Abstract
While the acute inhibitory effect of opioids on locus coeruleus (LC) neurons is mediated primarily by the activation of G protein-gated inwardly-rectifying K+ (GIRK) channels, the 3′-5′-cyclic adenosine monophosphate (cAMP)-system has been implicated in the effects of chronic morphine exposure. Presently, the impact of chronic morphine treatment on GIRK-dependent and GIRK-independent mechanisms underlying the opioid-induced inhibition of LC neurons is unclear. Here, opioid-induced postsynaptic inhibition was studied in LC neurons from wild-type and GIRK2/GIRK3-/- mice at baseline and following chronic morphine treatment. The postsynaptic inhibition of LC neurons caused by the opioid agonist [Met]5 enkephalin (ME) was unaffected by chronic morphine treatment in mice of both genotypes. Furthermore, chronic morphine treatment had no effect on the forskolin augmentation of the ME-induced current in wild-type LC neurons, and only a minor effect on the ME-induced current in LC neurons from GIRK2/GIRK3-/- mice. Chronic morphine treatment did, however, lead to an increased frequency of spontaneous excitatory postsynaptic currents (EPSCs) in the LC. Interestingly, while forskolin augmented the EPSC frequency similarly in untreated and morphine-treated wild-type mice, as well as untreated GIRK2/GIRK3-/- mice, it failed to increase the frequency of EPSCs in morphine-treated GIRK2/GIRK3-/- mice. Altogether, the findings suggest that chronic morphine treatment exerts little impact on ion channels and signaling pathways that mediate the postsynaptic inhibitory effects of opioids, but does enhance excitatory neurotransmission in the mouse LC.
Keywords: Kir3, opioid, tolerance, dependence, withdrawal
Introduction
Studies of the locus coeruleus (LC) have highlighted mechanisms underlying acute and chronic effects of opiates (Nestler & Aghajanian, 1997; Nestler, 2001; Williams et al., 2001). Acutely, mu opioid receptor (MOR) agonists hyperpolarize LC neurons (Pepper & Henderson, 1980; Williams et al., 1982). The hyperpolarization of LC neurons involves multiple channel types, the most dominant of which appears to be the G protein-gated inwardly-rectifying K+ channel (GIRK/Kir3 channel) (Torrecilla et al., 2002).
MOR agonists also inhibit adenylyl cyclase in LC neurons (Duman et al., 1988). The activity of the cAMP system is enhanced during prolonged morphine exposure, an adaptation that parallels tolerance and may explain the recovery of LC neuron firing rates to pre-treatment levels (Duman et al., 1988; Nestler & Tallman, 1988; Rasmussen et al., 1990). In addition, application of MOR antagonists following chronic morphine exposure leads to hyper-stimulation of the cAMP system in LC neurons, a phenomenon implicated in the elevated firing rates of LC neurons seen in some studies during opiate withdrawal (Kogan et al., 1992; Lane-Ladd et al., 1997; Ivanov & Aston-Jones, 2001). Similar findings have been reported in other brain regions, supporting the contention that cAMP signaling contributes to the behavioral manifestations of opiate dependence, withdrawal, and reinforcement (Nestler, 2001; Williams et al., 2001).
Though consensus has emerged that adaptations in the cAMP pathway occur during chronic morphine exposure, the impact on neuronal excitability is uncertain. Some data argue that modifications intrinsic to LC neurons explain the enhanced activity noted during withdrawal, while other studies implicate enhanced afferent excitatory input. For example, antisense or dominant-negative suppression of the cAMP-response element binding (CREB) protein in LC neurons opposed the elevated firing rates and behavioral manifestations induced by opiate withdrawal (Lane-Ladd et al., 1997; Han et al., 2006). Nevertheless, the opiate withdrawal syndrome was observed following neurochemical lesion of the LC (Chieng & Christie, 1995; Caille et al., 1999), and glutamate release was elevated in the LC during opiate withdrawal (Akaoka & Aston-Jones, 1991; Aghajanian et al., 1994). Furthermore, glutamate receptor antagonism substantially blocked the withdrawal-induced enhancement of LC neuron firing rates (Akaoka & Aston-Jones, 1991). The latter observations are consistent with studies in other brain regions (Bonci & Williams, 1997; Chieng & Williams, 1998; Ingram et al., 1998; Jolas et al., 2000; Hack et al., 2003; Bie et al., 2005).
The relative contributions of intrinsic and extrinsic modifications to the LC resulting from chronic morphine administration remain unclear. Previously, we reported that the postsynaptic inhibitory effect of opioids (ME) was reduced in LC neurons from GIRK2/GIRK3-/- mice (Torrecilla et al., 2002). Here, we examined the impact of chronic morphine administration on ME-induced current in LC neurons from wild-type and GIRK2/GIRK3-/- mice. We reasoned that adaptations to other opioid-modulated currents would be better resolved in the absence of the GIRK conductance. Our findings indicate that chronic morphine treatment does not alter the opioid-induced postsynaptic inhibition of LC neurons, but rather leads to a significant increase in excitatory input to the LC.
Materials and Methods
Experimental subjects
All experiments were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University and conducted according to guidelines established by the National Institutes of Health governing the use of animals in research. GIRK2/GIRK3-/- mice were generated as described previously (Torrecilla et al., 2002). Wild type and GIRK2/GIRK3-/- male and female mice (3-8 weeks) were implanted with morphine pellets (25 mg morphine base, obtained from NIDA) under isoflurane anesthesia on Days 1 (1 pellet) and 3 (1-2 pellets). This protocol has been used extensively to induce tolerance and dependence in rats and mice (Christie et al., 1986; Kolesnikov et al., 1993; Shoji et al., 1999; Georges et al., 2006; Mouledous et al., 2007). At the time of pellet implantation, each subject was given an intraperitoneal injection of lactated saline to prevent dehydration; we have observed that this approach leads to improved health of the animals during the study. All animals used in this study exhibited clear signs of opiate exposure following pellet implantation, including Straub tail and elevated motor activity. A second group of mice was implanted with placebo pellets (morphine-free pellets) following the same schedule. Data from the placebo group were indistinguishable from data from untreated mice. Data were pooled from these control groups, and are referred to throughout as deriving from untreated or control mice.
