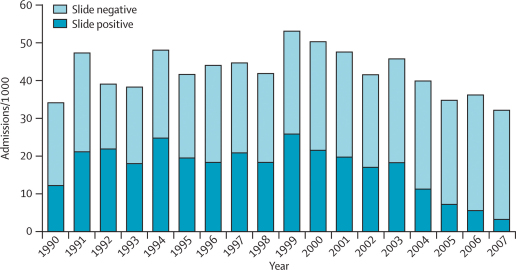Summary
Background
As efforts to control malaria are expanded across the world, understanding the role of transmission intensity in determining the burden of clinical malaria is crucial to the prediction and measurement of the effectiveness of interventions to reduce transmission. Furthermore, studies comparing several endemic sites led to speculation that as transmission decreases morbidity and mortality caused by severe malaria might increase. We aimed to assess the epidemiological characteristics of malaria in Kilifi, Kenya, during a period of decreasing transmission intensity.
Methods
We analyse 18 years (1990–2007) of surveillance data from a paediatric ward in a malaria-endemic region of Kenya. The hospital has a catchment area of 250 000 people. Clinical data and blood-film results for more than 61 000 admissions are reported.
Findings
Hospital admissions for malaria decreased from 18·43 per 1000 children in 2003 to 3·42 in 2007. Over 18 years of surveillance, the incidence of cerebral malaria initially increased; however, malaria mortality decreased overall because of a decrease in incidence of severe malarial anaemia since 1997 (4·75 to 0·37 per 1000 children) and improved survival among children admitted with non-severe malaria. Parasite prevalence, the mean age of children admitted with malaria, and the proportion of children with cerebral malaria began to change 10 years before hospitalisation for malaria started to fall.
Interpretation
Sustained reduction in exposure to infection leads to changes in mean age and presentation of disease similar to those described in multisite studies. Changes in transmission might not lead to immediate reductions in incidence of clinical disease. However, longitudinal data do not indicate that reductions in transmission intensity lead to transient increases in morbidity and mortality.
Funding
Wellcome Trust, Kenya Medical Research Institute.
Introduction
Malaria transmission intensity across Africa ranges from unstable epidemic transmission to high perennial transmission and can be characterised by 100-fold differences in entomological measures of transmission.1 The clinical epidemiology of malaria under different conditions of transmission has been described in ecological comparisons between sites.2–8 Consistent differences have been shown in the presentation of severe malaria and the age of people affected. Under conditions of high transmission, the burden of disease rests disproportionately on very young children and severe disease is most commonly malarial anaemia. Immunity to malaria is acquired quickly, and severe malaria is rarely seen in children older than 5 years. With lower transmission, the burden of disease is distributed throughout childhood, and the proportion of children who develop cerebral malaria—characterised by impaired consciousness, seizures, and coma—increases.
The relation between transmission intensity and the overall incidence of disease is more complex. The incidence of disease seems to be insensitive to differences in transmission over a wide range of intermediate values, decreasing only when transmission drops below a threshold value.2,9,10 Disease incidence might also decrease slightly under conditions of very high transmission.
The higher mean age of children with malaria in areas of low transmission, coupled with an increase in case fatality with age after infancy (caused partly by the higher incidence of cerebral malaria in older children), led to speculation of a possible increase in mortality as transmission decreases. Analysis of mortality data from multiple endemic areas indicates that mortality either increases linearly with transmission, reaches a plateau at moderate transmission, or possibly reaches a peak at moderate transmission and decreases slightly at very high transmission.11–13 Whichever scenario is true, these observations apply to communities observed for short intervals with little or no change in transmission. Where transmission is falling, children will have acquired immunity under conditions of higher transmission in preceding years. Longitudinal surveillance in a single site is therefore essential for predicting and monitoring the effects of malaria control measures designed to reduce exposure to infection.
Okiro and colleagues described a recent downward trend of hospital admission for malaria in three contiguous districts of coastal Kenya.14 Intensive community surveillance in a small, well-defined population in one of those districts showed that significant reductions in exposure to infection preceded a decrease in admissions.15 We now analyse 18 years of detailed hospital surveillance data in a large malaria endemic area of the Kenyan coast. We describe changes in incidence of, and age of patients with, severe malaria and test the hypothesis that changes in transmission cause changes in the proportion and incidence of the severe forms of disease.
Methods
Study area and population
Kilifi district lies on the coast of Kenya, stretches along 65 km of coastline, extends 90 km inland from the coast at the widest point, and covers diverse ecological zones. The district is subdivided into 36 administrative units known as locations. Two-thirds of the population live in 14 locations surrounding Kilifi District Hospital, from which 80% of paediatric admissions come. In 2002, these 14 locations were incorporated into a demographic surveillance site; comprising the catchment area for Kilifi District Hospital, we refer to them as the study area. Kilifi District Hospital is located centrally within the district and serves as the first referral centre for patients requiring hospitalisation. The hospital operates a paediatric ward with 35 beds and a smaller paediatric high-dependency unit. Children up to 14 years of age are admitted to the paediatric ward, although 94% of children admitted are less than 8 years old.
