Summary
In recent years substantial progress has been made in identifying culture conditions and specific molecular factors that maintain human embryonic stem cells (hESCs) in a self-renewing, pluripotent state. As science and medicine move closer to producing viable hESC-based therapeutics, effective methods of isolating and maintaining undifferentiated hESCs using clinically acceptable good manufacturing practices must be developed. In recent years, progress toward this goal has included identification of molecular factors that induce or repress hESC self-renewal and the development of defined media that support long-term hESC expansion. In addition, the recent discovery of novel means to derive pluripotent cells that avoid embryo destruction, including induced pluripotent stem (iPS cells), may mitigate ethical concerns associated with the use of hESCs.
Introduction
Human embryonic stem cells (hESCs), first derived from the blastocyst inner cell mass in 1998 [1], have become an attractive cell type for regenerative therapies, in vitro diagnostic technologies, and developmental models. Because of their infinite self-renewal capability and pluripotent nature, hESCs have the potential to generate large quantities of all somatic cell types, including terminally differentiated cells and their progenitors. However, one key to translating hESCs into viable applications is establishing defined, reproducible methods to derive and maintain the cells in vitro without compromising their differentiation potential or exposing them to potentially pathogenic agents that could subsequently transmit diseases upon clinical use. Until the past couple of years, hESCs were only maintained in undefined conditions, leading to heterogeneity in cells and culture conditions, and diminishing the utility of these cells in clinical applications. This review will describe how recent insights into the molecular mechanisms of human ES cell (hESC) self-renewal have enabled the development of defined conditions for culturing and deriving hESCs and other pluripotent cells, and will identify challenges that remain in hESC culture system development.
Advances in hESC culture conditions
Feeder Cells
Originally, primary mouse embryonic fibroblasts (MEFs), and later immortalized MEFs (STO line), were used for the derivation and propagation of hESCs in an undifferentiated state [1,2]. Due to concerns about hESC contact with xenogeneic cells, a number of human cell types have been subsequently examined as alternative feeder cells for hESC culture. Fibroblasts from different stages of development (fetal, neonatal, adult) and tissue sources (skin, muscle, placenta, uterus) are reportedly capable of supporting hESC undifferentiated growth, although certain cells support hESC maintenance better than others (for a review of feeder cells for hESC culture see [3]). In addition to allogeneic cell sources, fibroblast-like cells that spontaneously differentiate in hESC cultures can also be isolated and propagated to serve as an autogeneic source of feeder cells [4]. The differences in the performance of various feeder cells to support the pluripotency and self-renewal of hESCs can be attributed to the production of different types and amounts of secreted molecules. For example, MEFs typically produce more activin A and less basic fibroblast growth factor (bFGF) than human foreskin fibroblasts, which produce variable amounts of bFGF among different lines [5].
Culture Media Composition
Soon after the initial derivation of hESC lines, the first feeder-free culture system was described. In lieu of feeder layers, hESCs were grown for extended passages on Matrigel-coated substrates in the presence of MEF-conditioned medium [6]. Recent interrogation of the complex compositions of media conditioned by fibroblasts has provided insight into specific factors capable of supporting hESC pluripotency in vitro and facilitated the transition from feeder cells to defined media. By performing comparative proteomic analysis on media before and after conditioning by human fetal, neonatal and mouse embryonic fibroblasts, 175 proteins were uniquely identified, of which 34 were shared among the 3 cell types [7]. Many of the proteins identified, such as Activin/Inhibin, insulin-like growth factor-1 (IGF1) and BMP/TGF-beta1, were independently identified to play an active role in pluripotent hESC growth. Molecules secreted into the media by primary MEFs and a MEF line incapable of supporting hESCs in an undifferentiated state were also compared to identify pluripotent maintenance factors. As a result, a mixture of 6 recombinant proteins capable of supporting hESC growth (pigment epithelium-derived factor, plasminogen activator inhibitor, insulin-like growth factor binding proteins 2 and 7, monocyte chemoattractant protein 1, and interleukin 6) could be substituted for the more complex MEF conditioned media when culturing cells on fibronectin [8].