Electrophysiology
Electrophysiological studies were performed on days 5-6 after pellet implantation. Mice were anesthetized with halothane and their brains were removed rapidly. Coronal and horizontal sections (240 μm) were used for whole-cell voltage-clamp experiments. Voltage-clamp experiments were performed at 34°C using solutions and conditions described previously (Torrecilla et al., 2002). Intracellular recordings were performed as described (Williams et al., 1982). In brief, slices (280 μm) were cut in the horizontal plane, and electrodes (50-60 MΩ) were filled with KCl (2 M). [Met]5enkephalin (ME), UK-14304, forskolin, and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) were purchased from Sigma (St. Louis, MO, USA) and were applied by superfusion. Forskolin was applied to slices at both 10 and 30 μM; no significant differences were seen with respect to electrophysiological outcomes with these two concentrations. Slices from both control and morphine-treated mice were incubated in morphine-free solution for at least 1 hr prior to recordings. Whole-cell currents, synaptic events, and membrane potentials were acquired with an Axopatch-1D amplifier (Axon Instruments, Inc.; Foster City, CA, USA). Data were low-pass filtered at 5 kHz, digitized with an ITC-16 Computer Interface (Instrutech Corporation; Long Island, NY, USA), and sampled at 10 kHz with Axograph 4.9 (Axon Instruments, Inc.). Whole-cell currents and spontaneous EPSCs were measured in voltage-clamp mode with the membrane potential (Vhold) held at -60 mV. Current-voltage relationships were obtained by stepping the membrane potential from −50 to −130 mV in −10 mV increments (100 ms/step). EPSC frequencies were determined from 30 s of continuous recordings made in presence of picrotoxin (100 μM) to isolate excitatory activity. Frequency information was extracted using a template function (Axograph X; Berkeley, CA, USA), with deflections < 7 pA excluded from analysis.
Data analysis
Data analysis was performed with PRISM software (GraphPad Software, San Diego, CA). All results are presented as the mean ± SEM. EC50 values for agonist-induced hyperpolarization of LC neurons were determined by fitting concentration-response curves with a logistic function using KaleidaGraph (Synergy Software, Reading PA). Statistical significance was assessed using analysis of variance (two-way ANOVA) for multiple comparisons and Student's t test for pair-wise comparisons of the action of forskolin. Spontaneous EPSC frequency and amplitude under each condition were pooled and plotted as cumulative histograms, and analyzed with the Kolmogorov-Smirnov test. The level of significance was set at p<0.05.
Results
Previously, we reported that the ME-induced current in LC neurons from GIRK2/GIRK3-/- mice was significantly smaller than that seen in wild-type controls (Torrecilla et al., 2002). The small residual current in slices from GIRK2/GIRK3-/- mice was partially blocked by barium leaving a residual current of ∼15 pA that was insensitive to membrane potential. This residual current may be the result of the inhibition of a cAMP-dependent inward current reported previously in rat LC neurons (Alreja & Aghajanian, 1993). This possibility was tested by measuring whole-cell currents induced by ME in LC neurons from wild-type and GIRK2/GIRK3-/- mice prior to and 10 min after the application of forskolin, which stimulates adenylyl cyclase in a receptor-independent manner (Osborne & Williams, 1996).
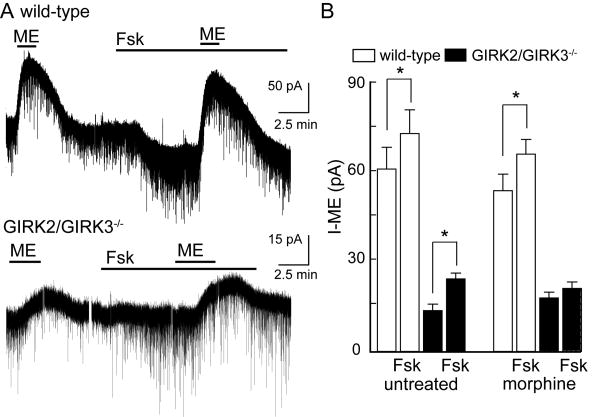
ME induced an outward current in LC neurons from wild-type mice (61±7 pA at -60 mV, n=15; Fig. 1A,B). Subsequent application of forskolin produced a small inward current and an accompanying increase in excitatory synaptic input (analyzed in detail below). In the presence of forskolin, the current induced by ME in LC neurons from wild-type mice was significantly enhanced (74±8 pA, n=15, p<0.001). The ME-induced outward current was smaller in LC neurons from GIRK2/GIRK3-/- mice (14±2 pA at -60 mV, n=21; p<0.001). Similar to experiments involving wild-type animals, forskolin increased the ME-induced current by 10-15 pA in LC neurons from GIRK2/GIRK3-/- mice (Fig. 1A,B). Thus, there was no evidence of a significant genotype-dependent difference in cAMP-dependent signaling in LC neurons.
Figure 1. Opioid-induced outward currents in LC neurons from wild type and GIRK2/GIRK3-/- mice.
A) Current traces showing the outward current induced by ME (30 μM) in the absence and presence of forskolin (Fsk, 30 μM) in slices from wild-type mouse (top trace) and a GIRK2/GIRK3-/- mouse (bottom trace). The holding potential (Vhold) was -60 mV. Forskolin (Fsk) induced a small inward current and increased spontaneous excitatory input. B) Summary showing the amplitude of the current induced by ME (30 μM) in slices from wild-type (white bars) and GIRK2/GIRK3-/- (black bars) mice, and the impact of chronic morphine treatment and Fsk on the ME-induced current (Vhold = -60 mV). In the interest of clarity, only within-genotype differences are noted on the plot; genotype-dependent differences were clearly evident and noted in the text. Symbols: * p<0.05 vs. Fsk, within genotype.
