Abstract
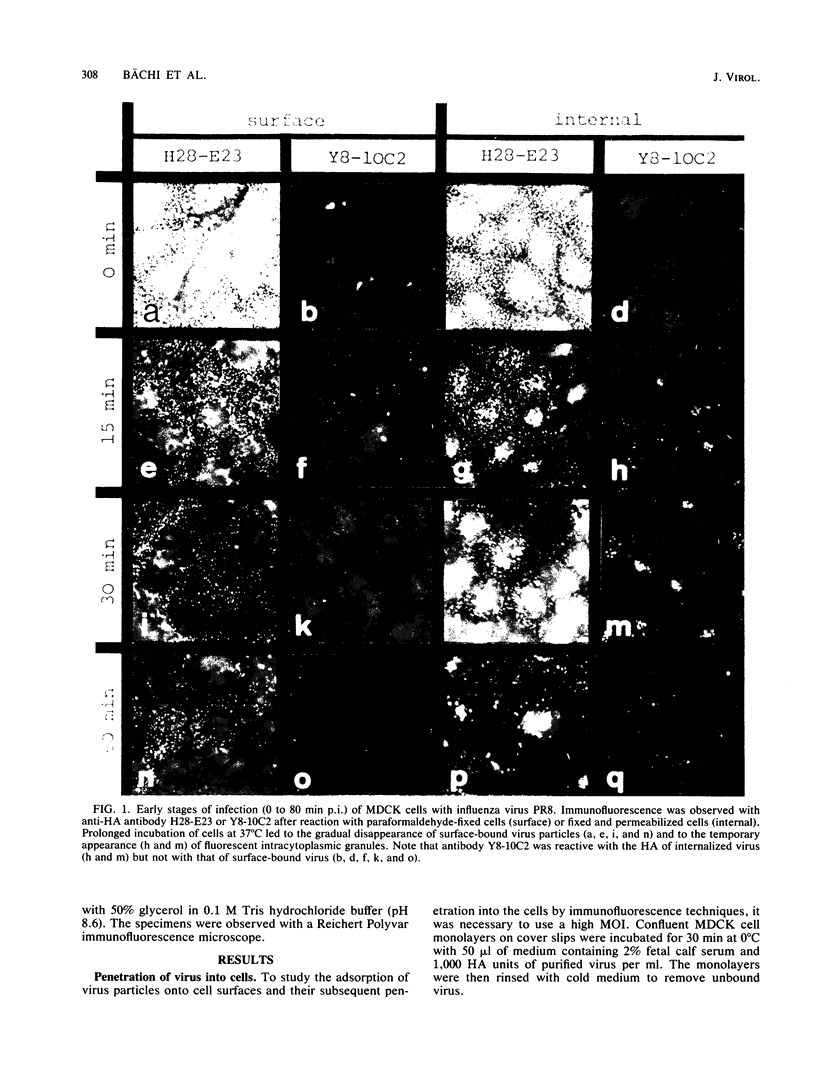
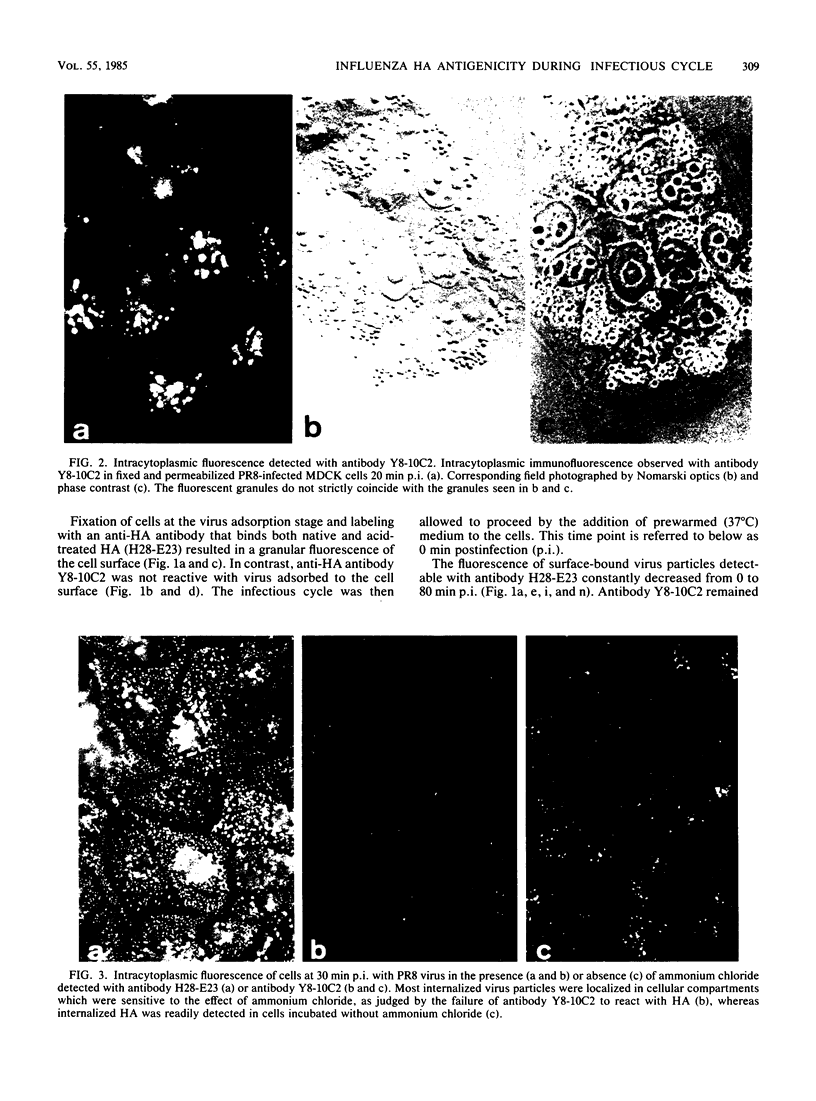
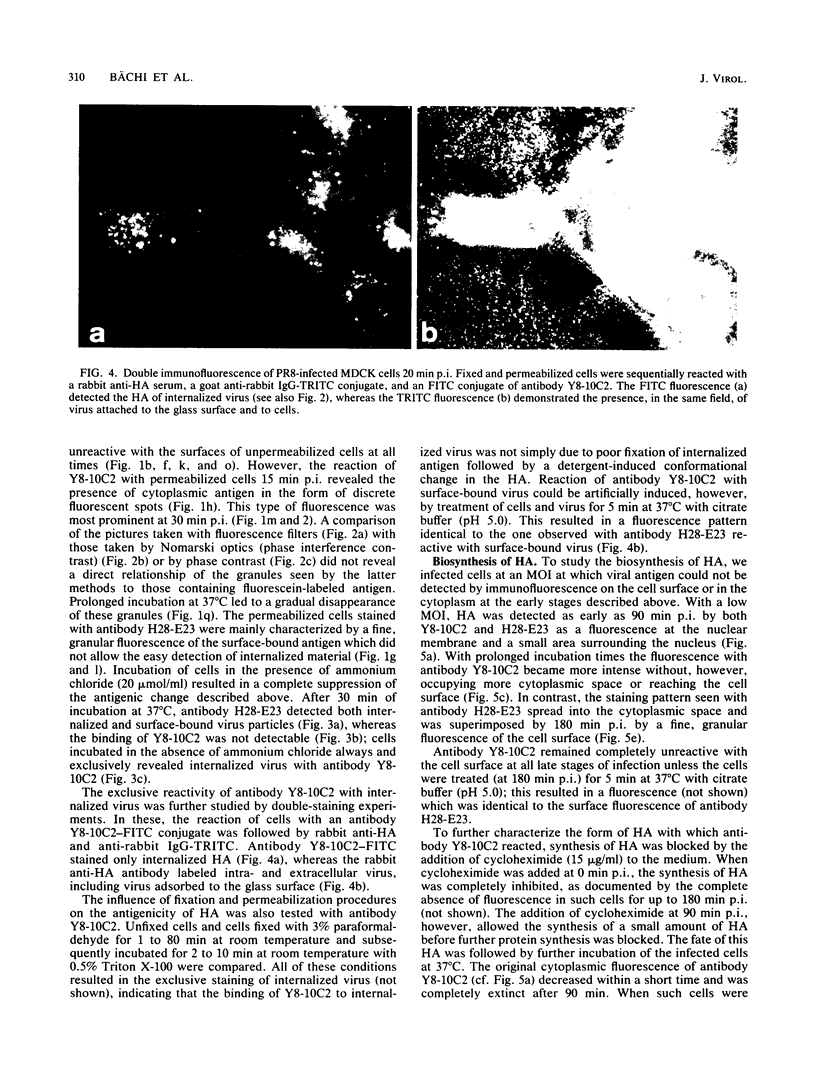
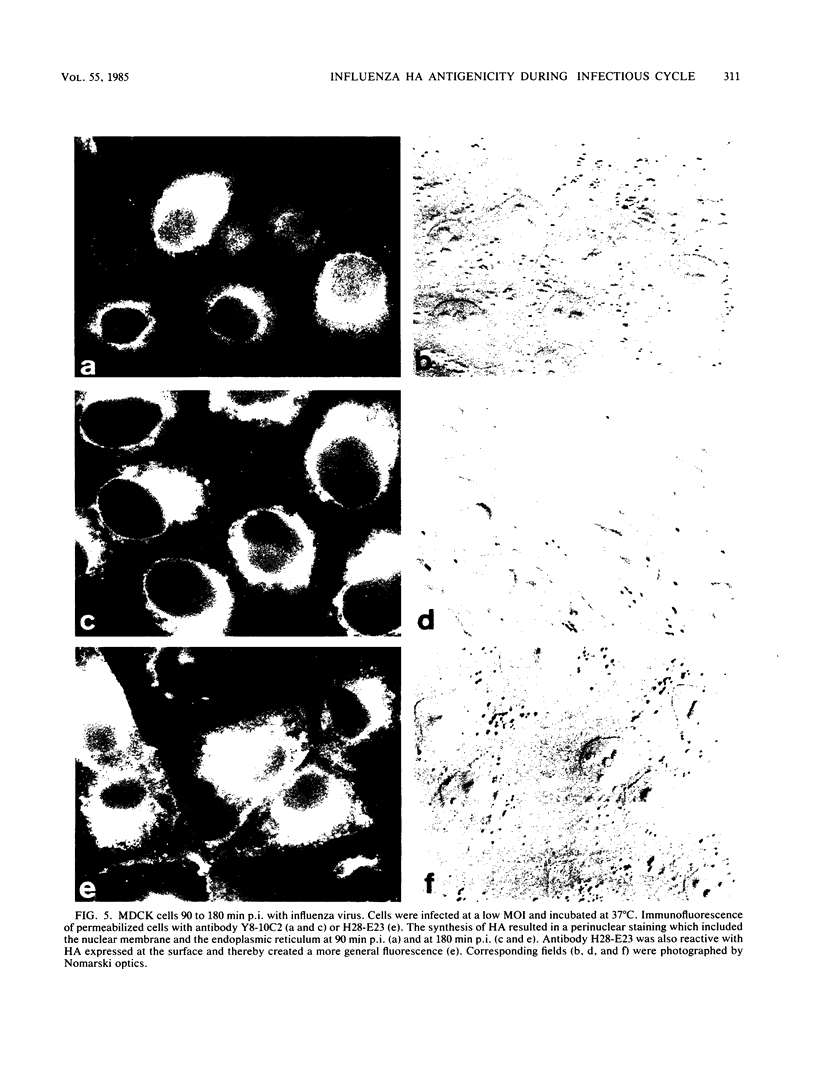
Monoclonal antibodies specific for the influenza virus A/PR/8/34 hemagglutinin (HA) were used to examine the structure of the HA glycoprotein by immunofluorescence techniques during infection of MDCK cells. One antibody (Y8-10C2), shown previously to detect conformational alterations in the HA coinciding with the acid induction of viral fusion activity, bound to internalized virus but not to virus adsorbed to the cell surface. The binding of Y8-10C2 was completely inhibited by ammonium chloride treatment of the cells. These findings are consistent with the idea that Y8-10C2 detects conformational changes in the HA which accompany the acid-induced fusion of viral and endosomal membranes. The same antibody also bound to newly synthesized HA but not to later forms of cytoplasmic HA or to HA incorporated into the cell membrane during virus maturation. A possible common denominator of the antigenic changes detected by antibody Y8-10C2 during virus entry and replication may be alterations in the structural relationship among the three HA monomers which form the mature HA molecule.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bächi T., Eichenberger G., Hauri H. P. Sendai virus hemolysis: influence of lectins and analysis by immune fluorescence. Virology. 