About 250 000 people currently live in the study area, most living off subsistence farming and fishing. Malaria is endemic, and transmission increases after the long rains from April to June and the short rains from October to November each year. The main malaria vectors are Anopheles gambiae sensu lato, with minor contributions from Anopheles funestus.16 The annual entomological inoculation rate varied across the study area from less than 0·1 to 60 in the mid-1990s.16,17
Bednet use also varies across the study area. In the early 1990s, coverage was estimated to be less than 6%.18 A trial of insecticide-treated nets was started in part of northern Kilifi in June, 1993, and insecticide treated nets were distributed to residents in a defined geographical area representing about 30% of the population,19 although use in other areas remained low.20 In 2005, insecticide-treated nets became available through government clinics at highly subsidised cost, and in September, 2006, a free distribution campaign by the District Health Management Team increased coverage across Kilifi district from about 0·25 to 0·5 insecticide-treated nets per person.14
Before 1998, the first-line antimalarial therapy was chloroquine. Increases in resistance to chloroquine over several years prompted a national policy change to sulfadoxine–pyrimethamine as the first-line therapy in 1998.21 Shortly after the switch, a shopkeeper training programme was initiated in northern Kilifi to increase antimalarial access in the community through the informal retail sector.22 In September, 2006, artemether–lumefantrine replaced sulfadoxine–pyrimethamine in government clinics and is offered to paediatric patients, free of charge, through the public-health sector.23
Data collection
The hospital keeps records of clinical examination, location of residence, laboratory investigations, discharge diagnoses, and outcome for each child admitted in a central database.24,25 A routine blood slide is taken on all admissions and is examined by microscopy for malaria parasites. Haemoglobin concentrations are also measured for all admissions. Clinical intake examination includes an assessment of the level of consciousness and children are given a Blantyre coma score.26 Blantyre coma scores are available for all children between 2002 and 2007 and for those admitted to the high-dependency unit between 1990 and 2001. Records from the high-dependency unit could not be located for the last 6 months of 1992, and children admitted to the high-dependency unit in 1998 could not be matched to the ward registers, so these years were omitted from the analysis of severe disease. Only children who were resident in the study area at the time of admission were included in the analysis.
Hospitalised malaria was defined as any admission with a positive blood film. Severe malarial anaemia was defined as a parasite-positive blood film on intake and a haemoglobin concentration of less than 5 g/dL. Cerebral malaria was defined as a parasite-positive blood film on intake and a Blantyre coma score of less than 3. The definitions were not mutually exclusive. Although impaired consciousness might not be due to parasites in some patients, this is the most objective definition of cerebral malaria and can be uniformly applied to all admissions. Studies and data collection methods were approved by the Kenya Medical Research Institute's ethical review committee.
Parasite densities from children admitted to Kilifi District Hospital for malaria and from children admitted because of trauma (taken as control individuals) were used to model the relation between parasite density and clinical disease. The method has been described previously.27 A logistic regression model was used where log(p)=a+bxτ, p is the probability of admission, x is the density of parasitaemia, and τ is the power function that maximises the likelihood estimation. The coefficient b in the logistic model can then be used to calculate the risk of malaria for each individual. The risks of a group of individuals are summed to estimate the proportion of the group with true malaria. The model was repeated in two time periods; several years before changes in incidence of slide-positive admissions (1998–2003) and the years after initial changes in incidence (2004–07). Confidence intervals were estimated by bootstrapping with 1000 iterations.
Analysis
Population data and growth rate from the 1999 population and housing census were obtained from the Kenya National Bureau of Statistics for each location. The study area population was multiplied by the proportion of children under 14 years of age (46%, H Gatakaa Kenya Medical Research Institute–Wellcome Trust, personal communication). The number of children under 14 in other years was estimated by assuming a 3·1% growth rate and extrapolating backwards and forwards from 1999. Annual incidence was calculated by dividing the total number of hospital events (admissions, deaths, etc) by the total number of children under 14 years of age resident in the study area for each year.
Statistical analyses were done with Stata 9.0. Trends in incidence by year were investigated with linear regression. The residuals of linear regression of age on year showed slight deviations from normality, so trends were investigated with quintile regression. However, results did not differ between the two methods. Confidence intervals for parasite prevalence were calculated with a binomial distribution.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between 1800 and 4400 children from the study area were admitted to the paediatric ward and had blood films examined for parasites each year (table 1). An initial increase in slide-positive admissions between 1990 and 1991 reflects an increase in hospital use and all-cause admissions during that period (figure 1). Between 1990 and 2003, the incidence of admissions for malaria fluctuated with the total admissions, whereas the proportion of all admissions with parasites was stable at 40–50% (data not shown). No significant trend in incidence was apparent from 1990 to 2003 (p=0·68). In 2004, the incidence of hospital admissions for malaria began to fall (p=0·008 for 1990–2007). The incidence of slide-positive admissions in 2007 was less than a fifth of that in 2003 (table 1), and only 383 (11%) of 3620 admissions were slide-positive.