We next asked whether chronic morphine treatment caused an adaptation in the opioid-induced current. Following treatment of wild-type mice, the amplitude of the ME-induced current (55±6 pA at −60 mV, n=11) and the augmentation of the ME-induced current by forskolin were indistinguishable from those recorded in slices from untreated wild-type mice (Fig. 1B). Similarly, in slices taken from morphine-treated GIRK2/GIRK3-/- mice, the ME-induced current (18±2 pA at -60 mV, n=18) was not significantly different from that measured in LC neurons from untreated GIRK2/GIRK3-/- mice. The ME-induced current, however, was not significantly enhanced by forskolin in slices from morphine-treated GIRK2/GIRK3-/- mice (Fig. 1B).
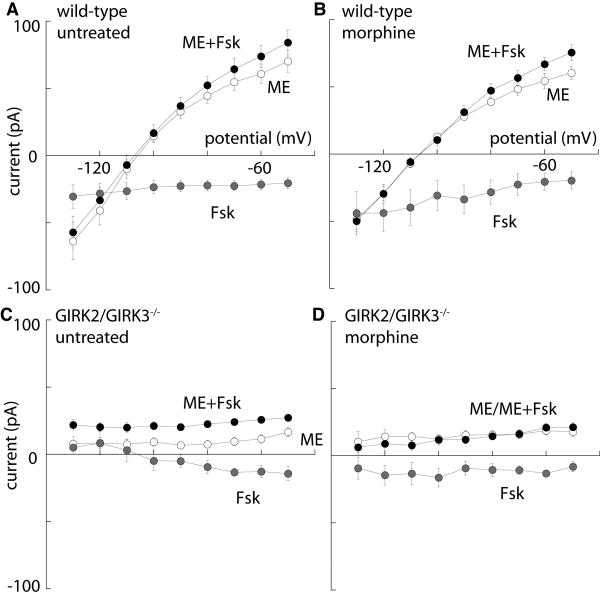
The current/voltage relationships in the absence and presence of forskolin were measured to determine the effect of forskolin on the reversal potential of the ME-induced current and to determine the voltage dependence of the current induced by forskolin (Fig 2). In slices from untreated wild-type mice, forskolin produced an increase in the slope of the ME-induced current with no change in reversal potential (Fig. 2A). A similar result was obtained in slices taken from animals that were treated with morphine (Fig. 2B). The current induced by forskolin was inward at all potentials and was not different between slices from untreated and morphine-treated animals (Fig. 2A,B). In slices from untreated and morphine-treated GIRK2/GIRK3-/- mice, the amplitude of the ME-induced current was not sensitive to membrane potential (Fig. 2C,D). Forskolin evoked a voltage-independent increase in the outward ME current in LC neurons from untreated GIRK2/GIRK3-/- mice (Fig. 2C), but had no effect on the ME-induced current in slices from morphine-treated GIRK2/GIRK3-/- mice (Fig. 2D). The observation that forskolin had no apparent effect on the ME-induced current suggests that there was no dramatic up-regulation in the cAMP signaling pathway following chronic morphine treatment in GIRK2/GIRK3-/- mice.
Figure 2. Current-voltage relationships in LC neurons from untreated and morphine-treated mice.
The voltage dependence of the whole-cell currents measured following application of ME (30 μM; white circles), Fsk alone (gray circles), or ME in the presence of Fsk (ME+Fsk; black circles) are plotted for LC neurons from untreated and morphine-treated wild-type (A,B) and GIRK2/GIRK3-/- (C,D) mice. While the small Fsk-induced current measured at Vhold = -60 mV was similar across the four groups tested, some differences in the I-V profiles were evident (e.g., compare C and D). Also note that the I-V plots for the ME-induced current measured in the absence or presence of Fsk were largely overlapping in panel D, reflecting the lack of effect of Fsk on ME-induced current in LC neurons from morphine-treated GIRK2/GIRK3-/- mice.
Though pharmacologic manipulations suggested that recording conditions were suitable for measuring the influence of cAMP-dependent signaling on the ME-induced current in mouse LC neurons, the dialysis of the intracellular milieu during whole-cell recording experiments may adversely impact cAMP-dependent processes. Indeed, spontaneous LC neuron firing has been observed to run down under standard whole-cell recording conditions (Alreja & Aghajanian, 1995). To address this concern, we next used an intracellular recording technique to measure the hyperpolarization induced by ME in LC neurons from untreated and morphine-treated mice.
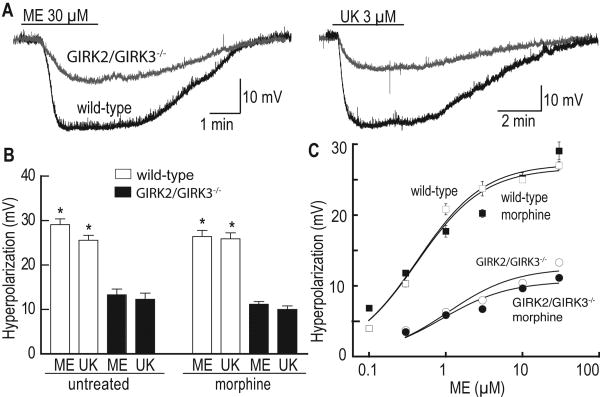
To measure the ME-induced hyperpolarization of LC neurons, holding current (0-200 pA) was applied to inhibit spontaneous firing and to hold the membrane potential near -60 mV. Under these conditions, ME induced a dose-dependent hyperpolarization of LC neurons in slices from untreated wild-type mice, with a maximal response measured at 30 μM (29±1 mV) and an EC50 of 0.64±0.09 μM (n=14) (Fig. 3). Consistent with the voltage-clamp data, the ME-induced hyperpolarization was significantly blunted in LC neurons from GIRK2/GIRK3-/- mice (13.3±1.2 mV at 30 μM ME, n=9; Fig. 3A,B). The EC50 for ME-induced hyperpolarization in slices from the GIRK2/GIRK3-/- mice (1.96±0.29 μM) was about 3-fold greater than that measured in slices from wild-type mice, arguing that the residual conductance mediating this hyperpolarization in LC neurons from GIRK2/GIRK3-/- mice is slightly less sensitive to opioid receptor stimulation than the GIRK channel. After chronic treatment of wild-type mice with morphine, there was no change in the maximal ME-induced hyperpolarization (26±1 mV, n=7) or the EC50 (0.59±0.13 μM; Fig. 3C). In GIRK2/GIRK3-/- mice treated chronically with morphine, there was also no significant change in the maximal ME-induced hyperpolarization (14± 1 mV, n=9) or the EC50 (3.29±0.43 μM).
