Abstract
We have developed a simple method for isolating and purifying plasma membrane proteins from various cell types. This one-step affinity-chromatography method uses the property of the lectin concanavalin A (ConA) and the technique of magnetic-bead separation to obtain highly purified plasma membrane proteins from crude membrane preparations or cell lines. ConA is immobilized onto magnetic beads by binding biotinylated ConA to streptavidin magnetic beads. When these ConA magnetic beads were used to enrich plasma membranes from a crude membrane preparation, this procedure resulted in 3.7-fold enrichment of plasma membrane marker 5′-nucleotidase activity with 70% recovery of the activity in the crude membrane fraction of rat liver. In agreement with the results of 5′-nucleotidase activity, immunoblotting with antibodies specific for a rat liver plasma membrane protein, CEACAM1, indicated that CEACAM1 was enriched about threefold relative to that of the original membranes. In similar experiments, this method produced 13-fold enrichment of 5′-nucleotidase activity with 45% recovery of the activity from a total cell lysate of PC-3 cells and 7.1-fold enrichment of 5′-nucleotidase activity with 33% recovery of the activity from a total cell lysate of HeLa cells. These results suggest that this one-step purification method can be used to isolate total plasma membrane proteins from tissue or cells for the identification of membrane biomarkers.
Isolation of plasma membranes from cells or tissues is the first step in the characterization and purification of plasma membrane proteins. Current methods for plasma membrane purification depend on density gradient centrifugation to separate plasma membranes from other organelles in cell homogenates. Density gradient centrifugation used in isolating plasma membranes uses differences in sedimentation velocities to separate particles of different densities in lysed cell solutions [1]. This procedure is time-consuming because multiple steps of centrifugation are needed to obtain a crude plasma membrane preparation. It is also inaccurate because of the inconsistent nature of cell lysis and centrifugation settings. Often, much of the plasma membrane is lost in the early steps of centrifugation, and some organelles may remain in the plasma membrane fraction. As a result, these methods not only are lengthy but also yield only a small percentage of the plasma membranes [1-4]. The low recovery also presents difficulty in obtaining sufficient plasma membranes for the purification of membrane proteins from limited sources, such as primary cells isolated from organs.
Several methods have been developed to improve purification of plasma membranes. One method uses polylysine-coated acrylamide beads to bind plasma membranes from HeLa cell lysates [5]. This method requires preparation of the polylysine-coated beads. Use of magnetic beads with immobilized monoclonal antibodies against specific membrane proteins for isolation of highly pure plasma membrane has also been reported [6]. However, this method requires a large amount of purified monoclonal antibodies and can be applied only to the cells that express the specific membrane proteins.
Most plasma membrane proteins carry sugar residues on the protein segments that are exposed on the cell surface. The total amount of carbohydrates in the plasma membrane varies between 2% and 10% of the membrane's total weight. In the plasma membrane, the sugar residues are exposed on the outside of the cells, whereas in internal membranes, they face inward, toward the lumen of the membrane-bounded compartment.
The lectins are a group of carbohydrate-binding proteins that have different sugar-binding specificities: they bind a specific sequence of sugar moieties and can be used to affinity purify plasma membrane proteins that contain those specific sugar moieties from cell lysates. The most commonly used lectin for binding glycosylated membrane proteins is concanavalin A (ConA), which binds the α-D-glucose and α-D-mannose present in high-mannose glycopeptides [7]. It is likely that lectin-affinity chromatography can be used to isolate membranes from other organelles.
Magnetic beads, mentioned above, have been developed for various applications in biology. For example, chemically derived magnetic beads can be coupled with various proteins, and they have become a new form of affinity matrix. In contrast to the conventional matrices (i.e., agarose or acrylamide), magnetic beads can be conveniently separated from the mixture by using a magnet. The conventional agarose- or acrylamide-affinity matrices cannot be used to isolate membranes because they sediment with the organelles (e.g., nuclei) that have relatively high densities. The use of magnetic beads can overcome this problem because they are drawn toward the magnet and thus can be used to separate organelles independent of their densities. By simply holding the tubes near the magnet, the magnetic beads can be recovered at the sides of the tubes, allowing easy recovery of the beads from the mixture. Thus, magnetic beads can be used as a substitute for centrifugation. This property is likely to have great advantages in separating organelles.
