Abstract
The subventricular zone (SVZ) of the lateral ventricle contains neural stem and progenitor cells that generate neuroblasts which migrate to the olfactory bulb where they differentiate into interneurons. Ischemic stroke induces neurogenesis in the SVZ and these cells migrate to the boundary of the ischemic lesion. This article reviews current data on cytokinetics, signaling pathways and vascular niche that are involved in processes of proliferation, differentiation, and migration of neural progenitor cells after stroke.
Introduction
Focal cerebral ischemia causes morbidity and mortality, and therapies are being sought to improve functional neurological recovery after stroke (Liu et al., 1998; Jin et al., 2001a; Kee et al., 2001; Yagita et al., 2001; Yoshimura et al., 2001; Zhang et al., 2001a; Arvidsson et al., 2002; Iwai et al., 2002; Parent et al., 2002; Schmidt and Reymann, 2002; Iwai et al., 2003; Tonchev et al., 2003; Zhu et al., 2003; Tanaka et al., 2004; Zhang et al., 2004; Thored et al., 2006; Yamashita et al., 2006). Studies in experimental stroke demonstrate that focal cerebral ischemia promotes neurogenesis in the subventricular zone (SVZ) and subgranular zone (SGZ) of the dentate gyrus and induces SVZ neuroblast migration towards the ischemic boundary (Liu et al., 1998; Jin et al., 2001a, b; Kee et al., 2001; Yagita et al., 2001; Yoshimura et al., 2001; Zhang et al., 2001a; Arvidsson et al., 2002; Iwai et al., 2002; Parent et al., 2002; Schmidt and Reymann, 2002; Iwai et al., 2003; Tonchev et al., 2003; Zhu et al., 2003; Tanaka et al., 2004; Tureyen et al., 2004; Zhang et al., 2004; Thored et al., 2006; Yamashita et al., 2006). More importantly, stroke-induced neurogenesis has also recently been demonstrated in the adult human brain, even in advanced age patients (Jin et al., 2006; Macas et al., 2006; Minger et al., 2007). These findings have led to a hope for a neurorestorative treatment of stroke which aims to manipulate endogenous neurogenesis and thereby enhance brain repair. This review article focuses on proliferation and differentiation of neural stem and progenitor cells in the SVZ of adult rodents after focal cerebral ischemia. The signaling pathways that mediate neurogenesis after stroke will be examined. Considerable evidence supports the concept of a vascular niche where neural stem and progenitor cells reside and which regulates stem cell self-renewal, progenitor differentiation and neuroblast migration (Leventhal et al., 1999; Lin et al., 2000; Morris et al., 2000; Palmer et al., 2000; Zhang et al., 2000; Zhang et al., 2001b; Jin et al., 2002; Zhang and Chopp, 2002; Zhang et al., 2002; Shen et al., 2004; Greenberg and Jin, 2005; Ohab et al., 2006; Thored et al., 2007; Teng et al., 2008). Thus, the effect of brain microenvironment on neurogenesis after stroke will be reviewed.
The adult rodent SVZ
During cortical neurogenesis in the rodent, neural stem cells reside in the ventricular zone (VZ) and generate cortical neurons (Hinds and Ruffett, 1971; Chenn and McConnell, 1995). As embryonic neurogenesis comes to an end, the VZ is replaced by an ependymal layer while the subventricular zone (SVZ) shrinks and persists in the adult (Morshead et al., 1998). Radial glia located in the VZ are neural stem cells and a subpopulation of radial glia transform into adult SVZ astrocytes during the early postnatal stage (Miyata et al., 2001; Noctor et al., 2001; Tramontin et al., 2003; Weissman et al., 2003; Anthony et al., 2004). This astrocyte population forms neural stem cells in the adult SVZ (Tramontin et al., 2003). Based on morphology and phenotypes of these cells, the adult SVZ is composed of types A, B, and C cells (Doetsch et al., 1997; Garcia-Verdugo et al., 1998) (Fig. 1). Type A cells are neuroblasts and type B cells are astrocytes that form a glial boundary between migrating neuroblasts and the underlying striatum (B2) as well as between migrating neuroblasts and the ependymal cells (B1). Type C cells are the most actively proliferating cells, while type B2 cells derived from radial glia are relatively quiescent neural stem cells with a cell cycle of approximately 15 days (Morshead et al., 1994; Doetsch et al., 1997; Doetsch et al., 1999b; Tramontin et al., 2003). These neural stem cell and progenitor cells generate neurons and glia throughout adulthood (Luskin, 1993; Lois and Alvarez-Buylla, 1994). Transient amplifying cells (type C cells) and neuroblasts (type A cells) constitute 34%, while relatively quiescent neural stem cells (type B2 cells) are approximately 2% of the total population of SVZ cells (Morshead et al., 1994; Doetsch et al., 1997). More than 30,000 neuroblasts are generated daily in the rodent SVZ (Alvarez-Buylla et al., 2001; Lledo et al., 2006). Neuroblasts form chains which are ensheathed by astrocytes and migrate through the rostral migratory stream (RMS) to the olfactory bulb where they differentiate into interneurons (Fig. 1) (Lois et al., 1996; Alvarez-Buylla et al., 2001; Lledo et al., 2006). Using different genetic approaches, several groups recently demonstrated that the adult SVZ contains different populations of neural stem cells (Willaime-Morawek et al., 2006; Merkle et al., 2007; Ventura and Goldman, 2007; Young et al., 2007). Using Cre-lox fate mapping in transgenic mice, Young et al show that all parts of the telencephalic neuroepithelium, including the medial ganglionic eminence (MGE), lateral ganglionic eminence (LGE) and the cerebral cortex, contribute multipotent, self-renewing stem cells to the adult SVZ(Young et al., 2007). LGE and cortical populations of stem cells generate tyrosine hydroxylase and calretinin positive interneurons to the olfactory bulb, whereas LGE-derived stem cells exclusively produce calbindin positive interneurons in the olfactory glomeruli (Young et al., 2007). By following EmaxIREScre; LacZ/EGFP cells, Willaime-Morawek et al demonstrate that embryonic cortical neural stem cells contribute to neural stem cells in dorsal regions of adult SVZ (Willaime-Morawek et al., 2006). Using an adenovirus that expresses Cre under the control of the mouse GFAP promoter (Ad:GFAP-Cre), studies from Alvarez-Buylla’s laboratory demonstrate that adult neural stem cells are a restricted and diverse population of progenitor cells (Merkle et al., 2007). Dorsal regions of the SVZ of the lateral ventricle produce mostly tyrosine hydroxylase positive periglomerular cells, whereas ventral parts of the SVZ mainly generate calbindin-expressing periglomerular cells(Merkle et al., 2007). Radial glia in the dorsolateral SVZ labeled with adenovirus expressing Cre recombinase in postnatal day 1 generate tyrosine hydroxylase positive periglomerular cells 8 weeks after delivery of the virus (Ventura and Goldman, 2007).