Table 1.
Epidemiological summary of malaria in children age less than 14 years, in Kilifi, Kenya, 1990–2007
| Population <14 years | Admissions | Slide-positive admissions | Slide-positive deaths | SMA | CM | Ratio CM/SMA | Mean age of slide positive (years) | Mean age of slide negative (years) | Parasite prevalence | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1990 | 51 939 | 34·21 | 12·36 | 0·87 | 2·02 | 0·54 | 0·27 | 2·3 | 2·15 | 0·16 |
| 1991 | 55 475 | 47·32 | 21·23 | 1·08 | 5·25 | 0·96 | 0·18 | 2·47 | 2·02 | 0·28 |
| 1992 | 59 012 | 39·13 | 21·96 | 0·93 | 3·97 | .. | .. | 2·42 | 2·05 | 0·39 |
| 1993 | 62 548 | 38·37 | 18·16 | 0·67 | 3·09 | 0·42 | 0·13 | 2·4 | 1·93 | 0·35 |
| 1994 | 66 084 | 47·97 | 24·91 | 0·88 | 5·84 | 1·21 | 0·21 | 2·4 | 2·12 | 0·33 |
| 1995 | 69 620 | 41·67 | 19·52 | 0·49 | 3·3 | 0·95 | 0·29 | 2·37 | 1·95 | 0·34 |
| 1996 | 73 156 | 44·02 | 18·45 | 0·52 | 3·28 | 1·01 | 0·31 | 2·1 | 1·88 | 0·27 |
| 1997 | 76 692 | 44·7 | 20·95 | 1·02 | 4·75 | 1·51 | 0·32 | 2·37 | 1·87 | 0·28 |
| 1998 | 80 229 | 41·89 | 18·48 | 0·79 | 3·73 | .. | .. | 2·46 | 1·93 | 0·31 |
| 1999 | 83 765 | 53·09 | 25·92 | 0·81 | 4·14 | 1·1 | 0·27 | 2·72 | 1·98 | 0·29 |
| 2000 | 87 301 | 50·3 | 21·75 | 0·63 | 2·78 | 1·17 | 0·42 | 2·66 | 1·98 | 0·24 |
| 2001 | 90 837 | 47·59 | 19·85 | 0·54 | 1·81 | 0·89 | 0·49 | 2·76 | 2·01 | 0·19 |
| 2002 | 94 195 | 41·59 | 17·17 | 0·64 | 2·04 | 2·05 | 1·01 | 2·93 | 2·02 | 0·19 |
| 2003 | 97 827 | 45·71 | 18·43 | 0·51 | 2·59 | 1·59 | 0·62 | 2·86 | 2·06 | 0·24 |
| 2004 | 101 747 | 39·86 | 11·27 | 0·33 | 1·52 | 1·07 | 0·7 | 2·78 | 2·05 | 0·13 |
| 2005 | 105 337 | 34·87 | 7·31 | 0·16 | 1·04 | 0·64 | 0·61 | 3·21 | 2·2 | 0·11 |
| 2006 | 108 121 | 36·27 | 5·76 | 0·16 | 0·75 | 0·58 | 0·78 | 3·44 | 2·05 | 0·07 |
| 2007 | 112 054 | 32·31 | 3·42 | 0·12 | 0·37 | 0·39 | 1·07 | 3·59 | 2·18 | 0·01 |
Data are incidence per 1000. Cerebral malaria (CM) incidence was not available for 1992 and 1998. SMA=severe malarial anaemia.
Figure 1.
Hospital admissions per 1000 children resident in the study area
Malaria attributable fractions calculated from logistic regression suggested that 61% of slide-positive admissions were attributable to malaria infection during 1998–2003, just before changes in the incidence of slide-positive admissions (table 2). Because the mean incidence of admissions with positive slides fell by 12·9 per 1000 by 2004–2007, an attributable fraction of 61% would predict that overall incidence of admissions would fall by 7·8 per 1000. In fact, the overall drop in admissions, 10·3 per 1000, was slightly higher than this prediction (table 2).
Table 2.
Difference in all admissions and slide-positive admissions before and after the decline in slide-positive admissions
|
Mean incidence per 1000 |
MAF (95% CI) | ||
|---|---|---|---|
| All admissions | Slide-positive admissions | ||
| 1998–2003 | 45·4 | 19·7 | 0·61 (0·56–0·65) |
| 2004–07 | 35·1 | 6·8 | 0·70 (0·66–0·74) |
| Difference | 10·3 | 12·9 | .. |
| Expected difference (95% CI)* | 7·8 (7·2–8·4) | .. | .. |
Expected difference in all admissions is the change in slide-positive admissions multiplied by the malaria attributable fraction from the preceding years (ie, 12·9×0·61). MAF=malaria attributable fraction.