Using the property of ConA and the technique of magnetic bead separation, we have developed a new method for plasma membrane isolation and purification. This procedure is simpler than the traditional approach, does not require expensive centrifugation equipment, and provides good yields of highly purified plasma membrane proteins from crude membrane preparations or from cell lysates. Thus, this one-step method will expedite the identification of plasma membrane proteins from tissue or cells for use in identifying membrane biomarkers.
Materials and methods
Materials
Biotinylated ConA was purchased from Vector Laboratories (Burlingame, CA). Streptavidin magnetic beads were from BioClone, Inc. (San Diego, CA). CHAPS was from Pierce (Rockford, IL). Frozen rat livers were purchased from Pel Freeze (Rogers, AR). PC-3 human prostate cancer and HeLa cervical cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA).
Preparation of ConA magnetic beads
ConA was immobilized onto the streptavidin magnetic beads by binding biotinylated ConA to them. The beads were washed according to the procedures provided by the manufacturer. In brief, 1 mL of streptavidin magnetic beads in suspension (contains 0.05 mL settled beads) was placed into a 1.5-mL Eppendorf test tube. The magnetic beads were separated from the solution by holding the tube near a magnet (Dynal MPC-L, Invitrogen), and the solution was discarded by pipetting. The beads were then washed twice with 1 mL of water followed by 1 mL of Tris-buffered saline (TBS) containing 150 mM NaCl and 50 mM Tris HCl, pH 7.4.
To immobilize ConA onto the streptavidin-coupled magnetic beads, the beads were resuspended in 210 μL of TBS, and 70 μL of biotinylated ConA (2.5 mg/mL) was added. This suspension was mixed on a rocker for 1 h to allow binding of biotinylated ConA to streptavidin. The beads were then washed sequentially with 1 mL of TBS, 1 mL of TBS with 1% Triton-X 100, and three times with 1 mL of TBS. They were then resuspended in 450 μL of TBS and stored at 4 °C until use.
Preparation of crude plasma membranes from rat liver
Rat liver plasma membranes were prepared by using a modification of Neville's procedure [8], as described by Marshak et al. [9]. In brief, two rat livers (∼20 g) were minced and homogenized in 500 mL of ice-cold solution containing 1 mM CaCl2, protease inhibitors (Roche, Indianapolis, IN), and 50 mM HEPES, pH 7.4. After being filtered through two layers of cheesecloth, the homogenates were spun in a centrifuge at 1500 g for 10 min. The supernatant was removed and the loose pellets collected. The pellets were resuspended by homogenizing them in buffer (50 mM HEPES, pH 7.4) in a Dounce homogenizer, and the solution was brought to 44% (w/w) by adding 1.1 volumes of 69% (w/w) sucrose to the homogenate. The homogenates in 44% sucrose were transferred to SW28 polyallomer tubes (Beckman Coulter) and overlaid carefully with 42.3% sucrose until the material came to 4 mm below the rim of the tube. The samples were spun at 25,000 rpm at 4 °C for 2 h in a Beckman ultracentrifuge. Then, the layer of membranes that appeared on the top of the tube was collected.
Enrichment of plasma membrane from crude rat liver membrane preparation
A volume of 500 μL of crude rat liver plasma membranes, prepared as described above, was added to the prepared ConA magnetic beads, and the membranes were allowed to bind with ConA magnetic beads on the rocker for 1 h. After binding, the magnetic beads were separated from the solution by holding the tube near a magnet; the unbound membranes (i.e., the flow-through fraction) were removed. The beads were washed five times with 1 mL of TBS, and the bound proteins were eluted from the ConA magnetic beads by incubating with 500 μL of 0.25 M methyl α-D-mannoside in TBS with 0.5% CHAPS for 10 min. The elution step was repeated twice to increase recovery.
Purification of plasma membrane from rat liver lysate
One rat liver was minced and homogenized as described above. The homogenate (50 mL) was spun in a centrifuge at 1500 g for 10 min. Then the pellet was resuspended in 1 mL of 50 mM HEPES, pH 7.4, by homogenization with a Dounce homogenizer. This resuspension was incubated with ConA magnetic beads to purify the plasma membranes as described above.
