Abstract
Contraction of the large Igh and Igκ loci brings all V genes, spanning >2.5 Mb in each locus, in proximity to DJH or Jκ genes. CTCF is a transcription factor that regulates gene expression by long-range chromosomal looping. We therefore hypothesized CTCF may be crucial for the contraction of the Ig loci, but no CTCF sites have been described in any V loci. Utilizing ChIP-Chip, we demonstrated many CTCF sites in the VH and Vκ regions. However, CTCF enrichment in the Igh locus, but not the Igκ locus, was largely unchanged throughout differentiation, suggesting that CTCF binding alone cannot be responsible for stage-specific looping. Since cohesin can colocalize with CTCF, we performed ChIP for the cohesin subunit Rad21, and found lineage and stage-specific Rad21 recruitment to CTCF in all Ig loci. The differential binding of cohesin to CTCF sites at may promote multiple loop formation and thus effective V(D)J recombination.
Keywords: B cells, Gene rearrangement, Gene regulation, Transcription Factors
INTRODUCTION
Diversity in the Ig antibody repertoire is achieved through V(D)J recombination, a lineage specific process that is highly regulated during B-cell development. IgH rearrangement in pro-B cells begins with DH to JH rearrangement followed by rearrangement of a VH gene segment to DHJH. The IgH is assembled prior to the light chains and Igκ rearrangement precedes Igλ rearrangement. Strict regulation of accessibility allows for the lineage-specific and developmentally ordered rearrangement of V(D)J gene segments. The mechanisms controlling VH to DHJH rearrangement are exceptionally complex because over 100 functional murine VH genes span a 2.5 Mb region. Likewise, the 96 functional Vκ genes cover 3.1 Mb. The question arises how all the V genes acquire access to the small J cluster (<2 kb) in either the Igh or Igκ loci. 3D FISH studies have demonstrated that the Igh and Igκ loci undergo significant contraction to position gene segments in proximity for rearrangement at the appropriate time for rearrangement (1-4). In pro-B cells, the VH genes are brought into close proximity to DH gene segments via multiple loop structures, thus facilitating rearrangement through these long-range chromosomal interactions (4). The contraction and looping of the loci raises the question of which nuclear factors could be controlling these interactions. One potential nuclear protein that participates in long-range chromosomal interactions is CTCF (CCCTC-binding factor).
CTCF is a ubiquitously expressed 11-zinc finger nuclear protein that is associated with all known vertebrate insulators (5). Chromatin insulators or boundary elements create distinct chromosomal domains, preventing outside influences on the insulated region (5). The enhancer-blocking activity of CTCF prevents the interactions between enhancers and promoters separated by the insulator. Global-mapping of CTCF binding has shown that CTCF binds in regions that could separate different chromosomal domains, consistent with the idea that CTCF may insulate the spread of repressive chromatin modifications into neighboring active domains (6-8). One of the ways that CTCF may function as an insulator and regulate gene expression is through the facilitation of long-range intrachromosomal and interchromosomal looping (9-11).
CTCF has been reported to associate with a number of factors including YY1 (12), the chromodomain helicase CHD8 (13), and cohesin subunits (14-17). Cohesin proteins have an established role of facilitating cohesion of sister chromatids during cell division (18), however, emerging roles for cohesin also include regulation of gene expression and chromatin insulator functions. Cohesin consists of four core subunits named Smc1, Smc3, Rad21, and Scc3. Recently, through mapping of binding sites, four groups have demonstrated that cohesin co-localizes with CTCF at many sites (14-17), with CTCF being the DNA-binding protein. Partial genome-wide mapping of cohesin binding sites in mouse and human cells, revealed that 77-89% of the cohesin-binding sites overlapped with binding sites for CTCF, whereas 60-65% of CTCF sites also bind cohesin (14, 17). The association of cohesin binding with CTCF sites may be tissue specific since subsets of cohesin binding sites showed tissue specific enrichment of cohesin (14).
