Abstract
The oligodendrocyte precursor cell (OPC) arises from the subventricular zone (SVZ) during early vertebrate development to migrate and proliferate along axon tracts before differentiating into the myelin-forming oligodendrocyte. We demonstrate that the spatial and temporal regulation of oligodendrocyte differentiation depends intimately on the axonal microenvironment and the density of precursor cells along a specified axonal area. Differentiation does not require dynamic axonal signaling, but instead is induced by packing constraints resulting from intercellular interactions. Schwann cells and even artificial beads bound to the axonal surface can mimic these constraints and promote differentiation. Together, these results describe the coordinately controlled biophysical interaction of oligodendrocyte precursors within an axonal niche leading to self-renewal and differentiation.
Keywords: myelination, neuronal–glial interactions, mechanotransduction
Damage to the myelin membrane, as a result of nerve injury or disease, significantly impairs the ability of the nervous system to communicate and can lead to a host of debilitating symptoms, as well as an ultimate loss of function. In the CNS, demyelination is accompanied by the loss of oligodendrocytes, the terminally differentiated cells responsible for the formation of the myelin sheath. After the initial onset of demyelination, OPCs are induced to differentiate and remyelinate, effectively replacing lost oligodendrocytes. Unfortunately, the capacity for remyelination is limited, and ultimately fails in the presence of chronic demyelination. It remains unclear why the CNS cannot sustain this initial ability to repair the myelin sheath. One possible explanation is that adult OPCs eventually lose their ability to differentiate into remyelinating oligodendrocytes (1). It is plausible that the continuous presence of a demyelinating environment is responsible for inhibiting the differentiation process. If this supposition is true, then it is imperative to identify the environmental conditions conducive to the ongoing production of oligodendrocytes.
Examining oligodendrocyte generation during development could prove useful for determining the role of the environment in the induction of differentiation. Developing OPCs are proliferative and self-renewing cells that originate in the SVZ and migrate along axons throughout the CNS. During development, an OPC must decide how many times it will divide, and where it will migrate. Also, an OPC must choose whether to remain as a precursor cell into adulthood or to differentiate into a myelinating oligodendrocyte. Such complex decisions are likely to be heavily influenced by the nature of the surrounding environment and by the behavior of neighboring cells. Based on these assumptions, it is our goal to identify the environmental factors that influence the decision of an OPC to differentiate into an oligodendrocyte. To accomplish this goal, we first looked at the developing rat spinal cord to examine the temporal regulation of oligodendrocyte differentiation in vivo.
Temporal Regulation of Oligodendrocyte Differentiation.
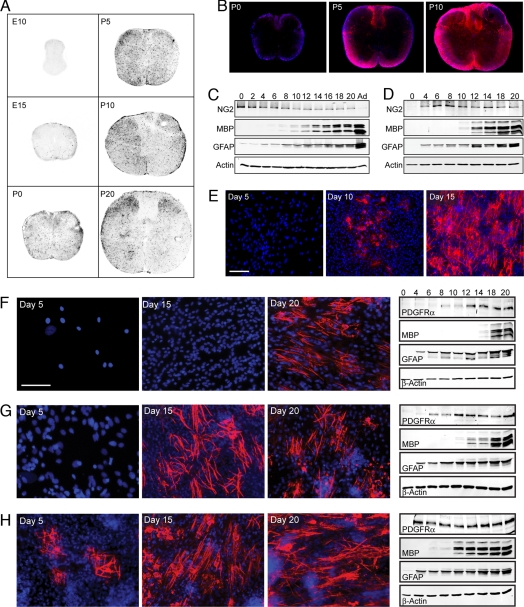
OPCs appear in the rat spinal cord as early as embryonic day (E)15. These precursor cells can be seen to increase in number as they migrate throughout the spinal cord (Fig. 1A). OPCs continue to proliferate until shortly after birth, at which time the number of OPCs becomes relatively constant (Fig. 1 A and C). Beginning around postnatal day (P)8, we observe a gradual decline in the number of OPCs (Fig. 1 A and C). This decrease in OPC numbers is followed by the appearance of differentiated oligodendrocytes, which are undetectable in the rat spinal cord until approximately one week postnatal (Fig. 1 B and C). These in vivo observations suggest that developing OPCs make a temporally synchronized transition from proliferating progenitors to differentiated oligodendrocytes. By using an in vitro coculture system involving sensory dorsal root ganglion (DRG) neurons and purified OPCs (2), we can consistently replicate this temporally specific pattern of differentiation. In our system, we find that after seeding a standard density of OPCs (200,000), the precursor cells undergo a fixed period of proliferation before differentiation. Differentiation begins approximately 10 days after the OPCs are seeded onto the neurons (Fig. 1 D and E). This sequence of events closely approximates the pattern of OPC development that we observe in the rat spinal cord. What mechanisms coordinate the initiation of oligodendrocyte differentiation that we see both in our cocultures and in vivo? In other words, how is the timing of oligodendrocyte differentiation synchronized between OPCs?
