Abstract
Since the first isolation of HIV-1 from a patient with generalized lymphadenopathy in 1983, great progress has been made in understanding the viral life cycle and the functional nuances of each of the nine genes encoded by HIV-1. Considerable attention has been paid to four small HIV-1 open reading frames, vif, vpr, vpu and nef. These genes were originally termed “accessory” because their deletion failed to completely disable viral replication in vitro. More than twenty years afte the cloning and sequencing of HIV-1, a great deal of information is available regarding the multiple functions of the accessory proteins and it is well accepted that, collectively, these gene products modulate the host cell biology to favor viral replication, and that they are largely responsible for the pathogenesis of HIV-1. Expression of Vpr, in particular, leads to cell cycle arrest in G2, followed by apoptosis. Here we summarize our current understanding of Vpr biology with a focus on Vpr-induced G2 arrest and apoptosis.
Introduction
HIV-1 Vpr
The first clue as to the role of Vpr in the viral life cycle came from the observation that truncation of the open reading frame resulted in a slower-replicating virus (Hattori et al., 1990; Ogawa et al., 1989; Wong-Staal et al., 1987) and hence its name, viral protein, regulatory. HIV-1 Vpr is a small 96 amino acid (14 kDa) protein that is delivered in two modes during infection. De novo expressed Vpr appears during the late phase of infection (Schwartz et al., 1991), while Vpr protein is also packaged into nascent virus particles via an interaction with Gag p6 (reviewed in (Tungaturthi et al., 2003)).
The precise contribution of Vpr to HIV-1 pathogenesis in vivo is difficult to determine, in part because Vpr is highly conserved. However, SIV studies from primate models suggest that the functions of Vpr are critical to AIDS progression. In the HIV-2/SIVmac/SIVsm lineage, the functions associated with HIV-1 Vpr have segregated into two genes, vpr and vpx (Tristem et al., 1992). While deletion of either SIVmac vpr or vpx alone only modestly reduces in vivo viral replication, deletion of both genes generates a severely crippled virus incapable of causing disease (Gibbs et al., 1995; Lang et al., 1993).
In vitro analysis has illustrated various Vpr functions that could potentially contribute to HIV-1 pathogenesis in vivo. These functions are transactivation of the HIV-1 long terminal repeat (LTR), nuclear import of preintegration complexes (PICs), induction of cell cycle arrest in G2, and apoptosis in infected cells.
Structure of HIV-1 Vpr
Several approaches to model the structure of Vpr by NMR and circular dichroism have been used with success (Henklein et al., 2000; Schuler et al., 1999; Wecker et al., 2002; Wecker and Roques, 1999). The first view of Vpr structure came from studies in which the N and C terminal halves of Vpr were analyzed separately by NMR (Schuler et al., 1999; Wecker and Roques, 1999). As had been predicted, amino acids 1–51 of Vpr form a α helix turn-α helix structure, while amino acids 52–96 form another α helix rich in leucine resides that has been proposed to form a leucine zipper-like structure (Bourbigot et al., 2004; Schuler et al., 1999; Wecker and Roques, 1999). Analysis of full-length Vpr in semi-hydrophobic solvent (CD3CN), confirmed earlier predictions showing the presence of the three amphipathic α helices folded around a hydrophobic core (Morellet et al., 2003). These helices are flanked by a flexible, negatively charged N-terminal domain, and a flexible, positively charged C-terminal domain rich in arginine residues (Morellet et al., 2003). Specific C-terminal arginines, such as R80, have been proposed to play a role in Vpr signaling and protein/protein interactions leading to G2 arrest and apoptosis (Gaynor and Chen, 2001; Lum et al., 2003).
Vpr sequence variation
The vpr gene is highly conserved in vivo, not only in HIV-1 isolates from different geographical origins, but also through the evolution of primate lentiviruses (Fletcher et al., 1996; Planelles et al., 1996; Tristem et al., 1998). However, due to the limited genetic variation of vpr in vivo, a direct link with viral pathogenesis remains largely theoretical. Two studies have provided compelling evidence that there is strong selection in favor of the vpr gene in vivo. In a vaccine study conducted in chimpanzees (Fultz et al., 1992), two animals were challenged with a virus stock derived from HIVIIIB, which encodes a truncated, unstable and non-functional Vpr protein. Goh and colleagues (Goh et al., 1998) performed a retrospective, longitudinal analysis of the vpr gene sequence in this animals virus populations, which revealed restoration of the truncated open reading frame in both chimpanzees at 6 to 8 weeks and at 2 years post-inoculation, respectively (Goh et al., 1998). The second study resulted from an unfortunate accident in which a laboratory worker became infected with a stock of HIVIIIB, which also contained the above inactivating mutation in vpr (Reitz et al., 1994; Weiss et al., 1988). Sequence analysis of virus from peripheral blood cells from this individual two years after infection revealed that the vpr gene reverted to full-length (Goh et al., 1998). Thus, there is positive selection for Vpr function in vivo.
More recently, various surveys of vpr sequences in long-term non-progressors (LTNP) populations have found non-synonymous nucleotide substitutions in vpr. The Vpr mutation Q3R was identified in viruses isolated from an LTNP patient who demonstrated high levels of viremia, yet did not show significant loss of CD4+ lymphocytes (Somasundaran et al., 2002). Later, another Vpr mutation, R77Q, was found in 80% of virus isolates from a cohort of LTNPs (Lum et al., 2003). The R77Q mutation was also present in a cohort of progressor patients, although with a lower frequency (33%) (Lum et al., 2003). Interestingly, both the R77Q and Q3R mutants induce G2 arrest, but induce apoptosis less efficiently, in comparison with wild-type Vpr (Lum et al., 2003; Somasundaran et al., 2002). In both studies, it was tempting to speculate that the reduction in Vpr-associated cytotoxicity might account for the nonprogressive clinical course in patients (Lum et al., 2003; Somasundaran et al., 2002). However, the association of R77Q with a non-progressive clinical phenotype has recently been questioned in two separate studies (Fischer et al., 2004; Rodes et al., 2004). Both studies sequenced virus from cohorts of LTNPs and compared these sequences with those from a progressor cohort, and observed that the R77Q mutation was found in both progressor and non-progressor cohorts at statistically equivalent frequencies (Fischer et al., 2004; Rodes et al., 2004). A more recent analysis of the GenBank database revealed that R77 predominates in subtype B, whereas Q77 predominates in clades A, C, D, G, H and groups O and N viruses (Rajan et al., 2006). In contrast, subtype F and K strains often contain H77 (Rajan et al., 2006). Rajan et al. also observed that a virus carrying Vpr Q77 induced less cytopathicity if it had R5 tropism, but not if it had X4 tropism (Rajan et al., 2006).
A recent study has analyzed the impact of the Vpr(R77Q) (Andersen et al., 2006) and Vpr(Q3R) (JA and VP, unpublished results) polymorphisms on Vpr function in vitro . The levels of induction of both G2 arrest and apoptosis were compared between wild-type Vpr and Vpr(R77Q) or Vpr(Q3R) and found that expression of either mutant resulted in normal, and not lower, levels of apoptosis induction. Two other surveys of viral sequences in LTNP populations found vpr to be highly conserved, and did not identify amino acid substitutions predicted to impact Vpr function (Alexander et al., 2000; Zhang et al., 1997).
Vpr from other primate lentiviruses
Vpr is conserved in five of the primate lentiviral lineages, including HIV-1/SIVcpz, HIV-2/SIVmac/SIVsm, SIVagm, SIVsyk, and SIVmnd (Tristem et al., 1998). SIV isolates from other primates including red-capped mangabey, mona, and mustached have been found to express Vpr although it is unclear whether Vpr from these SIV strains is functionally analogous to HIV-1 Vpr (Barlow et al., 2003; Beer et al., 2001; Courgnaud et al., 2003; Takemura and Hayami, 2004).
An interesting exception to the conservation of Vpr in the primate lentiviral lineages is the HIV-2/SIVmac/SIVsm lineage, in which the functions of Vpr have segregated into two genes, termed Vpr, and Vpx . Tristem et al. proposed that Vpx arose as a result of homologous recombination between SIVagm and an ancestor of HIV-2 (Tristem et al., 1992; Tristem et al., 1998). Both HIV-2 and SIVmac Vpr induce G2 arrest, but unlike Vpr from other lineages, these Vpr proteins do not assist in PIC nuclear import, a role taken over by Vpx (Fletcher et al., 1996; Planelles et al., 1996). Moreover, HIV-2 Vpx appears to exert a novel function by binding to the MHC class II invariant chain (Ii) and causing Ii degradation (Pancio et al., 2000). The cell surface presentation of exogenously-derived peptides by MHC class II molecules on the surfaces of antigen-presenting cells depends on the association between Ii and MHC class II within the ER and Golgi. Pancio et al. reported that cells stably expressing Vpx showed a marked decrease in Ii levels (Pancio et al., 2000), which could lead to a malfunction in MHC class II antigen presentation.
