Abstract
Integrity of the extracellular matrix (ECM) is essential for maintaining the normal structure and function of connective tissues. ECM is secreted locally by cells and organized into a complex meshwork providing physical support to cells, tissues, and organs. Initially thought to act only as a scaffold, the ECM is now known to provide a myriad of signals to cells regulating all aspects of their phenotype from morphology to differentiation. Matricellular proteins are a class of ECM related molecules defined through their ability to modulate cell–matrix interactions. Matricellular proteins are expressed at high levels during development, but typically only appear in postnatal tissue in wound repair or disease, where their levels increase substantially. Members of the CCN family, tenascin-C, osteopontin, secreted protein acidic rich in cysteine (SPARC), bone sialoprotein, thrombospondins, and galectins have all been classed as matricellular proteins. Periostin, a 90 kDa secreted homophilic cell adhesion protein, was recently added to matricellular class of proteins based on its expression pattern and function during development as well as in wound repair. Periostin is expressed in connective tissues including the periodontal ligament, tendons, skin and bone, and is also prominent in neoplastic tissues, cardiovascular disease, as well as in connective tissue wound repair. This review will focus on the functional role of periostin in tissue physiology. Fundamentally, it appears that periostin influences cell behaviour as well as collagen fibrillogenesis, and therefore exerts control over the structural and functional properties of connective tissues in both health and disease. Periostin is a novel matricellular protein with close homology to Drosophila fasciclin 1. In this review, the functional role of periostin is discussed in the context of connective tissue physiology, in development, disease, and wound repair.
Keywords: Periostin, Matricellular protein, Connective tissue, Marfan’s syndrome, Wound repair
Introduction
The extracellular matrix (ECM) is a key regulator of cell behaviour, providing molecular signals to resident cell populations that are essential for maintenance of normal connective tissue structure and function (Berrier and Yamada 2007; Culav et al. 1999; Lukashev and Werb 1998; Stamenkovic 2003). ECM is composed of many different proteins, including the structural proteins fibronectin, collagens, laminins, vitronectin, as well as specialized proteins such as proteoglycans, glycoproteins, growth factors, and matrix metalloproteinases (Stamenkovic 2003; Tayebjee et al. 2003). It is the amount, type, and composition of the ECM that give connective tissues their unique properties (Culav et al. 1999; Lukashev and Werb 1998). The ECM is a dynamic structure, continually remodeling in response to mechanical stimuli, integrin signaling, and pathology (Berk et al. 2007; Gallagher et al. 2007; Hinz and Gabbiani 2003; Larsen et al. 2006). As molecular techniques have advanced, it has been possible to learn more about ECM remodeling and the functions of each protein subclasses. In particular genetic knockout mice have proven an excellent model for investigating ECM molecules (Muller 1999). Such models have highlighted that during development and wound repair, synthesis of matrix components can be highly transient, providing organizational cues to specific cell populations in a tightly controlled, time dependent manner. Adhesion of cells to ECM through integrin receptors regulates their shape, proliferation, intracellular signaling and differentiation, thus maintaining normal tissue function (Humphries et al. 2004; Lock et al. 2008; Zelenka 2004).
During wound repair and certain pathologies, changes occur in the composition of the ECM, providing signals to the cells that mediate repair or if misregulated, can result in development of various pathologies (Berk et al. 2007; Darby and Hewitson 2007; Grzesik and Narayanan 2002; Midwood et al. 2004; Raines 2000). In 2000, Paul Bornstein proposed that there was a family of secreted ECM proteins that could be linked through their common functionality. They termed these proteins “matricellular” proteins to highlight their influence on cell–matrix interactions. Matricellular proteins are important during development, but are typically restricted to tissue remodeling and wound repair in the normal adult. Matricellular proteins interact with cell surface receptors such as integrins and are able to bind growth factors as well as to the structural components of the matrix such as collagen(Baril et al. 2007; Butcher et al. 2007; Gillan et al. 2002; Shimazaki et al. 2008). Based on this definition, several proteins have now been identified as matricellular proteins (Bornstein 2000), including connective tissue growth factors (Leask and Abraham 2006), thrombospondins (Bornstein et al. 2000; Chen et al. 2000), and galectins (Elola et al. 2007; He and Baum 2006). A comprehensive list of known matricellular proteins is shown in Table 1 and functions of matricellular proteins in Table 2. However, in this review, the focus will be on periostin, a relatively recent addition to the matricellular protein family, despite being first identified 15 years ago as osteoblast specific factor-2 (OSF-2; Takeshita et al. 1993).
Table 1.
List of known matricellular proteins
| Matricellular proteins | |
|---|---|
| Bone sialoprotein | Periostin |
| CCN2 | SPARC (Osteonectin) |
| Cyr61 | Tenascin-C |
| Galectin 1, 2, 3, 4, 8, and 9 | Thrombospondin-1 and 2 |
| Nov | WISP-1, 2 and 3 |
| Osteopontin | |
Table 2.
Functions of matricellular proteins
| Known effects of matricellular proteins cell physiology | |
|---|---|
| Cell adhesion | Cell de-adhesion |
| ECM synthesis | Collagen fibrillogenesis |
| Proliferation | Apoptosis |
| Differentiation | De-differentiation |
| Migration | Growth factor production |
| Morphology | Biomineralization |
Periostin: a novel matricellular protein
Periostin was originally identified as an 811-amino acid protein secreted by murine osteoblasts, which had structural homology to the insect axonal guidance protein fasciclin-1(Takeshita et al. 1993). Originally termed osteoblast specific factor-2 (OSF-2), it was renamed periostin due localized expression in the periosteum and the periodontal ligament (Kruzynska-Frejtag et al. 2004). In humans, the periostin gene is located on chromosome 13, at map position 13q13.3, and the protein is 835 amino acids in size. Periostin is a disulfide linked 90-kDa heparin-binding N terminus-glycosylated protein, containing four tandem fasciclin (Fas1) domains (Kudo et al. 2007; Litvin et al. 2004). Norris and colleagues, in 2008, were the first to propose that periostin should be classed as a matricellular protein, based on its apparent biological functions in the developing murine cardiac system (Norris et al. 2008a).