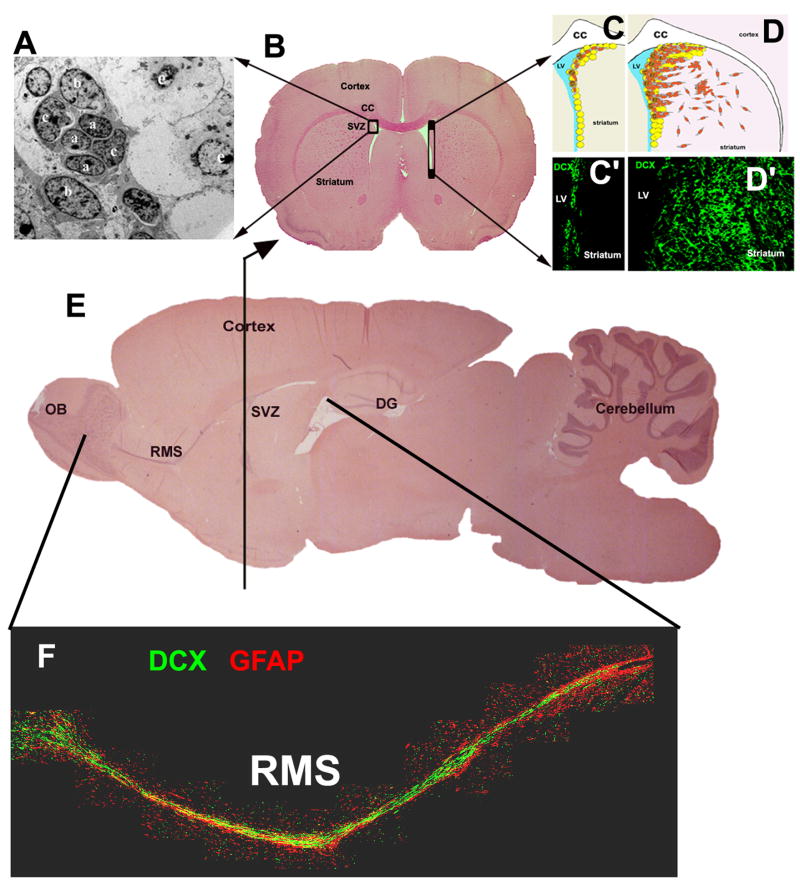
Figure 1.
Neurogenesis in the non-ischemic and ischemic SVZ of adult rodent brain. Depictions of sagittal (E and F) and coronal (B) views of rat brain in the SVZ which contains types A, B, and C cells (A). Schematic coronal view of neural stem/progenitor cells (
 in C and D) and neuroblasts (
in C and D) and neuroblasts (
 in C and D) in the SVZ of the right lateral ventricle of non-ischemic (C) and ischemic (D) hemispheres as well as a corresponding view of neuroblasts identified by doublecortin (DCX) positive cells (C′ and D′, green). Neuroblasts in the SVZ (C and C′ green) migrate through the rostral migratory stream (RMS, E and F, green color) into the olfactory bulb (OB, E and F, green color). However, after stroke, many neuroblasts in the SVZ migrate towards the ischemic striatum (D and D′, green color). Red color in panel F is GFAP positive astrocytes. CC = corpus callosum and DG = dentate gyrus.
in C and D) in the SVZ of the right lateral ventricle of non-ischemic (C) and ischemic (D) hemispheres as well as a corresponding view of neuroblasts identified by doublecortin (DCX) positive cells (C′ and D′, green). Neuroblasts in the SVZ (C and C′ green) migrate through the rostral migratory stream (RMS, E and F, green color) into the olfactory bulb (OB, E and F, green color). However, after stroke, many neuroblasts in the SVZ migrate towards the ischemic striatum (D and D′, green color). Red color in panel F is GFAP positive astrocytes. CC = corpus callosum and DG = dentate gyrus.
