Abstract
Human cytomegalovirus (HCMV) forms two different membrane protein complexes, gH/gL/gO and gH/gL/UL128/UL130/UL131, that function in different cell types. gH/gL/gO appears to be important for HCMV entry into or spread between fibroblasts, processes that occur at neutral pH. We demonstrated that HCMV entry into epithelial and endothelial cells requires gH/gL/UL128–131 and involves endocytosis and low pH. A complex of all five HCMV proteins, gH, gL, UL128, UL130, and UL131, is the functionally important mediator of this entry pathway into epithelial/endothelial cells. Here, we report that expression of gH/gL/UL128–131 in ARPE-19 epithelial cells causes the cells to be resistant to HCMV infection. Another HCMV glycoprotein, gB, did not interfere, and expression of all five gH/gL/UL128–131 proteins was required for this interference. gH/gL/UL128–131 interference was at the stage of virus entry into cells rather than the initial adsorption onto cell surfaces or after-entry defects. By contrast, expression of gH/gL/UL128–131 in primary human fibroblasts did not block HCMV infection. Previously, interference by retrovirus and herpes-simplex-virus entry mediators resulted from sequestration or obstruction of receptors. We concluded that epithelial cells express gH/gL/UL128–131 receptors that mediate HCMV entry. Fibroblasts either lack the gH/gL/UL128–131 receptors, the receptors are more numerous, or fibroblasts express other functional receptors.
Keywords: HCMV glycoproteins, receptor interference
The β-herpesvirus human cytomegalovirus (HCMV) causes substantial morbidity and mortality in immunologically naive children, immunosuppressed transplant patients, and immunodeficient AIDS patients. Congenital HCMV infection is common and can lead to serious developmental defects involving the CNS and often leads to deafness (1, 2). In immunosuppressed transplant patients, HCMV infection can result in pneumonia, gastrointestinal disease, hepatitis, retinitis, and encephalitis. Moreover, acute or chronic HCMV infection and the associated inflammation in blood vessels can increase rejection of transplanted tissues (3). The wide spectrum of HCMV diseases is because of, in part, a capacity to replicate in many different cell types, including epithelial and endothelial cells, microglial cells, neurons, monocyte-macrophages, and fibroblasts. Epithelial cells represent portals of HCMV entry into and exit from the body and are major targets of virus infection in the gut and in the retina (1, 4, 5). HCMV replication in vascular endothelial cells produces virus particles that spread directly to monocyte-macrophages and lymphocytes, which disseminate the virus throughout the body (6, 7). Fibroblasts are also extensively infected in vivo and are the principle cell type used to propagate HCMV in vitro (5).
HCMV entry into cells is poorly understood. As with other herpesviruses, HCMV adsorbs onto heparan sulfate glycosaminoglycans (GAGs), a process that increases cell-surface concentrations of virus (8). Integrins and epidermal growth factor receptors (EGFRs) have been described as HCMV receptors. HCMV infection of human embryonic lung (HEL) fibroblasts and breast cancer cells correlated with EGFR expression and entry into HEL cells was blocked with EGFR antibodies (9). However, it has also been argued that EGFRs are not important for HCMV entry (10). Integrins, especially those including α2, α6, αv, β1, and β3 polypeptides, enhance HCMV entry into human fibroblasts (11). HCMV infection of human fibroblasts was reduced by integrin-specific antibodies. Additionally, a mouse fibroblast cell line lacking β1 integrin was resistant to HCMV and murine CMV (MCMV), and transient expression of β1 rendered these cells more susceptible to HCMV and MCMV. HCMV glycoprotein gB contains a disintegrin-like domain, and peptides encompassing this domain blocked HCMV entry, consistent with the notion that gB binds integrins (11). By contrast, other work suggested that gB binds EGFRs and gH binds integrins, as coreceptors for fibroblast entry (12).
The studies supporting a role for EGFRs and integrins in HCMV entry involved a HCMV laboratory strain, AD169, that does not infect epithelial and endothelial cells. Laboratory strains of HCMV, including AD169, harbor genetic mutations, deletions, and rearrangements in the UL128–150 genes (13). A subset of these genes, UL128, UL130, and UL131, are specifically required for infection of epithelial and endothelial cells (13–15). The UL128–131 genes encode three small proteins with signal sequences that bind the HCMV glycoprotein gH/gL (16–18), forming complexes distinct from gH/gL complexes that contain another HCMV glycoprotein, gO (17–19). We showed that the gH/gL/UL128–131 complex functions to mediate entry into epithelial and endothelial cells through a process that involves endocytosis and low pH-dependent fusion (16, 20). Omission of any one of the five members of the gH/gL/UL128–131 complex reduces assembly and endoplasmic reticulum (ER) export as well as entry into epithelial/endothelial cells. By contrast, entry into fibroblasts occurs by direct fusion with the plasma membrane and does not require gH/gL/UL128–131 (20, 21). UL128-, UL130-, and UL131-specific antibodies block infection of epithelial and endothelial cells but do not block infection of fibroblasts (17, 18). Moreover, antibodies specific to gH block infection of fibroblasts (1), consistent with the existence of other gH/gL complexes that facilitate entry into fibroblasts. Together, these data suggest that gH/gL/UL128–131-mediated entry into epithelial/endothelial cells via endocytosis and low pH involves cellular machinery that is different from that used when HCMV enters fibroblasts at neutral pH. Moreover, EGFRs and integrins cannot explain gH/gL/UL128–131-mediated entry into epithelial/endothelial cells. Lab strain AD169, which binds these receptors, does not express gH/gL/UL128–131. Additionally, integrins and EGFRs are broadly expressed on both fibroblasts and epithelial/endothelial cells (22, 23).