Figure 3. ME-induced hyperpolarization in LC neurons from untreated and morphine-treated mice.
A) The superimposed traces on the left show the hyperpolarization induced by 30 μM ME in LC neurons from a wild-type (black trace) and GIRK2/GIRK3-/- mouse (grey trace). The superimposed traces on the right show the hyperpolarization induced by 3 μM UK-14304 in LC neurons from wild-type (black trace) and GIRK2/GIRK3-/- (grey trace) mice. B) Summary plot showing the hyperpolarization induced by ME (30 μM) and UK-14304 (UK, 3 μM) in LC neurons from untreated and morphine-treated wild-type (white bar) and GIRK2/GIRK3-/- (black bar) mice. C) Concentration-response curves describing the hyperpolarization induced by ME in LC neurons from untreated (white symbols) and morphine-treated (black symbols) wild-type and GIRK2/GIRK3-/- mice. Symbols: * p<0.05 vs. GIRK2/GIRK3-/- mice.
The α2-adrenoceptor agonist UK-14304 (3 μM) was applied at the end of each experiment to probe for opioid receptor-specific or effector-specific changes induced by chronic morphine exposure. The hyperpolarization induced by UK-14304 (3 μM) was significantly smaller in LC neurons from untreated GIRK2/GIRK3-/- mice (12.3±1.4 mV, n=7; p<0.001) as compared to wild-type controls (25.4±1.0 mV, n=19; Fig. 3A,B). Furthermore, there was no change in the hyperpolarization induced by UK-14303 following chronic morphine treatment for either genotype (Fig. 3B).
Consistent with findings from the voltage-clamp study, forskolin increased the amplitude of the ME-induced hyperpolarization (data not shown). For these experiments, a sub-saturating concentration of ME (1 μM) was used in experiments involving slices from wild-type mice such that the amplitude of the hyperpolarization would not be limited by the approach of the membrane potential toward the potassium equilibrium potential. The ME test concentration was increased to (10 μM) in experiments involving LC neurons from GIRK2/GIRK3-/- mice since the hyperpolarization was not dependent on the change in voltage and because the amplitude of the hyperpolarization induced by ME (1 μM) was very small. In slices from untreated wild-type mice, the hyperpolarization induced by ME increased from 19.1±2.0 mV in control to 21.7±2.2 mV after treatment with forskolin (n=17, p<0.01). In slices from GIRK2/GIRK3-/- mice, the hyperpolarization induced by ME was 10.0±1.4 mV in control, and this increased to 12.2±1.7 mV in the presence of forskolin (n=5, p<0.05). Following chronic morphine treatment, forskolin increased the ME-induced hyperpolarization in LC neurons from both wild-type (13.7±2.0 mV vs. 15.3±2.2 mV, n=6; p<0.01) and GIRK2/GIRK3-/- (9.7±1.4 mV vs. 12.1±1.8 mV, n=9; p<0.01) mice. The forskolin-induced augmentation of the ME-induced hyperpolarization seen in LC neurons from morphine-treated GIRK2/GIRK3-/- mice was somewhat unexpected given the lack of forskolin effect on residual ME-induced current in LC neurons from these mice. Though there is no clear-cut explanation for the difference in results, there are several possibilities given the technical differences in the experiments. Alternatively, changes in membrane potential may constitute a more sensitive measure of opioid actions in LC neurons.
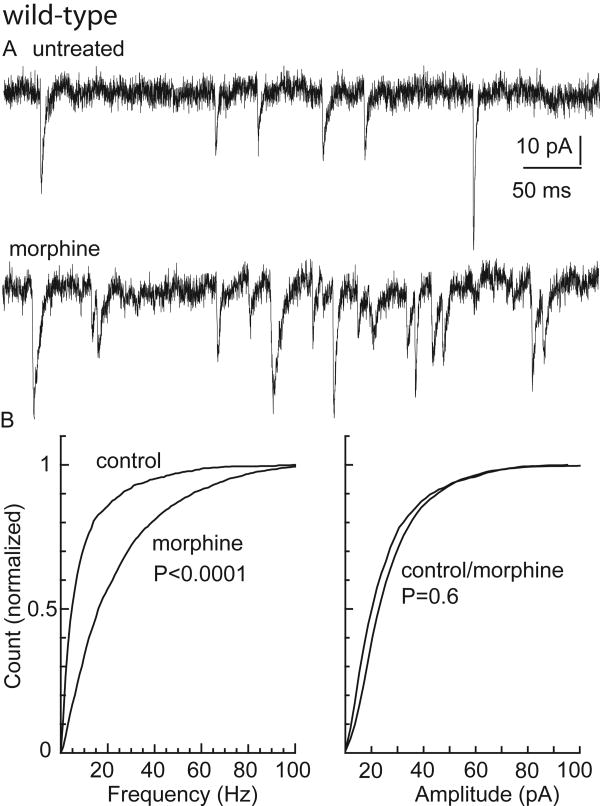
Though the subject of some debate, enhanced excitatory transmission has been implicated in the elevated excitability of rat LC neurons seen during opiate withdrawal (Akaoka & Aston-Jones, 1991; Aghajanian et al., 1994; Ivanov & Aston-Jones, 2001). To determine whether such modifications occur in the mouse, spontaneous EPSCs were measured in LC neurons from wild-type and GIRK2/GIRK3-/- mice at baseline and following chronic morphine treatment. Mouse LC neurons exhibited spontaneous EPSCs that were abolished by the glutamate receptor antagonist NBQX (5 μM, data not shown). The frequency, but not amplitude, of spontaneous EPSCs was elevated in slices from morphine-treated wild-type mice (Fig. 4). There was a similar elevation in the frequency of spontaneous EPSCs measured in slices from morphine-treated GIRK2/GIRK3-/- mice (Fig. 5). Thus, an increase in excitatory input to LC neurons was evident in slices from both wild-type and GIRK2/GIRK3-/- mice during withdrawal from chronic morphine treatment.