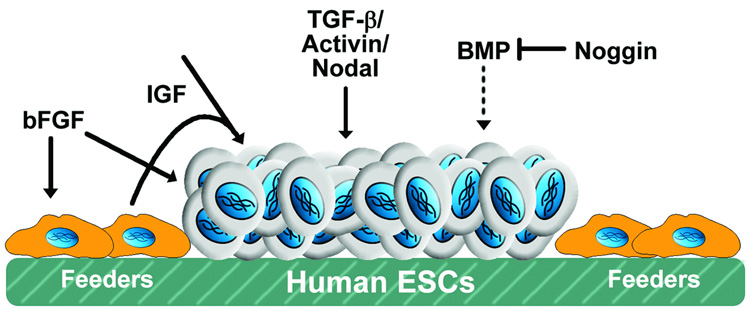
Based in part on analysis of conditioned media, a number of individual soluble factors, as well as combinations of molecules, have been identified to be important regulators of pluripotent stem cell self-renewal (Figure 1). Traditionally, bFGF has been the primary supplement added to hESC culture media according to original derivation and culture protocols for undifferentiated cell growth. Several years ago, several independent groups reported that bFGF was necessary and sufficient for hESC growth under feeder-free conditions, even without MEF-conditioned media [9–12]. By increasing the concentration of bFGF to 100 ng/ml (~6 nM, nearly 25x the amount added to MEF CM), hESCs could be sustained in a pluripotent state similar to CM [13]. The requirement of bFGF concentrations substantially above the Kd (< 1 nM) may be an effect of the low thermal stability of bFGF in culture conditions or alternatively may permit signaling through lower-affinity interactions [13].
Figure 1.
Molecules regulating pluripotent stem cell renewal. Growth factors such as bFGF, IGF and TGF-β/Activin/Nodal stimulate pluripotent stem cell growth via direct and indirect mechanisms, whereas inhibitors of BMP signaling, such as noggin, prevent premature differentiation.
In addition to bFGF, TGFβ/Activin/Nodal signaling via the SMAD2/3 pathway has also been demonstrated to play a significant, if not critical role in regulating the pluripotency of hESCs in the absence of feeders layers [14–16]. The combination of Activin with bFGF can sufficiently maintain hESC self-renewal even in the absence of feeder cells, CM, and serum replacement [17], and Activin A treatment induces expression of numerous pluripotent genes, such as Oct4 and Nanog, and stimulatory growth factors, such as Nodal, Wnt3, bFGF and FGF8 [18]. Inhibiting the bioactivity of potent morphogens, such as bone morphogenic proteins, by a specific antagonist, like Noggin, may also enhance the maintenance of hESCs in a pluripotent state [12,19]. More recently, analysis of specific growth factor receptor expression and signal transduction implicated the IGF signaling pathway as a direct mediator of hESC growth, whereas bFGF appeared to play an indirect role [20,21].
Although most hESC self-renewal studies have focused on the effects of growth factor proteins, recent evidence indicates that lipid molecules, abundant in serum and knockout serum replacement, are also important factors capable of maintaining hESC pluripotency. The addition of sphingosine-1-phosphate (S1P), in combination with platelet derived growth factor (PDGF), to serum-free media inhibited the spontaneous differentiation of hESCs grown on MEFs [22]. Likewise, supplementation of hESC culture media with sphingolipid metabolites, such as S1P or ceramide, appear to benefit the maintenance of hESC cultures by enhancing proliferation, as well as preventing hESC apoptosis and perhaps selectively promoting apoptosis of differentiating cells [23–25]. Due to their hydrophobic nature, introduction of lipids to aqueous media requires a hydrophilic carrier molecule, often albumin, in order to be remain soluble. A recent comparison of hESC cultures treated with either lipid-rich or lipid-poor fractions of bovine serum albumin (BSA) clearly demonstrated that only the lipid-rich BSA stimulated self-renewal of karyotypically normal hESCs in a pluripotent state [26].