Important insights into how HCMV enters cells can be obtained from the more extensively studied α-herpesvirus, herpes simplex virus (HSV) (reviewed in ref. 24). The HSV receptor-binding glycoprotein, gD, interacts with gD receptors and triggers the formation of larger complexes composed of gB, gD, and gH/gL (25, 26). gB and gH/gL apparently make up the HSV membrane fusion machinery, so that, once triggered by gD binding to its receptors, gH/gL can cause hemi-fusion of virion and cellular membranes, although gB is necessary to promote full fusion (27). Some of the first and most compelling evidence that HSV gD acts as a receptor-binding protein came from observations that gD-transfected cells were resistant to infection with HSV (28, 29). This process, termed interference, apparently involves sequestration, surface down-regulation, or masking of gD receptors that are essential for HSV entry (30). These results were similar to earlier observations of receptor interference where retrovirus-infected cells were resistant to infection by related retroviruses that used the same entry receptors (31). Therefore, interference provides strong evidence for cellular receptors or entry mediators that are bound by specific viral proteins.
To further characterize HCMV entry into epithelial cells, we expressed gH/gL/UL128–131 in epithelial cells and fibroblasts by using adenovirus (Ad) vectors. HCMV entry into epithelial cells expressing gH/gL/UL128–131 was inhibited by >90% compared with control cells. Interference required all five gH/gL/UL128–131 proteins, and there was no interference because of expression of HCMV gB. Notably, gH/gL/UL128–131 did not interfere with HCMV infection of fibroblasts. These results provide strong evidence for the existence of gH/gL/UL128–131 receptors on the surfaces of epithelial cells that mediate HCMV entry.
Results
Expression of the HCMV gH/gL/UL128–131 Complex Renders Epithelial Cells Resistant to HCMV Infection.
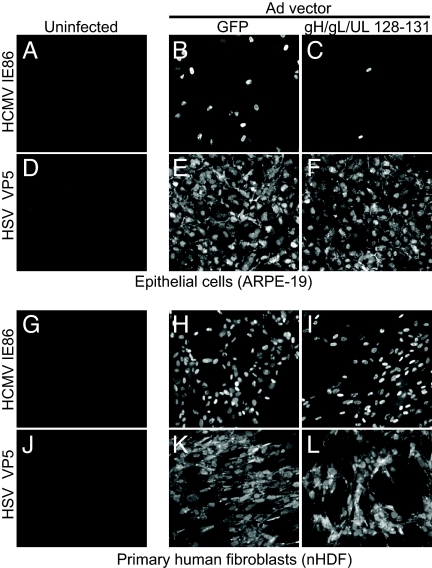
The HCMV proteins UL128, UL130, and UL131 form a complex with gH/gL that facilitates entry into epithelial and endothelial cells, but is dispensable for entry into fibroblasts (14–18, 20). To test whether gH/gL/UL128–131 might interfere with HCMV entry, we expressed the gH, gL, UL128, UL130, and UL131 proteins in human ARPE-19 retinal pigment epithelial cells by using nonreplicating Ad vectors described in ref. 16. Cells expressing gH/gL/UL128–131 or GFP from a control Ad vector (AdGFP) for 12 h were infected with HCMV TR, a wild-type clinical strain, and then analyzed by immunofluorescence for HCMV immediate-early protein 86 (IE86) after an additional 48 h. Thirty to forty percent of the cells treated with AdGFP were infected with HCMV TR as judged by immediate-early (IE) expression (Fig. 1B). By contrast, only 2% of the epithelial cells expressing gH/gL/UL128–131 could be infected with HCMV (Fig. 1C). Expression of gH/gL/UL128–131 did not affect the susceptibility of these cells to HSV infection, indicating that the observed interference was specific to HCMV and was not because of reduced cell viability (Fig. 1 E and F).
Fig. 1.
Inhibition of HCMV infection of epithelial cells, but not human fibroblasts, by expression of gH/gL/UL128–131. ARPE-19 cells (A–F) or primary human fibroblasts (G–L) were left untreated (A, D, G, and J) or were transduced for 12 h with 50 PFU per cell of AdGFP (B, E, H, and K) or 10 PFU per cell each of AdgH, AdgL, AdUL128, AdUL130, and AdUL131 (C, F, I, and L). Cells were then infected with HCMV TR (3 PFU per cell) and stained for HCMV IE86 48 h later (A–C and G–I) or inoculated with 1 PFU per cell of HSV-1 for 12 h and then stained with HSV capsid protein VP5-specific antibodies (D–F and J–L).
