Abstract
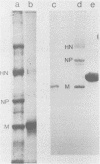
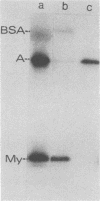
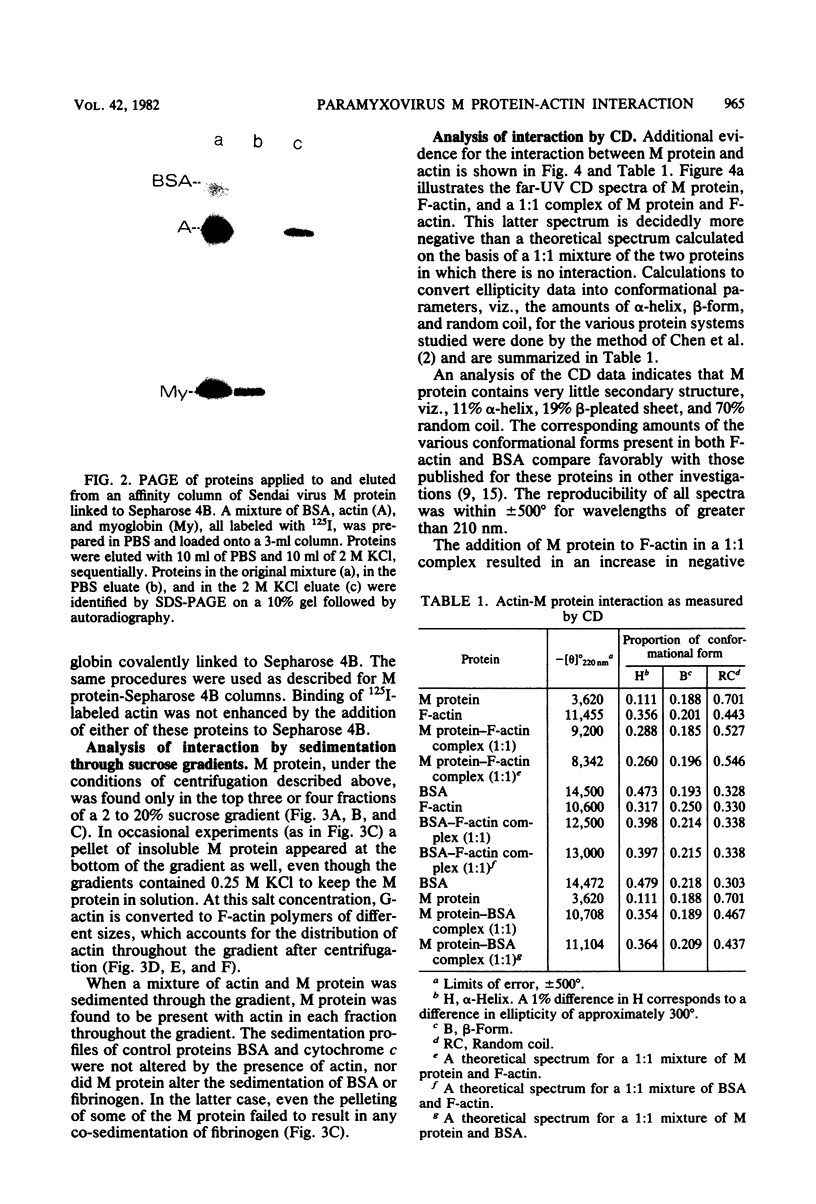
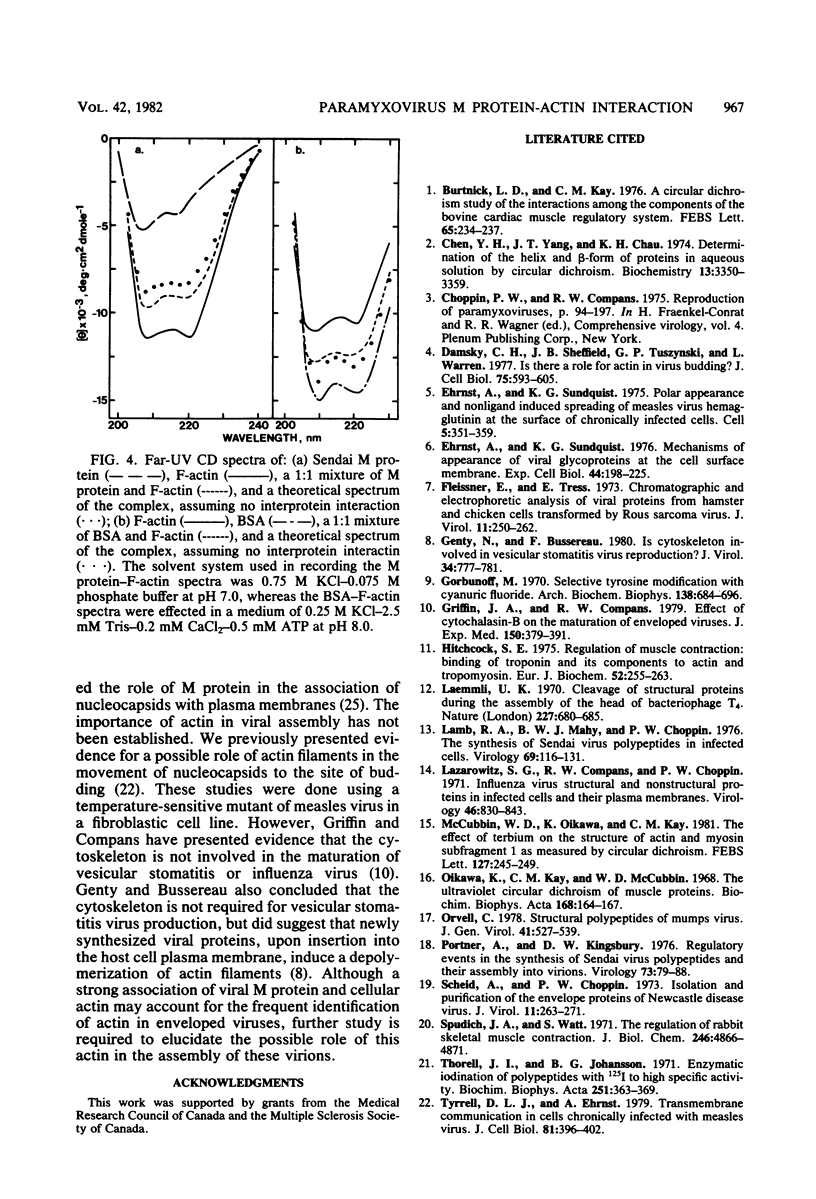
Evidence for an interaction of the membrane (M) protein of Newcastle disease and Sendai viruses with cellular actin was obtained by three different techniques. M protein linked to Sepharose 4B was found to bind actin, but not myoglobin or bovine serum albumin, and to selectively remove actin from a mixture of these three proteins. Sedimentation of a mixture of M protein and F-actin through a sucrose gradient resulted in sedimentation of M protein with actin. Control proteins, bovine serum albumin and cytochrome c, did not sediment with actin. In circular dichroism studies, M protein added to actin in a 1:1 complex resulted in a significant increase in negative ellipticity at 220 nm, which corresponds to an increase in alpha-helix and a decrease in beta-structure and random coil. This is indicative of an interaction between M protein and actin. It is possible that the frequent identification of cellular actin in a number of enveloped viruses may be attributed to the interaction of actin and M protein or its equivalent.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burtnick L. D., Kay C. M. A circular dichroism study of the interactions among the components of the bovine cardiac muscle regulatory system. FEBS Lett. 1976 Jun 1;65(2):234–237. doi: 10.1016/0014-5793(76)80487-5. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Damsky C. H., Sheffield J. B., Tuszynski G. P., Warren L. Is there a role for actin in virus budding? J Cell Biol. 1977 Nov;75(2 Pt 1):593–605. doi: 10.1083/jcb.75.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnst A., Sundqvist K. G. Polar appearance and nonligand induced spreading of measles virus hemagglutinin at the surface of chronically infected cells. Cell. 1975 Aug;5(4):351–359. doi: 10.1016/0092-8674(75)90053-7. [DOI] [PubMed] [Google Scholar]
