Abstract
Objectives
The invasive growth of circulating tumor cells (CTCs) propagates cancer metastasis. The aims of this study were to evaluate the association of invasive CTCs, detected by a novel cell invasion assay, with disease stage, CA-125 level and patient survival.
Methods
Peripheral blood samples from 71 patients undergoing evaluation for ovarian malignancy were assessed for the presence of invasive CTCs using a cell invasion assay that enriches and identifies tumor cells with a cell adhesion matrix (CAM). Invasive CTCs were identified as cells exhibiting CAM invasion (CAM+) and expressing standard epithelial markers (Epi+).
Results
43 (60.6%) patients had detectable CTCs: 0/5 benign patients, 1/10 (10%) early stage, 39/52 (73.1%) late stage and 3/4 (75%) unstaged patients (p-value < 0.001). CTC counts ranged from 0-149 CTCs/ml with stage III/IV patients exhibiting significantly higher mean counts (41.3 CTCs/ml) than stage I/II patients (6.0 CTCs/ml) and benign patients (0 CTCs/ml, p-value = 0.001). A positive correlation between CTC count and CA-125 level was observed (Spearman correlation coefficient r = 0.309, p-value = 0.035). Kaplan-Meier curves revealed a significant decrease in disease-free survival in patients with detectable CTCs (median survival 15.0 months vs. 35.0 months, log-rank p-value = 0.042). Tumor grade and tumor histology did not influence CTC detection.
Conclusions
Invasive CTCs can be detected in a majority of epithelial ovarian cancer patients and may predict shorter disease-free survival. Furthermore, higher CTC counts may reflect later stage disease and higher CA-125 levels.
Introduction
Ovarian cancer is the second most commonly diagnosed gynecologic malignancy and the leading cause of death from a gynecologic malignancy in the United States [1]. One reason that ovarian cancer is so lethal is because over 75% of cases are stage III/IV by the time of diagnosis. The preponderance of advanced stage is associated with poorer survival outcomes [2]. Furthermore, although a majority of optimally cytoreduced, late stage disease patients achieve a complete clinical response after completing chemotherapy, most of those patients eventually relapse[3]. Thus methods that help diagnose ovarian cancer earlier and which can detect metastasis sooner have the potential to improve survival.
The idea that hematogeneous spread of ovarian cancer could play an important role in ovarian cancer progression was first explored in 1990 by Cain [4]. Since then, several studies have investigated the association of tumor cell detection in bone marrow with survival prognostication [5-8], however only two have evaluated the survival effect of tumor cells in peripheral blood (circulating tumor cells or CTCs) [8,9]. Marth et al's study identified CTCs using immunomagnetic beads conjugated to an epithelial glycoprotein (EGP-2). Samples that demonstrated at least 2 cells with antibody-bound beads were considered positive. Judson et al's study used immunomagnetic beads conjugated to a mixture of epithelial markers (anti-cytokeratin 8 and 18, TFS02, CK-7, CK-10 and EGFR) to enrich tumor cells, and then used microscopic evaluation to identify and count CTCs based on specific cytoplasmic staining and tumor cell morphology. These antibody-based methods resulted in low CTC detection rates ranging from 12%-18.7%, and the presence of CTCs defined by anti-epithelial antibody markers alone was not found to affect survival or disease recurrence. Furthermore, the majority of CTCs detected in these previous studies may not have been viable since only a few CTCs retain the ability for invasive growth needed for metastatic progression [10].
In the current study, a novel cell invasion assay modified from a previously described method [11] was used to detect viable and invasive CTCs. This method enriches CTCs based on a tumor cell's unique ability to invade and ingest a Cell Adhesion Matrix (CAM) and distinguishes CTCs from non-tumor and non-viable cells. When blood containing CTCs is exposed to CAM, CTCs bind to, invade and ingest CAM, while the majority of non-tumor and dead tumor cells do not. If CAM is fluorescently labeled, fluorescence microscopic visualization of CAM fragments (CAM+) by a cell that is simultaneously co-stained with epithelial markers (Epi+) can identify a CTC in vitro. Our study evaluated the clinical characteristics and survival prognosticative value of these CAM+/Epi+ CTCs in ovarian cancer patients. Specifically, the study aimed to: 1) determine how clinical parameters, such as disease stage, tumor grade, tumor histology and CA-125 level, affect the presence CAM+/Epi+ cells and 2) assess the association between overall and disease-free survival time and the presence of CAM+/Epi+ cells.