Purification of plasma membrane from PC-3 cells using ConA magnetic beads
PC-3 cells were grown to confluence in 10-cm plates in RPMI 1640 medium with 10% fetal bovine serum and 1% penicillin-streptomycin. Cells from 10 plates were used to isolate the plasma membranes. Cells were collected by washing each plate with 10 mL TBS three times and then scraping the cells off the plate with 1 mL TBS. The scraped cell suspensions were collected in a 50-mL tube and spun in a clinical centrifuge to collect the cells. The supernatant was removed, and the cell pellet was resuspended in 0.75 mL of TBS. This resuspension was next homogenized with 30 strokes in a 2-mL Dounce homogenizer. Finally, the PC-3 cell lysate was added to the streptavidin magnetic beads, prepared as described above, and mixed on a rocker for 1 h. After binding, the beads were washed and eluted as described above.
Purification of plasma membrane from HeLa cells using ConA magnetic beads
HeLa cells were grown to confluence in 10-cm plates in Modified Eagle's Medium with 10% fetal bovine serum and 1% penicillin-streptomycin. Cells from 15 plates were used to isolate plasma membranes. After being washed with TBS, the cells were collected by scraping each plate with 1 mL of TBS. The scraped cell suspensions were collected in a 15-mL tube and spun in a clinical centrifuge to collect the cells. The supernatant was removed, and the cell pellet was saved. To prepare the cell lysate for binding to the magnetic beads, the pellet was resuspended in 0.5 mL of TBS and homogenized in a 2-mL Dounce homogenizer. Then the cell lysate was added to the streptavidin magnetic beads prepared as described above and mixed on a rocker for 1 h. After binding, the streptavidin magnetic beads were collected by holding the tube near a magnet, and the unbound fraction (i.e., the flow-through fraction) was removed. The beads were washed five times with 1 mL of TBS, and the proteins that bound to the ConA magnetic beads were eluted as described above.
5′-Nucleotidase assay
5′-Nucleotidase activity was determined by measuring the inorganic phosphate released from AMP. The assay was performed in a 96-well microtiter plate. Proteins (10 to 40 μg) were incubated in glycine buffer (75 mM, pH 9.0) containing 5 mM MgSO4, with or without AMP (final concentration, 2.5 mM), in a total volume of 100 μL for 20 min at 37 °C. At the end of the incubation, 100 μL of phosphate reaction solution containing 10% ascorbic acid and 0.42% ammonium molybdate in 1 N H2SO4 was added, and the color-reaction products were measured at 820 nm in a microtiter plate reader (Synergy HT, Bio-Tek, Winooski, VT). The 5′-nucleotidase activity was determined by subtracting the values obtained in the absence of AMP from those obtained in the presence of AMP and comparing them with a phosphate standard curve.
SDS-PAGE and Western blot analyses
Equal amounts of protein from the total cell lysates, flow-through, washes, and elutions were run on 4-12% SDS-PAGE. The gel was either fixed with 50% methanol and 7% acetic acid and stained with Gelcode (Pierce, Rockford, IL) following the manufacturer's procedures or transferred to nitrocellulose membranes for Western blotting. The rat liver membrane preparation was blotted with antibody against CEACAM1 [10]. A rabbit polyclonal antibody against pancadherin (Sigma, St. Louis, MO) was used for immunoblotting of the membrane proteins isolated from PC-3 and HeLa cells. The intensities of the respective bands were quantified by using the Image J program.
Protein concentration determination
Protein concentrations were measured by testing 10 μL of each sample in 200 μL of Coomassie Blue Plus Protein assay reagent (Pierce), using bovine serum albumin as a protein standard. The reaction was performed in a 96-well microtiter plate, and the color was read at 595 nm.
Results
Binding of ConA to streptavidin magnetic beads
ConA was immobilized onto magnetic beads (1-2 μm) through the binding of biotinylated ConA with streptavidin magnetic beads. Biotinylated ConA contains an average of one biotin per ConA molecule, and the streptavidin magnetic beads have a binding capacity of approximately 2,000 pmol of biotin per mL of the magnetic beads. Because the molecular mass of ConA is 104 kDa, each mL of magnetic beads can bind approximately 208 μg of biotinylated ConA. The efficiency of the immobilization was determined by comparing the amount of protein in the flow-through fraction with that in the original solution before binding. Of the 180 μg of biotinylated ConA in the binding solution, 42 ± 3 μg were found in the flow-through, yielding a coupling efficiency of 76%.