Since CTCF sites are known to be important in the formation of chromosomal loops and domains, we hypothesized that we should find many CTCF sites in the IgV loci, and that they should be bound in a stage-specific manner if CTCF is responsible for creating the multiple loop structure seen at the Igh locus. Our ChIP-Chip studies identified CTCF binding sites across the Igh, Igκ, and Igλ loci in different stages of lymphocyte development, but not always in a stage-specific pattern. However, CTCF colocalizes with the cohesin subunit Rad21 in a largely developmental stage-specific and lineage-specific manner, suggesting that this CTCF-cohesin complex may be involved in locus contraction.
MATERIALS AND METHODS
Mice and cell lines
RAG-/- mice on a C57BL/6 background, RAG-/- mice bearing a rearranged IgH gene (μ+RAG-/-), and C57BL/6 mice, were bred and maintained at TSRI in accordance with protocols approved by the TSRI IACUC. The B6 MEF cells were obtained from K. Mowen (TSRI).
Purification of pro-B, pre-B cells and thymocytes
Bone marrow cells were isolated from 4- 6-week-old RAG-/- and μ+RAG-/- mice and were enriched for CD19+ cells with MACS microbeads (Miltenyi Biotec). The CD19+ cells from RAG-/- mice and from μ+RAG-/- mice were used as the source of pro-B cells and pre-B cells, respectively, for all the data in this study. Thymocytes were harvested from 4- to 6-week-old C57BL/6 mice.
ChIP and qPCR
ChIP and qPCR were performed as previously described (19) using antibodies against CTCF (Millipore #07-729), RAD21 (Abcam, #ab992), and IgG as a negative control. Real-time PCR was performed by using the Quantitec SYBR PCR Kit (Qiagen) and the ABI Prism 7900 Sequence Detection System (Applied Biosystems). Enrichment of target genes in ChIP preparations was normalized to actin. ChIPs were performed 2-4 times for each cell type from independent samples. Means ± SEMs were calculated from the average of 2-3 PCR repeats for each primer set from each ChIP.
ChIP-on-Chip
Affymetrix GeneChip Mouse Tiling 2.0R Array E and Array F were used according to the Affymetrix protocol. Each ChIP-Chip analysis is the average of two ChIP-Chips from two independent samples.
Online Supplemental Material
Extended Methods explains ChIP primer design and ChIP-Chip procedures. Results from ChIP assay for YY1 are shown in Figure S1.
RESULTS AND DISCUSSION
Identification of CTCF binding sites across the Igh locus by ChIP-Chip
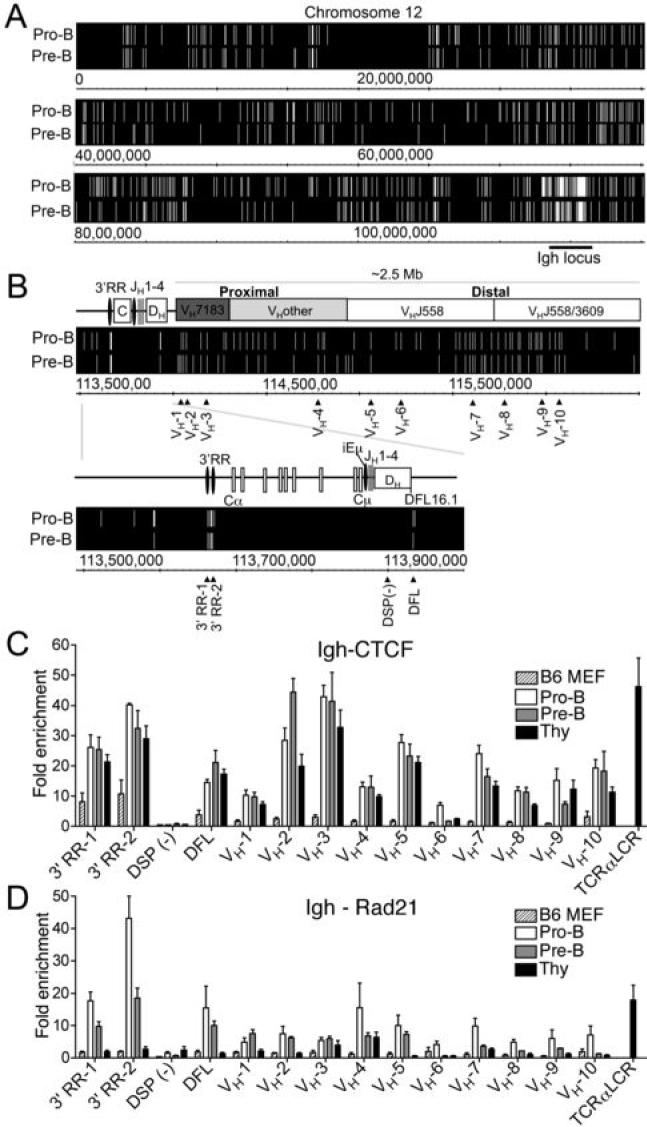