Due to an explosion of papers in the last 2 years, expression of periostin has now been confirmed in many other tissues and pathologies. Thus far, periostin has been found in bone (Horiuchi et al. 1999; Litvin et al. 2004; Nakazawa et al. 2004; Oshima et al. 2002), skin (Norris et al. 2007; Roy et al. 2007; Tilman et al. 2007), periodontal ligament (Horiuchi et al. 1999; Kii and Kudo 2007; Kruzynska-Frejtag et al. 2004; Lallier and Spencer 2007; Suzuki et al. 2004), muscle injury (Goetsch et al. 2003; Kudo et al. 2004), vascular injury(Lindner et al. 2005), myocardial infarction (Dorn 2007; Iekushi et al. 2007; Oka et al. 2007; Shimazaki et al. 2008), epithelial ovarian cancer(Gillan et al. 2002), colorectal cancer (Tai et al. 2005), and pulmonary vascular remodeling (Li et al. 2004; Woodruff et al. 2007). Furthermore, periostin expression is known to be prominent in fibrotic conditions, including sub-epithelial fibrosis in bronchial asthma (Takayama et al. 2006), as well as in bone marrow fibrosis (Oku et al. 2008). Many of the initial research performed on periostin was descriptive in nature, confirming periostin expression in different tissues and pathologies. However, with the derivation of the periostin knockout mouse model (Kii et al. 2006; Rios et al. 2005), the functions of periostin in development, wound repair, and disease, are beginning to be revealed.
Phenotype of the periostin knockout mouse
The first description of the periostin knockout mouse was by Rios and colleagues in 2005 (Rios et al. 2005). The phenotype of the periostin knockout mouse is of great interest due to the number of tissues affected. As with other matricellular proteins, periostin deficiency does not result in embryonic lethality, although approximately 14% of the pups die postnatally prior to weaning. Periostin expression is most common in collagen rich connective tissues. Deletion of periostin results in severe growth retardation, suggesting periostin is essential for postnatal development. Histological analysis of the periostin knockout mice demonstrated a lack of trabecular bone, severe incisor enamel defects, periodontal disease, cartilage and cardiac valve defects. However, when the mice are placed on a soft diet, growth retardation is attenuated suggesting that this maybe due to eating difficulties as a result of the lesions in the periodontium (Rios et al. 2005).
Aside from the CCN2 knockout (Kuiper et al. 2007), mice that lack matricellular proteins tend to have mild phenotypes that become more severe under injury or disease conditions (Bornstein and Sage 2002). Deletion of matricellular proteins such as TSP-1 and -2, SPARC, galectins, or tenascin-C affect many tissue types, but more commonly these mice exhibit an altered response to tissue injury (Bornstein et al. 2000, 2004; Elola et al. 2007; Gruber et al. 2005; Park et al. 2004; Yan and Sage 1999; Yang et al. 2000). However, the loss of periostin appears to be more severe, with significant damage occurring in connective tissues during postnatal development. Interestingly, all matricellular proteins are known to play a major role in normal wound repair, but the role of periostin is not as clear.
Wound repair
Wound repair is a serious of overlapping events that begin immediately after wounding (platelet aggregation) until matrix contraction results in tissue closure (Midwood et al. 2004). While fibrin, collagen, and fibronectin provide structural support to the matrix during wound repair, matricellular proteins act by providing specific signals to the constituent cell populations, modulating their phenotype (Alford and Hankenson 2006; Kyriakides and Bornstein 2003). Each protein is expressed at different stages of wound repair and some patterns are more transient than others. However, to date, the expression profile of periostin in the wound repair process has yet to be elucidated. Thus far, expression of periostin in tissue repair has been investigated predominantly within the vascular and cardiac systems (Dorn 2007; Kuhn et al. 2007; Lindner et al. 2005; Litvin et al. 2007; Norris et al. 2008a; Shimazaki et al. 2008), and to a lesser extent in chronic dermal wounds (Roy et al. 2007), muscle (Goetsch et al. 2003), and bone fracture (Nakazawa et al. 2004). Interestingly, the regulatory processes, including matricellular protein expression, responsible for normal development of bone, cartilage and cardiac tissue also play a major role in their pathogenesis in adults.
Periostin was initially identified in periosteum and bone (Horiuchi et al. 1999; Takeshita et al. 1993), and in bone fracture, periostin mRNA is upregulated twofold and localizes to preosteoblastic cells within the periosteum, as well as in undifferentiated mesenchymal cells close to the fracture site (Nakazawa et al. 2004). The periostin mRNA signal peaks at day 7, and is significantly reduced by day 14, where the undifferentiated mesenchymal cells no longer express periostin mRNA. It seems likely that periostin plays a role in recruitment of pre-osteoblast cells into the provisional callus during fracture healing (Kojima et al. 2007; Nakazawa et al. 2004). However, the importance of periostin for mesenchymal cell physiology is not limited to only bone and periosteum.
In periostin knockout mice, large numbers of undifferentiated mesenchymal cells remain in the heart tissue after development (Butcher et al. 2007; Norris et al. 2008b), suggesting that periostin maybe required in the differentiation of mesenchymal progenitors to cardiomyocytes. Periostin has been observed to increase the number of cardiomyocytes actively replicating DNA in rats after myocardial infarction (Kuhn et al. 2007). In particular, periostin is expressed by cardiac fibroblasts where it interacts with integrins on cells likely modulating their behaviour during the remodeling process following infarct (Shimazaki et al. 2008). However, it is still not clear if periostin acts on cardiomyocytes directly, or support cells only such as the cardiac fibroblasts (Dorn 2007).
