Abstract
Neurogenesis persists in two germinal regions in the adult mammalian brain, the subventricular zone of the lateral ventricles and the subgranular zone in the hippocampal formation. Within these two neurogenic niches, specialized astrocytes are neural stem cells, capable of self-renewing and generating neurons and glia. Cues within the niche, from cell–cell interactions to diffusible factors, are spatially and temporally coordinated to regulate proliferation and neurogenesis, ultimately affecting stem cell fate choices. Here, we review the components of adult neural stem cell niches and how they act to regulate neurogenesis in these regions.
Keywords: stem cell, niche, astrocyte, subventricular zone, subgranular zone
1. Stem cells and their niches
Stem cells have two essential properties: self-renewal and multipotency. Self-renewal is the ability to generate an identical daughter cell and multipotency is the capacity to generate all cell types of a tissue. Although self-renewal often occurs via asymmetric divisions, to yield another stem cell and a more differentiated cell, stem cells may also undergo symmetric divisions to generate two stem cells or two differentiated daughter cells. Thus, stem cell self-renewal can occur at the single cell level or the population level, such that the stem cell population is maintained (Spradling et al. 2001). In many different organs, stem cells divide relatively infrequently to generate transit-amplifying cells, which in turn divide to rapidly expand their number before generating more mature progeny. This hierarchy of division and differentiation allows amplification of the number of mature cells that can be derived from a single stem cell, while minimizing the possibility of mutations due to DNA replication in the genome of long-lived stem cells (Reya et al. 2001). Interestingly, studies from several niches have revealed that transit-amplifying cells also retain the ability to act as stem cells when challenged by appropriate stimulation (Doetsch et al. 2002; Marshman et al. 2002; Raff 2003; Brawley & Matunis 2004; Kai & Spradling 2004).
Specialized microenvironments or niches support the lifelong self-renewal of stem cells and their production of differentiated cells (Spradling et al. 2001; Fuchs et al. 2004). Importantly, stem cells themselves extensively interact with and participate in the niche. Furthermore, niches may in fact be dynamic structures that alter their location and characteristics over time concomitant with tissue remodelling. Comparison of stem cell niches in Drosophila germ-line lineages, and in the mammalian haematopoietic system, intestinal epithelium, skin/hair follicle and nervous system has revealed common and unique features (figure 1; reviewed in Spradling et al. 2001; Doetsch 2003b; Fuchs et al. 2004; Li & Xie 2005). Cell–cell interactions (figure 1a) and diffusible signals (figure 1b) are key elements allowing feedback control of stem cell activation and differentiation from progeny and/or niche support cells. An emerging feature of several stem cell niches is the intimate association with endothelial cells, which regulate stem cell self-renewal and differentiation (figure 1c). Within a niche, stem cells are frequently anchored to a basal lamina or stromal cells (figure 1c,d) that can provide a substrate for oriented cell division. The basal lamina is also an important regulator of the accessibility of growth factors and other signals, as associated extracellular matrix (ECM) molecules and glycoproteins can both concentrate and sequester factors in inactive or active forms (figure 1c). Cell anchoring may orient cell division resulting in the segregation of key determinants into one or both daughter cells depending on the plane of division (figure 1d). Here, we review the role of the above components in the in vivo niches for adult neurogenesis.
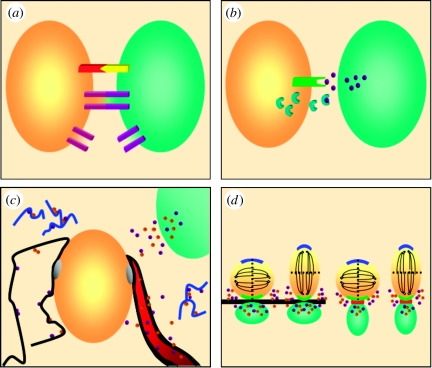
Figure 1.
Components of the stem cell niche. (a) Cell–cell and cell–extracellular environment interactions. Membrane-associated receptors and ligands (red and yellow) mediate cell–cell contacts and cell fate decisions, including self-renewal and differentiation. In addition, gap junctions (purple), intercellular channels that allow the passage of ions and metabolites, both coordinate behaviour between coupled cells and can tether cells together. Hemi-channels allow communication between the cell and the environment. (b) Diffusible factors. Diffusible factors (purple spheres) can direct stem cells to either self-renew or generate differentiated progeny. The availability of diffusible factors that bind to receptors (green) in turn can be regulated by ligand inhibitors (blue half-moons), which can sequester these factors and prevent signalling. (c) Basal lamina and blood vessels. An ECM-rich basal lamina (black), which can be associated with blood vessels (red) or cells, has several functions in stem cell niches, including anchoring cells to the niche (grey spheres), sequestering and presenting diffusible signals (purple and brown spheres), and linking cells and the ECM. In addition, proteolytic fragments of ECM components (blue squiggles) may regulate stem cells. Endothelial cells and the vasculature are emerging as integral components of stem cell niches, where they can regulate stem cell fate decisions through either diffusible signals and/or direct cell–cell contact. (d) Oriented cell division. Both cell–cell contact (via adherens junctions, red) and tethering of cells to the basal lamina (black) can influence cell fate by orienting the plane of cell division, such that key intracellular determinants are symmetrically or asymmetrically distributed. Oriented segregation of these factors may influence cell fate. Short-range diffusible factors (purple and brown spheres) secreted by stromal cells (green) that promote self-renewal only influence immediately adjacent cells, allowing differentiating daughter cells to escape the niche.
2. Adult neural stem cell niches
Neural stem cells persist in the adult mammalian brain and exhibit the two fundamental properties of stem cells; they undergo self-renewal and are multipotent, generating neurons and macroglia (astrocytes and oligodendrocytes). While neural stem cells can be cultured in vitro with growth factors as either adherent cultures or non-adherent cultures called neurospheres (reviewed in Gage 2000; Temple 2001), their biology is in large part defined by their in vivo niche.
Neurogenesis occurs in two principal brain regions in adult mammals: the subventricular zone (SVZ), adjacent to the lateral ventricles (figure 2), and the subgranular zone (SGZ) of the hippocampal formation (figure 3) (reviewed in Doetsch & Hen 2005). The SVZ consists of a thin layer of dividing cells that extends along the length of the lateral walls of the lateral ventricles and is largely separated from the cerebrospinal fluid (CSF) by a layer of multi-ciliated ependymal cells. Newly generated neuroblasts traverse a network of chains which extends throughout the SVZ to join the rostral migratory stream (RMS) that leads to the olfactory bulb. There they differentiate into two kinds of inhibitory interneurons, granule and periglomerular cells, and functionally integrate into the existing circuitry (Belluzzi et al. 2003; Carleton et al. 2003). The SGZ is located between the hilus and the granule cell layer of the dentate gyrus. Newly generated granule neurons born in the SGZ migrate only a short distance to the granule cell layer, where they extend dendrites to the molecular layer and an axon along the mossy fibre path and integrate functionally into the circuitry of the dentate gyrus (van Praag et al. 2002; Jessberger & Kempermann 2003; Ge et al. 2006).
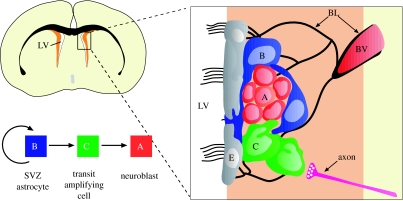
Figure 2.
Cell types and anatomy of the adult SVZ niche. Schema of frontal section of the adult mouse brain showing the SVZ (orange) adjacent to the lateral ventricle (LV). SVZ astrocytes in this region (B, blue) are stem cells which generate migrating neuroblasts (A, red) destined for the olfactory bulb via a rapidly dividing transit-amplifying cell (C, green). Region in box is expanded at right to show the relationship of cells in this region and some elements of the SVZ niche. Multi-ciliated ependymal cells (E, grey) line the walls of the lateral ventricle. Chains of neuroblasts travel through tunnels formed by processes of SVZ astrocytes. Transit-amplifying cells are found in small clusters adjacent to the chains. Signals released from axons (pink) regulate proliferation and survival in this region. A specialized basal lamina (BL, black) extends from perivascular cells and contacts all cell types. Endothelial cells, blood vessels (BV) and the basal lamina are all likely key components of the niche.
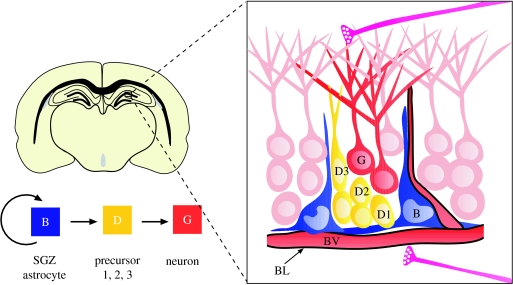
Figure 3.
Cell types and anatomy of the adult SGZ niche. Schema of frontal section of the adult mouse brain showing the SGZ at the interface between the hilus (area below blood vessel) and the granule cell layer (light pink cells) of the dentate gyrus. SGZ astrocytes (B, blue) divide to generate intermediate precursors (type D cells; nomenclature according to Seri et al. 2004, yellow), which progressively generate more differentiated progeny (type D1→type D2→type D3), which mature into granule neurons (G, red). Neurogenesis occurs in pockets adjacent to blood vessels and although a specialized basal lamina has not yet been described in this region, the vascular basal lamina likely plays an important role in the niche. Afferent axons (pink) from the entorhinal cortex and axons from subcortical regions as well as from local inhibitory interneurons project to the SGZ.
Strikingly, in both regions, a subset of astrocytes, glial cells classically associated with support functions in the brain, are the in vivo primary precursors for adult neurogenesis (reviewed in Doetsch 2003a). These cells have been defined as astrocytes based on their ultrastructural features, markers they express and electrophysiological properties. An emerging hypothesis is that stem cells are contained within the astrocyte lineage (Alvarez-Buylla et al. 2001; Doetsch 2003a). During development, radial glia are the in vivo primary precursors of neurons and glia (Miyata et al. 2001; Noctor et al. 2001, 2002, 2004; Malatesta et al. 2003; Anthony et al. 2004). Post-natally, radial glia transition into astrocytes (Schmechel & Rakic 1979; Voigt 1989; Alves et al. 2002; Merkle et al. 2004), some of which are retained as stem cells in adult neurogenic niches (Merkle et al. 2004). Interestingly, until post-natal day 11, astrocytes from throughout the brain can generate neurospheres, but thereafter this capacity becomes limited to adult SVZ astrocytes (Laywell et al. 2000). An important unanswered question is whether all astrocytes (or a subset) in the adult brain retain latent neurogenic potential or whether specialized stem cell astrocytes are only found in neurogenic niches in the adult brain.