Materials Methods
Patients
The study was conducted at Stony Brook University Hospital after approval from the Committees on Research Involving Human Subjects was obtained. Patients undergoing evaluation for suspected ovarian malignancy (initial diagnosis or recurrence) from January 1, 2001 to December 31, 2007 were recruited. Blood samples were collected once written, informed consent was obtained. Patients with pathologically or cytologically confirmed epithelial ovarian cancer or primary peritoneal cancer were included in the study. Disease staging was defined using the FIGO classification system. Disease recurrence was confirmed by examination, CT scan, biopsy, tumor markers and/or repeat surgery.
Sample collection
Five to twenty milliliters of blood were collected at the time of surgery, prior to incision (or at the time of follow-up visit for non-operative candidates). Three ml aliquots were used for quantification of CTCs. Blood was collected in Vacutainer™ tubes (Becton Dickinson, Franklin Lakes, NJ; green top, lithium heparin as anticoagulant) and processed within one to four hours from collection.
Tumor cell enrichment and labeling
Blood samples were subjected to Ficoll density gradient centrifugation to obtain the mono-nucleated cells (MNC). To label tumor cells that ingest fluorescent CAM fragments (CAM+), MNCs from 0.5 ml whole blood aliquots were seeded onto one well of a red fluorescent CAM-coated 16-well chamber slide (Vita-Assay™, Vitatex Inc., Stony Brook, NY) for 12 hours and the non-adherent cells were washed away with PBS to generate the remaining red fluorescent CAM-labeled cells. Cells were cultured with CCC media (1:1 mixture of Dulbecco's modified Eagle's medium and RPMI1640 medium supplemented with 10% calf serum, 10% Nu-serum, 2 mM L-glutamine, 1 unit/ml penicillin, and 10 μg/ml streptomycin). To stain cells with green fluorescent antibodies, cells were fixed with 3.5% paraformaldehyde/PBS, permeablized with 0.1% Triton X-100, and stained using fluorescein-conjugates of antibodies against epithelial cell lineage markers (Epi+), including EpCAM (clone Ber-Ep4, Dako, Carpinteria, CA); ESA (clone B29.1, Biomeda, Foster City, CA); and pan-cytokeratins 4,5,6,8,10,13, and 18 (CK) (clone C11, Sigma, St. Louis, MO). In addition, CAM-enriched cells were stained with an anti-CD45 antibody (clone T29/33, DakoCytomation, Carpinteria, CA) and followed by red color alkaline-phosphatase-anti-alkaline-phosphatase (APAAP) conjugated secondary antibodies (DakoCytomation, Carpinteria, CA) for internal control of cells with common leukocyte markers. A Nikon E-400 inverted fluorescence microscope equipped with Microfire digital camera system and Image Pro Plus software was used to examine and record the images.
