Abstract
Objectives
To identify factors associated with developing severe respiratory syncytial virus (RSV) pneumonia and their commonality with all-cause lower respiratory tract infection (LRTI), in order to isolate those risk factors specifically associated with RSV-LRTI and identify targets for control.
Methods
A birth cohort of rural Kenyan children was intensively monitored for acute respiratory infection (ARI) over three RSV epidemics. RSV was diagnosed by immunofluorescence of nasal washings collected at each ARI episode. Cox regression was used to determine the relative risk of disease for a range of co-factors.
Results
A total of 469 children provided 937 years of follow-up, and experienced 857 all-cause LRTI, 362 RSV-ARI and 92 RSV-LRTI episodes. Factors associated with RSV-LRTI, but not RSV-ARI, were severe stunting (z-score ≤−2, RR 1.7 95%CI 1.1–2.8), crowding (increased number of children, RR 2.6, 1.0–6.5) and number of siblings under 6 years (RR 2.0, 1.2–3.4). Moderate and severe stunting (z-score ≤−1), crowding and a sibling aged over 5 years sleeping in the same room as the index child were associated with increased risk of all-cause LRTI, whereas higher educational level of the primary caretaker was associated with protection.
Conclusion
We identify factors related to host nutritional status (stunting) and contact intensity (crowding, siblings) which are distinguishable in their association with RSV severe disease in infant and young child. These factors are broadly in common with those associated with all-cause LRTI. The results support targeted strategies for prevention.
Keywords: respiratory syncytial virus, risk factors, disease, Kenya
Introduction
Pneumonia is the leading cause of morbidity and mortality in the developing world (Bryce et al. 2005; WHO 2006a; Greenwood et al. 2007) and respiratory viruses make a major contribution to this disease burden (Selwyn 1990; Monto 2002). Among the viruses, RSV is a major contributor to community acquired pneumonia (Weber et al. 1998b, 2002; Robertson et al. 2004; Nokes et al. 2008). However, as with most viral infections, RSV is generally characterized by a self-limiting mild illness episode, and only a few patients will progress to severe or life-threatening conditions. The factors which influence this progression from infection to severe disease are not well understood.
Pneumonia has multiple aetiologies, and the risk factors for RSV-associated disease may be common to all pneumonias, specific to viral pneumonias or may be agent-specific. Past studies report a number of possible risk factors for increased viral disease severity, most of which are common to RSV. These may be environmental [higher household population density (Aaby 1988; Weber et al. 1999; Suwanjutha et al. 2002), attending school (Monto & Sook 1971; Hall et al. 1976), increased smoke exposure (Gardner et al. 1984; Cruz et al. 1990)], host factors [e.g. born prematurely (Nielsen et al. 2003), genetic susceptibility (Karron et al. 1999), poor nutritional status (Vardas et al. 1999; Loscertales et al. 2002; Djelantik et al. 2003)] and pathogen (Mufson et al. 1988; Sullender 2000) specific factors. Severe RSV in developing countries has been strongly associated with crowding (Weber et al. 1999), but this is not so for malnutrition, and in a number of instances malnutrition has been associated with protection (Adegbola et al. 1994; Nwankwo et al. 1994; Loscertales et al. 2002; Djelantik et al. 2003). Importantly all previous studies have the limitation of not distinguishing risk factors that are specific to disease as opposed to infection resulting in any level of severity.
We undertook a study to examine a range of potential risk factors for RSV disease (RSV-LRTI) in infants and young children followed from birth, within families under surveillance for ARI from a rural Kenyan community. Risk factors for progression from mild RSV infection to a LRTI were isolated by the extraction of factors identified for total RSV episodes irrespective of severity, and contrasted with factors associated with all-cause LRTI.
Methods
The study was conducted in Kilifi, a rural district on the coast of Kenya with a tropical climate and seasonal rains (March–July and October–December). The community is served by a district hospital (KDH) based in Kilifi town. Ethical permission was provided by the Kenya National Ethical Review Committee and Coventry Research Ethics Committee, UK. The terminology used for respiratory disease throughout the text is described in Table 1.
Table 1.
Terminology used for disease types
| Term | Description and synonyms |
|---|---|
| LRTI | Lower respiratory tract infection. Used synonymously with pneumonia. Includes all cases identified as mild, severe or very severe pneumonia. |
| All-cause LRTI | Non-specific lower respiratory tract infection. |
| RSV-ARI | Clinical (i.e. symptomatic) RSV antigen positive episode. Irrespective of severity. |
| RSV-LRTI | RSV-associated LRTI (pneumonia) and equivalent to RSV disease. |
| Disease progression | Development of LRTI from upper respiratory tract infection. |
Birth cohort study
Full details of the birth cohort study have been described previously (Nokes et al. 2004, 2008; Okiro 2007). Briefly study participants were recruited between January 2002 and May 2003, from KDH maternity ward and the maternal child health clinic (if <2 weeks old), and if their homes were within easy access to the hospital. Written informed consent was obtained for participation. Surveillance continued until each child had experienced three RSV epidemics. These epidemics were clearly defined, occurring on an approximately annual basis, and lasting for between 14 and 21 weeks (mean 17 weeks) (Nokes et al. 2008). Households were visited by trained study field workers (FW) weekly during, and monthly outside of RSV epidemics. Potential cases of LRTI identified by a FW during home visits were referred to the clinic, and given one way bus fares. These referral cases were recognized by cough or difficulty in breathing (on the day or a history over the preceding week) in association with fast breathing for age (50 or more breaths per minute in infants, and 40 or more breaths per minute otherwise), or (alone or accompanied by) the presence of lower chest wall indrawing (World Health Organization 2005) or difficulty in breathing alone if observed on the day of the visit. ARI surveillance through presentation at the research out-patient (OP) clinic at KDH was maintained throughout the follow-up period either by self (passive) referral, or FW (active) referral at home visits. To enhance passive surveillance (self referral), mothers were encouraged to bring their child to the research clinic if they identified any symptoms of respiratory infection. At the OP clinic, the severity of respiratory disease was ascribed following a review by a study clinician, which would include (a repeat) measurement of respiratory rate. Transport costs were reimbursed, and definitive medicines were provided without charge. At each contact with study participants identification of symptoms consistent with ARI on the day or during the preceding week, and an absence of RSV infection for the prior 14 days, prompted the collection of a nasal specimen by nasal washing. Specimens were examined for RSV antigen by direct immunofluorescence test (DFA, Chemicon). The severity of respiratory disease was ascribed following a clinical review using a standard proforma and based on WHO guidelines (Nokes et al. 2004; WHO 2005).
Risk factor survey
Between June and November 2004 a cross-sectional risk factor survey was carried out on households of all birth-cohort children remaining under surveillance. The purpose of the study was explained to the parents or guardians and verbal consent sought before the interview commenced. A household was defined as all individuals who normally eat at the same meal. Individuals 15 years of age or older were considered adults. The questionnaire was based on previous risk-factor surveys conducted in sub-Saharan Africa (Ballard & Neumann 1995; Weber et al. 1999; Broor et al. 2001) addressing household characteristics, and demographic, socioeconomic and environmental factors, but tailored to the specific setting of the study community. Data related to key asset indicators including primary caretaker (PCT) education level, occupation of the major income provider (MIP), housing characteristics (type of walls, sanitation), source of drinking water, family size and sleeping patterns in relation to the birth cohort child and nutritional status was collected. Crowding and contact intensity was measured by the total household size, number of sibling children in the household, and sleeping proximity.