- Ehrnst A., Sundqvist K. G. The mechanisms of appearance of viral glycoproteins at the cell surface membrane. Exp Cell Biol. 1976;44(3-6):198–225. doi: 10.1159/000163112. [DOI] [PubMed] [Google Scholar]
- Fleissner E., Tress E. Chromatographic and electrophoretic analysis of viral proteins from hamster and chicken cells transformed by Rous sarcoma virus. J Virol. 1973 Feb;11(2):250–262. doi: 10.1128/jvi.11.2.250-262.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty N., Bussereau F. Is cytoskeleton involved in vesicular stomatitis virus reproduction? J Virol. 1980 Jun;34(3):777–781. doi: 10.1128/jvi.34.3.777-781.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunoff M. J. Selective tyrosine modification with cyanuric fluoride. Arch Biochem Biophys. 1970 Jun;138(2):684–696. doi: 10.1016/0003-9861(70)90397-8. [DOI] [PubMed] [Google Scholar]
- Griffin J. A., Compans R. W. Effect of cytochalasin B on the maturation of enveloped viruses. J Exp Med. 1979 Aug 1;150(2):379–391. doi: 10.1084/jem.150.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock S. E. Regulation of muscle contraction: bindings of troponin and its components to actin and tropomyosin. Eur J Biochem. 1975 Mar 17;52(2):255–263. doi: 10.1111/j.1432-1033.1975.tb03993.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- McCubbin W. D., Oikawa K., Kay C. M. The effect of terbium on the structure of actin and myosin subfragment 1 as measured by circular dichroism. FEBS Lett. 1981 May 18;127(2):245–249. doi: 10.1016/0014-5793(81)80216-5. [DOI] [PubMed] [Google Scholar]
