Abstract
Invariant natural killer T (iNKT) cells constitute a subpopulation of T cells that recognize glycolipids presented by CD1d molecules. They are characterized by their prompt production of interleukin-4 (IL-4) and interferon-γ (IFN-γ), which enables them to modulate diverse immune responses. Recently, we enlarged this concept by identifying a distinct IL-17-producing iNKT cell subset, named iNKT17 cells. The mechanisms leading to the acquisition of this new iNKT cell activity are unknown. Herein we show that IL-17-producing iNKT cells are already present in the thymus, predominantly among a subset regarded so far as an immature stage of thymic iNKT cell development, the CD1d tetramerposCD44posNK1.1negCD4neg cells. Using EGFP reporter mice, we demonstrate that the transcription factor ROR-γt is critical for the thymic differentiation of this subset because only ROR-γtpos iNKT cells are capable of massively secreting IL-17. Moreover, IL-17-producing CD1d tetramerposCD44posNK1.1negCD4neg thymic iNKT cells have reached a mature differentiation stage because they fail to generate other cell subsets in fetal thymic organ culture. Conversely, thymic ROR-γtneg iNKT cell precursors give rise to progeny, but acquire neither ROR-γt expression nor the ability to secrete IL-17. In conclusion, our findings demonstrate an alternative thymic pathway leading to the development of iNKT17 cells that requires ROR-γt expression.
Keywords: thymic precursors, α-GalCer, CD1d, cytokines
Naïve CD4pos T lymphocytes proliferate and differentiate into 2 distinct effector subsets, T helper type 1 (Th1) and Th2 cells, characterized by their distinct cytokine profile. Th1 cells produce large amounts of interferon-γ (IFN-γ), whereas Th2 cells are a source of interleukin-4 (IL-4), IL-5, and IL-13 (1, 2). More than 2 years ago, a new effector population named Th17 was identified by its capacity to produce IL-17A (or IL-17), IL-17F, and IL-21 (3, 4). Cytokines, such as IL-6 and TGF-β, act in synergy to induce the differentiation of Th17 cells in mice, whereas IL-21 and IL-23 contribute to their amplification and stabilization (3–7).
IL-17 is the founding member of this newly identified cytokine family composed of 6 proteins: IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (IL-25), and IL-17F (5). It is characterized by its potent proinflammatory functions mediated by pleiotropic activities, namely the ability to induce proinflammatory cytokines (IL-6 and TNF-α) and chemokines (KC, MCP-1, and MCP-2), as well as proliferation, maturation, and chemotaxis of neutrophils that will ultimately mediate tissue infiltration, pathogen engulfment and destruction. Th17 cells recognize peptide antigens (Ag), like their Th1 and Th2 counterparts, and undergo a process of differentiation before acquiring their particular function (6, 7). By contrast, the invariant natural killer T (iNKT) cells, which respond specifically to glycolipids, can produce IL-17 without prior polarization (8).
iNKT cells constitute a unique population of innate-like T lymphocytes that coexpress a highly restricted T cell receptor (TCR) repertoire composed of a single invariant Vα14Jα18 chain in mice and a Vα24Jα18 chain in humans, preferentially paired with a limited number of TCR Vβ chains (9, 10). This semi-invariant TCR reflects a positive selection by glycolipid antigens presented by the nonpolymorphic MHC class I-like molecule, CD1d (11, 12). iNKT cells are well known for their involvement in various infectious, autoimmune, and allergic diseases as well as antitumor responses because of their prompt production of cytokines, such as IL-4, IFN-γ, TNF-α, IL-3, and GM-CSF (9, 10, 13–17). We have identified more recently a functionally distinct peripheral iNKT cell subset that constitutes an important source of IL-17 and is implicated in airway neutrophilia (8). These cells differ from their classical counterpart by the lack of NK1.1 expression.
It has been established that iNKT cells acquire their ability to produce IL-4 during the developmental stages that take place in the thymus whereas they become capable of generating IFN-γ mainly after thymic emigration once they display the NK1.1 marker (18). This finding raises the question whether the iNKT17 cells that we identified as NK1.1neg (8), turn into IL-17-producing cells during thymic or post-thymic development. To address this issue, we assessed the functional capacities of iNKT cells sorted from the thymus at different phenotypically defined stages of differentiation. We show that IL-17 is massively produced by the CD44highNK1.1negCD4neg subset, which indicates that this faculty is encoded in the developmental program of thymic iNKT cell differentiation. Using an EGFP reporter mouse, we found that the ability to produce large amounts of IL-17 is conferred by the expression of ROR-γt (retinoic acid receptor-related orphan receptor γt) in thymic CD44highNK1.1negCD4neg iNKT cells. Hence, our data provide evidence for an alternative iNKT17 cell differentiation pathway in the thymus, in which ROR-γt expression plays an important role.