Toward Defined, Humanized Culture Conditions
The use of hESCs in clinical trials and therapeutic applications will require good manufacturing practices (GMP) during both derivation and subsequent culture, including validation and documentation of manufacturing and testing protocols (see [27] for a recent review of GMP regulations as they pertain to hESC-based therapies). Since hESC lines can incorporate animal components, including the nonhuman sialic acid Neu5Gc, during culture in the presence of animal feeder cells or animal-derived serum components [28], deriving and culturing hESC lines in defined, humanized conditions would be ideal. However, lines that have been exposed to animal components may lose Neu5Gc following culture in humanized conditions [29,30], suggesting that contaminated lines may still be useful in clinical applications if they are proven to be free of infectious or pathogenic agents.
Allogeneic human fibroblasts, as well as hESC-derived cells, offer xeno-free feeder options for the derivation of hESC lines [3]. Recently, human feeder cells have been used in conjunction with medium containing human serum to derive xeno-free hESC lines [31,32]. Ludwig et al. described the first defined, completely animal-component free hESC derivation. Their medium contained a combination of small molecules and proteins including bFGF, TGFβ1, LiCl, GABA, and pipecolic acid and hESCs were cultured on a matrix consisting of human collagen IV, vitronectin, laminin, and fibronectin [33]. Although these lines acquired chromosomal abnormalities, including XXY and chromosome 12 trisomy, it remains unclear to what extent the defined culture conditions contributed to these abnormalities.
Advances in scaling hESC culture
Clinical trials or therapies using hESC-derived cells will require the ability to culture large quantities of hESCs under GMP conditions. For example, treatment of type 1 diabetes mellitus may require greater than 108 beta cells per transplant, with multiple transplants required per patient [34]. An improved understanding of the factors regulating hESC self-renewal and differentiation has led to development of more defined conditions for robust, scalable hESC culture.
Scaling hESC genetic manipulation, purification, and enzymatic dissociation has been complicated by low rates of clonal expansion. Inhibition of Rho-associated kinase (ROCK) can improve cloning efficiency from <1% to approximately 27% [35]. Likewise, neurotrophins and pleiotrophin appear to improve clonal recovery by inhibiting apoptosis [36,37]. Culturing hESCs in hypoxic conditions also improves clonal recovery [38].
Reliable methods to monitor and suppress abnormal karyotype acquisition will be a crucial quality control factor in scaling hESC cultivation. Chromosomal abnormalities are commonly acquired after long-term passaging [39,40], but little is known about the mechanisms by which these abnormalities arise or ways to repress them. Mechanical dissociation of cell colonies during passaging has been suggested to decrease the rate of karyotypic variations as compared to enzymatic dissociation [41], but mechanical dissociation of individual colonies is unlikely to be efficient for large-scale culture.
Development of scalable hESC culture systems remains in its infancy. A recently-developed robotic system for plating, feeding, and harvesting hESCs in MEF co-culture exhibited similar growth and differentiation rates as manual culture methods [42]. Suspension culture expansion of hESCs potentially offers a more robust, scalable system than adherent plate-based expansion. However, effective self-renewal of hESCs in suspension has not yet been demonstrated. Suspension cultures of murine embryonic stem cells (mESCs) attached to microcarriers or in multicellular aggregates have established that cell density and shear force can be manipulated to repress aggregation and spontaneous differentiation during expansion [43–45]. Translation of these findings to hESCs may be complicated by differences in self-renewal cues between mESCs and hESCs.
Advances in human pluripotent cell derivation
Derivation of new hESC lines has been hindered because of ethical concerns regarding the destruction of human embryos. However, new pluripotent cell lines are necessary for understanding the basic biology of hESC growth and differentiation and for translating scientific advances to clinical therapies. Recent progress in hESC derivation has led to protocols that do not require embryo destruction, including fusion of existing hESC lines with somatic cells, use of parthenogenesis to generate blastocysts, somatic cell nuclear transfer, and reprogramming of adult cells to induced pluripotent stem (iPS) cells. In addition to reducing the ethical concerns surrounding hESC research and development, these novel methods advance the potential of developing of patient-specific pluripotent cell lines and generating lines suitable for clinical applications.