In the SVZ, stem cell astrocytes (type B cells, glial fibrillary acidic protein expressing; GFAP+) divide relatively infrequently to generate neuroblasts (type A cells, GFAP−/Dlx2+/doublecortin (Dcx)+) via a rapidly dividing transit-amplifying cell (type C cells, GFAP−/Dlx2+) (Doetsch et al. 1999a). Oligodendrocytes can also be generated in the adult SVZ both under normal conditions (Ahn & Joyner 2005) and after demyelination (Nait-Oumesmar et al. 1999; Picard-Riera et al. 2002), but the cell type that generates them remains undefined. Whereas SVZ astrocytes have been shown to be stem cells both in vitro and in vivo (Doetsch et al. 1999a; Laywell et al. 2000; Imura et al. 2003; Morshead et al. 2003; Garcia et al. 2004; Sanai et al. 2004; Ahn & Joyner 2005), there is some debate as to whether the adult SGZ contains neural stem cells or only committed neurogenic precursors (Seaberg & van der Kooy 2002; Bull & Bartlett 2005). Within the SGZ, GFAP+/nestin+ astrocytes are the primary precursors that divide to generate intermediate amplifying GFAP−/nestin+ cells which in turn generate GFAP−/nestin−/Dcx+ cells that mature into differentiated granule neurons (Seri et al. 2001, 2004; Kronenberg et al. 2003; Kempermann et al. 2004; Steiner et al. 2004; Encinas et al. 2006). As cells progress along these lineages from SVZ and SGZ astrocytes to differentiated neurons, they sequentially acquire distinct electrophysiological properties (Carleton et al. 2003; Filippov et al. 2003; Fukuda et al. 2003) and express a series of transcription factors that determine their phenotype (Pleasure et al. 2000; Doetsch et al. 2002; Kronenberg et al. 2003; Stenman et al. 2003; Parras et al. 2004; Seri et al. 2004; Hack et al. 2005; Kohwi et al. 2005; Waclaw et al. 2006). These molecular programmes become stabilized through a combination of epigenetic, transcriptional and post-transcriptional mechanisms (reviewed in Hsieh & Gage 2004; Cheng et al. 2005). Intriguingly, while common molecular mechanisms may regulate stem cell proliferation and differentiation in the embryo and the adult, some regulatory mechanisms may be unique to post-natal and/or adult neurogenesis. For example, mice deficient in the polycomb repressor Bmi1 (Molofsky et al. 2003), the orphan nuclear receptor Tlx (Shi et al. 2004) and the conditional mutants of sonic hedgehog signalling components (Machold et al. 2003) first reveal deficits at post-natal stages. Thus, a unique complement of regulatory mechanisms may be active in adult neurogenic regions in addition to the conserved embryonic pathways.
Stem cells harvested from non-neurogenic regions can generate neurons and astrocytes when cultured in vitro, but only make glia in vivo (reviewed in Gage 2000). In addition, primary cells from neurogenic areas transplanted into non-neurogenic regions exhibit very limited neurogenesis (reviewed in Herrera et al. 1999; Lim et al. 2000; Temple 2001). An inhibitory environment that is refractory to neurogenesis is therefore present throughout most of the brain. In contrast, upon transplantation into the SVZ, RMS or SGZ, cultured neural stem cells derived from non-neurogenic regions can generate neurons appropriate to the region (Suhonen et al. 1996; Shihabuddin et al. 2000; reviewed in Temple 2001). Thus, adult neurogenic niches have an instructive role in directing neuronal production and stem cell maintenance and shield ongoing neurogenesis from possible external inhibitory influences. Although the components of adult neurogenic niches that mediate these processes are still being elucidated, it is clear that both neural and non-neural cell types are key players (reviewed in Doetsch 2003b). Neurogenesis occurs in close proximity to blood vessels and may be associated with angiogenesis. Both regions are enriched in ECM proteins and a prominent basal lamina has been described in the SVZ as well as perivascular connective tissue comprising macrophages and fibroblasts. Neurotransmitter signalling probably plays a key role, as both regions are innervated by axons from distant brain regions. Finally, the proximity of unique structures, such as meningeal projections and the CSF, are likely important sources of signals.
3. Stem cell astrocytes and niche astrocytes
Astrocytes have classically been considered support cells in the brain, with multiple roles including forming a capsule around the surface of the brain via their endfeet, buffering extracellular potassium ion concentrations, interacting with endothelial cells to form the blood–brain barrier and taking up neurotransmitters at the synaptic cleft. However, recent work has revealed that astrocytes are dynamic regulators of many brain processes, including synaptogenesis and synaptic efficacy (Ullian et al. 2001; Christopherson et al. 2005), support adult neurogenesis (Pixley 1992; Lim & Alvarez-Buylla 1999; Song et al. 2002; Kornyei et al. 2005) and act as neural stem cells in the adult brain (Doetsch et al. 1999a; Laywell et al. 2000; Imura et al. 2003; Morshead et al. 2003; Garcia et al. 2004; Sanai et al. 2004; Ahn & Joyner 2005). Thus, astrocytes are emerging as key mediators of brain development, function and plasticity, highlighting the critical need to better characterize the heterogeneity and developmental specification of different subpopulations of astrocytes both within adult neurogenic regions and throughout the brain (Bachoo et al. 2004; Bonaguidi et al. 2005; Muroyama et al. 2005; Imura et al. 2006; Lim et al. 2006; Sakaguchi et al. 2006).
Within adult neurogenic niches, in addition to their role as stem cells, astrocytes are uniquely poised to be sensors and regulators of the environment. They have long processes that envelop and contact all cell types and structures in the niche (Doetsch et al. 1997; Seri et al. 2004), including blood vessels and the basal lamina (Mercier et al. 2002), allowing them to integrate diverse signals from many sources. Furthermore, astrocytes are often coupled via gap junctions and can form a syncytium (reviewed in Giaume & McCarthy 1996; Giaume & Venance 1998), which may allow them to propagate signals locally or throughout the entire niche, thereby regulating activation and differentiation of stem cells. Astrocytes also contribute to the neurogenic niche through contact-mediated cues and by secreting diffusible signals (Pixley 1992; Lim & Alvarez-Buylla 1999; Taupin et al. 2000; Song et al. 2002; Kornyei et al. 2005; Lie et al. 2005). It is still unknown whether the dual roles of astrocytes as stem cells and niche support cells are segregated into distinct astrocyte populations or whether individual astrocytes can have both roles.
Within the SVZ and SGZ, astrocytes are heterogeneous at the morphological, ultrastructural and molecular levels, including growth factor receptor expression, proliferation capacity and electrophysiological properties (Doetsch et al. 1997, 2002; Seri et al. 2001, 2004; Filippov et al. 2003; Fukuda et al. 2003; Kronenberg et al. 2003; Garcia et al. 2004). At least two populations of astrocytes have been defined in the SGZ: (i) radial astrocytes, which extend a process into the granule cell layer, are nestin-positive and divide and (ii) horizontal astrocytes, which extend basal processes under the granule cell layer, are nestin-negative and S100+ (Kronenberg et al. 2003; Seri et al. 2004). In the SVZ, morphologically distinct astrocytes have also been described. At the ultrastructural level, two types of astrocytes (type B1 and B2) are present that differ in their location as well as in their cytoplasmic and nuclear structure and proliferation (Doetsch et al. 1997). At the light microscope, both highly stellate astrocytes and unipolar and bipolar astrocytes are present, which differ in their proliferation profiles (Garcia et al. 2004; E. Drapeau & F. Doetsch 2004, unpublished data). Intriguingly, occasionally, the process of an SVZ astrocyte intercalates between ependymal cells and comes into contact with the lateral ventricle, and thus is exposed directly to the CSF (Doetsch et al. 1999b). The number of astrocytes in contact with the lateral ventricle is increased during regeneration and infusion of growth factors (Doetsch et al. 1999b, 2002; Conover et al. 2000). SVZ astrocytes in contact with the ventricle express a single 9+0 primary cilium, which is also found on neuroepithelial stem cells during development in mammals (Sotelo & Trujillo-Cenóz 1958; Stensaas & Stensaas 1968), on radial glia (Tramontin et al. 2003; Spassky et al. 2005) and on primary precursors in adult songbirds (Alvarez-Buylla et al. 1998). This primary cilium may be important for transduction of signals in the CSF and for stem cell function. Despite these molecular, biophysical and morphological differences, it is still unclear whether the different subpopulations of astrocytes in both adult neurogenic regions represent functionally distinct astrocytes or are at different stages in the lineage.
4. Ependymal cells: support cells in the niche
Ependymal cells line the walls of the ventricles in the adult brain and are thus at the interface between the CSF and the brain tissue. They act both as a structural barrier and as a sensor of CSF components and osmotic pressure. Ependymal cells actively regulate the absorption of ions and transport of factors from the CSF into the parenchyma. Numerous growth factors that affect adult neurogenesis, including transforming growth factor-α (TGF-α; Seroogy et al. 1993), basic fibroblast growth factor (bFGF) (Hayamizu et al. 2001) and amphiregulin (Falk & Frisen 2002), are made by the choroid plexus, epithelial cells within the ventricles that produce the CSF. Ependymal cells may distribute and create gradients of factors produced by the choroid plexus through the directional beating of their cilia (Del Bigio 1995); thereby, they have been proposed to guide the migration of SVZ neuroblasts (Sawamoto et al. 2006). Importantly, within the SVZ, ependymal cells are closely apposed to astrocytes (Doetsch et al. 1997) as well as the specialized basal lamina (Mercier et al. 2002), creating the potential for transducing signals in this area. Although both ependymal cells and SVZ astrocytes are derived from radial glia (Merkle et al. 2004; Spassky et al. 2005), ependymal cells do not appear to be endogenous neural stem cells (Chiasson et al. 1999; Doetsch et al. 1999a; Laywell et al. 2000, Capela & Temple 2002; Spassky et al. 2005) as has been suggested (Johansson et al. 1999). However, they contribute to the niche in many ways, including as a source of secreted pro-neurogenic factors and of CSF components.