- Oikawa K., Kay C. M., McCubbin W. D. The ultraviolet circular dichroism of muscle proteins. Biochim Biophys Acta. 1968 Sep 10;168(1):164–167. doi: 10.1016/0005-2795(68)90248-1. [DOI] [PubMed] [Google Scholar]
- Orvell C. Structural polypeptides of mumps virus. J Gen Virol. 1978 Dec;41(3):527–539. doi: 10.1099/0022-1317-41-3-527. [DOI] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Regulatory events in the synthesis of Sendai virus polypeptides and their assembly into virions. Virology. 1976 Aug;73(1):79–88. doi: 10.1016/0042-6822(76)90062-3. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Isolation and purification of the envelope proteins of Newcastle disease virus. J Virol. 1973 Feb;11(2):263–271. doi: 10.1128/jvi.11.2.263-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. L., Ehrnst A. Transmembrane communication in cells chronically infected with measles virus. J Cell Biol. 1979 May;81(2):396–402. doi: 10.1083/jcb.81.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. L., Norrby E. Structural polypeptides of measles virus. J Gen Virol. 1978 May;39(2):219–229. doi: 10.1099/0022-1317-39-2-219. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Nagai Y'Yoshii S., Maeno K., Matsumoto T. Membrane (M) protein of HVJ (Sendai virus): its role in virus assembly. Virology. 1976 May;71(1):143–161. doi: 10.1016/0042-6822(76)90101-x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Nagai Y., Maeno K., Iinuma M., Hamaguchi M., Matsumoto T., Nagayoshi S., Hoshino M. Studies on the role of M protein in virus assembly using a ts mutant of HVJ (Sendai virus). Virology. 1979 Jan 15;92(1):139–154. doi: 10.1016/0042-6822(79)90220-4. [DOI] [PubMed] [Google Scholar]