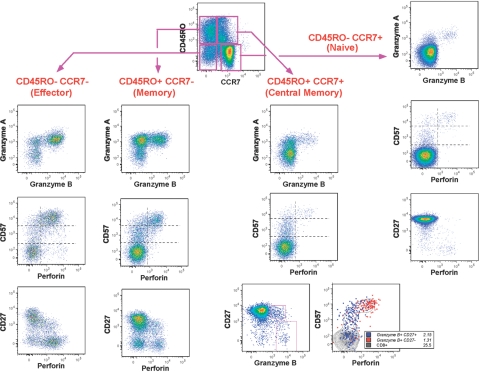
Fig. 2.
CE content relates to cellular differentiation. Patterns of CE expression are shown for various CD8+ T cell subsets from a representative donor. The top left graph shows the expression of CCR7 and CD45RO on CD8+ T cells, defining naïve cells, effector cells, and two memory subsets. For each cell type except central memory, three graphs show expression of gB versus gA (top graphs in column groups of three), Perf versus CD57 (middle graphs in column groups of three), and Perf versus CD27 (bottom graphs in column groups of three). CEs were rarely expressed within cells from the “naïve” CD45RO– CCR7+ population. Non-naïve subsets were heterogeneous for CE expression. Central memory cells had comparatively smaller gA+ gB+ populations than effector cells but had more gA+ gB– cells. Effector cells were mostly gA+ gB+ Perf+. For central memory cells, the bottom two graphs show gB versus CD27 on the left and Perf versus CD57 on the right, where two gB/CD27-defined subsets are shown as overlay on total CD8+ T cells. The few cells that coexpress Perf and CD57 are those cells that do not express CD27 (shown in red). These are likely not “true” central memory cells.
Fig. 3.
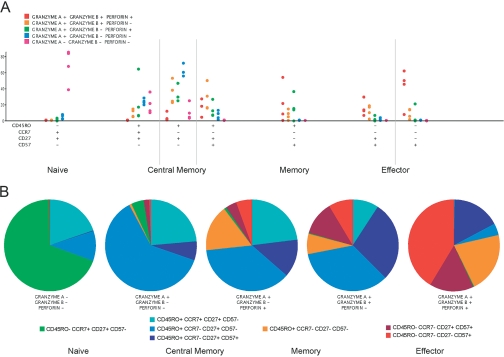
Frequency of CE-expressing cells within various CD8+ T cell subsets, across four healthy donors. (A) Using 10-color flow cytometry, cell subsets were identified according to their expression of CD45RO, CCR7, CD27, and CD57 and roughly split into naïve, central memory, memory, and effector cell populations, as shown along the x-axis. The frequency of cells within each naïve or memory/effector subset expressing a variety of CE combinations is shown for each cell subset. These data are shown from left to right and are color-coded according to the top left key. Within naïve cells, most cells lacked expression of gA, gB, and Perf. Within effector cell populations, most cells expressed gA, gB, and Perf. (B) Data are displayed by CE subset with one pie chart for each subset. The pie slices show the median frequency of cells within the CE subset expressing each combination of naïve/memory-defining markers, as shown in the bottom keys. Cool colors are used for naïve and central memory, and warm colors are used for memory and effector. The cells not expressing CEs clearly consist of mostly naïve and central memory cells, and the gA+ gB+ Perf+ populations are largely memory and effector cells.
Central memory T cells, as defined by their expression of CD45RO with CCR7 or CD27, were heterogeneous in their expression of CEs (Figs. 2 and 3A). Among central memory cells expressing all three markers (CD45RO+ CCR7+ CD27+), ∼20% did not express a CE. The remainder of these cells expressed gA, with or without Perf, but only rarely was gB or CD57 expressed (Fig. 3A). Thus, cells expressing all three CEs simultaneously were not observed in this subset. In central memory subsets not expressing CCR7 (CD45RO+ CCR7– CD27+ CD57–, Fig. 3A), gB+ cells were more frequent than in CCR7+ central memory cells, and gA+ gB+ Perf+ cells were detectable. Once a high level of CD57 expression was acquired (CD45RO+ CCR7– CD27+ CD57bright, Fig. 3A), the proportion of cells expressing gA alone was reduced, and the proportion of cells expressing all three CEs was increased compared with other central memory-like cells. Thus, among cells that would typically be defined as central memory T cells, there appeared to be an ordered progression of CE expression from CCR7+ cells, which lacked CE expression or expressed only gA, to CCR7– CD57bright central memory cells that frequently expressed gB and Perf. Whether the latter are more closely related to central memory or effector T cells remains to be determined.