Ependymal cells and astrocytes are coupled by gap junctions homotypically (ependyma–ependyma and astrocyte–astrocyte) and heterotypically (ependyma—astrocyte; Zahs 1998), raising the possibility that local signals can be coordinated over long ranges within the SVZ. These intercellular channels allow the passage of ions, metabolites and second messengers between neighbouring cells (Kumar & Gilula 1996; Sohl et al. 2005). Connexin 43 (Cx43), a component of gap junctions that is predominantly found in glial cells in the brain (reviewed in Theis et al. 2005) is expressed in both adult neurogenic regions (Miragall et al. 1997; Menezes et al. 2000; Theis et al. 2003; Peretto et al. 2005; P. A. Riquelme & F. Doetsch 2005, unpublished data) and may be involved in gap junction coupling, hemi-channel signalling and cell–cell adhesion. Unlike gap junctional coupling, hemi-channels allow direct communication between the cells and the extracellular environment (Bennett et al. 2003). Hemi-channels have been implicated in the initiation and propagation of calcium waves between clusters of cells during cortical neurogenesis (Weissman et al. 2004). As such, Cx43 in adult neurogenic regions may coordinate the communication between neighbouring and distant cells within the niche either through gap junctions or hemi-channels. Cx43 may also act as a cell–cell adhesion unit to tether cells as has been suggested for glioma cells (Lin et al. 2002). Connexin 26, which is also expressed in the SVZ (Mercier & Hatton 2001), may have similar roles.
Ependymal cells also create a favourable environment for neurogenesis, together with astrocytes, through the secretion of noggin, an antagonist of bone morphogenic protein (BMP) signalling (Lim et al. 2000; Peretto et al. 2004). BMPs are members of the TGF-β family that are important for development. Within the adult SVZ, BMP signalling favours the production of astrocytes, but this pro-glial fate is reversed to a pro-neurogenic fate through the sequestration of BMP ligands by noggin (Lim et al. 2000). In addition to noggin, ependymal cells also express CXCR4 (Stumm et al. 2002), a chemokine receptor for SDF1 that is important for migration and survival (Dziembowska et al. 2005), EphA7 (Holmberg et al. 2005), a member of the Eph family of tyrosine kinase receptors that guide many developmental processes, and pigment epithelium-derived factor (PEDF; Ramirez-Castillejo et al. 2006; see §5). How these molecules and others yet to be identified interact merits further study.
5. Vasculature and adult neural stem cell niches
An important component of adult neurogenic niches is the vasculature. Within the SGZ, neurogenesis occurs in close proximity to blood vessels, with proliferative clusters containing neural progenitors, glial cells, newborn neurons and endothelial cells (Palmer et al. 2000), suggesting that neurogenesis and angiogenesis are coordinated processes. Indeed, common signals, which are active in adult neurogenic niches, regulate the development of the vasculature and the nervous system (reviewed in Carmeliet 2003). Thus, angiogenesis and neurogenesis are extensively interconnected and likely reciprocally influence each other in adult neurogenic regions.
Endothelial cells are emerging as critical niche cells that regulate stem cell self-renewal and neurogenesis. Co-culture of embryonic neural stem cells or adult SVZ cells with endothelial cells leads to enhanced stem cell self-renewal as well as increased neurogenesis from these expanded stem cells upon differentiation (Shen et al. 2004). The diffusible signals that effect these changes are as yet largely unidentified. One factor secreted by both endothelial cells and ependymal cells is PEDF, which has been proposed to regulate SVZ astrocyte self-renewal (Ramirez-Castillejo et al. 2006). Endothelial cells also secrete leukaemia inhibitory factor (LIF; Mi et al. 2001) and brain derived neurotrophic factor (BDNF; Leventhal et al. 1999; Louissaint et al. 2002), factors known to influence proliferation and/or differentiation in adult neurogenic regions. Given LIF's role, together with BMP signalling, in promoting self-renewal in embryonic stem cells (Ying et al. 2003) as well as its role in astrocyte differentiation (Ross et al. 2003), it will be intriguing to see how LIF, BMP and noggin signalling converges within adult neurogenic niches, where astrocytes have neural stem cell properties. In vitro, endothelial cells in part support SVZ-derived neuron outgrowth, survival and migration through the release of BDNF (Leventhal et al. 1999). In vivo evidence for a connection between endothelial cell-derived BDNF and neuronal maturation comes from adult songbirds, in which new neurons are seasonally added to the higher vocal centre (HVC; Nottebohm 2005). Testosterone implantation induces angiogenic bursts eliciting BDNF production by endothelial cells prior to neuronal recruitment into the HVC (Louissaint et al. 2002). Thus, angiogenesis and neuronal maturation are coordinated in HVC.
A common factor regulating both neurogenesis and angiogenesis is vascular endothelial growth factor (VEGF), which is implicated in neurogenesis in the SVZ and SGZ (Jin et al. 2002; Fabel et al. 2003; Cao et al. 2004). VEGF infusion into the lateral ventricle leads to an increase in SVZ proliferation and neurogenesis, likely through VEGFR2/Flk-1 (Jin et al. 2002). Whether this is a direct or indirect effect on neural progenitors or cell survival is unknown. In the SGZ, exercise-induced neurogenesis in the hippocampus acts in part through VEGFR2/Flk-1 signalling (Cao et al. 2004). A web of factors active in adult neural stem cell niches also regulates angiogenesis, including sonic hedgehog, BMPs, Ephs/ephrins, Notch and FGF, nitric oxide and erythropoietin (reviewed in Carmeliet 2003; Alvarez-Buylla & Lim 2004, Brines & Cerami 2005; Matarredona et al. 2005). In addition, blood vessels are conduits for the delivery of paracrine factors, such as hormones (sex hormones, glucocorticoids and prolactin) and cytokines, from distant sources (reviewed in Gould et al. 2000; Lennington et al. 2003). These ‘long-distance’ cues may act directly on neural stem cells and progenitors, endothelial cells or both to regulate angiogenesis and neurogenesis.
6. Basal lamina and extracellular matrix
The ECM and associated molecules are integral components of stem cell niches creating a favourable microenvironment and architecture. They regulate signalling in the niche by providing, storing and compartmentalizing growth factors and cytokines indispensable for cell proliferation and differentiation, as well as acting as a substrate for anchoring cells.
One common component of many stem cell niches is a basal lamina. Within the SVZ, a unique basal lamina, rich in laminin and collagen-1, extends from perivascular macrophages as ‘fractones’ (Mercier et al. 2002). Each fractone consists of a base attached to perivascular macrophages, a stem crossing the SVZ and bulbs located underneath ependymal cells. The branched configuration of fractones allows for extensive interaction with all SVZ cells, especially with SVZ astrocytes and ependymal cells. This basal lamina, as well as other ECM components abundantly expressed in the SVZ, is probably a key mediator of stem cells and their progeny. Fractones may represent sites at which growth factors and other signalling molecules interact with stem cells and progenitors to regulate their proliferation, activation and differentiation by modulating the availability of signalling molecules within the stem cell niche. The source of these factors is diverse: ependymal cells, CSF, endothelial cells and SVZ cells. It will be fascinating to see whether fractones are dynamic, perhaps indicating that adult neural stem cell niches undergo constant remodelling. It is not known whether similar structures are present in the SGZ.
Other ECM components known at present to be in adult neurogenic niches are chondroitin sulphate proteoglycans (CSPG; Gates et al. 1995; Thomas et al. 1996; Bruckner et al. 2003), heparan sulphate proteoglycans (HSPG; Fuxe et al. 1994), tenascin-C (Gates et al. 1995; Ferhat et al. 1996; Jankovski & Sotelo 1996; Thomas et al. 1996; Deller et al. 1997; Heck et al. 2004; Peretto et al. 2005), laminins (Mercier et al. 2002; Heck et al. 2004) and collagen 1 (Mercier et al. 2002), which together probably modulate accessibility of growth factors, cytokines and other signalling molecules. Integrins are receptors that provide structural links between the ECM and the cytoskeleton, allowing for oriented cell division. In addition, they cooperate with growth factor receptors to enhance signal transduction (Comoglio et al. 2003). As such, the integrins coordinate spatial positioning within the niche with downstream cellular signalling and probably play a key role in maintaining adult neural stem cell niches, as they do in other stem cell niches (reviewed in Fuchs et al. 2004).
The cell surface carbohydrate Lewis X (LeX)/CD15/SSEA1 (fucose N-acetyl lactosamine), an epitope found on several stem cell populations and other cell types, is expressed by all neurosphere-forming cells in the SVZ (Capela & Temple 2002), the majority of which correspond to transit-amplifying cells and a subset of astrocytes (Doetsch et al. 2002). In vivo, LeX is also expressed by astrocytes in contact with blood vessels (Capela & Temple 2002), suggesting that LeX may capture factors from the vasculature and/or other niche cells. Indeed, LeX binds both FGFs and Wnts (Dvorak et al. 1998; Jirmanova et al. 1999; Capela & Temple 2006). LeX ectodomains are shed in vitro (Capela & Temple 2002); such ectodomains may modulate signalling in neurogenic niches as has been shown for proteolytic fragments of ECM which are endogenous inhibitors of angiogenesis (Sottile 2004). It will be interesting to see whether proteolytic fragments and carbohydrate ectodomains also play a role in adult neurogenic regions.
7. Long-range and local inputs in the niche: neurotransmitter regulation of neurogenesis
Both adult neurogenic regions are richly innervated by axonal inputs of local and distant origins. Release of neurotransmitters and other factors, such as nitric oxide and sonic hedgehog (Shh), by afferent inputs may regulate precursors at different stages of the stem cell lineage.