Other studies on interspecies differences in Vpr have focused on SIVagm Vpr and HIV-1 Vpr, which share 31% amino acid identity and are functionally conserved in virion encapsidation, cell cycle arrest, and transactivation of the LTR (Accola et al., 1999; Campbell and Hirsch, 1997; Philippon et al., 1999; Planelles et al., 1996; Stivahtis et al., 1997; Zhu et al., 2001). However, some differences between SIVagm Vpr and HIV-1 Vpr have been observed. One marked difference is that while HIV-1 lacking Vpr is able replicate in vitro, Vpr-null SIVagm is severely crippled and fails to replicate in vitro (Campbell and Hirsch, 1997). In addition, LTR transactivation and apoptosis induced by SIVagm Vpr appear to be at least partially independent of G2 arrest, in contrast with the interdependence that occurs in the context of HIV-1 Vpr (Zhu et al., 2001).
G2 Cell Cycle Arrest and Vpr
G2 arrest and DNA damage
Cell cycle arrest in G2 has been characterized in detail in the context of DNA damage so it is in this field that we understand the molecular pathways leading to, and the cellular consequences of, cell cycle checkpoint activation. Cells have evolved a variety of response pathways to protect the integrity of their genomes and have coordinated these pathways with cell cycle progression and apoptosis. The objectives of these pathways are to excise damaged DNA, rejoin DNA strand breaks, or directly reverse lesions with the ultimate goal of preventing propagation of DNA mutations in S phase or through cell division. Accordingly, checkpoint activation leads to cell cycle arrest prior to DNA replication (G1/S arrest) or prior to mitosis (G2/M arrest) to allow time for repair. If the damage is irreparable, the cell dies by apoptosis.
ATM and ATR: Regulators of the G2/M checkpoint in response to genotoxic stress
The cascade of events leading to cell cycle arrest is initiated when sentinel proteins within the nucleus recognize alterations in DNA/chromatin structure. These early-responding proteins include ataxia telangiectasia-mutated and Rad3-related (ATR), and ataxia telangiectasia-mutated (ATM). ATR and ATM can be categorized based on the type of lesion they respond to although some overlap in function does occur. In general, ATM is primarily activated by double strand breaks (DSBs), while ATR responds to a wider range lesions, but most notably, stalled replication forks (reviewed in (Harrison and Haber, 2006)).
The gene that encodes ATM is mutated in the autosomal recessive disorder, ataxia telangiectasia (A-T). A-T patients suffer from chromosomal instability, cancer susceptibility, growth retardation, cerebellar degeneration and immunodeficiency. In the absence of DNA damage, ATM exists as an inactive dimer with the kinase domain of each molecule concealed within its homodimeric binding region. Exposure of cells to ionizing radiation leads to ATM autophosphorylation at residue 1981 and dissociation of the dimer, which exposes the kinase domain and thus allows ATM to phosphorylate substrates (reviewed in (Ward and Chen, 2004)). Recruitment of ATM to sites of damage is facilitated by a group of cofactors including Mre11, Rad50, and Nbs1 (Bakkenist and Kastan, 2003; Falck et al., 2005).
The first identified target of ATM phosphorylation was Ser-15 of p53, a residue important for p53 activation (Siliciano et al., 1997). Once activated, p53 triggers upregulation of several target genes including p21waf1/CIP1, which inactivates cyclin E/Cdk2 complexes and prevents G1-to-S-phase progression (reviewed in (Agarwal et al., 1998)). Other targets of ATM include Chk2 (Thr-68), and BRCA1 (Ser-1423), which are involved in activation of cell cycle checkpoints, apoptosis and DNA repair (Cortez et al., 1999; Matsuoka et al., 1998).
In contrast to ATM, early studies in mammalian cell systems proposed a model in which the kinase activity of ATR remains constant regardless of the presence or absence of damage (Falck et al., 2005; Zou and Elledge, 2003). The ATR model, based on these studies, predicts that the kinase properties of ATR are focused as ATR protein is recruited to sites of DNA damage via an interaction with ATR-interacting protein (ATRIP)/replication protein A (RPA)-coated single stranded DNA (ssDNA). One significant extension of this model is that it predicts a mechanism by which the ATR-dependent checkpoint is activated: the aberrant persistence of RPA at sites of stalled replication (as opposed to transient RPA coating of ssDNA at sites of ongoing replication) triggers ATR checkpoint activation. However, this model has been challenged by recent work in Xenopus in which depletion of RPA did not abrogate the ability of ATR to respond to replication stress (Kumagai et al., 2006). Rather, topoisomerase IIβ binding protein 1 (TopBP1) was found to directly modulate the activity of ATR in response to replication stress (Kumagai et al., 2006). These data suggest an alternative model in which ATR kinase activity can be modulated by TopBP1 independent of the association between ATR/ATRIP and RPA.
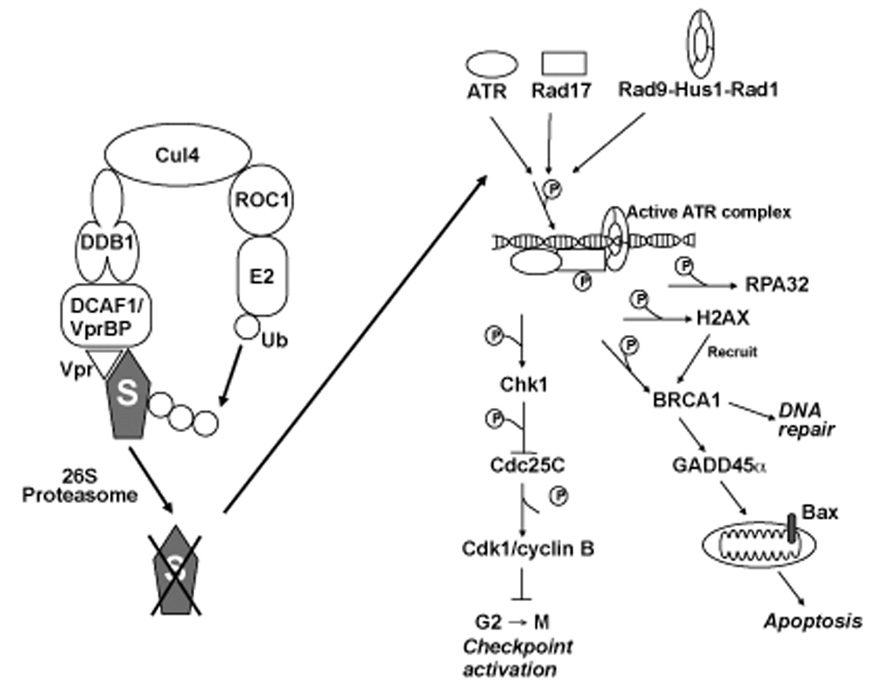
Activated ATR phosphorylates a number of targets implicated in control of the G2 checkpoint including H2AX, Rad17, BRCA1, p53, and Chk1 (Bao et al., 2001; Chen, 2000; Foray et al., 2003; Guo et al., 2000; Tibbetts et al., 1999; Tibbetts et al., 2000; Ward and Chen, 2001; Zou et al., 2002). Phosphorylation of these targets by ATR can have wide-ranging effects (Figure 1). For instance, phosphorylation of BRCA1 initiates the upregulation of genes involved in DNA repair, cell cycle arrest, apoptosis, and gene expression (reviewed in (Lane, 2004)), such as Gadd45α (Jin et al., 2000). In addition, phosphorylation of Chk1 leads to phosphorylation/inactivation of Cdc25c, which in turn allows for activation of Wee1 and consequent inactivation of Cdk1, resulting in G2 arrest (reviewed in (Zhou and Elledge, 2000)).
Figure 1.
Overview of the signaling pathways thought to be responsible for the cytostatic and pro-apoptotic effects of Vpr.
Vpr-induced G2 arrest and viral replication
Several reports in 1995 described Vpr as a cytostatic protein that arrests cells in the G2 phase of the cell cycle (He et al., 1995; Jowett et al., 1995; Re et al., 1995; Rogel et al., 1995). The in vivo significance of Vpr-induced G2 arrest has been difficult to determine, but in vitro studies suggest that the viral promoter, or long terminal repeat (LTR), is highly active in the G2 phase of the cell cycle (Goh et al., 1998; Hrimech et al., 1999; Zhu et al., 2001). Therefore, G2 arrest may promote optimal transcription from the LTR, which in turn promotes an increase in viral output. One study found that the decrease in LTR transactivation observed with Vpr-deficient virus could be recovered by the addition of pharmacological inducers of G2 arrest, which supports the notion that G2 arrest alone, and not some other property of Vpr, is sufficient for LTR transactivation (Goh et al., 1998). In addition, G2 arrest fosters a cellular environment in which cap-dependent translation is diminished in favor of translation from internal ribosome entry sites (IRESs), common to many viruses including the well-charcterized picornaviruses and cricket paralysis viruses (reviewed in (Gallego, 2002)). One study from Brasey et al. described the presence of an IRES element in the 5’ leader of HIV-1, which showed peak translational activity in G2 (Brasey et al., 2003). Thus, G2 arrest induced by Vpr may promote virus production by upregulating both transcription (via the LTR) and translation (via the putative HIV-1 IRES) of viral products. Recently, Sakai et al. reported that deletion of Vpr, in addition to Vif, rendered HIV-1 incapable of causing G2 arrest and eliminated the cytopathic properties of the virus, which suggests a link between viral cytopathicity and cell cycle arrest (Sakai et al., 2006).