Figure 4. Excitatory input to LC neurons in untreated and morphine-treated wild-type mice.
A) Representative current traces showing spontaneous EPSCs in slices from untreated (top) and morphine-treated (bottom) wild-type mice (Vhold = -60 mV). B) Cumulative histograms of frequency (left panel, >2000 EPSCs; 13 slices from 7 animals) and amplitude (right panel, >2000 EPSCs; 15 slices from 7 animals) showing that the frequency of spontaneous EPSCs is increased in slices from morphine-treated animals, while there was no significant change in the amplitude distribution.
Figure 5. Excitatory input to LC neurons in untreated and morphine-treated GIRK2/GIRK3-/- mice.
A) Representative current traces showing spontaneous EPSCs in slices from untreated (top) and morphine-treated (bottom) GIRK2/GIRK3-/- mice (Vhold = -60 mV). B) Cumulative histograms of frequency (left panel, >2000 EPSCs; 20 slices from 8 animals) and amplitude (right panel, >2000 EPSCs; 18 slices from 8 animals) showing that the frequency of spontaneous EPSCs is increased in slices from morphine-treated animals, while there was no significant change in the amplitude distribution.
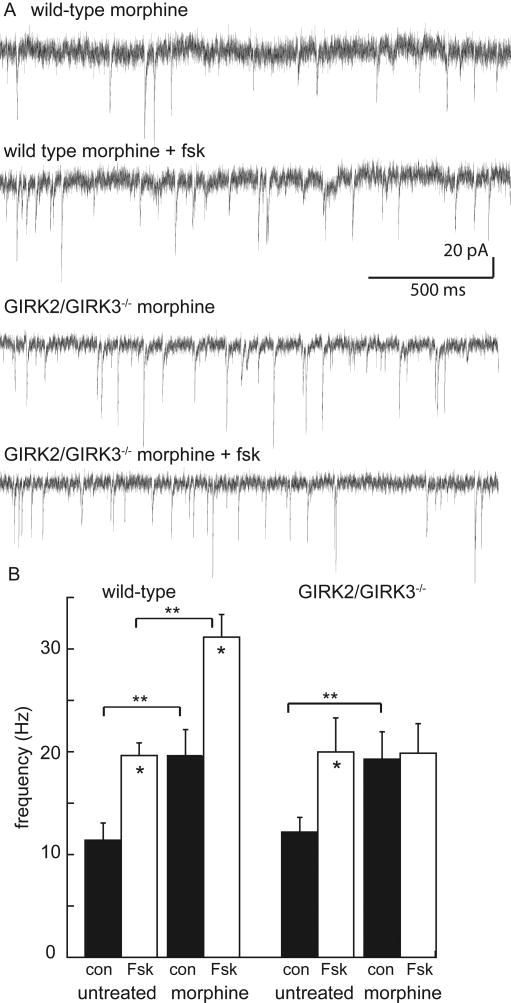
The mean frequency and amplitude of spontaneous EPSCs measured in LC neurons were indistinguishable between untreated wild-type (10.6±1.8 Hz & 22.8±1.6 pA, n=12) and GIRK2/GIRK3-/- (12.3±1.5 Hz & 17.7±1.1 pA, n=20 unpaired t-test p>0.05) mice, suggesting that constitutive ablation of GIRK2 and GIRK3 did not elevate excitatory input to the LC (Fig. 6, Fig. S1). Forskolin (10 μM) increased the EPSC frequency in slices from both untreated wild-type (19.1±1.6 Hz, n=12; p<0.01) and GIRK2/GIRK3-/- (17.9±2.8 Hz, n=13; p<0.01) mice (Fig. 6B). Forskolin had no effect on the amplitude of spontaneous EPSCs in slices from morphine-treated animals of either genotype (Fig. S1). These observations support the contention that significant genotype-dependent differences in cAMP-dependent signaling contributing to excitatory neurotransmission were not evident at baseline. Following chronic morphine treatment, forskolin increased the frequency of spontaneous EPSCs in slices from wild-type animals, but had no significant effect in slices from GIRK2/GIRK3-/- mice (Fig 6B, Fig. S1).
Figure 6. Forskolin increases the frequency of spontaneous EPSCs.
A) Representative traces from morphine-treated wild-type and GIRK2/GIRK3-/- mice (Vhold = -60 mV). The traces show the increase in EPSC frequency induced by Fsk in the slice from a morphine-treated wild-type mouse (top two traces) and the lack of action of Fsk in a slice taken from a morphine-treated GIRK2/GIRK3-/- mouse (lower two traces). B) Summary plot of the frequencies (in Hz) of spontaneous EPSCs measured in LC neurons from untreated and morphine-treated wild-type (left panel) and GIRK2/GIRK3-/- (right panel) mice, in the absence (black rectangle, con) or presence (white rectangle) of Fsk (10 μM). Symbols: * p<0.05 vs. con group, within genotype and morphine-treatment groups; ** p<0.01 vs. untreated group, within Fsk treatment group.