Fig. 1.
Temporal regulation of oligodendrocyte differentiation. (A) Immunostaining in rat spinal cord sections from E10 through P20. OPCs, identified by immunostaining for PDGFRα, appear initially at E15 and then proliferate as they migrate throughout the spinal cord. (B) Immunostaining in rat spinal cord sections from P0 through P10. Astrocytes (blue) are identified by immunostaining for GFAP. Oligodendroyctes (red), identified by immunostaining for MBP, do not appear in the spinal cord until P5, almost two weeks after the initial appearance of OPCs. (C and D) Western blot analysis of rat spinal cords (C) and OPC-DRG cocultures (D) probed for proteins expressed by OPCs (NG2), oligodendrocytes (MBP), and astrocytes (GFAP). Actin serves as a loading control. Cocultures replicate the temporal expression pattern seen in vivo, in which the appearance of oligodendrocytes is delayed compared with the appearance of OPCs. (E) In OPC-DRG cocultures, immunostaining for MBP labels differentiated oligodendrocytes (red). Nuclei are stained using DAPI (blue). Differentiation begins 10 days after a standard density of 200,000 OPCs is seeded onto neurons. After 15 days, a robust and reproducible amount of myelination is observed. The onset of differentiation is preceded by a period of OPC proliferation, a pattern that approximates the behavior of OPCs in the spinal cord. (F–H) Immunostaining and Western blot analysis of OPC-DRG cocultures, in which OPCs are seeded at low density (20,000 OPCs) (F), standard density (200,000 OPCs) (G), and high density (2 million OPCs) (H) onto DRG neurons. Immunostaining for MBP labels differentiated oligodendrocytes (red). Nuclei are stained by using DAPI (blue). Western blot analysis of cocultures are probed for proteins expressed by OPCs (PDGFRα), oligodendrocytes (MBP), and astrocytes (GFAP). Actin serves as a loading control. These results demonstrate that an increase in cell density accelerates the onset of oligodendrocyte differentiation, but does not affect the differentiation of OPCs into astrocytes.
Induction of Differentiation Requires a Critical Density of OPCs.
It is possible that the global synchronicity of this cell fate decision can be explained by previous studies that suggest that OPCs differentiate based on the presence of an intrinsic timer (3). Essentially, OPCs are programmed to undergo a set period of division and then differentiate. Related OPCs possess highly similar programs and are coordinated in the timing of their differentiation, even when they are relocated to separate environments. It is important to note that the intrinsic timer was identified in studies performed at clonal density in the absence of other cell types (4, 5). In contrast, an OPC in our system is subject to the potential external influences of axons and neighboring OPCs. Can an intrinsic program explain the timing of oligodendrocyte differentiation in the presence of these extrinsic influences? To test this possibility, we seeded neurons with three different densities of OPCs (Fig. 1 F–H). If the initiation of differentiation is regulated by an intrinsic timer, then OPCs in all three experimental conditions should differentiate simultaneously. Instead, we find that the time at which differentiation is initiated depends on the density at which OPCs are seeded. OPCs seeded at high density (2 million OPCs) start to differentiate after only five days in culture (Fig. 1H), whereas OPCs seeded at our standard density (200,000 OPCs) and low density (20,000 OPCs) must be cultured for approximately two (Fig. 1G) and three weeks (Fig. 1F), respectively, before differentiation begins. These results suggest that an intrinsic timer is not responsible for the initiation of differentiation in our coculture system. Instead, it appears that a critical density of OPCs must be reached in order for differentiation to begin. This conclusion is supported by Western blot analysis (Fig. 1 F–H), showing the expression profiles of the OPC marker, platelet-derived growth factor receptor α (PDGFRα), and the oligodendrocyte marker, myelin basic protein (MBP). Regardless of the initial density of OPCs seeded, the expression of PDGFRα must reach a threshold level before MBP expression is detected. In addition, these findings demonstrate that in our cocultures, the extent of oligodendrocyte differentiation is not proportional to the number of OPCs initially seeded. Together, these studies support the hypothesis that extrinsic factors must regulate the onset of oligodendrocyte differentiation.
Differentiation and Myelination in the Absence of Dynamic Axonal Signaling.