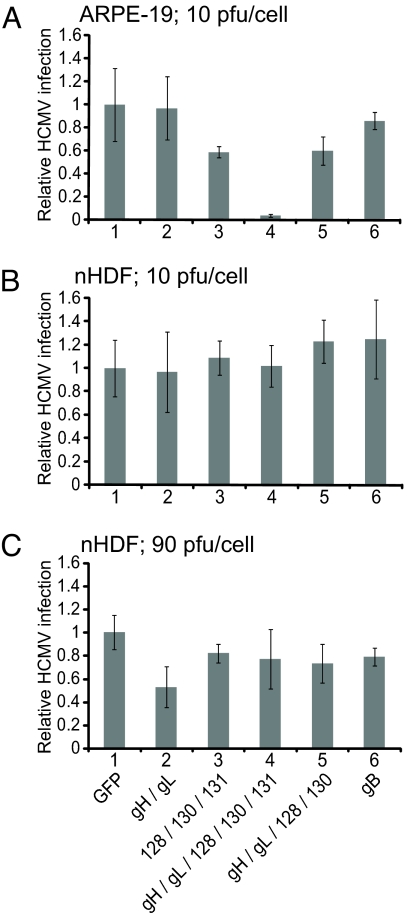
It was of interest whether subcomponents of the gH/gL/UL128–131 complex, e.g., gH/gL, might also interfere with HCMV infection. Expression of the complete gH/gL/UL128–131 complex in ARPE-19 epithelial cells reduced the number of HCMV-infected cells by 96% compared with expression of GFP (Fig. 2A, columns 1 and 4). By contrast, expression of gH/gL alone had little effect on HCMV infection (Fig. 2A, column 2). Expression of UL128/UL130/UL131 or gH/gL/UL128/UL130 reduced HCMV infection by ≈40% (Fig. 2A, columns 3 and 5). Increasing the expression of UL128/UL130/UL131 or gH/gL/UL128/UL130 by using higher doses of the Ad vectors did not significantly increase interference (data not shown). Therefore, only expression of the complete gH/gL/UL128–131 complex results in substantial interference. These results correlate with our previous observations that complexes lacking any one of the five proteins were retained in the ER (16) and are thus likely not incorporated into the virion envelope.
Fig. 2.
Comparison of the interference mediated by gH/gL/L128–131, gH/gL, gB, and other HCMV proteins in epithelial cells and fibroblasts. APRE-19 (A) or primary human fibroblasts (nHDF) (B and C) cells were transduced for 12 h with the indicated combinations of Ad vectors. In A and B, each Ad vector was delivered at 10 PFU per cell, whereas in C, each Ad vector was used at 90 PFU per cell. The total dose of Ad vectors in each dish of cells was made the same by the addition of AdGFP. The cells were infected with HCMV TR (3 PFU per cell) and stained for HCMV IE86 protein 48 h later, costaining nuclei with DAPI. For each condition, at least 350 cells were counted in each of three random fields, and the average number of IE86 positive cells was normalized to the average number of infected cells observed in monolayers expressing GFP only. Error bars indicate the standard deviation from the mean. Results shown are representative of three separate experiments.
The gB homologues of HCMV and other herpesviruses contribute to virion attachment onto cells through interactions with heparan sulfate GAGs and may interact with more specific, saturable receptors (32, 33). However, in contrast to gH/gL/UL128–131, gB expression in ARPE-19 cells did not interfere with HCMV infection (Fig. 2A, column 6), and increased doses of AdgB did not result in interference (data not shown).
Expression of gH/gL/UL128–131 Complexes in Fibroblasts Does Not Interfere with HCMV Infection.
Given that UL128–131 proteins are dispensable for entry into fibroblasts, we tested whether gH/gL/UL128–131 expression in fibroblasts might interfere with HCMV infection. Virtually all of the cells in monolayers of human foreskin fibroblasts transduced with AdGFP were infected with HCMV (Fig. 1H), and this was also the case with fibroblasts expressing gH/gL/UL128–131 (Figs. 1I and 2B, column 4). Moreover, there was no interference with gH/gL, UL128–131, or gH/gL/UL128/130 (Fig. 2B, columns 2, 3, 5, and 6). Again, gB did not interfere with HCMV infection of fibroblasts (Fig. 2B, column 6).