A wealth index was constructed from data on household asset ownership (e.g. owning a bicycle) and characteristics (e.g. house and toilet type) using principal component analysis (Filmer & Pritchett 2001). Weights (scoring coefficients) derived from the first principal component were used to assign each household a wealth index from which socioeconomic groups were defined as follows: the top 33% were referred to as ‘least poor’, the next 33% as ‘poor’ and the bottom 34% as ‘most poor’.
Anthropometric measurements were obtained at birth and at 3-month intervals thereafter for cohort children. A WHO macro [igrowup_STATA macro (WHO 2006b) ] was used to calculate z-scores (the standardized deviation from the median of a reference population) for three anthropometric indicators: weight-for-age (waz-underweight), length or height-for-age (haz-stunting), weight-for-length or height (whz-wasting).
Data analysis
Data were double entered onto FileMaker (FileMaker Pro 5.5 v1) with internal consistency checks, and analysed using Stata (v8.2, STATACorp, Texas). Longitudinal data on infection history were combined with cross-sectional data from the risk factor survey. Observation time included days from date of recruitment until the last study visit, or until lost to follow-up, excluding days absent from the district. Each child had multiple record visit data over the follow-up period. Certain variables were reassigned at intervals of 3 months (nutritional status) or at each epidemic period (number of siblings sleeping in house, rooms and beds).
For the purpose of analysis only clinical data obtained from CO reviews were used (as opposed to that of the FW) and a diagnosis of LRTI was assigned to children with acute cough or difficulty in breathing in association with any one or more of the following (i) raised respiratory rate for age (respiratory rate of ≥40 breaths/min for children aged >12 months, ≥50 breaths/min for ages greater than 1 month, and ≥60 for a child of any age), (ii) lower chest wall indrawing or (iii) inability to feed, reduced conscious level or hypoxia (O2 saturation <90% by Oximetry), the latter group only if confirmed by the clinician’s own diagnosis of LRTI or bronchiolitis. The outcome variables were: (i) all-cause LRTI, (ii) RSV-ARI and (iii) RSV-LRTI (as defined in Table 1).
Univariate analysis was performed to describe the study population and identify risk factors for inclusion in multivariate analysis. Predictors were considered for inclusion in the multiple regression models using the log-rank test of equality of survival distribution across strata (for categorical variables) or a univariate Cox proportional hazard regression for the continuous variables. Predictors were considered for inclusion if the test had a P-value of 0.25 or less, and for groups of collinear variables (e.g. household contact measures) only those with the strongest univariate association were included. Significant variables were included in the multivariate models using a non-automated forward stepwise regression starting from the variable with the highest test statistic. Variables that no longer showed significance (P ≥ 0.05) were removed. For highly correlated variables (r ≥ 0.8) only the variable remaining significant in the multivariate model was included. The Cox shared frailty model was used with the all-cause LRTI outcome because of significant multiple failures per individual (θ = 0.326, P < 0.001). The standard Cox model with adjusted standard errors adjusting for clustering within individual was used for RSV-ARI and RSV-LRTI. Analysis time was calendar time, eliminating the potential confounding effect of seasonality in RSV and all-cause LRTI. Time-varying covariate(s) were specified through multiple observations per subject, ensuring risk sets at each failure were associated with the correct value of the risk factor. The results are reported as relative risks (hazard ratios) with 95% confidence intervals.
Results
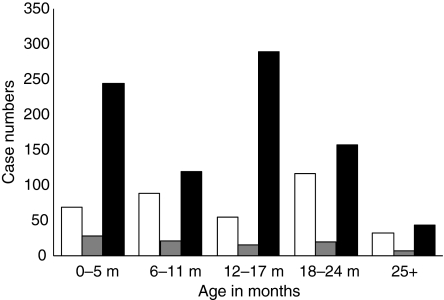
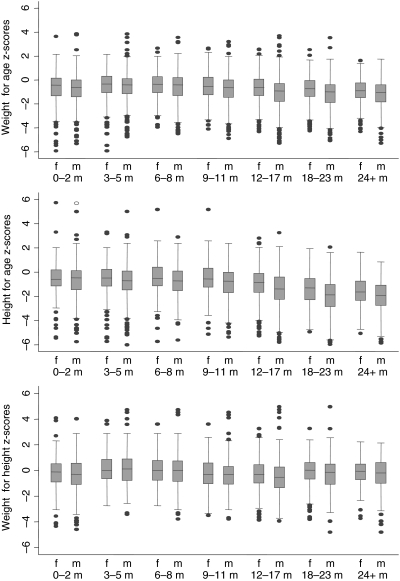
The birth cohort was monitored over four calendar years until each child had lived through three epidemics of RSV infection. From the 469 children under surveillance at the time of the risk factor survey, 29 979 separate visits (observations) for the detection of all-cause LRTI, RSV-ARI and RSV-LRTI were made. The observations per child were between 23 and 104 with a mean and median of 71. There were 362 episodes of clinical RSV-ARI detected among 940 person-years of observation; 283 single infections and 79 children were identified as being symptomatically infected by RSV more than once (68 twice, 8 three times, 2 four times and 1 five times). There were 857 episodes of all-cause LRTI; 128 (37%) children had only one episode of LRTI, 216 (63%) had two or more episodes. Of the all-cause LRTI episodes, 92 were associated with RSV; 86 (93%) children had a single episode of RSV-LRTI, with 6 children having two episodes. Population characteristics for the cohort are detailed in Table 2. There were more cases of RSV infection and RSV-LRTI in females than males; 55% and 52%, respectively, while cases of all-cause LRTI were equally distributed by sex. The age distribution of cases for all the three outcomes is shown in Figure 1. The mean age of a child with a RSV infection was 13.9 months (median age, 13 months) while that for a RSV-associated LRTI was 11.7 months (median age, 10 months). The mean age of a child with all-cause LRTI was 11.8 months (median age, 12 months). Figure 2 illustrates the different nutritional indices summarized by age progressively through follow-up.
Table 2.