Results and Discussion
α-GalCer Stimulation Induces Thymic IL-17 Production.
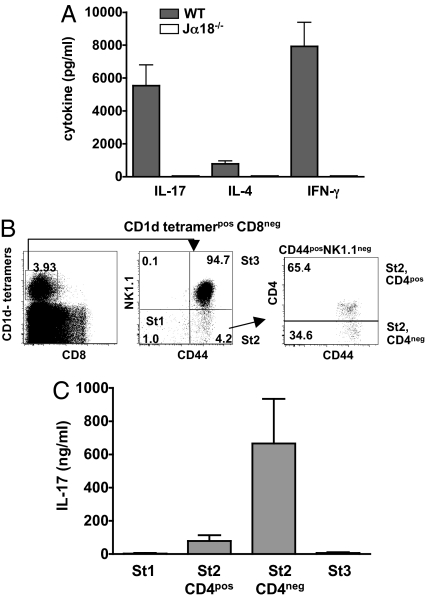
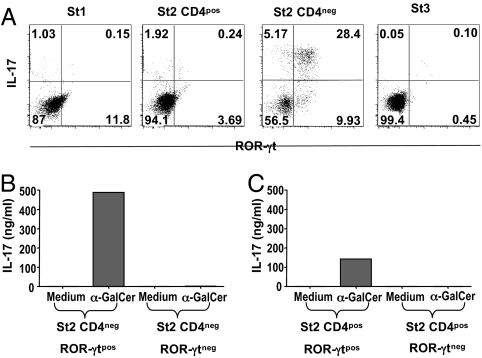
We recently characterized a distinct subset of iNKT cells that produce high levels of IL-17 and are therefore termed iNKT17 cells (8). To address the question of whether this population is already present in the thymus, we stimulated iNKT cell-enriched thymic CD8neg cells with α-GalCer and found that they did effectively produce substantial amounts of IL-17 in addition to IL-4 and IFN-γ (Fig. 1A).
Fig. 1.
Thymic iNKT cells produce IL-17. (A) iNKT cell-enriched (CD8neg) thymocytes recovered from C57BL/6 (WT) or Jα18−/− mice were stimulated for 72 h at a concentration of 106 cells /ml with α-GalCer in the presence of irradiated macrophages from Jα18−/− mice. IL-17, IL-4 and IFN-γ were measured in the supernatants. (B and C) CD1d tetramerposCD8neg thymocytes were sorted into 4 distinct subsets or stages (St), namely CD44negNK1.1neg (St 1), CD44posNK1.1negCD4pos (St 2, CD4pos), CD44posNK1.1negCD4neg (St 2, CD4neg), and CD44posNK1.1pos (St 3) as illustrated in the FACS profiles (B). These sorted cells were further stimulated with α-GalCer in the presence of irradiated macrophages from Jα18−/− mice as APCs. IL-17 was measured in the supernatants 3 days later. No cytokines were detected in the absence of α-GalCer or APCs and stimulated APCs were also negative (data not shown). Data represent the mean ± SD of 4 individual experiments.
CD44posNK1.1negCD4neg Thymic iNKT Cells Are the Major Source of IL-17.