The SVZ receives significant axonal input from the substantia nigra, the main source of dopamine in the brain. Dopamine release from these inputs affects proliferation of the transit-amplifying C cells (Hoglinger et al. 2004). Loss of dopamine signalling leads to a decrease in the number of proliferating cells and subsequent neurogenesis (Baker et al. 2004; Hoglinger et al. 2004; Freundlieb et al. 2006), which can be rescued by giving dopamine-depleted animals dopamine analogues or D2-like receptor agonists (Hoglinger et al. 2004). Unlike dopamine, gamma aminobutyric acid (GABA) is a locally produced, non-synaptically released neurotransmitter that influences the SVZ niche. In contrast to its role as an inhibitory neurotransmitter in the adult brain, GABA signalling is excitatory in the adult SVZ (Wang et al. 2003; Bolteus & Bordey 2004; Liu et al. 2005), as in the developing brain (reviewed in Ben-Ari 2002; Owens & Kriegstein 2002). In addition to influencing the rate of neuronal migration in the SVZ (Bolteus & Bordey 2004), release of GABA by migrating neuroblasts negatively regulates the proliferation of GFAP+ stem cell SVZ astrocytes (Liu et al. 2005). Whether GABA also regulates the proliferation of transit-amplifying C cells remains to be determined. In addition, NADPH+ neurons in the striatum extensively innervate the SVZ and may regulate proliferation and neurogenesis of SVZ cells through the secretion of nitric oxide (Packer et al. 2003; Matarredona et al. 2004; Moreno-Lopez et al. 2004).
The SGZ receives inputs originating from distant brain regions, including the entorhinal cortex and basal forebrain/septum, and locally from interneurons within the hippocampus, which influence neurogenesis either directly or indirectly (Cameron et al. 1995; Lai et al. 2003; Fontana et al. 2005; Tozuka et al. 2005; Ge et al. 2006). As in the SVZ, GABA is excitatory for progenitors and newly generated neurons; however, it is released synaptically, probably by local inhibitory interneurons (Overstreet Wadiche et al. 2005; Tozuka et al. 2005; Wang et al. 2005; Ge et al. 2006). GABA decreases progenitor proliferation, promotes neuronal differentiation and is critical for the maturation of newly generated neurons (Tozuka et al. 2005; Ge et al. 2006; Overstreet-Wadiche et al. 2006). Like GABA, glutamatergic signalling regulates proliferation and neurogenesis in the SGZ, although it is unclear whether this is a direct or indirect effect. While in vivo studies have produced conflicting reports (Cameron et al. 1995, 1998; Gould & Cameron 1997; Deisseroth et al. 2004), in vitro studies suggest that glutamate signalling activates pro-neurogenic programmes in progenitors to stimulate neuronal production (Deisseroth et al. 2004). How glutamatergic and GABAergic signalling converges in vivo remains to be elucidated. In addition, it will be interesting to determine whether newborn or mature granule neurons feedback on to progenitors to regulate neuronal production.
Monoaminergic inputs, including serotonergic, dopaminergic and noradrenergic afferents, affect proliferation in both adult neurogenic regions (Kulkarni et al. 2002; Santarelli et al. 2003; Banasr et al. 2004; Hoglinger et al. 2004; Encinas et al. 2006). In contrast, cholinergic innervation is implicated in neuronal survival (Cooper-Kuhn et al. 2004). As such, a combination of multiple afferent inputs from distant and local brain regions regulates adult neurogenesis. Defining at which stages of the stem cell lineage different neurotransmitters and receptor subtypes regulate proliferation and differentiation will be important.
8. Cell–cell contact and diffusible signals in the niche
Intrinsic genetic programmes as well as extracellular signals underlie stem cell fate choices. Whether a cell undergoes self-renewal or differentiation is the result of the spatial and temporal convergence of niche cues and the intrinsic state of the cell. The architectural elements of the niche have been discussed in detail above. Feedback signals from newly generated progeny can also regulate neural stem cells via either cell–cell contact or diffusible signals. When neuroblasts and transit-amplifying cells are depleted by an anti-mitotic treatment, SVZ astrocytes divide to rapidly regenerate the SVZ network of chains (Doetsch et al. 1999a,b), perhaps reflecting loss of feedback inhibition from the neuroblasts onto their ancestors, such as loss of GABA signalling (Liu et al. 2005). The first molecular cues that regulate stem cell lineages in the adult brain have begun to be identified (figure 4).
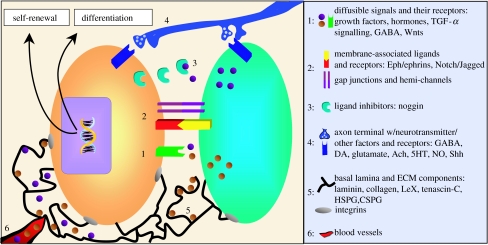
Figure 4.
Neural stem cell regulation. Neural stem cells in the two adult neurogenic niches, the SVZ and SGZ, can be regulated by (1) diffusible factors (EGF, FGF, TGF-α, VEGF, PEDF, hormones, BMPs, ATP, Wnts and GABA) and their receptors, (2) cell–cell and cell–extracellular environment interactions via membrane-associated ligands and their receptors (Eph/ephrin and Notch/Jagged) or gap junctions and hemi-channels, (3) ligand inhibition (noggin), (4) release of neurotransmitters and other factors from axons (GABA, dopamine (DA), glutamate, acetylcholine (ACh), serotonin (5HT), NO and Shh), (5) basal lamina (sequestration and presentation of diffusible factors, such as growth factors) and ECM proteins (laminin, collagen-1, tenascin-C, LeX, heparan sulphate and chondroitin sulphate proteoglycans), and (6) endothelial cell/blood vessel-mediated cues. The cell types that are able to direct neural stem cell fate choices include cells within the niche (both support cells and stem cells and their progeny) (green), neurons (axonal projection; blue) and endothelial cells/blood vessels (red). Structural elements that likely regulate neural stem cell fate decisions include fractones (in the SVZ) and basal laminae (black lines). In addition, CSF and meningeal projections (not pictured in this figure) are likely important components of the niche. These elements may work in concert or independently to promote neural stem cell self-renewal or differentiation. The key to the right lists some of the factors known to be present in adult neurogenic niches.
Notch is a transmembrane protein whose signalling regulates stem cell self-renewal in different niches (reviewed in Molofsky et al. 2004). Notch is activated upon binding Delta or Jagged, both membrane ligands, which causes cleavage of the intracellular tail of Notch and its translocation to the nucleus (reviewed in Gaiano & Fishell 2002). Over-expression of activated Notch1 in the embryonic brain or of activated Notch1 or 3 in adult cultured hippocampal progenitors leads to the generation of astrocytes (Gaiano et al. 2000; Chambers et al. 2001; Tanigaki et al. 2001). This raises the possibility that Notch is also involved in stem cell/progenitor maintenance in adult neurogenic niches, where Notch1 and Jagged1 are expressed (Stump et al. 2002; Irvin et al. 2004; Nyfeler et al. 2005). However, whether Notch activation promotes the acquisition of a stem cell astrocyte fate or differentiation into non-stem cell niche astrocytes awaits further evaluation.
Eph/ephrins are another large class of membrane-associated receptors and ligands with diverse roles during development, including axon guidance, cell migration and synaptogenesis (reviewed in Pasquale 2005). In the adult SVZ, different ephrins and Ephs are expressed by each SVZ cell type (Conover et al. 2000; Holmberg et al. 2005; Ricard et al. 2006). Eph/ephrin signalling regulates proliferation of different cell types in the adult SVZ (Conover et al. 2000; Holmberg et al. 2005; Katakowski et al. 2005; Ricard et al. 2006) and may be an important component of feedback regulation in this region.
Another molecule involved in proliferation in adult neurogenic regions is Shh. During development, Shh acts as a morphogen, playing a crucial role in ventral patterning along the entire extent of the neuraxis, and as a mitogen, stimulating granule cell precursor proliferation in the cerebellum (reviewed in Ruiz i Altaba et al. 2002). In the adult SVZ, Shh regulates the proliferation of SVZ astrocytes (type B cells) and transit-amplifying type C cells (Machold et al. 2003; Ahn & Joyner 2005; Palma et al. 2005). Shh also affects proliferation in the SGZ (Lai et al. 2003; Machold et al. 2003), although it is unknown which cell type Shh acts on. As mentioned above, conditional mice deficient in shh signalling components first exhibit deficits in neural stem cell niches post-natally. The cellular source of Shh has not yet been identified, although for the hippocampal formation, it has been proposed that Shh is anterogradely transported to the SGZ via the fimbria–fornix (Lai et al. 2003; Machold et al. 2003). Importantly, the signals that trigger Shh release in both niches are unknown.
Members of the Wnt family of soluble ligands play critical roles in various physiological processes during development and in the adult. Wnt signalling regulates stem cell self-renewal in several stem cell niches (reviewed in Kleber & Sommer 2004). In adult neurogenic niches, Wnt signalling has thus far been shown to regulate neurogenesis in the SGZ (Lie et al. 2005). Increasing or decreasing Wnt activity in vivo leads to an increase or decrease of SGZ neurogenesis, respectively. Interestingly, astrocytes are the source of Wnts, highlighting their multiple roles in the niche.
Two mitogens widely used to culture neural stem cells in vitro either as neurospheres or as adherent monolayers are epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) (Reynolds & Weiss 1992; Palmer et al. 1995). These assays test the potential of a cell to act as a stem cell, but do not necessarily reflect its in vivo behaviour. Although neurospheres were believed to arise from the relatively quiescent in vivo SVZ stem cells, the majority of EGF-responsive neurospheres arise from the rapidly dividing transit-amplifying cells (Doetsch et al. 2002), which retain the capacity to act as stem cells when exposed to exogenous growth factors. These findings emphasize the need to study stem cells and their progeny in vivo to elucidate their roles in adult neurogenesis. Dissection of the EGF- and bFGF-responsive SVZ cell types has revealed that signalling through the EGF- and FGF-receptors occurs at distinct stages in the stem cell lineage. bFGF likely acts on more quiescent SVZ astrocytes (Zheng et al. 2004) and has been proposed, based on analysis of bFGF null mice, to maintain the pool of neural stem cells (Zheng et al. 2004). In contrast, EGF-responsive cells are transit-amplifying C cells and a subset of SVZ astrocytes (Doetsch et al. 2002). This differential regulation may allow expansion of activated stem cells and transit-amplifying cells without depleting the more quiescent stem cells. The endogenous ligand for the EGF-R is likely TGF-α, which is expressed in the choroid plexus and striatum (Seroogy et al. 1993). Consistent with this, TGF-α null mice exhibit decreased proliferation and neurogenesis in the SVZ (Tropepe et al. 1997). Precise dissection of the roles of bFGF and TGF-α will require inducible genetic systems that allow one to circumvent possible developmental defects. Within adult neurogenic niches, other secreted factors, such as glycosylated cystatin which is expressed by some SGZ astrocytes, act synergistically with bFGF to influence neurogenesis (Taupin et al. 2000). Another signal that may synergize with bFGF is ATP (Mishra et al. 2006). The ecto-ATPase NTPDase2, which regulates ATP signalling, is present in both SVZ and SGZ astrocytes (Braun et al. 2003; Shukla et al. 2005). The in vivo role of nucleotide signalling in adult neurogenesis is yet to be explored.
