Abstract
The NLR (nucleotide-binding domain leucine-rich repeat containing) family of proteins has been demonstrated to function as regulators of innate immune response against microbial pathogens. Stimulation of NOD1 and NOD2, two prototypic NLRs, results in the activation of MAPK and NF-κB. On the other hand, a different set of NLRs induces caspase-1 activation through the assembly of an inflammasome. This review discusses recent findings regarding the signaling pathways utilized by NLR proteins in the control of caspase-1 and NF-κB activation, as well as the non-redundant role of NLRs in pathogen clearance. The review also covers advances regarding the cellular localization of these proteins and the implications this may have on pathogen sensing and signal transduction.
Introduction
Nearly 20 years ago, Charles Janeway coined the term PAMP (pathogen associate molecular pattern), to define the essential chemical determinants broadly expressed on different classes of pathogens that could interact with pattern recognition receptors (PRRs) on ‘innate’ cells of the immune system and activate them for the initiation of immune functions. Since then, a number of different PRRs have been discovered and demonstrated to sense a wide-range of PAMPs, such as lipopolysaccharides (LPS), peptidoglycan (PGN), flagellin and microbial nucleic acids. PRRs can be found in the extracellular space, integrated in cellular membranes or in the cytosol. Perhaps the best-known PRRs are the Toll-like receptors (TLRs), which are localized either at the cell surface or within endosomes [1]. Cytoplamic PRRs include: RIG-1 and MDA-5, which are found in the cytosol and are involved in viral recognition [1], and the nucleotide binding oligomerization domain (Nod)-like receptor (NLR) family [2]. Our substantial understanding of how PAMPs interact with PRRs to promote host immune defense can be attributed to research in the field of TLR biology. However, in recent years, there is an emerging functional role of NLRs in complementing and synergizing with TLRs in innate immunity. NLR orthologs can be found in plants (R genes), which are required for anti-pathogen response in plants. Given the importance of R proteins in plant cell defense, one could hypothesize that mammalian NLRs are functionally analogous to plant R proteins.
Nod-like receptors
The NOD domain was first found in apoptotic protease activating factor 1 (APAF1) and its nematode homologue CED-4, which are regulators of developmental and p53-dependent programmed cell death [3,4]. Subsequently, two NOD-containing molecules, NOD1 (CARD4) and NOD2 (CARD15), were identified through database searches for APAF1/CED4 homologues. At present, the human NLR family is composed of 23 proteins, and there are at least 34 NLR genes in mice. Although primarily expressed in immune cells, some NLRs are also present in non-immune cells. NLR proteins are defined by a tripartite structure consisting of 1) a variable N-terminal protein-protein interaction domain, defined by the caspase recruitment domain (CARD), pyrin domain (PYD), or the baculovirus inhibitor domain (BIR), 2) a centrally located NOD domain that facilitates self-oligomerization during activation [5], and 3) a C-terminal leucine-rich repeat (LRR) responsible for binding/detecting PAMPs. The modular design of NLRs proteins are structurally reminiscent to plant R proteins, a family of molecules that mediates anti-pathogen responses. In plants, detection of avirulence (avr) proteins delivered by pathogenic bacteria triggers rapid activation of host defense. Detection of avr proteins by R proteins can occur either by direct detection of the corresponding avr protein (the ‘receptor-ligand’ model) or by perceiving alterations in normal cellular function(s) that are targets of avr protein action in the promotion of pathogen virulence (the ‘guard hypothesis’) [6–8]. Given the importance of R proteins in plant cell defense and the structural similarity between R proteins and NLRs, one could hypothesize that NLRs are cytosolic PRRs ‘sensing’ microbial products in the cytosol analogous to the plant R proteins.