Focal cerebral ischemia induces neurogenesis
Transient and permanent occlusion of the middle cerebral artery results in increased neurogenesis in the ipsilateral SVZ, and neuroblasts in the SVZ migrate towards the ischemic boundary regions of the striatum and cortex (Liu et al., 1998; Jin et al., 2001a; Kee et al., 2001; Yagita et al., 2001; Yoshimura et al., 2001; Zhang et al., 2001a; Arvidsson et al., 2002; Iwai et al., 2002; Parent et al., 2002; Schmidt and Reymann, 2002; Iwai et al., 2003; Tonchev et al., 2003; Zhu et al., 2003; Tanaka et al., 2004; Zhang et al., 2004; Thored et al., 2006). Some of the neuroblasts in the striatal ischemic boundary express markers of spiny neostriatal neurons(Arvidsson et al., 2002; Parent et al., 2002). Infusion of an anti-mitotic agent (cytosine-β-D-arabiofuranoside, Ara-C) to the brain almost completely ablates type A and type C cells, but not the relatively quiescent type B cells in the non-ischemic and ischemic SVZ (Doetsch et al., 1999a; Arvidsson et al., 2002; Chen et al., 2004). However, 7 days after termination of Ara-C infusion, type A and C cells are entirely repopulated in the SVZ after stroke, while repopulation of neuroblasts in the SVZ takes 14 days in non-ischemic SVZ. The cell lineage is from type B to C to A (Alvarez-Buylla and Lim, 2004). Therefore, after ablation of type A and C cells with Arac-C, type B cells divide to generate new type C cells that in turn generate type A cells to repopulate the constitutively proliferating population (Alvarez-Buylla and Lim, 2004). Stroke promotes the repopulation process, suggesting that type A to C cells are involved in stroke-induced neurogenesis (Thored et al., 2006; Chen et al., 2004). Neurogenesis triggered by stroke persists for at least 6 months (unpublished data) (Thored et al., 2006; Chen et al., 2004).
Proliferation of neural progenitor cells after stroke
The proportion of proliferating cells and the length of the cell cycle are two critical parameters of the cytokinetics for neocortical neurogenesis (Nowakowski et al., 1989; Takahashi et al., 1993; Caviness et al., 2003). In the adult rat, approximately 15 to 21% of the SVZ cell population is actively dividing (Schultze and Korr, 1981; Smith and Luskin, 1998; Zhang et al., 2006b). The cell cycle length of actively dividing SVZ cells in the adult rat is 18–21 hr and remains relatively constant throughout the animal’s lifetime (Schultze and Korr, 1981; Smith and Luskin, 1998; Zhang et al., 2006b). Stroke changes the length of the cell cycle and the proportion of proliferating SVZ cells, which contribute to stroke-induced neurogenesis (Zhang et al., 2006b; Zhang et al., 2007b). Analysis of growth fraction and cell cycle phases of actively proliferating SVZ cells is performed by cumulative and single S phase labeling with 5-bromo-2′-deoxyuridine (BrdU) (Nowakowski et al., 1989). In vivo studies reveal that stroke substantially increases the percentage of dividing SVZ cells, starting 2 days (24%) and reaches a maximum at 7 days (31%) after stroke. Fourteen days after stroke, the proportion of dividing cells returns to the level observed 2 days after stroke. Concurrently, the cell cycle length of SVZ cells changes dynamically over a period of 2 to 14 days after stroke, with the shortest length of 11h at 2 days after stroke, which is significantly shorter than the cell cycle length of 19 hr in non-stroke SVZ cells (Zhang et al., 2006b). Four days after stroke, the cell cycle length gradually increases and by 14 days after stroke reaches the length of non-ischemic progenitor cells (Zhang et al., 2007b). Alteration of the G1 phase of SVZ cells contributes to stroke-induced changes of the cell cycle length (Zhang et al., 2007b). Interestingly, dynamic changes of the cell cycle kinetics are correlated to the proportion of daughter cells that remain and leave the cell cycle over a period of 2 to 14 days after stroke. Reduction of the G1 phase of the cell cycle 2 to 4 days after stroke is associated with an increase of dividing daughter cells that remain within the cell cycle to rapidly expand the SVZ, whereas lengthening the G1 phase 4 to 14 days after stroke is accompanied by augmentation of daughter cells that exit the cell cycle to differentiate into neurons (Zhang et al., 2007b). These data indicate that stroke triggers dynamic changes of the G1 phase of actively dividing SVZ cell cycle resulting in early expansion of a neural progenitor pool and later neuronal differentiation, which leads to augmentation of neurogenesis. During cortical neurogenesis, the cell cycle length is associated with neural progenitor cell progression from proliferation to neurogenic divisions and lengthening the G1 phase of the neuroepthelial cell cycle triggers neuronal differentiation (Calegari et al., 2005; Gotz and Huttner, 2005; Takahashi et al., 1996; Calegari et al., 2005; Huttner and Kosodo, 2005). Thus, stroke may trigger actively proliferating SVZ neural progenitor cells in the adult rodent to recapture the cell cycle kinetics of embryonic neural progenitor cells.
Stroke induces radial glial cells
In addition to neural progenitor cells, stroke induces ependymal cell proliferation and these dividing ependymal cells express many radial glial markers including the cdc2-kinase phosphorylated form of vimentin (4A4), astrocyte-specific glutamate transporter (GLAST), and brain-lipid-binding protein (BLBP) (Shibata et al., 1997; Kamei et al., 1998; Hartfuss et al., 2001; Zhang et al., 2001a; Zhang et al., 2007c). This finding can be placed into context with studies that demonstrate the lineage relationship between radial glia and ependymal cells. Ependymal cells originate from radial glia between E14 and E16 (Spassky et al., 2005). It was suggested that ependymal cells are neural stem cells, but other studies show that ependymal cells adjacent to the lateral ventricular wall do not have characteristics of neural stem cells (Chiasson et al., 1999; Johansson et al., 1999; Rietze et al., 2001). During embryonic development, radial glial cells act as neural stem cells (Miyata et al., 2001; Noctor et al., 2001; Weissman et al., 2003; Anthony et al., 2004). In the adult mammalian brain, radial glial cells differentiate into astrocytes and are no longer present (Voigt, 1989). Stroke-induced radial glial cells in the ipsilateral SVZ are localized to actively proliferating type A and C cells (Zhang et al., 2007c), suggesting that radial glial cells may serve as neural stem cells to produce type C cells which differentiate into neuroblasts. Intraventricular infusion of EGF promotes adult ependymal cells in the lateral wall of the lateral ventricles adopting radial glial cell morphology(Gregg and Weiss, 2003), although adult ependymal cells are postmitotic and do not divide under normal conditions (Spassky et al., 2005). Thus, it is possible that stroke induces ependymal cells to transform into radial glial cells.