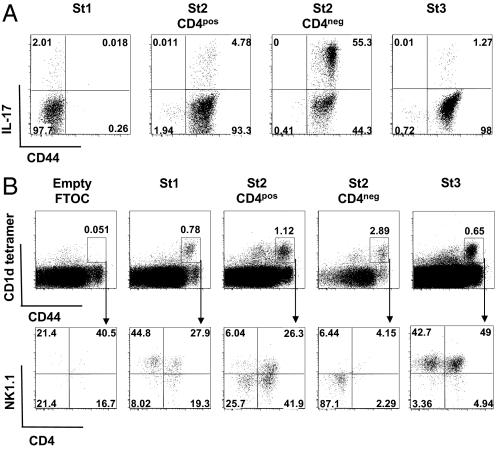
The understanding of thymic iNKT cell development has considerably progressed in recent years. It is generally acknowledged that precursors of this lineage diverge from mainstream thymocyte development at the CD4posCD8pos double-positive (DP) stage to undergo a series of expansion and differentiation steps giving rise to peripheral IL-4- and IFN-γ-producing iNKT cells (18). During their progression to the mature CD24low phenotype, 3 intermediate differentiation stages have been distinguished ranging from CD44lowNK1.1neg to CD44highNK1.1neg, and CD44highNK1.1pos cells (18–20). Based on this definition, we sorted thymic CD8neg CD1d-tetramerpos iNKT cells into 4 subsets, namely CD44lowNK1.1neg (stage 1), CD44highNK1.1negCD4pos (stage 2, CD4pos), CD44highNK1.1negCD4neg (stage 2, CD4neg), and CD44highNK1.1pos (stage 3) (Fig. 1B) to assess their functional characteristics. IL-17 production was very low in stages 1 and 3 whereas most of the activity was concentrated in the CD44posNK1.1negCD4neg fraction (stage 2, CD4neg) (Fig. 1C). We used intracellular cytokine staining to assess IL-17 on the single cell level and confirmed that producer cells are actually predominant in the CD44posNK1.1negCD4neg iNKT subset because >50% of cells was positive (Fig. 2A).
Fig. 2.
Characterization of IL-17-producing thymic iNKT cells. (A) Intracellular IL-17 staining was performed after in vitro stimulation of enriched thymic iNKT cells with PMA+ionomycin for 4 h in the presence of brefeldin A. Representative FACS profile of CD44 and IL-17 expression by gated CD1d tetramerposCD8neg St1 (CD44negNK1.1neg), St2, CD4pos (CD44posNK1.1negCD4pos), St2, CD4neg (CD44posNK1.1negCD4neg), or St3 (CD44posNK1.1pos). (B) Thymic lobes were collected from Jα18−/− fetuses at day 17 of gestation. Thymic iNKT cells corresponding to the 4 differentiation stages were electronically sorted and added to the thymic lobes until day 14 when they were recovered. Representative FACS profile of CD4 and NK1.1 expression (Lower) among gated CD1d tetramerposCD44pos cells (Upper). Data are representative of 3 independent experiments. Percentages of each subset are indicated in quadrants.
IL-17-Producing CD44posNK1.1negCD4neg iNKT Thymocytes Represent a Mature Stage of iNKT Cell Development.
Little is known about CD44posNK1.1negCD4neg iNKT cells and their functions. It has been reported that they derive from HSAlowNK1.1negCD4pos iNKT cell precursors, similarly to their CD4pos counterpart (21–23), but it has not been established whether they can give rise to progeny. To address this issue, we seeded fetal thymic organ cultures (FTOC) from iNKT cell-deficient Jα18−/− mice with the 4 thymic iNKT cell subsets represented in Fig. 1B and analyzed their differentiation potentials under these conditions. Our results are consistent with the conclusion that the CD44posNK1.1negCD4neg (stage 2, CD4neg) iNKT cells represent a mature differentiation stage because they failed to develop in FTOC, similarly to mature CD44posNK1.1pos (stage 3) iNKT cells, whereas CD44negNK1.1neg (stage 1) and CD44posNK1.1negCD4pos (stage 2, CD4pos) precursors gave rise to mature NK1.1pos progeny (Fig. 2B).
IL-17-Producing iNKT Cells Express ROR-γt.
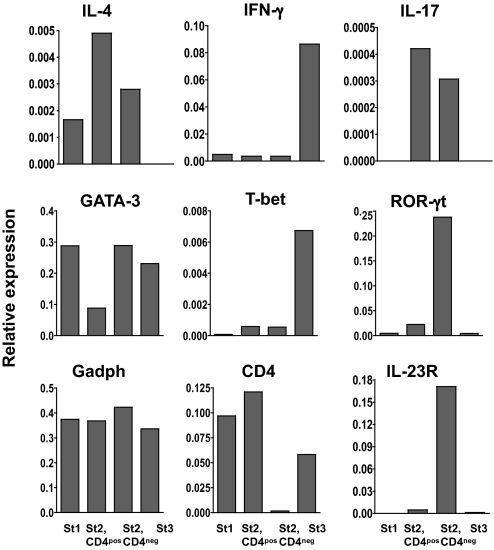
We performed qPCR array profiling to determine the expression of genes typically associated with iNKT cell differentiation and to identify the cytokine profile generated by the 4 thymic iNKT cell subsets described in Fig. 1B. These subsets were sorted and immediately analyzed without further manipulation (Fig. 3). IL-4 and GATA-3 mRNAs were expressed at different levels whereas both IFN-γ and T-bet transcripts were highly up-regulated in mature NK1.1pos cells (stage 3). IL-17 mRNAs were detected only at the stage 2 whether these cells were CD4neg or CD4pos whereas IL-23R mRNA expression was basically observed only among stage2 CD4neg cells. Analysis of mRNA encoding ROR-γt, which is required for the differentiation of conventional Th17 cells (24), revealed high expression in CD44posNK1.1negCD4neg iNKT cells (stage 2, CD4neg), but also among stage 2, CD4pos, prompting us to verify whether ROR-γt and IL-17 production was also associated in thymic iNKT cells.