NOD1 and NOD2 activation and signaling
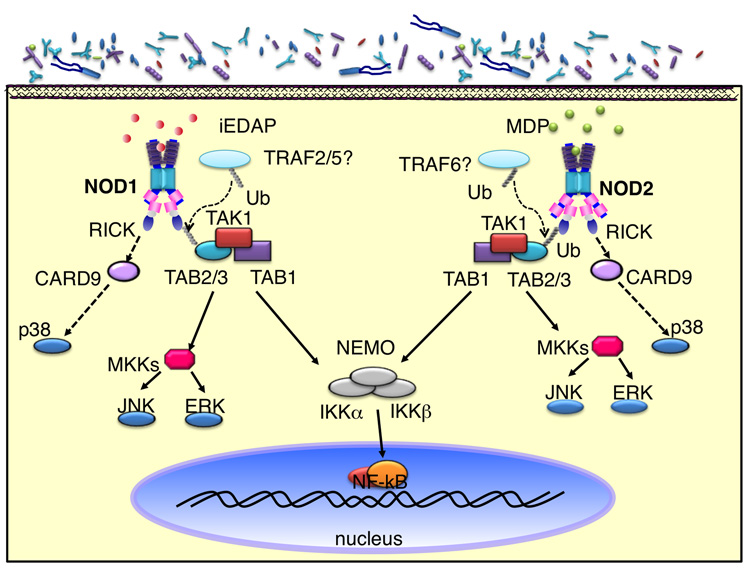
NOD1 and NOD2 can be viewed as the ‘germinal’ and the most well-studied members of the NLR family [9,10]. NOD1 is ubiquitously expressed, while NOD2 expression is restricted to monocytes, macrophages, dendritic cells and intestinal Paneth cells [2]. Initial biochemical characterization of NOD1 and NOD2 revealed that both NOD1 and NOD2 induced NF-κB activation in a TLR-independent fashion [9]. Subsequent analysis demonstrated that NOD1 and NOD2 recognize different structural core motifs derived from peptidoglycan (PGN), a component of bacterial cell walls. NOD1 activity is triggered by γ-D-glutamyl-meso-diaminopimelic acid (meso-DAP) (Figure 1), which is unique to PGN structures from all Gram-negative bacteria and certain Gram-positive bacteria, including the genus Listeria and Bacillus [11,12]. In contrast, NOD2 is activated by muramyl dipeptide (MDP) (Figure 1), a peptidogylcan motif present in all Gram-positive and –negative bacteria [13,14]. Upon ligand recognition, NOD1 and NOD2 undergo conformational changes and self-oligomerization. This is followed by the recruitment and activation of the serine threonine kinase RICK (RIP2), essential for the activation of NF-κB and MAPKs [5,15–17]. Similar to TLR signaling, recent studies have demonstrated that K63-linked regulatory ubiquitination of RICK is essential for the recruitment of TAK1 [18,19], a kinase required for the activation of the MAPK and IKK complex [20]. As in the case of TLRs, this modification can be removed by the deubiquitinating enzyme A20, thus dampening NOD1/NOD2-induced NF-κB activation [18,19]. Although, both NOD1 and NOD2 induce similar K63-linked ubiquitination of RICK for NF-κB activation, Nod2-signaling appears to preferentially utilize the E3 ligase TRAF6, whereas TRAF2 and TRAF5 were shown to be important for NOD1-mediated signaling. Nevertheless, NOD1- and NOD2-mediated activation of NF-κB results in the upregulated transcription and production of inflammatory mediators.
Figure 1. NOD1/NOD2 activation and signaling.
NOD1 and NOD2 sense peptidoglycan-derived motifs in the cytosol and form a complex with RICK. K63-linked regulatory ubiquitination of RICK leads to the recruitment of TAK1. The activation and recruitment of TAK1 complex to RICK further recruits the IKK complex. The interaction between RICK and NEMO ultimately results in the activation of IKKs and MKK by TAK1.
In contrast to the molecular events leading to NOD1 and NOD2-induced NF-κB activation, signaling intermediates involved in NOD1 and NOD2-mediated MAPK (p38, JNK, Erk) activation are not as well-characterized, but involve similar upstream component such as RICK and TAK1 [16,21,22]. Recently it was demonstrated that ectopically expressed CARD9, an essential protein in anti-fungal responses and TLR signaling, interacts with NOD2 after challenge with L. monocytogenes. Furthermore, CARD9-deficient macrophages exhibited impaired phosphorylation of p38 after MDP stimulation, suggesting that CARD9 is another essential intermediate in the NOD2 signaling cascade, that acts downstream of TAK1 [23].