Sources of embryos for hESC derivation
The initial hESC lines were derived via harvest of the inner cell mass from blastocyst stage embryos and subsequent subculture on MEF feeder cells [1]. While most hESC derivation methods still utilize the entire inner cell mass of the blastocyst, lines can also be created from the morula or arrested embryos, albeit at a lower efficiency than from blastocysts [46,47]. Embryos with a low morphological grade do not efficiently yield hESC lines if they are arrested prior to the blastocyst stage (<1% efficiency), but at the blastocyst stage 8.5% of poor quality embryos formed hESC lines in one study [48]. Chung et al. derived multiple hESC lines using single cell biopsies from morula-stage embryo without affecting the ability of the biopsied embryos to develop to the blastocyst stage in vitro [49]. This technique, similar to that used in preimplantation genetic diagnosis (PGD), has not yet been used to generate an hESC line for an embryo that implanted and formed a complete organism. Given the labor-intensive nature of this process and the risk that blastomere biopsy may pose to the developing embryos, it is unlikely to become a widespread strategy for generating patient-specific hESC lines, but it may be beneficial for creating lines for embryos that undergo PGD.
Inducing fusion of murine embryonic stem cells with adult thymocytes reprogrammed the adult nucleus to an embryonic state [50]. Similar approaches successfully fused existing hESC lines with human foreskin fibroblasts and hESC-derived myeloid precursors to generate pluripotent cells that express hESC-specific genes and exhibit embryonic DNA methylation patterns [51,52]. The tetraploid nature of these cells makes them unsuitable for clinical applications unless the hESC chromosomes can be removed before or after fusion.
Revazova et al. demonstrated that blastocysts produced by chemically-induced parthenogenesis, asexual development of an unfertilized oocyte, can be used to derive hESC lines [53]. Parthenogenesis of human oocytes has also been used to generate human HLA homozygous hESC from HLA heterozygous donors [54]. While parthenogenesis results in a high incidence of genetic abnormalities (i.e. deleterious recessive mutations in the donor oocyte), this method does not require embryo destruction and may be suitable for generating HLA-matched lines which could be banked for patient-specific regenerative therapies.
Somatic cell nuclear transfer (SCNT) has been used to clone embryos and generate ESC lines from these embryos in a number of species, including primates [55]. A cloned human blastocyst-stage embryo was created by transfer of the nucleus from an undifferentiated hESC into a human oocyte [56]. More recently, SCNT of adult fibroblast nuclei into human oocytes was used to generate multiple cloned blastocysts with 23% efficiency. However, hESC lines have not yet been derived from these cloned blastocysts [57]. Hwang et al. reported, then retracted, generation of hESC lines from blastocysts formed by SCNT; interestingly, one of these lines was later demonstrated to be the result of parthenogenesis [58].
Induced Pluripotent Stem (iPS) Cells
A promising new source of patient-specific pluripotent cells is iPS cells (Table 1). These cells do not possess the same ethical concerns of hESC lines since they do not require the destruction of blastocysts. Yu et al. illustrated that primary and transformed human dermal fibroblasts can be reprogrammed to a pluripotent state by expression of OCT4, SOX2, NANOG, and LIN28 [59]. Simultaneously, Takahashi et al. demonstrated that four transcription factors, Oct4, Sox2, Klf4, and c-Myc, also induced pluripotency in human dermal fibroblasts [60]. MYC expression was not required to generate human iPS lines, but did improve efficiency [61]. Expression of hTERT and the SV40 large T antigen in addition to OCT4, SOX2, KLF4, and c-MYC also enhanced iPS cell generation from primary adult fibroblasts [62]. Human iPS cells express the hESC markers SSEA-3, SSEA-4, TRA-1-60, TRA-1-81, alkaline phosphatase, NANOG, OCT3/4, SOX2 [59,60]. Furthermore, iPS cells exhibit high telomerase activity, histone methylation patterns similar to those found in hESCs, and pluripotency as determined by embryoid body analysis, directed differentiation, and teratoma formation [59,60]. Global gene expression analysis of various iPS clones and hESC lines identified minor differences between iPS cells and hESCs and between different iPS clones, although these differences were no greater than those found between distinct hESC lines [59,60]. Further characterization of iPS lines, including assessing growth and differentiation potential, and the development of efficient induction factor delivery methods that reduce the heterogeneity of transgene expression will be required to establish the promise of these cells in cell-based therapeutics.