1978 Apr;85(2):518–530. doi: 10.1016/0042-6822(78)90458-0. [DOI] [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Daniels R. S., Douglas A. R., Skehel J. J., Wiley D. C. Analyses of the antigenicity of influenza haemagglutinin at the pH optimum for virus-mediated membrane fusion. J Gen Virol. 1983 Aug;64(Pt 8):1657–1662. doi: 10.1099/0022-1317-64-8-1657. [DOI] [PubMed] [Google Scholar]
- Gerhard W., Yewdell J., Frankel M. E., Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981 Apr 23;290(5808):713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- Green J., Griffiths G., Louvard D., Quinn P., Warren G. Passage of viral membrane proteins through the Golgi complex. J Mol Biol. 1981 Nov 15;152(4):663–698. doi: 10.1016/0022-2836(81)90122-4. [DOI] [PubMed] [Google Scholar]
- Helenius A., Marsh M. Endocytosis of enveloped animal viruses. Ciba Found Symp. 1982;(92):59–76. doi: 10.1002/9780470720745.ch4. [DOI] [PubMed] [Google Scholar]
- Huang R. T., Wahn K., Klenk H. D., Rott R. Fusion between cell membrane and liposomes containing the glycoproteins of influenza virus. Virology. 1980 Jul 30;104(2):294–302. doi: 10.1016/0042-6822(80)90334-7. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Lenard J., Miller D. K. pH-dependent hemolysis by influenza, Semliki, Forest virus, and Sendai virus. Virology. 1981 Apr 30;110(2):479–482. doi: 10.1016/0042-6822(81)90079-9. [DOI] [PubMed] [Google Scholar]