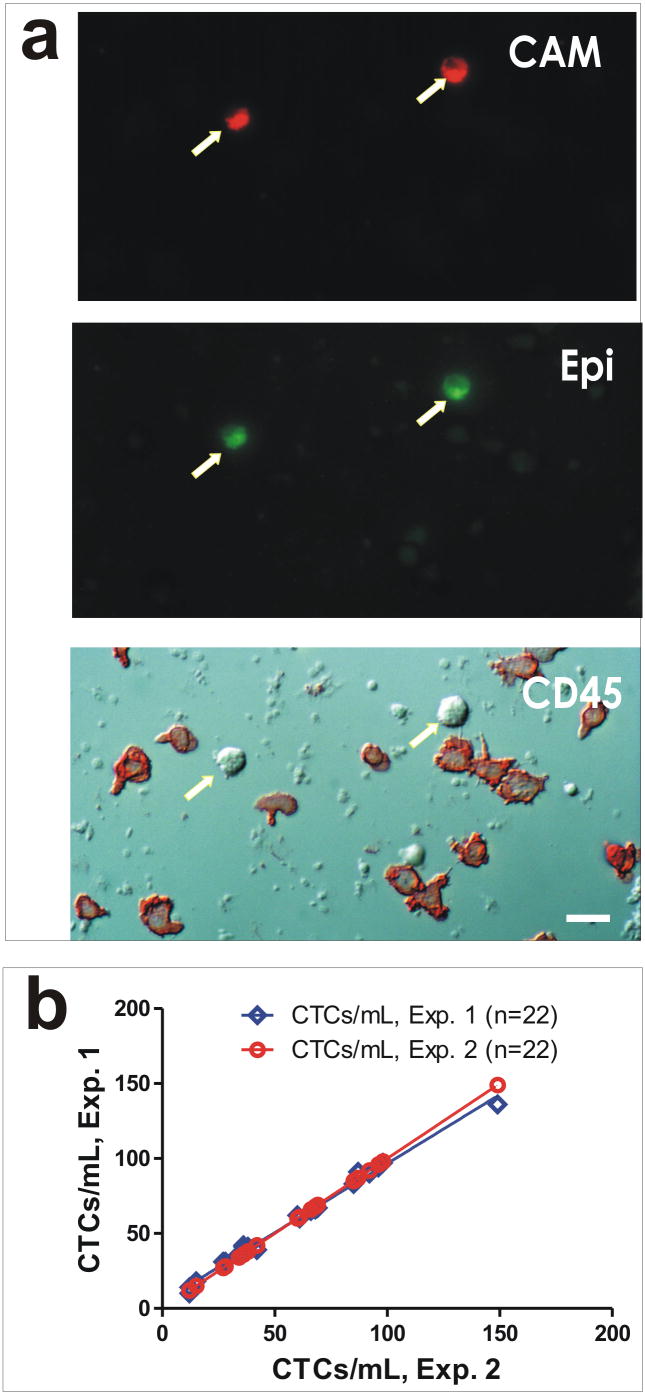
Cells that demonstrated ingested fluorescent CAM fragments (CAM+) and positive immuno-staining with epithelial cell lineage markers (Epi+) were counted as CTCs (Fig. 1a). To normalize fluorescent intensity, CD45-positive cells that scored as contaminating normal hematologic cells were used as the fluorescence baseline for positive signal. To evaluate the reproducibility of CTC detection by the CAM invasion assay, split samples were used that showed high experimental reproducibility (Fig. 1b, r2 = 0.99). To evaluate tumor cell capture by CAM invasiveness, we conducted two additional series of experiments: (1) spiking experiments involved the recovery of tumor cells that were spiked into whole blood from healthy donors (Supplementary Fig. 1S), and (2) culturing of CAM-captured cells that proliferated and differentiated into colonies of tumor cell morphology in vitro (Supplementary Fig. 2S). Any sample that had at least one CTC counted was considered “CTC-positive”. Counts were performed by trained personnel and confirmed by a second observer.
Figure 1.
CTC detection by the CAM invasion assay. a) CTCs and hematologic cells from a stage IIIc ovarian cancer patient that ingested fluorescent CAM fragments (CAM+) and positive immuno-staining with epithelial cell lineage markers (Epi+). Arrows indicate CTCs in the identical field seen under different microscopic panels; hematologic cells are shown as color stained in red with antibody against CD45 in the lowest panel. b) Concordance experiment to test experimental variability of split samples analyzed under identical conditions.
Clinical Data Collection
In this retrospective cohort study, all clinical data was abstracted from patients' medical chart using a frozen file date of February 1, 2008. Tumor grade and histology were collected from pathology and cytology reports. CA-125 levels were measured up to 30 days prior to CTC blood collection, without any intervening treatment. Survival length was calculated from the date of blood sample collection to date of death (for overall survival) or recurrence/progression (for disease-free survival), or most recent documented contact, if the patient was still alive with no evidence of disease. Since patients found to have benign disease were not followed by the gynecology oncology practice, their survival data was excluded from survival analyses.