The Notch and sonic hedgehog signaling pathways regulate stroke-induced neurogenesis
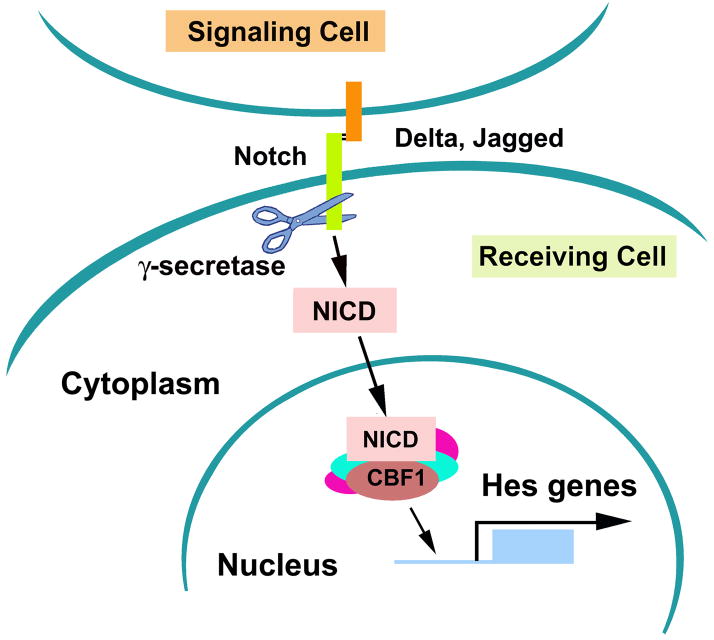
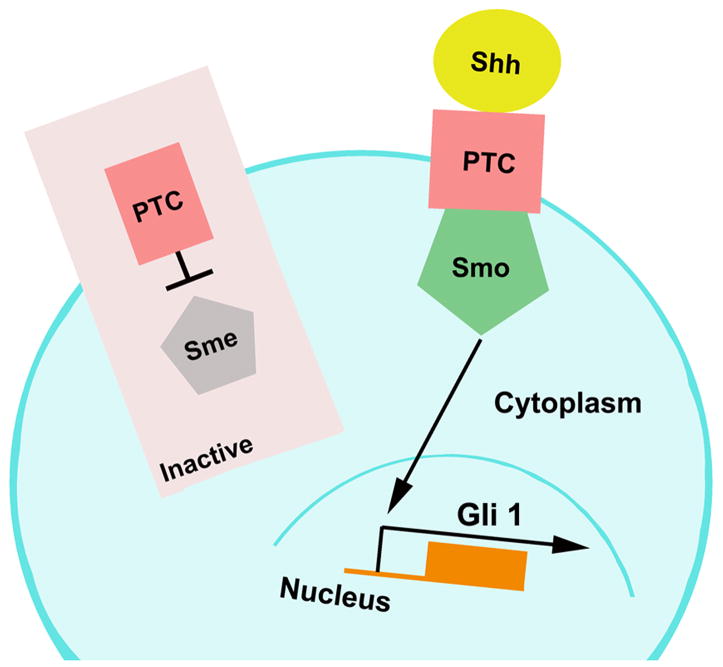
The Notch receptors are transmembrane proteins activated by Delta and Jagged ligands (Gaiano et al., 2000; Hitoshi et al., 2002; Androutsellis-Theotokis et al., 2006; Guentchev and McKay, 2006). On activation, Notch intracellular cellular domain (NICD) is cleaved by presenilin-1 and the γ-secretase enzyme complex and translocates into the nucleus (Gaiano et al., 2000; Hitoshi et al., 2002; Androutsellis-Theotokis et al., 2006; Guentchev and McKay, 2006). Within the nucleus, the NICD forms a complex with C-promoter binding factor 1 (CBF1), which activates transcription factors of hairy and enhancer of split (Hes) family (Gaiano et al., 2000; Hitoshi et al., 2002; Androutsellis-Theotokis et al., 2006; Guentchev and McKay, 2006) (Fig. 2). During development, the Notch signaling pathway maintains a neural progenitor pool and promotes the generation of astrocytes through the regulation of the cell cycle and the interaction with ciliary neurotrophic factor, respectively (Nagao et al., 2007). Notch signals are expressed in neural stem and progenitor cells in the SVZ of adult rodent (Mizutani et al., 2007). In vitro studies reveal that stroke upregulates Notch and Hes1 expression in SVZ neural progenitor cells and promotes translocation of NICD into the nucleus (unpublished data). Blockage of the Notch pathway either with a γ-secretase inhibitor or siRNA against Notch suppresses stroke-induced progenitor cell proliferation (unpublished data). During differentiation of neural progenitor cells, inactivation of Notch signals promotes the generation of neurons in ischemic neural progenitor cells(unpublished data). Studies in vivo show that intraventricular infusion of fibroblast growth factor-2 (FGF2) and Notch ligand delta-like 4 (Dll4) after stroke significantly increase the number of proliferating neural progenitor cells in the SVZ (Androutsellis-Theotokis et al., 2006). These in vitro and in vivo data suggest that activation of Notch signals augments expansion of a pool of neural progenitor cells after stroke. However, little is known whether activation of Notch signals mediates stroke-induced radial glia as has been shown at E9.5 that activated Notch promotes radial glial identity(Gaiano et al., 2000). The Notch pathway is coupled with the sonic hedgehog (Shh) pathway in regulating neural stem cells (Androutsellis-Theotokis et al., 2006). In the adult rodent brain, Shh acts as a mitogen in cooperation with epidermal growth factor (EGF) to regulate proliferation of neural stem cells in the adult SVZ (Palma et al., 2005) (Fig. 3). Exogenous Shh increases neurogenesis in the SVZ, whereas blockage of Shh signaling reduces SVZ cell proliferation(Charytoniuk et al., 2002; Palma et al., 2005; Rafuse et al., 2005). In vivo genetic fate-mapping studies with Gli1-CreERT2 mice reveal that the Shh signaling pathway regulates neural stem and progenitor cells in the SVZ of the adult mouse(Ahn and Joyner, 2005). Inactivation of Shh signals depletes neural stem cells (type B1 cells) and actively proliferating type C cells in the SVZ (Balordi and Fishell, 2007). The Shh signaling pathway mediates erythropoietin (EPO)- and carbamylated erythropoietin (CEPO)-induced neurogenesis (Wang et al., 2007). EPO regulates neurogenesis in the SVZ of adult normal and ischemic mice through its receptor EPOR in adult SVZ (Shingo et al., 2001; Tsai et al., 2006; Chen et al., 2007). Administration of EPO significantly augments ischemia-induced neurogenesis (Wang et al., 2004; Wang et al., 2006) and blockage of the Shh pathway with cyclopamine, a specific inhibitor of Smo, or siRNA against Gli1 suppresses EPO- and CEPO-increased neurogenesis (Wang et al., 2007) (unpublished data). Therefore, therapies targeting these signaling pathways may amplify endogenous neurogenesis after stroke.