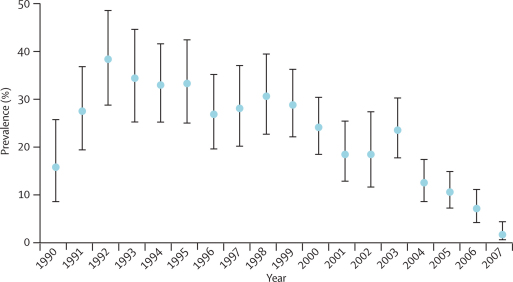
Between 130 and 350 children from the study area were admitted each year for accidents such as fractures, snake bites, burns, and poisoning. The mean age of this group is 4·0 years (95% CI 3·85–4·07). Parasitaemia among these children is unrelated to the cause for their admission and is therefore expected to reflect exposure to infection in the community. Parasite prevalence decreased from 1992 to 2007 (p<0·0001; figure 2) indicating a reduction in exposure to infection among children across the hospital catchment area.
Figure 2.
Annual parasite prevalence in patients admitted for trauma
Bars are 95% CIs.
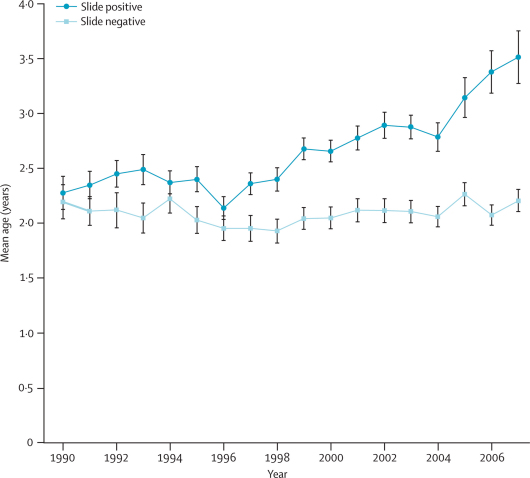
Over the surveillance period, the mean age of slide-positive admissions rose significantly (p<0·0001), but the mean age for slide-negative admissions remained stable (table 1; figure 3). The increase in age of slide-positive patients was incrementally distributed over the last 10 years or so.
Figure 3.
Mean age in years of slide-positive and slide-negative hospital admissions by year
Bars are 95% CIs.
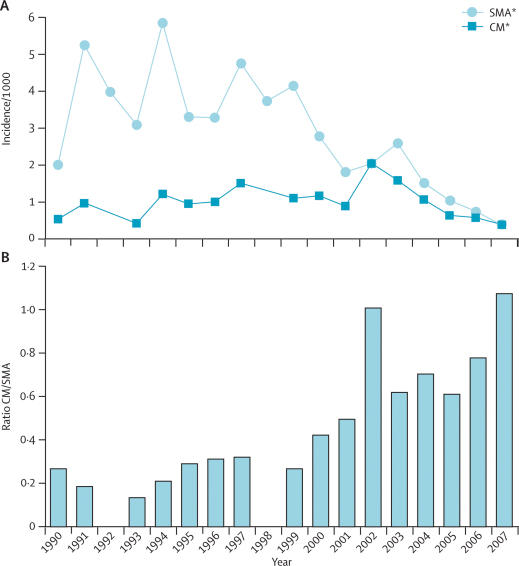
Annually, a mean of 28% of slide positive admissions fit the criteria for severe disease, either severe malarial anaemia (haemoglobin <5 g/dL) or cerebral malaria (Blantyre coma score <3). At the beginning of the surveillance period, the number of cases of severe malarial anaemia outnumbered those of cerebral malaria by more than four to one (table 1). The incidence of cerebral malaria increased gradually from 1990 to 2002, whereas the incidence of severe malarial anaemia began to decrease in 1997 (figure 4). In 2002, the incidence of cerebral malaria equalled that of severe malarial anaemia. When numbers of slide-positive admissions began to fall in 2004, admissions of both forms of severe malaria decreased in parallel, but the ratio of cerebral malaria to severe malarial anaemia continues to rise (figure 4). In 2007, the incidence of cerebral malaria barely exceeded that of severe malarial anaemia.
Figure 4.
Annual incidence (A) and ratio (B) of cerebral malaria and severe malarial anaemia
Data for cerebral malaria were unavailable in 1992 and 1998. SMA=severe malarial anaemia. CM=cerebral malaria.