- Louvard D., Reggio H., Warren G. Antibodies to the Golgi complex and the rough endoplasmic reticulum. J Cell Biol. 1982 Jan;92(1):92–107. doi: 10.1083/jcb.92.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Kawasaki K., Ohnishi S. Interaction of influenza virus hemagglutinin with target membrane lipids is a key step in virus-induced hemolysis and fusion at pH 5.2. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4133–4137. doi: 10.1073/pnas.78.7.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Ohnishi S. Activation of influenza virus by acidic media causes hemolysis and fusion of erythrocytes. FEBS Lett. 1980 Dec 29;122(2):283–287. doi: 10.1016/0014-5793(80)80457-1. [DOI] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981 Dec;91(3 Pt 1):601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen N. L., Nayak D. P., Jones L. V., Compans R. W. Chimeric influenza virus hemagglutinin containing either the NH2 terminus or the COOH terminus of G protein of vesicular stomatitis virus is defective in transport to the cell surface. Proc Natl Acad Sci U S A. 1984 Jan;81(2):395–399. doi: 10.1073/pnas.81.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Paskiet K. T., Salas P. J., Bard E. Intracellular transport of influenza virus hemagglutinin to the apical surface of Madin-Darby canine kidney cells. J Cell Biol. 1984 Jan;98(1):308–319. doi: 10.1083/jcb.98.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Bayley P. M., Brown E. B., Martin S. R., Waterfield M. D., White J. M., Wilson I. A., Wiley D. C. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci U S A. 1982 Feb;79(4):968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Brown L. E., Jackson D. C. Changes in the antigenicity of the hemagglutinin molecule of H3 influenza virus at acidic pH. Virology. 1983 Apr 30;126(2):587–599. doi: 10.1016/s0042-6822(83)80015-4. [DOI] [PubMed] [Google Scholar]