Figure 2.
The Notch signaling pathway. When ligands including Delta-like and Jagged families from signaling cells bind to Notch receptor in signaling receiving cells, NICD is cleaved by the γ-secretase enzyme complex. The NICD translocates into the nucleus and associates with CBF1 and others to activate target genes including Hes family members.
Figure 3.
The Shh signaling pathway. In the absence of Sonic hedgehog (Shh), Patched 1 (Ptc1) represses the activation of Smoothened (Smo) and the downstream transduction cascade (Inactivation). Binding of Shh to the Ptc 1 activates Smo and this complex regulates Gli activity in nucleus.
Wnt and bone morphogenic protein (BMP) genes regulate neurogenesis in the adult brain(Lim et al., 2000; Lie et al., 2005). Overexpression of BMP7 in ependymal cells inhibits neural progenitor cell proliferation and neuroblast production, demonstrating that BMPs potently inhibit neurogenesis(Lim et al., 2000). In contrast, the Wnt pathway promotes neurogenesis in the dentate gyrus(Lie et al., 2005). Stroke changes expression of Wnt and BMP family genes in SVZ neural progenitor cells of adult rodent (Liu et al., 2007; Morris et al., 2007). However, how these genes regulate proliferation and differentiation of neural progenitor cells after stroke remains to be determined.
Cerebral vascular niche for stroke-induced neurogenesis
The vascular niche affects the neurogenic behavior of neural stem and progenitor cells (Leventhal et al., 1999; Palmer et al., 2000; Shen et al., 2004). Endothelial cells release an array of neurotrophic factors including brain derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) (Leventhal et al., 1999; Palmer et al., 2000; Shen et al., 2004). These factors stimulate the self renewal of adult neural stem cells, inhibit their differentiation and promote their production of neurons (Leventhal et al., 1999; Palmer et al., 2000; Shen et al., 2004). Stroke induces angiogenesis and neurogenesis, which are coupled (Lin et al., 2000; Morris et al., 2000; Zhang et al., 2000; Zhang et al., 2001b; Jin et al., 2002; Zhang and Chopp, 2002; Zhang et al., 2002; Greenberg and Jin, 2005; Ohab et al., 2006; Teng et al., 2008; Thored et al., 2007). Under physiological conditions, neuroblasts born in the SVZ of the adult rodent travel the RMS to the olfactory bulb (Doetsch et al., 1997; Luskin et al., 1997; Garcia-Verdugo et al., 1998). However, after stroke neuroblasts generated in the SVZ migrate to the ischemic boundary where angiogenesis occurs, and during migration neuroblasts are closely associated with cerebral vessels (Jiang et al., 2005; Ohab et al., 2006; Thored et al., 2007). Activated endothelial cells of cerebral vessels secrete stromal-derived factor 1α (SDF-1α) to attract neuroblasts expressing CXCR4, a receptor for SDF-1α (Hill et al., 2004; Imitola et al., 2004; Robin et al., 2004; Tran et al., 2004; Thored et al., 2006), and blockage of CXCR4 abrogates migration of neuroblasts to the ischemic boundary (Imitola et al., 2004; Robin et al., 2004; Ohab et al., 2006; Thored et al., 2006). In vivo blockage of angiogenesis with intraventricular infusion of a neutralized antibody against angiopoietin receptor, tyrosine kinase with immunoglobulin and epidermal growth factor homology domains 2 (Tie2), or systemic administration of endostatin substantially attenuates migration of neuroblasts newly born in the SVZ to the ischemic region (Ohab et al., 2006). In addition to migration, endothelial cells activated by ischemia promote neural progenitor cell proliferation and neuronal differentiation. Co-culture of cerebral endothelial cells harvested from the ischemic boundary and neural progenitor cells derived from non-ischemic SVZ significantly increases the number of proliferating neural progenitor cells(Teng et al., 2008). Moreover, the endothelial cells augment the neuronal but not astrocytic population(Teng et al., 2008). Furthermore, neural progenitor cells harvested from the ischemic SVZ promote in vitro angiogenesis measured by a capillary-like tube formation assay(Teng et al., 2008). VEGF critically mediates this coupling process since blockage of VEGFR2 with a VEGFR2 antagonist suppresses the coupling (Teng et al., 2008). These in vitro data provide insight into recent in vivo findings that neuroblasts in angiogenic areas persist for at least 6 months after stroke (Thored et al., 2007) (unpublished data). We speculate that in addition to angiogenesis which guides neuroblasts to the ischemic region, neuroblasts promote maturation of new vessels, whereas activated endothelial cells trigger in situ neuroblast proliferation (Zhang et al., 2007a). Cell and pharmacologically based therapies have recently demonstrated that increased angiogenesis leads to enhancement of neurogenesis (Chopp and Li, 2002; Jin et al., 2002; Chen et al., 2003; Zhang et al., 2003; Shyu et al., 2004; Taguchi et al., 2004; Wang et al., 2004).