In vascular remodeling, which can be induced through balloon injury, periostin mRNA levels strongly increase (Lindner et al. 2005). Periostin expression after injury was localized to smooth muscle cells of the neointima and the adventitia. This is similar to the expression pattern of other matricellular proteins including tenascin-C after balloon injury (Majesky 1994; Wallner et al. 2002), suggesting that periostin may perform similar functions in such situations. Significantly, over expression of periostin enhances smooth muscle cell migration in vitro (Li et al. 2006), and may have a de-adhesive activity similar to tenascin-C and other matricellular proteins (Murphy-Ullrich 2001). While matricellular proteins appear important in arterial remodeling, in vein grafts molecules such as tenascin-C appear to contribute to intimal hyperplasia and ultimate graft failure (Wallner et al. 1999). It will be of great interest to assess if periostin is also expressed in vein grafts during remodeling in the arterial system.
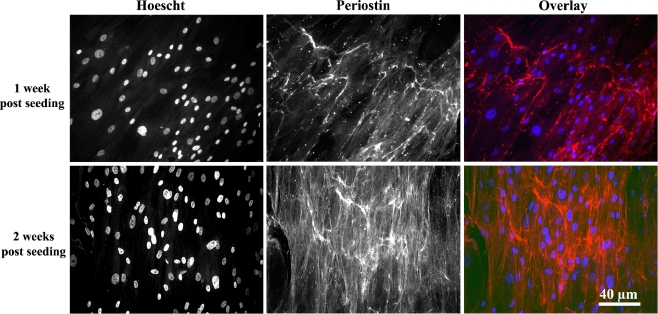
From the information highlighted above, it appears that periostin, like other matricellular proteins, can be considered to play a fundamental role in tissue remodeling. Periostin is known to interact with integrins, influencing cell–matrix interactions, adhesion, proliferation and differentiation processes (Kudo et al. 2007). The focus in our laboratory is on wound healing in the periodontium, particularly in the presence of biomaterials (Hamilton and Brunette 2007; Hamilton et al. 2006, 2007; Schuler et al. 2006). We have recently shown secretion of periostin in to the ECM by human gingival fibroblasts cultured on titanium in vitro, but only in the presence of ascorbic acid (Fig. 1). This is suggestive that periostin may closely associate with collagen synthesis on titanium. Indeed, it appears that periostin is essential for certain parts of the collagen assembly process.
Fig. 1.
Extracellular localization of periostin protein in ECM secreted by human gingival fibroblasts cultured on titanium
Periostin–ECM interactions: influence on collagen fibrillogenesis
Collagen fibrils are the ECM component allowing connective tissues to withstand tensile forces, and tissues such as ligaments, tendons, bone, cartilage, and skin contain large numbers of collagen fibrils thus allowing dispersal of such forces (Canty and Kadler 2005; Culav et al. 1999). Collagen fibrillogenesis is a complex multi-step process that is still poorly understood (Canty and Kadler 2005). Although it was initially thought that secreted collagen might self assemble, evidence is now quickly accumulating that other ECM proteins are required. Matricellular proteins in particular appear to be of importance in collagen assembly (Bornstein et al. 2000, 2004; ; Yang et al. 2000). For example, deletion of the thrombospondin-2 gene has shown to disrupt collagen fibrillogenesis (Bornstein et al. 2000), and in SPARC null mice, collagen fibrils are significantly smaller (Martinek et al. 2007).
Expression of periostin is common in collagen rich tissues, suggesting that it may influence collagen fibrillogenesis (Borg and Markwald 2007). Interestingly, periostin protein is routinely present in adult animals in tissues such as the periodontal ligament (Horiuchi et al. 1999; Tomokiyo et al. 2008; Wilde et al. 2003), unlike many of the other matricellular proteins, further suggesting that it plays a key role in adult tissues. Norris et al. 2007, investigated the role of periostin in collagen I fibrillogenesis in murine connective tissues including periodontal ligament, tendon, skin and atrioventricular valves. Using co-immunoprecipitation techniques, they identified that periostin directly binds to collagen type I, and in periostin knockout mice, collagen fiber diameter and cross-linking are significantly reduced. Furthermore, biomechanical testing of skin samples from knockout and wild type mice highlighted a reduced modulus of elasticity and lower ultimate stress in samples from periostin knockouts. They concluded that periostin appeared to influence maturation and assembly of collagen I fibrils. This hypothesis is backed further from the observations that periostin null mice also appear to be unable to support normal valvular remodeling or maturation of the cardiac skeleton (Butcher et al. 2007; Norris et al. 2008b; Snider et al. 2008). The hypothesis that periostin plays an important role in collagen fibrillogenesis has significant implications for connective tissue disease, where defects in collagen and elastin production result in chronic fibrotic conditions (Abraham et al. 1982; Leask et al. 2004; Uitto 1979; Uitto and Lichtenstein 1976).
Connective tissue disease and periostin expression: insights from the periostin−/− mouse phenotype
Connective tissue diseases are disorders featuring abnormalities commonly involving the ECM proteins collagen and elastin. Several chronic connective tissue diseases have been identified, including Marfan’s syndrome (fibrillin defect), scleroderma (collagen over-production), Ehlers–Danlos (defect in collagen synthesis), and pseudoxanthoma elasticum (elastin defect). Matricellular proteins have already been implicated in such disease conditions; CCN2 in scleroderma (Leask et al. 2004), and thrombospondin-2 in rheumatoid arthritis (Park et al. 2004).