Recently, infected lymphocytes (identified by expression of Gag p24) isolated from recent HIV-1 seroconverters and stained with propidium iodide for DNA content were found to be arrested in G2, indicating that G2 arrest is not merely an in vitro phenomenon (Zimmerman et al., 2006).
Mechanisms to explain Vpr-induced G2 arrest
Early work on Vpr-induced G2 arrest found that the effect of Vpr on cells was similar to the effects of the alkylating agent, nitrogen mustard, in that both caused a decrease in Cdk1 kinase activity leading to a cell cycle arrest that could be partially reversed by methylxanthines (Poon et al., 1997). In another early study it was found that Vpr-induced cell cycle arrest was not dependent on ATM or p53, and concluded that the mechanism of Vpr-induced arrest differed from canonical DNA damage response pathways (Bartz et al., 1996). While this conclusion may have been appropriate based on the understanding of DNA damage pathways at the time, over the following years the emergence of novel DNA damage-sensing proteins has revealed new pathways on which Vpr acts to induce cell cycle arrest.
Our laboratory described ATR (Figure 1) as being required for HIV-1 Vpr-induced G2 arrest (Roshal et al., 2003). Using small interfering RNAs (siRNA) specific for ATR, or a dominant-negative ATR construct, Roshal et al. were able to effectively downregulate the levels of ATR protein or its function (Roshal et al., 2003). Under these conditions, Vpr-induced G2 arrest was relieved and transactivation of the LTR was eliminated (Roshal et al., 2003). In a subsequent report, it was shown that downstream components of the ATR pathway are activated by Vpr, including the phosphorylated histone 2A variant-X (γ-H2AX), and BRCA1 (Andersen et al., 2005; Zimmerman et al., 2004).
Recently, Zimmerman et al. reported that HIV-1 infection in primary human CD4+ lymphocytes results in ATR-dependent G2 arrest and accumulation of RPA, which suggests the presence of stalled replication forks (Zimmerman et al., 2006). Consistent with a model in which Vpr activates the ATR DNA damage response pathway, Yuan et al. found that Vpr activates the negative regulator of Cdk1, Wee1 (Yuan et al., 2003). They further showed that Vpr is unable to induce G2 arrest in cells in which Wee1 has been depleted by siRNA (Yuan et al., 2003). In addition, Lai et al. demonstrated that Vpr causes foci formation of the ATR-associated molecules 53BP1 and RPA, in addition to Chk1 phosphorylation at a residue specifically phosphorylated by ATR (Lai et al., 2005). Interestingly, the authors also demonstrated that a GFP-tagged Vpr protein could be observed binding to chromatin in intact cells, while C-terminal mutants of Vpr, defective in causing G2 arrest, were unable to bind chromatin, suggesting that these two functions of Vpr may be linked (Lai et al., 2005). While these data suggest that Vpr may directly cause damage to DNA, analysis of DNA damage by pulse field gel electrophoresis (PFGE) failed to reveal the presence of DSBs by Vpr (Lai et al., 2005). These data were in conflict by results obtained by Tachiwana et al., who detected Vpr-induced DSBs by PFGE (Tachiwana et al., 2006), and it is unclear, given the similar experimental systems used by these groups, how the apparent contradiction can be explained. However, the dispensability of ATM in Vpr-induced G2 arrest (Bartz et al., 1996; Zimmerman et al., 2004), and the lack of ATM activation/phosphorylation in Vpr-expressing G2-arrested cells (Lai et al., 2005) strongly support a model in which Vpr activates the checkpoint by a mechanism that does not involve DSBs. In addition, the presence of RPA foci and the strict requirement for ATR and its downstream targets (Lai et al., 2005; Zimmerman et al., 2006), raises the possibility that Vpr, either directly through interaction with chromatin or replication machinery, or indirectly via interference with nuclear structure or the proteosome pathway, causes replication stress.
A possible clue to explain Vpr-induced G2 arrest was provided by studies by de Noronha et al. in which they captured, by live-cell microscopy, the presence of dynamic herniations of the nuclear envelope, concomitant with changes in the subcellular distrubution of Cyclin B1, Wee1, and Cdc25C in Vpr-expressing cells (de Noronha et al., 2001). Nuclear herniations were observed as the Vpr-expressing cells transitioned through S phase. These herniations were found to contain DNA, and defects in lamin structure were observed around the nuclear lesions. However de Noronha et al. were unable to detect a direct interaction between Vpr and nuclear lamins, nor an increase in Vpr protein level around or within the nuclear herniations. Importantly, nuclear herniations were not observed in cells expressing Vpr mutants defective in inducing G2 arrest. Additionally, nuclear herniations were not observed in cells treated with pharmacological inducers of G2 arrest and apoptosis, suggesting a difference in the mechanism by which Vpr induces G2 arrest compared to genotoxic agents (de Noronha et al., 2001).
What could be the link between disruptions in nuclear architecture and G2 arrest by Vpr? Nuclear herniations would likely correlate with or cause alterations in chromatin structure and therefore affect the processivity of DNA replication. Indeed, disruption of nuclear lamin structure impacts the DNA replication checkpoint response (Liu et al., 2006; Manju et al., 2006). A model in which Vpr causes replication stress by inducing herniations would be consistent with the body of literature demonstrating that Vpr specifically activates ATR pathway. However, it remains to be seen whether the nuclear herniations are the Vpr-induced stimulus to activate ATR or, alternatively, a downstream result of Vpr-induced ATR activation.
It is noteworthy that Vpr possesses a great affinity for the nuclear envelope and several groups reported that Vpr specifically interacts with components of the nuclear pore complex (Fouchier et al., 1998; Le Rouzic et al., 2002; Popov et al., 1998; Vodicka et al., 1998). The possibility that Vpr directly acts at this level to perturb the nuclear architecture and consequently induces G2 arrest remains open. It remains to be seen whether Vpr mutants can be identified which dissect Vpr’s cytostatic activity from its localization at the nuclear envelope.
Vpr binding partners involved in G2 arrest
In vitro mutational analysis of Vpr has shown that the determinants of G2 arrest are primarily located in the C-terminal region of Vpr although the biological significance of this region, in terms of binding partners or signaling, has not been elucidated. Vpr has been shown to bind to numerous targets in vitro, with several implicated in the induction of G2 arrest. However, no consensus as to the biologically relevant Vpr-binding protein has been met until recently. In the following paragraphs, we will summarize many of the arguments surrounding the putative Vpr binding proteins and conclude by summarizing recent data from several groups that demonstrate a role for Vpr in modulating the activity of Cullin RING ubiquitin ligases toward induction of G2 arrest.
Vpr was found in complex with the glucocorticoid receptor (GR). A third protein found in the Vpr/GR complex, termed Vpr-interacting protein (VIP), was identified through a yeast two-hybrid approach and was proposed to be associated with G2 arrest induction by Vpr (Mahalingam et al., 1998). Although the physiological role of VIP remains undetermined, it was proposed to be a member of the eukaryotic initiation factor 3 (eIF3) complex, which regulates transcription and is essential for G1/S and G2/M progression. However, Sherman et al. found that G2 arrest and GR-binding are independent phenotypes of Vpr based on experiments in which Vpr mutants defective for G2 arrest retained their ability to bind the GR (Sherman et al., 2000). A recent report by Muthumani et al. demonstrated that the Vpr/GR interaction prevents PARP-1 accumulation in the nucleus and therefore may primarily affect gene transcription by interfering with NF-κB (Muthumani et al., 2006).
Vpr was shown to bind uracil DNA glycosylase (UNG) and the human homologue of rad 23 A (HHR23A) (Gragerov et al., 1998; Withers-Ward et al., 1997). Both of these proteins are involved in the DNA repair process so they were naturally suspected of playing a role in Vpr-induced G2 arrest. However, subsequent analysis showed that there is no correlation between the association of HHR23A or UNG with Vpr and the induction of G2 arrest (Kaiser and Emerman, 2006; Mansky et al., 2001). In addition, Goh et al. reported that Vpr binds and inactivates the positive regulator of Cdk1, Cdc25, and mutation of the Cdc25-binding region within Vpr rendered Vpr deficient in G2 arrest induction (Goh et al., 2004). However, the observation that Vpr activates molecules upstream of Cdc25, such as ATR and Chk1, suggests that inactivation of Cdc25 may be a consequence of Vpr-mediated activation of upstream kinases (specifically, Chk1) rather than a direct effect of Vpr.