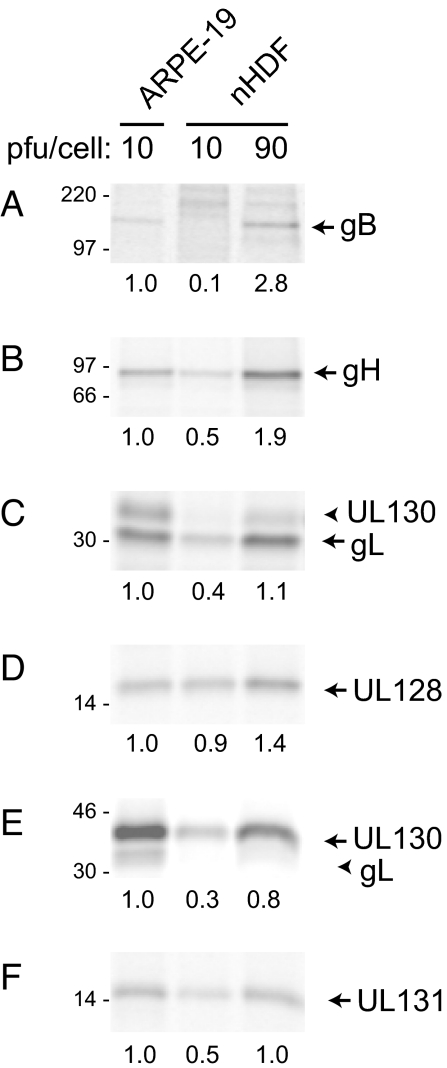
The level of HCMV proteins expressed by these Ad vectors can vary between cell types and could explain the lack of interference in fibroblasts. Therefore, we compared the expression of gB, gH, gL, UL128, UL130, and UL131 in fibroblasts and epithelial cells. When fibroblasts were transduced with 10 plaque forming units (PFU) per cell (as assessed on 293 cells), expression was generally lower compared with similarly transduced ARPE-19 epithelial cells (Fig. 3). However, when fibroblasts were transduced with 90 PFU per cell of each Ad vector, expression of HCMV proteins was generally equal to or greater than expression in epithelial cells infected with 10 PFU per cell. When interference experiments were repeated with the higher Ad vector doses in fibroblasts, there was no interference with gH/gL/UL 128-131, but some interference (40%) with gH/gL alone (Fig. 2C). From these data, we concluded that neither gB, nor gH/gL/UL128–131 interferes with HCMV infection of fibroblasts, and this is unlikely to relate to lower levels of expression.
Fig. 3.
Ad vector expression of gB and gH/gL/UL128–131 in epithelial cells and fibroblasts. ARPE-19 epithelial cells and primary human fibroblasts (nHDF) were transduced for 48 h with 10 or 90 PFU per cell of AdgB (A) or AdgH + AdgL + AdUL128 + AdUL130 + AdUL131 (B–F). Cells were labeled with [35S]-methionine/cysteine, and HCMV glycoproteins were immunoprecipitated and analyzed by SDS/PAGE. Note that gL and UL130 coprecipitate (C and E, arrowheads). Numbers below each gel indicate the relative band intensities based on phosphorimager analysis.
Because gH/gLUL128–131 also facilitates entry into endothelial cells as well as epithelial cells, similar experiments were conducted with human umbilical vessel endothelial cells and several other endothelial cell lines. Unfortunately, with all of these endothelial cell lines, we observed extensive cell rounding and detachment related to the combination of Ad vector infection and subsequent HCMV infection, which precluded analysis of interference (data not shown).
Expression of gH/gL/UL128–131 in Epithelial Cells Blocks HCMV Entry but Not Adsorption onto Cell Surfaces.
HCMV mutants lacking UL128–131 genes are able to adsorb onto the surfaces of epithelial and endothelial cells, but these particles do not enter cells (16, 20). To determine whether gH/gL/UL128–131 expression reduced the adsorption or initial binding of HCMV onto ARPE-19 epithelial cells, radiolabeled HCMV TR particles were incubated with cells for 1 h at 4°C. The amount of radioactivity that remained associated with the cells after several washes was determined as described (20). Adsorption of HCMV onto cells expressing gH/gL/UL128–131 was not different from that observed with cells expressing GFP (Fig. 4).
Fig. 4.
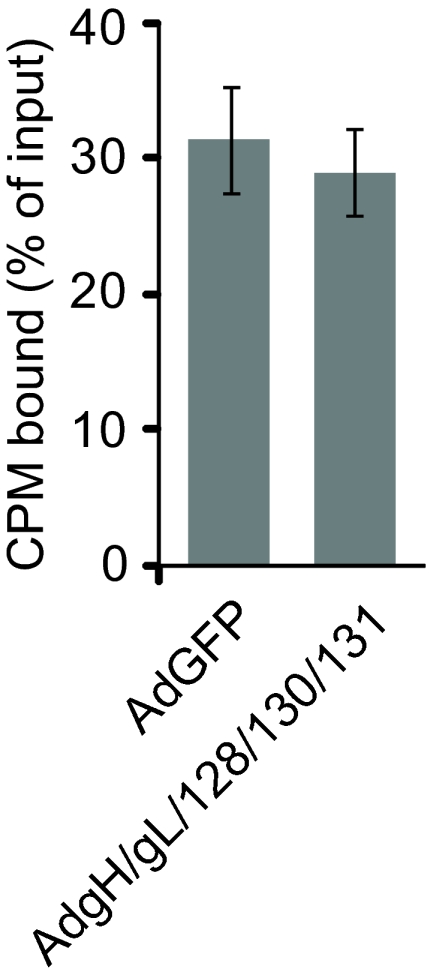
Adsorption of HCMV particles onto epithelial cells expressing gH/gL/UL128–131. ARPE-19 cells were transduced for 12 h with 50 PFU per cell of AdGFP or 10 PFU per cell of each of AdgH, AdgL, AdUL128, AdUL130, and AdUL131. Cells were incubated with purified 3H–thymidine-labeled HCMV TR particles (representing ≈0.4 PFU per cell) at 4°C for 2 h and washed extensively, and then radioactivity bound to cells was quantified. Shown is the average number of cell-associated radioactive counts (as a percentage of input) in three experiments. Error bars represent the standard deviation from the mean.