Other memory cell populations frequently expressed gB and Perf (Fig. 3A), and such cells were typically CD57bright. The CE content of CD45RO+ memory cells was different than central memory-like T cells, as the latter contained fewer cells expressing all three CEs and more cells expressing gA and gB without Perf. Notably, when these cells were CD57+, they were mostly (if not all) CD57dim. Within effector cell populations (CD45RO– CCR7– CD57+, Fig. 3A), there were two distinct phenotypes, which differed by their expression of CD27 and levels of gA+ gB+ Perf+ cells. Effector cells lacking CD27 contained many more cells expressing all three CEs than CD27+ effector cell populations. The latter typically expressed CD27 dimly; this rarely described cell population is found to be elevated after vaccinia vaccination and thus, may have some unique antiviral activity [33]. Again, regardless of CD27 expression, Perfbright cells were typically CD57bright, and Perfdim and Perf– cells were CD57dim or CD57–.
When the data are displayed by CE phenotype (rather than by maturation marker), the relationship between CE expression and cellular differentiation becomes more apparent (Fig. 3B). The population lacking CEs (gA– gB– Perf–) consists mostly of naïve cells and to a lesser extent, central memory cells that do not express CD57. Cells expressing only gA are nearly exclusively CD57– central memory-like T cells. In contrast, cells expressing gA, along with gB or Perf, include memory and effector cells among a large proportion of central memory cells (many of which express CD57). In cell populations where all CEs are expressed, the proportion of effector and memory cells is much higher, and nearly all cells express CD57.
In summary, within CD8+ T cell subsets, expression of CEs relates to the expression of markers of cellular differentiation: Less differentiated, naïve T cells express no CEs, and nearly all highly differentiated effector T cells (which are largely terminally differentiated CD57bright cells) express the three CEs simultaneously (Fig. 3B). These results suggest a link between CE content and cell differentiation in CD8+ T cells.
Similar results were observed in CD4+ T cell populations, although the proportion of CE-expressing cells was much lower (Fig. 4). Naïve CD4+ T cells did not express gA, gB, or CD57, and a small proportion of central memory CD4+ T cells expressed low levels of gA (without expression of gB or CD57, Fig. 4). Effector memory cells were the most likely to express gA or gB, and effector memory cells expressing a CE generally expressed gA and gB along with CD57 (Fig. 4). Further analysis showed that when these cells were CD57bright, they also expressed high levels of Perf (data not shown). Thus, although most CD4+ T cells do not exhibit the potential for cytolytic activity, a small population of CE-expressing CD4+ T cells exists, follows the common rules for CE expression, and consists mostly of differentiated memory cells. Notably, CD4+ Perfbright CD57bright cells are not found within regulatory (forkhead box P3+ CD25+ CD127–) T cells or TH17 populations (as measured after Staphylococcus aureus enterotoxin B stimulation and detection of linear forms of Perf).
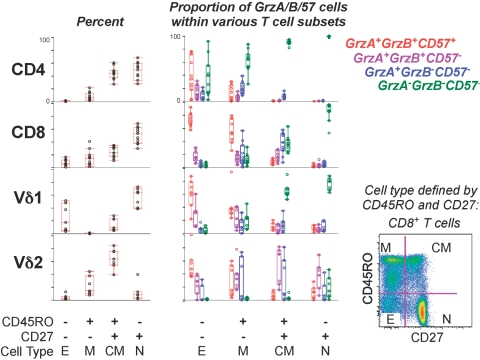
Fig. 4.
Frequency of CE-expressing cells within CD4+ αβ, CD8+ αβ, and γδ T cell subsets in 10 healthy donors. Cell subsets were defined by expression of CD45RO and CD27 as shown in the bottom right graph; however, results were similar to those shown for the more finely defined CD8+ T cell subsets (i.e., including CCR7 to define subsets) in Figure 3. The left plots show the percent of the indicated T cell subset expressing or not expressing CD45RO and/or CD27. The middle plots show the proportion of each naïve/memory subset that is expressing the CE markers as shown in the right legend. Naïve (N) cells typically lacked CE expression, and effector cells (E) commonly expressed gA, gB, and CD57. CM, Central memory; M, other memory.
The expression of CEs in γδ+ T cells is consistent with that observed for αβ+ T cells. As we (and others) have shown previously [34], the majority of γδ+ T cells in the blood uses the vδ1 or vδ2 TCR genes, and these subsets differ in naïve and memory cell composition. Vδ1 T cell populations mirror αβ T cell populations with respect to naïve cell representation [34], although these populations contain a smaller proportion of memory (CD45RO+ CD27–) and a greater proportion of effector (CD45RO– CD27–) cells than αβ T cells (Fig. 4). Consistent with this, the Vδ1+ population consists of gA– gB– naïve (CD45RO– CD27+) cells or gA+ gB+ effector cells, which also express Perf (Figs. 1 and 4). In contrast, most Vδ2 cells are central memory (CD45RO+ CD27+) or memory cells (Fig. 4) and exhibit the diverse CE content (Figs. 1 and 4) that is characteristic of central memory and memory CD8+ αβ+ T cells (Figs. 2 and 3). In summary, the two major γδ T cell subsets found in adult peripheral blood, which express distinct differentiation markers, differ in CE content in a manner analogous to that observed across naïve and memory αβ+ T cell compartments.