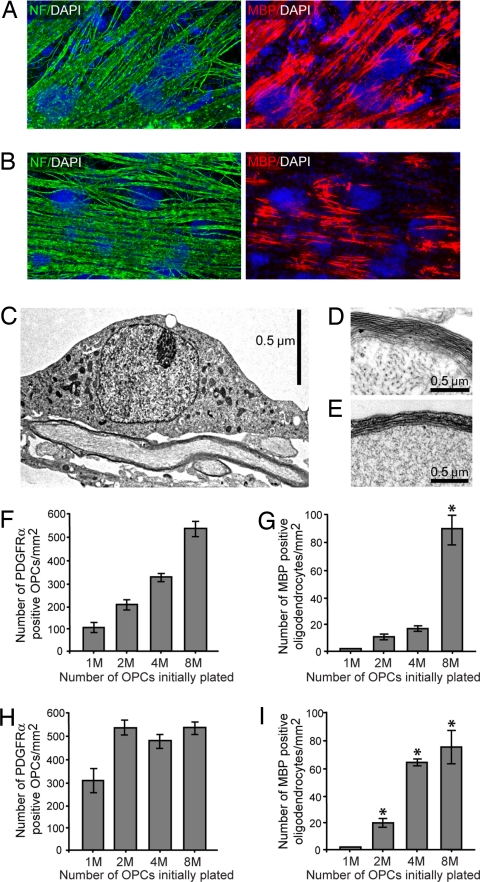
It is likely that neurons serve as extrinsic regulators of OPC development. What role does the axon have in the process of oligodendrocyte differentiation? Similar to previous studies (6), we find that oligodendrocyte differentiation is not density dependent in the absence of axons (data not shown). These results suggest that interactions between axons and OPCs may be responsible for the density-dependent coordination of oligodendrocyte differentiation. To test this possibility, we treated our cocultures with either conditioned medium from axons or with purified axonal membranes (data not shown). Because these classic experiments failed to induce oligodendrocyte differentiation, we took an alternative approach to clarify the role of the axon. To eliminate dynamic changes in axonal signaling, we fixed axons with paraformaldehyde before seeding OPCs. We find that high-density OPCs seeded onto fixed axons differentiate with the same timing and robustness as OPCs on live axons (Fig. 2 A and B). To our surprise, the OPCs seeded onto fixed axons could also form compact myelin (Fig. 2 C–E). These results clearly indicate that dynamic interactions between oligodendrocytes and axons are not required for either differentiation or myelination.
Fig. 2.
The induction of differentiation at a critical density of OPCs does not require dynamic axonal signaling. (A and B) Immunostaining of cocultures five days after seeding a high density of OPCs. Axons (green) are identified by immunostaining for NF. (A) Live axons. (B) Axons fixed with 4% paraformaldehyde to eliminate dynamic axonal signaling. Immunostaining with MBP (red) demonstrates that fixed axons support oligodendrocyte differentiation and myelination in a manner comparable to live axons. Nuclei are stained by using DAPI (blue). (C–E) Electron micrographs of oligodendrocyte myelination. The compact, multilayered myelin formed by oligodendrocytes on live axons (C and D) is also seen in fixed axon cocultures (E). (F–I) Quantification of the critical density of OPCs required to induce population-wide differentiation after five days in culture. A density of ≈500–600 PDGFRα+ OPCs on axons per millimeter squared is required for the induction of oligodendrocyte differentiation on both fixed axons (F and G) and on live axons (H and I). Here, we define population-wide differentiation as ≈60–100 MBP+ oligodendrocytes per millimeter squared. Note that because OPCs seeded onto fixed axons (F and G) fail to proliferate, population-wide differentiation is induced only when 8 million (8M) OPCs are initially plated. In contrast, OPCs seeded onto live axons (H and I) at an initial density of 2M, 4M, or 8M cells will all reach the critical density required for differentiation. Note that after five days, OPCs seeded onto live axons at an initial density of 2M have just reached the critical density and are just beginning to differentiate. Error bars represent standard deviation. MBP+ oligodendrocytes were quantified by counting 20 fields/coverslip, 3 coverslips/density. P < 0.01 versus 1M density cultures (Student–Newman–Keuls post hoc comparison after one-way ANOVA).
Inhibition of Proliferation Is Not Sufficient to Induce Differentiation.