Model parameters and selected characteristics of the study population
| Number of events for each outcome | |||||
|---|---|---|---|---|---|
| Parameters | Percent of population† | Pyo | RSV ARI | RSV-LRTI | All-cause LRTI |
| Current age (months)‡ | |||||
| 0–5 | 21.0¶ | 200.5 | 69 | 28 | 245 |
| 6–11 | 22.4 | 213.2 | 88 | 21 | 120 |
| 12–17 | 22.1 | 211.3 | 55 | 16 | 290 |
| 18 or more | 34.5 | 315.2 | 150 | 27 | 202 |
| Multiple babies | |||||
| 1 child | 93.8 | 880.5 | 337 | 83 | 790 |
| Twins | 5.8 | 55.1 | 22 | 8 | 50 |
| Triplets | 0.4 | 4.6 | 3 | 1 | 17 |
| Educational level of PCT (years) | |||||
| No schooling | 25.4 | 241.0 | 92 | 25 | 249 |
| 1–7 | 39.2 | 370.2 | 156 | 31 | 353 |
| 8–12 | 31.1 | 289.5 | 105 | 34 | 241 |
| >12 | 4.3 | 3839.5 | 9 | 2 | 14 |
| PCT age group (years) | |||||
| 13–20 | 14.9 | 136.5 | 49 | 26 | 125 |
| 21–30 | 48.8 | 458.7 | 186 | 24 | 428 |
| 31–40 | 27.7 | 262.4 | 92 | 28 | 226 |
| 41–50 | 6.6 | 63.0 | 23 | 14§ | 51 |
| 51–63 | 1.9 | 19.6 | 12 | 27 | |
| Child’s care | |||||
| Mother | 81.2 | 762.2 | 300 | 73 | 670 |
| Other family members | 12.2 | 114.3 | 41 | 13 | 129 |
| House help | 4.3 | 39.5 | 7 | 3 | 20 |
| School | 0.2 | 2.4 | 1 | 0 | 0 |
| Mother/family member | 2.1 | 21.7 | 13 | 3 | 38 |
| Smokers in HH | |||||
| None | 71.9 | 673.1 | 255 | 66 | 597 |
| 1 | 23.5 | 223.8 | 83 | 21 | 216 |
| 2 or more | 4.7 | 43.3 | 24 | 5 | 44 |
| Family assisted†† | |||||
| No | 19.4 | 756.7 | 273 | 69 | 665 |
| Yes | 80.6 | 183.5 | 89 | 23 | 192 |
| House and toilet type | |||||
| Mudwall no toilet | 29.9 | 288.0 | 124 | 26 | 287 |
| Blockwall no toilet | 3.2 | 31.1 | 15 | 7 | 31 |
| Mudwall latrine | 30.9 | 290.4 | 121 | 34 | 303 |
| Blockwall latrine | 29.2 | 268.8 | 88 | 24 | 211 |
| Mudwall flush toilet | 0.6 | 4.7 | 1 | 0 | 0 |
| Block flush toilet | 6.2 | 57.2 | 13 | 1 | 25 |
| Main cooking fuel | |||||
| Gas/paraffin | 3.8 | 35.9 | 11 | 0 | 16 |
| Charcoal | 23.5 | 211.4 | 66 | 12 | 158 |
| Firewood | 70.2 | 669.6 | 276 | 79 | 660 |
| Firewood/charcoal | 2.6 | 23.3 | 9 | 1 | 23 |
| Job category description of MIP | |||||
| Non-skilled | 37.3 | 354.2 | 145 | 35 | 359 |
| Trade | 16.6 | 156.6 | 74 | 21 | 146 |
| Skilled | 35.4 | 330.0 | 115 | 27 | 284 |
| Professional | 10.7 | 99.4 | 28 | 9 | 68 |
| Weight for age z-score‡ | |||||
| >−1 | 61.6¶ | 579.8 | 214 | 49 | 507 |
| −1.99 to −1 | 24.3 | 228.2 | 92 | 27 | 205 |
| ≤−2 | 14.0 | 132.1 | 56 | 16 | 145 |
| Height for age z-score‡ | |||||
| >−1 | 49.3¶ | 458.3 | 162 | 38 | 363 |
| −1.99 to −1 | 26.1 | 247.0 | 97 | 26 | 239 |
| ≤−2 | 24.7 | 234.9 | 103 | 28 | 255 |
| Weight for height z-score‡ | |||||
| >−1 | 76.9¶ | 725.0 | 280 | 71 | 652 |
| −1.99 to −1 | 17.2 | 160.3 | 56 | 13 | 136 |
| ≤−2 | 5.9 | 54.9 | 26 | 8 | 69 |
| Family children | |||||
| 1–5 | 72.7 | 681.4 | 257 | 60 | 598 |
| 6–10 | 24.1 | 231.2 | 92 | 26 | 214 |
| 11 or more | 3.2 | 27.6 | 13 | 6 | 45 |
| Siblings <6 years | |||||
| None | 28.6 | 262.8 | 88 | 16 | 185 |
| 1–2 | 56.5 | 536.9 | 212 | 58 | 517 |
| 3–4 | 12.6 | 119.5 | 53 | 15 | 129 |
| 5 or more | 2.4 | 21.0 | 9 | 3 | 26 |
| Number of siblings <6 years sleeping in same room as index‡ | |||||
| None | 55.8¶ | 524.3 | 190 | 50 | 425 |
| 1–2 | 44.1 | 404.8 | 164 | 39 | 421 |
| 3 | 1.2 | 11.0 | 8 | 3 | 11 |
| Number of siblings 6 years or over sleeping in same room as index‡ | |||||
| None | 67.0¶ | 629.8 | 250 | 63 | 551 |
| 1 | 19.4 | 183.8 | 70 | 19 | 203 |
| 2–3 | 11.7 | 108.4 | 34 | 8 | 90 |
| 4 | 1.9 | 18 | 8 | 2 | 13 |
| Number of siblings <6 years going to school‡ | |||||
| None | 83.4¶ | 780.7 | 289 | 77 | 732 |
| 1 | 13.8 | 130.8 | 53 | 10 | 87 |
| 2–3 | 2.8 | 28.7 | 20 | 5 | 38 |
Index refers to the birth cohort child.
Pyo, person years of observation; PCT, primary caretaker; MIP, major income provider; HH, household.
Proportion of the study population with this characteristic. Excludes factors that were reassigned during study period.
Time changing variables.
PCT aged 41 to 63 years.
Proportion of total observation time spent in specific category.
Family assisted means the family receives financial assistance from relatives living away from home.
Figure 1.
Descriptive characteristics of cases identified during the study. The bars represent the absolute numbers of cases of RSV-ARI (white bars), RSV-LRTI (grey bars) and all-cause LRTI (black bars) by specific age category.
Figure 2.
Weight for age, height for age, weight for height z–scores of children in the study by sex and age. The box plot depicts the interquartile range as a box and the median as a line in the box. Bars, upper and lower adjacent values and dots represent outliers.
Factors significantly associated with the risk of acquiring RSV-ARI and RSV-LRTI or all-cause LRTI by univariate analysis are presented in Table 3. Increased risk arose with higher number of children in the household and number of siblings under 6 years of age as well as more male siblings. Having one or more siblings sleeping in the same room as the birth cohort child was associated with increased risk of all-cause LRTI while having one or more siblings <6 years sleeping in the same bed as the birth cohort child was associated with increased risk of RSV-ARI. Moderate-to-severe malnutrition was associated with increased risk of RSV-LRTI (haz ≤ −2) and LRTI (waz and whz ≤ −2 and haz ≤ −1). Having two or more smokers in the household was correlated with increased risk of infection. Living in a mud-walled house, using firewood as the main cooking fuel and being a child of a multiple birth was associated with increased risk of RSV-ARI and all-cause LRTI. Having an older PCT was also associated with increased risk of infection and RSV-LRTI. Several factors were related to protection: living in a household classified as ‘least poor’, having a hired house help, a flush toilet, a major income provider with a professional job or a PCT with higher than high school level education.
Table 3.