As more is learned about connective tissue diseases, insights are gained into the possible involvement of periostin (Erkan et al. 2007; Oku et al. 2008; Takayama et al. 2006). In this review, we will specifically deal with Marfan’s syndrome (Callewaert et al. 2008). Although the condition has been genetically linked to defects in fibrillin (Nasuti et al. 2004), the exact mechanisms underlying the condition are still not well understood (Boileau et al. 2005; Collod-Beroud and Boileau 2002; Robinson and Booms 2001; Whiteman et al. 2006). In vitro studies have shown that type I collagen secreted by cells isolated from Marfan’s patients is more soluble (Francis et al. 1976; Priest et al. 1973). This is indicative of lower levels of collagen cross-linking, which has already been identified in periostin knockout mice (Norris et al. 2007). This is backed by other research that suggest that the Marfan’s phenotype may be due to the expression of a variety of primary structure alterations in the chains of type I collagen that interfere with normal crosslink formation (Byers et al. 1981). In our laboratory, we have recently performed analysis of periostin knockout mice skulls, confirming the observations by Norris et al, of severe periodontal disease, significant reduction in bone density, and incisor defects (Figs. 2 and 3). Our analysis has also described for the first time that bones are missing in the orbit of the mice, or fail to properly fuse (Fig. 4). In Marfan’s patients, craniofacial abnormalities are common (De Coster et al. 2004; Pirinen 1998; Westling et al. 1998), and we hypothesize that the periostin knockout mouse has a Marfan’s like phenotype. Whether periostin impacts on collagen synthesis and assembly directly or through fibrillin is not yet known, but the prospect is intriguing. Furthermore, as Marfan’s syndrome, periostin deficient mice have severe defects in their heart’s valves, especially the mitral valve (Rios et al. 2005). The valve leaflets become extremely floppy and do not close tightly, allowing blood to leak backwards across the valve, back into the ventricles. This provides further evidence that the periostin knockout mouse suffers from a Marfan’s-like phenotype. Further analysis of the periostin knockouts could provide important information for human connective tissue diseases, particularly those where collagen synthesis and assembly is defective.
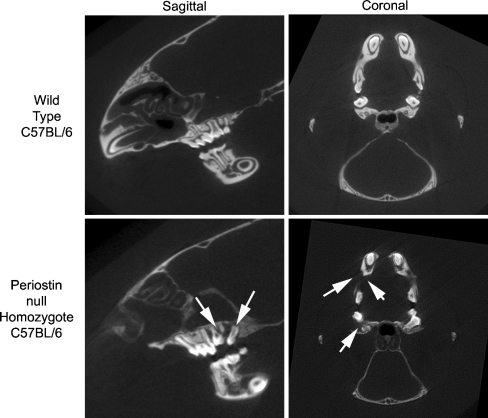
Fig. 2.
Six-week old periostin knockout C57BL/6 and litter matched wild types were analyzed using microCT imaging. In wild type mice, the molars are well arranged, with healthy periodontal ligament evident in the sagittal view. Periostin knockout mice have significant defects around the bone and periodontal ligaments, particularly in the back molars (arrow, sagittal view). In the coronal view, bone loss is evident in the jaw (arrows) when periostin knockout mice are compared with wild type litter matched controls
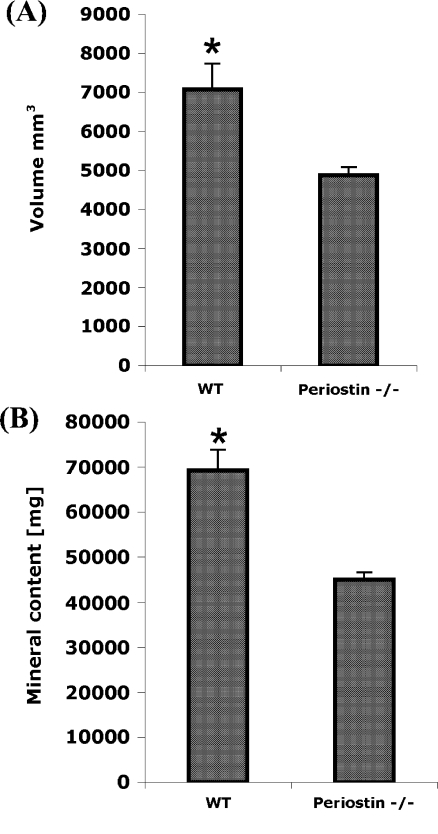
Fig. 3.
Analysis of the bone volume and mineral content reveals that the loss of periostin influences formation of bone, which is consistent with the findings of Rios et al. 2005
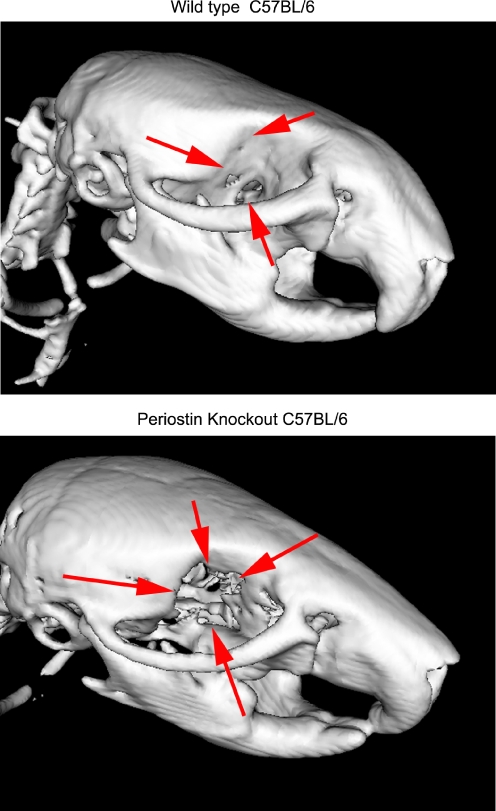
Fig. 4.
MicroCT analysis of wild type C57BL/6 and periostin knockout C57BL/6 mice at a scanning distance of 40 μm. Overall skull shape differed between the mice types, and the orbital bones appear to be missing entirely in the periostin knockout (see arrows) which is a characteristic of Marfan’s syndrome
Conclusions
Periostin is a novel secreted protein with very diverse functions that appear necessary for postnatal development. The expression of periostin is most common in collagen rich connective tissues, where it appears essential for proper ECM synthesis, particularly with respect to collagen I fibrillogenesis. Mice deficient in periostin show a phenotype similar to Marfan’s syndrome, suggesting that periostin may be involved in this pathology, in addition to fibrillin-1.