Given the cellular localization of TLRs and NLRs, one would predict that the functions of TLRs and NLRs are mutually exclusive. However, there is ample evidence to suggest that these PRRs can have complementary as well as non-redundant functions. The synergistic effect of TLR and NLR signaling can be demonstrated when stimulating cells with peptidoglycan [24,25] or with TLR and NOD2 agonists [26]. Furthermore, complementary roles of NLRs and TLRs in an inflammatory response have been demonstrated with the two-step process leading to IL-1β production and secretion. TLR-mediated NF-κB is required for the production of pro-IL-1β while the cleavage of pro-IL-1β into its biologically active form is dependent on NLR-mediated caspase-1 activation [27]. This sequential pathway, differentially regulated by TLRs and NLRs, may serve as a safeguard against host-immunopathology resulting from excessive IL-1β production.
More recently, it has become apparent that NLRs and TLRs signaling pathways may have non-redundant roles. In situations whereby cells are rendered refractory to TLR agonists, e.g. within the intestinal epithelium or following the induction of LPS tolerance, NOD1/2 signaling was not dampened but rather increased by the enhanced expression of RICK [28]. In such a scenario, it was demonstrated that after the induction of tolerance, host resistance against L. monocytogenes infection was greatly reduced in the absence of NOD1 and NOD2 signaling [28].
Cellular Localization of NOD1 and NOD2
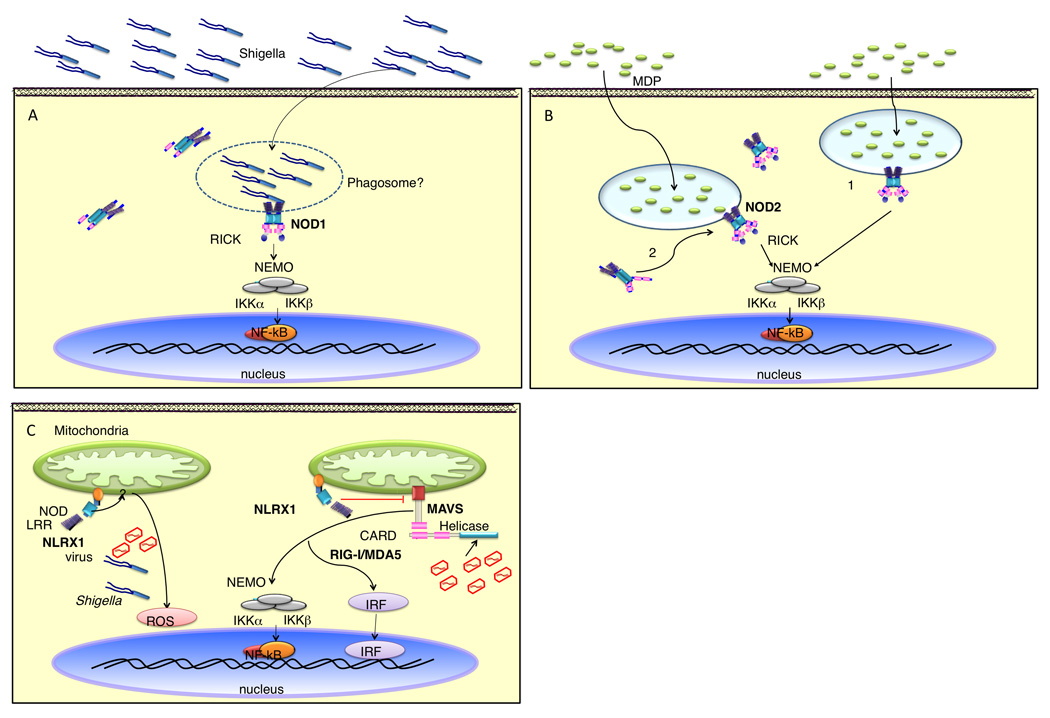
Despite very low expression of endogenous proteins, in vitro enforced protein expression of NLRs has shed some light to the intracellular distribution of these molecules. In addition to their cytosolic localization, both NOD1 and NOD2 were also found to be associated with the plasma membrane [29,30]. The plasma membrane association of NOD1 and NOD2 has been linked to activation of downstream signaling events since point-mutations of NOD1 and NOD2 that interfered with their capacity to become membrane-associated resulted in blunted NOD1- and NOD2-mediated NF-κB activation [29]. However, the latter studies are difficult to interpret because the same mutations also interfered with MDP recognition [31]. In the case of NOD1, it was shown that NOD1 can localize to the entry foci formed by Shigella flexneri (Figure 2a) [30]. One could speculate that the plasma membrane localization of NOD1 may play a role not only for the detection of invading pathogens that inject or release NOD1 agonists, but also for maintaining tissue homeostasis against commensal flora