Fig. 3.
ROR-γt mRNA expression in thymic iNKT cell subsets. Histograms represent the relative mRNA expression in the 4 distinct subsets from thymic CD1d tetramerposCD8neg cells, namely CD44negNK1.1neg (St1), CD44posNK1.1negCD4pos (St2, CD4pos), CD44posNK1.1negCD4neg (St2, CD4neg), and CD44posNK1.1pos (St3), processed immediately for RNA extraction after sorting. The mRNA expression profile was assessed by a PCR array (SuperArray Bioscience Corporation) and data represent relative values established by comparison with 4 housekeeping genes: Hprt, Gusb, Hsp90, and Gadph (only the latter are represented in the figure).
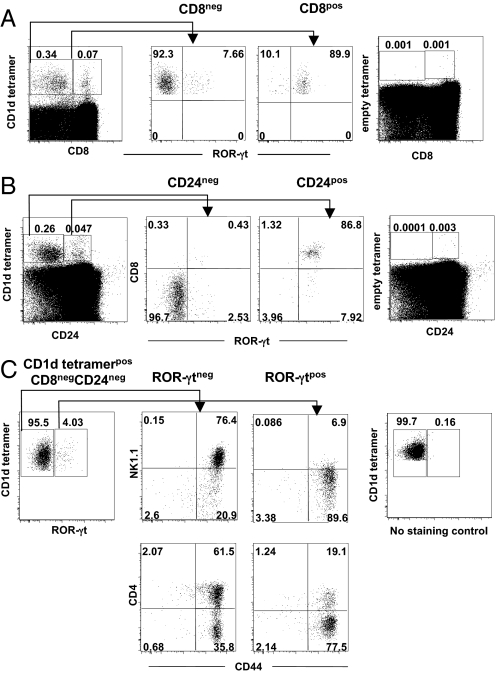
It has been reported before that most thymic double-positive precursors express ROR-γt before engaging into the differentiation process during which receptor expression is gradually lost (21, 22). We confirmed this notion in experiments performed with Rorc(γt)-GfpTG mice because the majority of immature CD8posCD1d tetramerpos or CD24posCD1d tetramerpos iNKT cells expressed ROR-γt (up to 90%) in contrast with their more mature CD8negCD1d tetramerpos (Fig. 4A) or CD24negCD1d tetramerpos (Fig. 4B) iNKT cell counterparts. Nevertheless, a small fraction of CD8negCD1d tetramerpos iNKT cells conserved their ROR-γt expression, and most of them were CD44posNK1.1negCD4neg (Fig. 4C), similar to IL-17-producing thymic iNKT cells (Fig. 2 A and B). It can therefore be concluded that nearly all ROR-γtpos iNKT cells and IL-17-producing iNKT cells share the same phenotype. It is noteworthy that stage 1 CD44negNK1.1neg cells were present among gated ROR-γtpos CD8neg iNKT cells, suggesting that a fraction of iNKT cell precursors retains ROR-γt expression to give rive rise ultimately to more mature ROR-γtpos iNKT cells.
Fig. 4.
Characterization of thymic iNKT ROR-γtpos cells. (A and B) Expression of ROR-γt among gated CD1d tetramerpos CD8pos or CD8neg (A Left) or CD1d tetramerpos CD24pos or CD24neg (B Left) iNKT cells using Rorc(γt)-GfpTG mice. (A Right and B Right) Negative controls with empty tetramers. (C Left) CD4, CD44, and NK1.1 expression was analyzed among gated CD1d tetramerpos CD8neg CD24neg ROR-γtpos or ROR-γtneg iNKT cells. (C Right) Negative control with FITC channel in wild-type mice. Data are representative of 3 independent experiments. Percentages of each subset are indicated in quadrants.