To identify sites bound by CTCF we performed ChIP-Chip analyses using the Affymetrix Mouse Tiling Array E that contains the Igh locus, and the data was analyzed with MAT software (20). ChIP-Chip analyses revealed that there is a higher density of bound CTCF in the Igh locus relative to all other regions of chromosome 12 in both pro-B and pre-B cells (Fig. 1A). A previous report identified a robust CTCF binding site in the 3' regulatory region (3' RR) of the Igh locus that is occupied in pro-B cells (21), however no other CTCF binding sites within the murine Igh locus have previously been described. The only other CTCF sites in the entire Igh locus other than within the VH locus are two closely positioned sites, located ~3.2 and 5.6 kb 5' of DFL16.1, the most 5' DH gene (Fig. 1B). We propose that this site may function as an insulator/barrier that could separate the D and J regions from the V regions in pre-pro-B cells by looping to the 3' RR. This hypothesis is based in part on data from the studies of Jhunjhunwala et al. (4). The CTCF site upstream of DFL16.1 is near their h5 probe, which is buried within the C region probes in pre-pro-B cells, near probe h2 (3' RR) (4). In pro-B cells, there is a dramatic conformational change in the Igh locus. The distal and proximal VH regions move close to each other, and the Dh probe h5 leaves the constant region, and moves close to the VH locus, especially to the distal VH probe h10 (4). We suggest that the CTCF site just upstream of DFL may bind to the CTCF sites at the 3' RR during the pre-pro-B cell stage, when only D to J rearrangement occurs, forming a DJCH domain that excludes the VH genes. We propose that the accessibility of the VH locus depends upon this CTCF site at DFL moving to connect with the VH locus.
FIGURE 1. CTCF binding sites in the Igh locus.
A, ChIP-chip analysis of CTCF binding in pro-B and pre-B cells across chromosome 12 with the Igh locus underlined. Lines indicate sites of CTCF binding from two independent experiments using MAT software (see Extended Methods). B, Map of the murine Igh locus and CTCF ChIP-chip analysis in pro-B cells and pre-B cells. Primer locations (▴) and negative control primers (-) are indicated. C, qPCR of CTCF and RAD21 ChIPs with primer sets for CTCF sites in the Igh locus. Results represent the mean ± SEM of 2-4 ChIPs.
The remaining 53 CTCF binding sites in pro-B cells are all within the VH locus. There are 8 sites bound by CTCF in the proximal region, with 6 sites near VH7183 genes and 2 close to VHQ52 genes. In the middle VH region there are 12 sites, and finally there are 33 sites in the 1.5 Mb VHJ558/3609 region. The sites in the VH7183/Q52 region are located within a short distance 3' of the RSS. All of the CTCF binding sites in the J558/3609 region were either far upstream of the VH coding regions or intergenic.
If CTCF were the limiting factor required for contraction of the Igh locus in pro-B cells, we would predict that we would see fewer sites bound in pre-B cells, since the Igh locus begins decontraction at this stage (1). However, our ChIP-Chip analyses with pre-B cells revealed a roughly similar pattern of bound CTCF across the Igh locus compared to pro-B cells, with the exception of a gap in CTCF binding sites in the middle of the VH locus (Fig. 1B).