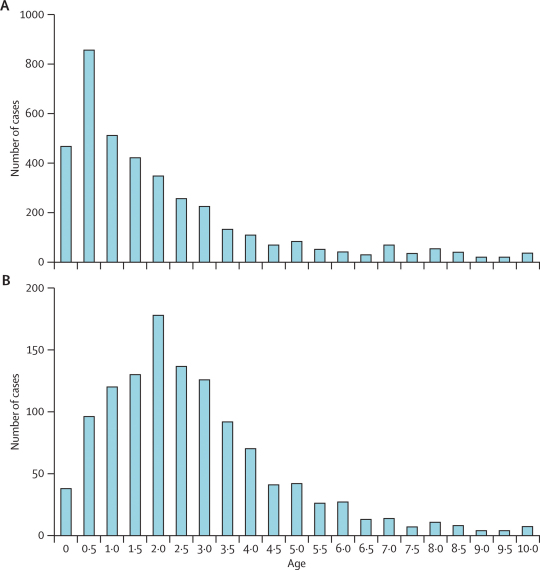
The mean age of children with severe malarial anaemia was 2·1 years and increased slightly but not significantly over the 18 years (2·5 years). The mean age for cerebral malaria remained constant (3·1 years) and was significantly higher than that for severe malarial anaemia in each year (p<0·005 for all 16 comparisons). The incidence of severe malarial anaemia peaked in 6 month olds, whereas incidence of cerebral malaria peaked at 2 years of age, leading to distinct age distributions for each syndrome (figure 5).
Figure 5.
Age distributions for children admitted with severe malarial anaemia (A) and cerebral malaria (B)
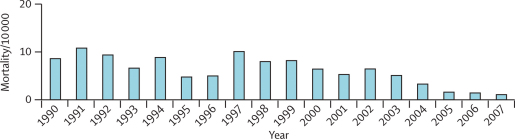
Mortality among slide-positive admissions fluctuated from year to year (figure 6), although there was a small but significant decrease over the surveillance period (p<0·0001). As with the incidence of slide-positive admissions, no significant trend in mortality of slide-positive admissions was detected before 2003. However, in 2004 malaria mortality was lower than for any of the previous 14 years (table 1). The number of deaths continued to decrease from 2004 to 2007 (table 1) in parallel with the decrease in slide-positive admissions. In 2007, there were fewer than 15 deaths of children who were slide positive, compared with 78 in 1997.
Figure 6.
Mortality of slide-positive cases per 10 000 children resident in the study area
Overall, case-fatality for non-severe malaria admissions (ie, slide positive but neither severe malarial anaemia nor cerebral malaria) was low: 2·1% (400/18 829) compared with 8·3% (3176/38 100) for slide-negative admissions. The case fatality for both forms of severe disease was higher than for non-severe slide-positive admissions: 5·9% (214/3652) for severe malarial anaemia and 14·0% (167/1194) for cerebral malaria. The case fatality for non-severe slide-positive admissions fell significantly with time from 6·5% in 1990 to 1·3% in 2007 (p<0.0001), whereas there was no significant trend in case fatality of cerebral malaria or severe malarial anaemia.
Infants younger than 6 months were at the greatest risk of death (5·9%), but that risk fell to 3·8% by age 1 year. Case fatality remained stable in early childhood (4·2% in children age 1–5 years), and decreased slightly in children age 6–8 years (2·7%), before increasing again in children age 9–14 years (4·1%).
Discussion
Transmission of malaria has been changing for over 10 years, as evidenced by declining prevalence of parasitaemia among trauma admissions (an indicator of parasite prevalence in the community), increasing mean age of malaria admissions, and shifting presentation of severe disease; however, the absolute incidence of malaria disease presenting to hospital only began to change 4–5 years ago. Once this change began, the effect has been substantial as evidenced by a reduction in deaths from malaria of more than 75%. The fall in transmission is not specific to Kilifi and seems to involve the entire coastal region of Kenya.14 Our results are consistent with comparisons between multiple sites2,3,9 and provide further evidence that reduction of transmission leads to a change in pattern of severe disease but might not lead to immediate reductions in disease burden. The threshold parasite prevalence of 20%, above which disease incidence remained constant and below which incidence fell sharply, was identical to that predicted in studies across multiple sites.9
The pathology of severe disease is partly dependent on the age of individuals independent of previous exposure.28,29 In children age less than 2 years, severe malaria typically manifests as life-threatening anaemia. In older children, there is an increased risk of cerebral malaria, characterised by impaired consciousness, seizures, and coma.30 In previous studies, possible differences between host and parasite populations could not be excluded as potential confounders in the differences observed between sites. In Kilifi, increasing mean age of patients who were slide positive on admission over the past 10 years was accompanied by an increase in the proportion of severe disease presenting as cerebral malaria. Although the overall mean age of hospital admissions for malaria increased, the mean age of children with severe malarial anaemia was consistently lower than that of children with cerebral malaria. This finding supports the hypothesis that the pathology of severe malaria is related to intrinsic factors of host age rather than only to extrinsic factors, such as parasite genetics or transmission intensity.