Univariate analysis of risk factors associated with infection and disease
| Relative risk (95% CI) | ||||
|---|---|---|---|---|
| Putative risk factor | Categories | RSV-ARI | RSV-LRTI | All-cause LRTI |
| Socioeconomic status | Most poor | – | – | – |
| Poor | 1.02 (0.83–1.26) | 1.49 (0.94–2.37) | 1.02 (0.81–1.28) | |
| Least poor | 0.75 (0.60–0.95) | 0.80 (0.48–1.34) | 0.70 (0.55–0.89) | |
| Current age (months) | 0–5 | – | – | – |
| 6–11 | 1.44 (0.74–2.80) | 1.20 (0.38–3.77) | 0.58 (0.43–0.77) | |
| 12–17 | 1.16 (0.54–2.45) | 1.44 (0.38–5.39) | 1.85 (1.40–2.43) | |
| 18 or more | 0.98 (0.48–2.00) | 1.06 (0.28–4.05) | 0.85 (0.59–1.21) | |
| Multiple delivery | 1 child | – | – | – |
| Twins | 1.05 (0.73–1.51) | 1.54 (0.79–3.01) | 1.02 (0.75–1.34) | |
| Triplets | 1.77 (1.06–2.93) | 1.99 (0.47–8.50) | 3.85 (3.35–4.42) | |
| Age group of PCT (years) | 13–22 | – | – | – |
| 23–30 | 1.14 (0.85–1.51) | 2.15 (0.84–5.47) | 0.99 (0.74–1.31) | |
| 31–40 | 0.98 (0.71–1.35) | 2.43 (0.94–6.27) | 0.93 (0.68–1.28) | |
| 41–50 | 0.96 (0.62–1.48) | 3.65 (1.34–9.98)† | 0.88 (0.58–1.32) | |
| 51–63 | 1.73 (1.16–2.59) | 1.36 (0.96–1.92) | ||
| Education level of PCT (years) | No schooling | – | – | – |
| 1–7 | 1.11 (0.88–1.39) | 0.84 (0.49–1.44) | 0.94 (0.80–1.11) | |
| 8–12 | 0.96 (0.76–1.21) | 1.14 (0.68–1.92) | 0.80 (0.63–1.03) | |
| >12 | 0.61 (0.36–1.04) | 0.51 (0.13–2.04) | 0.34 (0.21–0.55) | |
| Literate PCT | No | – | – | – |
| Yes | 1.00 (0.83–1.21) | 0.96 (0.63–1.46) | 0.82 (0.67–0.98) | |
| Family assisted financially | No | – | – | – |
| Yes | 1.34 (1.11–1.63) | 1.40 (0.91–2.15) | 1.20 (0.97–1.49) | |
| Weight-age-z score | >−1 | – | – | – |
| −1.99 to −1 | 0.99 (0.80–1.24) | 1.37 (0.86–2.17) | 1.06 (0.90–1.25) | |
| ≤−2 | 1.01 (0.79–1.29) | 1.37 (0.82–2.28) | 1.30 (1.08–1.56) | |
| Height-age-z score | >−1 | – | – | – |
| −1.99 to−1 | 1.07 (0.85–1.36) | 1.43 (0.87–2.36) | 1.32 (1.12–1.56) | |
| ≤−2 | 1.18 (0.95–1.47) | 1.85 (1.15–2.97) | 1.60 (1.36–1.89) | |
| Weight-height-z score | >−1 | – | – | – |
| −1.99 to −1 | 0.82 (0.63–1.07) | 0.70 (0.40–1.26) | 0.91 (0.76–1.10) | |
| ≤−2 | 0.97 (0.69–1.36) | 1.17 (0.58–2.37) | 1.41 (1.10–1.81) | |
| Number of family children | 1–5 | – | – | – |
| 6–10 | 1.04 (0.84–1.27) | 1.32 (0.85–2.04) | 1.08 (0.86–1.37) | |
| 11 or more | 1.09 (0.87–1.36) | 2.51 (1.32–4.76) | 1.80 (1.31–2.47) | |
| Number siblings under 6 years | None | – | – | – |
| 1–2 | 1.17 (0.93–1.47) | 1.78 (1.06–2.98) | 1.37 (1.16–1.62) | |
| 3–4 | 1.32 (1.00–1.73) | 2.00 (1.00–3.97) | 1.50 (1.21–1.88) | |
| 5 or more | 1.34 (0.87–2.06) | 2.39 (0.81–7.09) | 1.70 (1.28–2.26) | |
| Number of male siblings | 0–2 | – | – | – |
| 3–6 | 1.09 (0.88–1.34) | 1.36 (0.87–2.10) | 1.22 (0.97–1.54) | |
| 7 or more | 1.36 (0.98–1.88) | 3.28 (1.76–6.17) | 1.15 (0.65–2.04) | |
| Index child’s care | Mother | – | – | – |
| Another family member | 0.91 (0.68–1.21) | 1.12 (0.63–1.98) | 1.24 (0.99–1.56) | |
| House help | 0.45 (0.24–0.81) | 0.78 (0.27–2.31) | 0.56 (0.36–0.86) | |
| School | 1.13 (1.01–1.26) | – | – | |
| Mother/family member | 1.52 (0.95–2.43) | 1.28 (0.48–3.38) | 1.89 (1.22–2.93) | |
| House type | Block walled | – | – | – |
| Mud walled | 1.29 (1.07–1.56) | 1.15 (0.76–1.76) | 1.37 (1.12–1.67) | |
| House ownership | Owner occupied | – | – | – |
| Rented | 0.83 (0.64–1.07) | 0.52 (0.28–0.96) | 0.87 (0.67–1.14) | |
| Not owned/ rented | 0.94 (0.62–1.42) | 0.55 (0.15–2.00) | 0.62 (0.35–1.10) | |
| Toilet type | No toilet | – | – | – |
| Flush | 0.52 (0.34–0.78) | 0.16 (0.02–1.13) | 0.40 (0.24–0.67) | |
| Latrine | 0.85 (0.71,1.03) | 1.03 (0.68,1.57) | 0.93 (0.76,1.14) | |
| Main fuel used for cooking | Charcoal | – | – | – |
| Gas/paraffin | 1.07 (0.61–1.88) | – | 1.61 (0.96–2.68) | |
| Firewood | 1.37 (0.81–2.33) | 2.08 (1.18–3.66) | 2.16 (1.35–3.46) | |
| Firewood/charcoal | 1.26 (0.53–2.97) | 0.82 (0.13–5.29) | 2.27 (1.10–4.69) | |
| Water source site | Public | – | – | – |
| Own source | 1.19 (0.85–1.66) | 0.22 (0.06–0.86) | 2.12 (1.50–3.00) | |
| Shared | 1.17 (0.79–1.72) | 0.71 (0.38–1.31) | 2.07 (1.37–3.11) | |
| job description of MIP | Non-skilled | – | – | – |
| Trade | 1.17 (0.93–1.48) | 1.42 (0.84–2.40) | 0.94 (0.71–1.24) | |
| Skilled | 0.85 (0.68–1.06) | 0.84 (0.53–1.34) | 0.86 (0.68–1.07) | |
| Professional | 0.71 (0.52–0.96) | 0.97 (0.46–2.03) | 0.69 (0.53–0.91) | |
| Number of smokers in Household | None | – | – | – |
| 1 | 0.99 (0.78–1.24) | 0.93 (0.56–1.53) | 1.08 (0.87–1.35) | |
| 2 or more | 1.47 (1.13–1.93) | 1.21 (0.47–3.11) | 1.15 (0.81–1.64) | |
| Number of adults sleeping in index’s room | 0–1 | – | – | – |
| 2 | 1.02 (0.84–1.25) | 0.66 (0.44–1.00) | 0.82 (0.66–1.02) | |
| 3 or more | 0.87 (0.55–1.39) | 0.82 (0.34–1.95) | 0.88 (0.52–1.49) | |
| Number of siblings <6 years living in same house as index | None | – | – | – |
| 1 | 1.07 (0.87–1.30) | 1.00 (0.65–1.55) | 1.10 (0.89–1.35) | |
| 2–4 | 1.01 (0.78–1.31) | 1.73 (1.02–2.93) | 1.41 (1.06–1.86) | |
| Number of siblings 6 years or over living in same house as index | None | – | – | – |
| 1–2 | 1.02 (0.84–1.25) | 0.92 (0.59–1.44) | 1.14 (0.92–1.42) | |