Since ChIP-Chip analysis is not quantitative, qPCR is necessary to determine possible differences between CTCF bound to sites in pro-B, pre-B, and thymocytes. Using qPCR, we compared the relative enrichment of CTCF at specific binding sites in pro-B cells, pre-B cells, and thymocytes (Fig. 1C). Our results indicated that CTCF binding to the VH locus is largely similar in B lineage progenitors, and even in thymocytes which do not undergo VH rearrangement (Fig. 1C), suggesting that CTCF is not the cell-type specific factor that changes to promote looping of the VH region in pro-B cells. However, CTCF may be part of a complex that includes other proteins that do bind in a cell-type specific manner.
RAD21 colocalizes with CTCF throughout the Igh locus preferentially in pro-B cells
Since cohesin colocalizes with CTCF at the majority of sites genome-wide (14-17), we hypothesized that cohesin could be the stage-specific factor to facilitate Igh looping. To test this, we performed qPCR at all the CTCF sites for which we had designed qPCR primers. In pro-B cells, we found extensive Rad21 binding at CTCF binding sites throughout the Ig loci (Fig. 1D). In pre-B cells, the recruitment of Rad21 at CTCF binding sites is reduced at all CTCF sites other than the most proximal portion of the VH locus (Fig. 1D). Rad21 enrichment at the CTCF sites is much lower in thymocytes compared to both pro-B and pre-B cells, however CTCF binding is observed at the known CTCF site in the TCRα LCR that we used as a positive control. From this data, it appears that many CTCF sites are involved in lineage specific recruitment of Rad21.
Another potential CTCF cofactor that could be involved in looping is YY1, since YY1-/- pro-B cells do not undergo Igh locus contraction (22). We identified CTCF sites that had adjacent YY1 consensus sites, however we did not see binding of YY1 by ChIP at these sites (Supplementary Fig. S1). Furthermore, it has been demonstrated that YY1 is a required cofactor along with CTCF for X chromosome inactivation (12). It is possible that YY1 is not a cofactor with CTCF, or it could be part of a large multi-protein complex and not accessible to the antibody we used in the ChIP studies.
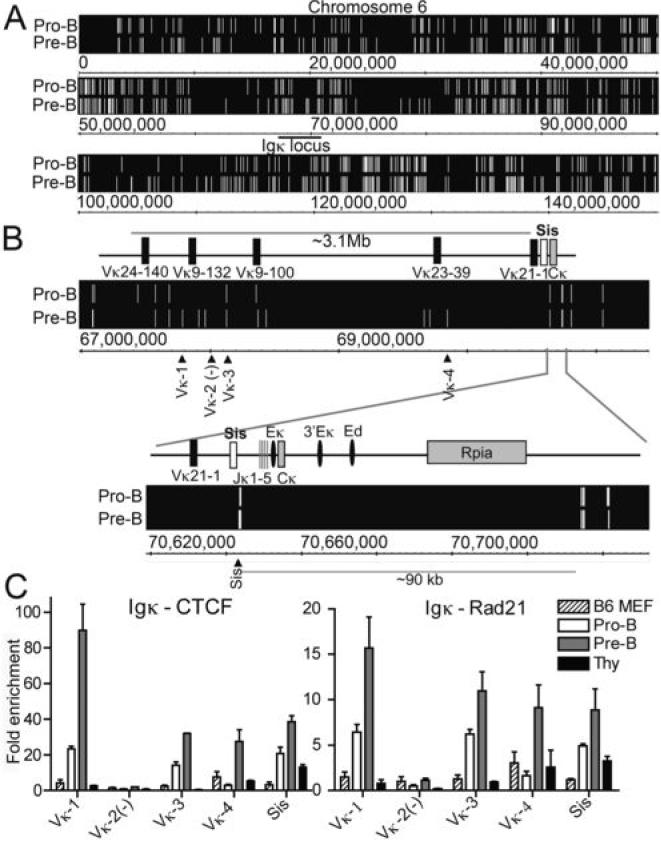
Identification of CTCF binding sites throughout the Igκ locus and cohesin colocalization
In pro-B cells, the kappa locus is extended but in the pre-B cell stage, the Igκ alleles undergo contraction in preparation for Igκ rearrangement (1). The Igκ locus contains ~96 functional Vκ genes that span over 3.1 Mb and this region must contract in order to bring all of the Vκ gene segments in proximity with one of the 4 functional Jκ genes that are contained within a small 1.3 kb region. Using ChIP-chip on Array F we identified CTCF sites throughout the kappa locus that are occupied in pro-B and pre-B cells (Fig. 2A). Despite the comparable size of the Igh and Igκ loci and similar number of V genes, there are fewer sites bound by CTCF in the kappa locus (Fig. 2B) than in the Igh locus (Fig. 1B). There are 11 CTCF sites in the Vκ region that are occupied in pre-B cells (Fig. 2B). In pro-B cells, only 4 sites are occupied in the distal region (Fig. 4A). The only other CTCF