Enrichment of plasma membranes by ConA magnetic beads from a crude membrane preparation
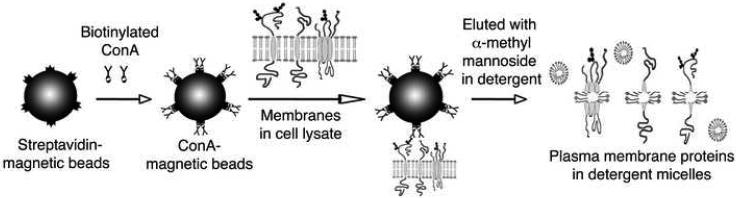
The membrane-preparation method developed by Neville [8] and modified by Marshak et al. [9], as described in Materials and Methods, is commonly used to isolate crude plasma membranes from rat liver. This membrane-isolation procedure gave us a high yield of plasma membranes: 30-50 mg of crude plasma membranes was usually obtained from one rat liver. However, the membranes were still contaminated with other cellular components. Thus, we next tested whether the ConA magnetic beads can be used to further enrich plasma membranes from this crude membrane preparation. We incubated 3.7 mg of crude membrane with ConA magnetic beads for 1 h at room temperature. Then the membranes that did not bind to the ConA magnetic beads were removed. After several washes with TBS, the membrane proteins that bound to the ConA magnetic beads were eluted with TBS buffer containing α-methylmannoside and 0.5% CHAPS. A scheme of the procedure for the purification of plasma membrane proteins is shown in Fig. 1.
Fig. 1.
Outline of the one-step plasma membrane protein purification procedure using concanavalin A (ConA) magnetic beads. Biotinylated ConA was immobilized onto streptavidin magnetic beads. The ConA magnetic beads were used to bind plasma membranes through the binding of glycosylated proteins present on the plasma membranes in the cell lysate. The plasma membrane proteins were eluted from ConA magnetic beads by using detergents and α-methylmannoside, which is a competitive sugar for binding to ConA.
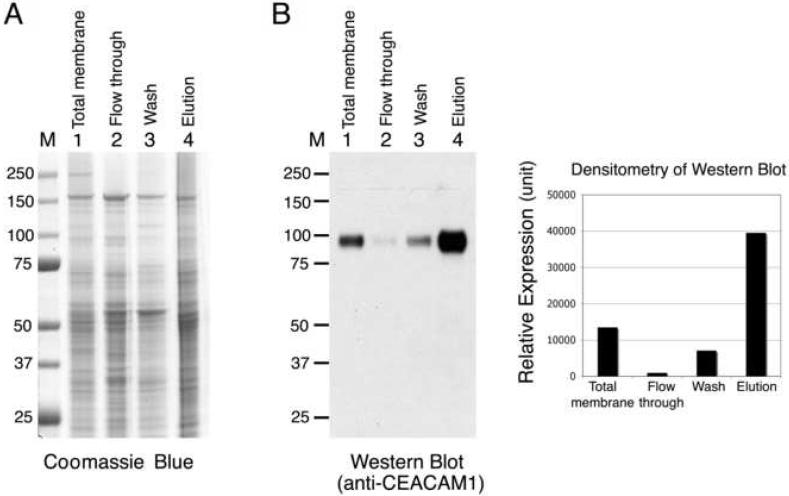
The enzymatic activity of the plasma membrane protein 5′-nucleotidase was used to monitor the purification. Measurement of protein concentration indicated that 0.7 mg of protein was recovered in the eluted fraction. As shown in Table 1, this procedure resulted in 3.7-fold enrichment of 5′-nucleotidase activity with 70% recovery of the activity from the crude membrane fraction. We further used Western blot analysis to determine the degree of purification of the plasma membrane proteins. An equal amount of protein from each purification step was loaded onto SDS-PAGE gels, and the proteins were stained with Coomassie blue. As shown in Fig. 2A, the protein profile of the eluted fraction differed somewhat from those of the flow-through and wash fractions. Immunoblotting with antibodies specific for a rat liver plasma membrane protein, CEACAM1, showed that CEACAM1 was enriched in the elution fraction relative to the level in the crude membrane fraction (Fig. 2B). The relative amounts of CEACAM1, as determined by densitometry of the Western blots, indicated that CEACAM1 was enriched to about three times the amount in the original membranes (Fig. 2B); this was consistent with the results obtained from 5′-nucleotidase activity (3.7 fold; Table 1).
Table 1.