Stroke induces neurogenesis in humans
Although cellular composition and cytoarchitecture of the adult human SVZ differ from the that of the adult rodent SVZ, neurogenesis is present in the human SVZ (Quinones-Hinojosa et al., 2006; Curtis et al., 2007a; Curtis et al., 2007b). Stroke induces neurogenesis in the adult human SVZ and ischemic boundary (Jin et al., 2006; Macas et al., 2006; Minger et al., 2007). Analysis of postmortem brain tissue from advanced age humans having suffered from ischemic stroke reveals increases of proliferating SVZ cells and neuroblasts (Macas et al., 2006). Immunostaining of brain biopsy tissue from stroke patients shows that cells in the ischemic boundary regions, which are preferentially localized in the vicinity of blood vessels, express markers associated with newborn neurons (Jin et al., 2006). These data are consistent with experimental results showing that stroke induces neurogenesis in aged animals, although basal neurogenesis is attenuated in these animals (Jin et al., 2004; Maslov et al., 2004; Luo et al., 2006; Zhang et al., 2006a). Coupling of angiogenesis and neurogenesis has also been observed in the adult human(Pereira et al., 2007). Using MRI, a study shows that exercise specifically changes cerebral blood volume (CBV) in the adult human dentate gyrus where exercise-induced neurogenesis has been demonstrated in the animal (Pereira et al., 2007). Experimental studies in aged animals have shown that pharmaceutical agents such as statins and sildenafil substantially enhance angiogenesis and neurogenesis and improve functional outcome during stroke recovery (Chen et al., 2006; Zhang et al., 2006a).
Future studies
Studies of signaling pathways and cytokinetics in adult neural progenitor cells provide insight into mechanisms of stroke-induced neurogenesis. It is important to elucidate how the signaling pathways are spatially and temporally coordinated to control proliferation, differentiation, and migration of neural stem and progenitor cells after stroke. Cell and pharmacological based therapies have demonstrated that enhancement of endogenous neurogenesis is associated with improvement of neurological function during stroke recovery, which suggests that adult neurogenesis contributes to improved functional outcome. However, we are presently unable to identify the improvement in neurological function specifically attributed to stroke-induced neurogenesis because enhanced functional outcome after stroke likely results from a composite of events including angiogenesis, neurogenesis, and axonal as well as dendritic plasticity. Using inducible Cre to specifically knockout migrating neuroblasts in the adult mice after stroke could provide insight into the direct effect of neurogenesis on functional outcome during stroke recovery. It is also essential for future studies to determine which neuronal types are generated by neural progenitor cells and to clarify the formation of neuronal circuitry between new-born neurons and resident neurons in ischemic brain.
Acknowledgments
This work was supported by NINDS grants PO1 NS23393, PO1 NS42345, and RO1NS38292.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007;27:5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calegari F, Haubensak W, Haffner C, Huttner WB. Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J Neurosci. 2005;25:6533–6538. doi: 10.1523/JNEUROSCI.0778-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS, Jr, Goto T, Tarui T, Takahashi T, Bhide PG, Nowakowski RS. Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb Cortex. 2003;13:592–598. doi: 10.1093/cercor/13.6.592. [DOI] [PubMed] [Google Scholar]
- Charytoniuk D, Traiffort E, Hantraye P, Hermel JM, Galdes A, Ruat M. Intrastriatal sonic hedgehog injection increases Patched transcript levels in the adult rat subventricular zone. Eur J Neurosci. 2002;16:2351–2357. doi: 10.1046/j.1460-9568.2002.02412.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Zhang R, Katakowski M, Gautam SC, Xu Y, Lu M, Zhang Z, Chopp M. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004;1005:21–28. doi: 10.1016/j.brainres.2003.11.080. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Li A, Zhang C, Ding J, Roberts C, Lu M, Kapke A, Chopp M. Vascular endothelial growth factor mediates atorvastatin-induced mammalian achaete-scute homologue-1 gene expression and neuronal differentiation after stroke in retired breeder rats. Neuroscience. 2006;141:737–744. doi: 10.1016/j.neuroscience.2006.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Asavaritikrai P, Prchal JT, Noguchi CT. Endogenous erythropoietin signaling is required for normal neural progenitor cell proliferation. J Biol Chem. 2007;282:25875–25883. doi: 10.1074/jbc.M701988200. [DOI] [PubMed] [Google Scholar]
- Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–641. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Eriksson PS, Faull RL. Progenitor cells and adult neurogenesis in neurodegenerative diseases and injuries of the basal ganglia. Clin Exp Pharmacol Physiol. 2007a;34:528–532. doi: 10.1111/j.1440-1681.2007.04609.x. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen JF, Dragunow M, Faull RL, Eriksson PS. Human Neuroblasts Migrate to the Olfactory Bulb via a Lateral Ventricular Extension. Science. 2007b doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999a;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999b;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo JM, Doetsch F, Wichterle H, Lim DA, Alvarez-Buylla A. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol. 1998;36:234–248. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- Gregg C, Weiss S. Generation of functional radial glial cells by embryonic and adult forebrain neural stem cells. J Neurosci. 2003;23:11587–11601. doi: 10.1523/JNEUROSCI.23-37-11587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentchev M, McKay RD. Notch controls proliferation and differentiation of stem cells in a dose-dependent manner. Eur J Neurosci. 