In multisite comparisons, low transmission was associated with higher mean age of hospitalisation and, as a result, a higher proportion of cerebral malaria than found in areas of high transmission.2,7,8 The case fatality of hospitalised clinical malaria has been reported to increase with age after the first year of life,8,9 partly because of the high case fatality of cerebral malaria compared with that of severe malarial anaemia.8,31,32 If the total number of patients hospitalised with clinical malaria remains constant, or even increases, as transmission decreases from high to intermediate levels, then increasing age at hospitalisation might lead to higher incidence of cerebral malaria and malaria mortality.11 Our study did not support this scenario, consistent with results from shorter periods of observation after trials with insecticide-treated bednets.33,34 Although the mean age of severe and uncomplicated malaria rose, and the proportion of cerebral malaria increased as predicted, we did not find increasing overall mortality or severe morbidity as a result. A small increase in the number of deaths in patients with cerebral malaria was offset by a decrease in incidence of severe malarial anaemia and the improved survival of moderate malaria admissions. Although incidence of cerebral malaria increased between 1990 and 2002, this presentation remains rare.
Older children exposed to higher levels of transmission in early life might have a lower incidence of cerebral malaria than those who grow up in an area of low, stable endemicity. Indeed, by contrast with other studies,8,9 case fatality for hospitalised malaria was lower in older children than in younger children in our study. The difference in the relation between age and case fatality indicates greater heterogeneity of exposure in children born during the surveillance period, than in earlier studies with observations made over shorter time intervals in several sites. More generally, our results describe a community undergoing a gradual change in transmission and may be different than those generated by an abrupt change in exposure as a result of widespread deployment of a highly effective intervention followed by a gradual equilibration at lower transmission. The incidence of cerebral malaria might increase as children with less exposure grow up and experience episodes of severe disease. However, on the basis of current trends, the proportion of severe disease presenting as cerebral malaria will probably increase, but the incidence will likely continue to fall.
The reduction in all hospital admissions was small compared with the reduction in slide-positive admissions, indicating that some slide-positive admissions are coincidental coinfections. The malaria attributable fraction calculations confirmed this. After adjusting for coincidental parasitaemias, all admissions decreased more than would be expected. The attributable-fraction model might underestimate the true proportion of malaria cases or infection might increase susceptibility to other disease and reduction of infection could reduce total admissions by more than just the true malaria cases.
The reasons for the changing pattern of malaria epidemiology and declining disease burden are most likely multifactorial. Bednet distribution in parts of northern Kilifi in the early 1990s19 could have initially contributed to the fall in transmission in that area.15 Bednet use 5 years later remained high in the bednet trial area (69%), although most nets were untreated and in bad repair.35 Bednet coverage in most of the catchment population was much lower; the 5% coverage reported in southern Kilifi20 is unlikely to have lowered transmission. The number of insecticide-treated nets in the district at the time of the greatest change in disease burden was estimated to be less than one for every ten people.14 Coverage increased to about one for every two people between 2005 and 2007, but this was after the steep fall in malaria admissions. Increases in the mean age of children with malaria, indicating reduced exposure to infection, seem to be contemporary with the replacement of chloroquine with sulfadoxine–pyrimethamine as the first-line therapy 10 years ago. The widespread use of an effective, longlasting antimalarial possibly contributed to the reduction in transmission. What seems clear is that the substantial changes in the epidemiology and burden of disease observed in Kilifi began before large-scale distribution of insecticide-treated bednets and availability of artemisinin-combination therapies. Other areas in east Africa have described similar reductions in burden of disease correlated to the scale-up of control efforts,36–38 but this does not seem to be the case in Kilifi. Although effective treatment and prevention will play a crucial part in maintaining and further reducing the burden of malaria in Kilifi, the initial cause of the reduction remains unknown.
Other external factors unrelated to specific malaria interventions might have changed over the 18 years of surveillance and affected the presentation of disease at the hospital. These factors include those that affect the risk of infection, reflected in declining parasite prevalence observed here, as well as factors that affect the probability of presenting at the hospital with clinical disease. Agriculture practices, economic development, housing construction, and environmental management of breeding sites for mosquitoes, among others, likely changed over time and might have contributed to the reduced risk of infection in the community. Changing rates of hospital use due to expanding transport networks, user fees, the effectiveness of treatment at the primary care level, or changes in referral practices, could have influenced the probability of presenting at the hospital in either direction. Overall, there is evidence for gradually increasing use of hospitals, although changes have been small. Slight increases in hospital use could have increased the apparent incidence of malaria, leading to an underestimate of the reductions in malaria incidence. Although presentation with trauma might be influenced by factors affecting hospital use, parasite prevalence among those patients would not be. In other words, prevalence in trauma patients is likely to reflect transmission independent of external factors that affect disease presentation.
Observations from 18 years of longitudinal surveillance show non-linear relations between incidence and presentation of disease, age, and mortality. The changes in the pattern of clinical disease are consistent with long-term, sustained reductions in transmission. Significant increases in the mean age of slide-positive hospital admissions, and a rise in the proportion of cerebral malaria preceded changes in the overall incidence of disease. Continued surveillance is needed to describe changes in the burden of disease among those children growing up with much less exposure than those born only 5 years or 10 years ago. Emphasis on use of insecticide-treated bednets, early treatment, and other control measures must be increased to maintain reductions in disease burden and prevent a potential resurgence of malaria in a population with far less immunity than before.