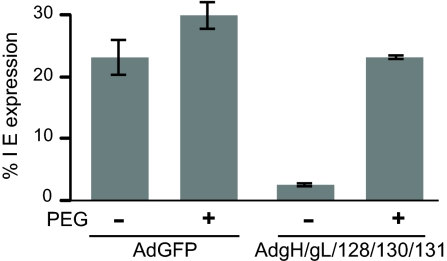
To determine whether gH/gL/UL128–131 expression blocked entry of HCMV into epithelial cells, cells were treated with polyethylene glycol (PEG), which can overcome defects in entry by causing fusion between closely apposed viral and cellular membranes. We showed that PEG treatment facilitated entry of HCMV UL128–131 mutants into epithelial and endothelial cells (16, 20). Here, ARPE-19 cells expressing gH/gL/UL128–131 or GFP were inoculated with HCMV TR at 4°C (to prevent endocytosis) and then exposed briefly to 44% PEG or PBS. In the absence of PEG treatment, <3% of the gH/gL/UL128–131-expressing cells became infected with HCMV. However, when gH/gL/UL128–131-expressing cells were treated with PEG, the number of HCMV IE-expressing cells was increased by 8-fold (Fig. 5). Note that IE expression was lower (24%) when HCMV was incubated with cells at 4°C compared with conditions in which HCMV is incubated with cells at 37°C (40%). Together, these data demonstrate that gH/gL/UL128–131 expression in ARPE-19 blocks HCMV entry without altering the adsorption onto cell surfaces.
Fig. 5.
Expression of gH/gL/UL128–131 blocks entry into epithelial cells. ARPE-19 cells were transduced for 12 h with 50 PFU per cell of AdGFP or 10 PFU per cell each of AdgH, AdgL, AdUL128, AdUL130, and AdUL131 and then incubated on ice with HCMV TR (3 PFU per cell) for 2 h. The cells were then warmed to 37°C, treated with either 44% PEG or PBS for 30 sec, washed extensively, and incubated for an additional 48 h at 37°C. The cells were fixed and stained for HCMV IE86 protein, and nuclei were stained with DAPI. Infected cells were quantified as described in Fig. 2. Results shown are representative of three separate experiments.
Discussion
The use of alternative entry mediators or receptors is emerging as a common theme among herpesviruses. Herpesvirus receptors are frequently cell-type specific and can also influence the mechanism of entry, whether by fusion at the cell surface or fusion with endosomal membranes (34–36). A well characterized example is that of Epstein–Barr virus (EBV) (reviewed in ref. 37). The EBV gH/gL complex can be modified by the addition of gp42. The gH/gL/gp42 complex binds to MHC class II molecules on the surface of B cells and facilitates entry through endocytosis, whereas unmodified gH/gL binds to an unidentified molecule on epithelial cells and facilitates fusion at the cell surface.
A recent important advance was made in attempts to understand HCMV entry with observations of two different gH/gL complexes: gH/gL/gO and gH/gL/UL128–131 (18). gH/gL/UL128–131 is required for entry into epithelial and endothelial cells, but not for fibroblast entry (14–18, 20). Here, we report that expression of gH/gL/UL128–131 in retinal epithelial cells interferes with HCMV infection. Minimal interference was observed when just gH/gL or UL128–131 were expressed, compared with expression of the full complex. These data provide further evidence that gH/gL/UL128–131 functions as a complex requiring all five proteins (16). Experiments involving radiolabeled virus particles and PEG treatment demonstrated that interference did not block virus adsorption onto cells but instead inhibited subsequent stages of HCMV entry into cells. Thus, gH/gL/UL128–131-mediated interference appears to be similar to the interference mediated by the HSV receptor-binding protein gD (28–30) and retrovirus interference involving common receptors (31). Our observations support the hypothesis that gH/gL/UL128–131 promotes entry into epithelial cells by binding receptors or entry mediators, molecules that can be masked, sequestered, or down-regulated by gH/gL/UL128–131 expression.
By contrast, expression of gH/gL/UL128–131 and gH/gL in primary fibroblasts did not interfere with HCMV entry, despite levels of expression similar to that in epithelial cells. These results suggest that fibroblasts do not express gH/gL/UL128–131 receptors or, perhaps, express other receptors in addition to gH/gL/UL128–131 receptors. Alternatively, it is possible that fibroblasts express significantly higher levels of gH/gL/UL128–131 receptors, so that saturation was not achieved, even at the relatively high doses of gH/gL/UL128–131 expressed by the Ad vectors. However, given that UL128–131 is not required for entry into fibroblasts, we favor the hypothesis that HCMV entry into fibroblasts involves viral receptor-binding proteins other than gH/gL/UL128–131 as well as distinct cellular receptors.