Interestingly, transit-amplifying cells are emerging as key nodes in the stem cell lineage. Many signals, including dopamine (Hoglinger et al. 2004), soluble amyloid precursor protein (Caille et al. 2004), nitric oxide (Estrada et al. 1997; Matarredona et al. 2005) and Shh (Ahn & Joyner 2005; Palma et al. 2005), converge on the transit-amplifying cells and act cooperatively with signalling through the EGF receptor. As transit-amplifying cells are rapidly dividing, it is critical to regulate their proliferation to prevent runaway growth. Furthermore, this mode of regulation allows the relatively quiescent stem cells to divide infrequently, stopping them from accumulating mutations with division.
The structural elements and molecules important for stem cell self-renewal and differentiation, as well as for embryonic stem cells, are rapidly being elucidated. It will be fascinating to see if these pathways are conserved in other niches and, importantly, in different species. Within adult neurogenic niches, astrocytes are stem cells in the adult SVZ in both rodents (reviewed in Doetsch 2003a) and humans (Sanai et al. 2004), yet there may be fundamental differences in how neural stem cells from the two species interpret these molecular signals and ultimately affect their output. Uncovering these pathways, as well as the complex interactions within the niche, will provide invaluable insight into stem cell biology and into the potential use of neural stem cells for restorative neurogenesis in disease or trauma.
Acknowledgments
We thank Artem Kaplan and Masoud Tavazoie for critical reading of the manuscript. P.A.R. was supported by P50 AG08702 and E.D. by the Fondation pour la Recherche Medicale. F.D. is a Packard Foundation Fellow and Irma T. Hirschl Fellow and work in the laboratory is partially supported by the Jerry and Emily Spiegel Laboratory for Cell Replacement Therapies and the Anne and Bernard Spitzer Fund for Cell Replacement Therapy.
Footnotes
One contribution of 14 to a Theme Issue ‘Stem cells and brain repair’.
References
- Ahn S, Joyner A.L. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. doi:10.1038/nature03994 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo J.M, Tramontin A.D. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2001;2:287–293. doi: 10.1038/35067582. doi:10.1038/35067582 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, García-Verdugo J.M, Mateo A, Merchant-Larios H. Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J. Neurosci. 1998;18:1020–1037. doi: 10.1523/JNEUROSCI.18-03-01020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim D.A. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. doi:10.1016/S0896-6273(04)00111-4 [DOI] [PubMed] [Google Scholar]
- Alves J.A, Barone P, Engelender S, Froes M.M, Menezes J.R. Initial stages of radial glia astrocytic transformation in the early postnatal anterior subventricular zone. J. Neurobiol. 2002;52:251–265. doi: 10.1002/neu.10087. doi:10.1002/neu.10087 [DOI] [PubMed] [Google Scholar]
- Anthony T.E, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. doi:10.1016/S0896-6273(04)00140-0 [DOI] [PubMed] [Google Scholar]
- Bachoo R.M, et al. Molecular diversity of astrocytes with implications for neurological disorders. Proc. Natl Acad. Sci. USA. 2004;101:8384–8389. doi: 10.1073/pnas.0402140101. doi:10.1073/pnas.0402140101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S.A, Baker K.A, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur. J. Neurosci. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. doi:10.1111/j.1460-9568.2004.03486.x [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. doi:10.1038/sj.npp.1300320 [DOI] [PubMed] [Google Scholar]
- Belluzzi O, Benedusi M, Ackman J, Loturco J.J. Electrophysiological differentiation of new neurons in the olfactory bulb. J. Neurosci. 2003;23:10 411–10 418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. doi:10.1038/nrn920 [DOI] [PubMed] [Google Scholar]
- Bennett M.V, Contreras J.E, Bukauskas F.F, Saez J.C. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. doi:10.1016/j.tins.2003.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolteus A.J, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J. Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. doi:10.1523/JNEUROSCI.1999-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi M.A, McGuire T, Hu M, Kan L, Samanta J, Kessler J.A. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development. 2005;132:5503–5514. doi: 10.1242/dev.02166. doi:10.1242/dev.02166 [DOI] [PubMed] [Google Scholar]
- Braun N, Sevigny J, Mishra S.K, Robson S.C, Barth S.W, Gerstberger R, Hammer K, Zimmermann H. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur. J. Neurosci. 2003;17:1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. doi:10.1046/j.1460-9568.2003.02567.x [DOI] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. doi:10.1126/science.1097676 [DOI] [PubMed] [Google Scholar]
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat. Rev. Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. doi:10.1038/nrn1687 [DOI] [PubMed] [Google Scholar]
- Bruckner G, Grosche J, Hartlage-Rubsamen M, Schmidt S, Schachner M. Region and lamina-specific distribution of extracellular matrix proteoglycans, hyaluronan and tenascin-R in the mouse hippocampal formation. J. Chem. Neuroanat. 2003;26:37–50. doi: 10.1016/s0891-0618(03)00036-x. doi:10.1016/S0891-0618(03)00036-X [DOI] [PubMed] [Google Scholar]
- Bull N.D, Bartlett P.F. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J. Neurosci. 2005;25:10 815–10 821. doi: 10.1523/JNEUROSCI.3249-05.2005. doi:10.1523/JNEUROSCI.3249-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille I, Allinquant B, Dupont E, Bouillot C, Langer A, Muller U, Prochiantz A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131:2173–2181. doi: 10.1242/dev.01103. doi:10.1242/dev.01103 [DOI] [PubMed] [Google Scholar]
- Cameron H.A, McEwen B.S, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J. Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron H.A, Hazel T.G, McKay R.D.G. Regulation of neurogenesis by growth factors and neurotransmitters. J. Neurobiol. 1998;36:287–306. doi:10.1002/(SICI)1097-4695(199808)36:2<287::AID-NEU13>3.0.CO;2-B [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga D.S, Liu Y, Fong D.M, Young D, During M.J. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. 2004;36:827–835. doi: 10.1038/ng1395. doi:10.1038/ng1395 [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. doi:10.1016/S0896-6273(02)00835-8 [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment and binds Wnt-1. Dev. Biol. 2006;291:300–313. doi: 10.1016/j.ydbio.2005.12.030. doi:10.1016/j.ydbio.2005.12.030 [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu L.T, Lansford R, Alvarez-Buylla A, Lledo P.M. Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat. Rev. Genet. 2003;4:710–720. doi: 10.1038/nrg1158. doi:10.1038/nrg1158 [DOI] [PubMed] [Google Scholar]
- Chambers C.B, Peng Y, Nguyen H, Gaiano N, Fishell G, Nye J.S. Spatiotemporal selectivity of response to Notch1 signals in mammalian forebrain precursors. Development. 2001;128:689–702. doi: 10.1242/dev.128.5.689. [DOI] [PubMed] [Google Scholar]
- Cheng L.C, Tavazoie M, Doetsch F. Stem cells: from epigenetics to microRNAs. Neuron. 2005;46:363–367. doi: 10.1016/j.neuron.2005.04.027. doi:10.1016/j.neuron.2005.04.027 [DOI] [PubMed] [Google Scholar]
- Chiasson B.J, Tropepe V, Morshead C.M, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J. Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson K.S, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. doi:10.1016/j.cell.2004.12.020 [DOI] [PubMed] [Google Scholar]
- Comoglio P.M, Boccaccio C, Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol. 2003;15:565–571. doi: 10.1016/s0955-0674(03)00096-6. doi:10.1016/S0955-0674(03)00096-6 [DOI] [PubMed] [Google Scholar]
- Conover J.C, Doetsch F, Garcia-Verdugo J.M, Gale N.W, Yancopoulos G.D, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat. Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. doi:10.1038/80606 [DOI] [PubMed] [Google Scholar]
- Cooper-Kuhn C.M, Winkler J, Kuhn H.G. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J. Neurosci. Res. 2004;77:155–165. doi: 10.1002/jnr.20116. doi:10.1002/jnr.20116 [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer T.D, Malenka R.C. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. doi:10.1016/S0896-6273(04)00266-1 [DOI] [PubMed] [Google Scholar]
- Del Bigio M.R. The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia. 1995;14:1–13. doi: 10.1002/glia.440140102. doi:10.1002/glia.440140102 [DOI] [PubMed] [Google Scholar]
- Deller T, Haas C.A, Naumann T, Joester A, Faissner A, Frotscher M. Up-regulation of astrocyte-derived tenascin-C correlates with neurite outgrowth in the rat dentate gyrus after unilateral entorhinal cortex lesion. Neuroscience. 1997;81:829–846. doi: 10.1016/s0306-4522(97)00194-2. doi:10.1016/S0306-4522(97)00194-2 [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat. Neurosci. 2003a;6:1127–1134. doi: 10.1038/nn1144. doi:10.1038/nn1144 [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr. Opin. Genet Dev. 2003b;13:543–550. doi: 10.1016/j.gde.2003.08.012. doi:10.1016/j.gde.2003.08.012 [DOI] [PubMed] [Google Scholar]
- Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr. Opin. Neurobiol. 2005;15:121–128. doi: 10.1016/j.conb.2005.01.018. doi:10.1016/j.conb.2005.01.018 [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo J.M, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim D.A, Garcia-Verdugo J.M, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999a;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. doi:10.1016/S0092-8674(00)80783-7 [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo J.M, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl Acad. Sci. USA. 1999b;96:11 619–11 624. doi: 10.1073/pnas.96.20.11619. doi:10.1073/pnas.96.20.11619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo J.M, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. doi:10.1016/S0896-6273(02)01133-9 [DOI] [PubMed] [Google Scholar]