Acknowledgments
Acknowledgments
This work is published with the permission of the Director of Kenya Medical Research Institute. We wish to thank all the clinical, field, and laboratory staff at the Kenya Medical Research Institute–Wellcome Trust Programme who made this study possible. WPO is funded by the Fogarty International Center. CRJCN is funded by the Wellcome Trust. RWS is a Wellcome Trust Principal Research Fellow (#079080).
Contributors
WPO conceived the study, did data analysis, and drafted the Article. PB did data analysis, assisted with interpretation of results, and edited the paper. TWM assisted with study design and interpretation of results. EO contributed to interpretation of the results and editing of the paper. RS contributed to study design, interpretation of the results and editing of the paper. CN contributed to study design, data collection, interpretation of the results and editing of the paper. KM contributed to study design, interpretation of results, and revising of the paper.
Conflict of interest statement
We declare that we have no conflict of interest.
References
- 1.Hay SI, Rogers DJ, Toomer JF, Snow RW. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, internet access and review. Trans R Soc Trop Med Hygiene. 2000;94:113–127. doi: 10.1016/s0035-9203(00)90246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snow RW, Omumbo JA, Lowe B. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 3.Trape JF, Quinet MC, Nzingoula S. Malaria and urbanization in Central Africa—the example of Brazzaville. 5. Pernicious attacks and mortality. Trans R Soc Trop Med Hygiene. 1987;81:34–42. doi: 10.1016/0035-9203(87)90475-5. [DOI] [PubMed] [Google Scholar]
- 4.Rogier C, Tall A, Diagne N, Fontenille D, Spiegel A, Trape JF. Plasmodium falciparum clinical malaria: lessons from longitudinal studies in Senegal. Parassitologia. 1999;41:255–259. [PubMed] [Google Scholar]
- 5.Snow RW, Deazevedo IB, Lowe BS. Severe childhood malaria in 2 areas of markedly different falciparum transmission in. East Africa Acta Tropica. 1994;57:289–300. doi: 10.1016/0001-706x(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 6.Idro R, Aloyo J, Mayende L, Bitarakwate E, John CC, Kivumbi GW. Severe malaria in children in areas with low, moderate and high transmission intensity in Uganda. Trop Med Int Health. 2006;11:115–124. doi: 10.1111/j.1365-3156.2005.01518.x. [DOI] [PubMed] [Google Scholar]
- 7.Modiano D, Sirima BS, Sawadogo A. Severe malaria in Burkina Faso: influence of age and transmission level on clinical presentation. Am J Trop Med Hygiene. 1998;59:539–542. doi: 10.4269/ajtmh.1998.59.539. [DOI] [PubMed] [Google Scholar]
- 8.Reyburn H, Mbatia R, Drakeley C. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA. 2005;293:1461–1470. doi: 10.1001/jama.293.12.1461. [DOI] [PubMed] [Google Scholar]
- 9.Marsh K, Snow RW. Malaria transmission and morbidity. Parassitologia. 1999;41:241–246. [PubMed] [Google Scholar]
- 10.Trape JF, Rogier C. Combating malaria morbidity and mortality by reducing transmission. Parasitol Today. 1996;12:236–240. doi: 10.1016/0169-4758(96)10015-6. [DOI] [PubMed] [Google Scholar]
- 11.Snow RW, Marsh K. Will reducing Plasmodium falciparum transmission alter malaria mortality among African children? Parasitol Today. 1995;11:188–190. doi: 10.1016/0169-4758(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 12.Smith TA, Leuenberger R, Lengeler C. Child mortality and malaria transmission intensity in Africa. Trends Parasitol. 2001;17:145–149. doi: 10.1016/s1471-4922(00)01814-6. [DOI] [PubMed] [Google Scholar]
- 13.Snow RW, Marsh K. The consequences of reducing transmission of Plasmodium falciparum in Africa. Adv Parasitol. 2002;52:235–264. doi: 10.1016/s0065-308x(02)52013-3. [DOI] [PubMed] [Google Scholar]
- 14.Okiro EA, Hay SI, Gikandi PW. The decline in paediatric malaria admissions on the coast of Kenya. Malar J. 2007;6:151. doi: 10.1186/1475-2875-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Meara WP, Mwangi T, Williams T, McKenzie FE, Snow R, Marsh K. Relationship between exposure, clinical malaria, and age in an area of changing transmission intensity. Am J Trop Med Hygiene. 2008;79:185–191. [PMC free article] [PubMed] [Google Scholar]
- 16.Mbogo CNM, Snow RW, Khamala CPM. Relationships between Plasmodium falciparum transmission by vector populations and the incidence of severe disease at 9 sites on the Kenyan coast. Am J Trop Med Hygiene. 1995;52:201–206. doi: 10.4269/ajtmh.1995.52.201. [DOI] [PubMed] [Google Scholar]
- 17.Mbogo CM, Mwangangi JM, Nzovu J. Spatial and temporal heterogeneity of anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hygiene. 2003;68:734–742. [PubMed] [Google Scholar]