Discussion
While a consensus has emerged that adaptations in cAMP-signaling underlie the cellular and behavioral consequences of chronic morphine administration, many questions remain. This study began by asking whether chronic morphine treatment resulted in altered opioid-induced postsynaptic currents in the mouse LC. Among the ion channels involved in the inhibitory effect of opioids on LC neurons, GIRK channels appear to mediate most of the postsynaptic inhibition (Torrecilla et al., 2002). Nevertheless, opioids also inhibit voltage-gated calcium channels in LC neurons, and both barium-sensitive and insensitive components of the residual opioid-induced current are discernable in LC neurons from GIRK2/GIRK3-/- mice (Torrecilla et al., 2002). The present work shows that exogenous activation of adenylyl cyclase can also impact the postsynaptic opioid-induced current in LC neurons. One key finding in this study is that neither the postsynaptic inhibitory effect of ME, nor the forskolin-induced augmentation of the ME-induced inhibitory current, was significantly impacted by chronic morphine treatment. Thus, alterations occurring in the LC as a consequence of prolonged morphine exposure do not appear to significantly influence the signaling pathways and channels mediating the postsynaptic inhibitory effect of opioids.
Parallel studies with mice lacking functional GIRK channels permitted the direct evaluation of modifications to less-pronounced components of the opioid-induced current that resulted from chronic morphine treatment. Though there was a slight discrepancy in the forskolin-induced augmentation of opioid inhibition measured in LC neurons from GIRK2/GIRK3-/- mice using voltage-clamp and intracellular recording techniques, both approaches indicated that any postsynaptic modification involving the cAMP-system following chronic morphine treatment is either minimal or uncoupled from the opioid-induced postsynaptic inhibition. Recent microarray studies have identified many genes exhibiting altered regulation in the rodent LC following chronic morphine treatment, including several directly involved in synaptic transmission, such as the glutamate receptor subunit GluR1 (McClung et al., 2005). Given the apparent lack of effect of chronic morphine treatment on postsynaptic opioid-dependent signaling, altered regulation of these targets is more likely to explain any change in the intrinsic excitability of LC neurons linked to chronic morphine exposure and precipitated withdrawal.
As the GIRK knockout mice used in this study were both global and constitutive in nature, it is important to consider that findings made with these mice may reflect a combination of developmental alterations or mechanisms designed to compensate for the loss of the gene(s) in question. Indeed, mice lacking GIRK2 exhibit blunted postsynaptic inhibition throughout the central nervous system (Luscher et al., 1997; Slesinger et al., 1997; Cruz et al., 2004; Koyrakh et al., 2005; Marker et al., 2006; Labouebe et al., 2007), and display a wide variety of behavioral phenotypes (Blednov et al., 2001; Blednov et al., 2002; Blednov et al., 2003; Morgan et al., 2003; Marker et al., 2004; Costa et al., 2005; Marker et al., 2005). Nevertheless, the lesion in LC neurons from GIRK2/GIRK3-/- mice appears relatively selective for GIRK channels. For example, previous work showed that ME-induced suppression of voltage-gated calcium channels is preserved in LC neurons from GIRK2/GIRK3-/- mice (Torrecilla et al., 2002). The forskolin-dependent augmentation of the opioid-induced current is normal in LC neurons from untreated GIRK2/GIRK3-/- mice. Furthermore, baseline excitatory input and the augmentation of this input by forskolin are normal in slices taken from GIRK2/GIRK3-/- mice. Thus, the most peculiar findings in this study were the lack of an action of forskolin on postsynaptic opioid-induced current in, and excitatory input to, LC neurons from morphine-treated GIRK2/GIRK3-/- mice. While the different actions of forskolin found in untreated and morphine-treated GIRK2/GIRK3-/- mice suggest that an alteration occurred in the cAMP-system, one might have reasonably predicted that forskolin would have larger actions after chronic morphine treatment. It is possible, however, that the tone of the cAMP-system is elevated in LC neurons from morphine-treated GIRK2/GIRK3-/- mice. In this case, the stimulatory effect of forskolin endpoints would be muted.
There has been some debate as to whether the elevated firing rate of LC neurons noted during opiate withdrawal reflects intrinsic modifications or alterations in afferent input. Support for intrinsic modifications has come from multiple approaches, including the failure of various inhibitors of synaptic transmission to normalize the elevated LC neuron firing rates during withdrawal (Ivanov & Aston-Jones, 2001), and manipulation of CREB levels and activity in individual LC neurons. Indeed, LC neurons expressing a constitutively-active version of CREB show elevated firing rates, while LC neurons injected with antisense oligonucleotides targeting CREB or expressing dominant-negative forms of CREB show decreased firing rates (Lane-Ladd et al., 1997; Han et al., 2006). On the other side of the debate, elevated glutamate levels have been measured in the rat LC during withdrawal (Aghajanian et al., 1994), and glutamate receptor antagonists have been shown to reduce LC firing rates to pre-treatment levels (Akaoka & Aston-Jones, 1991).
The present work supports the idea that elevated excitatory transmission facilitates the activity of LC neurons during withdrawal. Indeed, the most notable modification resulting from chronic morphine treatment seen in this study was the increase in excitatory afferent input to the LC. The frequency of spontaneous EPSCs measured in LC neurons was elevated significantly following chronic morphine treatment in both wild-type and GIRK2/GIRK3-/- mice. The enhanced excitatory input seen during withdrawal is predicted to contribute to elevated LC neuron firing rates in both wild-type and GIRK2/GIRK3-/- mice, particularly in vivo where all afferent connections are intact. Nevertheless, a recent study reported that following chronic morphine treatment, LC neuron firing rates were elevated in slices from wild-type but not GIRK2/GIRK3-/- mice (Cruz et al., 2008). While this discrepancy could be explained by a compensatory enhancement in inhibitory input to the LC of GIRK2/GIRK3-/- mice, we have found the number of spontaneous inhibitory postsynaptic currents observed in mouse LC neurons to be quite low in slice studies, irrespective of genotype. Resolution of this issue will require examination of LC neuron firing rates in vivo, both at baseline and during withdrawal. Such studies will also provide new insight into the relevance of the LC to withdrawal behavior, as GIRK2/GIRK3-/- mice exhibit a severely-attenuated naloxone-precipitated withdrawal syndrome, a phenotype that can be rescued by chemical ablation of the LC (Cruz et al., 2008).