Within NK cells, we observed a similar relationship between CE content and cell differentiation. NK cells expressing high levels of CD56 without CD16 (CD16–CD56bright) have been described previously as immature, based on their low ex vivo cytotoxicity [35] and their ability to differentiate into highly cytolytic CD16+ CD56+ cells upon exposure to IL-2 [35]. The majority of these cells expressed gA but not gB or Perf (Fig. 5). In contrast, NK cells expressing CD16 (CD16+ CD56+ or CD16+ CD56–) generally expressed all three CEs. Interestingly, the vast majority of Perf+ cells was CD57+ (Fig. 5), suggesting a common link between CD57 expression and CE content that is shared by NK and T cells (Fig. 5). These data also suggest that CD57 expression correlates with NK differentiation in a manner similar to that reported for T cells.
Fig. 5.
CE expression within NK cell subsets. (A) The three NK cell subsets defined by their expression of CD16 and CD56 are shown in three rows, and the expression of gB versus gA and Perf versus CD57 is shown for each. The NK subsets expressed varying levels and combinations of CEs. (B) The top plot shows the frequency of each NK subset for seven healthy donors. The middle and bottom plots show the proportion of each NK subset expressing the CEs indicated in the keys.
CE expression by antigen specificity
Previous studies have used peptide stimulation and intracellular cytokine staining to investigate CE content within antigen-specific T cells [36]; however, this technique is highly dependent on the marker used to identify the response (e.g., not all antigen-responsive cells express IFN-γ). Moreover, in preliminary experiments, we found that peptide stimulation could also induce partial degranulation of cytotoxic cells (data not shown). Therefore, we measured CE content in HLA-A2+ individuals (HIV+ and HIV–) for whom detectable levels of CMV-, EBV-, and HIV-specific T cells could be measured ex vivo using pMHCI multimers.
Although CMV- and EBV-specific populations followed the characteristic patterns of CE expression (Fig. 6), these populations differed in the proportion of CE+ cells and diversity of CE content, suggesting that maturation of CMV- and EBV-specific T cells is not similar. Within CMV-specific cells, the vast majority of cells expressed all three CEs (Fig. 6, A and B); however, more gA+ gB– cells were observed in the EBV-specific population. These differences in CE content are consistent with the maturation phenotype observed for CMV- and EBV-specific cells, with the latter containing mostly central memory-like cells and few effector/effector memory-type cells. (Note that in other experiments, we confirmed that CD45RO– CD27+ cells in EBV- and CMV-specific T cell populations were not naïve cells but rather, a diverse memory cell population that expressed varying levels of CCR7, CD57, CD127, and programmed death 1.)
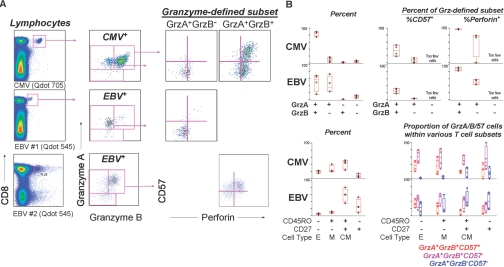
Fig. 6.
CE expression by antigen specificity. (A) Cells were stained with pMHCI multimers to detect T cells specific for CMV and EBV. The top and middle rows of graphs show data for one individual, and the bottom row shows data for another individual. The right graphs show the expression of Perf versus CD57 for the granzyme-defined subsets shown in the middle column of graphs. (B) CMV- and EBV-specific T cells followed the characteristic patterns of CE expression described for overall CD8+ T cell populations; however, these populations differed in CE content. The upper left graphs shows the proportion of CMV- and EBV-specific cells expressing the CEs shown on the x-axis. The upper right graphs show the proportion of the gA-expressing (Grz-defined) subsets that express CD57 or Perf. The gA– gB– CD57– subsets are not shown as a result of low frequency. The lower left graphs show the proportion of CMV- and EBV-specific cells expressing the memory markers shown on the x-axis. The lower right graphs show the proportion of the naïve/memory subsets that express the CEs as shown in the key. These differences could be accounted for by differences in maturation of CMV- and EBV-specific cells. CMV-specific cell populations, which contained more CD45RO– CD27– effector memory cells, also had higher levels of gA+ gB+ CD57+ cells (red bars) than EBV-specific T cells.