Could differentiation result from the presence of a permissive environment rather than from induction by an instructive cue? Our findings suggest that the onset of differentiation is somehow inextricably tied to the process of OPC proliferation. Perhaps differentiation is merely the result of inhibited proliferation that occurs once a critical density of OPCs on axons is reached. After all, it is plausible that achieving a high density of OPCs could inhibit proliferation by depleting available mitogens or through contact-mediated interactions. If this hypothesis is true, then an experimental inhibition of proliferation should induce oligodendrocyte differentiation. To test this possibility, we seeded our standard density of OPCs onto fixed axons [supporting information (SI) Fig. S1]. It is known that axons secrete OPC mitogens such as PDGF (7). In our cocultures, axonal fixation eliminates the secretion of these mitogenic factors, and greatly reduces the extent of OPC proliferation (Fig. S1). These findings are in line with previous studies demonstrating that axonal fixation selectively halts proliferation, but does not affect the viability of glial cells (8). Despite the inhibition of proliferation in our fixed axon cocultures, the OPCs failed to differentiate in advance of OPCs on live axons. In fact, even after 20 days, very few differentiated oligodendrocytes are detectable in these fixed axon cocultures (Fig. S1). These results suggest that the absence of mitogenic factors prevents OPCs from reaching the critical density required for differentiation. Also, we tested whether it was possible to delay differentiation by increasing the amount of available mitogens. The addition of exogenous PDGF to live axon cultures does not inhibit OPC differentiation and may actually advance the differentiation process (Fig. S2). Taken together, these results demonstrate that the induction of differentiation at a critical density of OPCs is not the result of an inhibition of proliferation.
Spatial and Geometric Constraints Induce Differentiation.
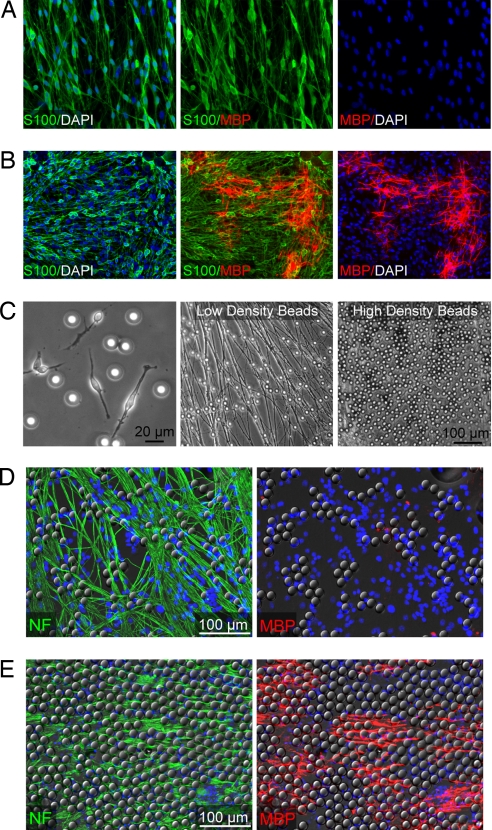
Why do OPCs need to reach a critical density to differentiate? Quantification of the critical density suggests that differentiation is induced once OPCs reach a density of ≈500–600 PDGFRα+ cells per millimeter squared. This density does not depend on the number of OPCs initially seeded and is highly conserved between live and fixed axon cultures (Fig. 2 F–I). Also, quantification of MBP+ cells suggests that the number of differentiated oligodendrocytes is not proportional to the number of OPCs initially seeded. These results confirm that a significant amount of differentiation is induced only after a critical density of OPCs is reached. Is it possible that the induction of differentiation at a critical density requires density-dependent interactions between OPCs? To test this possibility, we attempted to induce differentiation in cocultures containing both OPCs and Schwann cells seeded onto live axons. When our standard density of OPCs is seeded onto neurons either alone (Fig. 1 E and G) or in the presence of a low density of Schwann cells (Fig. 3A), we fail to see any differentiated oligodendrocytes after five days in culture. However, when seeded with a high density of Schwann cells, we see a significant amount of differentiation after five days (Fig. 3B). These findings suggest that differentiation is not controlled by the expression of a signal exclusive to OPCs. Instead, the addition of a high density of Schwann cells is sufficient to mimic the critical density required for the initiation of differentiation.
Fig. 3.
Spatial constraints along an axon are sufficient to induce differentiation. (A and B) Immunostaining of OPC-DRG live axon cocultures five days after seeding a standard density of 200,000 OPCs. As shown by staining for S100 (green), cocultures are also seeded with either a low (A) or high (B) density of Schwann cells. Oligodendrocytes (red) are identified by immunostaining for MBP. Nuclei are stained by using DAPI (blue). (B) OPCs are induced to differentiate only in the presence of a high density of Schwann cells. (C) Phase contrast microscopic images of polystyrene beads. Beads are 20 μm in diameter, comparable in size to the cell body of an OPC (Left) and Schwann cell (data not shown). Beads were coated with an antibody to an axonal protein (p75NTR) and then conjugated at either low density (Center) or high density (Right) along the length of axons. (D and E) Immunostaining of OPC-DRG cocultures five days after seeding 200,000 OPCs and either a low (D) or high (E) density of polystyrene beads. OPCs are induced to differentiate only in the presence of a high density of beads (E). Oligodendrocytes are identified by immunostaining for MBP (red). Axons are identified by immunostaining for NF (green). Nuclei are stained by using DAPI (blue). Beads are visualized by using DIC microscopy.