Table 1.
Genes involved in reprogramming human somatic cells to the pluripotent state
| Gene | Function | Necessary for iPS cell generation? |
|---|---|---|
| OCT4 | Homeodomain transcription factor in the POU family. Required for pluripotency. | Yes |
| SOX2 | HMG box-containing transcription factor. Required for pluripotency. | Yes. |
| NANOG | Homeobox transcription factor. Required for pluripotency. | No. Expression can be induced in reprogrammed cells by other factors [60]. Improves clone recovery [59]. |
| KLF4 | Kruppel-like transcription factor. | No. |
| LIN28 | mRNA binding protein. Expressed in undifferentiated hESCs. | No. Improves clone recovery [59]. |
| c-MYC | Proto-oncogene that encodes a transcription factor. | No. Improves reprogramming efficiency [61]. |
Conclusions
In the past two years, the field of human pluripotent stem cells has experienced a series of innovations in the derivation and culture practices of these cells. Rapid progress made in the field thus far is expected to accelerate as increasing numbers of investigators and companies invest in the development of pluripotent human stem cell therapies. A variety of molecular factors involved in pluripotent stem cell self-renewal have been identified - determining the synergistic effects of these molecules and the convergence of independent signaling pathways affecting the growth and maintenance of hESCs will enable a better mechanistic understanding of pluripotent stem cell regulation. In addition, the recent demonstrations of the ability to derive pluripotent cells from different embryonic and adult cell sources will provide unique opportunities to comparatively examine the key factors and conditions that facilitate expansion of cells in a pluripotent state. Such advances are expected to enable the efficient scale-up of human pluripotent cells for clinical applications in the future.
Acknowledgements
We apologize to colleagues whose valuable contributions to this field were not able to be included because of space constraints. TCM is supported by grants from the National Science Foundation (CBET 0651739, EEC 9731643) and the National Institutes of Health (R21EB007316). SPP acknowledges National Science Foundation grant EFRI-0735903 and National Institute of Biomedical Imaging and Bioengineering grant 1R01EB007534.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Park JH, Kim SJ, Oh EJ, Moon SY, Roh SI, Kim CG, Yoon HS. Establishment and maintenance of human embryonic stem cells on STO, a permanently growing cell line. Biol Reprod. 2003;69:2007–2014. doi: 10.1095/biolreprod.103.017467. [DOI] [PubMed] [Google Scholar]
- 3.Stacey GN, Cobo F, Nieto A, Talavera P, Healy L, Concha A. The development of 'feeder' cells for the preparation of clinical grade hES cell lines: challenges and solutions. J Biotechnol. 2006;125:583–588. doi: 10.1016/j.jbiotec.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Stojkovic P, Lako M, Stewart R, Przyborski S, Armstrong L, Evans J, Murdoch A, Strachan T, Stojkovic M. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 2005;23:306–314. doi: 10.1634/stemcells.2004-0137. [DOI] [PubMed] [Google Scholar]
- 5.Eiselleova L, Peterkova I, Neradil J, Slaninova I, Hampl A, Dvorak P. Comparative study of mouse and human feeder cells for human embryonic stem cells. Int J Dev Biol. 2008;52:353–363. doi: 10.1387/ijdb.082590le. [DOI] [PubMed] [Google Scholar]