Among HIV RK9-specific T cells, CE expression followed the same patterns observed for other types of cells (Fig. 7A). When CE content in RK9-specific T cells was compared with the overall CD8+ T cell population, the proportions of granzyme-expressing cells were similar (Fig. 6B). However, despite a fairly large number of gA+ gB+ cells with the RK9-specific T cell population (Fig. 7A), the proportion of Perf-expressing cells among gA+ gB+ RK9-specific T cells was lower than within the overall CD8+ T cell population (Fig. 7B). These differences were not statistically significant, in part as a result of our selection of HIV-infected individuals with a variety of CD4+ T cell counts and HIV viral loads; however, the result suggests that HIV infection may be accompanied by antigen-specific deficits in Perf production.
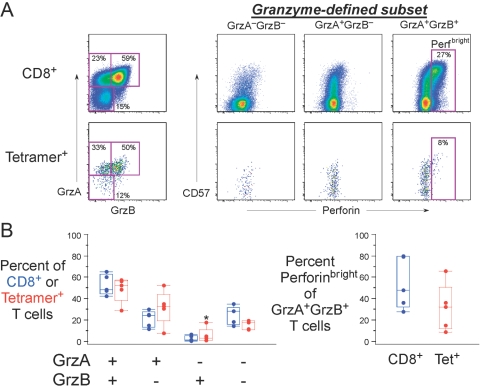
Fig. 7.
HIV-specific T cells have variable expression of granzymes and Perf. (A) Representative data from a HIV-infected subject with responses to the A03-restricted RK9 peptide. Upper graphs show overall CD8+ T cells, and lower graphs show RK9 pMHCI multimer+ cells. The left graphs show the expression of the granzymes, and the right graphs show the expression of Perf versus CD57 for the granzyme-defined subsets. The gate identifies bright Perf-staining cells. RK9-specific CD8+ T cells were typically gA+ or gB+ but rarely expressed Perf. (B) CE expression within overall CD8+ T cells (blue) or RK9-specific T cells (red) is shown for five HIV+ individuals with varying CD4+ T cell counts and viral load. The right graph shows the proportion of the gA/gB-coexpressing cells that expresses bright levels of Perf. Although trends were not statistically significant, it appeared that the RK9-specific CD8+ T cell population was enriched for gA+ gB+ cells but had lower levels of Perfbright cells than the overall CD8+ T cell population. Note that there is one individual (*) who has a relatively high proportion of RK9-specific cells expressing gB without gA. In all other individuals examined in this study, this subset was absent or present at very low frequency.
DISCUSSION
Cells involved in adaptive and innate immune responses contain a variety of CEs; however, little is known about the expression patterns of these molecules. Here, we demonstrate that CEs are expressed in a characteristic manner, which is common to a wide variety of cell types. Across T and non-T cell populations, we observed that gB was never expressed without gA and that high levels of Perf were found primarily in cells containing gA and gB.
As the cells we studied (i.e., αβ T cells, γδ T cells, and NK cells) use different receptors to recognize foreign antigens, our results suggest that expression of cytotoxic enzymes is regulated by cell signaling molecules downstream from early activation signals. Transcription factors such as eomesodermin (EOMES) and T-box expressed in T cells (T-bet), which are known to induce granzyme expression [37, 38] and are found across T and non-T cell populations [39], may govern CE expression patterns. Indeed, ectopic expression of EOMES can induce expression of Perf and gB by cells that normally lack cytotoxic activity (such as TH2 CD4+ cells) [38]. Similarly, CD8+ T cells from T-bet knockout mice are much less efficient at killing peptide-loaded target cells in vitro than cells from wild-type mice [37]. Furthermore, T-bet knockout mice are more susceptible to infection by pathogens that require CD8+ T cell immunity, such as lymphocytic choriomenengitis virus [37]. Interestingly, however, some capacity for cytotoxicity remains in the absence of T-bet [40], which indicates that additional mechanisms may govern CE expression.
Although the mechanisms governing CE expression are not fully known, our data (and the work of Takata and Takiguchi [31]) suggest that they are closely related to cellular differentiation. Among CD8+ αβ T cells, naïve cells express little or no CEs compared with more differentiated cells types. CE expression within central memory cells was considerably more diverse than other cell populations, perhaps as a result of the multiple cell-surface proteins that can be used to identify these cells. When central memory cells were defined by their expression of CCR7 and CD27, these populations consisted primarily of CE-negative or gA+ cells lacking CD57 expression; however, CCR7– CD27+ central memory cells consisted of CD57– and CD57+ populations, with the latter containing more cells expressing all three CEs. These results highlight the problem with simple classification schemes for describing T cell maturity: Functional attributes, such as CE content, might differ dramatically among cells broadly grouped together as “central memory cells.” In any case, assuming that CD27 and CCR7 accurately identify central memory cells, the diversity of CE content that we observed might reflect complex differentiation pathways for the generation of these cells. Some central memory subsets, such as those expressing CCR7 without CEs, may be generated from naïve cells during an immune response, and others (such as those expressing CD57 and all three CEs or gA alone) may survive the contraction of effector cells after an immune response. Further analysis of the functional attributes of highly specific T cell subsets will be required to address this issue.