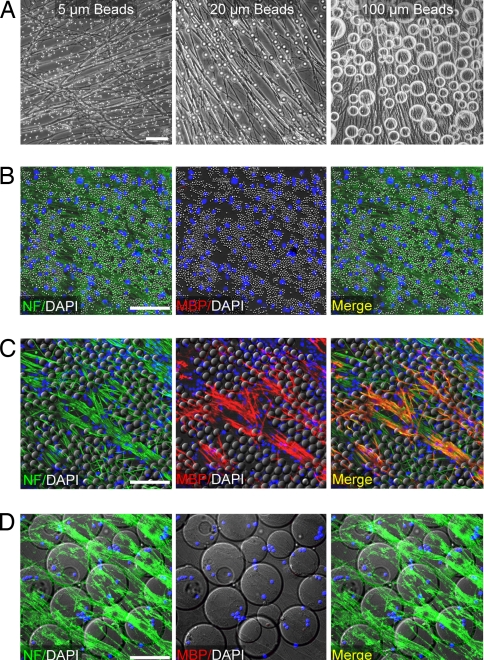
Because Schwann cells and OPCs originate from distinct cell lineages and do not interact in vivo, it is not clear why Schwann cells would express a signal that induces OPCs to differentiate. Therefore, we propose an alternative explanation for the ability of Schwann cells to induce oligodendroycte differentiation. We hypothesize that an OPC differentiates not because of instructive intercellular signaling, but instead because of a restriction in axonal space proximal to the OPC. To test this hypothesis, Schwann cells were fixed and seeded onto live axons along with a standard density of OPCs (Fig. S3). When seeded at a high density, fixed Schwann cells were sufficient to induce oligodendrocyte differentiation after only five days. These results suggest that secreted factors do not control the ability of Schwann cells to induce differentiation, adding support to the hypothesis that differentiation is induced through a restriction of available axonal space. To rule out the possibility that a membrane-bound factor is required to induce oligodendrocyte differentiation, we conjugated axons with polystyrene beads measuring 20 μm in diameter, which is comparable in size to both OPCs and Schwann cells (Fig. 3C). Coating the beads with an antibody against an axonal protein allowed us to bind the beads at low and high density along the surface of live axons (Fig. 3C). When a standard density of OPCs was seeded onto neurons with a low density of beads, no oligodendrocytes were observed after 5 days in culture (Fig. 3D). However, we find that an increase in the density of beads along axons is sufficient to induce differentiation on either live (Fig. 3E) or fixed axons (Fig. S4). These findings indicate that the induction of differentiation at a critical density of OPCs depends on a restriction of available axonal space. However, in the absence of axons, the polystyrene beads do not influence differentiation (data not shown). Also, we find that the induction of differentiation depends not only on the density of beads along an axon, but also on their dimensions. Either an increase or decrease in the diameter of the beads eliminates the potential to induce differentiation (Fig. 4). These results suggest that the induction of oligodendrocyte differentiation depends on both the spatial and geometric parameters of the surrounding microenvironment.
Fig. 4.
The induction of differentiation depends on the geometry of the microenvironment. (A) Phase contrast microscopic images of beads measuring either 5 (Left), 20 (Center), or 100 (Right) μm in diameter. Beads were coated with an antibody to an axonal protein (p75NTR) and then conjugated at high density along the length of axons. (B–D) Immunostaining of OPC-DRG cocultures five days after seeding a standard density of 200,000 OPCs and a high density of beads measuring either 5 (B), 20 (C), or 100 (D) μm in diameter. OPCs are induced to differentiate only in the presence of the 20-μm beads (C). Oligodendrocytes are identified by immunostaining for MBP (red). Axons are identified by immunostaining for NF (green). Nuclei are stained by using DAPI (blue). Beads are visualized by using DIC microscopy.
Discussion
Our results suggest that physical characteristics of the microenvironment can influence cell fate decisions. We hypothesize that spatial and geometric constraints along an axon may induce differentiation through lateral compression of OPCs. How does a mechanical stimulus promote a change in cell fate? One possibility is that contractile forces can physically alter the size or shape of an OPC. Changes in cell shape have previously been shown to regulate processes such as cell survival (9) and cell fate decisions (10). It has been suggested that changes in cell shape may facilitate structural rearrangement within the cell (11). This intracellular reorganization could initiate interactions between upstream mediators and downstream effectors that are responsible for the induction of differentiation (12). Also, it has been demonstrated that changes in cell shape can be directly linked to structural changes in the nucleus (13). In our system, we observe that an increase in cell density correlates with a decrease in nuclear size, as shown by staining with DAPI. It is possible that changes to the size and structure of the nucleus induce transcriptional activity necessary for oligodendrocyte differentiation. Perhaps an increase in cell density affects transcription factors known to regulate differentiation, such as Nkx2.2, Sox10, Olig2, and the recently identified Yin Yang 1 (14–17). Understanding the relationship between intrinsic and extrinsic factors could help identify environmental conditions that will promote oligodendrocyte differentiation in the presence of chronic demyelination.