site in the entire kappa locus is associated with the recombination silencer Sis located in the Vκ-Jκ intron. The next closest CTCF site is 70 kb 3' of the 3'Eκ, past the unrelated Rpia gene (Fig. 2B). Using qPCR we found that in the Vκ locus, the CTCF sites closest to Vκ9-132 and Vκ23-39 have higher CTCF binding in pre-B cells compared to other cell types (Fig. 2B). At the CTCF site in Sis, we observe CTCF binding in all three cell types, however it is highest in pre-B cells. Thus, in contrast to the Igh locus where there was similar binding of CTCF in thymocytes, pro-B and pre-B cells for most of the primer locations, at the Igκ locus there is differential CTCF enrichment at different sites among the different cell types (Fig. 2C). This suggests that lineage-specific recruitment of CTCF may be more important in the Igκ locus compared to the Igh locus. Rad21 recruitment to all four analyzed CTCF sites is highest in pre-B cells, and is reduced in pro-B cells and thymocytes (Fig. 2C).
FIGURE 2. CTCF binding sites in the Igκ locus.
A ChIP-chip analysis of CTCF binding in pro-B and pre-B cells across chromosome 6. The location of the Igκ locus is underlined. B, Map of the murine Igκ locus and ChIP-chip analysis of the kappa displaying the entire kappa locus (top) and a close-up of the Sis, Jκ, Cκ, Eκ region (bottom). C, qPCR of CTCF and RAD21 ChIPs with primer sets at CTCF sites in the Igκ locus. Results represent the mean ± SEM of 2-3 ChIPs.
Sis is a cis-acting element that negative regulates rearrangement of the Igκ locus (23). Sis has been demonstrated to recruit individual kappa alleles to heterochromatin by association with Ikaros, a repressor protein that binds to heterochromatin. A 3.7 kb fragment containing Sis, and also containing this CTCF site, has been deleted in a YAC transgenic minilocus, and the deletion results in a 5-fold increase in Vκ-Jκ recombination (23). The pre-B cells that contained the deleted Sis element displayed less association with heterochromatin. Although Liu et al. demonstrated by ChIP that there are other sites with Ikaros bound in the Jκ and Cκ regions, these sites do not result in heterochromatin targeting in the absence of Sis, suggesting that other unidentified proteins besides Ikaros may be involved in the heterochromatin recruitment mediated by Sis. Since the CTCF site we identified is in the middle of the Sis region, and is flanked on both sides by Ikaros motifs, it is interesting to speculate that CTCF may cooperate with Ikaros for heterochromatin recruitment. However, unlike the Igh locus, where we suggest that the CTCF site flanking DFL16.1 may bind to CTCF sites within the VH regions and facilitate looping, the Sis deletion resulted in increased Igκ rearrangement, suggesting that this particular CTCF site is not required for kappa locus contraction. It should be considered, however, that the increased rearrangement may be due to decreased recruitment to the repressive heterochromatin. Ikaros has been shown to play a role in locus contraction at the Igh locus, since Ikaros knockout mice reconstituted with RAG activity display reduced Igh locus contraction (24). Whether CTCF and Ikaros cooperate for Igh locus contraction is unknown.