Statistical Analysis
Continuous variables were summarized by means and standard deviation; categorical variables were listed as frequencies and percentages. Chi-square or Fisher's Exact Test were used to compare categorical data; for continuous data, because the distribution was not normal, non-parametric statistics (Kruskal-Wallis and Mann-Whitney U) were used. Spearman's correlation was used to analyze the association between CTC count and CA-125 level and CTC counts of split samples. Kaplan-Meier curves for overall and disease-free survival were estimated and compared using Log-rank test. All analyses were performed using SPSS version 15.0. Results with two-tailed P-values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
Sixty-six ovarian and primary peritoneal cancer patients and 5 benign patients met inclusion criteria and were analyzed in this study. Overall, 43 (60.6%) patients had detectable CTCs. Patients who had detectable CTCs had a larger proportion of stage III/IV cases: 90.7% vs. 46.4% stage I/II cases (Table 1, p-value <0.001). Additionally, a larger number of subsequent disease recurrences were observed in patients with detectable CTCs: 76.2% vs. 39.3% (Table 1, p-value 0.003). Otherwise, no significant difference in age, race, BMI, smoking history, cancer type, tumor grade and type, debulking status was observed. If the baseline characteristics of patients with CTC-low (0-31.5 CTCs/ml) and CTC-high (>31.5 CTC/ml) counts are compared, no significant difference is found for any category. Of the patients with malignancy, 13 (19.7%) subsequently received no chemotherapy, 19 (28.8%) received chemotherapy regimens consisting of a taxane + carboplatinum, 27 (40.9%) received regimens consisting of a taxane + carboplatinum + an additional agent, 3 (4.5%) received other regimens and 4 (6.1%) received intraperitoneal chemotherapy. Reasons that patients did not receive chemotherapy included advanced disease with patient receiving palliative care only, patient dying prior to receiving chemotherapy or patients with low-grade stage IA or IB disease who chose not to have chemotherapy. No significant difference was found between chemotherapy regimens subsequently received between patients initially found to have high CTC counts (> 31.5 CTC/ml) and those that had low CTC counts (< 31.5 CTCs/ml), Chi-square p-value 0.246.
Table 1.
Baseline Characteristics
| CTC Negative
N(%) or mean (SD) |
CTC Positive
N(%) or mean (SD) |
P-value | |
|---|---|---|---|
| Total | 28 (39.4%) | 43 (60.6%) | |
| Age (yrs) | 64.3 (11.3) | 60.9 (11.4) | 0.32 |
| Race | 0.56 | ||
| White | 26 (92.9%) | 42 (97.7%) | |
| Non-white | 2 (7.1%) | 1 (2.3%) | |
| BMI units | 28.3 (6.6) | 26.7 (4.4) | 0.50 |
| + Smoking history | 14 (50.0%) | 25 (59.5%) | 0.55 |
| Recurrence at time of blood draw | 2 (7.1%) | 5 (11.6%) | 0.59 |
| Subsequent recurrence (as of 2/1/08) | 11 (39.3%) | 32 (76.2%) | 0.003* |
| Stage | <0.001* | ||