Treatment of demyelinating conditions may also require elucidation of the relationship between the various environmental stimuli that have been shown to regulate differentiation. In our studies we find that the presence of the axon is required for the induction of differentiation through geometric and spatial constraints. Our results are in line with previous studies, which suggest that the nature of the cellular substrate can influence the effect of a mechanical stimulus on cell fate decisions (11). For instance, the fate of mesenchymal stem cells can be modulated by the flexibility of the substrate on which the cells are cultured (18). It is possible that biophysical characteristics of the axon, such as its size, shape, and tensile strength could be instrumental in the density-dependent induction of differentiation. After all, the caliber of the axon has previously been shown to influence multiple aspects of the myelination process. Studies suggest that axon diameter can regulate whether axons are myelinated (19), the thickness of the myelin membrane, and the distribution of internodes along an axon (20). However, recent studies suggest that in the peripheral nervous system the regulation of myelin sheath thickness may be due solely to the fact that an increase in axon diameter correlates with an increase in the expression of the membrane-bound axonal factor neuregulin 1 (NRG1) type III (21, 22). As illustrated by these findings, it is important to determine whether it is biophysical or biochemical attributes (or a combination of both) that make the axon a necessary component of density-dependent differentiation.
It is possible that the induction of differentiation through environmental constraints depends not on the structural dimensions of the axon but, instead, on the expression of membrane-bound axonal signals. Perhaps these signals facilitate the ability of an OPC to transduce mechanical forces into the biochemical initiation of oligodendrocyte differentiation. This type of context-dependent regulation could represent an alternative explanation for the role of an axonal substrate in the density-dependent induction of oligodendrocyte differentiation. It is possible that the mechanical induction of differentiation depends on the formation of an adherens junction between the axon and the OPC. This theory is supported by studies that implicate ECM receptors and cell–cell adhesion molecules as key mediators of mechanotransduction (23). Interestingly, adhesion molecules have previously been shown to have a role in oligodendrocyte differentiation and myelination (24–28). ECM receptors such as integrins are also known to modulate the effects of extrinsic growth factors on various aspects of oligodendrocyte development (29, 30). Therefore, it is plausible that the neuronal-glial interaction facilitates the formation of an adhesion complex that is responsible for the integration of various chemical and mechanical factors regulating differentiation (31). This hypothesis could help explain how the axon can regulate differentiation through the expression of putative factors such as Jagged-1 (32, 33) and Lingo-1 (34, 35), as well as through its role in mechanotransduction. Because novel differentiation factors continue to be identified, it is essential to understand how a multitude of diverse extrinsic factors can be properly synthesized in the coordination of a single cell fate decision. Identifying the mechanisms responsible for the integration of extrinsic signals may be crucial for the establishment of an environment promoting remyelination.
Materials and Methods
Immunopanning Protocol.
OPCs were purified from 6 to 7 day old (P6-P7) rat brain cortices with a panning protocol adapted from one previously described (2). Briefly, Petri dishes containing a goat anti-mouse IgG+IgM secondary antibody solution (Jackson Laboratories) were incubated overnight. Dishes were rinsed and incubated with primary antibody solutions containing either GalC or A2B5 hybridoma supernatants. Rat brain cerebral hemispheres were first diced and then dissociated with papain at 37°C. After trituration, cells were resuspended in a panning buffer and then incubated at room temperature sequentially on three immunopanning dishes: IgG+IgM, GalC, and A2B5. A2B5+ OPCs were released from the final panning dish by using trypsin (Sigma).
Purified OPC/DRG Cocultures.
OPC-DRG cocultures were prepared as described previously (2). Briefly, DRG neurons from E13–15 Sprague–Dawley rats were dissociated, plated, and purified on collagen-coated coverslips in the presence of NGF (100 ng/ml). Neurons were maintained for two to three weeks before the addition of OPCs. Either the TrkA-Fc receptor chimera (1 μg/ml; Regeneron Pharmaceuticals) or the anti-TrkA antibody (RTA, 50 μg/ml) was added to the DRG cultures one week before the addition of OPCs. OPCs were seeded at either low (20,000 OPCs), medium (200,000 OPCs), or high (2 million OPCs) density onto coverslips containing purified DRG neurons. Coverslips were incubated in a small volume of MEM overnight to facilitate OPC attachment. The day after, coverslips were transferred into wells with MEM containing 10% FBS and either anti-NGF or TrkA-Fc. All OPC/DRG coculture experiments were conducted by using MEM containing 10% FBS and either anti-NGF or TrkA-Fc, with the exception of the experimental condition in Fig. S2, in which 50 ng/ml of PDGF-AA (PeproTech) was added to the medium.