Enrichment of plasma membrane from crude rat liver membrane, rat liver, PC-3 and HeLa cell lysates
| Protein fraction | Total protein (mg) | Total 5′-nucleotidase activity (nmol AMP/min) | Specific activity (nmol AMP/mg protein/min) | Fold purification | Yield (%) |
|---|---|---|---|---|---|
| Crude rat liver membrane | |||||
| Total membranes | 3.7 | 80.0 | 21.6 | 1.0 | 100.0 |
| Flow-through | 0.5 | 5.0 | 10.0 | 0.5 | 6.3 |
| Wash | 0.8 | 4.0 | 5.0 | 0.2 | 5.0 |
| Elution | 0.7 | 56.0 | 80.0 | 3.7 | 70.0 |
| Rat liver | |||||
| Lysate | 25.6 | 269.3 | 10.5 | 1.0 | 100.0 |
| Flow-through | 18.0 | 157.6 | 8.8 | 0.8 | 58.5 |
| Elution | 1.0 | 85.8 | 85.8 | 8.2 | 31.9 |
| PC-3 cell | |||||
| Total lysate | 8.6 | 62.4 | 7.3 | 1.0 | 100.0 |
| Flow-through | 7.5 | 31.6 | 4.2 | 0.6 | 51.0 |
| Wash | 0.4 | 2.4 | 6.0 | 0.8 | 4.0 |
| Elution | 0.3 | 28.0 | 93.3 | 13.0 | 45.0 |
| HeLa cell | |||||
| Total lysate | 4.5 | 6.1 | 1.4 | 1.0 | 100.0 |
| Flow through | 3.8 | 3.5 | 0.9 | 0.6 | 57.4 |
| Wash | 0.4 | 0.5 | 1.3 | 0.9 | 8.2 |
| Elution | 0.2 | 2.0 | 10.0 | 7.1 | 32.8 |
Fig. 2.
Purification of plasma membrane proteins from a crude membrane preparation. Rat liver plasma membrane, prepared as described by the method developed by Neville [8] and modified by Marshak et al. [9] (see Methods), was purified with the concanavalin A (ConA) magnetic beads method, as described in Materials and Methods. Equal amounts of protein (4 μg) from the original total membrane, flow-through, wash, and elution fractions were loaded onto SDS-PAGE gels. The gels were (A) stained with Coomassie blue or (B) transferred to nitrocellulose membranes and immunoblotted with anti-CEACAM1 antibody, and the relative amount of CEACAM1 was determined by densitometric quantification of the signals in the Western blots. M, molecular weight markers.
Purification of plasma membranes from rat liver lysate
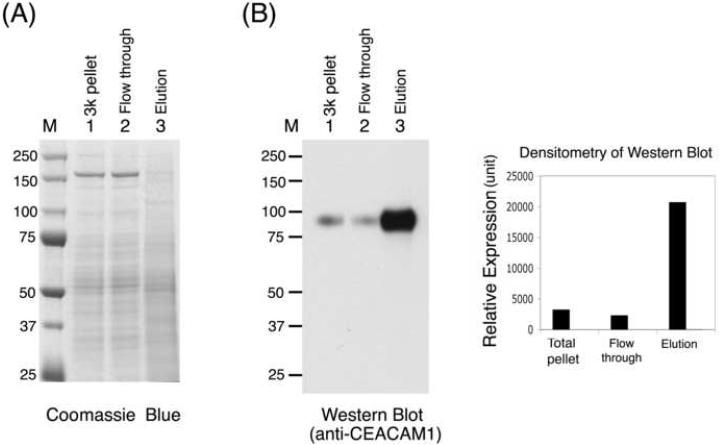
We next tested whether the ConA magnetic beads can be used to purify plasma membranes directly from rat liver lysate. Rat liver lysate prepared as described in Materials and Methods was incubated with ConA magnetic beads. From 25.6 mg of total lysate protein, about 1 mg of proteins was eluted from ConA magnetic beads. This resulted in 8.2-fold enrichment of 5′-nucleotidase activity with 32% recovery of the activity from the liver lysate (Table 1). Western blotting with anti-CEACAM1 antibody confirmed that CEACAM1 was enriched in the eluted fraction to about 6.4 times the amount in the liver lysate (Fig. 3).
Fig. 3.
Enrichment of plasma membrane from rat liver lysate. The rat liver lysate was prepared as described in Materials and Methods. Equal amounts of protein (4 μg) from the total lysate, flow-through, wash, and elution fractions were loaded on SDS-PAGE gels. The gels were (A) stained with Coomassie blue or (B) transferred to nitrocellulose membranes and immunoblotted with anti-CEACAM1 antibody, and the relative amount of CEACAM1 was determined by densitometric quantification of the signals in the Western blots. M, molecular weight markers. 3k pellet, the pellet fraction from 3,000 xg centrifugation.