| Benign | 5 (17.9%) | 0 (0%) | |
| I | 6 (21.4%) | 1 (23.0%) | |
| II | 3 (10.7%) | 0 (0%) | |
| III | 9 (32.1%) | 28 (65.1%) | |
| IV | 4 (14.3%) | 11 (25.6%) | |
| Unstaged | 1 (3.6%) | 3 (7.0%) | |
| Grade | 0.69 | ||
| Low | 0 (0%) | 2 (4.9%) | |
| Moderate | 2 (8.7%) | 2 (4.9%) | |
| High | 15 (65.2%) | 27 (65.9%) | |
| ungraded | 6 (26.1%) | 10 (24.4%) | |
| Histology | 0.45 | ||
| Papillary Serous | 7 (30.4%) | 12 (27.9%) | |
| Serous | 7 (30.4%) | 18 (41.9%) | |
| Endometrioid | 1 (4.3%) | 1 (2.3%) | |
| Clear Cell | 4 (17.4%) | 2 (4.7%) | |
| Other | 4 (17.4%) | 10 (23.3%) | |
| Cancer Type | 0.29 | ||
| Ovarian | 25 (92.6%) | 33 (84.6%) | |
| Primary Peritoneal | 2 (7.4%) | 6 (15.4%) | |
| Debulking | 0.30 | ||
| Optimal | 16 (76.2%) | 35 (87.5%) | |
| Suboptimal | 5 (23.8%) | 5 (12.5%) | |
| Elevated CA-125 | 15 (78.9%) | 26 (96.3%) | 0.15 |
significant p-value < 0.05
Clinical Characteristics of CTCs
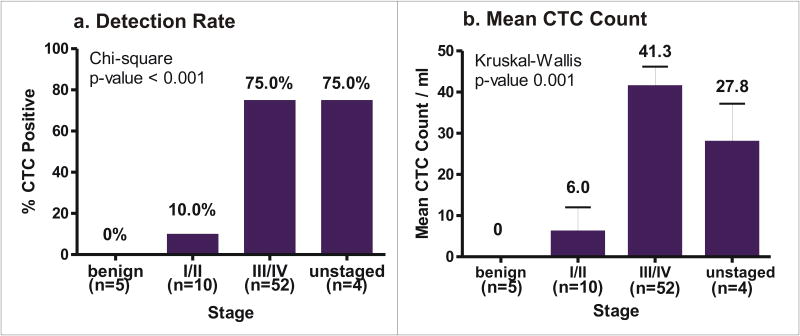
Analyses were performed evaluating the effect of various clinical indices on CTC detection rates and mean counts. Overall, patients with more advanced disease had higher CTC detection rates: 39/52 (75.0%) versus 1/10 (10.0%) in stage I/II patients (Figure 2a, p-value <0.001). Additionally, advanced stage patients demonstrated higher mean CTC counts: 41.3 CTCs/ml versus 6.0 CTCs/ml in stage I/II patients (Figure 2b, p-value <0.001). No significant difference was found between subgroups of tumor grade and tumor histology (data not shown). A positive correlation was noted between CA-125 level and CTC count (Spearman correlation coefficient r=0.289, p-value 0.046).
Figure 2.
CTCs by stage. a) CTC detection rates. Percentage of patients within a stage that had detectable CTCs. Absolute number of patients with detectable CTCs are given in (). b) Mean CTC counts. Error bars indicate SEM.
Survival of Patients with CTCs
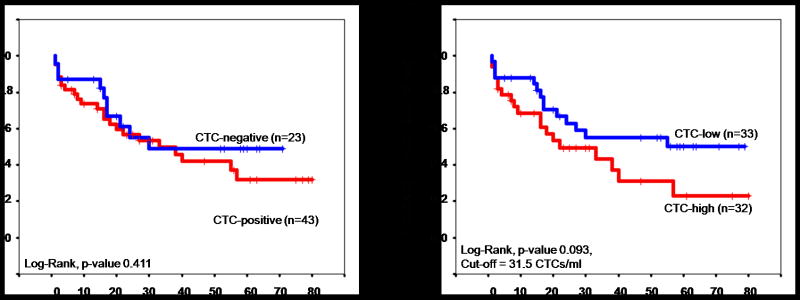
Patients were followed for a median of 18 months (range: 1-80 months). At the conclusion of the study, of the 66 patients with a malignancy, 21 (32%) were alive with no evidence of disease, 12 (18%) were still alive with disease, 31 (47%) were dead of disease, and 2 (3%) were dead of intercurrent disease. Survival times were compared between patients with and without detectable CTCs, and between patients with high versus low CTC counts. The sample median of 31.5 CTCs/ml was used to determine a cut-off point where ovarian cancer patients who had CTC counts of 0-31.5 CTCs/ml were considered “CTC-low” patients while patients who had CTC counts > 31.5 CTC/ml were considered “CTC-high” patients. When evaluating overall survival, no significant difference was found between patients with and without CTCs (Figure 3a, p-value 0. 411) or between patients with high versus low CTC counts (Figure 3b, p-value 0.093). However, a separation of the survival curves started at approximately 40 months.