Acknowledgements
Dr Hamilton would like to thank Dr Simon Conway for graciously providing periostin knockout mice. He would also like to thanks Dr David Holdsworth for performing the microCT analysis, and Pastor Salano for his help in analyzing the data.
Abbreviations
- ECM
Extracellular matrix
- CCN2
connective tissue growth factor
- FA
focal adhesion
- HGF
human gingival fibroblast
- OSF-2
osteoblast specific factor-2
- RCO
rat calvarial osteoblast
- SPARC
secreted protein acidic rich in cysteine
- TSP
thrombospondin
Footnotes
This review also appears on line under CCNExpress section of the Newsletters page on ICCNS Website at http://ccnsociety.com.
References
- Abraham PA, Perejda AJ, Carnes WH, Uitto J (1982) Marfan syndrome. Demonstration of abnormal elastin in aorta. J Clin Invest 70:1245–1252 doi:10.1172/JCI110723 [DOI] [PMC free article] [PubMed]
- Alford AI, Hankenson KD (2006) Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone 38:749–757 doi:10.1016/j.bone.2005.11.017 [DOI] [PubMed]
- Baril P, Gangeswaran R, Mahon PC, Caulee K, Kocher HM, Harada T et al (2007) Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene 26:2082–2094 doi:10.1038/sj.onc.1210009 [DOI] [PubMed]
- Berk BC, Fujiwara K, Lehoux S (2007) ECM remodeling in hypertensive heart disease. J Clin Invest 117:568–575 doi:10.1172/JCI31044 [DOI] [PMC free article] [PubMed]
- Berrier AL, Yamada KM (2007) Cell–matrix adhesion. J Cell Physiol 213:565–573 doi:10.1002/jcp.21237 [DOI] [PubMed]
- Boileau C, Jondeau G, Mizuguchi T, Matsumoto N (2005) Molecular genetics of Marfan syndrome. Curr Opin Cardiol 20:194–200 doi:10.1097/01.hco.0000162398.21972.cd [DOI] [PubMed]
- Borg TK, Markwald R (2007) Periostin: more than just an adhesion molecule. Circ Res 101:230–231 doi:10.1161/CIRCRESAHA.107.159103 [DOI] [PubMed]
- Bornstein P (2000) Matricellular proteins: an overview. Matrix Biol 19:555–556 doi:10.1016/S0945-053X(00)00103-7 [DOI] [PubMed]
- Bornstein P, Sage EH (2002) Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 14:608–616 doi:10.1016/S0955-0674(02)00361-7 [DOI] [PubMed]
- Bornstein P, Kyriakides TR, Yang Z, Armstrong LC, Birk DE (2000) Thrombospondin 2 modulates collagen fibrillogenesis and angiogenesis. J Investig Dermatol Symp Proc 5:61–66 doi:10.1046/j.1087-0024.2000.00005.x [DOI] [PubMed]
- Bornstein P, Agah A, Kyriakides TR (2004) The role of thrombospondins 1 and 2 in the regulation of cell–matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol 36:1115–1125 doi:10.1016/j.biocel.2004.01.012 [DOI] [PubMed]
- Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR (2007) Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol 302:256–266 doi:10.1016/j.ydbio.2006.09.048 [DOI] [PMC free article] [PubMed]
- Byers PH, Siegel RC, Peterson KE, Rowe DW, Holbrook KA, Smith LT et al (1981) Marfan syndrome: abnormal alpha 2 chain in type I collagen. Proc Natl Acad Sci USA 78:7745–7749 doi:10.1073/pnas.78.12.7745 [DOI] [PMC free article] [PubMed]
- Callewaert B, Malfait F, Loeys B, De Paepe A (2008) Ehlers–Danlos syndromes and Marfan syndrome. Best Pract Res Clin Rheumatol 22:165–189 doi:10.1016/j.berh.2007.12.005 [DOI] [PubMed]
- Canty EG, Kadler KE (2005) Procollagen trafficking, processing and fibrillogenesis. J Cell Sci 118:1341–1353 doi:10.1242/jcs.01731 [DOI] [PubMed]
- Chen H, Herndon ME, Lawler J (2000) The cell biology of thrombospondin-1. Matrix Biol 19:597–614 doi:10.1016/S0945-053X(00)00107-4 [DOI] [PubMed]
- Collod-Beroud G, Boileau C (2002) Marfan syndrome in the third millennium. Eur J Hum Genet 10:673–681 doi:10.1038/sj.ejhg.5200876 [DOI] [PMC free article] [PubMed]
- Culav EM, Clark CH, Merrilees MJ (1999) Connective tissues: matrix composition and its relevance to physical therapy. Phys Ther 79:308–319 [PubMed]
- Darby IA, Hewitson TD (2007) Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol 257:143–179 doi:10.1016/S0074-7696(07)57004-X [DOI] [PubMed]
- De Coster PJ, Martens LC, De Paepe A (2004) Orofacial manifestations of congenital fibrillin deficiency: pathogenesis and clinical diagnostics. Pediatr Dent 26:535–537 [PubMed]
- Dorn GW 2nd (2007) Periostin and myocardial repair, regeneration, and recovery. N Engl J Med 357:1552–1554 doi:10.1056/NEJMcibr074816 [DOI] [PubMed]
- Elola MT, Wolfenstein-Todel C, Troncoso MF, Vasta GR, Rabinovich GA (2007) Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol Life Sci 64:1679–1700 doi:10.1007/s00018-007-7044-8 [DOI] [PMC free article] [PubMed]
- Erkan M, Kleeff J, Gorbachevski A, Reiser C, Mitkus T, Esposito I et al (2007) Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology 132:1447–1464 doi:10.1053/j.gastro.2007.01.031 [DOI] [PubMed]
- Francis G, Donnelly PV, Di Ferrante N (1976) Abnormally soluble collagen produced in fibroblasts cultures. Experientia 32:691–693 doi:10.1007/BF01919835 [DOI] [PubMed]
- Gallagher GL, Jackson CJ, Hunyor SN (2007) Myocardial extracellular matrix remodeling in ischemic heart failure. Front Biosci 12:1410–1419 doi:10.2741/2157 [DOI] [PubMed]
- Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD (2002) Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res 62:5358–5364 [PubMed]
- Goetsch SC, Hawke TJ, Gallardo TD, Richardson JA, Garry DJ (2003) Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genomics 14:261–271 [DOI] [PubMed]
- Gruber HE, Sage EH, Norton HJ, Funk S, Ingram J, Hanley EN Jr (2005) Targeted deletion of the SPARC gene accelerates disc degeneration in the aging mouse. J Histochem Cytochem 53:1131–1138 doi:10.1369/jhc.5A6687.2005 [DOI] [PubMed]
- Grzesik WJ, Narayanan AS (2002) Cementum and periodontal wound healing and regeneration. Crit Rev Oral Biol Med 13:474–484 [DOI] [PubMed]
- Hamilton DW, Brunette DM (2007) The effect of substratum topography on osteoblast adhesion mediated signal transduction and phosphorylation. Biomaterials 28:1806–1819 doi:10.1016/j.biomaterials.2006.11.041 [DOI] [PubMed]
- Hamilton DW, Wong KS, Brunette DM (2006) Microfabricated discontinuous-edge surface topographies influence osteoblast adhesion, migration, cytoskeletal organization, and proliferation and enhance matrix and mineral deposition in vitro. Calcif Tissue Int 78:314–325 doi:10.1007/s00223-005-0238-x [DOI] [PubMed]
- Hamilton DW, Chehroudi B, Brunette DM (2007) Comparative response of epithelial cells and osteoblasts to microfabricated tapered pit topographies in vitro and in vivo. Biomaterials 28:2281–2293 doi:10.1016/j.biomaterials.2007.01.026 [DOI] [PubMed]
- He J, Baum LG (2006) Galectin interactions with extracellular matrix and effects on cellular function. Methods Enzymol 417:247–256 doi:10.1016/S0076-6879(06)17017-2 [DOI] [PubMed]
- Hinz B, Gabbiani G (2003) Cell–matrix and cell–cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb Haemost 90:993–1002 [DOI] [PubMed]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H et al (1999) Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14:1239–1249 doi:10.1359/jbmr.1999.14.7.1239 [DOI] [PubMed]
- Humphries MJ, Travis MA, Clark K, Mould AP (2004) Mechanisms of integration of cells and extracellular matrices by integrins. Biochem Soc Trans 32:822–825 doi:10.1042/BST0320407 [DOI] [PubMed]
- Iekushi K, Taniyama Y, Azuma J, Katsuragi N, Dosaka N, Sanada F et al (2007) Novel mechanisms of valsartan on the treatment of acute myocardial infarction through inhibition of the antiadhesion molecule periostin. Hypertension 49:1409–1414 doi:10.1161/HYPERTENSIONAHA.106.080994 [DOI] [PubMed]
- Kii I, Kudo A (2007) Periostin function in the periodontal ligament and the periosteum. Clin Calcium 17:202–208 [PubMed]
- Kii I, Amizuka N, Minqi L, Kitajima S, Saga Y, Kudo A (2006) Periostin is an extracellular matrix protein required for eruption of incisors in mice. Biochem Biophys Res Commun 342:766–772 doi:10.1016/j.bbrc.2006.02.016 [DOI] [PubMed]
- Kojima T, Freitas PH, Ubaidus S, Suzuki A, Li M, Yoshizawa M et al (2007) Histochemical examinations on cortical bone regeneration induced by thermoplastic bioresorbable plates applied to bone defects of rat calvariae. Biomed Res 28:219–229 doi:10.2220/biomedres.28.219 [DOI] [PubMed]
- Kruzynska-Frejtag A, Wang J, Maeda M, Rogers R, Krug E, Hoffman S et al (2004) Periostin is expressed within the developing teeth at the sites of epithelial–mesenchymal interaction. Dev Dyn 229:857–868 doi:10.1002/dvdy.10453 [DOI] [PubMed]
- Kudo H, Amizuka N, Araki K, Inohaya K, Kudo A (2004) Zebrafish periostin is required for the adhesion of muscle fiber bundles to the myoseptum and for the differentiation of muscle fibers. Dev Biol 267:473–487 doi:10.1016/j.ydbio.2003.12.007 [DOI] [PubMed]
- Kudo Y, Siriwardena BS, Hatano H, Ogawa I, Takata T (2007) Periostin: novel diagnostic and therapeutic target for cancer. Histol Histopathol 22:1167–1174 [DOI] [PubMed]
- Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S et al (2007) Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 13:962–969 doi:10.1038/nm1619 [DOI] [PubMed]
- Kuiper EJ, Roestenberg P, Ehlken C, Lambert V, van Treslong-de Groot HB, Lyons KM et al (2007) Angiogenesis is not impaired in connective tissue growth factor (CTGF) knock-out mice. J Histochem Cytochem 55:1139–1147 doi:10.1369/jhc.7A7258.2007 [DOI] [PMC free article] [PubMed]
- Kyriakides TR, Bornstein P (2003) Matricellular proteins as modulators of wound healing and the foreign body response. Thromb Haemost 90:986–992 [DOI] [PubMed]
- Lallier TE, Spencer A (2007) Use of microarrays to find novel regulators of periodontal ligament fibroblast differentiation. Cell Tissue Res 327:93–109 doi:10.1007/s00441-006-0282-5 [DOI] [PubMed]
- Larsen M, Artym VV, Green JA, Yamada KM (2006) The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol 18:463–471 doi:10.1016/j.ceb.2006.08.009 [DOI] [PubMed]
- Leask A, Abraham DJ (2006) All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci 119:4803–4810 doi:10.1242/jcs.03270 [DOI] [PubMed]
- Leask A, Denton CP, Abraham DJ (2004) Insights into the molecular mechanism of chronic fibrosis: the role of connective tissue growth factor in scleroderma. J Invest Dermatol 122:1–6 doi:10.1046/j.0022-202X.2003.22133.x [DOI] [PubMed]