Vpr has also been show to bind Cyclophilin A (CypA), a peptidyl prolyl cis-trans isomerase, involved in maintenance of protein conformation by cis-trans interconversion of N-terminal peptide bonds. Zander et al. reported that CypA is required for the stability and function of Vpr (Zander et al., 2003). They further showed that Vpr mutated at proline 35 is unable to bind CypA nor induce G2 arrest (Zander et al., 2003). In an effort to investigate the significance of the Vpr-CypA interaction, our group demonstrated that abolishment of the interaction (either by using CypA-deficient cells, incubating with Cyclosporin A, or by mutating Vpr at proline 35) does not abrogate Vpr-induced G2 arrest (Ardon et al., 2006). Therefore, binding to CypA and inductuion of G2 arrest are independent activities of Vpr.
Another proposed Vpr binding partner is Hsp70, an abundant stress-regulated cellular protein (Bukrinsky and Zhao, 2004; Iordanskiy et al., 2004a; Iordanskiy et al., 2004b). Bukrinsky and colleagues reported that, in contrast to other Vpr-binding molecules, Hsp70 interacted with Vpr to inhibit, rather than induce, G2 arrest (Bukrinsky and Zhao, 2004). The authors proposed that Hsp70 is induced by HIV-1 infection as part of an innate antiviral response to oppose the pathogenic properties of Vpr. The authors also showed that Hsp70 inhibits replication of Vpr-proficient HIV-1, but not Vpr-deficient HIV-1 (Iordanskiy et al., 2004a; Iordanskiy et al., 2004b).
A recent report demonstrated that Vpr interacts with SAP145, a subunit of Sf3b required for the splicing of precursor mRNAs (Terada and Yasuda, 2006). In their study, Terada and Yasuda found that full-length Vpr, but not Vpr lacking the C terminus, bound SAP145 and that Vpr-SAP145 binding prevented an association between SAP145 and SAP49 (Terada and Yasuda, 2006). The authors also found that depletion of either SAP145 or SAP49 caused G2 arrest and the induction of γH2AX and BRCA1 nuclear foci formation (Terada and Yasuda, 2006), as has been observed in the context of expression of Vpr alone (Zimmerman et al., 2004). It is unclear how the Vpr/SAP145 interaction may lead to G2 arrest, as inhibition of SAP145 did not alter the splicing of genes required for cell cycle progression such as Cyclin B1 or Cdk1 (Terada and Yasuda, 2006). In a subsequent report, Aida and colleagues corroborated the Vpr/SAP145 interaction and found that SAP145 is required for Vpr-induced inhibition of processing of certain pre-mRNAs such as immunoglobulin M, and β-globin (Hashizume et al., 2007). However, the authors found that Vpr(R80A), a Vpr mutant defective for G2 arrest, could bind SAP145 and inhibit pre-mRNA processing (Hashizume et al., 2007), suggesting that the Vpr/SAP145 interaction per se is not sufficient to induce G2 arrest.
In 1993, Scheffner and colleagues published a seminal paper in which they demonstrated that E6 proteins from human papilloma virus (HPV) types 16 and 18 caused polyubiquitination and degradation of the tumor suppressor p53 (Scheffner et al., 1993). By causing degradation of p53, HPV is able to suppress cell cycle arrest and apoptosis of the infected cell, and thereby replicate under more favorable cellular conditions. This discovery was the first of several describing the ability of viral proteins to modulate the ubiquitin proteasome system (UPS). The list of viral proteins that can manipulate the UPS now includes the paramyxovirus V protein, the hepatitis B virus protein X, and the HIV-1 proteins, Vpu and Vif (Horvath, 2004; Leupin et al., 2005; Margottin et al., 1998; Mehle et al., 2004; Sheehy et al., 2003; Sitterlin et al., 2000; Yu et al., 2003), and as its most recent addition, HIV-1 Vpr (reviewed in (Dehart and Planelles, 2007))
After years of searching for a mechanism to explain Vpr-induced G2 arrest, it is somewhat perplexing that the most informative Vpr-binding partner was discovered in 1994 when a protein of unknown function was co-precipitated with Vpr (Zhao et al., 1994). This protein was originally named Vpr-interacting protein (RIP) or Vpr binding protein (VprBP; see Figure 1), and its cellular function remained enigmatic until 2006 when several groups identified a family of proteins, of which RIP/VprBP is a member, associated with damaged-DNA specific binding protein 1 (DDB1; see Figure 1) (Angers et al., 2006; He et al., 2006; Higa et al., 2006; Jin et al., 2006). This family of proteins interacts with, and confers substrate specificity to Cullin 4- and DDB1-based E3 ubiquitin ligase complexes (hereby referred to as Cul4-DDB1 E3 ligases). After the discovery of this protein family, RIP/VprBP was accordingly renamed DDB1- and Cullin 4A-associated factor (DCAF) -1.
Recently, several laboratories reported that Vpr associates with a Cul4A-containing E3 ligase complex via its association with DCAF-1 and/or DDB1 (Belzile et al., 2007; Dehart et al., 2007; Hrecka et al., 2007; Le Rouzic et al., 2007; Schrofelbauer et al., 2007; Tan et al., 2007; Wen et al., 2007). Knockdown of DCAF1 by RNA interference prevented Vpr co-immunoprecipitation with DDB1. These data form a model in which DCAF1 bridges Vpr to DDB1 and the larger E3 ligase complex. Based on this model, at least two possibilities could explain Vpr-induced G2 arrest: 1) Vpr directs the Cul4-DDB1 E3 ligase complex toward degradation of a cellular protein; or 2) Vpr inhibits the function of the Cul4-DDB1 E3 ligase. If the first possibility is correct, then two types of Vpr mutants should exist: Those that ablate DCAF1 binding, and those that ablate substrate binding. Indeed, mutation of Vpr at Q65 (Q65R) prevents binding of Vpr to DCAF1, while the Vpr mutant R80A retains the ability to bind DCAF but does not induce G2 arrest (presumably because it lacks the ability to bind the E3 ligase substrate). In addition, expression of Vpr R80A exerts a dominant negative (DN) effect (overrides G2 arrest) when coexpressed with wt Vpr. However, mutation of Q65 (Q65R) in the context of R80A eliminates the DN effect of Vpr R80A, suggesting that the DN phenotype of Vpr R80A requires its ability to bind DCAF1 (Dehart et al., 2007; Le Rouzic et al., 2007).
In further support of a model in which proteosomal activity is required for Vpr-induced G2 arrest, DeHart et al. demonstrated that inhibition of proteosomal activity either by pharmacological inhibition with epoxomicin or by overexpression of a dominant-negative ubiquitin mutant, Ub(K48R), abrogated Vpr-induced G2 arrest (Dehart et al., 2007). Together, these data support a model in which Vpr directs the Cul4-DDB1 E3 ligase complex to ubiquitinate and degrade a cellular protein(s) required for cell cycle progression. Obviously, the elucidation of such a protein(s) is currently an area of intense investigation and we anticipate that its discovery will open new and exciting avenues for the development of anti-HIV-1 therapeutics.
Vpr and Apoptosis
HIV-1 infection and apoptosis
The precise mechanism by which CD4+ T cells are lost over the course of an HIV-1 infection is poorly understood (Hazenberg et al., 2000; Roshal et al., 2001). Several studies on HIV-1 induced cell death reported disparate observations. Finko et al. and Muro-Cacho et al., examining lymph nodes of HIV-1 infected patients, reported that cell death was predominantly occurring in uninfected “bystander” cells (Finkel et al., 1995; Muro-Cacho et al., 1995). In contrast, Ho et al. and Wei et al. reported that HIV-1 replication directly leads to death of infected cells in vivo (Ho et al., 1995; Wei et al., 1995). Several mechanisms have been proposed to explain the loss of CD4+ T cells in HIV-1-infected patients, including direct killing by HIV-1 replication, CD8+ T cell-mediated killing of infected CD4+ lymphocytes, apoptosis caused by hyper-activation of the immune system, and apoptosis of infected cells induced by expression of viral genes (reviewed in (Roshal et al., 2001)). Given that these mechanisms are mutually exclusive, it is likely that a combination of them contributes to the decline in CD4+ T cells over the course of HIV-1 infection in the absence of treatment.
Apoptosis is a homeostatic process utilized by multicellular organisms to remove cells from tissue with minimal disturbance to tissue architecture or function. Cells utilize several apoptotic pathways that can be categorized as either extrinsic or intrinsic. Extrinisic apoptotic pathways involve ligation of death receptors such as Fas or TNF. The binding of death receptors to their cognate ligands leads to the recruitment of death domain-containing proteins such as FADD (Fas-activated death domain) and the apical caspase, caspase 8, to intracellular death receptor domains. From this platform, the proform of caspase 8 is cleaved and activated, at which point it can activate downstream caspases such as caspases 3 or 7, or cleave Bid to generate tBid, which translocates to the mitochondria to promote Bax/Bak oligomerization, leading to cytochrome c release (reviewed in (Danial and Korsmeyer, 2004)).