2006;23:2289–2296. doi: 10.1111/j.1460-9568.2006.04766.x. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Ruffett TL. Cell proliferation in the neural tube: an electron microscopic and golgi analysis in the mouse cerebral vesicle. Z Zellforsch Mikrosk Anat. 1971;115:226–264. doi: 10.1007/BF00391127. [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol. 2005;17:648–657. doi: 10.1016/j.ceb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Sato K, Omori N, Nagano I, Manabe Y, Shoji M, Abe K. Three steps of neural stem cells development in gerbil dentate gyrus after transient ischemia. J Cereb Blood Flow Metab. 2002;22:411–419. doi: 10.1097/00004647-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Iwai M, Sato K, Kamada H, Omori N, Nagano I, Shoji M, Abe K. Temporal profile of stem cell division, migration, and differentiation from subventricular zone to olfactory bulb after transient forebrain ischemia in gerbils. J Cereb Blood Flow Metab. 2003;23:331–341. doi: 10.1097/01.WCB.0000050060.57184.E7. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Zhang ZG, Ding GL, Zhang L, Ewing JR, Wang L, Zhang R, Li L, Lu M, Meng H, Arbab AS, Hu J, Li QJ, Pourabdollah Nejad DS, Athiraman H, Chopp M. Investigation of neural progenitor cell induced angiogenesis after embolic stroke in rat using MRI. Neuroimage. 2005;28:698–707. doi: 10.1016/j.neuroimage.2005.06.063. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001a;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001b;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Xie L, Sun Y, Mao XO, Wang Y, Simon RP, Greenberg DA. Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell. 2004;3:373–377. doi: 10.1111/j.1474-9728.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Inagaki N, Nishizawa M, Tsutsumi O, Taketani Y, Inagaki M. Visualization of mitotic radial glial lineage cells in the developing rat brain by Cdc2 kinase-phosphorylated vimentin. Glia. 1998;23:191–199. doi: 10.1002/(sici)1098-1136(199807)23:3<191::aid-glia2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Kee NJ, Preston E, Wojtowicz JM. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Exp Brain Res. 2001;136:313–320. doi: 10.1007/s002210000591. [DOI] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Lin TN, Wang CK, Cheung WM, Hsu CY. Induction of angiopoietin and Tie receptor mRNA expression after cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2000;20:387–395. doi: 10.1097/00004647-200002000-00021. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang RL, Gregg SR, Meng H, Chopp M. Comparison of in vivo and in vitro gene expression profiles in subventricular zone neural progenitor cells from the adult mouse after middle cerebral artery occlusion. Neuroscience. 2007;146:1053–1061. doi: 10.1016/j.neuroscience.2007.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5:139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Zigova T, Soteres BJ, Stewart RR. Neuronal progenitor cells derived from the anterior subventricular zone of the neonatal rat forebrain continue to proliferate in vitro and express a neuronal phenotype. Mol Cell Neurosci. 1997;8:351–366. doi: 10.1006/mcne.1996.0592. [DOI] [PubMed] [Google Scholar]
- Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26:13114–13119. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Minger SL, Ekonomou A, Carta EM, Chinoy A, Perry RH, Ballard CG. Endogenous neurogenesis in the human brain following cerebral infarction. Regen Med. 2007;2:69–74. doi: 10.2217/17460751.2.1.69. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Mizutani KI, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Morris DC, Davies K, Zhang Z, Chopp M. Measurement of cerebral microvessel diameters after embolic stroke in rat using quantitative laser scanning confocal microscopy. Brain Res. 2000;876:31–36. doi: 10.1016/s0006-8993(00)02543-9. [DOI] [PubMed] [Google Scholar]
- Morris DC, Zhang ZG, Wang Y, Zhang RL, Gregg S, Liu XS, Chopp M. Wnt expression in the adult rat subventricular zone after stroke. Neurosci Lett. 2007;418:170–174. doi: 10.1016/j.neulet.2007.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Craig CG, van der Kooy D. In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development. 1998;125:2251–2261. doi: 10.1242/dev.125.12.2251. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Nagao M, Sugimori M, Nakafuku M. Cross talk between notch and growth factor/cytokine signaling pathways in neural stem cells. Mol Cell Biol. 2007;27:3982–3994. doi: 10.1128/MCB.00170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Soundararajan P, Leopold C, Robertson HA. Neuroprotective properties of cultured neural progenitor cells are associated with the production of sonic hedgehog. Neuroscience. 2005;131:899–916. doi: 10.1016/j.neuroscience.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412:736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- Robin A, Zhang Z, Wang L, Zhang R, Katakowksi M, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Reymann KG. Proliferating cells differentiate into neurons in the hippocampal CA1 region of gerbils after global cerebral ischemia. Neurosci Lett. 2002;334:153–156. doi: 10.1016/s0304-3940(02)01072-8. [DOI] [PubMed] [Google Scholar]