In summary, chronic morphine treatment did not significantly influence the composite postsynaptic conductance or net inhibitory effect of opioids on LC neurons. Instead, enhanced excitatory transmission was the primary consequence of chronic morphine exposure. As such, these data support the contention that extrinsic adaptations induced by chronic morphine treatment play a significant role in the elevated excitability of LC neurons observed during opiate withdrawal.
Supplementary Material
Fsk increased the frequency of EPSCs in all groups except for morphine-treated GIRK2/GIRK3-/- mice. Forskolin had no significant effect on the amplitude of EPSCs for any group.
Acknowledgments
The authors would like to thank Dr. Christian Lüscher, as well as members of the Wickman and Williams laboratories, for reading and providing helpful feedback on this manuscript. The work was supported by National Institute of Health grants DA08163 (MT, JTW), MH61933 (KW), DA011806 (KW), and DA023793 (NQ).
Abbreviations
- cAMP
cyclic adenosine-5′-monophosphate
- CREB
cAMP-response element binding protein
- EPSC
excitatory postsynaptic current
- GIRK
G-protein-gated inwardly rectifying K+ channel
- LC
locus coeruleus
- ME
[Met]5-enkephalin
- MOR
mu opioid receptor
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione
References
- Aghajanian GK, Kogan JH, Moghaddam B. Opiate withdrawal increases glutamate and aspartate efflux in the locus coeruleus: an in vivo microdialysis study. Brain Res. 1994;636:126–130. doi: 10.1016/0006-8993(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Akaoka H, Aston-Jones G. Opiate withdrawal-induced hyperactivity of locus coeruleus neurons is substantially mediated by augmented excitatory amino acid input. J Neurosci. 1991;11:3830–3839. doi: 10.1523/JNEUROSCI.11-12-03830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alreja M, Aghajanian GK. Opiates suppress a resting sodium-dependent inward current and activate an outward potassium current in locus coeruleus neurons. J Neurosci. 1993;13:3525–3532. doi: 10.1523/JNEUROSCI.13-08-03525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alreja M, Aghajanian GK. Use of the whole-cell patch-clamp method in studies on the role of cAMP in regulating the spontaneous firing of locus coeruleus neurons. J Neurosci. 1995;59:67–75. doi: 10.1016/0165-0270(94)00195-m. [DOI] [PubMed] [Google Scholar]
- Bie B, Peng Y, Zhang Y, Pan ZZ. cAMP-mediated mechanisms for pain sensitization during opioid withdrawal. J Neurosci. 2005;25:3824–3832. doi: 10.1523/JNEUROSCI.5010-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Chang SR, Harris RA. GIRK2 deficient mice. Evidence for hyperactivity and reduced anxiety. Physiol Behav. 2001;74:109–117. doi: 10.1016/s0031-9384(01)00555-8. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Cooper R, Wallace D, Mane N, Harris RA. Hyperactivity and dopamine D1 receptor activation in mice lacking Girk2 channels. Psychopharmacology. 2002;159:370–378. doi: 10.1007/s00213-001-0937-6. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Alva H, Harris RA. A pervasive mechanism for analgesia: activation of GIRK2 channels. Proc Natl Acad Sci USA. 2003;100:277–282. doi: 10.1073/pnas.012682399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Williams JT. Increased probability of GABA release during withdrawal from morphine. J Neurosci. 1997;17:796–803. doi: 10.1523/JNEUROSCI.17-02-00796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille S, Espejo EF, Reneric JP, Cador M, Koob GF, Stinus L. Total neurochemical lesion of noradrenergic neurons of the locus ceruleus does not alter either naloxone-precipitated or spontaneous opiate withdrawal nor does it influence ability of clonidine to reverse opiate withdrawal. J Pharmacol Exp Ther. 1999;290:881–892. [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Lesions to terminals of noradrenergic locus coeruleus neurones do not inhibit opiate withdrawal behaviour in rats. Neurosci Lett. 1995;186:37–40. doi: 10.1016/0304-3940(95)11276-3. [DOI] [PubMed] [Google Scholar]
- Chieng B, Williams JT. Increased opioid inhibition of GABA release in nucleus accumbens during morphine withdrawal. J Neurosci. 1998;18:7033–7039. doi: 10.1523/JNEUROSCI.18-17-07033.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. Tolerance to opioids in single locus coeruleus neurons of the rat. NIDA Res Monogr. 1986;75:591–594. [PubMed] [Google Scholar]
- Christie MJ, Williams JT, Osborne PB, Bellchambers CE. Where is the locus in opioid withdrawal? Trends Neurosci. 1997;18:134–140. doi: 10.1016/s0165-6147(97)01045-6. [DOI] [PubMed] [Google Scholar]
- Costa AC, Stasko MR, Stoffel M, Scott-McKean JJ. G-protein-gated potassium (GIRK) channels containing the GIRK2 subunit are control hubs for pharmacologically induced hypothermic responses. J Neurosci. 2005;25:7801–7804. doi: 10.1523/JNEUROSCI.1699-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz HG, Berton F, Sollini M, Blanchet C, Pravetoni M, Wickman K, Lüscher C. Absence and rescue of morphine withdrawal in GIRK/Kir3 knock-out mice. J Neurosci. 2008;28:4069–4077. doi: 10.1523/JNEUROSCI.0267-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Lüscher C. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Duman RS, Tallman JF, Nestler EJ. Acute and chronic opiate-regulation of adenylate cyclase in brain: specific effects in locus coeruleus. J Pharmacol Exp Ther. 1988;246:1033–1039. [PubMed] [Google Scholar]