In the intrinsic pathway, the apoptotic signal originates from within the cell, which occurs, for instance, in the case of DNA damage. In this pathway, pro-apoptotic signals triggered by the intracellular damage activate pro-apoptotic Bcl-2 family members such as Bad, Bim, and Puma, which promote Bax/Bak oligomerization. Oligomerization of Bax and Bak facilitates the release of cytochrome c, and other pro-apoptotic proteins from the intermembrane space. Once cytoplasmic, cytochrome c binds to Apaf-1, which serves as a co-factor to oligomerize and activate the initiator caspase 9 to promote cell death. Active initiator caspases cleave and activate the zymogenic forms of executioner caspases, such as Caspase 3. Executioner caspases cleave substrates throughout the cell to either dismantle cellular components, or activate other zymogens involved in the apoptotic process (reviewed in (Danial and Korsmeyer, 2004)).
Vpr activates the intrinsic apoptosis signaling pathway
As mentioned above, one key point of difference between the extrinsic and intrinsic pathways lies at the level of apical caspase activation (activation of caspase 8 is unique to the extrinsic pathway). In an attempt to determine which of these pathways is activated by Vpr, Muthumani et al. reported that Vpr-expressing cells underwent apoptosis via the intrinsic pathway, characterized by cytochrome c release, and caspase 9 (Muthumani et al., 2002). Importantly, the authors did not observe activation of caspase 8, or expression of Fas or its ligand in response to Vpr (Muthumani et al., 2002). These data ruled out the possibility that the Vpr-induced apoptotic signal was detected by death receptors on the cell surface, and was consistent with the possibility that Vpr induces apoptosis through intracellular stress.
Relationship between cell cycle arrest and apoptosis by Vpr
Because the most overt phenotype of Vpr in vitro is probably cell cycle arrest, the suspicion that cell cycle arrest and apoptosis by Vpr might be causally related has garnered attention in the literature. In support of model in which cell cycle arrest and apoptosis occur independently, Nishizawa et al. reported that mutation of certain C terminal amino acids, such as isoleucine 74, rendered Vpr moderately impaired for apoptosis but left its cell cycle arrest function intact (Nishizawa et al., 2000). In addition, Waldhuber et al. reported that the Vpr-GFP fusion protein does not induce cell cycle arrest but effectively induces apoptosis (Waldhuber et al., 2003). However, a recent report challenges these findings (Andersen et al., 2006). Specifically, it was showed that Vpr mutated at isoleucine 74 induces apoptosis in immortalized CD4+ T cells at levels similar to wt Vpr. In addition, the Vpr-GFP fusion protein carrying an Arg to Ala mutation at position 80 of Vpr (a mutation that renders Vpr inactive for cell cycle arrest) still induced apoptosis, suggesting that apoptosis induced by Vpr-GFP may represent a gain-of-function phenotype unrelated to the nature of wild-type Vpr (Andersen et al., 2006).
Early reports supporting a link between cell cycle arrest and apoptosis demonstrated a temporal relationship between the two, in which G2 arrest peaked first, followed by apoptosis (Poon et al., 1997; Stewart et al., 1997; Stewart et al., 2000). Later, in an attempt to identify a pathway to link the two phenotypes, Zhu et al. showed that treatment of cells with caffeine, an inhibitor of ATR and ATM, relieved both cell cycle arrest and apoptosis by Vpr (Zhu et al., 2001). Later, the use of RNAi allowed for more specific targeting of proteins. Taking advantage of this, Yuan et al. showed that RNAi knockdown of Wee1, a Cdk1-inhibiting kinase downstream of ATR, alleviated both G2 arrest and apoptosis induced by Vpr (Yuan et al., 2004; Yuan et al., 2003).
It was later found that siRNA-mediated depletion of ATR dramatically abrogated apoptosis by Vpr (Andersen et al., 2006; Andersen et al., 2005). In contrast, depletion of ATM, or its downstream target, Chk2, had no effect. Andersen et al. also found that an essential downstream mediator of the pro-apoptotic signal initiated by ATR was the growth arrest and DNA damage protein 45α (GADD45α; Figure 1) (Andersen et al., 2005). It was also observed that BRCA1 was phosphorylated in an ATR-dependent manner at serine 1423 and that it formed BRCA1 nuclear foci (Andersen et al., 2005; Zimmerman et al., 2004). Indeed, the presence of activated BRCA1, a known p53-independent regulator of Gadd45α, may help to explain how Vpr kills cells independently of p53 status. Therefore, from the above studies it was concluded that Vpr induced G2 arrest and apoptosis induction are functionally linked, and both effects require the upstream contribution of the ATR kinase (Figure 1).
An alternative model to explain Vpr-induced apoptosis was proposed by Kroemer and colleagues. The authors found that recombinant Vpr or C terminal Vpr peptides associate with fractionated mitochondria via an interaction with the adenine nucleotide transporter (ANT), a component of the permeability transition pore that resides at the inner mitochondrial membrane (Jacotot et al., 2000; Roumier et al., 2002; Vieira et al., 2000). The addition of C terminal Vpr to mitochondria triggered mitochondrial membrane permeabilization (MMP), which allows release of pro-apoptotic proteins harbored in the inner membrane space, such as cytochrome c (Jacotot et al., 2000; Roumier et al., 2002; Vieira et al., 2000). Induction of MMP by Vpr was suppressed by overexpression of Bcl-2 (Jacotot et al., 2000).
Recent studies of ANT and cyclophilin D knockout mice suggest that these mitochondrial pore components may promote necrotic cell death such as that induced by high cellular calcium concentration or oxidative stress, but not apoptotic death induced by DNA damage (Baines et al., 2005; Kokoszka et al., 2004; Nakagawa et al., 2005). With the advent of RNA interference as a method of genetic manipulation, the role of ANT in Vpr-induced apoptosis was revisited (Andersen et al., 2006). In these studies, siRNA mediated depletion of ANT did not appreciably affect Vpr-induced apoptosis, whereas depletion of another mitochondrial gateway protein, Bax (Figure 1), very effectively blocked apoptosis. In addition, it was found that induction of apoptosis by Vpr correlated with a conformational change in Bax that is considered synonymous with its activation (Andersen et al., 2006). How can data supporting Vpr’s interaction with ANT be reconciled with data supporting the requirement of pro-apoptotic stress pathways upstream of the mitochondria? Interestingly, C terminal Vpr has recently been shown to induce apoptosis in non-dividing differentiated monocytes (Mishra et al., 2007). It is possible that the interaction with ANT may be relevant in cell types that display little or no cell cycle activity (macrophages, or neurons), while cells with high replicative activity (immortalized fibroblasts, or activated CD4+ T cells), may die more rapidly from cell cycle insults. It will be important ot explore these potential differences in the future.
Cytostatic activity of Vpr in yeast
Soon after the cell cycle arrest activity of Vpr was documented in human cells, Zhao et al. reported a similar activity in fission yeast (Schizosaccharomyces pombe) (Zhao et al., 1996), a model organism widely used in cell cycle studies. Overexpression of Vpr in yeast led to Cdc2 kinase inhibition and for most of the Vpr mutants examined, phenotypes were similar in mammalian and yeast cells (Chen et al., 1999; Zhao et al., 1996). These observations argued that Vpr targets an evolutionary conserved mechanism of cell cycle control. In this hypothesis, the existence of well-characterized yeast mutants and the relatively easy handling of fission yeast would be advantageous to further dissect Vpr-mediated cell cycle arrest as well as to set up large-scale screens for inhibitors of Vpr function. However, two lines of evidence may challenge the relevance of the fission yeast model in the study of Vpr activity. First, Vpr-mediated G2 arrest in yeast occurs independently of classical DNA damage checkpoints and does not require the Chk1 kinase (Elder et al., 2000; Masuda et al., 2000; Matsuda et al., 2006), in contrast to what has been observed in mammalian cells (Roshal et al., 2003). Second, the gene encoding DCAF1, which appears to be crucial for Vpr-mediated G2 arrest in human cells, shows no obvious ortholog in S. pombe. However it is intriguing that during evolution the tandem Cul4-DDB1 appeared in fission yeast whereas it is absent in budding yeast (Liu et al., 2003), where overexpression of Vpr inhibits growth but does not cause G2 arrest (Gu et al., 1997). Whether Vpr arrests S. pombe cell cycle by using a surrogate of DCAF1 to usurp the Cul4 ligase or by an unrelated mechanism remains an open question.
Conclusion
Recent progress has been made in understanding the basis for two of Vpr’s most prominent functions: G2 arrest and apoptosis. However, several issues remain to be solved. Specifically, clarification is needed to understand the nature of Vpr-mediated manipulation of the ubiquitin proteasome system. How does Vpr manipulate the cognate E3 Ubiquitin ligase it recruits? What is the target(s) whose degradation induces replication stress?. Another key issue will be to further validate the in vitro Vpr phenotypes in HIV-1 infected patients and determine whether targeting of these recently discovered pathways has the potential for a therapeutic benefit. As G2 arrest appears to be the gateway to at least two of Vpr’s additional functions (apoptosis, LTR transactivation), we propose that a therapy aimed at interfering with this cytostatic property of Vpr may offer clinical benefit.