of the gut. As for NOD2, it was demonstrated that, upon internalization by macrophages, MDP is localized to acidified vesicles (Figure 2b) [32]. Although it is unclear whether NOD2 is actively recruited to these structures, the colocalization of MDP to these vesicular structures suggests that NOD2-mediated NF-κB and MAPK activation may be emanating from these vesicles [32]. Recently, two groups independently identified NLRX1, as a NLR that localizes to mitochondria (Figure 2c) [33,34]. Although the function of NLRX1 is unclear, the association of NLRX1 with mitochondria appears to be a requisite for its function [33,34]. Therefore, the intracellular localization of NLRs is emerging as a critical determinant of its function.
Figure 2. Cellular localization of NOD1 and NOD2.
A) During S. flexneri infection NOD1 is recruited to the bacterial entry site. It is unclear whether NOD1 is recruited to the bacterial contact foci from the cytosol or the plasma membrane fraction. Nevertheless, the co-localization of NOD1 with the bacterial contact point suggests that PGN sensing and signaling by NOD1 occurs at this initial site of contact. B) MDP is internalized into an acidified endosomal compartment. NOD2 could be sensing MDP at the plasma membrane and becomes associated with the vesicle following invagination of the plasma membrane during vesicle formation (1). Alternatively, NOD2 could be actively recruited to the MDP-containing vesicle after internalization (2). The localization of MDP to vesicle suggests that NOD2 signaling may be emanating from these vesicular structures. C) NLRX1, a mitochondria-targeted NLR protein, might act as a negative regulator of MAVS signalling, diminishing virally induced RIG-like helicase interactions. In a different model, NLRX1 promotes ROS production at the mitochondria, which consequently helps to fight bacteria and viruses. How NLRX1 is activated remains unclear.
NLRs and the Inflammasome
In addition to activating NF-κB and MAPK, NLRs are also involved in the formation of the ‘inflammasome’. The inflammasome is a signaling platform scaffolded by NLR proteins and mediates the activation of caspase-1, which is required for the processing and maturation of the pro-inflammatory cytokines, IL-1β and IL-18. To date there are three known NLRs that participate in the formation of inflammasome complexes: NLRP1, NLRP3 and NLRC4. Common to these inflammasomes is the role of ASC as the adaptor protein that links these NLRs to caspase-1 (Figure 3) [35,36].
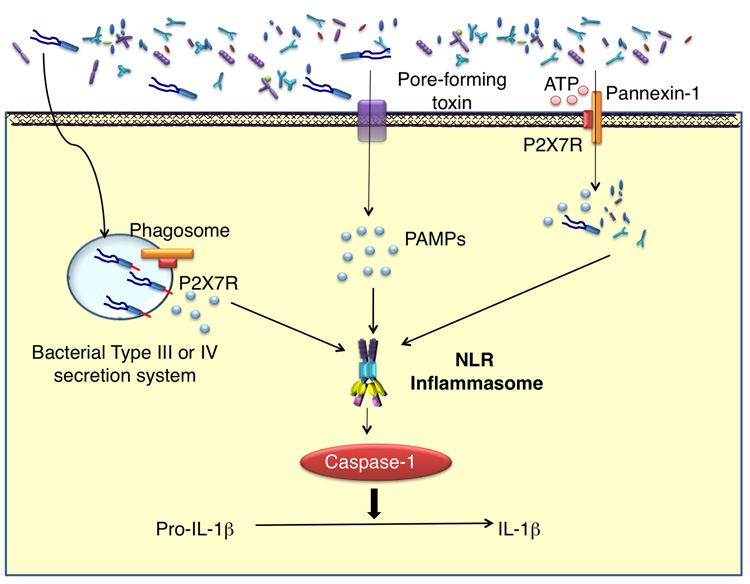
Figure 3. Productive IL-1β secretion is a two-step process.