| 3–4 | 0.87 (0.67–1.12) | 0.91 (0.49–1.69) | 0.86 (0.66–1.12) | |
| 5–7 | 0.98 (0.65–1.43) | 2.10 (1.08–4.10) | 1.45 (0.90–2.36) | |
| Number of siblings <6 years sleeping in same room as index | None | – | – | – |
| 1–3 | 1.08 (0.91–1.30) | 1.05 (0.71–1.55) | 1.30 (1.07–1.57) | |
| Number of siblings 6 years or over sleeping in same room as index | None | – | – | – |
| 1 | 0.96 (0.74–1.25) | 1.05 (0.64–1.71) | 1.27 (1.00–1.62) | |
| 2–3‡ | 0.81 (0.62–1.05) | 0.77 (0.39–1.53) | 0.95 (0.72–1.26) | |
| 4 | 0.83 (0.38–1.29) | |||
| Number of siblings <6 years sleeping in same bed as index | None | – | – | – |
| 1–3 | 1.16 (0.78–1.72) | 1.22 (0.99–1.50) | 1.21 (1.01–1.45) | |
| Number of siblings <6 years going to school | None | – | – | – |
| 1 | 0.88 (0.68–1.12) | 0.75 (0.40–1.41) | 0.77 (0.60–0.98) | |
| 2–3 | 1.40 (0.99–2.00) | 1.77 (0.76–4.12) | 1.69 (1.02–2.80) | |
| Number of siblings 6 years or over going to school | None | – | – | – |
| 1–2 | 0.97 (0.78–1.19) | 1.02 (0.61–1.70) | 1.13 (0.87–1.44) | |
| 3–5 | 0.97 (0.76–1.25) | 1.31 (0.78–2.20) | 1.12 (0.84–1.48) | |
| 6–9 | 1.17 (0.76–1.80) | 3.04 (1.48–6.22) | 1.46 (0.98–2.26) | |
Significant estimates (P < 0.05) are in bold.
MIP, major income provider; PCT, primary caretaker.
PCT aged 41 to 63 years.
2–4 siblings 6 years or over sleeping in same bed as index for RSV ARI and RSV LRTI.
Results from the multivariate regression are shown in Table 4. Factors independently associated with RSV-ARI are shown in column 1. Higher age of the PCT (>50) was the strongest independent predictor of increased risk. Exposure to tobacco smoke was also associated with an increased risk, whereas two indicators of higher socioeconomic status (SES), namely, block-walled house with a flush toilet and hired house help with child care, were associated with protection from RSV-ARI.
Table 4.
Risk factors independently predicting increased relative risks of infection and disease
| Relative risk (95% CI) | ||||
|---|---|---|---|---|
| Risk factor | Categories | RSV-ARI | RSV-LRTI | All cause -LRTI |
| Current age (month) | 0–5 | – | – | 1 |
| 6–11 | – | – | 0.55 (0.41–0.74) | |
| 12–17 | – | – | 1.72 (1.31–2.25) | |
| 18 or more | – | – | 0.74 (0.52–1.04) | |
| Multiple babies | 1 child | – | – | 1 |
| Twins | – | – | 0.80 (0.54–1.19) | |
| Triplets | – | – | 4.12 (1.55–10.9) | |
| Education level of PCT(years) | No schooling | – | – | 1 |
| 1–7 | – | – | 0.95 (0.76–1.19) | |
| 8–12 | – | – | 0.81 (0.64–1.19) | |
| >12 | – | – | 0.40 (0.21–0.76) | |
| Child’s care | Mother | 1 | 1 | – |
| Another family member | 0.77 (0.54–1.08) | 0.95 (0.53–1.71) | – | |
| House help | 0.54 (0.30–0.99) | 1.49 (0.56–4.00) | – | |
| School | 1.06 (0.87–1.30) | 0.00 (–) | – | |
| Mother / family member | 1.28 (0.80–2.05) | 1.26 (0.47–3.37) | – | |
| PCT age group (years) | 13–24 | 1 | 1 | – |
| 25–30 | 1.15 (0.87–1.52) | 1.03 (0.58–1.82) | – | |
| 31–40 | 1.09 (0.79–1.49) | 1.49 (0.88–2.53) | – | |
| 41–50 | 1.02 (0.66–1.57) | 2.19 (1.13–4.25)† | – | |
| 51–63 | 2.13 (1.27–3.59) | – | ||
| Number of smokers in HH | None | 1 | 1 | – |
| 1 | 0.92 (0.74–1.15) | 0.87 (0.53–1.45) | – | |
| 2 or more | 1.40 (1.07–1.84) | 0.81 (0.36–1.84) | – | |
| Height-for-age z-score | >−1 | – | 1 | 1 |
| −1.99 to−1 | – | 1.34 (0.83–2.17) | 1.27 (1.06–1.52) | |
| ≤−2 | – | 1.73 (1.08–2.76) | 1.38 (1.12–1.70) | |
| Family assisted | No | 1 | 1 | – |
| Yes | 1.20 (0.98–1.46) | 1.34 (0.86–2.09) | – | |
| House and toilet type | Mud wall no toilet | 1 | 1 | – |
| Block wall no toilet | 1.03 (0.73–1.46) | 3.62 (1.53–8.88) | – | |
| Mud wall with latrine | 0.98 (0.79–1.21) | 1.57 (0.96–2.57) | – | |
| Block wall with latrine | 0.83 (0.65–1.06) | 1.41 (0.79–2.53) | – | |
| Mud wall flush toilet | 0.53 (0.19–1.47) | 0.00 (−) | – | |
| Block flush toilet | 0.60 (0.40–0.91) | 0.25 (0.03–2.11) | – | |
| Main cooking fuel | Gas/paraffin | – | – | 1 |
| Charcoal | – | – | 1.34 (0.74–2.44) | |
| Firewood | – | – | 1.71 (0.96–3.05 | |
| Firewood/charcoal | – | – | 2.11 (0.97–4.60) | |
| Job description of MIP | Non-skilled | – | 1 | – |
| Trade | – | 1.64 (0.98–2.73) | – | |
| Skilled | – | 0.91 (0.56–2.09) | – | |
| Professional | – | 1.04 (0.48–2.27) | – | |
| Number of family children | 1–5 | – | 1 | 1 |
| 6–10 | – | 0.97 (0.57–1.66) | 0.96 (0.77–1.19) | |
| 11 or more | – | 2.58 (1.03–6.50) | 1.68 (1.07–2.63) | |
| Number of siblings under 6 years | None | – | 1 | – |
| 1–2 | – | 2.00 (1.17–3.42) | – | |
| 3–4 | – | 1.99 (0.81–4.91) | – | |
| 5 or more | – | 1.74 (0.54–5.63) | – | |
| Number of siblings <6 years sleeping in same room as index | None | – | – | 1 |
| 1 –2 | – | – | 1.19 (0.99–1.43) | |
| 3 | – | – | 1.69 (0.81–3.51) | |
| Number of siblings 6 years or more sleeping in same room as index | None | – | – | 1 |
| 1 | – | – | 1.29 (1.04–1.61) | |
| 2–3 | – | – | 0.83 (0.62–1.11) | |
| 4 | – | – | 0.63 (0.31–1.28) | |
| Number of siblings <6 years going to school | None | – | – | 1 |
| 1 | – | – | 0.80 (0.62–1.05) | |
| 2–3 | – | – | 1.52 (0.98–2.33) | |
Statistically significant results (P < 0.05) are in bold.