Isolation of plasma membranes from PC-3 and HeLa cells
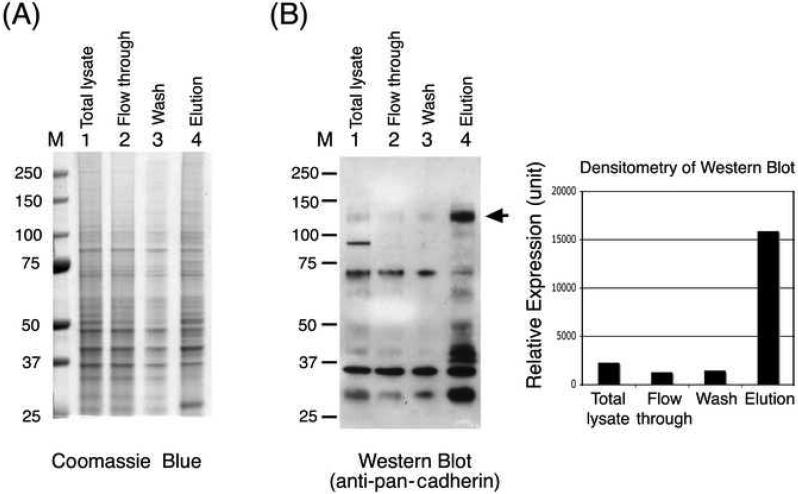
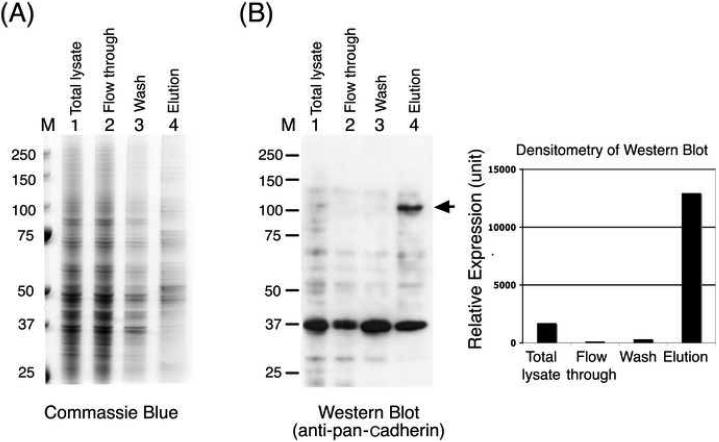
To determine whether ConA magnetic beads could be used to isolate plasma membranes from cell lines, we adapted the procedure for PC-3 and HeLa cell lines. Homogenized cells were incubated directly with ConA magnetic beads, and membrane proteins were eluted with TBS buffer containing α-methylmannoside and 0.5% CHAPS. For PC-3 cells, 5′-nucleotidase activity indicated that the plasma membrane marker was enriched to 13 times that in the total lysate, with a yield of 45% (Table 1). SDS-PAGE analysis showed that there were noticeable differences in the protein profiles between the flow-through and eluted fractions (Fig. 4A). Western blotting using an antibody against pan-cadherin, a family of cell surface-adhesion molecules [11], showed enrichment in the relative amounts of cadherins (with apparent molecular mass of 120 kDa) between the eluted and cell-lysate fractions (Fig. 4B). Semiquantitative densitometric determination of the intensities of the 120-kDa cadherin signals on Western blotting showed that the 120-kDa protein was enriched sevenfold. The slight discrepancy between the results of Western blotting and 5′-nucleotidase activity determination is likely due to the nonlinear nature of the enhanced chemiluminescence method.
Fig. 4.
Isolation of plasma membrane proteins from PC-3 cells. Plasma membrane proteins were purified from PC-3 cell lysates as described in Materials and Methods. Equal amounts of protein (4 μg) from the original total cell lysate, flow-through, wash, and elution fractions were loaded onto SDS-PAGE gels. The gels were (A) stained with Coomassie blue or (B) transferred to nitrocellulose and immunoblotted with anti-pan-cadherin antibody, and the relative amount of cadherins (120 kDa) was determined by densitometric quantification of the signals in the Western blots. M, molecular weight markers.