The measurement of CD57 in this setting represents an important extension of the work of Takata and Takiguchi [31]. Here, we demonstrate that high levels of Perf were uniformly accompanied by bright expression of CD57. Thus, the simultaneous expression of gA, gB, and high levels of Perf is now attributed to a subset of cells that can be measured with just one surrogate marker (CD57) rather than the combination of five markers described by the previous report [31]. For investigators limited to three- or four-color flow cytometry, this result has important implications. Moreover, the relationship between high CE content and bright CD57 expression suggests that in ex vivo PBMCs, cells with the most CEs have reduced proliferative capacity along with a history of more cell divisions [41]. In the setting of CMV infection, this may reflect chronic antigen exposure and a mechanism to prevent continual amplification of potentially damaging cytolytic cells. Indeed, we demonstrate that T cells specific for these chronic antigens were mostly gA+ gB+ Perfbright.
We also report that the relationship between cellular differentiation and CE content spans a variety of cell types from CD4+ αβ+ T cells to γδ T cells and NK cells. Within CD4+ T cells, previous reports attributed cytotoxicity to a small subset of cells [42] or cells that could only be generated in vitro [43,44,45,46,47,48,49]; however, those studies did not assess CE content. We found that of the CD4+ T cells that express CEs, many are terminal (CD57bright) effector cells expressing gA, gB, and Perf; this indicates that the relationship between cellular differentiation and CE content extends beyond classical (i.e., CD8+) cell types. Our results complement a recent report describing CMV-specific CD4+ T cells that contain CEs and upon stimulation, express three cytokines (IFN-γ, TNF-α, MIP-1β) simultaneously with CD107a (a marker of degranulation) [50]. Thus, it is likely that CE+ CD4+ T cells are capable of elucidating many functions, from cognate T cell help to direct cytotoxicity.
When we examined antigen-specific T cells, we found that CE patterns followed the rules of expression defined for other types of cells; however, CE content differed dramatically by antigen. In EBV-specific T cells, many cells expressing gA alone were found; however, the vast majority of CMV-specific T cells expressed gA, gB, and Perf. This is consistent with previous reports that CMV-specific cells are highly differentiated [32, 51] and might reflect the differing nature of latency in these two pathogens: EBV latency, unlike CMV latency, is characterized by complete clearance of antigen; therefore, some EBV-specific T cells may have rested down to a phenotype characterized by expression of gA alone. Further work will be required to determine if the expression of gA alone is a characteristic of resting memory cells that were once effectors.
We also examined, for the first time, expression of three CEs in the setting of HIV infection. Previous reports have demonstrated the importance of granule-mediated killing in the lysis of HIV-infected cells by CTL and that overall Perf expression is reduced in HIV-infected individuals [52,53,54,55], but these studies have not measured the three CEs simultaneously, and some have only measured mRNA levels (and thus, could not detect preformed CEs) [56]. Contrary to previous reports [57], we found that Perf was present in the total CD8+ T cell population, although it was largely absent in HIV-specific CD8+ T cells. This, along with our analysis of EBV- and CMV-specific cells from HIV donors, suggests that deficits in Perf content are not a common consequence of chronic infections [57] but are instead a unique characteristic of HIV-specific CD8+ T cells. In contrast, we found that granzyme content was similarly represented across HIV-specific and total CD8+ T cell populations, suggesting that the granzyme deficits reported previously for HIV-specific CD8+ T cells are, in fact, inherent to the total population of CD8+ T cells in HIV-infected individuals.
In summary, the experiments performed here set the stage for a better understanding of CE function and cytolytic activity. To date, the available studies of CE content have focused on single molecules or CD8+ αβ+ T cells, in part, because of the technical obstacles associated with simultaneous measurement of multiple CEs, cell subset markers, and differentiation antigens. By linking cell differentiation and in particular, bright expression of CD57 to CE content across a broad range of cells, this study may help provide surrogate markers for the presence of cytolytic cells in vaccine or disease settings.
Acknowledgments
The authors acknowledge the Flow Cytometry Core at the Vaccine Research Center (NIH) for its work in developing and maintaining the specialized flow cytometers used in this study. The authors are also grateful to Joanne Yu for preparing many of the antibody/fluorochrome conjugates used in this study. Finally, we thank Julie McElrath and the clinicians at the Seattle HIV Vaccine Trials Unit for providing the PBMC samples for the RK9 analyses.