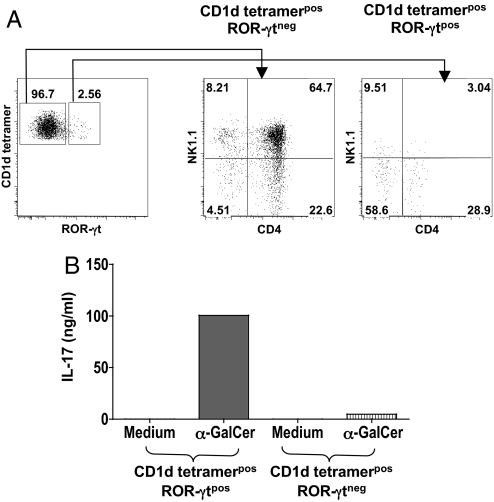
It remained to be determined whether ROR-γt expression and IL-17 production were effectively associated in iNKT cells. Intracellular staining of sorted populations showed that this was actually the case because nearly all IL-17-producing iNKT cells were both ROR-γtpos and CD44posNK1.1negCD4neg (Fig. 5A). Furthermore, sorted CD44posNK1.1negCD4neg ROR-γtpos iNKT cells produced high levels of IL-17 upon stimulation whereas their ROR-γtneg counterpart did not (Fig. 5B). CD44posNK1.1negCD4pos ROR-γtpos iNKT can also produce some IL-17, even though they are less efficient than the corresponding CD4neg subset (Fig. 5C).
Fig. 5.
The thymic iNKT CD44posNK1.1negCD4neg ROR-γtpos subset is the major source of IL-17. (A) Intracellular IL-17 staining was performed after in vitro stimulation of the 4 sorted thymic iNKT cell populations, namely CD44negNK1.1neg (St1), CD44posNK1.1negCD4pos (St2, CD4pos), CD44posNK1.1negCD4neg (St2, CD4neg), and CD44posNK1.1pos (St3) cells, with PMA+ionomycin during 4 h in the presence of brefeldin A. Numbers in quadrants indicate percentages of each subset. (B) Thymic iNKT St2, CD4pos and St2, CD4neg were sorted into ROR-γtpos or ROR-γtneg fractions and further stimulated with α-GalCer in the presence of irradiated macrophages from Jα18−/− mice as APCs. IL-17 was measured in the supernatants 3 days later. Data are representative of 3 independent experiments.
We have reported that iNKT17 cells are present in the periphery, which raises the question of whether they conserve their ROR-γt expression. We found that 2.5% iNKT cells in the spleen were ROR-γtpos (Fig. 6A) and among these, 91% lacked the NK1.1 marker. This observation is in keeping with our previous data (8), recently confirmed by other groups (25, 26), that iNKT17 cells are indeed NK1.1neg. Similar to their thymic counterpart, sorted splenic ROR-γtpos but not ROR-γtneg iNKT cells produce high levels of IL-17 after α-GalCer stimulation (Fig. 6B), designating ROR-γt as a useful marker to identify peripheral IL-17-producing iNKT cell subsets.
Fig. 6.
Analysis of ROR-γt expression and IL-17 production by iNKT splenocytes. (A) CD1d tetramerpos splenic iNKT cells were gated from fluorescent cells recovered from Rorc(γt)-GfpTG mice. The expression of CD4 and NK1.1 was analyzed among gated CD1d tetramerpos ROR-γtneg or ROR-γtpos subsets. (B) CD1d tetramerpos iNKT splenocytes were sorted into ROR-γtpos or ROR-γtneg fractions and further stimulated with α-GalCer in the presence of irradiated macrophages from Jα18−/− mice as APCs. IL-17 was measured in the supernatants 3 days later. Data are representative of 2 independent experiments.
IL-17-Producing ROR-γtpos iNKT Cells Evolve from an Alternative Pathway of Development of Thymic iNKT Cells.