Western Blot Analysis.
Samples from cocultures and rat spinal cords were prepared for Western blot analysis as previously described (36). The proteins were transferred to pure nitrocellulose membranes and probed with specific antibodies. Antibodies for Western blot analysis: rabbit polyclonal anti-PDGFRα antibody (Santa Cruz), rabbit polyclonal anti-NG2 antibody (Chemicon), rat monoclonal anti-MBP antibody (Chemicon), mouse monoclonal anti-GFAP antibody (Chemicon), mouse anti-MAG antibody (Chemicon), and mouse monoclonal anti-β-Actin (Sigma). The Alexa Fluor goat anti-rabbit, anti- mouse, and anti-rat 680 IgG antibodies were used as secondary antibodies for near-infrared fluorescent detection performed on the Odyssey Infrared Imaging System (LI-COR).
Immunostaining.
Immunostaining of rat spinal cord sections and cocultures was performed as previously described (35). Briefly, cocultures were fixed by using 4% paraformaldehyde and dehydrated and then permeabilized and blocked by incubation with 20% goat serum and 0.2% Triton X-100 in PBS. Differentiated oligodendrocytes and myelin were detected with a rat monoclonal anti-MBP antibody (Chemicon). OPCs were detected by using a rabbit polyclonal anti-PDGFRα antibody (Santa Cruz). Astroyctes were detected by using a mouse monoclonal anti-GFAP antibody (Chemicon). Axons were detected by using a mouse mAb to neurofilament (NF) (American Type Culture Collection). Schwann cells were detected by using a rabbit polyclonal antibody to S100 (DakoCytomation). The Alexa Fluor anti-rat 594, anti-rabbit 488, anti-mouse 350, 488, and 594 IgG antibodies (Invitrogen) were used as secondary antibodies for fluorescence detection. Cell nuclei were examined with DAPI.
Axonal Fixation.
Before the seeding of OPCs, coverslips containing purified DRG neuronal cultures were washed twice gently in PBS. Neurons were then fixed with 2 ml of a 4% paraformaldehyde (PFA) solution for 10 min. After the removal of PFA, neurons were gently rinsed multiple times with PBS and then transferred to fresh wells with MEM containing 10% FBS. All fixed axon coculture experiments were conducted by using MEM containing 10% FBS and either anti-NGF or TrkA-Fc.
Electron Microscopy.
High density OPCs cocultured with live or fixed DRGs for seven days were fixed in 2% glutaraldehyde, stained with 1% osmium tetroxide, and counterstained with 1% uranyl acetate overnight. Cocultures were subsequently rinsed with distilled water, dehydrated in ethanol, and embedded in resin (EMBed-812, Electron Microscopy Sciences). Ultrathin sections (70 nm) were obtained and visualized with a JEM 1400 Electron Microscope (JEOL).
Schwann Cell/OPC/DRG Cocultures.
Schwann cells were collected from P2 rats as described previously (36). Schwann cells were purified with cytosine arabinoside and then seeded at either low (200,000 Schwann cells) or high (2 million Schwann cells) density onto live purified DRG neurons; 200,000 OPCs were added to Schwann cell/DRG cocultures two-three days after Schwann cells were seeded. All Schwann Cell/OPC/DRG coculture experiments were conducted by using MEM containing 10% FBS and either anti-NGF or TrkA-Fc.
Bead/OPC/DRG Cocultures.
We incubated 5-, 20-, and 100-μm Protein A beads overnight with a mouse mAb (hybridoma supernatant) to p75 neurotrophin receptor (p75NTR). The beads were then washed multiple times with MEM. In initial experiments (Fig. 3), 20-μm beads were added at either low or high density to live purified DRG neurons. In subsequent experiments (Fig. 4), 5-, 20-, and 100-μm beads were each added at high density to live purified DRG neurons. The attachment of the beads to the axons was facilitated by the antibody-mediated interaction with neuronal p75NTR. Beads were incubated on the neurons for 1–3 h before the addition of 200,000 OPCs; 5- and 20-μm polystyrene beads were obtained from G. Kisker-Products for Biotechnology; 100-μm Sepharose beads were obtained from Zymed Laboratories, Inc. All Bead/OPC/DRG coculture experiments were conducted by using chemically defined medium (2) to avoid the potential for IgGs in serum-containing medium to disrupt the antibody-mediated binding of the beads to the axons.