MIP, major income provider; PCT, primary caretaker.
PCT aged 41 to 63 years.
Significant predictors of the risk of infection were included in the RSV-LRTI multivariate analysis as the baseline. Additional variables linked to RSV-LRTI from the univariate analysis were then fitted, and independent predictors reported in column 2 of Table 4. Of the predictors identified to increase the risk of clinical RSV-ARI, increased age of PCT (>40 years) and the house and toilet type (block wall with no toilet) were also associated with an increase in risk of RSV disease. Crowding (as measured by number of children in the home) and number of children under 6 years in the home were found to correlate with increased risk of RSV-LRTI. Moderate-to-severe stunting (height-for-age z-score ≤ −2) was also an independent predictor of RSV-LRTI.
To identify which risk factors for disease were specific to RSV, we determined predictors of all-cause LRTI in this study population (Table 4, column 3). Factors found to be independently associated with increased risk of all-cause LRTI were height-for-age z-score of ≤ −1, crowding (number of children in the home), and contact pattern (number of siblings over 6 years of age sleeping the same room as cohort child). Current age was also an independent predictor of all-cause LRTI associated with protection in those 6–11 months and increased risk in those 12–17 months. A multiple birth (triplets) was the strongest independent predictor of LRTI, although rare in the cohort (Table 2). A borderline significant factor was having 2–3 siblings <6 years attending school. A caretaker with a college education (>12 years of schooling) was associated with reduced risk of all-cause LRTI.
Discussion
Data collected over 4 years of follow-up of a large birth cohort in rural Kenya were analysed to determine risk factors specific for RSV-ARI and those specific to RSV disease, and these were compared with factors common to all-cause LRTI. Two main factors emerged as being independently associated with increased risk of severe disease (both RSV-LRTI and all-cause LRTI) as opposed to total ARI, namely, growth stunting and household crowding.
The data show that stunting (mild to moderate and severe), an indication of long-term malnutrition, was a more important factor for RSV-LTRI and all-cause LRTI than acute (short-term) malnutrition (wasting). This risk has previously been reported in a study involving Kenyan children (Ballard & Neumann 1995). It is thought that malnourished children may be susceptible to opportunistic infections; although concurrent RSV and bacterial infections are uncommon (Weber et al. 1998a; Madhi et al. 2001; Loscertales et al. 2002; Nokes et al. 2008). Results from several studies indicate a deficiency in the immune response in malnourished children (Neumann et al. 1975; Chandra 1983; Watson et al. 1985). One other study in South Africa (Vardas et al. 1999) also reported increased risk of RSV-LRTI in admissions with malnutrition. However, there are several reports of an absence of association between, or a protective effect of, malnutrition and the incidence of RSV disease (Adegbola et al. 1994; Nwankwo et al. 1994; Miranda-Novales et al. 1999; Loscertales et al. 2002; Djelantik et al. 2003). Conversely, malnutrition is a widely known risk factor for ARI and all-cause LRTI (Selwyn 1990; Tupasi et al. 1990; Ballard & Neumann 1995). These differences between studies are hard to reconcile because of different methodologies, notably whether hospital-based or community-based and differences in definition used for malnutrition and adjustments for covariates. Notwithstanding these differences, our database represents the largest ever RSV community study, and together with a strong analysis design and properly computed z-scores (WHO 2006b), give the results credibility. The influence of HIV as a confounder of this association is not precisely known. Overall HIV-1 prevalence in women attending to KDH antenatal care in 2004 was 4.8% (95% CI 3.4–6.6) (E. Sanders, personal communication).
The second important predictor of disease was crowding, as measured by number of children, and siblings under 6 years of age, which was associated with increased risk of RSV-LRTI and all-cause LRTI. The underlying mechanism may be increased contact intensity, resulting in an increase in size and/or duration of the exposure inoculum resulting from proximity of contact (intimacy of contact) (DeVincenzo 2005). The association between proximity of contact and disease severity has been well described for measles in Guinea Bissau (Aaby et al. 1983; Aaby & Coovadia 1985; Aaby 1988). Severity of measles infections increases when two or more children are sick simultaneously, and where the secondary infected child sleeps in close proximity to the child who introduced the infection into the household. Likewise several recent studies (Simoes 2003) found a positive association between crowding and number of siblings and occurrence of RSV-LRTI. An alternative mechanism could be related to contact frequency such that children in contact with more people are exposed to more inocula (sequential inoculation from numerous contacts), which increases their chance of severe disease.
Several factors related to intensity (family size) and pattern of contact (sleeping arrangements; in same house, room, bed or with school going sibling) between other children in the home and the cohort child were investigated. An association between the number of siblings sleeping in the same room as the cohort child and increased risk of all-cause LRTI was observed (Table 4). Several studies have shown a similar correlation between risk of RSV-LRTI and all-cause LRTI and number of people sleeping in the same room with the child or with siblings in school (Aaby et al. 1984; Holberg et al. 1991; Suwanjutha et al. 2002; Cardoso et al. 2004). This reflects the higher probability of transmission taking place due to prolonged exposure and closer contact; thought necessary for RSV transmission (Hall & Douglas 1981; Aaby & Coovadia 1985). Similarly, RSV infection and illness rates were found to be higher in mothers than fathers as presumably mothers had more intimate contact with the children than did the fathers (Berglund 1967) – again suggesting that virus dosage and exposure time may play an important part in the outcome of RSV infection.
Other factors significantly associated with individual outcomes in the multivariate analysis included house and toilet type which relates to SES, exposure to tobacco and environmental smoke from cooking fuel (borderline significance) which have previously been reported (Pandey et al. 1989; Cruz et al. 1990) as increasing the risk of RSV-ARI and LRTI exposure to cooking smoke is prevalent due to mothers carrying their young children while attending to household chores (field observations). Interestingly, the risk of all-cause LRTI decreased with increased level of education of the PCT. Unfortunately, less than 5% of the mothers included in this study had more than a high school education with 25% having had no schooling at all.
Clustering of outcomes was observed in these data with several children experiencing more than one episode of LRTI or RSV-specific LRTI pointing to the role of some host-specific factors. This clustering was significant for all-cause pneumonia with 63% of the study population experiencing two or more episodes of LRTI.