- Dvorak P, Hampl A, Jirmanova L, Pacholikova J, Kusakabe M. Embryoglycan ectodomains regulate biological activity of FGF-2 to embryonic stem cells. J. Cell Sci. 1998;111:2945–2952. doi: 10.1242/jcs.111.19.2945. Pt 19. [DOI] [PubMed] [Google Scholar]
- Dziembowska M, Tham T.N, Lau P, Vitry S, Lazarini F, Dubois-Dalcq M. A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors. Glia. 2005;50:258–269. doi: 10.1002/glia.20170. doi:10.1002/glia.20170 [DOI] [PubMed] [Google Scholar]
- Encinas J.M, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl Acad. Sci. USA. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. doi:10.1073/pnas.0601992103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada C, Gomez C, Martin-Nieto J, De Frutos T, Jimenez A, Villalobo A. Nitric oxide reversibly inhibits the epidermal growth factor receptor tyrosine kinase. Biochem. J. 1997;326:369–376. doi: 10.1042/bj3260369. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo C.J, Palmer T.D. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. doi:10.1111/j.1460-9568.2003.03041.x [DOI] [PubMed] [Google Scholar]
- Falk A, Frisen J. Amphiregulin is a mitogen for adult neural stem cells. J. Neurosci. Res. 2002;69:757–762. doi: 10.1002/jnr.10410. doi:10.1002/jnr.10410 [DOI] [PubMed] [Google Scholar]
- Ferhat L, Chevassus Au Louis N, Jorquera I, Niquet J, Khrestchatisky M, Ben-Ari Y, Represa A. Transient increase of tenascin-C in immature hippocampus: astroglial and neuronal expression. J. Neurocytol. 1996;25:53–66. doi: 10.1007/BF02284785. doi:10.1007/BF02284785 [DOI] [PubMed] [Google Scholar]
- Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang L.P, Yamaguchi M, Kettenmann H, Kempermann G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol. Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. doi:10.1016/S1044-7431(03)00060-5 [DOI] [PubMed] [Google Scholar]
- Fontana X, Nacher J, Soriano E, Del Rio J.A. Cell proliferation in the adult hippocampal formation of rodents and its modulation by entorhinal and fimbria–fornix afferents. Cereb. Cortex. 2005;16:301–312. doi: 10.1093/cercor/bhi120. doi:10.1093/cercor/bhi120 [DOI] [PubMed] [Google Scholar]
- Freundlieb N, Francois C, Tande D, Oertel W.H, Hirsch E.C, Hoglinger G.U. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J. Neurosci. 2006;26:2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. doi:10.1523/JNEUROSCI.4859-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. doi:10.1016/S0092-8674(04)00255-7 [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J. Neurosci. 2003;23:9357–9366. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Chadi G, Tinner B, Agnati L.F, Pettersson R, David G. On the regional distribution of heparan sulfate proteoglycan immunoreactivity in the rat brain. Brain Res. 1994;636:131–138. doi: 10.1016/0006-8993(94)90187-2. doi:10.1016/0006-8993(94)90187-2 [DOI] [PubMed] [Google Scholar]
- Gage F.H. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. doi:10.1126/science.287.5457.1433 [DOI] [PubMed] [Google Scholar]
- Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu. Rev. Neurosci. 2002;25:471–490. doi: 10.1146/annurev.neuro.25.030702.130823. doi:10.1146/annurev.neuro.25.030702.130823 [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye J.S, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. doi:10.1016/S0896-6273(00)81172-1 [DOI] [PubMed] [Google Scholar]
- Garcia A.D, Doan N.B, Imura T, Bush T.G, Sofroniew M.V. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. doi:10.1038/nn1340 [DOI] [PubMed] [Google Scholar]
- Gates M.A, Thomas L.B, Howard E.M, Laywell E.D, Sajin B, Faissner A, Gotz B, Silver J, Steindler D.A. Cell and molecular analysis of the developing and adult mouse subventricular zone of the cerebral hemispheres. J. Comp. Neurol. 1995;361:249–266. doi: 10.1002/cne.903610205. doi:10.1002/cne.903610205 [DOI] [PubMed] [Google Scholar]
- Ge S, Goh E.L, Sailor K.A, Kitabatake Y, Ming G.L, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. doi:10.1038/nature04404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, McCarthy K.D. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996;19:319–325. doi: 10.1016/0166-2236(96)10046-1. doi:10.1016/0166-2236(96)10046-1 [DOI] [PubMed] [Google Scholar]
- Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. doi:10.1002/(SICI)1098-1136(199809)24:1<50::AID-GLIA6>3.0.CO;2-4 [PubMed] [Google Scholar]
- Gould E, Cameron H.A. Early NMDA receptor blockade impairs defensive behavior and increases cell proliferation in the dentate gyrus of developing rats. Behav. Neurosci. 1997;111:49–56. doi: 10.1037//0735-7044.111.1.49. doi:10.1037/0735-7044.111.1.49 [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol. Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. doi:10.1016/S0006-3223(00)01021-0 [DOI] [PubMed] [Google Scholar]
- Hack M.A, Saghatelyan A, De Chevigny A, Pfeifer A, Ashery-Padan R, Lledo P.M, Gotz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat. Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Hayamizu T.F, Chan P.T, Johanson C.E. FGF-2 immunoreactivity in adult rat ependyma and choroid plexus: responses to global forebrain ischemia and intraventricular FGF-2. Neurol. Res. 2001;23:353–358. doi: 10.1179/016164101101198550. doi:10.1179/016164101101198550 [DOI] [PubMed] [Google Scholar]
- Heck N, Garwood J, Loeffler J.P, Larmet Y, Faissner A. Differential upregulation of extracellular matrix molecules associated with the appearance of granule cell dispersion and mossy fiber sprouting during epileptogenesis in a murine model of temporal lobe epilepsy. Neuroscience. 2004;129:309–324. doi: 10.1016/j.neuroscience.2004.06.078. doi:10.1016/j.neuroscience.2004.06.078 [DOI] [PubMed] [Google Scholar]
- Herrera D.G, Garcia-Verdugo J.M, Alvarez-Buylla A. Adult-derived neural precursors transplanted into multiple regions in the adult brain. Ann. Neurol. 1999;46:867–877. doi: 10.1002/1531-8249(199912)46:6<867::aid-ana9>3.0.co;2-z. doi:10.1002/1531-8249(199912)46:6<867::AID-ANA9>3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- Hoglinger G.U, Rizk P, Muriel M.P, Duyckaerts C, Oertel W.H, Caille I, Hirsch E.C. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. doi:10.1038/nn1265 [DOI] [PubMed] [Google Scholar]
- Holmberg J, Armulik A, Senti K.A, Edoff K, Spalding K, Momma S, Cassidy R, Flanagan J.G, Frisen J. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005;19:462–471. doi: 10.1101/gad.326905. doi:10.1101/gad.326905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Gage F.H. Epigenetic control of neural stem cell fate. Curr. Opin. Genet Dev. 2004;14:461–469. doi: 10.1016/j.gde.2004.07.006. doi:10.1016/j.gde.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Imura T, Kornblum H.I, Sofroniew M.V. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J. Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Nakano I, Kornblum H.I, Sofroniew M.V. Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes. Glia. 2006;53:277–293. doi: 10.1002/glia.20281. doi:10.1002/glia.20281 [DOI] [PubMed] [Google Scholar]
- Irvin D.K, Nakano I, Paucar A, Kornblum H.I. Patterns of Jagged1, Jagged2, Delta-like 1 and Delta-like 3 expression during late embryonic and postnatal brain development suggest multiple functional roles in progenitors and differentiated cells. J. Neurosci. Res. 2004;75:330–343. doi: 10.1002/jnr.10843. doi:10.1002/jnr.10843 [DOI] [PubMed] [Google Scholar]
- Jankovski A, Sotelo C. Subventricular zone-olfactory bulb migratory pathway in the adult mouse: cellular composition and specificity as determined by heterochronic and heterotopic transplantation. J. Comp. Neurol. 1996;371:376–396. doi: 10.1002/(SICI)1096-9861(19960729)371:3<376::AID-CNE3>3.0.CO;2-#. doi:10.1002/(SICI)1096-9861(19960729)371:3<376::AID-CNE3>3.0.CO;2-# [DOI] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur. J. Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. doi:10.1111/j.1460-9568.2003.02986.x [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao X.O, Xie L, Greenberg D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl Acad. Sci. USA. 2002;99:11 946–11 950. doi: 10.1073/pnas.182296499. doi:10.1073/pnas.182296499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirmanova L, Pacholikova J, Krejci P, Hampl A, Dvorak P. O-linked carbohydrates are required for FGF-2-mediated proliferation of mouse embryonic cells. Int. J. Dev. Biol. 1999;43:555–562. [PubMed] [Google Scholar]
- Johansson C.B, Momma S, Clarke D.L, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. doi:10.1016/S0092-8674(00)80956-3 [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. doi:10.1038/nature02436 [DOI] [PubMed] [Google Scholar]
- Katakowski M, Zhang Z, Decarvalho A.C, Chopp M. EphB2 induces proliferation and promotes a neuronal fate in adult subventricular neural precursor cells. Neurosci. Lett. 2005;385:204–209. doi: 10.1016/j.neulet.2005.05.060. doi:10.1016/j.neulet.2005.05.060 [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. doi:10.1016/j.tins.2004.05.013 [DOI] [PubMed] [Google Scholar]
- Kleber M, Sommer L. Wnt signaling and the regulation of stem cell function. Curr. Opin. Cell Biol. 2004;16:681–687. doi: 10.1016/j.ceb.2004.08.006. doi:10.1016/j.ceb.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Kohwi M, Osumi N, Rubenstein J.L, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J. Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. doi:10.1523/JNEUROSCI.1435-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornyei Z, Szlavik V, Szabo B, Gocza E, Czirok A, Madarasz E. Humoral and contact interactions in astroglia/stem cell co-cultures in the course of glia-induced neurogenesis. Glia. 2005;49:430–444. doi: 10.1002/glia.20123. doi:10.1002/glia.20123 [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt M.D, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. doi:10.1002/cne.10945 [DOI] [PubMed] [Google Scholar]
- Kulkarni V.A, Jha S, Vaidya V.A. Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation, of granule cell progenitors in the adult rat hippocampus. Eur. J. Neurosci. 2002;16:2008–2012. doi: 10.1046/j.1460-9568.2002.02268.x. doi:10.1046/j.1460-9568.2002.02268.x [DOI] [PubMed] [Google Scholar]
- Kumar N.M, Gilula N.B. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. doi:10.1016/S0092-8674(00)81282-9 [DOI] [PubMed] [Google Scholar]