- Schultze B, Korr H. Cell kinetic studies of different cell types in the developing and adult brain of the rat and the mouse: a review. Cell Tissue Kinet. 1981;14:309–325. doi: 10.1111/j.1365-2184.1981.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shibata T, Yamada K, Watanabe M, Ikenaka K, Wada K, Tanaka K, Inoue Y. Glutamate transporter GLAST is expressed in the radial glia-astrocyte lineage of developing mouse spinal cord. J Neurosci. 1997;17:9212–9219. doi: 10.1523/JNEUROSCI.17-23-09212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, Li H. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110:1847–1854. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- Smith CM, Luskin MB. Cell cycle length of olfactory bulb neuronal progenitors in the rostral migratory stream. Dev Dyn. 1998;213:220–227. doi: 10.1002/(SICI)1097-0177(199810)213:2<220::AID-AJA7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr Cell cycle parameters and patterns of nuclear movement in the neocortical proliferative zone of the fetal mouse. J Neurosci. 1993;13:820–833. doi: 10.1523/JNEUROSCI.13-02-00820.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neuronogenesis. J Neurosci. 1996;16:6183–6196. doi: 10.1523/JNEUROSCI.16-19-06183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Yamashiro K, Mochizuki H, Cho N, Onodera M, Mizuno Y, Urabe T. Neurogenesis after transient global ischemia in the adult hippocampus visualized by improved retroviral vector. Stroke. 2004;35:1454–1459. doi: 10.1161/01.STR.0000126480.40967.b3. [DOI] [PubMed] [Google Scholar]
- Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, Gregg SR, Wu Z, Jiang A, Lu M, Zlokovic BV, Chopp M. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28:764–771. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Tonchev AB, Yamashima T, Zhao L, Okano HJ, Okano H. Proliferation of neural and neuronal progenitors after global brain ischemia in young adult macaque monkeys. Mol Cell Neurosci. 2003;23:292–301. doi: 10.1016/s1044-7431(03)00058-7. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Garcia-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex. 2003;13:580–587. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, Liu X, Wu H, Carmichael ST. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tureyen K, Vemuganti R, Sailor KA, Bowen KK, Dempsey RJ. Transient focal cerebral ischemia-induced neurogenesis in the dentate gyrus of the adult mouse. J Neurosurg. 2004;101:799–805. doi: 10.3171/jns.2004.101.5.0799. [DOI] [PubMed] [Google Scholar]
- Ventura RE, Goldman JE. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J Neurosci. 2007;27:4297–4302. doi: 10.1523/JNEUROSCI.0399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt T. Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol. 1989;289:74–88. doi: 10.1002/cne.902890106. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Jiao ZX, Wang Y, Pourabdollah-Nejad DS, Letourneau Y, Gregg SR, Chopp M. Neurogenin 1 mediates erythropoietin enhanced differentiation of adult neural progenitor cells. J Cereb Blood Flow Metab. 2006;26:556–564. doi: 10.1038/sj.jcbfm.9600215. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Gregg SR, Zhang RL, Jiao Z, Letourneau Y, Liu X, Feng Y, Gerwien J, Torup L, Leist M, Noguchi CT, Chen ZY, Chopp M. The sonic hedgehog pathway mediates carbamylated EPO enhanced proliferation and differentiation of adult neural progenitor cells. J Biol Chem. 2007;282:32462–32470. doi: 10.1074/jbc.M706880200. [DOI] [PubMed] [Google Scholar]
- Weissman T, Noctor SC, Clinton BK, Honig LS, Kriegstein AR. Neurogenic radial glial cells in reptile, rodent and human: from mitosis to migration. Cereb Cortex. 2003;13:550–559. doi: 10.1093/cercor/13.6.550. [DOI] [PubMed] [Google Scholar]
- Willaime-Morawek S, Seaberg RM, Batista C, Labbe E, Attisano L, Gorski JA, Jones KR, Kam A, Morshead CM, van der Kooy D. Embryonic cortical neural stem cells migrate ventrally and persist as postnatal striatal stem cells. J Cell Biol. 2006;175:159–168. doi: 10.1083/jcb.200604123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita Y, Kitagawa K, Ohtsuki T, Takasawa K, Miyata T, Okano H, Hori M, Matsumoto M. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke. 2001;32:1890–1896. doi: 10.1161/01.str.32.8.1890. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Vutskits L, Pepper MS, Kiss JZ. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J Cell Biol. 2003;163:1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001a;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Wang Y, Zhang C, Chopp M. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res. 2006a;83:1213–1219. doi: 10.1002/jnr.20813. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Lu M, Wang Y, Yang JJ, Chopp M. Reduction of the cell cycle length by decreasing G(1) phase and cell cycle reentry expand neuronal progenitor cells in the subventricular zone of adult rat after stroke. J Cereb Blood Flow Metab. 2006b;26:857–863. doi: 10.1038/sj.jcbfm.9600237. [DOI] [PubMed] [Google Scholar]
- Zhang RL, LeTourneau Y, Gregg SR, Wang Y, Toh Y, Robin AM, Zhang ZG, Chopp M. Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007;27:3157–3162. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Roberts C, Letourneau Y, Lu M, Zhang L, Wang Y, Chopp M. Lengthening the G(1) phase of neural progenitor cells is concurrent with an increase of symmetric neuron generating division after stroke. J Cereb Blood Flow Metab. 2008;28:602–611. doi: 10.1038/sj.jcbfm.9600556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Wang Y, Letourneau Y, Liu XS, Zhang X, Gregg SR, Wang L, Chopp M. Stroke induces ependymal cell transformation into radial glia in the subventricular zone of the adult rodent brain. J Cereb Blood Flow Metab:advance online publication. 2007b;27:1201–1212. doi: 10.1038/sj.jcbfm.9600430. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M. Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med. 2002;12:62–66. doi: 10.1016/s1050-1738(01)00149-9. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Tsang W, Zhang L, Powers C, Chopp M. Up-regulation of neuropilin-1 in neovasculature after focal cerebral ischemia in the adult rat. J Cereb Blood Flow Metab. 2001b;21:541–549. doi: 10.1097/00004647-200105000-00008. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and Angiopoietin Expression With Disruption of Blood-Brain Barrier and Angiogenesis After Focal Cerebral Ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Zhu DY, Liu SH, Sun HS, Lu YM. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci. 2003;23:223–229. doi: 10.1523/JNEUROSCI.23-01-00223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]