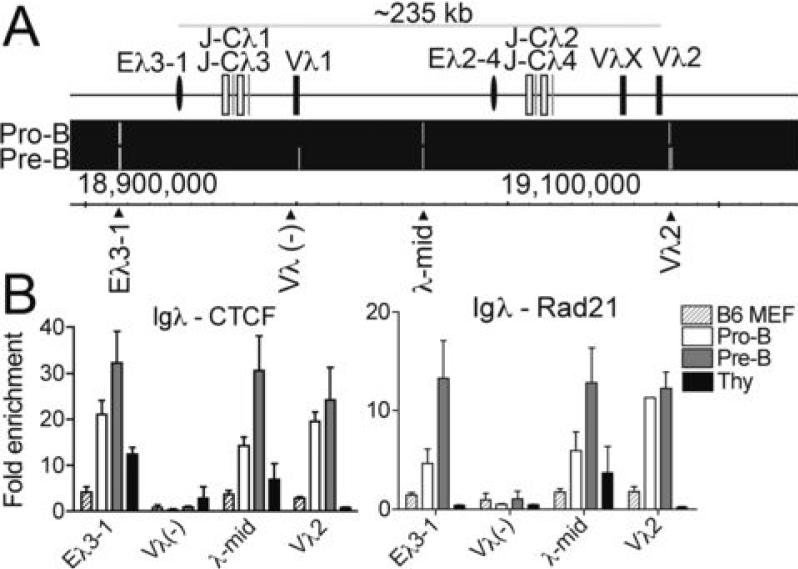
Identification of CTCF binding sites throughout the Igλ locus and cohesion colocalization
Compared to the Igh and Igκ loci, the murine lambda locus is simple and small (234 kb). There are two related clusters, each of which has an enhancer, two sets of J and C regions and either one or two Vλ genes. In both pro-B and pre-B cells, there are three strong CTCF sites bracketing the clusters (Fig. 3A). The CTCF at the 3' end of the λ locus has equal enrichment of CTCF and Rad21 in pro-B and pre-B cells, whereas the other two CTCF sites show highest binding in pre-B cells (Fig. 3B). Due to the small size of the λ locus, and since Vλ genes are close to their J genes, it is unlikely that contraction occurs at this locus. Thus, the CTCF sites at the 5' and 3' ends of the locus may serve as traditional insulator boundary elements.
FIGURE 3. CTCF binding sites in the Igλ locus.
A, Map of the murine IGλ locus and ChIP-Chip analysis of CTCF binding sites in pro-B and pre-B cells. B, qPCR of CTCF and RAD21 ChIPs with primer sets at CTCF sites in the IGλ. Results represent the mean ± SEM of 2-3 ChIPs.
Lineage specificity of CTCF binding
In this report, we identified CTCF binding sites within the three receptor loci (Igh, Igκ, and Igλ) located on three different chromosomes through ChIP-Chip. We observed similar levels of CTCF binding in pro-B cells compared to that of pre-B cells and thymocytes for most sites in the Igh locus, suggesting another factor such as cohesin is involved cell-type specific looping. Another study reported that 67% of the CTCF sites overlapped between two human cell types, primary fibroblast IMR90 and hematopoietic progenitor U937 cells, suggesting that many CTCF sites do not vary by cell-type (7). The functional activity of CTCF across different developmental stages or tissues could be regulated by posttranslational modifications (5), which would likely alter the interactions of CTCF with other proteins.
In comparison to the Igh locus, CTCF enrichment at sites in the light chains tends to display more of a site-specific and lineage specific pattern. Also, the extent of CTCF binding in MEFs was much lower across the all three receptor loci (Fig. 1C, 2C, and 3B), demonstrating cell type specificity. Interestingly, in accord with lower levels of CTCF binding, the Igh locus is significantly more extended in MEFs than in pro-B cells (4).