Acknowledgements
JLA was supported by NIH Training Grants T32 AI055434 and T32 AG000029. ELR was funded by INSERM. This work was supported, in part, by National Institutes of Health research grant R01AI49057 to VP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accola MA, et al. A conserved dileucine-containing motif in p6(gag) governs the particle association of Vpx and Vpr of simian immunodeficiency viruses SIV(mac) and SIV(agm) J Virol. 1999;73:9992–9999. doi: 10.1128/jvi.73.12.9992-9999.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal ML, et al. The p53 network. J Biol Chem. 1998;273:1–4. doi: 10.1074/jbc.273.1.1. [DOI] [PubMed] [Google Scholar]
- Alexander L, et al. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J Virol. 2000;74:4361–4376. doi: 10.1128/jvi.74.9.4361-4376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JL, et al. HIV-1 Vpr-Induced Apoptosis Is Cell Cycle Dependent and Requires Bax but Not ANT. PLoS Pathog. 2006;2:e127. doi: 10.1371/journal.ppat.0020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JL, et al. ATR and GADD45alpha mediate HIV-1 Vpr-induced apoptosis. Cell Death Differ. 2005;12:326–334. doi: 10.1038/sj.cdd.4401565. [DOI] [PubMed] [Google Scholar]
- Angers S, et al. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443:590–593. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- Ardon O, et al. Induction of G2 arrest and binding to cyclophilin A are independent phenotypes of human immunodeficiency virus type 1 Vpr. J Virol. 2006;80:3694–3700. doi: 10.1128/JVI.80.8.3694-3700.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- Bao S, et al. ATR/ATM-mediated phosphorylation of human Rad17 is required for genotoxic stress responses. Nature. 2001;411:969–974. doi: 10.1038/35082110. [DOI] [PubMed] [Google Scholar]
- Barlow KL, et al. Characterization of a novel simian immunodeficiency virus (SIVmonNG1) genome sequence from a mona monkey (Cercopithecus mona) J Virol. 2003;77:6879–6888. doi: 10.1128/JVI.77.12.6879-6888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz SR, et al. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer BE, et al. Characterization of novel simian immunodeficiency viruses from redcapped mangabeys from Nigeria (SIVrcmNG409 and -NG411) J Virol. 2001;75:12014–12027. doi: 10.1128/JVI.75.24.12014-12027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzile JP, et al. HIV-1 Vpr-Mediated G2 Arrest Involves the DDB1-CUL4A(VPRBP) E3 Ubiquitin Ligase. PLoS Pathog. 2007;3:e85. doi: 10.1371/journal.ppat.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbigot S, et al. The C-terminal domain of VPR adopts an antiparallel dimeric structure in solution via its leucine-zipper-like domain. Biochem J. 2004 doi: 10.1042/BJ20041759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasey A, et al. The leader of human immunodeficiency virus type 1 genomic RNA harbors an internal ribosome entry segment that is active during the G2/M phase of the cell cycle. J Virol. 2003;77:3939–3949. doi: 10.1128/JVI.77.7.3939-3949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M, Zhao Y. Heat-shock proteins reverse the G2 arrest caused by HIV-1 viral protein R. DNA Cell Biol. 2004;23:223–225. doi: 10.1089/104454904773819806. [DOI] [PubMed] [Google Scholar]
- Campbell BJ, Hirsch VM. Vpr of simian immunodeficiency virus of African green monkeys is required for replication in macaque macrophages and lymphocytes. J Virol. 1997;71:5593–5602. doi: 10.1128/jvi.71.7.5593-5602.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Ataxia telangiectasia-related protein is involved in the phosphorylation of BRCA1 following deoxyribonucleic acid damage. Cancer Res. 2000;60:5037–5039. [PubMed] [Google Scholar]
- Chen M, et al. Mutational analysis of Vpr-induced G2 arrest, nuclear localization, and cell death in fission yeast. J Virol. 1999;73:3236–3245. doi: 10.1128/jvi.73.4.3236-3245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez D, et al. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- Courgnaud V, et al. Identification of a new simian immunodeficiency virus lineage with a vpu gene present among different cercopithecus monkeys (C. mona, C. cephus, and C. nictitans) from Cameroon. J Virol. 2003;77:12523–12534. doi: 10.1128/JVI.77.23.12523-12534.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- de Noronha CM, et al. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science. 2001;294:1105–1108. doi: 10.1126/science.1063957. [DOI] [PubMed] [Google Scholar]
- Dehart JL, Planelles V. HIV-1 Vpr links proteasomal degradation and checkpoint activation. J Virol. 2007 doi: 10.1128/JVI.01628-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehart JL, et al. HIV-1 Vpr activates the G2 checkpoint through manipulation of the ubiquitin proteasome system. Virol J. 2007;4:57. doi: 10.1186/1743-422X-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder RT, et al. Cell cycle G2 arrest induced by HIV-1 Vpr in fission yeast (Schizosaccharomyces pombe) is independent of cell death and early genes in the DNA damage checkpoint. Virus Res. 2000;68:161–173. doi: 10.1016/s0168-1702(00)00167-2. [DOI] [PubMed] [Google Scholar]
- Falck J, et al. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- Finkel TH, et al. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- Fischer A, et al. Is the Vpr R77Q mutation associated with long-term non-progression of HIV infection? Aids. 2004;18:1346–1347. doi: 10.1097/00002030-200406180-00018. [DOI] [PubMed] [Google Scholar]
- Fletcher TM, et al. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-2/SIV(SM) Embo J. 1996;15:6155–6165. [PMC free article] [PubMed] [Google Scholar]
- Foray N, et al. A subset of ATM- and ATR-dependent phosphorylation events requires the BRCA1 protein. Embo J. 2003;22:2860–2871. doi: 10.1093/emboj/cdg274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier RA, et al. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J Virol. 1998;72:6004–6013. doi: 10.1128/jvi.72.7.6004-6013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz PN, et al. Vaccine protection of chimpanzees against challenge with HIV-1-infected peripheral blood mononuclear cells. Science. 1992;256:1687–1690. doi: 10.1126/science.256.5064.1687. [DOI] [PubMed] [Google Scholar]
- Gallego J. Internal initiation of translation by viral and cellular IRESs--a new avenue for specific inhibition of protein synthesis? Curr Opin Drug Discov Devel. 2002;5:777–784. [PubMed] [Google Scholar]
- Gaynor EM, Chen IS. Analysis of Apoptosis Induced by HIV-1 Vpr and Examination of the Possible Role of the hHR23A Protein. Exp Cell Res. 2001;267:243–257. doi: 10.1006/excr.2001.5247. [DOI] [PubMed] [Google Scholar]
- Gibbs JS, et al. Progression to AIDS in the absence of a gene for vpr or vpx. J Virol. 1995;69:2378–2383. doi: 10.1128/jvi.69.4.2378-2383.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh WC, et al. The human immunodeficiency virus Vpr protein binds Cdc25C: implications for G2 arrest. Virology. 2004;318:337–349. doi: 10.1016/j.virol.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Goh WC, et al. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- Gragerov A, et al. HHR23A, the human homologue of the yeast repair protein RAD23, interacts specifically with Vpr protein and prevents cell cycle arrest but not the transcriptional effects of Vpr. Virology. 1998;254:323–330. doi: 10.1006/viro.1998.9138. [DOI] [PubMed] [Google Scholar]
- Gu J, et al. Small heat shock protein suppression of Vpr-induced cytoskeletal defects in budding yeast. Mol Cell Biol. 1997;17:4033–4042. doi: 10.1128/mcb.17.7.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, et al. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 2000;14:2745–2756. doi: 10.1101/gad.842500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JC, Haber JE. Surviving the breakup: the DNA damage checkpoint. Annu Rev Genet. 2006;40:209–235. doi: 10.1146/annurev.genet.40.051206.105231. [DOI] [PubMed] [Google Scholar]
- Hashizume C, et al. Human immunodeficiency virus type 1 Vpr interacts with spliceosomal protein SAP145 to mediate cellular pre-mRNA splicing inhibition. Microbes Infect. 2007;9:490–497. doi: 10.1016/j.micinf.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Hattori N, et al. The human immunodeficiency virus type 2 vpr gene is essential for productive infection of human macrophages. Proc Natl Acad Sci U S A. 1990;87:8080–8084. doi: 10.1073/pnas.87.20.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazenberg MD, et al. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat Immunol. 2000;1:285–289. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- He J, et al. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YJ, et al. DDB1 functions as a linker to recruit receptor WD40 proteins to CUL4-ROC1 ubiquitin ligases. Genes Dev. 2006;20:2949–2954. doi: 10.1101/gad.1483206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henklein P, et al. Functional and structural characterization of synthetic HIV-1 Vpr that transduces cells, localizes to the nucleus, and induces G2 cell cycle arrest. J Biol Chem. 275:32016–32026. doi: 10.1074/jbc.M004044200. [DOI] [PubMed] [Google Scholar]