- Li P, Oparil S, Feng W, Chen YF (2004) Hypoxia-responsive growth factors upregulate periostin and osteopontin expression via distinct signaling pathways in rat pulmonary arterial smooth muscle cells. J Appl Physiol 97:1550–1558 discussion 1549 doi:10.1152/japplphysiol.01311.2003 [DOI] [PubMed]
- Li G, Oparil S, Sanders JM, Zhang L, Dai M, Chen LB et al (2006) Phosphatidylinositol-3-kinase signaling mediates vascular smooth muscle cell expression of periostin in vivo and in vitro. Atherosclerosis 188:292–300 doi:10.1016/j.atherosclerosis.2005.11.002 [DOI] [PMC free article] [PubMed]
- Lindner V, Wang Q, Conley BA, Friesel RE, Vary CP (2005) Vascular injury induces expression of periostin: implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol 25:77–83 [DOI] [PubMed]
- Litvin J, Selim AH, Montgomery MO, Lehmann K, Rico MC, Devlin H et al (2004) Expression and function of periostin-isoforms in bone. J Cell Biochem 92:1044–1061 doi:10.1002/jcb.20115 [DOI] [PubMed]
- Litvin J, Chen X, Keleman S, Zhu S, Autieri M (2007) Expression and function of periostin-like factor in vascular smooth muscle cells. Am J Physiol Cell Physiol 292:C1672–C1680 doi:10.1152/ajpcell.00153.2006 [DOI] [PubMed]
- Lock JG, Wehrle-Haller B, Stromblad S (2008) Cell–matrix adhesion complexes: master control machinery of cell migration. Semin Cancer Biol 18:65–76 doi:10.1016/j.semcancer.2007.10.001 [DOI] [PubMed]
- Lukashev ME, Werb Z (1998) ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol 8:437–441 doi:10.1016/S0962-8924(98)01362-2 [DOI] [PubMed]
- Majesky MW (1994) Neointima formation after acute vascular injury. Role of counteradhesive extracellular matrix proteins. Tex Heart Inst J 21:78–85 [PMC free article] [PubMed]
- Martinek N, Shahab J, Sodek J, Ringuette M (2007) Is SPARC an evolutionarily conserved collagen chaperone? J Dent Res 86:296–305 [DOI] [PubMed]
- Midwood KS, Williams LV, Schwarzbauer JE (2004) Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol 36:1031–1037 doi:10.1016/j.biocel.2003.12.003 [DOI] [PubMed]
- Muller U (1999) Ten years of gene targeting: targeted mouse mutants, from vector design to phenotype analysis. Mech Dev 82:3–21 doi:10.1016/S0925-4773(99)00021-0 [DOI] [PubMed]
- Murphy-Ullrich JE (2001) The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest 107:785–790 doi:10.1172/JCI12609 [DOI] [PMC free article] [PubMed]
- Nakazawa T, Nakajima A, Seki N, Okawa A, Kato M, Moriya H et al (2004) Gene expression of periostin in the early stage of fracture healing detected by cDNA microarray analysis. J Orthop Res 22:520–525 doi:10.1016/j.orthres.2003.10.007 [DOI] [PubMed]
- Nasuti JF, Zhang PJ, Feldman MD, Pasha T, Khurana JS, Gorman JH 3rd et al (2004) Fibrillin and other matrix proteins in mitral valve prolapse syndrome. Ann Thorac Surg 77:532–536 doi:10.1016/S0003-4975(03)01584-4 [DOI] [PubMed]
- Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R et al (2007) Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem 101:695–711 doi:10.1002/jcb.21224 [DOI] [PMC free article] [PubMed]
- Norris RA, Borg TK, Butcher JT, Baudino TA, Banerjee I, Markwald RR (2008a) Neonatal and adult cardiovascular pathophysiological remodeling and repair: developmental role of periostin. Ann N Y Acad Sci 1123:30–40 doi:10.1196/annals.1420.005 [DOI] [PubMed]
- Norris RA, Moreno-Rodriguez RA, Sugi Y, Hoffman S, Amos J, Hart MM et al (2008b) Periostin regulates atrioventricular valve maturation. Dev Biol 316:200–213 doi:10.1016/j.ydbio.2008.01.003 [DOI] [PMC free article] [PubMed]
- Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA et al (2007) Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 101:313–321 doi:10.1161/CIRCRESAHA.107.149047 [DOI] [PMC free article] [PubMed]
- Oku E, Kanaji T, Takata Y, Oshima K, Seki R, Morishige S, Imamura R, Ohtsubo K, Hashiguchi M, Osaki K et al. (2008) Periostin and bone marrow fibrosis. Int J Hematol 88(1):57–63 doi:10.1007/s12185-008-0095-2 [DOI] [PubMed]
- Oshima A, Tanabe H, Yan T, Lowe GN, Glackin CA, Kudo A (2002) A novel mechanism for the regulation of osteoblast differentiation: transcription of periostin, a member of the fasciclin I family, is regulated by the bHLH transcription factor, twist. J Cell Biochem 86:792–804 doi:10.1002/jcb.10272 [DOI] [PubMed]
- Park YW, Kang YM, Butterfield J, Detmar M, Goronzy JJ, Weyand CM (2004) Thrombospondin 2 functions as an endogenous regulator of angiogenesis and inflammation in rheumatoid arthritis. Am J Pathol 165:2087–2098 [DOI] [PMC free article] [PubMed]
- Pirinen S (1998) Genetic craniofacial aberrations. Acta Odontol Scand 56:356–359 doi:10.1080/000163598428310 [DOI] [PubMed]
- Priest RE, Moinuddin JF, Priest JH (1973) Letter: collagen of Marfan syndrome is abnormally soluble. Nature 245:264–266 doi:10.1038/245264a0 [DOI] [PubMed]
- Raines EW (2000) The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int J Exp Pathol 81:173–182 doi:10.1046/j.1365-2613.2000.00155.x [DOI] [PMC free article] [PubMed]
- Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A et al (2005) Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol 25:11131–11144 doi:10.1128/MCB.25.24.11131-11144.2005 [DOI] [PMC free article] [PubMed]
- Robinson PN, Booms P (2001) The molecular pathogenesis of the Marfan syndrome. Cell Mol Life Sci 58:1698–1707 doi:10.1007/PL00000807 [DOI] [PMC free article] [PubMed]
- Roy S, Patel D, Khanna S, Gordillo GM, Biswas S, Friedman A et al (2007) Transcriptome-wide analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc Natl Acad Sci U S A 104:14472–14477 doi:10.1073/pnas.0706793104 [DOI] [PMC free article] [PubMed]
- Schuler M, Owen GR, Hamilton DW, de Wild M, Textor M, Brunette DM et al (2006) Biomimetic modification of titanium dental implant model surfaces using the RGDSP-peptide sequence: a cell morphology study. Biomaterials 27:4003–4015 doi:10.1016/j.biomaterials.2006.03.009 [DOI] [PubMed]
- Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M et al (2008) Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med 205:295–303 doi:10.1084/jem.20071297 [DOI] [PMC free article] [PubMed]
- Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A et al (2008) Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res 102:752–760 doi:10.1161/CIRCRESAHA.107.159517 [DOI] [PMC free article] [PubMed]
- Stamenkovic I (2003) Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 200:448–464 doi:10.1002/path.1400 [DOI] [PubMed]
- Suzuki H, Amizuka N, Kii I, Kawano Y, Nozawa-Inoue K, Suzuki A et al (2004) Immunohistochemical localization of periostin in tooth and its surrounding tissues in mouse mandibles during development. Anat Rec A Discov Mol Cell Evol Biol 281:1264–1275 doi:10.1002/ar.a.20080 [DOI] [PubMed]
- Tai IT, Dai M, Chen LB (2005) Periostin induction in tumor cell line explants and inhibition of in vitro cell growth by anti-periostin antibodies. Carcinogenesis 26:908–915 doi:10.1093/carcin/bgi034 [DOI] [PubMed]
- Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S et al (2006) Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 118:98–104 doi:10.1016/j.jaci.2006.02.046 [DOI] [PubMed]
- Takeshita S, Kikuno R, Tezuka K, Amann E (1993) Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J 294(Pt 1):271–278 [DOI] [PMC free article] [PubMed]
- Tayebjee MH, MacFadyen RJ, Lip GY (2003) Extracellular matrix biology: a new frontier in linking the pathology and therapy of hypertension? J Hypertens 21:2211–2218 doi:10.1097/00004872-200312000-00002 [DOI] [PubMed]
- Tilman G, Mattiussi M, Brasseur F, van Baren N, Decottignies A (2007) Human periostin gene expression in normal tissues, tumors and melanoma: evidences for periostin production by both stromal and melanoma cells. Mol Cancer 6:80 doi:10.1186/1476-4598-6-80 [DOI] [PMC free article] [PubMed]
- Tomokiyo A, Maeda H, Fujii S, Wada N, Shima K, Akamine A (2008) Development of a multipotent clonal human periodontal ligament cell line. Differentiation 76:337–347 doi:10.1111/j.1432-0436.2007.00233.x [DOI] [PubMed]
- Uitto J (1979) Biochemistry of the elastic fibers in normal connective tissues and its alterations in diseases. J Invest Dermatol 72:1–10 doi:10.1111/1523-1747.ep12530093 [DOI] [PubMed]
- Uitto J, Lichtenstein JR (1976) Defects in the biochemistry of collagen in diseases of connective tissue. J Invest Dermatol 66:59–79 doi:10.1111/1523-1747.ep12481404 [DOI] [PubMed]
- Wallner K, Li C, Fishbein MC, Shah PK, Sharifi BG (1999) Arterialization of human vein grafts is associated with tenascin-C expression. J Am Coll Cardiol 34:871–875 doi:10.1016/S0735-1097(99)00272-7 [DOI] [PubMed]
- Wallner K, Shah PK, Sharifi BG (2002) Balloon catheterization induces arterial expression of new tenascin-C isoform. Atherosclerosis 161:75–83 doi:10.1016/S0021-9150(01)00627-X [DOI] [PubMed]
- Westling L, Mohlin B, Bresin A (1998) Craniofacial manifestations in the Marfan syndrome: palatal dimensions and a comparative cephalometric analysis. J Craniofac Genet Dev Biol 18:211–218 [PubMed]
- Whiteman P, Hutchinson S, Handford PA (2006) Fibrillin-1 misfolding and disease. Antioxid Redox Signal 8:338–346 doi:10.1089/ars.2006.8.338 [DOI] [PubMed]
- Wilde J, Yokozeki M, Terai K, Kudo A, Moriyama K (2003) The divergent expression of periostin mRNA in the periodontal ligament during experimental tooth movement. Cell Tissue Res 312:345–351 doi:10.1007/s00441-002-0664-2 [DOI] [PubMed]
- Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S et al (2007) Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A 104:15858–15863 doi:10.1073/pnas.0707413104 [DOI] [PMC free article] [PubMed]
- Yan Q, Sage EH (1999) SPARC, a matricellular glycoprotein with important biological functions. J Histochem Cytochem 47:1495–1506 [DOI] [PubMed]
- Yang Z, Kyriakides TR, Bornstein P (2000) Matricellular proteins as modulators of cell–matrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell 11:3353–3364 [DOI] [PMC free article] [PubMed]
- Zelenka PS (2004) Regulation of cell adhesion and migration in lens development. Int J Dev Biol 48:857–865 doi:10.1387/ijdb.041871pz [DOI] [PubMed]