Our data suggests that cohesin binding may be cell type specific at the majority of CTCF sites at all Ig loci. CTCF and cohesin together may be critical for the V locus loop formation which is required for locus contraction. This permits the large V loci to move in proximity to the (D)J regions to accomplish the rearrangement process at appropriate stages of differentiation. Given that there are many CTCF and Rad21 binding sites within the VH locus, these may well facilitate the multiple loop formation in rosettes seen at the Igh locus (4). The absence of CTCF sites between the 5' end of the DH region and the 3' RR is also in accordance with the 3D-FISH studies of locus contraction (4). In addition, there may be other proteins that work together with CTCF and cohesion to direct lineage-specific looping of receptor loci.
There are many layers controlling the accessibility of the Ig receptor V genes for rearrangement including chromosomal movement away from the nuclear periphery, histone post-translational modifications, germline transcription, contraction and looping of the V loci, association with heterochromatin, and binding of specific transcription factors. The dynamics of Ig receptor loci recombination is a highly regulated, ordered, and lineage specific process. This analysis of CTCF and cohesin binding sites throughout Ig receptor loci provides a step towards understanding the factors that mediate contraction of Ig receptor loci during lymphocyte development. This looping is necessary to produce a diverse repertoire of antibodies to enable effective protection against a wide variety of pathogens.
Supplementary Material
Acknowledgements
We gratefully acknowledge the assistance of Lana Schaffer and Steven Head of the DNA Array Core Facility in the hybridization and analysis of the ChIP-chip experiments. We thank Dr. Kees Murre for helpful comments on the manuscript, and Dr. Kerri Mowen for the MEFs.
This work was supported by NIH grants R01 A129672 and R01 A152313. S. Degner was supported by NIH training grant T32 HL07195.
Abbreviations used
- 3'RR
3' regulatory region
- ChIP
chromatin immunoprecipitation
- CTCF
CCCTC-binding factor
- qPCR
quantitative real-time PCR
REFERENCES
- 1.Roldan E, Fuxa M, Chong W, Martinez D, Novatchkova M, Busslinger M, Skok JA. Locus `decontraction' and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat Immunol. 2005;6:31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosak ST, Skok JA, Medina KL, Riblet R, Le Beau MM, Fisher AG, Singh H. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science. 2002;296:158–162. doi: 10.1126/science.1068768. [DOI] [PubMed] [Google Scholar]
- 3.Sayegh CE, Jhunjhunwala S, Riblet R, Murre C. Visualization of looping involving the immunoglobulin heavy-chain locus in developing B cells. Genes Dev. 2005;19:322–327. doi: 10.1101/gad.1254305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jhunjhunwala S, van Zelm MC, Peak MM, Cutchin S, Riblet R, van Dongen JJ, Grosveld FG, Knoch TA, Murre C. The 3D structure of the immunoglobulin heavy-chain locus: implications for long-range genomic interactions. Cell. 2008;133:265–279. doi: 10.1016/j.cell.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace JA, Felsenfeld G. We gather together: insulators and genome organization. Curr Opin Genet Dev. 2007;17:400–407. doi: 10.1016/j.gde.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie X, Mikkelsen TS, Gnirke A, Lindblad-Toh K, Kellis M, Lander ES. Systematic discovery of regulatory motifs in conserved regions of the human genome, including thousands of CTCF insulator sites. Proc Natl Acad Sci U S A. 2007;104:7145–7150. doi: 10.1073/pnas.0701811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim TH, Abdullaev ZK, Smith AD, Ching KA, Loukinov DI, Green RD, Zhang MQ, Lobanenkov VV, Ren B. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell. 2007;128:1231–1245. doi: 10.1016/j.cell.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Ling JQ, Li T, Hu JF, Vu TH, Chen HL, Qiu XW, Cherry AM, Hoffman AR. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science. 2006;312:269–272. doi: 10.1126/science.1123191. [DOI] [PubMed] [Google Scholar]