References
- Trapani J A, Smyth M J. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- Van Parijs L, Abbas A K. Role of Fas-mediated cell death in the regulation of immune responses. Curr Opin Immunol. 1996;8:355–361. doi: 10.1016/s0952-7915(96)80125-7. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tanaka M, Brannan C I, Jenkins N A, Copeland N G, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Bai J, Odin J A. Apoptosis and the liver: relation to autoimmunity and related conditions. Autoimmun Rev. 2003;2:36–42. doi: 10.1016/s1568-9972(02)00125-8. [DOI] [PubMed] [Google Scholar]
- Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- Trapani J A, Jans D A, Jans P J, Smyth M J, Browne K A, Sutton V R. Efficient nuclear targeting of granzyme B and the nuclear consequences of apoptosis induced by granzyme B and perforin are caspase-dependent, but cell death is caspase-independent. J Biol Chem. 1998;273:27934–27938. doi: 10.1074/jbc.273.43.27934. [DOI] [PubMed] [Google Scholar]
- Sarin A, Williams M S, Alexander-Miller M A, Berzofsky J A, Zacharchuk C M, Henkart P A. Target cell lysis by CTL granule exocytosis is independent of ICE/Ced-3 family proteases. Immunity. 1997;6:209–215. doi: 10.1016/s1074-7613(00)80427-6. [DOI] [PubMed] [Google Scholar]
- Darmon A J, Nicholson D W, Bleackley R C. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature. 1995;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- Chiu V K, Walsh C M, Liu C C, Reed J C, Clark W R. Bcl-2 blocks degranulation but not Fas-based cell-mediated cytotoxicity. J Immunol. 1995;154:2023–2032. [PubMed] [Google Scholar]
- Nakajima H, Park H L, Henkart P A. Synergistic roles of granzymes A and B in mediating target cell death by rat basophilic leukemia mast cell tumors also expressing cytolysin/perforin. J Exp Med. 1995;181:1037–1046. doi: 10.1084/jem.181.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froelich C J, Orth K, Turbov J, Seth P, Gottlieb R, Babior B, Shah G M, Bleackley R C, Dixit V M, Hanna W. New paradigm for lymphocyte granule-mediated cytotoxicity. Target cells bind and internalize granzyme B, but an endosomolytic agent is necessary for cytosolic delivery and subsequent apoptosis. J Biol Chem. 1996;271:29073–29079. doi: 10.1074/jbc.271.46.29073. [DOI] [PubMed] [Google Scholar]
- Browne K A, Blink E, Sutton V R, Froelich C J, Jans D A, Trapani J A. Cytosolic delivery of granzyme B by bacterial toxins: evidence that endosomal disruption, in addition to transmembrane pore formation, is an important function of perforin. Mol Cell Biol. 1999;19:8604–8615. doi: 10.1128/mcb.19.12.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J D, Hengartner H, Podack E R, Cohn Z A. Purification and characterization of a cytolytic pore-forming protein from granules of cloned lymphocytes with natural killer activity. Cell. 1986;44:849–859. doi: 10.1016/0092-8674(86)90007-3. [DOI] [PubMed] [Google Scholar]
- Raja S M, Wang B, Dantuluri M, Desai U R, Demeler B, Spiegel K, Metkar S S, Froelich C J. Cytotoxic cell granule-mediated apoptosis. Characterization of the macromolecular complex of granzyme B with serglycin. J Biol Chem. 2002;277:49523–49530. doi: 10.1074/jbc.M209607200. [DOI] [PubMed] [Google Scholar]
- Catalfamo M, Henkart P A. Perforin and the granule exocytosis cytotoxicity pathway. Curr Opin Immunol. 2003;15:522–527. doi: 10.1016/s0952-7915(03)00114-6. [DOI] [PubMed] [Google Scholar]
- Walsh C M, Matloubian M, Liu C C, Ueda R, Kurahara C G, Christensen J L, Huang M T, Young J D, Ahmed R, Clark W R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Street S E, Trapani J A, MacGregor D, Smyth M J. Suppression of lymphoma and epithelial malignancies effected by interferon γ. J Exp Med. 2002;196:129–134. doi: 10.1084/jem.20020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth M J, Thia K Y, Street S E, MacGregor D, Godfrey D I, Trapani J A. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth M J, Street S E, Trapani J A. Cutting edge: granzymes A and B are not essential for perforin-mediated tumor rejection. J Immunol. 2003;171:515–518. doi: 10.4049/jimmunol.171.2.515. [DOI] [PubMed] [Google Scholar]