- White J., Helenius A., Gething M. J. Haemagglutinin of influenza virus expressed from a cloned gene promotes membrane fusion. Nature. 1982 Dec 16;300(5893):658–659. doi: 10.1038/300658a0. [DOI] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Membrane fusion activity of influenza virus. EMBO J. 1982;1(2):217–222. doi: 10.1002/j.1460-2075.1982.tb01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J., Waterfield M. Evidence from studies with a cross-linking reagent that the haemagglutinin of influenza virus is a trimer. Virology. 1977 Jun 15;79(2):446–448. doi: 10.1016/0042-6822(77)90371-3. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Gerhard W., Bachi T. Monoclonal anti-hemagglutinin antibodies detect irreversible antigenic alterations that coincide with the acid activation of influenza virus A/PR/834-mediated hemolysis. J Virol. 1983 Oct;48(1):239–248. doi: 10.1128/jvi.48.1.239-248.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Kuroda K., Kawasaki K., Yamashina S., Maeda T., Ohnishi S. Infectious cell entry mechanism of influenza virus. J Virol. 1982 Jul;43(1):284–293. doi: 10.1128/jvi.43.1.284-293.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Ohnishi S. Uncoating of influenza virus in endosomes. J Virol. 1984 Aug;51(2):497–504. doi: 10.1128/jvi.51.2.497-504.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]