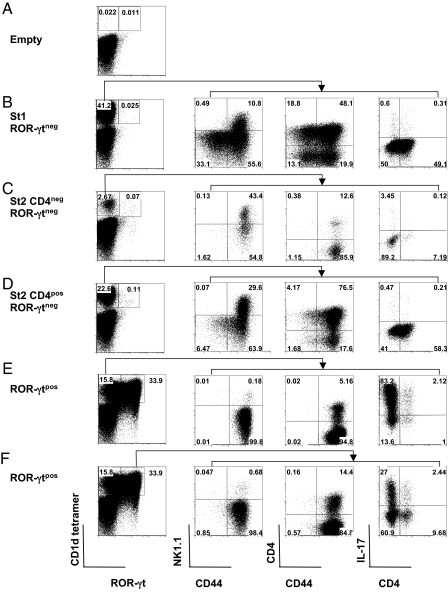
We addressed the question whether thymic ROR-γtneg iNKT cells could reacquire the expression of ROR-γt lost after the double-positive differentiation stage or inversely, whether ROR-γtpos iNKT cells could eventually become negative. Because of the low number of thymic ROR-γtpos iNKT cells, we developed a culture system that, similar to FTOC, could sustain iNKT cell differentiation. We took advantage of the fact that IL-7 induces preferential iNKT cell expansion (27) to set up cocultures between total thymocytes from Jα18−/− mice, used as feeder cells, and CD44negNK1.1neg (stage 1), CD44posNK1.1negCD4neg (stage 2, CD4neg), or CD44posNK1.1negCD4pos (stage 2, CD4pos) cells purified from sorted thymic CD1d tetramerpos ROR-γtneg iNKT cells. This system performed as well as FTOC, because differentiation from both stage 1 (Fig. 7B) and 2 (Fig. 7 C and D) was readily obtained. Even though ROR-γtneg iNKT populations generated more mature progeny, they failed to acquire ROR-γt and to produce IL-17 (Fig. 7 B–D). It can therefore be assumed that the developmental signals requisite for the maturation of these cells are inadequate in terms of ROR-γt expression or IL-17 production. By contrast, sorted ROR-γtpos iNKT cells maintain their massive IL-17 production (Fig. 7 E and F), despite the down-regulation that occurs in some cells after culture and stimulation (Fig. 7F). These data suggest that once the cells have been instructed to produce IL-17 through a ROR-γt-dependent process that is probably initiated in vivo after the double-positive (CD4posCD8pos) or CD24pos stage, a continuous high expression of ROR-γt is no longer necessary to produce IL-17, at least in these IL-7-dependent in vitro conditions.
Fig. 7.
ROR-γt is necessary for IL-17 production by iNKT cells. Total thymocytes from Jα18−/− mice were cultured in the presence of IL-7 alone (A) or together with thymic CD1d tetramerposCD8neg iNKT cells from Rorc(γt)-GfpTG mice sorted into St1 (CD44negNK1.1neg) ROR-γtneg (B), St2 (CD44posNK1.1neg) CD4neg ROR-γtneg (C), St2 (CD44posNK1.1neg) CD4pos ROR-γtneg (D), or ROR-γtpos subsets (E and F). Percentages of CD1d tetramerpos ROR-γtpos and ROR-γtneg cells generated within 4 days are indicated in the first column. (B–F) The expression of NK1.1, CD4, and CD44 was analyzed among gated CD1d tetramerpos ROR-γtneg (B–D and F) or ROR-γtpos populations (E). A fraction of the cells recovered after culture was further stimulated with PMA+ionomycin for 4 h in the presence of brefeldin A. (B–F) The percentage of IL-17pos cells was then analyzed among gated CD1d tetramerpos ROR-γtneg (B–D) or ROR-γtpos (E) cells as shown in the last column. Numbers in quadrants indicate the respective percentages. Data are representative of 2 independent experiments.
Taken together, our findings show that ROR-γt is essential for the alternative developmental program that generates highly effective iNKT17 cells. Our findings are consistent with the notion that some iNKT cells are predetermined to become IL-17 producers, although the intra and/or extracellular signals that induce or sustain ROR-γt expression during the maturation process remain unknown. In this context, we assessed whether the generation of IL-17-producing iNKT cells was affected by the absence of factors shown to be required for conventional Th17 cell development, such as IL-6. Using IL-6−/−Rorc(γt)-GfpTG mice we observed no modification in IL-17 production by thymic iNKT ROR-γtpos cells (data not shown), proving that IL-6 is not required for the generation of iNKT17 cells.