The magnitude of a risk at the population level is related to its prevalence. For instance, although children in households with 11 or more occupants are at increased risk of severe RSV disease (RR 2.6), only 3.3% of households have this many occupants. In contrast, having 1–2 siblings under 6 years is of lower risk (RR 2) but 56.5% of the population fall into this category.
A possible limitation of this study is that diagnosis of RSV infection on the basis of antigen detection alone has lower sensitivity compared with the use of assay combinations (Hall et al. 1976; Glezen et al. 1986). However, the sensitivity of the immunofluorescence antigen test is related to the concentration of antigen in the sample, so that infections undiagnosed are more likely to be milder. Consequently, the risk factors we have found are strictly associated with RSV infection detectable by our methods. The strength of the study was the re-specification of time-varying covariates during the study period reducing possible misclassification of exposure.
In conclusion, our results strongly suggest poor host nutritional status (severe stunting) and household size (high contact intensity and sibling numbers) as significant risk factors for severe RSV disease, and that these are broadly common to all-cause LRTI. These data not only reinforce previously suspected associations but also, through careful study design, provide more specific evidence for the relationship with disease progression. These data have implications for our understanding of RSV transmission and control. The notion of differences in the risk of acquiring disease according to contact age and intensity suggests the importance of these factors in transmission and control particularly in relation to who acquires infection from whom. In the light of this, vaccination programmes targeted to school children, who constitute the siblings within households, may show promise by providing indirect protection to the infant. Additional benefits would be achieved from education of mothers on reducing the intensity of contacts between siblings and the naïve young infant who is at most risk of severe disease. Furthermore, an increased risk of pneumonia in those with chronic poor nutritional status reinforces the need for community-based interventions directed towards improved diet, supplementation (vitamin supplements or fortified milk) and parental education (promoting breastfeeding), already acknowledged to have significant positive benefits on all outcomes.
Acknowledgments
We are indebted to all the enrolled children and their guardians, all staff of the RSV team (field, ward and lab) and the research out-patients clinic. We are grateful to Prof Bob Snow, Dr Mike English and Dr Lisa J White for their helpful comments on earlier versions of this paper, and Prof Laura Green for advice on statistical analysis. The study is published with permission of the Director of KEMRI. Support for this study was provided by The Wellcome Trust (061584, 076278).
References
- Aaby P. Malnutrition and overcrowding/intensive exposure in severe measles infection: review of community studies. Reviews of Infectious Diseases. 1988;10:478–491. doi: 10.1093/clinids/10.2.478. [DOI] [PubMed] [Google Scholar]
- Aaby P, Coovadia H. Severe measles: a reappraisal of the role of nutrition, overcrowding and virus dose. Medical Hypotheses. 1985;18:93–112. doi: 10.1016/0306-9877(85)90042-8. [DOI] [PubMed] [Google Scholar]
- Aaby P, Bukh J, Lisse IM, Smits AJ. Measles mortality, state of nutrition, and family structure: a community study from Guinea-Bissau. Journal of Infectious Diseases. 1983;147:693–701. doi: 10.1093/infdis/147.4.693. [DOI] [PubMed] [Google Scholar]
- Aaby P, Bukh J, Lisse IM, Smits AJ. Overcrowding and intensive exposure as determinants of measles mortality. American Journal of Epidemiology. 1984;120:49–63. doi: 10.1093/oxfordjournals.aje.a113874. [DOI] [PubMed] [Google Scholar]
- Adegbola RA, Falade AA, Sam BE, et al. The etiology of pneumonia in malnourished and well-nourished Gambian children. The Pediatric Infectious Disease Journal. 1994;13:975–982. doi: 10.1097/00006454-199411000-00008. [DOI] [PubMed] [Google Scholar]
- Ballard TJ, Neumann CG. The effects of malnutrition, parental literacy and household crowding on acute lower respiratory infections in young Kenyan children. Journal of Tropical Pediatrics. 1995;41:8–13. doi: 10.1093/tropej/41.1.8. [DOI] [PubMed] [Google Scholar]
- Berglund B. Respiratory syncytial virus infections in families. A study of family members of children hospitalized for acute respiratory disease. Acta Paediatrica Scandinavica. 1967;56:395–404. doi: 10.1111/j.1651-2227.1967.tb15398.x. [DOI] [PubMed] [Google Scholar]
- Broor S, Pandey RM, Ghosh M, et al. Risk factors for severe acute lower respiratory tract infection in under-five children. Indian Pediatrics. 2001;38:1361–1369. [PubMed] [Google Scholar]
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE, WHO Child Health Epidemiology Reference Group WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- Cardoso MR, Cousens SN, De Goes Siqueira LF, Alves FM, D’angelo LA. Crowding: risk factor or protective factor for lower respiratory disease in young children? BMC Public Health. 2004;4:19. doi: 10.1186/1471-2458-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra RK. Nutrition, immunity, and infection: present knowledge and future directions. Lancet. 1983;1:688–691. doi: 10.1016/s0140-6736(83)91980-3. [DOI] [PubMed] [Google Scholar]
- Cruz JR, Pareja G, De Fernandez A, Peralta F, Caceres P, Cano F. Epidemiology of acute respiratory tract infections among Guatemalan ambulatory preschool children. Reviews of Infectious Diseases. 1990;12:S1029–S1034. doi: 10.1093/clinids/12.supplement_8.s1029. [DOI] [PubMed] [Google Scholar]
- DeVincenzo JP. Factors predicting childhood respiratory syncytial virus severity: what they indicate about pathogenesis. The Pediatric Infectious Disease Journal. 2005;24:S177–S183. doi: 10.1097/01.inf.0000187274.48387.42. [DOI] [PubMed] [Google Scholar]
- Djelantik IG, Gessner BD, Soewignjo S, et al. Incidence and clinical features of hospitalization because of respiratory syncytial virus lower respiratory illness among children less than two years of age in a rural Asian setting. The Pediatric Infectious Disease Journal. 2003;22:150–157. doi: 10.1097/01.inf.0000048908.43063.c6. [DOI] [PubMed] [Google Scholar]
- Filmer D, Pritchett LH. Estimating wealth effects without expenditure data – or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- Gardner G, Frank AL, Taber LH. Effects of social and family factors on viral respiratory infection and illness in the first year of life. Journal of Epidemiology and Community Health. 1984;38:42–48. doi: 10.1136/jech.38.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen W, Taber L, Frank A, Kasel J. Risk of primary infection and reinfection with respiratory syncytial virus. American Journal of Diseases of Children. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- Greenwood B, Weber M, Mulholland K. Childhood pneumonia – preventing the world’s biggest killer of children. Bulletin of the World Health Organization. 2007;85 doi: 10.2471/BLT.07.044032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Douglas RG. Modes of transmission of respiratory syncytial virus. Journal of Pediatrics. 1981;99:100–103. doi: 10.1016/s0022-3476(81)80969-9. [DOI] [PubMed] [Google Scholar]