TLR signaling induces the synthesis of pro-IL-1β. Bacteria and/or bacterial products enter the cytosol either through the actions of pore-forming toxins or ATP-mediated activation of the pannexin-1 pore. Activation of NLRs by cytosolic PAMPs promotes the formation of caspase-1 activating ‘inflammasome’. Active caspase-1 then processes the IL-1β precursor into the bioactive form for secretion.
In vivo, NLRP1c senses Bacillus anthracis lethal toxin, which is delivered in the cytoplasm by receptor-mediated endocytosis [37]. NLRC4 has been demonstrated to mediate caspase-1 activation, independent of TLR5, following cytosolic delivery of flagellin. Various intracellular bacteria have the ability to activate caspase-1 in a NLRC4-dependant manner: Salmonella typhimurium [38,39], Pseudomonas aeruginosa [40,41] and Legionella pneumophila [42]. Because activation of caspase-1 by S. typhimurium and L. pneumophila flagellin depends on functional type-III and –IV secretion systems, respectively, it is hypothesized that flagellin and/or other bacterial products might leak into the cytosol through pores generated by these bacterial secretion systems. As will be discussed below, the activation of caspase-1 by NLRC4 does not require ATP nor low intracellular potassium concentrations, in contrast to the NLRP3 inflammasome [43,44]. NLRP3 mediates activation of caspase-1 in response to a variety of structurally unrelated stimuli, not limiting to those of bacterial origin. The repertoire of agonists capable of inducing NLRP3-dependent caspase-1 activation include: nucleic acids such as bacterial and viral RNA [45,46], monosodium urate crystals [47], changes in ion concentrations, particularly potassium, following exposure to bacterial pore-forming toxins such as nigericin and maitotoxin [48] and reactive oxygen species produced following asbestos and silica exposure [49]. Insights into how NLRP3 is able to respond to such a wide-range of agonists came from the observation that ATP stimulation of primed macrophages, which is mediated via the purigenic P2X7 receptor, not only enhanced caspase-1 activation and IL-1β production, but also induced pore formation by the hemichannel protein pannexin-1 [50,51]. This ATP-induced pore formation then either permits bacterial products to enter the cytosol and/or alters intracellular potassium levels thereby activating NLRP3 and caspase-1 (Figure 3) [27]. Based on these experiments one could hypothesize that activators of NLRP3 are all markers of cells in stress, and therefore NLRP3 may serve as a general sensor for cellular stress or injury. Furthermore, there appears to be complementation between NLRP3- and NOD2-signaling for caspase-1 activation and IL-1β secretion following MDP stimulation. [27,32].