- Ramshaw I A, Ramsay A J, Karupiah G, Rolph M S, Mahalingam S, Ruby J C. Cytokines and immunity to viral infections. Immunol Rev. 1997;159:119–135. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Mullbacher A, Waring P, Tha Hla R, Tran T, Chin S, Stehle T, Museteanu C, Simon M M. Granzymes are the essential downstream effector molecules for the control of primary virus infections by cytolytic leukocytes. Proc Natl Acad Sci USA. 1999;96:13950–13955. doi: 10.1073/pnas.96.24.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullbacher A, Wallich R, Moyer R W, Simon M M. Poxvirus-encoded serpins do not prevent cytolytic T cell-mediated recovery from primary infections. J Immunol. 1999;162:7315–7321. [PubMed] [Google Scholar]
- Mullbacher A, Hla R T, Museteanu C, Simon M M. Perforin is essential for control of ectromelia virus but not related poxviruses in mice. J Virol. 1999;73:1665–1667. doi: 10.1128/jvi.73.2.1665-1667.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullbacher A, Ebnet K, Blanden R V, Hla R T, Stehle T, Museteanu C, Simon M M. Granzyme A is critical for recovery of mice from infection with the natural cytopathic viral pathogen, ectromelia. Proc Natl Acad Sci USA. 1996;93:5783–5787. doi: 10.1073/pnas.93.12.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusel J W, Wesselschmidt R L, Shresta S, Russell J H, Ley T J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Ebnet K, Hausmann M, Lehmann-Grube F, Mullbacher A, Kopf M, Lamers M, Simon M M. Granzyme A-deficient mice retain potent cell-mediated cytotoxicity. EMBO J. 1995;14:4230–4239. doi: 10.1002/j.1460-2075.1995.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A, Costelloe E O, Johnson B J, Groves P, Buttigieg K, Fitzpatrick D R. The genes for perforin, granzymes A–C and IFN-γ are differentially expressed in single CD8(+) T cells during primary activation. Int Immunol. 2002;14:605–613. doi: 10.1093/intimm/dxf028. [DOI] [PubMed] [Google Scholar]
- Grossman W J, Verbsky J W, Tollefsen B L, Kemper C, Atkinson J P, Ley T J. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- Johnson B J, Costelloe E O, Fitzpatrick D R, Haanen J B, Schumacher T N, Brown L E, Kelso A. Single-cell perforin and granzyme expression reveals the anatomical localization of effector CD8+ T cells in influenza virus-infected mice. Proc Natl Acad Sci USA. 2003;100:2657–2662. doi: 10.1073/pnas.0538056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata H, Takiguchi M. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J Immunol. 2006;177:4330–4340. doi: 10.4049/jimmunol.177.7.4330. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay P K, Price D A, Harper T F, Betts M R, Yu J, Gostick E, Perfetto S P, Goepfert P, Koup R A, De Rosa S C, Bruchez M P, Roederer M. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat Med. 2006;12:972–977. doi: 10.1038/nm1371. [DOI] [PubMed] [Google Scholar]
- Precopio M L, Betts M R, Parrino J, Price D A, Gostick E, Ambrozak D R, Asher T E, Douek D C, Harari A, Pantaleo G, Bailer R, Graham B S, Roederer M, Koup R A. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa S C, Andrus J P, Perfetto S P, Mantovani J J, Herzenberg L A, Herzenberg L A, Roederer M. Ontogeny of γ δ T cells in humans. J Immunol. 2004;172:1637–1645. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- Shibuya A. Development and functions of natural killer cells. Int J Hematol. 2003;78:1–6. doi: 10.1007/BF02983233. [DOI] [PubMed] [Google Scholar]
- Rock M T, Yoder S M, Wright P F, Talbot T R, Edwards K M, Crowe J E., Jr Differential regulation of granzyme and perforin in effector and memory T cells following smallpox immunization. J Immunol. 2005;174:3757–3764. doi: 10.4049/jimmunol.174.6.3757. [DOI] [PubMed] [Google Scholar]
- Sullivan B M, Juedes A, Szabo S J, von Herrath M, Glimcher L H. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci USA. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E L, Mullen A C, Martins G A, Krawczyk C M, Hutchins A S, Zediak V P, Banica M, DiCioccio C B, Gross D A, Mao C A, Shen H, Cereb N, Yang S Y, Lindsten T, Rossant J, Hunter C A, Reiner S L. Control of effector CD8+ T cell function by the transcription factor eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- Glimcher L H, Townsend M J, Sullivan B M, Lord G M. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- Juedes A E, Rodrigo E, Togher L, Glimcher L H, von Herrath M G. T-bet controls autoaggressive CD8 lymphocyte responses in type 1 diabetes. J Exp Med. 2004;199:1153–1162. doi: 10.1084/jem.20031873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J M, Karandikar N J, Betts M R, Ambrozak D R, Hill B J, Crotty L E, Casazza J P, Kuruppu J, Migueles S A, Connors M, Roederer M, Douek D C, Koup R A. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- Suni M A, Ghanekar S A, Houck D W, Maecker H T, Wormsley S B, Picker L J, Moss R B, Maino V C. CD4(+)CD8(dim) T lymphocytes exhibit enhanced cytokine expression, proliferation and cytotoxic activity in response to HCMV and HIV-1 antigens. Eur J Immunol. 2001;31:2512–2520. doi: 10.1002/1521-4141(200108)31:8<2512::aid-immu2512>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Norris P J, Rosenberg E S. Cellular immune response to human immunodeficiency virus. AIDS. 2001;15:S16–S21. doi: 10.1097/00002030-200102002-00004. [DOI] [PubMed] [Google Scholar]