Figure 3.
Overall Survival.
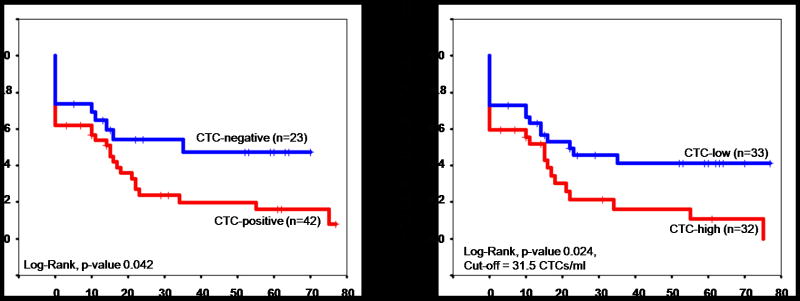
A significant decrease in disease-free survival was observed in patients with detectable CTCs. CTC-positive patients demonstrated a median disease-free survival time of 15 months, versus 35 months in CTC-negative patients (Figure 4a, p-value 0.042). A similar decrease was noted in patients with high CTC counts: 15.0 months median survival versus 22.0 months median survival in CTC-low patients (Figure 4b, p-value 0.017). If stage I, and stage I&II patients are removed from analyses, similar overall and disease-free survival trends are seen although the significance is lost with the decreased sample size.
Figure 4.
Disease-Free Survival.
Discussion
Currently, no consensus has been reached on the clinical relevance of CTCs in ovarian cancer patients. Traditionally, it was believed that epithelial ovarian cancers spread primarily by direct extension into the abdominal cavity. While recent studies have established the presence of tumor-like epithelial cells in peripheral blood of ovarian cancer patients [8,9], the clinical significance of these studies' findings is unclear.
Some research has shown that epithelial cells may be present in blood of healthy populations, either as non-specific epithelial antigen binding to leukocytes, or as benign epithelial cells in circulation [13-15]. Additionally, recent work investigating the role of Epithelial Mesenchymal Transition in cancer metastasis has found a heterogeneous downregulation of epithelial surface antigens in invasive epithelial tumor cells [16,17], thus reliance on a few standard epithelial markers alone for identification of tumor cells may not be reliable. Furthermore, current detection techniques do not distinguish viable cells from non-viable cells. The lifespan of a CTC is limited by anoikis [13,18] thus a proportion of epithelial-recovered CTCs may represent dead or dying cells which may not contribute greatly to cancer spread.
To address these concerns, we evaluated the feasibility of using a CAM invasion assay that enriches cells based on their invasive properties in addition to their expression of epithelial cell markers, to detect CTCs. We reason that detection of these invasive CTCs may offer an alternative method to assess metastatic progression that may be of prognostic value. In this exploratory study, we found that invasive CTCs were detected in the majority of epithelial ovarian cancer patients and significantly correlated with advanced stage disease, higher CA-125 level and shorter disease-free survival. This seems to indicate that as ovarian cancer progresses, a greater number of invasive CTCs are present. Whether this increase is a result of increased production or release of tumor cells into blood, or decreased CTC death or clearance remains to be determined. Regardless, these findings suggest a clinical utility for quantifying invasive CTCs in monitoring cancer progression.