It is noteworthy that gH and gL are essential for replication in fibroblasts (38) and yet Ad vector expression of gH/gL, without UL128–131, did not substantially interfere with HCMV infection of fibroblasts. It is possible that HCMV entry into fibroblasts involves gH/gL/gO or other gH/gL complexes (decorated with other viral proteins) and fibroblast ligands specific to these gH/gL complexes. Unfortunately, we have been unable to study interference with gH/gL/gO to date, because we have been unable to construct Ad vectors expressing substantial quantities of gO. However, inactivating mutations in the UL128–131 genes can occur in as few as five passages on fibroblasts (39), and we have noted that HCMV TR mutants lacking UL128–150 or UL131 replicate more efficiently in fibroblasts than the wild type (M. Chase, unpublished results). Therefore, it is conceivable that the UL128–131 proteins compete for the assembly of gH/gL/gO or other fibroblast-specific gH/gL complexes. The loss of UL128–131 might increase the amounts of gH/gL/gO or other gH/gL complexes in the virion envelope and promote infection and spread in human fibroblasts.
It is clear that HCMV gO mutants are defective for replication and/or virus spread in fibroblasts; plaques are much smaller compared with wild type HCMV (38, 40). However, it is not clear whether gH/gL/gO complexes promote virus entry into fibroblasts. Recently, Jiang et al. (41) constructed a gO-null HCMV and characterized growth on fibroblasts and endothelial cells. In fibroblasts, the gO mutant exhibited defects in both envelopment and cell-to-cell spread. The defects in cell-to-cell spread were less pronounced in endothelial cells. It is possible that gH/gL/gO functions both in assembly and in fibroblast-to-fibroblast spread, by analogy with HSV gD and gE/gI that promote both secondary envelopment and cell-to-cell spread (42).
HCMV gB has been described as a receptor-binding protein for integrins (11) and for EGFR (9) with fibroblasts. In our experiments, expression of gB in either fibroblasts or epithelial cells did not cause interference. These results suggest that HCMV gB cannot bind to saturable entry mediators or cannot down-regulate these in either cell type, although other explanations are possible.
It is important to consider gH/gL/UL128–131-mediated interference in terms of how a virus enters epithelial cells. HCMV enters fibroblasts by fusion with the plasma membrane at neutral pH, but enters epithelial/endothelial cells by endocytosis followed by low pH-dependent fusion (20, 21). Thus, gH/gL/UL128–131 receptors might be concentrated in coated pits on epithelial cell surfaces or other structures taken up by endocytosis, whereas fibroblast receptors might be more evenly distributed on the cell surface. The derivation of HCMV may also influence receptor usage and routes of infection. Wang and Shenk (43) reported that HCMV produced by epithelial cells more extensively infected epithelial cells in the presence of lysosomotropic agents compared with HCMV derived from fibroblasts. However, anti-UL130 antibodies blocked epithelial-derived and fibroblast-derived HCMV, suggesting that gH/gL/UL128–131 was essential in both cases.
In summary, gH/gL/UL128–131 expression in epithelial cells effectively blocked HCMV entry, but this interference was not observed with fibroblasts. These observations provide strong evidence that gH/gL/UL128–131 receptors are important for entry into epithelial cells. Entry into fibroblasts apparently involves other receptor-binding proteins and receptors. Moreover, these epithelial gH/gL/UL128–131 receptors appear to be distinct from described HCMV receptors, EGFRs, and integrins, which are apparently bound by the gB of strains that lack gH/gL/UL128–131 (9, 11). The identification of these putative receptors will be required for further development of this model.
Materials and Methods
Cells.
Neonatal human dermal fibroblasts (nHDF) from Cascade Biologics were grown in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 12% FBS (HyClone). Retinal pigment epithelial cells (ARPE-19) were obtained from American Type Culture Collection and grown in a 1:1 mix of DMEM and Ham's F-12 medium (Invitrogen) supplemented with 10% FBS.
Human Cytomegaloviruses.
HCMV TR is a wild-type, clinical strain that was derived from the retina and was cloned into a bacterial artificial chromosome after limited passage on fibroblasts (44). Stocks were prepared by infecting nHDF cells by using 0.1 PFU per cell for 10–16 days. To enrich for HCMV particles, infected cells were sonicated, and large cellular debris was removed by centrifugation at 6000 × g for 15 min. Virus particles were then concentrated by centrifugation through a 20% sorbitol cushion at 50,000 × g for 1 h. Pellets were resuspended in DMEM plus 10% FBS and stored at −70°C. HCMV stocks were titered by plaque assay on nHDF cells. Inoculation of ARPE-19 cells was performed under centrifugal enhancement (20), and, where indicated, cells were treated with 44% PEG for 30 sec to promote entry.
Ad Vectors.
Nonreplicating (E1-) Ad vectors expressing HCMV gH, gL, UL128, UL130, or UL131 have been described (16). An Ad vector expressing HCMV TR strain gB was produced by the same methods. All Ad vectors expressed HCMV proteins from a promoter that was transactivated by a transcription factor supplied by coinfection with an Ad vector, Adtet-trans (16, 45).
Immunofluorescence.