Supplementary Material
Acknowledgments.
We thank Drs. Martin Raff and Michel Cayouette for insightful discussions and Dr. Ben Ng for his technical expertise. We also thank the Langen and Chen Labs for assistance in electron microscopy. J.R.C. was supported by the National Multiple Sclerosis Society Career Transition Award (TA 3008A2/T), The Christopher Reeve Foundation (CB2–0606–2), and the Baxter Foundation Award. S.S.R. was supported by a National Institutes of Health Cellular, Biochemical and Molecular (CBM) Predoctoral Training grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805640105/DCSupplemental.
References
- 1.Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- 2.Chan JR, et al. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004;43:183–191. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raff M. The mystery of intracellular developmental programmes and timers. Biochem Soc Trans. 2006;34:663–670. doi: 10.1042/BST0340663. [DOI] [PubMed] [Google Scholar]
- 4.Gao FB, Durand B, Raff M. Oligodendrocyte precursor cells count time but not cell divisions before differentiation. Curr Biol. 1997;7:152–155. doi: 10.1016/s0960-9822(06)00060-1. [DOI] [PubMed] [Google Scholar]
- 5.Temple S, Raff MC. Clonal analysis of oligodendrocyte development in culture: Evidence for a developmental clock that counts cell divisions. Cell. 1986;44:773–779. doi: 10.1016/0092-8674(86)90843-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Miller RH. Density-dependent feedback inhibition of oligodendrocyte precursor expansion. J Neurosci. 1996;16:6886–6895. doi: 10.1523/JNEUROSCI.16-21-06886.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calver AR, et al. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869–882. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- 8.Salzer JL, Bunge RP, Glaser L. Studies of Schwann cell proliferation. III. Evidence for the surface localization of the neurite mitogen. J Cell Biol. 1980;84:767–778. doi: 10.1083/jcb.84.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CS, et al. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 10.McBeath R, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 11.Ingber DE. Tensegrity: The architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 12.Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: The shape of things to come. Biochem J. 1999;339:481–488. [PMC free article] [PubMed] [Google Scholar]
- 13.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi Y, et al. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- 15.Stolt CC, et al. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16:165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]
- 17.He Y, et al. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–230. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 19.Voyvodic JT. Target size regulates calibre and myelination of sympathetic axons. Nature. 1989;342:430–433. doi: 10.1038/342430a0. [DOI] [PubMed] [Google Scholar]
- 20.Trapp BD, Kidd GJ. Axo-glial septate junctions. The maestro of nodal formation and myelination? J Cell Biol. 2000;150:F97–F100. doi: 10.1083/jcb.150.3.f97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taveggia C, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michailov GV, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 23.Alenghat FJ, Ingber DE. Mechanotransduction: All signals point to cytoskeleton, matrix, and integrins. Sci STKE. 2002;2002:PE6. doi: 10.1126/stke.2002.119.pe6. [DOI] [PubMed] [Google Scholar]
- 24.Tait S, et al. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo-glial junction. J Cell Biol. 2000;150:657–666. doi: 10.1083/jcb.150.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14:199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- 26.Charles P, et al. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc Natl Acad Sci USA. 2000;97:7585–7590. doi: 10.1073/pnas.100076197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fewou SN, et al. Down-regulation of polysialic acid is required for efficient myelin formation. J Biol Chem. 2007;282:16700–16711. doi: 10.1074/jbc.M610797200. [DOI] [PubMed] [Google Scholar]
- 28.Schnadelbach O, et al. N-cadherin is involved in axon-oligodendrocyte contact and myelination. Mol Cell Neurosci. 2001;17:1084–1093. doi: 10.1006/mcne.2001.0961. [DOI] [PubMed] [Google Scholar]
- 29.ffrench-Constant C, Colognato H. Integrins: Versatile integrators of extracellular signals. Trends Cell Biol. 2004;14:678–686. doi: 10.1016/j.tcb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Baron W, Colognato H, ffrench-Constant C. Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia. 2005;49:467–479. doi: 10.1002/glia.20132. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz MA, Ginsberg MH. Networks and crosstalk: Integrin signalling spreads. Nat Cell Biol. 2002;4:E65–E68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- 33.Genoud S, et al. Notch1 control of oligodendrocyte differentiation in the spinal cord. J Cell Biol. 2002;158:709–718. doi: 10.1083/jcb.200202002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mi S, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- 35.Lee X, et al. NGF regulates the expression of axonal LINGO-1 to inhibit oligodendrocyte differentiation and myelination. J Neurosci. 2007;27:220–225. doi: 10.1523/JNEUROSCI.4175-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan JR, et al. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science. 2006;314:832–836. doi: 10.1126/science.1134069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.