Our study is the first to document decreased disease-free survival in ovarian cancer patients with detectable CTCs. We postulate two reasons for this finding: 1) the follow-up period in our study extended beyond the observation period reported in previous studies and 2) the cells enriched by CAM may represent the invasive tumor cell population responsible for cancer spread. Previous studies had follow-up periods of 0-39 months [8,9]. In contrast, our study had a follow-up period 0-80 months. In fact, 21 of our subjects had follow-up periods of 35-80 months. Furthermore, our study showed that cumulative overall survival started to differentiate at around 40 months, when the previous studies ended. Thus the lack of survival benefit observed in previous studies may have occurred because the follow-up period in the previous studies ended before the longer-term survival benefit could be observed. Perhaps if their studies extended beyond 35 months, a survival benefit in patients without CTCs would have been observed as well. Thus the presence of CTCs may have a greater role in prognosticating longer-term survival and perhaps with longer follow-up or a larger sample size, a significant difference in overall survival may be observed.
Interestingly, an early study published by Tarin et al in 1984 [12] evaluated the effect of the direct infusion of malignant ascites into the venous circulation of 29 inoperable cancer patients receiving palliative peritoneovenous shunting. Fifteen patients ultimately underwent autopsy evaluation of which only seven demonstrated evidence of hematogenous metastases and distant metastases. Furthermore, these metastases had been clinically asymptomatic. Although limited by its small sample size, Tarin's work would seem to suggest that circulation of non-hematopoietic tumor cells in blood may not propagate disease metastases or play a strong clinical role. However, the inability of peritoneovenously shunted ascites tumor cells to significantly spread disease hematogenously in Tarin's study could be because the tumor cells found in ascites may not share the same characteristics as CTCs that enter circulation of their own accord. In particular, tumor cells which exit primary tumor sites, invade blood vessel walls and enter circulation could be derived from the population of cancer stem or progenitor cells that have undergone processes (including Epithelial Mesenchymal Transition) that support aggressive and invasive cell behavior [13] that tumor cells in ascites might not have. It is likely that this is the type of aggressive and invasive behavior that the CAM assay targets.
The idea that CAM enriched cells may represent invasive tumor cells responsible for metastasis is further supported by work performed in our laboratory showing that CAM-enriched CTCs from ovarian cancer patients readily proliferate in culture and demonstrate disinhibited growth patterns (see Supplementary Fig. 2S). Thus it may be the aggressive and invasive behavior of tumor cells in circulation that both disseminate metastases and prognosticate disease recurrence.
Although limited by its small, homogenous sample size, the results of this pilot study illustrate the relationship between invasive CTCs and ovarian cancer disease staging and survival outcomes and raise the clinical potential of measuring CTCs in ovarian cancer. Ultimately, measuring CTCs may offer clinicians an improved method to identify aggressive disease earlier. While the prognostic relevance of CTCs in the blood of ovarian cancer patients with early-stage disease without overt metastases is not currently known, studies in larger cohorts of women undergoing surgery for initial diagnosis and staging of ovarian cancer may elucidate the role that CTCs play in early as well as late stage disease and may be able to help identify patients at greater risk for worse outcomes. Furthermore, if CTCs can be reliably detected in early stage disease, an opportunity for early disease detection of ovarian cancer may arise—an area that is currently lacking but much needed. Additionally, CTC measurement may provide researchers with an opportunity to improve metastasis detection and disease recurrence by assessing ovarian cancer spread directly and earlier than current techniques (CT/PET scan), which only detect established tumor growths. Lastly, having a functional technique to harvest metastasizing tumor cells may provide researchers with a population of viable and aggressive cells to examine gene expression profiles or gene mutations in cancer. The information resulting from these studies may offer more insight into the mechanism of metastasis in ovarian cancer, chemotherapy resistance and disease survival. This may provide an opportunity for more personalized medicine where physicians may be guided in treatment options using information from an individual's CTC profile. Our hope is that our findings may open more doors for further research into the role of CTCs in ovarian cancer progression, both from a basic sciences standpoint and a clinical one.
Supplementary Material
Footnotes
Conflict of Interest Statement:
Although we do not feel that the following information has biased our research in any capacity, we would like to disclose the following information:
Wen-Tien Chen is the founder of Vitatex Inc., the company who manufactures the cell invasion assay (Vita-Assay™) used in this work.