- Lai K, Kaspar B.K, Gage F.H, Schaffer D.V. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 2003;6:21–27. doi: 10.1038/nn983. doi:10.1038/nn983 [DOI] [PubMed] [Google Scholar]
- Laywell E.D, Rakic P, Kukekov V.G, Holland E.C, Steindler D.A. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc. Natl Acad. Sci. USA. 2000;97:13 883–13 888. doi: 10.1073/pnas.250471697. doi:10.1073/pnas.250471697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennington J.B, Yang Z, Conover J.C. Neural stem cells and the regulation of adult neurogenesis. Reprod. Biol. Endocrinol. 2003;1:99. doi: 10.1186/1477-7827-1-99. doi:10.1186/1477-7827-1-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman S.A. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol. Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. doi:10.1006/mcne.1999.0762 [DOI] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: structure and function. Annu. Rev. Cell Dev. Biol. 2005;17:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. doi:10.1146/annurev.cellbio.21.012704.131525 [DOI] [PubMed] [Google Scholar]
- Lie D.C, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. doi:10.1038/nature04108 [DOI] [PubMed] [Google Scholar]
- Lim D.A, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc. Natl Acad. Sci. USA. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. doi:10.1073/pnas.96.13.7526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D.A, Tramontin A.D, Trevejo J.M, Herrera D.G, Garcia-Verdugo J.M, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. doi:10.1016/S0896-6273(00)00148-3 [DOI] [PubMed] [Google Scholar]
- Lim D.A, Suarez-Farinas M, Naef F, Hacker C.R, Menn B, Takebayashi H, Magnasco M, Patil N, Alvarez-Buylla A. In vivo transcriptional profile analysis reveals RNA splicing and chromatin remodeling as prominent processes for adult neurogenesis. Mol. Cell Neurosci. 2006;31:131–148. doi: 10.1016/j.mcn.2005.10.005. doi:10.1016/j.mcn.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Lin J.H, et al. Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells. J. Neurosci. 2002;22:4302–4311. doi: 10.1523/JNEUROSCI.22-11-04302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar T.F, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. doi:10.1038/nn1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman S.A. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. doi:10.1016/S0896-6273(02)00722-5 [DOI] [PubMed] [Google Scholar]
- Machold R, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. doi:10.1016/S0896-6273(03)00561-0 [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack M.A, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. doi:10.1016/S0896-6273(03)00116-8 [DOI] [PubMed] [Google Scholar]
- Marshman E, Booth C, Potten C.S. The intestinal epithelial stem cell. Bioessays. 2002;24:91–98. doi: 10.1002/bies.10028. doi:10.1002/bies.10028 [DOI] [PubMed] [Google Scholar]
- Matarredona E.R, Murillo-Carretero M, Moreno-Lopez B, Estrada C. Nitric oxide synthesis inhibition increases proliferation of neural precursors isolated from the postnatal mouse subventricular zone. Brain Res. 2004;995:274–284. doi: 10.1016/j.brainres.2003.10.010. doi:10.1016/j.brainres.2003.10.010 [DOI] [PubMed] [Google Scholar]
- Matarredona E.R, Murillo-Carretero M, Moreno-Lopez B, Estrada C. Role of nitric oxide in subventricular zone neurogenesis. Brain Res. Brain Res. Rev. 2005;49:355–366. doi: 10.1016/j.brainresrev.2005.01.001. doi:10.1016/j.brainresrev.2005.01.001 [DOI] [PubMed] [Google Scholar]
- Menezes J.R, Froes M.M, Moura Neto V, Lent R. Gap junction-mediated coupling in the postnatal anterior subventricular zone. Dev. Neurosci. 2000;22:34–43. doi: 10.1159/000017425. doi:10.1159/000017425 [DOI] [PubMed] [Google Scholar]
- Mercier F, Hatton G.I. Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: roles in stem cell proliferation and morphological plasticity? J. Comp. Neurol. 2001;431:88–104. doi: 10.1002/1096-9861(20010226)431:1<88::aid-cne1057>3.0.co;2-d. doi:10.1002/1096-9861(20010226)431:1<88::AID-CNE1057>3.0.CO;2-D [DOI] [PubMed] [Google Scholar]
- Mercier F, Kitasako J.T, Hatton G.I. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J. Comp. Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. doi:10.1002/cne.10342 [DOI] [PubMed] [Google Scholar]
- Merkle F.T, Tramontin A.D, Garcia-Verdugo J.M, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc. Natl Acad. Sci. USA. 2004;101:17 528–17 532. doi: 10.1073/pnas.0407893101. doi:10.1073/pnas.0407893101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Haeberle H, Barres B.A. Induction of astrocyte differentiation by endothelial cells. J. Neurosci. 2001;21:1538–1547. doi: 10.1523/JNEUROSCI.21-05-01538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miragall F, Albiez P, Bartels H, de Vries U, Dermietzel R. Expression of the gap junction protein connexin43 in the subependymal layer and the rostral migratory stream of the mouse: evidence for an inverse correlation between intensity of connexin43 expression and cell proliferation activity. Cell Tiss. Res. 1997;287:243–253. doi: 10.1007/s004410050749. doi:10.1007/s004410050749 [DOI] [PubMed] [Google Scholar]
- Mishra S.K, et al. Extracellular nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Development. 2006;133:675–684. doi: 10.1242/dev.02233. doi:10.1242/dev.02233 [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. doi:10.1016/S0896-6273(01)00420-2 [DOI] [PubMed] [Google Scholar]
- Molofsky A.V, Pardal R, Iwashita T, Park I.K, Clarke M.F, Morrison S.J. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. doi:10.1038/nature02060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A.V, Pardal R, Morrison S.J. Diverse mechanisms regulate stem cell self-renewal. Curr. Opin. Cell Biol. 2004;16:700–707. doi: 10.1016/j.ceb.2004.09.004. doi:10.1016/j.ceb.2004.09.004 [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Romero-Grimaldi C, Noval J.A, Murillo-Carretero M, Matarredona E.R, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J. Neurosci. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. doi:10.1523/JNEUROSCI.1574-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead C.M, Garcia A.D, Sofroniew M.V, van der Kooy D. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur. J. Neurosci. 2003;18:76–84. doi: 10.1046/j.1460-9568.2003.02727.x. doi:10.1046/j.1460-9568.2003.02727.x [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Fujiwara Y, Orkin S.H, Rowitch D.H. Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature. 2005;438:360–363. doi: 10.1038/nature04139. doi:10.1038/nature04139 [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, van Evercooren A.B. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. doi:10.1046/j.1460-9568.1999.00873.x [DOI] [PubMed] [Google Scholar]
- Noctor S.C, Flint A.C, Weissman T.A, Dammerman R.S, Kriegstein A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. doi:10.1038/35055553 [DOI] [PubMed] [Google Scholar]
- Noctor S.C, Flint A.C, Weissman T.A, Wong W.S, Clinton B.K, Kriegstein A.R. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J. Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor S.C, Martinez-Cerdeno V, Ivic L, Kriegstein A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. doi:10.1038/nn1172 [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The neural basis of birdsong. PLoS Biol. 2005;3:e164. doi: 10.1371/journal.pbio.0030164. doi:10.1371/journal.pbio.0030164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyfeler Y, Kirch R.D, Mantei N, Leone D.P, Radtke F, Suter U, Taylor V. Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO J. 2005;24:3504–3515. doi: 10.1038/sj.emboj.7600816. doi:10.1038/sj.emboj.7600816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet Wadiche L, Bromberg D.A, Bensen A.L, Westbrook G.L. GABAergic signaling to newborn neurons in dentate gyrus. J. Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. doi:10.1152/jn.00633.2005 [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche L.S, Bensen A.L, Westbrook G.L. Delayed development of adult-generated granule cells in dentate gyrus. J. Neurosci. 2006;26:2326–2334. doi: 10.1523/JNEUROSCI.4111-05.2006. doi:10.1523/JNEUROSCI.4111-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens D.F, Kriegstein A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. doi:10.1038/nrn919 [DOI] [PubMed] [Google Scholar]
- Packer M.A, Stasiv Y, Benraiss A, Chmielnicki E, Grinberg A, Westphal H, Goldman S.A, Enikolopov G. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc. Natl Acad. Sci. USA. 2003;100:9566–9571. doi: 10.1073/pnas.1633579100. doi:10.1073/pnas.1633579100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma V, et al. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. doi:10.1242/dev.01567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T.D, Ray J, Gage F.H. FGF-2 responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol. Cell. Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. doi:10.1006/mcne.1995.1035 [DOI] [PubMed] [Google Scholar]
- Palmer T.D, Willhoite A.R, Gage F.H. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. doi:10.1002/1096-9861(20001002)425:4<479::AID-CNE2>3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- Parras C.M, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. doi:10.1038/sj.emboj.7600447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale E.B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. doi:10.1038/nrm1662 [DOI] [PubMed] [Google Scholar]
- Peretto P, Dati C, De Marchis S, Kim H.H, Ukhanova M, Fasolo A, Margolis F.L. Expression of the secreted factors noggin and bone morphogenetic proteins in the subependymal layer and olfactory bulb of the adult mouse brain. Neuroscience. 2004;128:685–696. doi: 10.1016/j.neuroscience.2004.06.053. doi:10.1016/j.neuroscience.2004.06.053 [DOI] [PubMed] [Google Scholar]
- Peretto P, Giachino C, Aimar P, Fasolo A, Bonfanti L. Chain formation and glial tube assembly in the shift from neonatal to adult subventricular zone of the rodent forebrain. J. Comp. Neurol. 2005;487:407–427. doi: 10.1002/cne.20576. doi:10.1002/cne.20576 [DOI] [PubMed] [Google Scholar]
- Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Evercooren A.B. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc. Natl Acad. Sci. USA. 2002;99:13 211–13 216. doi: 10.1073/pnas.192314199. doi:10.1073/pnas.192314199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pixley S. CNS glial cells support in vitro survival division, and differentiation of dissociated olfactory neuronal progenitor cells. Neuron. 1992;8:1191–1204. doi: 10.1016/0896-6273(92)90139-5. doi:10.1016/0896-6273(92)90139-5 [DOI] [PubMed] [Google Scholar]
- Pleasure S.J, Collins A.E, Lowenstein D.H. Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J. Neurosci. 2000;20:6095–6105. doi: 10.1523/JNEUROSCI.20-16-06095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. Adult stem cell plasticity: fact or artifact? Annu. Rev. Cell Dev. Biol. 2003;19:1–22. doi: 10.1146/annurev.cellbio.19.111301.143037. doi:10.1146/annurev.cellbio.19.111301.143037 [DOI] [PubMed] [Google Scholar]
- Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, Ferron S.R, Aroca-Aguilar J.D, Sanchez P, Mira H, Escribano J, Farinas I. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat. Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. doi:10.1038/nn1657 [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison S.J, Clarke M.F, Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. doi:10.1038/35102167 [DOI] [PubMed] [Google Scholar]
- Reynolds B, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. doi:10.1126/science.1553558 [DOI] [PubMed] [Google Scholar]
- Ricard J, Salinas J, Garcia L, Liebl D.J. EphrinB3 regulates cell proliferation and survival in adult neurogenesis. Mol. Cell Neurosci. 2006;31:713–722. doi: 10.1016/j.mcn.2006.01.002. doi:10.1016/j.mcn.2006.01.002 [DOI] [PubMed] [Google Scholar]
- Ross S.E, Greenberg M.E, Stiles C.D. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. doi:10.1016/S0896-6273(03)00365-9 [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat. Rev. Cancer. 2002;2:361–372. doi: 10.1038/nrc796. doi:10.1038/nrc796 [DOI] [PubMed] [Google Scholar]
- Sakaguchi M, et al. A carbohydrate-binding protein, Galectin-1, promotes proliferation of adult neural stem cells. Proc. Natl Acad. Sci. USA. 2006;103:7112–7117. doi: 10.1073/pnas.0508793103. doi:10.1073/pnas.0508793103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. doi:10.1038/nature02301 [DOI] [PubMed] [Google Scholar]
- Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. doi:10.1126/science.1083328 [DOI] [PubMed] [Google Scholar]
- Sawamoto K, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. doi:10.1126/science.1119133 [DOI] [PubMed] [Google Scholar]
- Schmechel D.E, Rakic P. A Golgi study of radial glia cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat. Embryol. 1979;156:115–152. doi: 10.1007/BF00300010. doi:10.1007/BF00300010 [DOI] [PubMed] [Google Scholar]
- Seaberg R.M, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J. Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo J.M, Mcewen B.S, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo J.M, Collado-Morente L, McEwen B.S, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J. Comp. Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. doi:10.1002/cne.20288 [DOI] [PubMed] [Google Scholar]
- Seroogy K.B, Lundgren K.H, Lee D.C, Guthrie K.M, Gall C.M. Cellular localization of transforming growth factor-a mRNA in rat forebrain. J. Neurochem. 1993;60:1777–1782. doi: 10.1111/j.1471-4159.1993.tb13403.x. doi:10.1111/j.1471-4159.1993.tb13403.x [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie S.K, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. doi:10.1126/science.1095505 [DOI] [PubMed] [Google Scholar]
- Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu R.T, Gage F.H, Evans R.M. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. doi:10.1038/nature02211 [DOI] [PubMed] [Google Scholar]
- Shihabuddin L.S, Horner P.J, Ray J, Gage F.H. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J. Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla V, Zimmermann H, Wang L, Kettenmann H, Raab S, Hammer K, Sevigny J, Robson S.C, Braun N. Functional expression of the ecto-ATPase NTPDase2 and of nucleotide receptors by neuronal progenitor cells in the adult murine hippocampus. J. Neurosci. Res. 2005;80:600–610. doi: 10.1002/jnr.20508. doi:10.1002/jnr.20508 [DOI] [PubMed] [Google Scholar]
- Sohl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat. Rev. Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens C.F, Gage F.H. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. doi:10.1038/417039a [DOI] [PubMed] [Google Scholar]
- Sotelo J.R, Trujillo-Cenóz O. Electron microscope study on the development of ciliary components of the neural epithelium of the chick embryo. Z. Zellforsch. 1958;49:1–12. doi: 10.1007/BF00335059. doi:10.1007/BF00335059 [DOI] [PubMed] [Google Scholar]
- Sottile J. Regulation of angiogenesis by extracellular matrix. Biochim. Biophys. Acta. 2004;1654:13–22. doi: 10.1016/j.bbcan.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle F.T, Flames N, Tramontin A.D, Garcia-Verdugo J.M, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. doi:10.1523/JNEUROSCI.1108-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. doi:10.1038/35102160 [DOI] [PubMed] [Google Scholar]
- Steiner B, Kronenberg G, Jessberger S, Brandt M.D, Reuter K, Kempermann G. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia. 2004;46:41–52. doi: 10.1002/glia.10337. doi:10.1002/glia.10337 [DOI] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J. Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensaas L.J, Stensaas S.S. An electron microscope study of cells in the matrix and intermediate laminae of the cerebral hemisphere of the 45 mm rabbit embryo. Z. Zellforsch. 1968;91:341–365. doi: 10.1007/BF00440763. doi:10.1007/BF00440763 [DOI] [PubMed] [Google Scholar]
- Stumm R.K, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J. Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump G, Durrer A, Klein A.L, Lutolf S, Suter U, Taylor V. Notch1 and its ligands Delta-like and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech. Dev. 2002;114:153–159. doi: 10.1016/s0925-4773(02)00043-6. doi:10.1016/S0925-4773(02)00043-6 [DOI] [PubMed] [Google Scholar]
- Suhonen J.O, Peterson D.A, Ray J, Gage F.H. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996;383:624–627. doi: 10.1038/383624a0. doi:10.1038/383624a0 [DOI] [PubMed] [Google Scholar]
- Tanigaki K, Nogaki F, Takahashi J, Tashiro K, Kurooka H, Honjo T. Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron. 2001;29:45–55. doi: 10.1016/s0896-6273(01)00179-9. doi:10.1016/S0896-6273(01)00179-9 [DOI] [PubMed] [Google Scholar]
- Taupin P, Ray J, Fischer W.H, Suhr S.T, Hakansson K, Grubb A, Gage F.H. FGF-2-responsive neural stem cell proliferation requires CCg, a novel autocrine/paracrine cofactor. Neuron. 2000;28:385–397. doi: 10.1016/s0896-6273(00)00119-7. doi:10.1016/S0896-6273(00)00119-7 [DOI] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. doi:10.1038/35102174 [DOI] [PubMed] [Google Scholar]
- Theis M, Sohl G, Speidel D, Kuhn R, Willecke K. Connexin43 is not expressed in principal cells of mouse cortex and hippocampus. Eur. J. Neurosci. 2003;18:267–274. doi: 10.1046/j.1460-9568.2003.02740.x. doi:10.1046/j.1460-9568.2003.02740.x [DOI] [PubMed] [Google Scholar]
- Theis M, Sohl G, Eiberger J, Willecke K. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 2005;28:188–195. doi: 10.1016/j.tins.2005.02.006. doi:10.1016/j.tins.2005.02.006 [DOI] [PubMed] [Google Scholar]
- Thomas L.B, Gates M.A, Steindler D.A. Young neurons from the adult subependymal zone proliferate and migrate along an astrocyte, extracellular matrix-rich pathway. Glia. 1996;17:1–14. doi: 10.1002/(SICI)1098-1136(199605)17:1<1::AID-GLIA1>3.0.CO;2-7. doi:10.1002/(SICI)1098-1136(199605)17:1<1::AID-GLIA1>3.0.CO;2-7 [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. doi:10.1016/j.neuron.2005.08.023 [DOI] [PubMed] [Google Scholar]
- Tramontin A.D, Garcia-Verdugo J.M, Lim D.A, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb. Cortex. 2003;13:580–587. doi: 10.1093/cercor/13.6.580. doi:10.1093/cercor/13.6.580 [DOI] [PubMed] [Google Scholar]
- Tropepe V, Craig C.G, Morshead C.M, van der Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J. Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian E.M, Sapperstein S.K, Christopherson K.S, Barres B.A. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. doi:10.1126/science.291.5504.657 [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder A.F, Christie B.R, Toni N, Palmer T.D, Gage F.H. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. doi:10.1038/4151030a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt T. Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J. Comp. Neurol. 1989;289:74–88. doi: 10.1002/cne.902890106. doi:10.1002/cne.902890106 [DOI] [PubMed] [Google Scholar]
- Waclaw R.R, Allen Z.J, II, Bell S.M, Erdelyi F, Szabo G, Potter S.S, Campbell K. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006;49:503–516. doi: 10.1016/j.neuron.2006.01.018. doi:10.1016/j.neuron.2006.01.018 [DOI] [PubMed] [Google Scholar]
- Wang D.D, Krueger D.D, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J. Physiol. 2003;550:785–800. doi: 10.1113/jphysiol.2003.042572. doi:10.1113/jphysiol.2003.042572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.P, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol. Cell. Neurosci. 2005;29:181–189. doi: 10.1016/j.mcn.2005.02.002. doi:10.1016/j.mcn.2005.02.002 [DOI] [PubMed] [Google Scholar]
- Weissman T.A, Riquelme P.A, Ivic L, Flint A.C, Kriegstein A.R. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. doi:10.1016/j.neuron.2004.08.015 [DOI] [PubMed] [Google Scholar]
- Ying Q.L, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. doi:10.1016/S0092-8674(03)00847-X [DOI] [PubMed] [Google Scholar]
- Zahs K.R. Heterotypic coupling between glial cells of the mammalian central nervous system. Glia. 1998;24:85–96. doi:10.1002/(SICI)1098-1136(199809)24:1<85::AID-GLIA9>3.0.CO;2-# [PubMed] [Google Scholar]
- Zheng W, Nowakowski R.S, Vaccarino F.M. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev. Neurosci. 2004;26:181–196. doi: 10.1159/000082136. doi:10.1159/000082136 [DOI] [PubMed] [Google Scholar]