It is possible that some specific endogenous antigens direct iNKT cell precursor cells preferentially toward iNKT17 cell differentiation during their positive selection at the double-positive CD4posCD8pos stage. However, until now, we were unable to detect any significant difference in terms of Vβ repertoire, whether or not iNKT cells produced IL-17 (8). Another possibility would be that some unspecified costimulatory molecules might intervene at some important branching point, to set off the program that favors iNKT17 cell development, eventually by maintaining the expression of ROR-γt. Hence, our data suggest that ROR-γt plays an important role in the program that determines iNKT17 differentiation in the thymus, in addition to its implication in the development of peripheral Th17 cells.
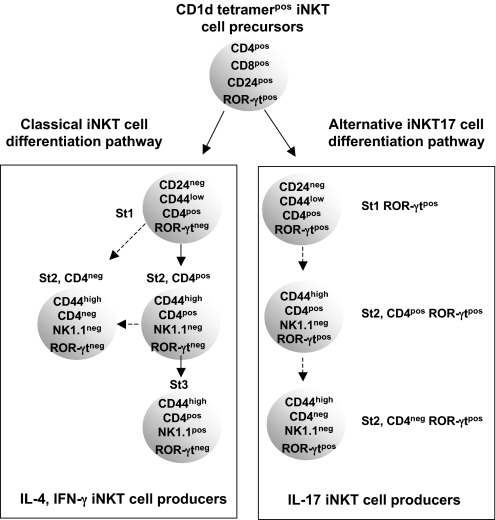
The specific physiologic or pathologic functions of iNKT17 cells remains to be elucidated even though they have already been implicated in certain models of lung inflammation (8, 28). Whatever the answer to these issues, our present study has uncovered an alternative thymic pathway from which IL-17-producing iNKT cells with the mature CD44posNK1.1negCD4negROR-γtpos phenotype will emerge (Fig. 8).
Fig. 8.
Proposed model for IL-17-producing iNKT cell differentiation. The majority of immature CD4posCD8posCD24posROR-γtpos iNKT cell precursors will lose ROR-γt expression when they differentiate into IL-4 and IFN-γ producers (classical pathway) whereas the minor fraction that remains ROR-γtpos will give rise to IL-17-producing iNKT cells (alternative pathway).
Materials and Methods
Animals.
Jα18−/− (29), Rorc(γt)-GfpTG (30), IL-6−/−Rorc(γt)-GfpTG (30), and C57BL/6 male mice from 4–8-weeks-of-age were used. Animal experiments were performed according to the French Institutional Committee.
iNKT Cell Enrichement and Sorting.
Enriched iNKT cells were obtained by depleting the thymus of CD8 cells and splenocytes of CD8, CD19, and CD62L cells labeled with the corresponding mAb (BD PharMingen) and with anti-rat Ig-coated magnetic beads (Dynabeads and Invitrogen), according to the manufacturers' protocols. Cells were then stained and iNKT cell subsets were sorted by using a FACSAria cell sorter (Becton Dickinson).
Immunofluorescence.
After iNKT cell enrichment, cells were incubated with CD1d-tetramer-APC (provided by NHI tetramer facilities), anti-NK1.1-PerCP-Cy5.5, anti-CD4-Pacific Blue, anti-CD8-PE or FITC (when cells from Rorc(γt)-GfpTG mice were not used), anti-CD24-PE, and anti-CD44-FITC or -PECy7 (BD PharMingen). For intracellular staining, cells were fixed in 4% PFA, washed, and permeabilized with 0.5% saponin (Sigma-Aldrich), then incubated with anti-IL-17-PE or isotype controls (Biosciences). In some experiments, anti-GFP Ab and anti-rabbit IgG-Alexa Fluor 488 were used. The cells were washed and analyzed in a FACSCanto II (Becton Dickinson) by using FlowJo software.
Cell Culture.
Thymic CD8neg or sorted iNKT cells were cultured at a final concentration of 5 × 106 or 5 × 105 cells per ml, respectively, with or without peritoneal macrophages from Jα18−/− mice as APCs, at a ratio of 1:2, and stimulated with 100 ng/ml α-GalCer (Alexis). All culture supernatants were harvested and stored at −80°C until cytokine detection by ELISA, as described (8). In some experiments, thymic iNKT cells were stimulated for 4 h with 10−8 M phorbol 12-myristate 13-acetate (PMA) (Sigma–Aldrich), 5 × 10−6 M ionomycin, and 10 μg/ml brefeldin A. Cells were then stained for intracellular cytokine detection.
Expansion of iNKT Cell Precursors.