- 6.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 7.Prowse AB, McQuade LR, Bryant KJ, Marcal H, Gray PP. Identification of potential pluripotency determinants for human embryonic stem cells following proteomic analysis of human and mouse fibroblast conditioned media. J Proteome Res. 2007;6:3796–3807. doi: 10.1021/pr0702262. [DOI] [PubMed] [Google Scholar]
- 8.Chin AC, Fong WJ, Goh LT, Philp R, Oh SK, Choo AB. Identification of proteins from feeder conditioned medium that support human embryonic stem cells. J Biotechnol. 2007;130:320–328. doi: 10.1016/j.jbiotec.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 10.Xu C, Rosler E, Jiang J, Lebkowski JS, Gold JD, O'Sullivan C, Delavan-Boorsma K, Mok M, Bronstein A, Carpenter MK. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005;23:315–323. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Li L, Menendez P, Cerdan C, Bhatia M. Human embryonic stem cells maintained in the absence of mouse embryonic fibroblasts or conditioned media are capable of hematopoietic development. Blood. 2005 doi: 10.1182/blood-2004-10-4065. [DOI] [PubMed] [Google Scholar]
- 12.Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nature Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 13.Levenstein ME, Ludwig TE, Xu RH, Llanas RA, VanDenHeuvel-Kramer K, Manning D, Thomson JA. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247.• The authors illustrated that 100 ng/ml basic fibroblast growth factor was sufficient to maintain hESC self-renewal in unconditioned medium.
- 14.James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 15.Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC, Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- 16.Saha S, Ji L, de Pablo JJ, Palecek SP. TGFbeta/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys J. 2008;94:4123–4133. doi: 10.1529/biophysj.107.119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 18.Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24:1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- 19.Wang G, Zhang H, Zhao Y, Li J, Cai J, Wang P, Meng S, Feng J, Miao C, Ding M, et al. Noggin and bFGF cooperate to maintain the pluripotency of human embryonic stem cells in the absence of feeder layers. Biochem Biophys Res Commun. 2005;330:934–942. doi: 10.1016/j.bbrc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 20.Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bosse M, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027.• This article demonstrates that IGF produced by autogenic feeder cells in response to bFGF treatment is a primary mitogen mitigating human ESC self-renewal.
- 21.Wang L, Schulz TC, Sherrer ES, Dauphin DS, Shin S, Nelson AM, Ware CB, Zhan M, Song CZ, Chen X, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110:4111–4119. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pebay A, Wong RC, Pitson SM, Wolvetang EJ, Peh GS, Filipczyk A, Koh KL, Tellis I, Nguyen LT, Pera MF. Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells. 2005;23:1541–1548. doi: 10.1634/stemcells.2004-0338. [DOI] [PubMed] [Google Scholar]
- 23.Inniss K, Moore H. Mediation of apoptosis and proliferation of human embryonic stem cells by sphingosine-1-phosphate. Stem Cells Dev. 2006;15:789–796. doi: 10.1089/scd.2006.15.789. [DOI] [PubMed] [Google Scholar]
- 24.Wong RC, Tellis I, Jamshidi P, Pera M, Pebay A. Anti-apoptotic effect of sphingosine-1-phosphate and platelet-derived growth factor in human embryonic stem cells. Stem Cells Dev. 2007;16:989–1001. doi: 10.1089/scd.2007.0057. [DOI] [PubMed] [Google Scholar]