- Higa LA, et al. CUL4-DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylation. Nat Cell Biol. 2006;8:1277–1283. doi: 10.1038/ncb1490. [DOI] [PubMed] [Google Scholar]
- Ho DD, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Horvath CM. Weapons of STAT destruction. Interferon evasion by paramyxovirus V protein. Eur J Biochem. 2004;271:4621–4628. doi: 10.1111/j.1432-1033.2004.04425.x. [DOI] [PubMed] [Google Scholar]
- Hrecka K, et al. Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0702102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrimech M, et al. Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection. J Virol. 1999;73:4101–4109. doi: 10.1128/jvi.73.5.4101-4109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanskiy S, et al. Heat-shock protein 70 exerts opposing effects on Vpr-dependent and Vpr-independent HIV-1 replication in macrophages. Blood. 2004a;104:1867–1872. doi: 10.1182/blood-2004-01-0081. [DOI] [PubMed] [Google Scholar]
- Iordanskiy S, et al. Heat shock protein 70 protects cells from cell cycle arrest and apoptosis induced by human immunodeficiency virus type 1 viral protein R. J Virol. 2004b;78:9697–9704. doi: 10.1128/JVI.78.18.9697-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacotot E, et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, et al. A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol Cell. 2006;23:709–721. doi: 10.1016/j.molcel.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Jin S, et al. BRCA1 activation of the GADD45 promoter. Oncogene. 2000;19:4050–4057. doi: 10.1038/sj.onc.1203759. [DOI] [PubMed] [Google Scholar]
- Jowett JB, et al. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser SM, Emerman M. Uracil DNA glycosylase is dispensable for human immunodeficiency virus type 1 replication and does not contribute to the antiviral effects of the cytidine deaminase Apobec3G. J Virol. 2006;80:875–882. doi: 10.1128/JVI.80.2.875-882.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoszka JE, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, et al. TopBP1 activates the ATR-ATRIP complex. Cell. 2006;124:943–955. doi: 10.1016/j.cell.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Lai M, et al. Activation of the ATR Pathway by Human Immunodeficiency Virus Type 1 Vpr Involves Its Direct Binding to Chromatin In Vivo. J Virol. 2005;79:15443–15451. doi: 10.1128/JVI.79.24.15443-15451.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TF. BRCA1 and Transcription. Cancer Biol Ther. 2004;3 doi: 10.4161/cbt.3.6.843. [DOI] [PubMed] [Google Scholar]
- Lang SM, et al. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993;67:902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rouzic E, et al. HIV1 Vpr Arrests the Cell Cycle by Recruiting DCAF1/VprBP, a Receptor of the Cul4-DDB1 Ubiquitin Ligase. Cell Cycle. 2007;6:182–188. doi: 10.4161/cc.6.2.3732. [DOI] [PubMed] [Google Scholar]
- Le Rouzic E, et al. Docking of HIV-1 Vpr to the nuclear envelope is mediated by the interaction with the nucleoporin hCG1. J Biol Chem. 2002;277:45091–45098. doi: 10.1074/jbc.M207439200. [DOI] [PubMed] [Google Scholar]
- Leupin O, et al. Hepatitis B virus X protein stimulates viral genome replication via a DDB1-dependent pathway distinct from that leading to cell death. J Virol. 2005;79:4238–4245. doi: 10.1128/JVI.79.7.4238-4245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, et al. Cop9/signalosome subunits and Pcu4 regulate ribonucleotide reductase by both checkpoint-dependent and -independent mechanisms. Genes Dev. 2003;17:1130–1140. doi: 10.1101/gad.1090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. DNA damage responses in progeroid syndromes arise from defective maturation of prelamin A. J Cell Sci. 2006;119:4644–4649. doi: 10.1242/jcs.03263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JJ, et al. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J Clin Invest. 2003;111:1547–1554. doi: 10.1172/JCI16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam S, et al. HIV-1 Vpr interacts with a human 34-kDa mov34 homologue, a cellular factor linked to the G2/M phase transition of the mammalian cell cycle. Proc Natl Acad Sci U S A. 1998;95:3419–3424. doi: 10.1073/pnas.95.7.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manju K, et al. Expression of disease-causing lamin A mutants impairs the formation of DNA repair foci. J Cell Sci. 2006;119:2704–2714. doi: 10.1242/jcs.03009. [DOI] [PubMed] [Google Scholar]
- Mansky LM, et al. Interaction of human immunodeficiency virus type 1 Vpr with the HHR23A DNA repair protein does not correlate with multiple biological functions of Vpr. Virology. 2001;282:176–185. doi: 10.1006/viro.2000.0791. [DOI] [PubMed] [Google Scholar]
- Margottin F, et al. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell. 1998;1:565–574. doi: 10.1016/s1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- Masuda M, et al. Genetic studies with the fission yeast Schizosaccharomyces pombe suggest involvement of wee1, ppa2, and rad24 in induction of cell cycle arrest by human immunodeficiency virus type 1 Vpr. J Virol. 2000;74:2636–2646. doi: 10.1128/jvi.74.6.2636-2646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, et al. HIV-1 Vpr induces G2 cell cycle arrest in fission yeast associated with Rad24/14-3-3-dependent, Chk1/Cds1-independent Wee1 upregulation. Microbes Infect. 2006;8:2736–2744. doi: 10.1016/j.micinf.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, et al. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- Mehle A, et al. Vif overcomes the innate antiviral activity of APOBEC3G by promoting its degradation in the ubiquitin-proteasome pathway. J Biol Chem. 2004;279:7792–7798. doi: 10.1074/jbc.M313093200. [DOI] [PubMed] [Google Scholar]
- Mishra S, et al. Activation of JNK-dependent pathway is required for HIV viral protein R-induced apoptosis in human monocytic cells: involvement of antiapoptotic BCL2 and c-IAP1 genes. J Biol Chem. 2007;282:4288–4300. doi: 10.1074/jbc.M608307200. [DOI] [PubMed] [Google Scholar]
- Morellet N, et al. NMR structure of the HIV-1 regulatory protein VPR. J Mol Biol. 2003;327:215–227. doi: 10.1016/s0022-2836(03)00060-3. [DOI] [PubMed] [Google Scholar]
- Muro-Cacho CA, et al. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- Muthumani K, et al. The HIV-1 Vpr and glucocorticoid receptor complex is a gain-of-function interaction that prevents the nuclear localization of PARP-1. Nat Cell Biol. 2006;8:170–179. doi: 10.1038/ncb1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumani K, et al. HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J Biol Chem. 2002;277:37820–37831. doi: 10.1074/jbc.M205313200. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, et al. Induction of apoptosis by the Vpr protein of human immunodeficiency virus type 1 occurs independently of G(2) arrest of the cell cycle. Virology. 2000;276:16–26. doi: 10.1006/viro.2000.0534. [DOI] [PubMed] [Google Scholar]
- Ogawa K, et al. Mutational analysis of the human immunodeficiency virus vpr open reading frame. J Virol. 1989;63:4110–4114. doi: 10.1128/jvi.63.9.4110-4114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancio HA, et al. Interaction of human immunodeficiency virus type 2 Vpx and invariant chain. J Virol. 2000;74:6168–6172. doi: 10.1128/jvi.74.13.6168-6172.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippon V, et al. Transactivation is a conserved function among primate lentivirus Vpr proteins but is not shared by Vpx. J Hum Virol. 1999;2:167–174. [PubMed] [Google Scholar]
- Planelles V, et al. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J Virol. 1996;70:2516–2524. doi: 10.1128/jvi.70.4.2516-2524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon B, et al. Human immunodeficiency virus type 1 vpr gene induces phenotypic effects similar to those of the DNA alkylating agent, nitrogen mustard. J Virol. 1997;71:3961–3971. doi: 10.1128/jvi.71.5.3961-3971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov S, et al. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J Biol Chem. 1998;273:13347–13352. doi: 10.1074/jbc.273.21.13347. [DOI] [PubMed] [Google Scholar]
- Rajan D, et al. Effect of R77Q, R77A and R80A changes in Vpr on HIV-1 replication and CD4 T cell depletion in human lymphoid tissue ex vivo. Aids. 2006;20:831–836. doi: 10.1097/01.aids.0000218546.31716.7f. [DOI] [PubMed] [Google Scholar]
- Re F, et al. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz MS, Jr, et al. Viral variability and serum antibody response in a laboratory worker infected with HIV type 1 (HTLV type IIIB) AIDS Res Hum Retroviruses. 1994;10:1143–1155. doi: 10.1089/aid.1994.10.1143. [DOI] [PubMed] [Google Scholar]
- Rodes B, et al. Differences in disease progression in a cohort of long-term non-progressors after more than 16 years of HIV-1 infection. Aids. 2004;18:1109–1116. doi: 10.1097/00002030-200405210-00004. [DOI] [PubMed] [Google Scholar]
- Rogel ME, et al. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshal M, et al. Activation of the ATR-mediated DNA damage response by the HIV-1 viral protein R. J Biol Chem. 2003;278:25879–25886. doi: 10.1074/jbc.M303948200. [DOI] [PubMed] [Google Scholar]