- Mahon B P, Katrak K, Nomoto A, Macadam A J, Minor P D, Mills K H. Poliovirus-specific CD4+ Th1 clones with both cytotoxic and helper activity mediate protective humoral immunity against a lethal poliovirus infection in transgenic mice expressing the human poliovirus receptor. J Exp Med. 1995;181:1285–1292. doi: 10.1084/jem.181.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littaua R A, Oldstone M B, Takeda A, Ennis F A. A CD4+ cytotoxic T-lymphocyte clone to a conserved epitope on human immunodeficiency virus type 1 p24: cytotoxic activity and secretion of interleukin-2 and interleukin-6. J Virol. 1992;66:608–611. doi: 10.1128/jvi.66.1.608-611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Burrows S R. Role of cytotoxic T lymphocytes in Epstein-Barr virus-associated diseases. Annu Rev Microbiol. 2000;54:19–48. doi: 10.1146/annurev.micro.54.1.19. [DOI] [PubMed] [Google Scholar]
- Jaye A, Magnusen A F, Sadiq A D, Corrah T, Whittle H C. Ex vivo analysis of cytotoxic T lymphocytes to measles antigens during infection and after vaccination in Gambian children. J Clin Invest. 1998;102:1969–1977. doi: 10.1172/JCI3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Vafai A, Lee J, Mahalingam R, Hayward A R. Specific lysis of targets expressing varicella-zoster virus gpI or gpIV by CD4+ human T-cell clones. J Virol. 1992;66:2664–2669. doi: 10.1128/jvi.66.5.2664-2669.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickham K, Munz C, Tsang M L, Larsson M, Fonteneau J F, Bhardwaj N, Steinman R. EBNA1-specific CD4+ T cells in healthy carriers of Epstein-Barr virus are primarily Th1 in function. J Clin Invest. 2001;107:121–130. doi: 10.1172/JCI10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza J P, Betts M R, Price D A, Precopio M L, Ruff L E, Brenchley J M, Hill B J, Roederer M, Douek D C, Koup R A. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Dunbar P R, Callan M, Klenerman P, Gillespie G M, Papagno L, Ogg G S, King A, Lechner F, Spina C A, Little S, Havlir D V, Richman D D, Gruener N, Pape G, wAters A, Easterbrook P, Salio M, Cerundolo V, McMichael A J, Rowland-Jones S L. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- Yang O O, Kalams S A, Trocha A, Cao H, Luster A, Johnson R P, Walker B D. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar P, Xu Z, Lieberman J. Viral-specific cytotoxic T lymphocytes lyse human immunodeficiency virus-infected primary T lymphocytes by the granule exocytosis pathway. Blood. 1999;94:3084–3093. [PubMed] [Google Scholar]
- Andersson J, Kinloch S, Sonnerborg A, Nilsson J, Fehniger T E, Spetz A L, Behbahani H, Goh L E, McDade H, Gazzard B, Stellbrink H, Cooper D, Perrin L. Low levels of perforin expression in CD8+ T lymphocyte granules in lymphoid tissue during acute human immunodeficiency virus type 1 infection. J Infect Dis. 2002;185:1355–1358. doi: 10.1086/340124. [DOI] [PubMed] [Google Scholar]
- Andersson J, Behbahani H, Lieberman J, Connick E, Landay A, Patterson B, Sonnerborg A, Lore K, Uccini S, Fehniger T E. Perforin is not co-expressed with granzyme A within cytotoxic granules in CD8 T lymphocytes present in lymphoid tissue during chronic HIV infection. AIDS. 1999;13:1295–1303. doi: 10.1097/00002030-199907300-00005. [DOI] [PubMed] [Google Scholar]
- Trabattoni D, Piconi S, Biasin M, Rizzardini G, Migliorino M, Seminari E, Boasso A, Piacentini L, Villa M L, Maserati R, Clerici M. Granule-dependent mechanisms of lysis are defective in CD8 T cells of HIV-infected, antiretroviral therapy-treated individuals. AIDS. 2004;18:859–869. doi: 10.1097/00002030-200404090-00003. [DOI] [PubMed] [Google Scholar]
- Zhang D, Shankar P, Xu Z, Harnisch B, Chen G, Lange C, Lee S J, Valdez H, Lederman M M, Lieberman J. Most antiviral CD8 T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood. 2003;101:226–235. doi: 10.1182/blood-2002-03-0791. [DOI] [PubMed] [Google Scholar]