- 25.Salli U, Fox TE, Carkaci-Salli N, Sharma A, Robertson GP, Kester M, Vrana K. Propagation of undifferentiated human embryonic stem cells with nano-liposomal ceramide. Stem Cells Dev. 2008 doi: 10.1089/scd.2007.0271. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Gonzalo FR, Belmonte JC. Albumin-associated lipids regulate human embryonic stem cell self-renewal. PLoS ONE. 2008;3:e1384. doi: 10.1371/journal.pone.0001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewitt ZA, Amps KJ, Moore HD. Derivation of GMP raw materials for use in regenerative medicine: hESC-based therapies, progress toward clinical application. Clin Pharmacol Ther. 2007;82:448–452. doi: 10.1038/sj.clpt.6100321. [DOI] [PubMed] [Google Scholar]
- 28.Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 29.Heiskanen A, Satomaa T, Tiitinen S, Laitinen A, Mannelin S, Impola U, Mikkola M, Olsson C, Miller-Podraza H, Blomqvist M, et al. N-glycolylneuraminic acid xenoantigen contamination of human embryonic and mesenchymal stem cells is substantially reversible. Stem Cells. 2007;25:197–202. doi: 10.1634/stemcells.2006-0444. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 31.Ellerstrom C, Strehl R, Moya K, Andersson K, Bergh C, Lundin K, Hyllner J, Semb H. Derivation of a xeno-free human embryonic stem cell line. Stem Cells. 2006;24:2170–2176. doi: 10.1634/stemcells.2006-0130. [DOI] [PubMed] [Google Scholar]
- 32.Skottman H, Dilber MS, Hovatta O. The derivation of clinical-grade human embryonic stem cell lines. FEBS Lett. 2006;580:2875–2878. doi: 10.1016/j.febslet.2006.03.083. [DOI] [PubMed] [Google Scholar]
- 33.Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177.• This article presents the first derivation and subsequent culture of a human pluripotent stem cell line under defined conditions. This study represents an advance toward developing clinically acceptable pluripotent cell lines.
- 34.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310.•• This study shows that inhibition of ROCK enhances survival of single human embryonic stem cells. This finding has implications in clonal analysis of hESCs.
- 36.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol. 2006;24:344–350. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- 37.Soh BS, Song CM, Vallier L, Li P, Choong C, Yeo BH, Lim EH, Pedersen RA, Yang HH, Rao M, et al. Pleiotrophin enhances clonal growth and long-term expansion of human embryonic stem cells. Stem Cells. 2007;25:3029–3037. doi: 10.1634/stemcells.2007-0372. [DOI] [PubMed] [Google Scholar]
- 38.Forsyth NR, Musio A, Vezzoni P, Simpson AH, Noble BS, McWhir J. Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities. Cloning Stem Cells. 2006;8:16–23. doi: 10.1089/clo.2006.8.16. [DOI] [PubMed] [Google Scholar]
- 39.Draper JS, Smith K, Gokhale P, Moore HD, Maltby E, Johnson J, Meisner L, Zwaka TP, Thomson JA, Andrews PW. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22:53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- 40.Baker DE, Harrison NJ, Maltby E, Smith K, Moore HD, Shaw PJ, Heath PR, Holden H, Andrews PW. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol. 2007;25:207–215. doi: 10.1038/nbt1285. [DOI] [PubMed] [Google Scholar]
- 41.Mitalipova MM, Rao RR, Hoyer DM, Johnson JA, Meisner LF, Jones KL, Dalton S, Stice SL. Preserving the genetic integrity of human embryonic stem cells. Nat Biotechnol. 2005;23:19–20. doi: 10.1038/nbt0105-19. [DOI] [PubMed] [Google Scholar]
- 42.Terstegge S, Laufenberg I, Pochert J, Schenk S, Itskovitz-Eldor J, Endl E, Brustle O. Automated maintenance of embryonic stem cell cultures. Biotechnol Bioeng. 2007;96:195–201. doi: 10.1002/bit.21061.• This work represents the first description of a completely automated approach to culture pluripotent stem cells.