HCMV- or HSV-infected cells were fixed with 2% formaldehyde, permeabilized by using PBS containing 0.5% Triton X-100 and 0.5% sodium deoxycholate, and then stained with rabbit polyclonal anti-HCMV IE86 antibody (R683) (20) or rabbit polyclonal anti-HSV VP5 antibody NC-1 (46), respectively, followed by Alexa Fluor 594-conjugated anti-rabbit antibodies. The relative numbers of infected cells were quantified by counting IE86+ or VP5+ cells versus nuclei stained with 0.4 μM 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI) as described (20).
Immunoprecipitation.
Ad-vector-transduced cells were dissociated from culture vessels and incubated in suspension with medium lacking methionine and cysteine for 1 h. Cells were then pelleted and resuspended in medium containing 500 μCi/ml [35S]-methionine/cysteine (Amersham). Cell extracts were made with Nonidet P-40 lysis buffer (0.5% Nonidet P-40 in Tris buffered saline supplemented with 1 mg/ml BSA and 1 mM phenylmethylsulfonyl fluoride), and clarified by sequential centrifugation at 1500 × g for 10 min and 100,000 × g for 30 min. Extracts were then precleared by incubation with mouse sera and protein A-agarose beads for 1–2 h. Immunoprecipitation involved the addition of ascites fluid or hybridoma supernatant for 2 h, followed by incubation with protein A-agarose for an additional 2 h. Quantification of precipitated protein was performed by phosphorimager analysis using a Thyphoon 9400 variable mode imager (GE Healthcare).
Purification of Radiolabled Virus Particles and Adsorption Assays.
Radiolabeled HCMV particles were purified from HCMV-infected nHDF incubated with 3H-thymidine (25 uCi/ml, Amersham) from 1 day until 6–9 days after infection as described (20). Radiolabeled virus particles were collected from culture supernatants. Cell debris was removed by sequential centrifugation at 800 × g for 25 min and 8000 × g for 30 min. Radiolabeled virus particles were then concentrated and purified by centrifugation at 50,000 × g for 1 h through a 20% sorbitol cushion. ARPE-19 cells were prechilled on ice, radiolabeled virus particles were applied, and cells were centrifuged at 800 × g for 2 h at 4°C. Unbound virus was removed by three washes with cold PBS. Then the cells were then scraped from wells, and radioactivity was quantified by liquid scintillation.
Acknowledgments.
We thank Bill Britt (University of Alabama School of Medicine, Birmingham, Alabama) for supplying important monoclonal antibodies; Tiffani Howard for graphics; and Michael Jarvis, Jay Nelson, and all of the members of the Johnson laboratory for advice and support. This work was supported by National Institutes of Health Grants AI055051 and EY11245 (to D.C.J.) and National Eye Institute Ruth L. Kirschstein National Research Service Award EY015965 (to B.J.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Britt WJ. In: Cytomegaloviruses: Biology and Immunology. Reddehase M, editor. Norfolk, UK: Caister Academic; 2006. pp. 1–28. [Google Scholar]
- 2.Pass RF. In: Fields Virology. Knipe DM, Howley PM, editors. Vol 2. Philadelphia: Lippincott Willliams & Davis; 2001. pp. 2675–2706. [Google Scholar]
- 3.Streblow DN, Orloff SL, Nelson JA. Acceleration of allograft failure by cytomegalovirus. Curr Opin Immunol. 2007;19:577–582. doi: 10.1016/j.coi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landolfo S, Gariglio M, Gribaudo G, Lembo D. The human cytomegalovirus. Pharmacol Ther. 2003;98:269–297. doi: 10.1016/s0163-7258(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 5.Sinzger C, et al. Fibroblasts, epithelial cells, endothelial cells, and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol. 1995;76(Pt 4):741–750. doi: 10.1099/0022-1317-76-4-741. [DOI] [PubMed] [Google Scholar]
- 6.Bentz GL, et al. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J Virol. 2006;80:11539–11555. doi: 10.1128/JVI.01016-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerna G, Baldanti F, Revello MG. Pathogenesis of human cytomegalovirus infection and cellular targets. Hum Immunol. 2004;65:381–386. doi: 10.1016/j.humimm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Compton T, Nowlin DM, Cooper NR. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, et al. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- 10.Isaacson MK, Feire AL, Compton T. Epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling. J Virol. 2007;81:6241–6247. doi: 10.1128/JVI.00169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feire AL, Koss H, Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci USA. 2004;101:15470–15475. doi: 10.1073/pnas.0406821101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Huang DY, Huong SM, Huang ES. Integrin αvβ3 is a coreceptor for human cytomegalovirus. Nat Med. 2005;11:515–521. doi: 10.1038/nm1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cha T, et al. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol. 1996;70:78–83. doi: 10.1128/jvi.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn G, et al. Human cytomegalovirus UL131–128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol. 2004;78:10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Shenk T. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol. 2005;79:10330–10338. doi: 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryckman BJ, et al. Characterization of the human cytomegalovirus gH/gL/UL128–131 complex that mediates entry into epithelial and endothelial cells. J Virol. 2008;82:60–70. doi: 10.1128/JVI.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adler B, et al. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J Gen Virol. 2006;87:2451–2460. doi: 10.1099/vir.0.81921-0. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci USA. 2005;102:18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber MT, Compton T. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J Virol. 1998;72:8191–8197. doi: 10.1128/jvi.72.10.8191-8197.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryckman BJ, et al. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol. 2006;80:710–722. doi: 10.1128/JVI.80.2.710-722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Compton T, Nepomuceno RR, Nowlin DM. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology. 1992;191:387–395. doi: 10.1016/0042-6822(92)90200-9. [DOI] [PubMed] [Google Scholar]