Tina Fan is the daughter-in-law of Wen-Tien Chen and served as a consultant to Vitatex Inc. for 9 months in 2006-2007. At the time she performed the work presented in this manuscript, she was no longer employed by Vitatex Inc., however her spouse maintains a position in the company.
Again, we would like to emphasize that despite these potential conflicts of interest, the utmost objectivity and adherence to the high ethical standards of scientific research were practiced in preparing this manuscript.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006 Nov;95 1:S161–S192. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004 Dec 9;351(24):2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 4.Cain JM, Ellis GK, Collins C, Greer BE, Tamimi HK, Figge DC, et al. Bone marrow involvement in epithelial ovarian cancer by immunocytochemical assessment. Gynecologic Oncology. 1990 Sep;38(3):442–5. doi: 10.1016/0090-8258(90)90088-3. [DOI] [PubMed] [Google Scholar]
- 5.Braun S, Schindlbeck C, Hepp F, Janni W, Kentenich C, Riethmuller G, et al. Occult tumor cells in bone marrow of patients with locoregionally restricted ovarian cancer predict early distant metastatic relapse. Journal of Clinical Oncology. 2001 Jan 15;19(2):368–75. doi: 10.1200/JCO.2001.19.2.368. [DOI] [PubMed] [Google Scholar]
- 6.Fehm T, Becker S, Bachmann C, Beck V, Gebauer G, Banys M, et al. Detection of disseminated tumor cells in patients with gynecological cancers. Gynecol Oncol. 2006 Dec;103(3):942–7. doi: 10.1016/j.ygyno.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 7.Schindlbeck C, Hantschmann P, Zerzer M, Jahns B, Rjosk D, Janni W, et al. Prognostic impact of KI67, p53, human epithelial growth factor receptor 2, topoisomerase IIalpha, epidermal growth factor receptor, and nm23 expression of ovarian carcinomas and disseminated tumor cells in the bone marrow. Int J Gynecol Cancer. 2007 Sep;17(5):1047–55. doi: 10.1111/j.1525-1438.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- 8.Marth C, Kisic J, Kaern J, Trope C, Fodstad O. Circulating tumor cells in the peripheral blood and bone marrow of patients with ovarian carcinoma do not predict prognosis. Cancer. 2002 Feb 1;94(3):707–12. doi: 10.1002/cncr.10250. [DOI] [PubMed] [Google Scholar]
- 9.Judson PL, Geller MA, Bliss RL, Boente MP, Downs LS, Jr, Argenta PA, et al. Preoperative detection of peripherally circulating cancer cells and its prognostic significance in ovarian cancer. Gynecol Oncol. 2003 Nov;91(2):389–94. doi: 10.1016/j.ygyno.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2000 Jan;6(1):100–2. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 11.Ghersi G, Zhao Q, Salamone M, Yeh Y, Zucker S, Chen WT. The protease complex consisting of dipeptidyl peptidase IV and seprase plays a role in the migration and invasion of human endothelial cells in collagenous matrices. Cancer Res. 2006 May 1;66(9):4652–61. doi: 10.1158/0008-5472.CAN-05-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B. Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Cancer Res. 1984 Aug;44(8):3584–92. [PubMed] [Google Scholar]
- 13.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007 Aug 18;253(2):180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Goeminne JC, Guillaume T, Symann M. Pitfalls in the detection of disseminated non-hematological tumor cells. Ann Oncol. 2000 Jul;11(7):785–92. doi: 10.1023/a:1008398228018. [DOI] [PubMed] [Google Scholar]
- 15.Crisan D, Ruark DS, Decker DA, Drevon AM, Dicarlo RG. Detection of circulating epithelial cells after surgery for benign breast disease. Mol Diagn. 2000 Mar;5(1):33–8. doi: 10.1007/BF03262020. [DOI] [PubMed] [Google Scholar]
- 16.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003 Dec;15(6):740–6. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007 Jun;39(3):305–18. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 18.Gassmann P, Haier J. The tumor cell-host organ interface in the early onset of metastatic organ colonisation. Clin Exp Metastasis. 2008;25(2):171–81. doi: 10.1007/s10585-007-9130-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.