Similar results were obtained with the HeLa cells. The 5′-nucleotidase activity in the plasma membrane was enriched to 7.1 times that in the total cell lysate, with a yield of 33% (Table 1). Western blotting with anti-pan-cadherin antibody confirmed that cadherins in the eluted fraction were enriched to 7.6 times that in the total cell lysate (Fig. 5). The different molecular masses of the cadherins detected in PC-3 (Fig. 4) and HeLa (Fig. 5) cells are likely due to different cadherin family proteins expressed in these cells that vary in molecular mass.
Fig. 5.
Isolation of plasma membrane proteins from HeLa cells. Plasma membrane proteins were purified from HeLa cell lysates as described in Materials and Methods. Equal amounts of protein (4 μg) from the original total cell lysate, flow-through, wash, and elution fractions were loaded onto SDS-PAGE gels. The gels were (A) stained with Coomassie blue or (B) transferred to nitrocellulose and immunoblotted with anti-pan-cadherin antibody, and the relative amount of cadherins (∼120 kDa) was determined by densitometric quantification of the signals in the Western blots. M, molecular weight markers.
Together, these data indicate that the use of ConA magnetic beads is effective in isolating plasma membrane proteins from cultured cell lines.
Discussion
The affinity chromatography method we developed for isolating total plasma membrane proteins using ConA magnetic beads is fast and simple, and it results in good recovery of plasma membrane proteins that are as pure as those obtained with other procedures [1,3]. In addition, this one-step purification method does not require ultracentrifugation. Thus, it will save the user the trouble of determining the densities of membrane vesicles and the expense of purchasing an ultracentrifuge.
In this procedure, we use a solution containing detergent CHAPS plus a competitive sugar (α-methylmannoside) to elute the plasma membrane proteins that bind to the ConA magnetic beads. The role of CHAPS is to solubilize the membrane into individual proteins. This results in the elution of plasma membrane proteins as solubilized forms in detergent micelles rather than as plasma membrane vesicles. The reason for including detergent in the elution is that we were unable to recover the plasma membranes by using α-methylmannoside alone (data not shown). It is possible that multiple proteins within one membrane vesicle are bound to multiple ConA molecules present on the ConA magnetic beads, making it difficult for the competing sugar to elute the membranes from the beads. It is also possible the binding sites between the plasma membranes and ConA magnetic beads are inaccessible to the competing sugar. Although we used the detergent CHAPS in this current study, other detergents, such as Triton X-100, may also be used to solubilize the membrane proteins. Because the plasma membrane proteins are already solubilized in detergents, they are ready for purification, immunoprecipitation, or other experiments.
We observed that about 50% of the plasma membrane marker activity, i.e., 5‵-nucleotidase, remained in the flow-through fraction after binding of the total cell lysate to the ConA magnetic beads (Table 1). For most plasma membrane proteins, glycosylation occurs on the protein sequences present on the outside of the cell surface. Homogenization of the cells produces both inside-out and right-side out plasma membrane vesicles. Glycoproteins present in the inside-out vesicles could not interact with ConA magnetic beads and are likely to remain in the flow-through fraction. This may explain why there was about 50% of plasma membrane marker activity left in the flow-through fraction. The Neville liver plasma membranes, on the other hand, were prepared from whole rat liver, not hepatocytes, and this method also selectively purified large pieces of membrane sheets (not vesicles) from low-speed centrifugation [8]. Thus, this may explain why the ConA-magnetic bead isolation method is more efficient for Neville liver membranes than it is for those from total cell lysates. Although we did not test this in the current study, it may be possible to overcome such membrane vesicle sidedness by binding the cells directly to the ConA magnetic beads before cell disruption.
We observed that HeLa cells had much less 5′-nucleotidase activity than PC-3 cells had; this is likely due to the differences in the levels of expression of this membrane marker between these two cell lines. Johnsen et al. [4] reported on the isolation of plasma membranes from HeLa cells using conventional differential centrifugation followed by density-gradient ultracentrifugation. They reported sevenfold purification because the specific activity of 5′-nucleotidase was 0.61 for total cell lysate and 4.28 nmol AMP hydrolyzed/mg protein/min for purified plasma membranes. Our one-step magnetic bead method yielded a comparable degree of purification (7.1-fold), but with much higher recovery (33%) than their method (6%).