Concluding remarks
In the past few years, many discoveries have been made in the field of NLRs regarding the functional roles of these proteins in innate immunity. Yet the question of how NLRs ‘sense’ their ‘ligands’ remains to be fully elucidated. Despite the similarities between NLRs and other LRR-containing pathogen-recognition molecules, there is a scarcity of evidence to support that NLRs directly bind and/or recognize pathogens or pathogen-derived products. The presence of LRRs in NLRs, analogous to mammalian TLRs and the plant R-proteins, would certainly suggest that LRR of NLRs are involved in binding pathogen products. This assumption may be true for some, but not all NLRs. LRR motifs-containing proteins are involved in many biologically important processes such as early mammalian development [52] and cell polarization [53]. In all these processes, the LRR domain is most likely mediating protein-protein interactions. Therefore the mere presence of LRRs does not necessarily endow NLRs with the capacity for pathogen recognition. On the other hand, there is convincing evidence that NLRs do indeed play important roles in innate immune responses against intracellular pathogens. Therefore, it may be accomodating to view NLRs as modifiers of host response to various pathogens.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 2.Inohara, Chamaillard, McDonald C, Nuñez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 3.Derry WB, Putzke AP, Rothman JH. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science. 2001;294:591–595. doi: 10.1126/science.1065486. [DOI] [PubMed] [Google Scholar]
- 4.Liu QA, Hengartner MO. The molecular mechanism of programmed cell death in C. elegans. Ann N Y Acad Sci. 1999;887:92–104. doi: 10.1111/j.1749-6632.1999.tb07925.x. [DOI] [PubMed] [Google Scholar]
- 5.Inohara N, Koseki T, Lin J, del Peso L, Lucas PC, Chen FF, Ogura Y, Nunez G. An induced proximity model for NF-kappa B activation in the Nod1/RICK and RIP signaling pathways. J Biol Chem. 2000;275:27823–27831. doi: 10.1074/jbc.M003415200. [DOI] [PubMed] [Google Scholar]
- 6.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 8.Van der Biezen EA, Jones JD. Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem Sci. 1998;23:454–456. doi: 10.1016/s0968-0004(98)01311-5. [DOI] [PubMed] [Google Scholar]
- 9.Inohara N, Ogura Y, Chen FF, Muto A, Nuñez G. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem. 2001;276:2551–2554. doi: 10.1074/jbc.M009728200. [DOI] [PubMed] [Google Scholar]
- 10.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nuñez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 11.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 12.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 13.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 14.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 15•.Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, et al. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155.This paper shows that NOD1 is recruited to S. flexneri point of contact and that NEMO, a downstream effector of NOD1 signaling, was also detectable at the bacterial-cell contact site.
- 16.Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, Inohara N, Nuñez G. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Kim YG, Shaw M, Kanneganti TD, Fujimoto Y, Fukase K, Inohara N, Nuñez G. Nod1/RICK and TLR signaling regulate chemokine and antimicrobial innate immune responses in mesothelial cells. J Immunol. 2007;179:514–521. doi: 10.4049/jimmunol.179.1.514. [DOI] [PubMed] [Google Scholar]
- 18•.Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Nuñez G, Inohara N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. EMBO J. 2008;27:373–383. doi: 10.1038/sj.emboj.7601962.This paper demonstrated that NOD1/NOD2 signaling requires RICK to be ubiquitinated at lysine 209. Ubiquitination of RICK was found to mediate the recruitment of TAK1, a kinase essential for NOD1-induced NF-κB activation.
- 19•.Hitotsumatsu O, Ahmad RC, Tavares R, Wang M, Philpott D, Turer EE, Lee BL, Shiffin N, Advincula R, Malynn BA, et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity. 2008;28:381–390. doi: 10.1016/j.immuni.2008.02.002.Similar to the previous paper, this group demonstrated that MDP induces ubiquitination of RICK in primary macrophages. In addition, it was shown that the ubiquitin-editing enzyme A20 dampens NOD2 induced signals in vitro and in vivo.
- 20.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 21.da Silva Correia J, Miranda Y, Leonard N, Hsu J, Ulevitch RJ. Regulation of Nod1-mediated signaling pathways. Cell Death Differ. 2007;14:830–839. doi: 10.1038/sj.cdd.4402070. [DOI] [PubMed] [Google Scholar]
- 22.Windheim M, Lang C, Peggie M, Plater LA, Cohen P. Molecular mechanisms involved in the regulation of cytokine production by muramyl dipeptide. Biochem J. 2007;404:179–190. doi: 10.1042/BJ20061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O, Qin XF, Dong C, Lin X. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8:198–205. doi: 10.1038/ni1426.This study demonstrates that CARD9, an essential protein in anti-fungal responses and TLR signaling, interacts with NOD2 after challenge with L. monocytogenes. Furthermore, CARD9-deficient macrophages exhibited impaired phosphorylation of p38 after MDP stimulation, suggesting that CARD9 is another essential intermediate in the NOD2 signaling cascade, that acts downstream of TAK1.