In some experiments, fetal thymus organ cultures (FTOCs) were carried out. In brief, thymic lobes were collected at day 17 of gestation from Jα18−/− mice. Hanging drops were prepared in Terasaki plates by adding to each well 30,000 sorted cells per thymic lobe. The plates were immediately inverted to form hanging drops and incubated for 48 h in a humidified incubator (5% CO2, 37°C). After incubation, the lobes were removed from the hanging drops, washed, and placed on a nuclepore filter (Nuclepore, Costar) resting on a grid in 2 ml of complete media, for 14 days. In another set of experiments, 2 × 104 sorted iNKT cells per ml were cocultured with total thymocytes from Jα18−/− mice in 24-well culture plates (20 × 106 cells per ml) and stimulated with IL-7 (20 ng/ml) The plates were incubated for 4 days. Dead cells were removed by FicollPaque centrifugation.
Gene Expression Analysis.
Total RNA for gene expression analysis by real time RT-PCR was extracted from 500–5,000 cells electronically sorted into vials, by using an RNeasy Micro Kit (Qiagen). High quality RNA (250–500 pg) was subjected to 1 linear mRNA amplification cycle by using the MessageBooster Kit for qRT-PCR (Epicentre Biotechnologies). Amplified mRNA (50–100 ng) was then converted to cDNA by using SuperScript III (Invitrogen) according to the manufacturer's protocol. The expression of 84 different genes was measured by using the Th17 RT2 Profiler PCR Array for mouse (SuperArray Bioscience Corporation) and confirmed for individual genes by using specific primer pairs (SuperArray Bioscience Corporation). Real-time PCR was performed in a PTC-200 thermocycler equipped with a Chromo4 detector (Bio-Rad Laboratories). Data were analyzed by using Opticon Monitor software (Bio-Rad Laboratories).
Acknowledgments.
We thank Sylvain Latour, Agnès Lehuen, and Kamel Benlagha for discussion, Rachel Bricard for technical assistance, and Jérôme Mégret and Corinne Garcia-Cordier for cell sorting. We also thank the National Institutes of Health Tetramer facilities for providing CD1d/PBS57 tetramers. This work was supported by the Centre National de la Recherche Scientifique, Université René Descartes - Paris V, and the Agence Nationale de Recherches Microbiologie-Maladie Emergentes ASTHMA-IL-17 (M.C.L.-d.-M.). A.C.K. received a postdoctoral fellowship from the Fondation pour la Recherche Médicale and Conselho Nacional de Desenvolvimento Cientifico e Tecnologico.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 Cells: Different Patterns of Lymphokine Secretion Lead to Different Functional Properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 3.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: An effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang S-K. IL-17 cytokine family. J Allergy Clin Immunol. 2004;114:1265–1273. doi: 10.1016/j.jaci.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 7.McGeachy MJ, Cua DJ. Th17 cell differentiation: The long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Michel M-L, et al. Identification of an IL-17-producing NK1.1neg iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronenberg M. Toward an understanding of NKT cell biology: Progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 10.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 11.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 13.Sharif S, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7:1057–1062. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 14.Leite-de-Moraes MC, et al. Ligand-activated natural killer T lymphocytes promptly produce IL-3 and GM-CSF in vivo: Relevance to peripheral myeloid recruitment. Eur J Immunol. 2002;32:1897–1904. doi: 10.1002/1521-4141(200207)32:7<1897::AID-IMMU1897>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 15.Lisbonne M, et al. Cutting edge: Invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003;171:1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 16.Akbari O, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 18.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 19.Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1(-)CD4(+) CD1d-dependent precursor stage. J Exp Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 21.Egawa T, et al. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Bezbradica JS, Hill T, Stanic AK, Van Kaer L, Joyce S. Commitment toward the natural T (iNKT) cell lineage occurs at the CD4+8+ stage of thymic ontogeny. Proc Natl Acad Sci USA. 2005;102:5114–5119. doi: 10.1073/pnas.0408449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J Exp Med. 2005;202:485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 25.Rachitskaya AV, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coquet JM, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci USA. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vicari A, et al. Interleukin 7 induces preferential expansion of V beta 8.2+CD4–8- and V beta 8.2+CD4+8- murine thymocytes positively selected by class I molecules. J Exp Med. 1994;180:653–661. doi: 10.1084/jem.180.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pichavant M, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205:385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui J, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 30.Lochner M, et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]