- Hall CB, Geiman JM, Biggar R, Kotok DI, Hogan PM, Douglas GR. Respiratory syncytial virus infections within families. The New England Journal of Medicine. 1976;294:414–419. doi: 10.1056/NEJM197602192940803. [DOI] [PubMed] [Google Scholar]
- Holberg CJ, Wright AL, Matinez FD. Risk factors for respiratory syncytial virus-associated lower respiratory illness in the first year of life. American Journal of Epidemiology. 1991;133:1135–1151. doi: 10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- Karron RA, Singleton RJ, Bulkow L, et al. Severe respiratory syncytial virus disease in Alaska native children. RSV Alaska Study Group. The Journal of Infectious Diseases. 1999;180:41–49. doi: 10.1086/314841. [DOI] [PubMed] [Google Scholar]
- Loscertales MP, Roca A, Ventura PJ, et al. Epidemiology and clinical presentation of respiratory syncytial virus infection in a rural area of southern Mozambique. The Pediatric Infectious Disease Journal. 2002;21:148–155. doi: 10.1097/00006454-200202000-00013. [DOI] [PubMed] [Google Scholar]
- Madhi SA, Venter M, Madhi A, Petersen MK, Klugman KP. Differing manifestations of respiratory syncytial virus-associated severe lower respiratory tract infections in human immunodeficiency virus type 1-infected and uninfected children. The Pediatric Infectious Disease Journal. 2001;20:164–170. doi: 10.1097/00006454-200102000-00010. [DOI] [PubMed] [Google Scholar]
- Miranda-Novales G, Solorzano-Santos F, Leanos-Miranda B, Vazquez-Rosales G, Palafox-Torres M, Guiscafre-Gallardo H. Blood culture and respiratory syncytial virus identification in acute lower respiratory tract infection. Indian Journal of Pediatrics. 1999;66:831–836. doi: 10.1007/BF02723847. [DOI] [PubMed] [Google Scholar]
- Monto AS. Epidemiology of viral respiratory infections. American Journal of Medicine. 2002;112(Suppl. 6A):4S–12S. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- Monto A, Sook K. The Tecumesh study of respiratory illness. III. incidence and periodicity of respiratory syncytial virus and Mycoplasma pneumoniae infections. American Journal of Epidemiology. 1971;94:290–301. doi: 10.1093/oxfordjournals.aje.a121322. [DOI] [PubMed] [Google Scholar]
- Mufson MA, Belshe RB, Orvell C, Norrby E. Respiratory syncytial virus epidemics: variable dominance of subgroups A and B strains among children 1981–1986. The Journal of Infectious Diseases. 1988;157:143–148. doi: 10.1093/infdis/157.1.143. [DOI] [PubMed] [Google Scholar]
- Neumann CG, Lawlor GJ, Stiehm ER, et al. Immunologic responses in malnourished children. The American Journal of Clinical Nutrition. 1975;28:89–104. doi: 10.1093/ajcn/28.2.89. [DOI] [PubMed] [Google Scholar]
- Nielsen HE, Siersma V, Andersen S, et al. Respiratory syncytial virus infection – risk factors for hospital admission: a case-control study. Acta Paediatrica. 2003;92:1314–1321. [PubMed] [Google Scholar]
- Nokes DJ, Okiro EA, Ngama M, et al. Respiratory syncytial virus epidemiology in a birth cohort from Kilifi district, Kenya: infection during the first year of life. The Journal of Infectious Diseases. 2004;190:1828–1832. doi: 10.1086/425040. [DOI] [PubMed] [Google Scholar]
- Nokes DJ, Okiro EA, Ngama M, et al. Respiratory syncytial virus infection and disease in infants and young children observed from birth in Kilifi District, Kenya. Clinical Infectious Disease Journal. 2008;46:50–57. doi: 10.1086/524019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwankwo MU, Okuonghae HO, Currier G, Schuit KE. Respiratory syncytial virus infections in malnourished children. Annals of Tropical Paediatrics. 1994;14:125–130. doi: 10.1080/02724936.1994.11747704. [DOI] [PubMed] [Google Scholar]
- Okiro E. Transmission Dynamics of Respiratory Syncytial Virus within the Household and in the Community. Milton Keynes: Open University; 2007. [Google Scholar]
- Pandey MR, Boleij JS, Smith KR, Wafula EM. Indoor air pollution in developing countries and acute respiratory infection in children. Lancet. 1989;1:427–429. doi: 10.1016/s0140-6736(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Robertson SE, Roca A, Alonso P, et al. Respiratory syncytial virus infection: denominator-based studies in Indonesia, Mozambique, Nigeria and South Africa. Bulletin of the World Health Organization. 2004;82:914–922. [PMC free article] [PubMed] [Google Scholar]
- Selwyn BJ. The epidemiology of acute respiratory tract infection in young children: comparison of findings from several developing countries. Reviews of Infectious Diseases. 1990;12(Suppl. 8):S870–S888. doi: 10.1093/clinids/12.supplement_s870. [DOI] [PubMed] [Google Scholar]
- Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. Journal of Pediatrics. 2003;143:S118–S126. doi: 10.1067/s0022-3476(03)00511-0. [DOI] [PubMed] [Google Scholar]
- Sullender WM. Respiratory syncytial virus genetic and antigenic diversity. Clinical Microbiology Reviews. 2000;13:1–15. doi: 10.1128/cmr.13.1.1-15.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanjutha S, Sunakorn P, Chantarojanasiri T, et al. Respiratory syncytial virus-associated lower respiratory tract infection in under-5-year-old children in a rural community of central Thailand, a population-based study. Journal of the Medical Association of Thailand. 2002;85(Suppl. 4):S1111–S1119. [PubMed] [Google Scholar]
- Tupasi TE, Mangubat NV, Sunico MES, et al. Malnutrition and acute respiratory tract infections in Filipino children. Reviews of Infectious Diseases. 1990;12:S1047–S1054. doi: 10.1093/clinids/12.supplement_8.s1047. [DOI] [PubMed] [Google Scholar]
- Vardas E, Blaauw D, Mcanerney J. The epidemiology of respiratory syncytial virus (RSV) infections in South African children. South African Medical Journal. 1999;89:1079–1084. [PubMed] [Google Scholar]
- Watson RR, Mcmurray DN, Martin P, Reyes MA. Effect of age, malnutrition and renutrition on free secretory component and IgA in secretions. The American Journal of Clinical Nutrition. 1985;42:281–288. doi: 10.1093/ajcn/42.2.281. [DOI] [PubMed] [Google Scholar]
- Weber MW, Dackour R, Usen S, et al. The clinical spectrum of respiratory syncytial virus disease in The Gambia. The Pediatric Infectious Disease Journal. 1998a;17:224–230. doi: 10.1097/00006454-199803000-00010. [DOI] [PubMed] [Google Scholar]
- Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Tropical Medicine and International Health. 1998b;3:268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- Weber MW, Milligan P, Hilton S, et al. Risk factors for severe respiratory syncytial virus infection leading to hospital admission in children in the Western Region of The Gambia. International Journal of Epidemiology. 1999;28:157–162. doi: 10.1093/ije/28.1.157. [DOI] [PubMed] [Google Scholar]
- Weber MW, Milligan P, Sanneh M, et al. An epidemiological study of RSV infection in the Gambia. Bulletin of the World Health Organization. 2002;80:562–568. [PMC free article] [PubMed] [Google Scholar]
- WHO . Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Illnesses with Limited Resources. Geneva: WHO; 2005. [Google Scholar]
- WHO . The Forgotten Killer of Children. Geneva: WHO; 2006a. [Google Scholar]
- WHO . WHO Child Growth Standards STATA Macro (igrowup.ado) Geneva: WHO; 2006b. [Google Scholar]