- 24.Netea MG, Ferwerda G, de Jong DJ, Jansen T, Jacobs L, Kramer M, Naber TH, Drenth JP, Girardin SE, Kullberg BJ, et al. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J Immunol. 2005;174:6518–6523. doi: 10.4049/jimmunol.174.10.6518. [DOI] [PubMed] [Google Scholar]
- 25.Uehara A, Yang S, Fujimoto Y, Fukase K, Kusumoto S, Shibata K, Sugawara S, Takada H. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell Microbiol. 2005;7:53–61. doi: 10.1111/j.1462-5822.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 27•.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nuñez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008.This study demonstrates that ATP stimulation of primed macrophages, which is mediated via the purigenic P2X7 receptor, not only enhanced caspase-1 activation and IL-1β production, but also induced pore formation by the hemichannel protein pannexin-1. This ATP-induced pore formation then either permits bacterial products to enter the cytosol and/or alters intracellular potassium levels thereby activating NLRP3 and caspase-1
- 28•.Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nuñez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012.This paper demonstrates that there is no cross-tolerization between TLR- and NOD1/NOD2-agonist. Furthermore, it was demonstrated following the induction of TLR tolerance, NOD1/2 signaling was not dampened but rather increased by the enhanced expression of RICK. After the induction of tolerance, host resistance against L. monocytogenes infection was greatly reduced in the absence of NOD1 and NOD2 signaling
- 29.Barnich N, Aguirre JE, Reinecker HC, Xavier R, Podolsky DK. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor-{kappa}B activation in muramyl dipeptide recognition. J Cell Biol. 2005;170:21–26. doi: 10.1083/jcb.200502153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Kufer TA, Kremmer E, Adam AC, Philpott DJ, Sansonetti PJ. The pattern recognition molecule Nod1 is localized at the plasma membrane at sites of bacterial interaction. Cell Microbiol. 2008;10:477–486. doi: 10.1111/j.1462-5822.2007.01062.x.This study investigates the localization of NOD1 during S. flexneri infection and demonstrates that NOD1 is recruited to the site of bacterial entry. Furthermore, this paper also demonstrate that NEMO is co-localized with NOD1 during S. flexneri infection.
- 31.Lecine P, Esmiol S, Metais JY, Nicoletti C, Nourry C, McDonald C, Nunez G, Hugot JP, Borg JP, Ollendorff V. The NOD2-RICK complex signals from the plasma membrane. J Biol Chem. 2007;282:15197–15207. doi: 10.1074/jbc.M606242200. [DOI] [PubMed] [Google Scholar]
- 32•.Marina-Garcia N, Franchi L, Kim YG, Miller D, McDonald C, Boons GJ, Nuñez G. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via Cryopyrin/NLRP3 independently of Nod2. J Immunol. 2008;180:4050–4057. doi: 10.4049/jimmunol.180.6.4050.it was demonstrated in this paper that, upon internalization by macrophages, MDP is localized to acidified vesicles. Although it is unclear whether NOD2 is actively recruited to these structures, the colocalization of MDP to these vesicular structures suggests that NOD2-mediated NF-κB and MAPK activation may be emanating from these vesicles.
- 33•.Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 34•.Tattoli I, Carneiro LA, Jehanno M, Magalhaes JG, Shu Y, Philpott DJ, Arnoult D, Girardin SE. NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production. EMBO Rep. 2008;9:293–300. doi: 10.1038/sj.embor.7401161.Both of these two papers independently identified NLRX1 as a mitochondrialtargeted NLR protein. Although the mechanism of NLRX1 function is unclear, the mitochondrial-localization of NLRX1 appears to be essential.
- 35.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 36.Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277:21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 37.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 38.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 39.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 40.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Nunez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 41.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 43.Franchi LL, Kanneganti TDT-D, Dubyak GRGR, Núñez GG. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J. Biol. Chem. 2007;282:18810. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 44.Pétrilli VV, Papin SS, Dostert CC, Mayor AA, Martinon FF, Tschopp JJ. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Diff. 2007;14:1583. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 45.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 46.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 47.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 48.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 49.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tong ZB, Nelson LM, Dean J. Mater encodes a maternal protein in mice with a leucine-rich repeat domain homologous to porcine ribonuclease inhibitor. Mamm Genome. 2000;11:281–287. doi: 10.1007/s003350010053. [DOI] [PubMed] [Google Scholar]
- 53.Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]