- Roshal M, et al. Apoptosis in AIDS. Apoptosis. 2001;6:103–116. doi: 10.1023/a:1009636530839. [DOI] [PubMed] [Google Scholar]
- Roumier T, et al. The C-terminal moiety of HIV-1 Vpr induces cell death via a caspase-independent mitochondrial pathway. Cell Death Differ. 2002;9:1212–1219. doi: 10.1038/sj.cdd.4401089. [DOI] [PubMed] [Google Scholar]
- Sakai K, et al. The Vif and Vpr accessory proteins independently cause HIV-1-induced T cell cytopathicity and cell cycle arrest. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0509417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, et al. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Schrofelbauer B, et al. HIV-1 Vpr function is mediated by interaction with the damage-specific DNA-binding protein DDB1. Proc Natl Acad Sci U S A. 2007;104:4130–4135. doi: 10.1073/pnas.0610167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W, et al. NMR structure of the (52–96) C-terminal domain of the HIV-1 regulatory protein Vpr: molecular insights into its biological functions. J Mol Biol. 1999;285:2105–2117. doi: 10.1006/jmbi.1998.2381. [DOI] [PubMed] [Google Scholar]
- Schwartz S, et al. Expression of human immunodeficiency virus type 1 vif and vpr mRNAs is Rev-dependent and regulated by splicing. Virology. 1991;183:677–686. doi: 10.1016/0042-6822(91)90996-o. [DOI] [PubMed] [Google Scholar]
- Sheehy AM, et al. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–1407. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- Sherman MP, et al. Human immunodeficiency virus type 1 Vpr contains two leucine-rich helices that mediate glucocorticoid receptor coactivation independently of its effects on G(2) cell cycle arrest. J Virol. 2000;74:8159–8165. doi: 10.1128/jvi.74.17.8159-8165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano JD, et al. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitterlin D, et al. UVDDB p127-binding modulates activities and intracellular distribution of hepatitis B virus X protein. Oncogene. 2000;19:4417–4426. doi: 10.1038/sj.onc.1203771. [DOI] [PubMed] [Google Scholar]
- Somasundaran M, et al. Evidence for a cytopathogenicity determinant in HIV-1 Vpr. Proc Natl Acad Sci U S A. 2002;99:9503–9508. doi: 10.1073/pnas.142313699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA, et al. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA, et al. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J Virol. 2000;74:3105–3111. doi: 10.1128/jvi.74.7.3105-3111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stivahtis GL, et al. Conservation and host specificity of Vpr-mediated cell cycle arrest suggest a fundamental role in primate lentivirus evolution and biology. J Virol. 1997;71:4331–4338. doi: 10.1128/jvi.71.6.4331-4338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachiwana H, et al. HIV-1 Vpr induces DNA double-strand breaks. Cancer Res. 2006;66:627–631. doi: 10.1158/0008-5472.CAN-05-3144. [DOI] [PubMed] [Google Scholar]
- Takemura T, Hayami M. Phylogenetic analysis of SIV derived from mandrill and drill. Front Biosci. 2004;9:513–520. doi: 10.2741/1242. [DOI] [PubMed] [Google Scholar]
- Tan L, et al. DDB1 and Cul4A are required for human immunodeficiency virus type 1 Vpr-induced G2 arrest. J Virol. 2007;81:10822–10830. doi: 10.1128/JVI.01380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y, Yasuda Y. Human immunodeficiency virus type 1 Vpr induces G2 checkpoint activation by interacting with the splicing factor SAP145. Mol Cell Biol. 2006;26:8149–8158. doi: 10.1128/MCB.01170-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts RS, et al. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 1999;13:152–157. doi: 10.1101/gad.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts RS, et al. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 2000;14:2989–3002. doi: 10.1101/gad.851000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristem M, et al. Evolution of the primate lentiviruses: evidence from vpx and vpr. Embo J. 1992;11:3405–3412. doi: 10.1002/j.1460-2075.1992.tb05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristem M, et al. Complex evolutionary history of primate lentiviral vpr genes. Virology. 1998;240:232–237. doi: 10.1006/viro.1997.8929. [DOI] [PubMed] [Google Scholar]
- Tungaturthi PK, et al. Role of HIV-1 Vpr in AIDS pathogenesis: relevance and implications of intravirion, intracellular and free Vpr. Biomed Pharmacother. 2003;57:20–24. doi: 10.1016/s0753-3322(02)00328-1. [DOI] [PubMed] [Google Scholar]
- Vieira HL, et al. Permeabilization of the mitochondrial inner membrane during apoptosis: impact of the adenine nucleotide translocator. Cell Death Differ. 2000;7:1146–1154. doi: 10.1038/sj.cdd.4400778. [DOI] [PubMed] [Google Scholar]
- Vodicka MA, et al. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhuber MG, et al. Studies with GFP-Vpr fusion proteins: induction of apoptosis but ablation of cell-cycle arrest despite nuclear membrane or nuclear localization. Virology. 2003;313:91–104. doi: 10.1016/s0042-6822(03)00258-7. [DOI] [PubMed] [Google Scholar]
- Ward I, Chen J. Early events in the DNA damage response. Curr Top Dev Biol. 2004;63:1–35. doi: 10.1016/S0070-2153(04)63001-8. [DOI] [PubMed] [Google Scholar]
- Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- Wecker K, et al. NMR structure of the HIV-1 regulatory protein Vpr in H2O/trifluoroethanol. Comparison with the Vpr N-terminal (1–51) and C-terminal (52–96) domains. Eur J Biochem. 2002;269:3779–3788. doi: 10.1046/j.1432-1033.2002.03067.x. [DOI] [PubMed] [Google Scholar]
- Wecker K, Roques BP. NMR structure of the (1–51) N-terminal domain of the HIV-1 regulatory protein Vpr. Eur J Biochem. 1999;266:359–369. doi: 10.1046/j.1432-1327.1999.00858.x. [DOI] [PubMed] [Google Scholar]
- Wei X, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- Weiss SH, et al. Risk of human immunodeficiency virus (HIV-1) infection among laboratory workers. Science. 1988;239:68–71. doi: 10.1126/science.3336776. [DOI] [PubMed] [Google Scholar]
- Wen X, et al. The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and Cullin4A-containing ubiquitin ligase complex using VprBP/DCAF1 as an adaptor. In press. J Biol Chem. 2007 doi: 10.1074/jbc.M703955200. (In press) [DOI] [PubMed] [Google Scholar]
- Withers-Ward ES, et al. Human immunodeficiency virus type 1 Vpr interacts with HHR23A, a cellular protein implicated in nucleotide excision DNA repair. J Virol. 1997;71:9732–9742. doi: 10.1128/jvi.71.12.9732-9742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F, et al. Human immunodeficiency virus: the eighth gene. AIDS Res Hum Retroviruses. 1987;3:33–39. doi: 10.1089/aid.1987.3.33. [DOI] [PubMed] [Google Scholar]
- Yu X, et al. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- Yuan H, et al. Increased levels of Wee-1 kinase in G(2) are necessary for Vpr- and gamma irradiation-induced G(2) arrest. J Virol. 2004;78:8183–8190. doi: 10.1128/JVI.78.15.8183-8190.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, et al. Depletion of Wee-1 kinase is necessary for both human immunodeficiency virus type 1 Vpr- and gamma irradiation-induced apoptosis. J Virol. 2003;77:2063–2070. doi: 10.1128/JVI.77.3.2063-2070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander K, et al. Cyclophilin A interacts with HIV-1 Vpr and is required for its functional expression. J Biol Chem. 2003;278:43202–43213. doi: 10.1074/jbc.M305414200. [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. Genetic characterization of vif, vpr, and vpu sequences from long-term survivors of human immunodeficiency virus type 1 infection. Virology. 1997;228:340–349. doi: 10.1006/viro.1996.8378. [DOI] [PubMed] [Google Scholar]
- Zhao LJ, et al. Biochemical mechanism of HIV-I Vpr function. Specific interaction with a cellular protein. J Biol Chem. 1994;269:15577–15582. [PubMed] [Google Scholar]
- Zhao Y, et al. Effect of human immunodeficiency virus type 1 protein R (vpr) gene expression on basic cellular function of fission yeast Schizosaccharomyces pombe. J Virol. 1996;70:5821–5826. doi: 10.1128/jvi.70.9.5821-5826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- Zhu Y, et al. Comparison of cell cycle arrest, transactivation, and apoptosis induced by the simian immunodeficiency virus SIVagm and human immunodeficiency virus type 1 vpr genes. J Virol. 2001;75:3791–3801. doi: 10.1128/JVI.75.8.3791-3801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ES, et al. Human Immunodeficiency Virus Type 1 Vpr-Mediated G2 Arrest Requires Rad17 and Hus1 and Induces Nuclear BRCA1 and {gamma}-H2AX Focus Formation. Mol Cell Biol. 2004;24:9286–9294. doi: 10.1128/MCB.24.21.9286-9294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ES, et al. Human immunodeficiency virus type 1 Vpr induces DNA replication stress in vitro and in vivo. J Virol. 2006;80:10407–10418. doi: 10.1128/JVI.01212-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, et al. Regulation of ATR substrate selection by Rad17-dependent loading of Rad9 complexes onto chromatin. Genes Dev. 2002;16:198–208. doi: 10.1101/gad.950302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]