- 22.Rhim JS, et al. A human vascular endothelial cell model to study angiogenesis and tumorigenesis. Carcinogenesis. 1998;19:673–681. doi: 10.1093/carcin/19.4.673. [DOI] [PubMed] [Google Scholar]
- 23.Xu KP, Yu FS. Cross talk between c-Met and epidermal growth factor receptor during retinal pigment epithelial wound healing. Invest Ophthalmol Vis Sci. 2007;48:2242–2248. doi: 10.1167/iovs.06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spear PG, Longnecker R. Herpesvirus entry: An update. J Virol. 2003;77:10179–10185. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atanasiu D, et al. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc Natl Acad Sci USA. 2007;104:18718–18723. doi: 10.1073/pnas.0707452104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avitabile E, Forghieri C, Campadelli-Fiume G. Complexes between herpes simplex virus glycoproteins gD, gB, and gH detected in cells by complementation of split enhanced green fluorescent protein. J Virol. 2007;81:11532–11537. doi: 10.1128/JVI.01343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian RP, Geraghty RJ. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc Natl Acad Sci USA. 2007;104:2903–2908. doi: 10.1073/pnas.0608374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campadelli-Fiume G, Arsenakis M, Farabegoli F, Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988;62:159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson RM, Spear PG. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989;63:819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geraghty RJ, Jogger CR, Spear PG. Cellular expression of alphaherpesvirus gD interferes with entry of homologous and heterologous alphaherpesviruses by blocking access to a shared gD receptor. Virology. 2000;268:147–158. doi: 10.1006/viro.1999.0157. [DOI] [PubMed] [Google Scholar]
- 31.Sommerfelt MA, Weiss RA. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990;176:58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- 32.Boyle KA, Compton T. Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein B. J Virol. 1998;72:1826–1833. doi: 10.1128/jvi.72.3.1826-1833.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bender FC, et al. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J Virol. 2005;79:11588–11597. doi: 10.1128/JVI.79.18.11588-11597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller N, Hutt-Fletcher LM. Epstein–Barr virus enters B cells and epithelial cells by different routes. J Virol. 1992;66:3409–3414. doi: 10.1128/jvi.66.6.3409-3414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein–Barr virus. Nat Med. 2002;8:594–599. doi: 10.1038/nm0602-594. [DOI] [PubMed] [Google Scholar]
- 36.Delboy MG, Patterson JL, Hollander AM, Nicola AV. Nectin-2-mediated entry of a syncytial strain of herpes simplex virus via pH-independent fusion with the plasma membrane of Chinese hamster ovary cells. Virol J. 2006;3:105. doi: 10.1186/1743-422X-3-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutt-Fletcher LM. Epstein–Barr virus entry. J Virol. 2007;81:7825–7832. doi: 10.1128/JVI.00445-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn W, et al. Functional profiling of a human cytomegalovirus genome. Proc Natl Acad Sci USA. 2003;100:14223–14228. doi: 10.1073/pnas.2334032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akter P, et al. Two novel spliced genes in human cytomegalovirus. J Gen Virol. 2003;84:1117–1122. doi: 10.1099/vir.0.18952-0. [DOI] [PubMed] [Google Scholar]
- 40.Hobom U, et al. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: Mutational analysis of human cytomegalovirus envelope glycoprotein genes. J Virol. 2000;74:7720–7729. doi: 10.1128/jvi.74.17.7720-7729.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang XJ, et al. UL74 of human cytomegalovirus contributes to virus release by promoting secondary envelopment of virions. J Virol. 2008;82:2802–2812. doi: 10.1128/JVI.01550-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farnsworth A, Goldsmith K, Johnson DC. Herpes simplex virus glycoproteins gD and gE/gI serve essential but redundant functions during acquisition of the virion envelope in the cytoplasm. J Virol. 2003;77:8481–8494. doi: 10.1128/JVI.77.15.8481-8494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D, et al. Human cytomegalovirus uses two distinct pathways to enter retinal pigmented epithelial cells. Proc Natl Acad Sci USA. 2007;104:20037–20042. doi: 10.1073/pnas.0709704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy E, et al. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc Natl Acad Sci USA. 2003;100:14976–14981. doi: 10.1073/pnas.2136652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomazin R, et al. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat Med. 1999;5:1039–1043. doi: 10.1038/12478. [DOI] [PubMed] [Google Scholar]
- 46.Cohen GH, et al. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J Virol. 1980;34:521–531. doi: 10.1128/jvi.34.2.521-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]