- 43.Cormier JT, zur Nieden NI, Rancourt DE, Kallos MS. Expansion of undifferentiated murine embryonic stem cells as aggregates in suspension culture bioreactors. Tissue Eng. 2006;12:3233–3245. doi: 10.1089/ten.2006.12.3233. [DOI] [PubMed] [Google Scholar]
- 44.Fok EY, Zandstra PW. Shear-controlled single-step mouse embryonic stem cell expansion and embryoid body-based differentiation. Stem Cells. 2005;23:1333–1342. doi: 10.1634/stemcells.2005-0112. [DOI] [PubMed] [Google Scholar]
- 45.Fernandes AM, Fernandes TG, Diogo MM, da Silva CL, Henrique D, Cabral JM. Mouse embryonic stem cell expansion in a microcarrier-based stirred culture system. J Biotechnol. 2007;132:227–236. doi: 10.1016/j.jbiotec.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 46.Strelchenko N, Verlinsky O, Kukharenko V, Verlinsky Y. Morula-derived human embryonic stem cells. Reprod Biomed Online. 2004;9:623–629. doi: 10.1016/s1472-6483(10)61772-5. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Stojkovic P, Przyborski S, Cooke M, Armstrong L, Lako M, Stojkovic M. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006;24:2669–2676. doi: 10.1634/stemcells.2006-0377. [DOI] [PubMed] [Google Scholar]
- 48.Lerou PH, Yabuuchi A, Huo H, Takeuchi A, Shea J, Cimini T, Ince TA, Ginsburg E, Racowsky C, Daley GQ. Human embryonic stem cell derivation from poor-quality embryos. Nat Biotechnol. 2008;26:212–214. doi: 10.1038/nbt1378. [DOI] [PubMed] [Google Scholar]
- 49.Chung Y, Klimanskaya I, Becker S, Li T, Maserati M, Lu SJ, Zdravkovic T, Ilic D, Genbacev O, Fisher S, et al. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell. 2008;2:113–117. doi: 10.1016/j.stem.2007.12.013.• This article demonstrates that pluripotent cell lines can be derived from single blastomeres without detrimental effects on the blastocyst development.
- 50.Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol. 2001;11:1553–1558. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- 51.Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1373. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- 52.Yu J, Vodyanik MA, He P, Slukvin II, Thomson JA. Human embryonic stem cells reprogram myeloid precursors following cell-cell fusion. Stem Cells. 2006;24:168–176. doi: 10.1634/stemcells.2005-0292. [DOI] [PubMed] [Google Scholar]
- 53.Revazova ES, Turovets NA, Kochetkova OD, Kindarova LB, Kuzmichev LN, Janus JD, Pryzhkova MV. Patient-specific stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2007;9:432–449. doi: 10.1089/clo.2007.0033.• The authors obtained pluripotent human cell lines by inducing human oocytes to undergo parthenogenesis.
- 54.Revazova ES, Turovets NA, Kochetkova OD, Agapova LS, Sebastian JL, Pryzhkova MV, Smolnikova V, Kuzmichev LN, Janus JD. HLA Homozygous Stem Cell Lines Derived from Human Parthenogenetic Blastocysts. Cloning Stem Cells. 2008;10:11–24. doi: 10.1089/clo.2007.0063. [DOI] [PubMed] [Google Scholar]
- 55.Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502. doi: 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- 56.Stojkovic M, Stojkovic P, Leary C, Hall VJ, Armstrong L, Herbert M, Nesbitt M, Lako M, Murdoch A. Derivation of a human blastocyst after heterologous nuclear transfer to donated oocytes. Reprod Biomed Online. 2005;11:226–231. doi: 10.1016/s1472-6483(10)60962-5. [DOI] [PubMed] [Google Scholar]
- 57.French AJ, Adams CA, Anderson LS, Kitchen JR, Hughes MR, Wood SH. Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblasts. Stem Cells. 2008;26:485–493. doi: 10.1634/stemcells.2007-0252. [DOI] [PubMed] [Google Scholar]
- 58.Kim K, Ng K, Rugg-Gunn PJ, Shieh JH, Kirak O, Jaenisch R, Wakayama T, Moore MA, Pedersen RA, Daley GQ. Recombination signatures distinguish embryonic stem cells derived by parthenogenesis and somatic cell nuclear transfer. Cell Stem Cell. 2007;1:346–352. doi: 10.1016/j.stem.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526.•• The authors demonstrates that human fibroblasts can be reprogrammed to a pluripotent state by expression of OCT4, SOX2, NANOG, and LIN28.
- 60.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019.•• The authors show that human fibroblasts can be reprogrammed to a pluripotent state by introducing Oct3/4, Sox2, c-Myc, and Klf4.
- 61.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 62.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]