- 18.Snow RW, Peshu N, Forster D, Mwenesi H, Marsh K. The role of shops in the treatment and prevention of childhood malaria on the coast of Kenya. Trans R Soc Trop Med Hygiene. 1992;86:237–239. doi: 10.1016/0035-9203(92)90290-s. [DOI] [PubMed] [Google Scholar]
- 19.Nevill CG, Some ES, Mungala VO. Insecticide-treated bednets reduce mortality and severe morbidity from malaria among children on the Kenyan coast. Trop Med Int Health. 1996;1:139–146. doi: 10.1111/j.1365-3156.1996.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 20.Mwangi T. Clinical epidemiology of malaria under differing levels of transmission. Open University; Oxford: 2003. [Google Scholar]
- 21.Shretta R, Omumbo J, Rapuoda B, Snow RW. Using evidence to change antimalarial drug policy in Kenya. Trop Med Int Health. 2000;5:755–764. doi: 10.1046/j.1365-3156.2000.00643.x. [DOI] [PubMed] [Google Scholar]
- 22.Marsh VM, Mutemi WM, Muturi J. Changing home treatment of childhood fevers by training shop keepers in rural Kenya. Trop Med Int Health. 1999;4:383–389. doi: 10.1046/j.1365-3156.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- 23.Amin AA, Zurovac D, Kangwana BB. The challenges of changing national malaria drug policy to artemisinin-based combinations in Kenya. Malaria J. 2007;6:72. doi: 10.1186/1475-2875-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkley JA, Lowe BS, Mwangi I. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 25.Berkley JA, Ross A, Mwangi I. Prognostic indicators of early and late death in children admitted to district hospital in Kenya: cohort study. BMJ. 2003;326:361. doi: 10.1136/bmj.326.7385.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–459. [PubMed] [Google Scholar]
- 27.Smith T, Schellenberg JA, Hayes R. Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med. 1994;13:2345–2358. doi: 10.1002/sim.4780132206. [DOI] [PubMed] [Google Scholar]
- 28.Baird JK, Masbar S, Basri H, Tirtokusumo S, Subianto B, Hoffman SL. Age-dependent susceptibility to severe disease with primary exposure to Plasmodium falciparum. J Infect Dis. 1998;178:592–595. doi: 10.1086/517482. [DOI] [PubMed] [Google Scholar]
- 29.Aponte J, Menendez C, Schellenberg D. Age interaction in the development of naturally acquired immunity to Plasmodium falciparum and its clinical presentation. PLoS Med. 2007;4:e242. doi: 10.1371/journal.pmed.0040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imbert P, Sartelet I, Rogier C, Ka S, Baujat G, Candito D. Severe malaria among children in a low seasonal transmission area, Dakar, Senegal: influence of age on clinical presentation. Trans R Soc Trop Med Hyg. 1997;91:22–24. doi: 10.1016/s0035-9203(97)90380-1. [DOI] [PubMed] [Google Scholar]
- 31.Taylor T, Olola C, Valim C. Standardized data collection for multi-center clinical studies of severe malaria in African children: establishing the SMAC network. Trans R Soc Trop Med Hyg. 2006;100:615–622. doi: 10.1016/j.trstmh.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsh K, Forster D, Waruiru C. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 33.Binka FN, Kubaje A, Adjuik M. Impact of permethrin impregnated bednets on child mortality in Kassena-Nankana district, Ghana: a randomized controlled trial. Trop Med Int Health. 1996;1:147–154. doi: 10.1111/j.1365-3156.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 34.Lindblade KA, Eisele TP, Gimnig JE. Sustainability of reductions in malaria transmission and infant mortality in western Kenya with use of insecticide-treated bednets: 4 to 6 years of follow-up. JAMA. 2004;291:2571–2580. doi: 10.1001/jama.291.21.2571. [DOI] [PubMed] [Google Scholar]
- 35.Mwangi TW, Ross A, Marsh K, Snow RW. The effects of untreated bednets on malaria infection and morbidity on the Kenyan coast. Trans R Soc Trop Med Hygiene. 2003;97:369–372. doi: 10.1016/s0035-9203(03)90056-3. [DOI] [PubMed] [Google Scholar]
- 36.Bhattarai A, Ali AS, Kachur SP. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graves PM, Osgood DE, Thomson MC. Effectiveness of malaria control during changing climate conditions in Eritrea, 1998–2003. Trop Med Int Health. 2008;13:218–228. doi: 10.1111/j.1365-3156.2007.01993.x. [DOI] [PubMed] [Google Scholar]
- 38.Killeen GF, Tami A, Kihonda J. Cost-sharing strategies combining targeted public subsidies with private-sector delivery achieve high bednet coverage and reduced malaria transmission in Kilombero Valley, southern Tanzania. BMC Infect Dis. 2007;7:121. doi: 10.1186/1471-2334-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]