- Georges F, Le Moine C, Aston-Jones G. No effect of morphine on ventral tegmental dopamine neurons during withdrawal. J Neurosci. 2006;26:5720–5726. doi: 10.1523/JNEUROSCI.5032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack SP, Vaughan CW, Christie MJ. Modulation of GABA release during morphine withdrawal in midbrain neurons in vitro. Neuropharmacology. 2003;45:575–584. doi: 10.1016/s0028-3908(03)00205-3. [DOI] [PubMed] [Google Scholar]
- Han MH, Bolanos CA, Green TA, Olson VG, Neve RL, Liu RJ, Aghajanian GK, Nestler EJ. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ. Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci. 1998;18:10269–10276. doi: 10.1523/JNEUROSCI.18-24-10269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Aston-Jones G. Local opiate withdrawal in locus coeruleus neurons in vitro. J Neurophysiol. 2001;85:2388–2397. doi: 10.1152/jn.2001.85.6.2388. [DOI] [PubMed] [Google Scholar]
- Jolas T, Nestler EJ, Aghajanian GK. Chronic morphine increases GABA tone on serotonergic neurons of the dorsal raphe nucleus: association with an up-regulation of the cyclic AMP pathway. Neuroscience. 2000;95:433–443. doi: 10.1016/s0306-4522(99)00436-4. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Nestler EJ, Aghajanian GK. Elevated basal firing rates and enhanced responses to 8-Br-cAMP in locus coeruleus neurons in brain slices from opiate-dependent rats. Eur J Pharmacol. 1992;211:47–53. doi: 10.1016/0014-2999(92)90261-2. [DOI] [PubMed] [Google Scholar]
- Kolesnikov YA, Pick CG, Ciszewska G, Pasternak GW. Blockade of tolerance to morphine but not to kappa opioids by a nitric oxide synthase inhibitor. Proc Natl Acad Sci USA. 1993;90:5162–5166. doi: 10.1073/pnas.90.11.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyrakh L, Lujan R, Colon J, Karschin C, Kurachi Y, Karschin A, Wickman K. Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J Neurosci. 2005;25:11468–11478. doi: 10.1523/JNEUROSCI.3484-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouebe G, Lomazzi M, Cruz HG, Creton C, Lujan R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Luscher C. RGS2 modulates coupling between GABA(B) receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, Nestler EJ. CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J Neurosci. 1997;17:7890–7901. doi: 10.1523/JNEUROSCI.17-20-07890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Marker CL, Stoffel M, Wickman K. Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia. J Neurosci. 2004;24:2806–2812. doi: 10.1523/JNEUROSCI.5251-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker CL, Lujan R, Loh HH, Wickman K. Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta- but not kappa-opioids. J Neurosci. 2005;25:3551–3559. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker CL, Lujan R, Colon J, Wickman K. Distinct populations of spinal cord lamina II interneurons G protein-gated potassium channels. J Neurosci. 2006;26:12251–12259. doi: 10.1523/JNEUROSCI.3693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ, Zachariou V. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. J Neurosci. 2005;25:6005–6015. doi: 10.1523/JNEUROSCI.0062-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AD, Carroll ME, Loth AK, Stoffel M, Wickman K. Decreased cocaine self-administration in Kir3 potassium channel subunit knockout mice. Neuropsychopharmacology. 2003;28:932–938. doi: 10.1038/sj.npp.1300100. [DOI] [PubMed] [Google Scholar]
- Mouledous L, Diaz MF, Gutstein HB. Extracellular signal-regulated kinase (ERK) inhibition does not prevent the development or expression of tolerance to and dependence on morphine in the mouse. Pharmacol Biochem Behav. 2007;88:39–46. doi: 10.1016/j.pbb.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Tallman JF. Chronic morphine treatment increases cyclic AMP-dependent protein kinase activity in the rat locus coeruleus. Mol Pharmacol. 1988;33:127–132. [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Osborne PB, Williams JT. Forskolin enhancement of opioid currents in rat locus coeruleus neurons. J Neurophysiol. 1996;76:1559–1565. doi: 10.1152/jn.1996.76.3.1559. [DOI] [PubMed] [Google Scholar]
- Pepper CM, Henderson G. Opiates and opioid peptides hyperpolarize locus coeruleus neurons in vitro. Science. 1980;209:394. doi: 10.1126/science.7384811. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Beitner-Johnson DB, Krystal JH, Aghajanian GK, Nestler EJ. Opiate withdrawal and the rat locus coeruleus: Behavioral, electrophysiological, and biochemical correlates. J Neurosci. 1990;10:2308–2317. doi: 10.1523/JNEUROSCI.10-07-02308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji Y, Delfs J, Williams JT. Presynaptic inhibition of GABA(B)-mediated synaptic potentials in the ventral tegmental area during morphine withdrawal. J Neurosci. 1999;19:2347–2355. doi: 10.1523/JNEUROSCI.19-06-02347.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slesinger PA, Stoffel M, Jan YN, Jan LY. Defective γ-aminobutyric acid type B receptor-activated inwardly rectifying K+ currents in cerebellar granule cells isolated from weaver and Girk2 null mutant mice. Proc Natl Acad Sci USA. 1997;94:12210–12217. doi: 10.1073/pnas.94.22.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci. 2002;22:4328–4334. doi: 10.1523/JNEUROSCI.22-11-04328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Egan TM, North RA. Enkephalin opens potassium channels on mammalian central neurones. Nature. 1982;299:74–77. doi: 10.1038/299074a0. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fsk increased the frequency of EPSCs in all groups except for morphine-treated GIRK2/GIRK3-/- mice. Forskolin had no significant effect on the amplitude of EPSCs for any group.