- 10.Splinter E, Heath H, Kooren J, Palstra RJ, Klous P, Grosveld F, Galjart N, de Laat W. CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev. 2006;20:2349–2354. doi: 10.1101/gad.399506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majumder P, Gomez JA, Chadwick BP, Boss JM. The insulator factor CTCF controls MHC class II gene expression and is required for the formation of long-distance chromatin interactions. J Exp Med. 2008;205:785–798. doi: 10.1084/jem.20071843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donohoe ME, Zhang LF, Xu N, Shi Y, Lee JT. Identification of a Ctcf cofactor, Yy1, for the X chromosome binary switch. Mol Cell. 2007;25:43–56. doi: 10.1016/j.molcel.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Ishihara K, Oshimura M, Nakao M. CTCF-dependent chromatin insulator is linked to epigenetic remodeling. Mol Cell. 2006;23:733–742. doi: 10.1016/j.molcel.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Parelho V, Hadjur S, Spivakov M, Leleu M, Sauer S, Gregson HC, Jarmuz A, Canzonetta C, Webster Z, Nesterova T, Cobb BS, Yokomori K, Dillon N, Aragon L, Fisher AG, Merkenschlager M. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell. 2008;132:422–433. doi: 10.1016/j.cell.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Rubio ED, Reiss DJ, Welcsh PL, Disteche CM, Filippova GN, Baliga NS, Aebersold R, Ranish JA, Krumm A. CTCF physically links cohesin to chromatin. Proc Natl Acad Sci U S A. 2008;105:8309–8314. doi: 10.1073/pnas.0801273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stedman W, Kang H, Lin S, Kissil JL, Bartolomei MS, Lieberman PM. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. EMBO J. 2008;27:654–666. doi: 10.1038/emboj.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wendt KS, Yoshida K, Itoh T, Bando M, Koch B, Schirghuber E, Tsutsumi S, Nagae G, Ishihara K, Mishiro T, Yahata K, Imamoto F, Aburatani H, Nakao M, Imamoto N, Maeshima K, Shirahige K, Peters JM. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature. 2008;451:796–801. doi: 10.1038/nature06634. [DOI] [PubMed] [Google Scholar]
- 18.McNairn AJ, Gerton JL. The chromosome glue gets a little stickier. Trends Genet. 2008;24:382–389. doi: 10.1016/j.tig.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Espinoza CR, Feeney AJ. The extent of histone acetylation correlates with the differential rearrangement frequency of individual VH genes in pro-B cells. J Immunol. 2005;175:6668–6675. doi: 10.4049/jimmunol.175.10.6668. [DOI] [PubMed] [Google Scholar]
- 20.Johnson WE, Li W, Meyer CA, Gottardo R, Carroll JS, Brown M, Liu XS. Model-based analysis of tiling-arrays for ChIP-chip. Proc Natl Acad Sci U S A. 2006;103:12457–12462. doi: 10.1073/pnas.0601180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrett FE, Emelyanov AV, Sepulveda MA, Flanagan P, Volpi S, Li F, Loukinov D, Eckhardt LA, Lobanenkov VV, Birshtein BK. Chromatin architecture near a potential 3' end of the IgH locus involves modular regulation of histone modifications during B-Cell development and in vivo occupancy at CTCF sites. Mol Cell Biol. 2005;25:1511–1525. doi: 10.1128/MCB.25.4.1511-1525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Schmidt-Supprian M, Shi Y, Hobeika E, Barteneva N, Jumaa H, Pelanda R, Reth M, Skok J, Rajewsky K. Yin Yang 1 is a critical regulator of B-cell development. Genes Dev. 2007;21:1179–1189. doi: 10.1101/gad.1529307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Widlak P, Zou Y, Xiao F, Oh M, Li S, Chang MY, Shay JW, Garrard WT. A recombination silencer that specifies heterochromatin positioning and ikaros association in the immunoglobulin kappa locus. Immunity. 2006;24:405–415. doi: 10.1016/j.immuni.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Reynaud D, Demarco IA, Reddy KL, Schjerven H, Bertolino E, Chen Z, Smale ST, Winandy S, Singh H. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat Immunol. 2008;9:927–936. doi: 10.1038/ni.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.