Other lectins than ConA may also be used in purifying membranes. For example, biotinylated wheat germ agglutinin, which recognizes N-acetylglucosamine, is also available commercially. In addition to immobilizing biotinylated lectins to streptavidin magnetic beads, it is possible to cross-link lectins directly onto magnetic beads that contain protein-reactive functional groups. For example, surface-activated Dynabeads, such as M-280 and M-450 tosylactivated, are available commercially (Invitrogen). It is also possible to take advantage of the binding specificity of the different lectins to isolate specific membranes with different glycosylation patterns.
In conclusion, we have developed a one-step purification method for isolating total plasma membrane proteins from tissue or cells. The new procedure results in good recovery of plasma membrane proteins. This will provide significant improvement in membrane protein purifications because low recovery often makes it impossible to obtain sufficient quantity of membrane proteins. Recently, there has been increased interest in identifying cell surface proteins as markers for different cell lineages and stem cells. Our one-step procedure will be of great use in isolating cell surface proteins for marker identification.
Acknowledgments
We thank Drs. Richard Burgess, Albert Courey, Karen Adelman, and Michael Marr, the instructors of the “Protein Purification and Characterization“ course in April 2008 at the Cold Spring Harbor Laboratories, for their encouragement.
The work was supported by National Institutes of Health Grant 2 R25 CA09481.
Footnotes
This work was performed during the Cold Spring Harbor Laboratory “Protein Purification and Characterization” course, April 2-15, 2008. The authors were course participants and contributed equally to the work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boone CW, Ford LE, Bond HE, Stuart DC, Lorenz D. Isolation of plasma membrane fragments from HeLa cells. J. Cell Biol. 1969;41:378–392. doi: 10.1083/jcb.41.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin SH, Wallace MA, Fain JN. Regulation of Ca2+−Mg2+−ATPase activity in hepatocyte plasma membranes by vasopressin and phenylephrine. Endocrinology. 1983;113:2268–2275. doi: 10.1210/endo-113-6-2268. [DOI] [PubMed] [Google Scholar]
- 3.Lin SH, Fain JN. Vasopressin and epinephrine stimulation of phosphatidylinositol breakdown in the plasma membrane of rat hepatocytes. Life Sci. 1981;29:1905–1912. doi: 10.1016/0024-3205(81)90523-3. [DOI] [PubMed] [Google Scholar]
- 4.Johnsen S, Stokke T, Prydz H. HeLa cell plasma membranes. I. 5′-Nucleotidase and ouabain-sensitive ATPase as markers for plasma membranes. J. Cell Biol. 1974;63:357–363. doi: 10.1083/jcb.63.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen CM, Kalish DI, Jacobson BS, Branton D. Membrane isolation on polylysine-coated beads. Plasma membrane from HeLa cells. J. Cell Biol. 1977;75:119–134. doi: 10.1083/jcb.75.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawson EL, Clifton JG, Huang F, Li X, Hixson DC, Josic D. Use of magnetic beads with immobilized monoclonal antibodies for isolation of highly pure plasma membranes. Electrophoresis. 2006;27:2747–2758. doi: 10.1002/elps.200600059. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya L, Ceccarini C, Lorenzoni P, Brewer CF. Concanavalin A interactions with asparagine-linked glycopeptides. Bivalency of high mannose and bisected hybrid type glycopeptides. J. Biol. Chem. 1987;262:1288–1293. [PubMed] [Google Scholar]
- 8.Neville DMJ. Isolation of an organ specific protein antigen from cell surface membrane of rat liver. Biochim. Biophys. Acta. 1968;154:540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- 9.Marshak DR, Kadonaga JT, Burgess RR, Knuth MW, Brennan WAJ, Lin S-H. Strategies for Protein Purification and Characterization: A Laboratory Course Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1996. [Google Scholar]
- 10.Cheung PH, Luo W, Qiu Y, Zhang X, Earley K, Millirons P, Lin SH. Structure and function of C-CAM1. The first immunoglobulin domain is required for intercellular adhesion. J. Biol. Chem. 1993;268:24303–24310. [PubMed] [Google Scholar]
- 11.Geiger B, Volberg T, Ginsberg D, Bitzur S, Sabanay I. Broad spectrum pan-cadherin antibodies, reactive with the C-terminal 24 amino acid residues of N-cadherin. J. Cell Sci. 1990;97:607–614. doi: 10.1242/jcs.97.4.607. [DOI] [PubMed] [Google Scholar]